Abstract
Nitrilase mediated synthesis of nicotinic acid (vitamin B3) from 3-cyanopyridine has emerged as promising viable alternative to its chemical synthesis. In the present investigation, the nitrilase production in Gordonia terrae MTCC8139 has been increased by two fold with inducer feeding approach [i.e. the addition of 0.5% (v/v) isobutyronitrile as inducer at 0, 16 and 24 h of incubation of the culture]. The use of hyper induced whole cell nitrilase of G. terrae as biocatalyst (10 U per ml reaction) to synthesize nicotinic acid from 3-cyanopyridine in a fed batch reaction at one litre scale resulted in accumulation of 1.65 M (202 g) nicotinic acid in 330 min. The catalytic productivity of hyper induced whole cell nitrilase was increased from 8.95 to 15.3 g/h/g dcw and the reaction time was reduced to half. This is the highest productivity of nicotinic acid in a nitrilase mediated process so far reported. The achievements of the present investigation will lead to mitigate the cost of nitrilase vis-a-vis nicotinic acid production at large scale.
Keywords: Gordonia terrae, Hyperinduction, Nicotinic acid, Whole cell nitrilase
Introduction
Nitrilases (EC 3.5.5.1) are enzymes that hydrolyze carbon nitrogen triple bond of cyano group of nitriles and transform nitrile into corresponding acids with the release of ammonia. These enzymes have been widely explored for the production of industrially valuable acids from nitriles (Lauder et al. 2020; Shen et al. 2021; Dai et al. 2022). Biocatalysts are enantio- and regioselective, operate under mild reaction conditions and thus have considerable benefits over energy-intensive chemical catalysts (Hauer, 2020; Wu et al. 2021). Nicotinic acid (vitamin B3 or niacin) is largely used in the food and pharmaceutical industries. It is a key component of medications to treat pellagra, cardiovascular diseases, cancer, diabetes and arthritis (Jin et al. 2013; Revuelta et al. 2016). In view of the ever-increasing demand for nicotinic acid, several chemical methods have been developed for its production. It is made chemically by the oxidation of nicotine, 3-methylpyridine and quinoline. These chemical processes require high temperature and pressure for reaction and results in substantial volume of undesired by-products that adds to the cost and time to downstream processing and effluent treatment. Furthermore, chemical catalysts are not 100 percent efficient. Efforts have been made in the past to find alternatives to chemical processes for the production of nicotinic acid (Chunk 2005; Jin et al. 2013). Enzymatic process that uses nitrilase to convert 3-cyanopyridine to nicotinic acid has been developed as an alternative to chemical routes of nicotinic acid production.
Nitrilases from Rhodococcus rhodochrous J1 (Mathew et al. 1988), Nocardia globerula NHB-2 (Sharma et al. 2006), Fusarium proliferatum ZJB-09150 (Jin et al. 2013), Gibberella intermedia CGMCC 4903 (Wu et al. 2013) and Alcaligenes faecalis MTCC 126 (Pai et al. 2014), Stenotrophomonas maltophilia AC21 (Badoei-Dalfard et al. 2016), Acidovorax facilis 72 W (Li et al. 2017) have been reported to hydrolyze 3-cyanopyridine to nicotinic acid. The enzymatic production of nicotinic acid is a more efficient and environmentally friendly as compared with chemical processes (Fig. 1). However, limited substrate tolerance, low product yield and slower conversion rates are some drawbacks of the nitrilase mediated process of nicotinic acid production. These limitations need to be addressed to make the enzymatic process cost effective. Hyperactive biocatalysts are necessary to overcome some of these constraints. Multiple feeding of culture with inducer (inducer feeding) during cultivation of microorganism for the production of the enzyme has been reported to enhance the production of the nitrillases (Sharma et al. 2011; Kumar et al. 2015; Thakur et al. 2018). In the present study, the inducer feeding approach was used to enhance the expression of nitrilase in Gordonia terrae MTCC 8139 and develop an improved process in terms of product yield and process time for the production of nicotinic acid from 3-cyanopyridine using the hyper induced whole cell nitrilase of G. terrae as biocatalyst. Most of the studies on nitrilase mediated production of nicotinic acid have focussed on upstream of the process and very little information is on separation and purification of the product from the reaction mixture (Badoei-Dalfard et al. 2016; Dong et al. 2017). In the present investigation an attempt has been also made to develop a simple downstream process for the recovery of nicotinic acid with high purity.
Fig. 1.
Nitrilase mediated synthesis of nicotinic acid from 3-cyanopyridine
Materials and methods
Chemicals and media components
The nitriles, acids and other compounds used as inducer/s, substrate/s in the enzyme reaction or product assay were procured from Sigma (United States) and Lancaster (England). The bacterial culture medium ingredients used in this investigation were acquired from Hi Media, India. HPLC (High Performance Liquid Chromatography) reagents were from Merck, India.
Bacterial strain and cultivation conditions
G. terrae MTCC 8139 (previously known as Rhodococcus sp. NDB 1165) was obtained from RL-II, Department of Biotechnology, Himachal Pradesh University, Shimla-171005, India and maintained on nutrient agar as described previously by Prasad et al (2007). The organism was cultivated according to Kumar and Bhalla (2013) for the production of nitrilase.
Nitrilase assay
The nitrilase activity of G. terrae resting cells was measured in 1 ml reaction containing 0.1 M potassium phosphate buffer (pH 8.0), 50 mM 3-cyanopyridine and whole cell nitrilase [equivalent to 0.25 mg dry cell weight (dcw)]. It was incubated at 40 °C for 15 min and the reaction was quenched with the addition of 1 ml of 0.1 N HCl. Ammonia produced by the nitrilase catalyzed hydrolysis of nitrile (3-cyanopyridine) was estimated according to the method of Fawcett and Scott (1960). The amount of the whole cell nitrilase (mg dcw) necessary to release 1 µmol of ammonia per min under the assay conditions was defined as one unit of nitrilase activity.
HPLC analysis of substrate and product
High performance liquid chromatography (HPLC) system (Shimadzu Corporation, Japan) was used to detect and quantify substrate (3-cyanopyridine) and product (nicotinic acid) using acetonitrile:water (65:35) as a mobile phase on a C18 column (250 × 4.6 mm) at 210 nm at a flow rate of 1 ml/min.
Screening of inducers for nitrilase induction in G. terrae
Nitriles (acetonitrile, acrylonitrile, propionitrile, isobutyronitrile, benzonitrile, 3-cyanopyridine, mandelonitrile) at a concentration of 0.4% (v/v) were added into culture medium (pH-8.0, yeast extract 5.0 g/L, peptone 5.0 g/L, K2HPO4 5.0 g/L, KH2PO4 2.0 g/L, MgSO4.7H2O 0.2 g/L, FeSO4.7H2O 0.03 g/L, CaCl2.2H2O 0.06 g/L, NaCl 1.0 g/L, glucose 10 g/L) at 0 h of incubation. These were incubated at 30 °C at 150 rpm for 40 h with control (without inducer). The biomass generation (dcw/ml) and nitrilase activity (U/mg dcw) of G. terrae in each case were measured.
Hyper induction of nitrilase with isobutyronitrile
To determine the optimum time and concentration of the inducer for hyper induction of nitrilase in G. terrae, the isobutyronitrile [0.1–1% (v/v)] was added in the production medium at 0, 16 and 24 h of incubation and the bacterial cell mass and the enzyme activity were measured at an interval of 8 h from 32 to 56 h of incubation.
Bioprocess development for bench-scale nicotinic acid synthesis from 3-cyanopyridine using hyper induced whole cell nitrilase of G. terrae
Time course of nicotinic acid formation with concomitant increase in whole cell nitrilase and 3-cyanopyridine concentration
Under the optimum reaction conditions at 1 ml scale (0.1 M potassium phosphate buffer, pH 8.0 and temperature 40 °C), the effect of increasing the quantity of whole cell nitrilase with concomitant increase in substrate concentration on the product formation was studied with following combinations of whole cell nitrilase (U/mL) and substrate (3-cyanopyridine) concentration (mM): 6 U/mL: 75 mM, 8 U/mL: 100 mM, 12 U/mL: 125 mM, and 16 U/mL: 150 mM.
Effect of whole cell enzyme loading
The whole cell nitrilase load was varied from 1.5 to 3.0 mg dcw equivalent to 6 U, 8 U, 10 U and 12 U of nitrilase per ml reaction at 75 mM substrate concentration to determine the optimum load of the biocatalyst for complete conversion of substrate to product in a possible short time. The reaction was carried out for 30 min at 40 °C in 0.1 M potassium phosphate buffer (pH 8.0) and the amount of substrate and product in the reaction was estimated by HPLC.
Fed batch reaction at 50 ml scale
A fed batch reaction at 50 ml scale in 250 ml Erlenmeyer flask was carried out under the reaction conditions optimized at 1 ml reaction scale. The substrate (75 mM) 3-cyanopyridine was fed at an interval of 15 min while the whole cell nitrilase in the reaction was 10 U/ml. It was incubated in a water bath shaker at 40 °C and 200 rpm. A total of 2 M substrate in 27 feeding over a period of 405 min was added and the accumulation of nicotinic acid was assessed through HPLC.
Fed batch reaction at 1L scale
Based on optimized process parameters at 50 ml reaction, the nicotinic acid production was carried out at 1L bench scale in a Bioflow C-32 fermenter (New Brunswick Scientific, USA). Twenty-two feedings of 75 mM substrate were given at an interval of 15 min over a period of 330 min.
Downstream processing of nicotinic acid
The cells were removed after the completion of fed batch reaction by centrifugation at 10,000g for 10 min. The obtained supernatant was collected and its pH was lowered to 4–5 pH by slow addition of concentrated HCl and steady stirring. As soon as the crystals of nicotinic acid started to appear in the mixture, it was put on ice for maximum precipitation of the nicotinic acid (Sharma et al. 2006). Using a vacuum pump, the precipitates were filtered through Whatman No. 1, dried in oven at 70 °C and the nicotinic acid recovered was weighed. The cells separated from the reaction were washed thrice with potassium phosphate buffer (0.1 M, pH 8.0) to remove the traces of product and their remaining nitrilase activity was assayed.
Instrumentation and characterization
The recovered product was analysed by HPLC. It was also characterised by fourier transfer infrared (FTIR) and nuclear magnetic resonance (NMR) spectroscopy at Panjab University, Chandigargh, India. FTIR spectra were obtained using Perkin Elmer FTIR spectrometer ranging from 400 to 4000 cm−1. 1D 1H and 13C NMR were taken in dimethyl sulfoxide (DMSO) using Bruker Avance Neo NMR spectrometer at 500 MHz.
Statistical analysis
All experiments were carried out in triplicates and average values were calculated. Error bar in figures represent the standard deviation from mean values. The obtained data was examined on Microsoft Excel, 2010 and SPSS Version 21.0 by applying one way ANOVA to assess the differences in mean and statistical significant differences amongst the attained average values established at p ≤ 0.05 and Dunnett’s post hoc test.
Results
Inducer feeding approach for enhancing nitrilase production
Several aliphatic, aromatic and aryl nitriles were screened as inducer for the nitrilase production and growth (biomass yield) of G. terrae and the results are shown in Table 1. Among these, isobutyonitrile induced highest nitrilase activity in the cells (2.2 U/mg dcw) vis-a-vis biomass yield of 3 mg/ml of the culture, whereas acetonitrile, propionitrile, benzonitrile and 3-cyanopyridine induced 1.5 U/mg dcw, 1.9 U/mg dcw, 1.3 U/mg dcw and 1.6 U/mg dcw nitrilase syntheses, respectively in this organism. No enzyme activity was found in the control (without inducer), indicating that this organism's nitrilase is inducible in nature. However, G. terrae's growth was entirely suppressed by acrylonitrile and mandalonitrile (Table1). This microorganism's stationary phase was delayed from 24 to 40 h after an inducer was added to the culture medium. Since isobutyronitrile proved to be a good inducer of nitrilase and biomass production in G. terrae, its concentration in the culture medium was changed from 0.1 to 1% (v/v) at 0, 16 and 24 h of incubation, respectively, to find the most appropriate concentration and time of inducer feeding for maximum of the enzyme in this bacterium. Two fold increase in whole cell nitrilase activity (4.5 U/mg dcw) of G. terrae was observed with 0.5% (v/v) isobutyronitrile (Table 2).
Table 1.
Screening of nitriles as an inducer for the production of nitrilase by G. terrae
| Nitrile | Inducer | Growth (mg/ml dcw) | Specific activity (U/mg dcw) |
|---|---|---|---|
| Aliphatic nitrile | Acetonitrile | 2.6 | 1.5 |
| Acrylonitrile | NG | – | |
| Propionitrile | 2.8 | 1.9 | |
| Isobutyronitrile | 3.0 | 2.2 | |
| Aromatic nitrile | Benzonitrile | 2.2 | 1.3 |
| Heterocyclic nitrile | 3-Cyanopyridine | 2.9 | 1.6 |
| Arylacetonitrile | Mandelonitrile | NG* | – |
| Control | No | 3.5 | – |
NG no growth
Table 2.
Effect of inducer (isobutyronitrile) concentration on the production of nitrilase by G. terrae at different incubation time periods
| Inducer concentration (%, v/v) at 0, 16 and 24 h | Nitrilase activity (U/mg dcw) at different incubation time | |||
|---|---|---|---|---|
| 32 h | 40 h | 48 h | 56 h | |
| 0.1 | 1.4 | 2.6 | 1.42 | 0.34 |
| 0.2 | 1.54 | 2.9 | 1.5 | 0.44 |
| 0.3 | 1.62 | 3.2 | 1.76 | 0.52 |
| 0.4 | 2.2 | 3.9 | 1.8 | 0.69 |
| 0.5 | 2.5 | 4.5 | 1.94 | 0.5 |
| 0.6 | 2.1 | 2.95 | 1.6 | 0.42 |
| 0.7 | 1.5 | 2.1 | 1.68 | 0.38 |
| 0.8 | 1.2 | 2.8 | 1.32 | 0.25 |
| 0.9 | 0.5 | 0.8 | 0.1 | 0.04 |
| 1.0 | 0.02 | 0.03 | 0.02 | 0.01 |
Bold values represent the maximum nitrilase activity at different incubation time with 0.5% inducer concentration
Bioprocess development
The nitrilase activity of G.terrae was maximum in 0.1 M potassium phosphate buffer of pH 8.0 at 40 °C with 50 mM 3-cyanopyridine. The enzyme activity was stable at 40 °C up to 14 h. Substrate and product inhibitions were encountered and complete inhibition of the nitrilase was noted at 500 mM 3-cyanopyridine in the reaction (not shown here). Based on these operational features of whole cell nitrilase of G. terrae, the bioprocess parameters for the synthesis of nicotinic acid at bench scale were optimized.
Whole cell nitrilase and 3-cyanopyridine ratio
The effect of increasing whole cell nitrilase load and substrate concentrations on nicotinic acid synthesis revealed that a reaction including 6 U/ml whole cell nitrilase and 75 mM 3-cyanopyridine had a high conversion rate (Fig. 2A).This experiment also indicated that a proportional increase in biocatalyst amount with substrate did not result in a corresponding higher conversion of substrate to product due to nitrilase inhibition caused by excess (> 75 mM) substrate loading.
Fig. 2.

Effect of A 3-cyanopyridine concentration and whole cell nitrilase load with time on accumulation of nicotinic acid B whole cell nitrilase load on the formation of nicotinic acid in the reaction containing 75 mM 3-cyanopyridine
Effect of whole cell nitrilase concentration
The rate of product (nicotinic acid) formation rose as the amount of whole cell biocatalyst increased (6 U/ml-12 U/ml) and the incubation duration was reduced. In 15 min at 40 °C with 10 U of whole cell nitrilase of G. terrae (per ml reaction), complete conversion of 75 mM 3-cyanopyridine was observed. Loading of higher amount of the enzyme (> 10 U/ml reaction) shortened the reaction time for complete conversion of added substrate to product (Fig. 2B).
Fed batch at 50 ml scale
The fed batch reaction at 50 ml scale with 10 U of whole cell nitrilase/ml reaction and 75 mM 3-cyanopyridine per feed resulted in complete conversion of 3-cyanopyridine up to 22 feeds after that substrate started to accumulate. After 27 feeds, status of product accumulation remained same i.e. 1700 mM nicotinic acid with 300 mM 3-cyanopyridine as such in the reaction (Fig. 3A). This revealed that the whole cell nitrilase of G. terrae is completely inhibited by product concentration beyond 1700 mM nicotinic acid.
Fig. 3.

Synthesis of nicotinic acid using 2.5 mg resting cells of G. terrae (per ml reaction) with feeding of 75 mM 3-cyanopyridine at an interval of 15 min in A 50 ml and B 1L fed batch reaction
Bench scale production of nicotinic acid
Based on the results of optimization of process parameters at 50 ml reaction, the conversion of 3-cyanopyridine to nicotinic acid was carried out at 1 L scale with 10 U of whole cell nitrilase of G. terrae (per ml of reaction) in 0.1 M of potassium phosphate buffer (pH 8.0) at 40 °C. A total of 1650 mM 3-cyanopyridine was fed in 22 feedings each at an interval of 15 min was completely converted to 202 g nicotinic acid in 330 min (Fig. 3B). The catalytical and volumetric productivity were calculated to be 15.3 g/h/g dcw and 202 g/L, respectively. The residual nitrilase activity of cells recovered after the reaction was 85% of the initial activity. The downstream processing of the reaction mixture for the recovery of nicotinic acid yielded a white pulverized product (Fig. 4).
Fig. 4.
Downstream process for recovery of nicotinic acid
Instrumental characterization of nicotinic acid
HPLC analysis
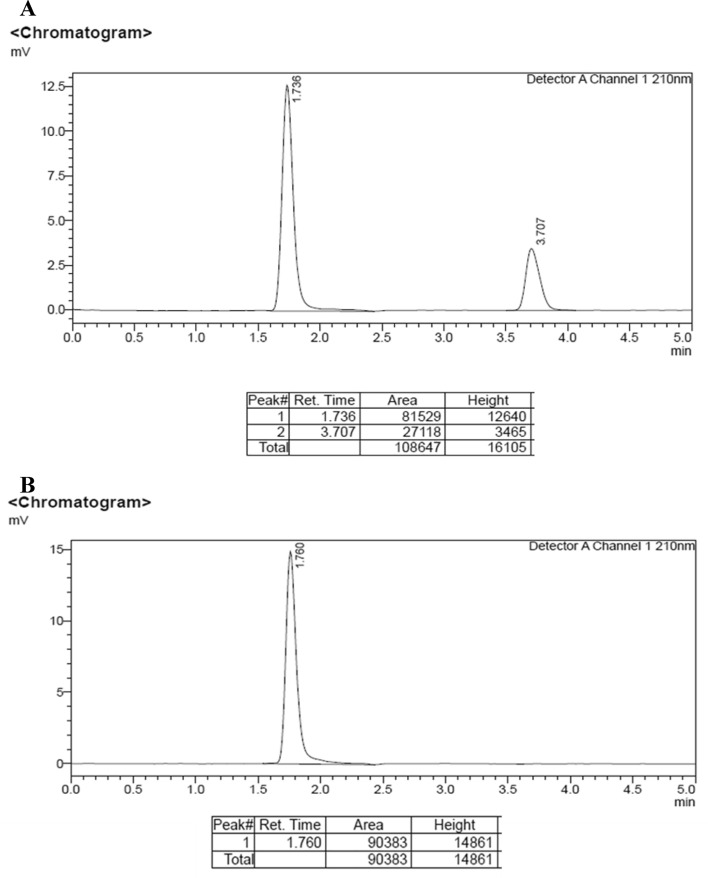
HPLC analysis of the product revealed it to be pure nicotinic acid (Fig. 5B) as compared with the standard peaks of substrate (3-cyanopyridine) and product (nicotinic acid) (Fig. 5A). The retention times (RT) of recovered product coincide with that of standard nicotinic acid (purchased from Sigma-Aldrich).
Fig. 5.
HPLC chromatogram of A standard of nicotinic acid (0.3 mM) and 3-cyanopyridine (0.3 mM), B the recovered product (RT 1.76)
FTIR analysis
The recovered product was identified as nicotinic acid and its purity was confirmed by FTIR. FTIR spectra were obtained using Perkin Elmer FTIR spectrometer ranging from 400 to 4000 cm−1 for recovered product and standard nicotinic acid (Sigma-Aldrich, USA) and compared. In the FTIR spectra of formed product showed the same pattern of peaks as that of standard nicotinic acid (Fig. 6A), confirming the formation of nicotinic acid with high purity. FTIR spectra of compound exhibited absorption bands at 2815–3076 cm−1 due to C–H stretching vibrations followed by the presence of vibrational peaks at 1866 cm−1 (combination bands of pyridine ring), 1693 cm−1 (C = O asymmetric stretching of COO–), 1597 cm−1 (C = C stretching), 1418 cm−1 (C = N stretching of pyridine ring), 1319 cm−1 (C = O symmetric stretching of COO–), 1290–1300 cm−1 (C–N stretching of pyridine ring), 1170–1120 cm−1 (C–O stretching of COO–), 1077–1032 cm−1 (C–H in plane bending vibrations) and 637–810 cm−1 (C–H out of plane bending vibrations) respectively. Absence of absorption peaks due to O–H stretching and bending vibrations indicated presence of acidic group as carboxylate anion (COO–), presence of which also supported by 1H NMR spectra.
Fig. 6.
Instrumental characterisation A FTIR spectrum of standard nicotinic acid (NAS) and the recovered product (NAP), B 13C and C 1H NMR spectra of nicotinic acid in DMSO
NMR analysis
13C NMR spectra of nicotinic acid showed peak at δ 166.26 ppm due to carboxylic carbon (C6) followed by the presence of peaks assigned to ring carbons of pyridine in the upfield region (i.e. lesser chemical shift) (Fig. 6B). The carbon atoms near to N-atom i.e. C5 (δ 153.12 ppm) and C1 (δ 150.17 ppm) absorbed more downfield in comparison to other ring C-atoms having peaks assigned at C3 (δ 136.91 ppm), C2 (δ 126.70 ppm) and C4 (δ 123.71 ppm) respectively. The absence of absorption peak in the region around δ 117 ppm due to –CN group suggested the complete conversion of substrate (3-cyanopyridine) to its enzymatic product nicotinic acid.
1H NMR spectra of nicotinic acid showed four NMR signals of aromatic protons of pyridine ring (Fig. 6C). Out of these four protons, signal of H(b) (δ 9.1 ppm) observed downfield as compared to other ring protons {H(c) (δ 8.8 ppm), H(d) (δ 8.3 ppm) and H(e) (δ 7.5 ppm)} and signal due to carboxylic proton H(a) either fall out of scale of given spectra or absent due to average view of H(a) in ionized state (COO-) of nicotinic acid. The four different signals of corresponding ring protons of heterocyclic ring indicated different chemical environment (non-equivalent) for aromatic protons [H (b, c, d & e)] and which suggested presence of substituted pyridine ring (–COOH for nicotinic acid) at position 3 with respect to N-atom.
Discussion
The main objective of the present investigation was to enhance the nitrilase production in G. terrae with periodic inducer feeding approach and to use the hyperinduced whole cell nitrilase to curtail process time for the conversion of 3-cyanopyridine to nicotinic acid. In contrast to the constitutive nitrilase of Klebsiella ozaenae (Stalker et al. 1988), Bacillus subtilis ZJB-063 (Zheng et al. 2008) and Alcaligenes sp. ECU0401 (Zhang et al. 2011), the nitrilase of G. terrae is inducible. Among the several aliphatic, aromatic and aryl nitriles tested as inducer, isobutyronitrile proved to be a very good inducer for the production of nitrilase by G. terrae. Similar observations were reported for nitrilase production in R. rhodochrous PA-34 (Bhalla et al. 1992), N. globerula NHB-2 (Sharma et al. 2011) and A. faecalis (Thakur et al. 2018). The growth of G. terrae was slowed in the presence of some nitriles as inducer in the production medium as compared to growth of the bacterium in the medium without inducer (Table 1). This may be due to some toxic effect of nitriles. The nitrilase of Nocardia globerula NHB-2 and A. faecalis followed a similar pattern (Sharma et al. 2011; Thakur et al. 2018). Literature survey reveals that nitrilases have little affinity towards some nitriles e.g. isobutyronitrile and isovaleronitrile and are poor substrates of these enzymes (Kobayashi et al. 1991; Kumar et al. 2015; Thakur et al. 2018). However, such nitriles turn out to be very effective inducers for nitrilases. Isobutyronitrile feeding at various time intervals resulted in increased nitrilase production from G. terrae and maximum level of nitrilase expression was attained in 40 h as compared with R. rhodochrous J1 (120 h, ε-caprolactam) (Nagasawa et al. 1990), R. rhodochrous K22 (120 h, isovaleronitrile) (Kobayashi et al. 1991), N. globerula NHB-2 (30 h, isobutyronitrile) (Sharma et al. 2006), G. terrae mutant E9 (36 h, isobutyronitrile) (Kumar et al. 2015), Alcaligenes sp. MTCC 10,675 (24 h, isobutyronitrile) (Bhatia et al. 2013) and A. faecalis MTCC 12,629 (21 h, isobutyronitrile) (Thakur et al. 2018). Inducer feeding approach in the present investigation resulted in two fold increase in nitrilase production in G. terrae while this strategy in earlier studies could enhance 1.1, 1.5, 1.2 and 1.2 folds nitrilase activity in P. putida, Alcaligenes sp. MTCC 10,675, G. terrae mutant E9 and A. faecalis MTCC 12629, respectively (Banerjee et al. 2006; Bhatia et al. 2013; Kumar et al. 2015; Thakur et al. 2018). Since inducers are poor substrates of nitrilases, therefore to utilize such nitriles as carbon and /or nitrogen source, the organism produces more nitrilase to hydrolyse inducer and use the products (ammonia and corresponding organic acid) for growth. As a result of this, the inducer level in the medium depletes and the enzymes production also decreases. Therefore, periodic feeding of culture with inducer maintains the required level of inducer in the culture medium for the organism to continuously produce enzyme which increases the production of enzyme as compared to experiments with one time addition of inducer in the culture.
It has been widely documented that activities of nitrilases are inhibited at higher concentration of substrate or product in the reaction (Prasad et al. 2007; Raj et al. 2007; Sharma et al. 2011; Kumar et al. 2015; Thakur et al. 2018). To address this limitation, the nitrilase mediated conversion of 3-cyanopyridine to nicotinic acid has been carried out in fed batch mode of reaction (Prasad et al. 2007; Sharma et al. 2011; Cantarella et al. 2012; Fan et al. 2017). Amount of whole cell nitrilase, substrate concentration per feed and time interval of substrate feed are important parameters which determine the process time to achieve maximum accumulation of nicotinic acid in fed batch reaction. As 3-cyanopyridine is added to the reaction it enters the cell and converted into nicotinic acid by the intracellular nitrilase and the nicotinic acid so formed diffuses back to the reaction mixture. The 3-cyanopyridine concentration at which nitrilase activity is inhibited varies with the source of nitrilase. Nitrilases from N. globerula NHB-2 (Sharma et al. 2011), F. proliferatum ZJB-09150 (Jin et al. 2013), S. maltophilia AC21 (Badoei-Dalfard et al. 2016) are inhibited at > 50 mM, > 60 mM and > 70 mM 3-cyanopyridine in the reaction, respectively. However, the whole cell nitrilase of G. terrae could tolerate 75 mM concentration of 3-cyanopyridine in the reaction for the transformation of 3-cyanopyridine to nicotinic acid. The use of hyper induced cells (that contained more enzyme/unit of dry cell mass) in the reaction shortened the substrate feed interval time resulting in curtailing reaction time from 660 to 330 min to achieve the maximum accumulation of nicotinic acid in the fed batch reaction. Increase in the product yield and decrease in the process time will reduce the cost of production of nicotinic acid. The hyper induced whole cell nitrilase of G. terrae yielded 202 g (1.65 M) nicotinic acid in 330 min with a catalytical productivity of 15.3 g/h/g dcw at 1 L scale of reaction. There was 71% increase in the catalytical productivity. In a previous study from this organism (then known as Rhodococcus sp. NDB 1165) 1.6 M 3-cyanopyridine was converted in to nicotinic acid using 2.0 mg dcw resting cells/ml reaction in 11 h with a catalytical productivity of 8.95 g/h/g dcw (Prasad et al. 2007). The productivitiy of nitrilases for nicotinic acid synthesis has been reported to be 172 g/L, 123.11 g/L, 116.2 g/L, 133 g/L from R. rhodochrous Jl (Mathew et al. 1988), hyper induced N. globerula NHB-2 (Sharma et al. 2011), S. maltophilia (Badoei-Dalfard et al. 2016) and Pseudomonas putida (Dong et al. 2017), respectively. To the best of our information, the present investigation reports highest production of nicotinic acid (202 g) among the nitrilase mediated processes at 1 L reaction.
The downstream process described above for the purification of the product from the reaction mixture is simple and straightforward. It involved solid (cells)-liquid separation by centrifugation and the product precipitation from the reaction milieu (supernatant) by decreasing its pH to isoelectric point of the nicotinic acid. Lowering its temperature by putting it on ice further enhanced and expedited the precipitation of the product. The final recovery of nicotinic acid by filtration in the present investigation is one of the simple methods for separation of solid–liquid in downstream processing of most of the fermentation products (Stanbury et al. 2016). Although the inducer feeding approach resulted in enhanced production of nitrilase activity/ml culture or per mg dry cell mass of G. terrae and use of such whole cell nitrilase improved the process in terms of reducing reaction time from 11 to 5 h 30 min yet substrate and product inhibition of nitrilase are the main limitations of the presently available nitrilases in reducing cost of nicotinic acid production. To achieve still higher production of nicotinic acid in nitrilase catalyzed processes, researchers need to focus on protein engineering, mutagenesis and metagenomic approaches to develop nitrilases that are not prone to inhibition by higher concentration of substrate or product; or engineer enzyme reaction system for continuous removal of product formed in the reaction.
Conclusion
Inducer feeding approach has enhanced the nitrilase production in G. terrae and this simple physiological intervention may find application to improve the production of other inducible microbial enzymes. The use of hyper induced whole cell nitrilase of G. terrae for the conversion of 3-cyanopyridine to nicotinic acid has reduced the time of the enzymatic reaction to half. The downstream process developed for recovery of nicotinic acid is simple and yielded the pure product. These are significant technological improvements of the process for the production and purification of nicotinic acid. However, to enhance volumetric yield of the product and make this process more economical, the present nitrilases need to be engineered such that these become free of substrate or product inhibition and operate under high concentration of 3-cyanopyridine and nicotinic acid in the reaction.
Acknowledgements
The authors gratefully acknowledge the fellowship grants from Indian Council of Medical Research (ICMR), New Delhi, India (F. No. 3/1/3/JRF-2017/HRD-LS/50606/02), Department of Biotechnology (DBT), Ministry of Science and Technology, New Delhi, India (F. No. BT/PR7262/BID/7/431/2013) and University Grants Commission (UGC), New Delhi, India (F. No. 18-1/2011 (BSR)/24th Feb 2014) to Monika, Sheetal and Tek Chand Bhalla, respectively.
Declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical approval
This article does not include any studies with human participants or animals.
References
- Badoei-Dalfard A, Karami Z, Ramezani-pour N. Bench scale production of nicotinic acid using a newly isolated Stenotrophomonas maltophilia AC21 producing highly-inducible and versatile nitrilase. J Mol Catal B Enzym. 2016;133:S552–S559. doi: 10.1016/j.molcatb.2016.11.019. [DOI] [Google Scholar]
- Banerjee A, Kaul P, Banerjee U. Purification and characterization of an enantioselective arylacetonitrilase from Pseudomonas putida. Arch Microbiol. 2006;184:407–418. doi: 10.1007/s00203-005-0061-9. [DOI] [PubMed] [Google Scholar]
- Bhalla TC, Miura M, Wakamoto A, Ohba A, Furuhashi K. Asymmetric hydrolysis of α-aminonitriles to optically active amino acids by a nitrilase of Rhodococcus rhodochrous PA-34. Appl Microbiol Biotechnol. 1992;37:84–190. doi: 10.1007/BF00178168. [DOI] [Google Scholar]
- Bhatia SK, Mehta PK, Bhatia RK, Bhalla TC. Optimization of arylacetonitrilase production from Alcaligenes sp. MTCC 10675 and its application in mandelic acid synthesis. Appl Microbiol Biotechnol. 2013;98:83–94. doi: 10.1007/s00253-013-5288-9. [DOI] [PubMed] [Google Scholar]
- Cantarella M, Cantarella L, Gallifuoco A, Spera A, Martinkova L. Nicotinic acid bio-production by Microbacterium imperiale CBS 489–74: effect of 3-cyanopyridine and temperature on amidase activity. Process Biochem. 2012;47:1192–1196. doi: 10.1016/j.procbio.2012.03.010. [DOI] [Google Scholar]
- Chuck R. Technology development in nicotinate production. Appl Catal A Gen. 2005;280:75–82. doi: 10.1016/j.apcata.2004.08.029. [DOI] [Google Scholar]
- Dai AD, Wu ZM, Zheng RC, Zheng YG. Constitutive expression of nitrilase from Rhodococcus zopfii for efficient biosynthesis of 2-chloronicotinic acid. 3Biotech. 2022;12:50–57. doi: 10.1007/s13205-022-03119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong TT, Gong JS, Gu BS, Zhang Q, Li H, Lu ZM, Lu ML, Shi JS, Xu ZH. Significantly enhanced substrate tolerance of Pseudomonas putida nitrilase via atmospheric and room temperature plasma and cell immobilization. Bioresourc Technol. 2017;244:1104–1110. doi: 10.1016/j.biortech.2017.08.039. [DOI] [PubMed] [Google Scholar]
- Fan H, Chen L, Sun H, Wang H, Ren Y, Wei D, Fan H, et al. A novel nitrilase from Ralstonia eutropha H16 and its application to nicotinic acid production. Bioprocess Biosyst Eng. 2017;40:1271–1281. doi: 10.1007/s00449-017-1787-x. [DOI] [PubMed] [Google Scholar]
- Fawcett JK, Scott JE. A rapid and precise method for determination of urea. J Clin Pathol. 1960;13:156–159. doi: 10.1136/jcp.13.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauer B. Embracing nature’s catalysts: a viewpoint on the future of biocatalysis. ACS Catal. 2020;10:8418–8427. doi: 10.1021/acscatal.0c01708. [DOI] [Google Scholar]
- Jin LQ, Liu ZQ, Xu JM, Zheng YG. Biosynthesis of nicotinic acid from 3-cyanopyridine by a newly isolated Fusarium proliferatum ZJB-09150. World J Microbiol Biotechnol. 2013;29:431–440. doi: 10.1007/s11274-012-1195-y. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Yanaka N, Nagasawa T, Yamada H. Hpyerinduction of an aliphatic nitrilase by Rhodococcus rhodochrous K22. FEMS Microbiol Lett. 1991;77:121–124. doi: 10.1111/j.1574-6968.1991.tb04333.x. [DOI] [Google Scholar]
- Kumar V, Bhalla TC. Transformation of p-hydroxybenzonitrile to p-hydroxybenzoic acid using nitrilase activity of Gordonia terrae. Biocatal Biotransform. 2013;31:42–48. doi: 10.3109/10242422.2012.757761. [DOI] [Google Scholar]
- Kumar V, KumarV TN, Bhalla TC. Bench scale synthesis of phydroxybenzoic acid using whole-cell nitrilase of Gordonia terrae mutant E9. Bioprocess Biosyst Eng. 2015;38:1267–1279. doi: 10.1007/s00449-015-1367-x. [DOI] [PubMed] [Google Scholar]
- Lauder K, Anselmi S, Finnigan JD, et al. Enantioselective synthesis of α-thiocarboxylic acids by nitrilase biocatalysed dynamic kinetic resolution of α-thionitriles. Chem A Eur J. 2020;26:10422–10426. doi: 10.1002/chem.202001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Dong W, Zhang Y, Liu K, Zhang W, Zhang M, Ma J, Jiang M. Enhanced catalytic efficiency of nitrilase from Acidovorax facilis 72W and application in bioconversion of 3-cyanopyridine to nicotinic acid. J Mol Catal B Enzym. 2017;133:S459–S467. doi: 10.1016/j.molcatb.2017.03.010. [DOI] [Google Scholar]
- Mathew CD, Nagasawa T, Kobayashi M, Yamada H. Nitrilase-catalyzed production of nicotinic acid from 3-cyanopyridine in Rhodococcus rhodochrous JI. Appl Microbiol Biotechnol. 1988;54:1030–1032. doi: 10.1128/aem.54.4.1030-1032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Nakamura T, Yamada H. e-Caprolactam, a new powerful inducer for the formation of Rhodococcus rhodochrous J1 nitrilase. Arch Micobiol. 1990;155:13–17. [Google Scholar]
- Pai O, Banoth L, Ghosh S, Ghisti Y, Banerjee UC. Biotransformation of 3-cyanopyridine to nicotinic acid by free and immobilized cells of recombinant Escherichia coli. Process Biochem. 2014;49:655–659. doi: 10.1016/j.procbio.2014.01.023. [DOI] [Google Scholar]
- Prasad S, Misra A, Jangir VP, Awasthi A, Raj J, Bhalla TC. A propionitrile-induced nitrilase of Rhodococcus sp NDB 1165 and its application in nicotinic acid synthesis. World J Microbiol Biotechnol. 2007;23:345–353. doi: 10.1007/s11274-006-9230-5. [DOI] [Google Scholar]
- Raj J, Singh N, Prasad S, Seth A, Bhalla TC. Bioconversion of benzonitrile to benzoic acid using free and agar entrapped cells of Nocardia globerula NHB-2. Acta Microbiol Immunol Hung. 2007;54:79–88. doi: 10.1556/amicr.54.2007.1.8. [DOI] [PubMed] [Google Scholar]
- Revuelta JL, Buey RM, Amaro RL, Vandamme EJ. Microbial biotechnology for the synthesis of (pro) vitamins, biopigments and antioxidants: challenges and opportunities. Microbiol Technol. 2016;9:564–567. doi: 10.1111/1751-7915.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma NN, Sharma M, Kumar H, Bhalla TC. Nocardia globerula NHB-2: bench scale production of nicotinic acid. Process Biochem. 2006;41:2078–2081. doi: 10.1016/j.procbio.2006.04.007. [DOI] [Google Scholar]
- Sharma NN, Monica S, Bhalla TC. An improved nitrilase mediated bioprocess for synthesis of nicotinic acid from 3-cyanopyridine with hyperinduced Nocardia globerula NHB-2. J Ind Microbiol Biotechnol. 2011;38:1235–1243. doi: 10.1007/s10295-010-0902-7. [DOI] [PubMed] [Google Scholar]
- Shen JD, Cai X, Liu ZQ, Zheng YG. Nitrilase: a promising biocatalyst in industrial applications for green chemistry. Crit Rev Biotechnol. 2021;41:72–93. doi: 10.1080/07388551.2020.1827367. [DOI] [PubMed] [Google Scholar]
- Stalker DM, Malyj LD, McBride KE. Purification and properties of a nitrilase specific for the herbicide bromoxynil and corresponding nucleotide sequence analysis of the bxn gene. J Biol Chem. 1988;263:6310–6314. doi: 10.1016/S0021-9258(18)68787-3. [DOI] [PubMed] [Google Scholar]
- Stanbury P, Whitaker A, Hall SJ. Principle of fermentation technology. Oxford: Butterworth-Heinemann; 2016. [Google Scholar]
- Thakur N, Kumar V, Thakur S, Sharma N, Bhalla TC. Biotransformation of 4-hydroxyphenylacetonitrile to 4-hydroxyphenylacetic acid using whole cell arylacetonitrilase of Alcaligenes faecalis MTCC 12629. Proces Biochem. 2018;73:117–123. doi: 10.1016/j.procbio.2018.07.012. [DOI] [Google Scholar]
- Wu Y, Gong JS, Lu ZM, Li H, Zhu XY, Li H, Shi JS, Xu ZH. Isolation and characterization of Gibberella intermedia CA3-1, a novel and versatile nitrilase-producing fungus. J Basic Microbiol. 2013;5:934–941. doi: 10.1002/jobm.201200143. [DOI] [PubMed] [Google Scholar]
- Wu US, Snajdrova R, Moore JC, Baldenius K, Bornscheuer UT. Biocatalysis: enzymatic synthesis for industrial applications. Angew Chem Int Ed. 2021;60:88–119. doi: 10.1002/anie.202006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZJ, Xu JH, He YC, Ouyang LM, Liu YY. Cloning and biochemical properties of a highly thermostable and enantioselective nitrilase from Alcaligenes sp. ECU0401 and its potential for (R)-(-)-mandelic acid production. Bioprocess Biosyst Eng. 2011;34:315–322. doi: 10.1007/s00449-010-0473-z. [DOI] [PubMed] [Google Scholar]
- Zheng YG, Chen J, Liu ZQ, Wu MH, Xing LY, Shen YC. Isolation, identification and characterization of Bacillus subtilis ZJB-063, a versatile nitrile-converting bacterium. Appl Microbiol Biotechnol. 2008;77:985–993. doi: 10.1007/s00253-007-1236-x. [DOI] [PubMed] [Google Scholar]