Abstract
Aqueous two-phase system (ATPS) composed of polyethylene glycol (PEG) and K2HPO4 solutions was used to extract saponin from sugar beet root. Extraction yield, purity and foam capacity of saponin were optimized according to response surface methodology (RSM). Analysis of liquid chromatography-mass spectrometry (LC–MS) showed that purified saponins were composed of hederagenin, akebonoic acid and oleanolic acid. Addition of 0.02 g sugar beet root saponin to one liter of malt beverage caused a considerable increase in foam volume and stability compared to malt beverage samples containing 0.1 g/L propylene glycol alginate (PGA). Malt beverages containing saponin showed higher turbidity, bitterness and overall sensory acceptance. Moreover, no significant changes in malt drink pH and °Brix were observed due to saponin addition. Adding lemon flavor caused a decrease in foam stability and sensory acceptance of malt beverage containing saponin compared to PGA containing ones. Less saponin content is suggested for flavored malt drinks.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05517-x.
Keywords: Saponin structure, Aqueous two-phase system, Foam, Malt beverage, Sensory evaluation
Highlights
Sugar beet saponin extracted via ATPS and analyzed by HPLC and LC-MS.
Three saponins including hederagenin, akebonoic acid and oleanolic acid was detected.
Addition of saponin to malt drink considerably increased foam stability.
Acceptable sensory properties were scored for saponin containing malt drinks.
Introduction
Sugar beet (B. vulgaris L.) belongs to the Chenopodiaceae family. A wide variety of sugar beets are cultivating in various climates and in different countries of the world to obtain root and leaves as well as for the purpose of sucrose and ethanol production in large scales (Duraisam et al., 2017). Sugar beet contains saponins mainly under the skin where they act as plant defense against disease and freezing damages.
Saponins are high molecular weight glycosides distributing in different parts of plants. The aglycone composed of triterpene glycosides (acidic saponins), steroidal glycosides (neutral saponins), or steroid-alkaloids (basic saponins). The glycone consists up of one or more than one sugar (mainly di-glucose, l-rhamnose, l-arabinose) and an uronic acid. The structure of sugar beet saponins extracted by chemical solvents has been introduced recently (Edelmann et al., 2020; Mikołajczyk-Bator et al., 2016b). The complex structure of saponins creates different physicochemical and phytochemical properties in plants and food formulations.
Aqueous two-phase system (ATPS) first described by Albertsson (1970) is a separation method to extract and purify bioactive substances (Albertsson, 1970). The separation is based on forming to immiscible phases after mixing two water miscible solutes upper than their critical concentrations (Phong et al., 2018). The most popular ATPSs are consisting of two polymer phases or one polymer phase and one salt phase. Polyethylene glycol (PEG)/ salt (usually sulfate and phosphate) systems are widely used because of their low cost and high differences in density, viscosity and hydrophobisity of the two phases (Han et al., 2008). There are several researches reporting the extraction of saponin using ATPS from different natural sources (Han et al., 2008; He et al., 2018; N. Wang, He, Li, Fang, & Li, 2019). However, there is no report regarding the utilization of ATPS method to extract sugar beet saponin.
Non alcoholic beer or malt beverage has gotten a considerable attention in recent years due to several reasons such as health benefits, religious bans and new driving /drinking rules. In non alcoholic malt beverage the production of alcohol is reduced or eliminated by restricting yeast activity or by separating alcohol through heating the wort or centrifugation process. However, the main drawback of non alcoholic malt beverage from consumer points of view is their poor organoleptic properties specially not favorable mouthfeel and weaker fullness. As an alternative to CO2 production by yeasts, carbonation is performed prior to bottling the non alcoholic beers (Sohrabvandi et al., 2010). Several factors determine the textural quality of beers including density, viscosity, foam and turbidity. Foam properties have got remarkable attentions due to its effect on consumer satisfaction. Schmidt et al. (2020) used propylene glycol alginate (PGA) as an additive in malt drink to increase foam stability. The stabilization was based on PGA interaction with amino acids on the foam bubble walls and resulting viscosity increment (Schmidt et al., 2020).
The aim of this study was first to optimize the saponin extraction from sugar beet root using ATPS. PEG/K2HPO4 system with three levels of each component was selected to determine the optimum extraction yield, purity and foam capacity of sugar beet saponin. The optimum purified saponins were structurally characterized and added to zero-alcohol malt beverage. The physicochemical, foaming and sensory properties of the resulting malt beverage were then evaluated.
Materials and methods
Plant materials and chemicals
The experiments were conducted on the root of the sugar Beta vulgaris ssp. vulgaris var. N harvested in the 2019 vegetative seasons. Sugar beet roots were supplied from Isfahan sugar beet fields (Isfahan, Iran). After harvesting, the fresh sugar beet roots were washed, dried and milled. The sugar beet root contained 74.9 ± 0.4% of moisture, 1.78 ± 0.06% of ash, 0.37 ± 0% of fat, 0.54 ± 0.05% of protein, 16 ± 0.3% of sucrose and 6.41 of fibers and other compounds (AOACInternational, 2000). Required chemicals including polyethylene glycol 4000 (PEG4000), dipotassium hydrogen phosphate (K2HPO4), ethanol 96%v/v, acetonitrile, formic acid, Quillaja saponaria saponins and other chemicals were obtained from Merck Co. (Germany).
Extraction of saponins
The ATPS was used based on the methods previously proposed to extract the saponins (Brezhneva et al., 2001; Han et al., 2008). To every 100 g of dry milled sugar beet, 1000 mL of distilled water was added and heated at 80–90 °C for 40 min. After cooling, the mixture was filtered through 100-mesh metal sieve and the permeate material (crude water extract) was concentrated. An ATPS was prepared by adding 0.8–1.2 g of PEG, 1.1–1.25 g of K2HPO4 and 200 µL of ethanol to every 12 mL of crude water extract. The range of PEG and K2HPO4 was determined based on the minimum and maximum amount of one component that made two phase system at a constant level of another one. The system was mixed to obtain homogeneous phase. Then, the mixture was centrifuged at 3000 g for 5 min to accelerate the phase separation. The salt-rich layer was collected and subjected to back-extraction for three times with 96%v/v ethanol. The ethanol extracted material was separated and stored at 4 °C overnight to let the salt be precipitated. The precipitate was discarded after centrifugation and saponin fraction was recovered from ethanol. The isolated extract was added to the HCl (pH = 1) with ratio of 1:10 v/v to precipitate saponins. Finally, the precipitated saponins were separated by centrifugation and kept in a cool and dry place for subsequent analysis.
Response surface methodology (RSM) was used to investigate the effect of PEG and K2HPO4 content on extraction yield, purity and foam capacity of sugar beet saponin. In this method three levels of PEG (0.8, 1 and 1.2 g) and three levels of K2HPO4 (1.1, 1.175 and 1.25 g) were determined in Design Expert software version 12. A central composite design (CCD) with 13 runs and 5 points was employed for processing experimental data.
Extraction yield
The amount of dry saponin (g) extracted from 100 g of dry sugar beet was measured as extraction yield (%).
Extraction purity
An HPLC device (Shimadzu, Japan) was applied to measure saponins concentration. HPLC apparatus included a C18-VP (25 × 4.6 mm) column and a UV–visible detector (SPD-6AV) adjusted at 210 nm. The acetonitrile-distilled water mixture with isocratic elution systems of ratio 50:50 with a flow rate of 1.2 mL/min was used as mobile phase. Standard and extracted saponins were dissolved in distilled water at a concentration of 1 mg/mL and centrifuged at 2500 g for 3 min to remove any particulate material. The samples were filtered through a Millipore filter (0.45 μm) prior to injection. The concentration of saponins was determined by standard injection and exit time peaks (2.2—2.3 min). Solutions with different concentrations of Quillaja saponaria saponin (50, 100, 250, 500 and 1000 ppm) were used as standard.
Foam properties of saponin solution
Foam properties were measured for concentrations of 0.1%w/w of sugar beet saponins. The saponin solutions were stirred for 40 min before the examination. Then, 10 mL of each solution was vortexed thoroughly for 30 s at room temperature. Foam capacity was expressed as the height (mm) of foam in Ø30 × 150 mm test tubes.
Structural characterization of saponins
High-performance liquid chromatography (HPLC) coupled with electrospray ionization (ESI) ion-trap mass spectrometry (MS) was used to determine the type of saponins. The dried sample (4 mg) was dissolved in water containing 25% acetonitrile and injected to the Alliance 2695 (Waters Alliance, Milford) device. The Atlantis T3-C18 3µ, 2.1 × 100 mm column adjusted at temperature of 35 °C was used. Mobile phase (A) consisted of acetonitrile with 0.1% formic acid and mobile phase (B) contained distilled water with 0.1% formic acid. The mobile phase flow rate and the injection volume were 200 µl/min and 10 µl, respectively. The gradient elution was programmed as follows: initially from 5% (A) and 95% (B), linear increase to 95% (A) and 5% (B) in 50 min, and constant at 95% (A) and 5% (B) for 20 min. Then, the system was returned to the initial conditions. The Quattro mass spectrometer with micro-API model (Waters, Milford) was equipped with an ESI and operated in negative-ion mode. The nebulizer was pure nitrogen gas with flow rate of 200 L/h and at 300 °C. The spray voltage, capillary voltage and vaporizer temperature were set to 3.50 kV, 0.2 V and 300 °C, respectively. The MS scanned ranges were set to 50–1000 m/z.
Production of malt beverage
Lemon and classic malt beverage samples were prepared in Behnoush Company, Iran. Accordingly, a certain amount of malt was milled, poured in water and cooked in a double-wall tank equipped with mixer. At this stage, barley malt starch hydrolyzes to oligosaccharides and the protein breaks down to peptides and amino acids. After hydrolysis stage, hop extract was added. The malt pulp was separated from the resulting liquid (Wort) through filtration and the Wort was transferred to another tank to get more precipitated. Afterward, the hot liquid was filtered and cooled by a plate cooler. Edible acids (ascorbic and citric acids) and other ingredients including sugar syrup and lemon flavor (in the case of flavored samples), saponin or PGA and CO2 were added. The amount of added saponin and PGA were respectively 0.02 g and 0.1 g in 1L of malt beverage sample. The resulting malt beverage samples were filled in bottles, capped and kept in refrigerator until analysis.
It is worth to note that the PGA concentration was selected based on industrial preference. The maximum permitted dose for daily intake of saponin is 50 mg per kg of bodyweight. The sugar beet saponin concentration was determined according to minimum saponin content that made a standard foam height (30 mm) of malt beverage remaining stable for at least 150 s and at most 250 s using a Nibem-t foam stability tester (Haffmans B.V., Netherland).
Physical properties of malt beverage
The pH of malt beverage was measured by a pH meter (Genway 3330 pH meter, England). The pH of saponin containing samples and control sample was measured after removing all CO2 gas by gently transferring malt beverage sample from one Erlenmeyer to another. Turbidity was measured by a Milwaukee Mi415 Turbidity meter (Italy). Since the CO2 gas interferes the turbidity analysis, it was removed before the measurements. The turbidity was recorded in EBC units. The total soluble solids (°Brix) was measured using a refractometer (model 010–95,000, France) after removing the malt beverage gas. The color of malt beverage samples was evaluated using a colorimeter (Nippon DENSHOKU, Japan). Parameters of l (l = 0, black; l = 100, white), a (− a = greenness; + a = redness) and b (− b = blueness; + b = yellowness) was measured to determine changes of color (ΔE) during the storage time according to Eq. 1:
| 1 |
Foam properties of malt beverage
Foam properties was measured using Nibem-t foam stability tester (Haffmans B.V., Netherland) at 18–20 ºC. The malt beverage sample was poured into 500 ml glass cylinder from a distance of 5 cm of the cylinder head. The initial foam volume was immediately recorded as foam capacity. The time taken for the foam to be minimized or eliminated was also recorded as the foam stability.
Bitterness of malt beverage
The malt beverage bitterness was measure according to Jurić et al. (2015) method with slight modifications. The amount of 10 ml of sample was transferred to a graduated cylinder and 0.5 ml of 6 N HCl and 20 ml of isooctane were added to it. The cylinder lid was closed and shacked for 15 min. The isooctane phase was transferred to the falcon and centrifuged for 3 min at 3000 rpm. The supernatant was kept in a dark place for 30 min and finally was read by the spectrophotometer (UV Mini- 1240 Shimadzu, Japan) at 275 nm. Bitterness was calculated using Eq. 2:(2).
where B is the bitterness (IBU) and A275 is the absorbance at 275 nm.
Sensory evaluation
Sensory evaluation was performed according to (Deng et al., 2018) and (ASBC & Chemists, 2014). In this test, 26 trained panelists including 13 men and 13 women aged between 19–32 years old evaluated the sensory properties of classic and lemon flavored malt beverage samples containing or non-containing saponins. Initially, three samples were provided to the panelists. Two of them were control samples and did not contain saponin and one of them contained saponins. This evaluation was aimed to find whether the evaluators were able to distinguish the control sample from the one containing saponins. In the next step, the panelists were requested to evaluate the sensory properties (odor, color, flavor, mouthfeel and general acceptance) of the samples. The samples were presented in codes with randomized digits. The panelists rinsed their palates with water before and between testing of each sample. Five-point hedonic scale method was used and the samples were assessed with 1–5 scores (5 = excellent, 4 = very good, 3 = good, 2 = average and 1 = bad).
Statistical analysis
Data related to optimization of extraction was analyzed using RSM design. Analysis of variance (ANOVA) was performed to define significance of the statistical model, coefficient estimation of each component and interaction between them. Numerical optimization of data was performed using Design Expert software, version 12. ANOVA of the data relating to physicochemical and sensory properties of malt beverage was performed using SAS 9.1. All the measurements were performed at least in three replications. The least significant difference (LSD) was used to compare the mean data with a confidence level of 95%.
Results and discussion
Optimization of saponin extraction
The effect of independent variables including PEG content (X1) and K2HPO4 content (X2) on extraction yield (Y1), purity (Y2) and foaming capacity (Y3) was studied using RSM method. It was revealed that the effect of all variables on responses followed quadratic model. ANOVA was used to determine p-value and F-value of variable components and the models (Supplementary Table 1). Lack of fit of all the models were not significant (p > 0.05) indicating that the models fit.
The effect of variables on extraction yield, purity and foam capacity of saponin is presented in Fig. 1. The results showed that increasing the amount of PEG in ATPS enhanced the extraction yield to an extent. However, the extraction yield was decreased when PEG content was surpassed to more than 1 g. The effect of K2HPO4 content on yield of extraction was significant (p < 0.05) increase at all the levels. Interaction of PEG and K2HPO4 contents was also significant (p < 0.05). Their interaction showed a peak of extraction yield when low PEG/ K2HPO4 was used. The K2HPO4 salt plays an important role in dissolving the saponin and separating it from other contaminants. On the other phase, PEG dissolves contaminant such as proteins (Han et al., 2008). However, at higher ratios of PEG/ K2HPO4 the saponin dispersed in polymer phase resulting in reduction of extraction yield. The purity of saponin was positively increased by increasing K2HPO4 content. However, the PEG and interaction of variables did not show significant effect (p > 0.05) on purity of saponin. Li et al. (2018) applied an ATPS based on choline chloride/1,4-butnediol and K2HPO4 solution to extract ginsenosides. They found that the extraction yield of ginsenosides increased with increasing K2HPO4 to 3.5 g followed by a decrease at upper levels of K2HPO4. Similar results were reported by Hu et al. (2019). In fact, larger number of salt ions compete with saponins to adsorb water molecules leading to remove the salvation spheres around the saponins and strengthen the salting-out effect. On the other hand, over concentrated hydrophobic phase did not participate in saponin separation (He et al., 2018).
Fig. 1.

3D plots showing the effect of PEG and K2HP4 contents on extraction yield (a), saponin purity (b) and foaming capacity of saponin (c)
Foaming capacity of saponin was significantly affected by variables and their interaction. In this case, similar to what was surveyed for extraction yield the highest foaming capacity was obtained at lower PEG to K2HPO4 ratio. The ability of foam formation is the most important feature of saponins which arises from their structure containing both polar (sugar) and non-polar (steroid or triterpene) groups. Like those of sugar beet root, saponins from many plant sources such as Quillaja saponaria, Gypsophila, Aesculus hippocastanum, Glycyrrhiza glabra can produce considerable volumes of foam in a low concentration (Böttcher & Drusch, 2016).
Numerical optimization of the models determined 0.89 g of PEG and 1.25 g of K2HPO4 as the most efficient levels of ATPS solutes that gave the maximum extraction yield (3.7 × 10–3%), purity (99.8%) and foam capacity (1.8 mm) of sugar beet saponin. The optimum point was verified by measuring the responses parameters and the resulted product was applied in malt beverage production.
Determination of structure of sugar beet saponins
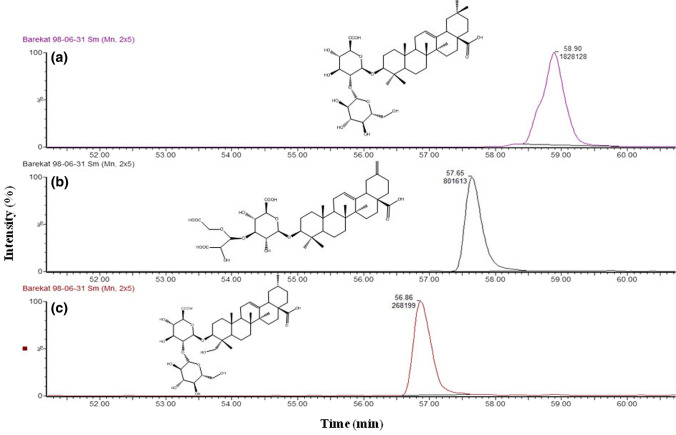
Saponins were extracted from sugar beet root (Normalbereich cultivar) and identified by LC–MS/MS. In this method, using negative-ion mode, saponins underwent fragmentation because of collision-induced dissociation. Mass spectrometry (MS) provides structural information on the units of aglycone and glycoside(s) of saponin. In this study, three fractions were observed in LC–MS chromatogram. Information on the chemical structure of saponin compounds extracted from sugar beet root is presented in Table 1. Figure 2. shows LC-chromatogram and chemical structure of saponins isolated from sugar beet root. The first peak was recorded at 56.86 min retention time which produced ions at m/z of 809–813. The structure of saponin I was assigned to hederagenin as aglycone part that was linked to a hexose and an uronic acid group (Mikołajczyk-Bator et al., 2016b; Mroczek, Kapusta, Janda, & Janiszowska, 2012). The second peak was observed at 57.65 min and according to m/z ranged from 777–778. Saponin II was attributed to akebonoic acid as aglycone group which was linked to one uronic acid and one acetal unit (Mikołajczyk-Bator et al., 2016a, 2016b). The third peak was observed at 58.90 min and the m/z was recorded at 791–796. It was contributed to saponin III containing aglycone of oleanoic acid and glycosides of hexose and uronic acid (Mikołajczyk-Bator et al., 2016b; Mroczek et al., 2012). The saccharide compounds detected in saponins sugar beet roots (B. vulgaris L.) were reported to be mainly glucuronic acid (GlcA), xylose (Xyl), glucose (Glc) and substituted sugar residues with acetal (Act) and dioxolane (Diox) (Edelmann et al., 2020). Mikołajczyk-Bator et al. (2016b) identified hederagenin, akebonoic acid, oleanolic acid as well as gypsogenin and norhederagenin saponins in sugar beets of the Hazara and Borina varieties. The presence of these two additional types of saponins which were not detected in our samples may be due to the differences in sugar beet cultivars, growth conditions, and saponins separation method. The results of HPLC also showed three peaks that were in accordance with peaks of Quillaja saponin as standard (Supplementary Fig. 1). The first, second and third output peaks were related to Hederagenin, Akebonoic acid and Oleanolic acid, respectively.
Table 1.
Chemical structure, amount and properties of sugar beet root extracted saponins
| Saponin number | tR [min] | Observed saponins | Observed m/z |
Average m/z | Formula |
|---|---|---|---|---|---|
| 1 | 56.86 |
Hederagenin Hexose, Uronic acid |
809–813 | 809/9565 | C42H65O15 |
| 2 | 57.65 |
Akebonoic acid Uronic acid, Acetal substituent |
777–778 | 777/8716 | C40H57O15 |
| 3 | 58.9 |
Oleanolic acid Hexose, Uronic acid |
793–794 | 793/9571 | C42H65O14 |
Fig. 2.
LC-chromatogram and chemical structure of saponins isolated from sugar beet root: (a) Oleanolic acid, (b) Akebonoic acid and (c) Hederagenin
Physicochemical properties of malt beverage samples
Physical properties of malt beverage
The °Brix of classical and flavored malt beverage samples (for both saponin and PGA containing samples) was observed to be 5 and 8, respectively during 15 weeks of storage. The flavored malt beverage sample showed higher °Brix due to sugar content. There were no significant changes in °Brix over storage time.
The turbidity of classical and flavored malt beverage samples containing saponin or PGA is presented in Table 2. Saponin containing samples exhibited higher turbidity compared to PGA containing samples. This could be due to interaction of saponins as a polyphenol source with proteins (Y. Wang & Ye, 2021). The flavored malt beverage samples showed higher turbidity compared to classical samples during the first week of storage. However, turbidity of classical samples surpassed during the rest of storage time. The greater turbidity in flavored malt beverage samples at first seven days might be due to higher viscosity of these samples aroused from presence of dissolved sucrose (Jurado Gonzalez & Sörensen, 2020). In this stage, high viscosity caused the suspended haze particles such as protein and polyphenols to disperse throughout the liquid medium. The greater viscosity also led the malt beverage samples to exhibit more stable texture so that the turbidity of flavored samples did not changed dramatically over time. However, the classical samples, due to having less viscose texture, showed more increase in turbidity during the storage time. In fact, haze particles got more freedom to disperse over time. For example, the interaction of special polyphenols and polypeptide chains is an important factor to produce turbidity in samples (Steiner et al., 2010). Lower viscosity of classical malt beverage gradually increased the reaction chance between haze compounds.
Table 2.
Turbidity, pH and ΔE of classical and flavored malt beverage samples containing saponin and PGA
| Classical malt beverage containing saponin |
Classical malt beverage containing PGA | Flavored malt beverage containing saponin | Flavored malt beverage containing PGA | ||
|---|---|---|---|---|---|
| Turbidity | |||||
| Storage time (week) | |||||
| 0 | 0.45 ± 0.05dC | 0.36 ± 0.01eC | 0.98 ± 0.01eA | 0.74 ± 0.01eB | |
| 1 | 0.70 ± 0.07cC | 0.39 ± 0.09deD | 0.99 ± 0.08deA | 0.79 ± 0.08eB | |
| 3 | 1.02 ± 0.11bA | 0.54 ± 0.06cdB | 1.02 ± 0.06deA | 1.00 ± 0.04deA | |
| 6 | 1.17 ± 0.09bA | 0.63 ± 0.03cC | 1.19 ± 0.01cA | 1.00 ± 0.04deB | |
| 10 | 1.72 ± 0.05aA | 1.54 ± 0.03bB | 1.33 ± 0.03bC | 1.11 ± 0.01bcD | |
| 15 | 1.78 ± 0.05aA | 1.59 ± 0.04aB | 1.41 ± 0.03aC | 1.15 ± 0.01aD | |
| pH | |||||
| 0 | 4.00 ± 0.03cA | 4.06 ± 0.02eA | 3.31 ± 0 dB | 3.21 ± 0fC | |
| 1 | 4.00 ± 0.03cA | 4.05 ± 0eA | 3.37 ± 0cB | 3.24 ± 0eC | |
| 3 | 4.07 ± 0.04bcB | 4.16 ± 0.02dA | 3.37 ± 0.02cC | 3.28 ± 0.01dD | |
| 6 | 4.15 ± 0.05bA | 4.24 ± 0.04cA | 3.42 ± 0.02bC | 3.37 ± 0cD | |
| 10 | 4.27 ± 0.06aB | 4.40 ± 0.03aA | 3.54 ± 0.01aC | 3.56 ± 0.03aC | |
| 15 | 4.17 ± 0.05abB | 4.31 ± 0.02bA | 3.34 ± 0.04cC | 3.43 ± 0.05bC | |
| ΔE | |||||
| 1 | 1.89 ± 0.02d | 3.24 ± 0.01e | 1.31 ± 0.05e | 0.39 ± 0.01e | |
| 3 | 4.51 ± 0.05c | 3.54 ± 0.05d | 2.27 ± 0.08d | 1.28 ± 0.01d | |
| 6 | 4.55 ± 0.05c | 4.46 ± 0.02c | 3.83 ± 0.05c | 2.65 ± 0.01c | |
| 10 | 4.99 ± 0.08b | 5.02 ± 0.08b | 4.39 ± 0.10b | 3.25 ± 0.05b | |
| 15 | 5.50 ± 0.09a | 6.54 ± 0.10a | 5.31 ± 0.12a | 3.85 ± 0.05a |
Different a, b, c…letters within the same column and different A, B, C… letters within the same row indicate a statistically significant difference (p < 0.05)
The pH ranged from 4.0–4.4 for classical malt beverage and 3.2–3.6 for flavored malt beverage sample during 15 weeks. Lower pH of flavored samples was attributed to the presence of organic acids such as ascorbic and citric acids in lemon flavor formulations. The pH of saponin and PGA containing samples did not show significant difference in most of cases.
The ΔE increased significantly during the storage time for all the samples. The color changes in malt beverage is mainly attributed to degradation of phenolic color compounds specially anthocyanins. However, the color changes was irrelevant to addition of saponin or flavor compounds.
Foam properties of malt beverage
Foam production in malt beverage is the result of interaction of malt extract protein with hops compounds. Proteins become denatured during extraction and malt boiling stages. Hops compound specially iso-α-acid and hydrophobic proteins form a non permeable stabilizer covering the gas bubbles after CO2 injection in malt drink (Bamforth, 2011). Foam properties of classical and flavored malt beverage samples containing saponin or PGA during 15 weeks of storage were presented in Fig. 3. Foam capacity and stability of samples containing 0.02 g/L saponin was significantly higher than samples containing 0.1 g/L PGA. High stability of sugar beet saponin foam during the storage time could attract the consumer acceptance. Aryan et al. (2021) added 0.02–0.05 g of Acanthophyllum laxiusculum saponin per kg of malt beverage and studied foaming properties of carbonated and non-carbonated beverage. They found that addition of saponin upper than 0.03 g/kg significantly enhanced foaming capacity and stability of malt beverage and in this regard saponin addition was more effective than carbonation. Sugar beet saponin exhibited more foaming capacity compared to Acanthophyllum laxiusculum extract in malt beverage as the former increased foaming capacity to about 127.5% at 0.02 g/kg level and the latter increased foaming capacity to 49.5% at 0.05 g/kg level.
Fig. 3.

Foam capacity (foam volume) and foam stability of malt beverage samples
Classical malt beverage samples showed higher foam capacity and stability compared to flavored ones that might refer to higher pH of classical samples. The surface activity of saponin was increased at higher pH due to loosing H+ and acting as ionic surfactant (Santini et al., 2019). It has been supposed that the saponin structure tend to elongate due to electrostatic repulsion of negative charges on saponin heads. The elongation let the saponins to absorb at the air–water interface (Böttcher & Drusch, 2017).
Bitterness
Hops are used in brewing process to produce bitter taste in malt beverage. Isomerization of hops alpha acids during boiling of the extract leads to the production of iso-alpha acids, which creates a bitter taste. Saponins also taste bitter and increase the bitterness when are added to malt beverage. The IBU value is used to reflect the bitterness of malt beverage. This calculation method measures iso α-acids, α-acid, β-acid, oxidative polar compounds (produced from oxygen degradation during storage) and polyphenols as total bitterness (Kishimoto et al., 2021). The bitterness of flavored malt beverage containing saponin was significantly higher than flavored samples containing PGA (2.25 ± 0.02 and 1.20 ± 0.02 IBU, respectively). The bitterness of classical malt beverage containing saponin and PGA were 22.22 ± 0.03 and 22.25 ± 0.05 IBU, respectively indicating that there was no significant difference between the bitterness of these samples. Our results were in accordance with those of Aryan et al. (2021) respect to insignificant increase of bitterness due to addition of 0.02–0.05 g saponin per kg of malt beverage.
Sensory evaluation.
The results of sensory evaluation are illustrated in Fig. 4. At the first stage of organoleptic test, the panelists were asked to distinguish the saponin containing sample from PGA containing ones. For classical malt beverage 19 and for flavored malt beverage 10 panelists recognized the saponin containing sample due to presence of higher foam volume. Odor, color and taste scores were not significant comparing classical malt beverage sample containing saponin or PGA (p > 0.05). However, mouthfeel and general acceptance significantly scored higher for classical sample contained saponin in comparison with PGA containing sample (p < 0.05). For flavored malt beverage samples, the panelist rated higher scores to the taste of PGA containing malt beverage and mouthfeel of saponin containing one (p < 0.05). Nevertheless, there was no significant difference between odor, color and general acceptance values of both flavored malt beverages (p > 0.05). In spite of classical malt drinks, the bitterness was not acceptable in flavored ones. The solution might be addition of less saponin or higher flavoring agent according to standard rules in order to reduce the perception of bitter taste.
Fig. 4.

Sensory evaluation of malt beverage samples
Three saponins including hedragenin, akebonoic acid and oleanolic acid were identified from sugar beet root. The optimum extraction condition was obtained at 0.89 g of PEG and 1.25 g of K2HPO4 in an ATPS. At this point, the extraction yield, purity and foam capacity of sugar beet saponin was determined 3.7 × 10–3%, 99.8% and 1.8 mm, respectively. Malt beverages containing saponin showed significantly higher foam capacity and stability, turbidity, bitterness and sensory evaluation scores. Adding lemon flavor decreased foam stability and general acceptances of drinks containing saponin in comparison with PGA containing one. The future studies may conduct the assistance of novel technologies which are safe for food applications beside ATPS extraction method.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by Isfahan University of Technology and Iran National Science Foundation. The authors would thank to Behnoosh company for their cooperation on sensory evaluation.
Abbreviations
- ATPS
Aqueous two-phase system
- RSM
Response surface methodology
- LC–MS/MS
Liquid chromatography-Mass Spectrometry/ Mass Spectrometry
- HPLC
High performance liquid chromatography
- PEG
Polyethylene glycol
- PGA
Propylene glycol alginate
Author contributions
AHM and SS performed the experiments, analyzed the data and wrote the original draft. AN and JK performed supervision and revised the manuscript.
Funding
The authors have not disclosed any funding.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ali Nasirpour, Email: ali.nasirpour@iut.ac.ir.
Sima Saeidy, Email: simasaeidy@yahoo.com.
References
- Albertsson PÅ. Partition of Cell Particles and Macromolecules in Polymer Two-Phase Systems. In: Anfinsen CB, Edsall JT, Richards FM, editors. Advances in Protein Chemistry. US: Cambridge; 1970. pp. 309–341. [DOI] [PubMed] [Google Scholar]
- AOAC International (2000) Agricultural chemicals, contaminants, drugs International, Vol. 1.
- Aryan S, Mortazavian AM, Mohammadi F, Mahdavi V, Moazami N, Jazaeri S. Physicochemical properties of saponin containing Acanthophyllum laxiusculum extract: example application in foam stability and qualitative parameters for malt beverage industry. J Food Sci Tech. 2021 doi: 10.1007/s13197-021-05169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Brewing Chemists . Methods of Analysis, Sensory Analysis– 10 Descriptive Analysis. St Paul, MN: The Society; 2014. [Google Scholar]
- Bamforth CW. 125th Anniversary Review: The Non-Biological Instability of Beer. J Inst Brew. 2011;117(4):488–497. doi: 10.1002/j.2050-0416.2011.tb00496.x. [DOI] [Google Scholar]
- Böttcher S, Drusch S. Interfacial Properties of Saponin Extracts and Their Impact on Foam Characteristics. Food Biophys. 2016;11(1):91–100. doi: 10.1007/s11483-015-9420-5. [DOI] [Google Scholar]
- Böttcher S, Drusch S. Saponins — Self-assembly and behavior at aqueous interfaces. Adv Colloid Interface Sci. 2017;243:105–113. doi: 10.1016/j.cis.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Brezhneva TA, Nikolaevskii VA, Selemenev VF, Slivkin AI, Muad AA, Khind T, Safonova EF. Isolation of Saponins from Sugar Beet Roots and Preliminary Characterization of Their Adaptogen Properties. Pharm Chem J. 2001;35(3):159–161. doi: 10.1023/A:1010462013789. [DOI] [Google Scholar]
- Deng Y, Bi H, Yin H, Yu J, Dong J, Yang M, Ma Y. Influence of ultrasound assisted thermal processing on the physicochemical and sensorial properties of beer. Ultrason Sonochem. 2018;40:166–173. doi: 10.1016/j.ultsonch.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Duraisam R, Salelgn K, Berekete AK. Production of Beet Sugar and Bio-ethanol from Sugar beet and it Bagasse: A Review. Int J Eng Trends Technol. 2017;43(4):222–233. doi: 10.14445/22315381/IJETT-V43P237. [DOI] [Google Scholar]
- Edelmann M, Dawid C, Hochreiter K, Ralla T, Stark T D, Salminen H, Weiss J, Hofmann T (2020) Molecularization of Foam-Active Saponins from Sugar Beet Side Streams (Beta vulgaris ssp. vulgaris var. altissima). J Agr Food Chem 68(39), 10962–10974. doi: 10.1021/acs.jafc.0c04603 [DOI] [PubMed]
- Han C, Hui Q, Wang Y. Hypoglycaemic activity of saponin fraction extracted from Momordica charantia in PEG/salt aqueous two-phase systems. Nat Prod Res. 2008;22(13):1112–1119. doi: 10.1080/14786410802079675. [DOI] [PubMed] [Google Scholar]
- He A, Dong B, Feng X, Yao S. Extraction of bioactive ginseng saponins using aqueous two-phase systems of ionic liquids and salts. Sep Purif Technol. 2018;196:270–280. doi: 10.1016/j.seppur.2017.05.041. [DOI] [Google Scholar]
- Hu X, Jin Y, Du J. Differences in protein content and foaming properties of cloudy beers based on wheat malt content. J Inst Brew. 2019;125(2):235–241. doi: 10.1002/jib.550. [DOI] [Google Scholar]
- Jurado Gonzalez P, Sörensen PM. Characterization of saponin foam from Saponaria officinalis for food applications. Food Hydrocolloids. 2020;101:105541. doi: 10.1016/j.foodhyd.2019.105541. [DOI] [Google Scholar]
- Jurić A, Ćorić N, Odak A, Herceg Z, Tišma M. Analysis of total polyphenols, bitterness and haze in pale and dark lager beers produced under different mashing and boiling conditions. J Inst Brew. 2015;121(4):541–547. doi: 10.1002/jib.254. [DOI] [Google Scholar]
- Kishimoto T, Teramoto S, Fujita A, Yamada O. Evaluation of Components Contributing to the International Bitterness Unit of Wort and Beer. J Am Soc Brew Chem. 2021 doi: 10.1080/03610470.2021.1878684. [DOI] [Google Scholar]
- Li P, Zhao P, Liu W, Jiang Y, Wang W, Bao L, Jin Y, Li X. Determination of common ginsenosides in Kang'ai injection by aqueous two-phase extraction with deep eutectic solvents and HPLC-UV/DAD. Microcheml J. 2018;137:302–308. doi: 10.1016/j.microc.2017.11.007. [DOI] [Google Scholar]
- Mikołajczyk-Bator K, Błaszczyk A, Czyżniejewski M, Kachlicki P. Characterisation and identification of triterpene saponins in the roots of red beets (Beta vulgaris L.) using two HPLC–MS systems. Food Chem. 2016;192:979–990. doi: 10.1016/j.foodchem.2015.07.111. [DOI] [PubMed] [Google Scholar]
- Mikołajczyk-Bator K, Błaszczyk A, Czyżniejewski M, Kachlicki P. Identification of saponins from sugar beet (Beta vulgaris) by low and high-resolution HPLC–MS/MS. J Chromatogr B. 2016;1029–1030:36–47. doi: 10.1016/j.jchromb.2016.06.038. [DOI] [PubMed] [Google Scholar]
- Mroczek A, Kapusta I, Janda B, Janiszowska W (2012) Triterpene Saponin Content in the Roots of Red Beet (Beta vulgaris L.) Cultivars. J Agr Food Chem, 60(50) 12397–12402. doi: 10.1021/jf303952x [DOI] [PubMed]
- Phong WN, Show PL, Chow YH, Ling TC. Recovery of biotechnological products using aqueous two phase systems. J Biosci Bioeng. 2018;126(3):273–281. doi: 10.1016/j.jbiosc.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Santini E, Jarek E, Ravera F, Liggieri L, Warszynski P, Krzan M. Surface properties and foamability of saponin and saponin-chitosan systems. Colloids Surf B. 2019;181:198–206. doi: 10.1016/j.colsurfb.2019.05.035. [DOI] [PubMed] [Google Scholar]
- Schmidt FL, Sebastian A, Tamayo R, de Brito ADC. Hydrocolloids as beers foam stabilizer. Afr J Food Sci. 2020;14(5):143–153. doi: 10.5897/AJFS2020.1922. [DOI] [Google Scholar]
- Sohrabvandi S, Mousavi SM, Razavi SH, Mortazavian AM, Rezaei K. Alcohol-free Beer: Methods of Production, Sensorial Defects, and Healthful Effects. Food Rev Int. 2010;26(4):335–352. doi: 10.1080/87559129.2010.496022. [DOI] [Google Scholar]
- Steiner E, Becker T, Gastl M. Turbidity and Haze Formation in Beer — Insights and Overview. J Inst Brew. 2010;116(4):360–368. doi: 10.1002/j.2050-0416.2010.tb00787.x. [DOI] [Google Scholar]
- Wang N, He F, Li W, Fang X, Li H (2019) Purification of the total steroidal saponins from fenugreek seeds (Trigonella foenum-graecum L.) using aqueous two-phase system and determination of diosgenin content using micellar electrokinetic chromatography method. Nat Prod Res 33(3), 453–456. 10.1080/14786419.2018.1455047 [DOI] [PubMed]
- Wang Y, Ye L (2021) Haze in Beer: Its Formation and Alleviating Strategies, from a Protein–Polyphenol Complex Angle. Foods 10(12). 10.3390/foods10123114 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.