Abstract
Edible bird’s nest beverage (B-nest-Bev) was produced from edible bird’s nest (B-nest) flakes using different thermal processes. Pasteurization of B-nest-Bev at a low temperature for a longer time (LTLT) or at a high temperature for a shorter time (HTST) resulted in lower CIE L*, CIE a*, CIE b*-values, and drained weight (p < 0.05) than sterilization (118 or 121 °C). Sterilized and pasteurized B-nest-Bev had similar soluble solid contents and pH (p < 0.05). Nevertheless, acidified beverages pasteurized via either LTLT or HTST process had a marked decrease in sialic acid content. In addition, drastic protein degradation occurred in pasteurized acidified beverages, regardless of the conditions used, ascertained by the disappearance of major protein bands. However, polymerization of proteins took place in sterilized samples, irrespective of the temperature used. After digestion in a gastrointestinal tract model system (GIMs), all samples had increased (p < 0.05) antioxidant activities including DPPH and ABTS radical scavenging activities, ferric reducing antioxidant power (FRAP) and oxygen radical absorbance capacity (ORAC). B-nest-Bev subjected to HTST pasteurization or sterilization at 121 °C had the best appearance and acceptability among all the treatments used. Therefore, thermal processes directly affected the properties and acceptability of B-nest-Bev.
Keywords: Edible bird's nest beverage, Thermal process, Pasteurization, Sterilization, Quality
Introduction
Thermal processing involves heating of food or food products at temperatures ranging from 50 to 160 °C to inactivate microbes and endogenous enzymes (Chantakun and Benjakul 2020a). According to the intensity of heat used, it can be classified as pasteurization (65–90 °C), sterilization (110–121 °C) or ultra-high-temperature (UHT) treatment (140–160 °C). Different processes render the finished products with varying quality, safety and shelf-life (Kong et al. 2007). Thermally processed products seek to ensure safety, by killing spoilage or pathogenic microorganisms. Beverages, which are popular for consumers, also require thermal processing for shelf-life extension. However, quality deterioration, including changes in color and texture, nutrient loss, and cooking loss, can occur with inappropriate processing conditions (Amit et al. 2017).
Edible bird’s nest (B-nest) or “Caviar of the East” is a famous and nutritious food that is well-known in Chinese traditional medicine (Chantakun et al. 2020). It is one of the most expensive foods. Edible bird’s nest beverage (B-nest-Bev) is the important product and is manufactured from dried B-nest. Hobbs (2004) documented that B-nest is cooked using double boiling method with rock sugar to make the Chinese cuisine, namely the bird’s nest beverage or soup, which has been known as an exclusive product. The world’s largest producer of B-nest is Indonesia, which has the largest colony of swiftlets currently, followed by Malaysia. In addition, Hong Kong is the largest importer of B-nest globally, followed by the Chinese community from North America (Hobbs 2004). In Thailand, the market size of these products is about 3.2 billion Thai Baht per year, equivalent to about 100 million USD (Chantakun and Benjakul 2020a). To prolong the shelf-life and ensure safety for consumption and to meet the export’s requirement, B-nest-Bev (B-nest in syrup) must be inevitably sterilized before domestic distribution or export (Amit et al. 2017). However, sterilization brings about degradation in the B-nest, leading to rejections by consumers. Since the major component of B-nest is protein (61.5–66.9%), it could undergo thermal degradation and solubilization of B-nest under harsh thermal process, leading to the rejection by consumers. Therefore, the pretreatment of B-nest has been developed to improve the resistance of B-nest to sterilization (Chantakun and Benjakul 2020a). Pasteurization is one of alternative processes to solve this problem, but the products must be stored at a low temperature (4–10 °C). Additionally, shelf-life is generally lesser than after sterilization (Escuder-Vieco et al. 2018). Normally, there are two types of pasteurization processes, namely high temperature short time (HTST, 76–90 °C) and low temperature long time (LTLT, 66–75 °C). The choice of process depends on the heat resistance of the product. To prolong the shelf-life, acidification of food or drink before pasteurization is recommended. An acidified beverage product (pH < 4.6) that had been pasteurized could be stored at room temperature for 6–12 months (Escuder-Vieco et al. 2018). Sinchaipanit and Kerr (2007) documented that the addition of acids could extend the shelf life of the beverages because of organic acid added. Such an undissociated acid has been known to exhibit an inhibitory effect toward the growth of microorganisms. Nowadays, in Thailand several processes (acidification in combination with pasteurization and sterilization) are employed in processing B-nest-Bev to serve different target consumers. However, sterilization is the main process for prolonging the shelf-life and ensuring the safety of the product. Recently, Chantakun and Benjakul (2020a) reported that B-nest flakes pretreated with sodium alginate solution combined with some divalent cross-linking agents could withstand the severe conditions (121 °C) of thermal sterilization. Sterilization can be conducted with different conditions such as temperature and time used, etc. Also, pasteurization having varying conditions such as HTST and LTLT, etc. could be implemented for preservation of the beverage products. Since different thermal processes could yield products with varying quality, this work therefore aimed to examine the effects of different thermal processes on quality and acceptability of B-nest-Bev.
Materials and methods
Chemicals
Food-grade calcium lactate was obtained from Shandong-Kaiteda Chemical Co. (Shandong, China) and food-grade sodium alginate was purchased from Arshine Pharmaceutical Co. (Changsha, China). Coomassie blue R-250, acetic acid, beta-mercaptoethanol, ethanol, ammonium sulfate and sodium dodecyl sulfate (SDS) were obtained from Merck (Darmstadt, Germany). Molecular protein standards were procured from GE Healthcare UK Limited (Buckinghams, UK). Microbial media were purchased from Sigma (St. Louis, MO, USA). All chemicals used for analyses were of analytical grade.
Collection of edible bird’s nest (B-nest) flakes
Dried B-nest flakes (from house B-nest) were purchased from My Great (Thailand) Company Limited, Hat-Yai, Songkhla, Thailand. The sample was sieved and stored as detailed by Chantakun and Benjakul (2020b).
Preparation of B-nest flakes and beverage
The solutions of 1% (w/v) sodium alginate and 1% (w/v) calcium lactate were used for pretreatment of B-nest flakes as detailed by Chantakun and Benjakul (2020a). Drying of the treated B-nest flakes in an air-conditioned room was performed as described by Chantakun and Benjakul (2020b). The resulting dried B-nest flakes were placed in polyethylene bags, sealed, and stored at 4–7 °C.
Beverage (B-nest-Bev) was prepared prior to thermal processing. Dried pre-treated B-nest flakes (0.7 g) were transferred into a glass bottle, filled with 40 mL of 14°Brix cane sugar syrup without and with acidification. To prepare the acidified syrup, pH of syrup was adjusted to 3.4 using 10% (w/v) citric acid solution. The pH of non-acidified syrup was 7.65. After filling the syrup, the bottles were then screw-capped.
Thermal processing
Pasteurization and sterilization processes were carried out using a Steamed Water Spray™ device (Steam Water Spray A-091 Pilot Retort, FMC Technology NV Food Processing Systems Division, Sint-Niklaas, Belgium). A computerized “Ellab” digital recorder (series: E-Val Flex System, Denmark), operated with 16 sensing thermocouple channels at a scanning rate of 1 per min, was used. Copper/constantan thermocouples of type T were placed to have the tip in the middle of the bottle (at the geometric center). For internal temperature monitoring, the heating medium temperature was determined and recorded using lead-free thermocouple as well as by using retort temperature monitoring devices.
Pasteurization of B-nest beverage (B-nest-Bev)
Pasteurization of B-nest-Bev (syrup without pH adjustment) or acidified B-nest-Bev (syrup with pH adjustment) was carried out for at least 24 h. All the bottles containing samples were transferred into a horizontal laboratory retort as mentioned above. The heating conditions were either high temperature short time (HTST) or low temperature long time (LTLT). The HTST process was applied at 90 °C for 15 min and the LTLT process was done at 75 °C for 180 min (10 min for come-up time), in which P value (P10/93.3) of 1.0 min was achieved (Ghani et al. 2001).
Sterilization of B-nest-Bev
Sterilization of B-nest-Bev as detailed by Chantakun and Benjakul (2020a) was adopted. The retort conditions were set at 118 °C for 25 min, or 121 °C for 11 min, in which the F0 value of 7.0 min was achieved. The temperatures of samples during thermal processing were monitored as detailed by Benjakul et al. (2018).
Analyses
All samples including pasteurized non-acidified and acidified (pH 3.4) B-nest-Bev subjected to LTLT or HTST processes as well as sterilized B-nest-Bev using temperatures of 118 or 121 °C were subjected to analyses.
Color
Color parameters (CIE L*, CIE a*, CIE b* and ∆E*-values) of all the beverages were examined as tailored by Benjakul et al. (2018).
Soluble solid content
The filtrates of all B-nest-Bev samples through Whatman filter paper (No.1) were determined for their soluble solid contents as detailed by Chantakun and Benjakul (2020b). Soluble solid content is reported as g/100 g sample.
pH value
A pH meter (Sartorius PB-10, G€ottingen, Germany) was used to measure the pH of all B-nest-Bev samples.
Drained weight
The weight of the beverage sample (whether liquid or solid) was first recorded. The sample was then transferred onto a weighed screen (mesh: 18), allowing it to drain for 5 min. The product remaining on the screen was weighed. The weight of the screen was subtracted, and drained weight of the product was calculated and was reported as g/100 g sample.
Sialic acid (SLA) content
A homogenate of the B-nest-Bev sample was firstly prepared by homogenizing the whole sample at 6,000 rpm for 3 min using IKA homogenizer model T25 D (IKA-Werke GmbH & Co. KG, Staufen, Germany). The homogenate (2 mL) was hydrolyzed with 0.5 M sodium bisulfate (NaHSO4) solution (1 mL) at 80 °C for 30 min. The hydrolysate was further mixed with o-phenylenediamine dihydrochloride solution (1 mL, 20 mg/mL) and heated in a water bath at 80 °C for 40 min for pre-column derivatization. SLA content was analyzed as described by Quek et al. (2018) using high performance liquid chromatography (HPLC) equipped with Water Sunfire C18 column (150 × 4.6 mm id., 5 µm). SLA quantification was done using N-acetylneuraminic acid (0–800 mg/L) as standard. SLA content was expressed as mg/L sample.
Protein patterns
Beverage samples were homogenized at 6,000 rpm for 3 min. The homogenate (2 mL) was mixed with 8 mL of 10% (w/v) sodium dodecyl sulfate (SDS). The mixture was heated in a water bath at 80 °C for 2 h and centrifuged for 10 min at 3000 × g using a centrifuge (Allegra 25-R, Beckman Instruments Inc., Palo Alto, CA, USA). SDS–PAGE analysis was performed following the method as modified by Singh et al. (2020).
Gastrointestinal tract model system (GIMs)
The homogenate (5.0 mL) of each sample was prepared as detailed above and then transferred into an amber bottle. To mimic human digestion, GIMs as described by Singh et al. (2020) was adopted. Firstly, the samples were added with freshly prepared pepsin solution (4% in 0.1 M HCl). Gastric phase digestion was performed at pH 2.0 for 60 min at 37 °C in a water bath shaker. To simulate duodenal phase, 0.9 M sodium bicarbonate was used to increase pH to 6.8 and then 0.3 mL of freshly prepared 4% bile salts and 0.08% pancreatin (0.3 mL) were added. Thereafter, the digestion was performed for 120 min at 37 °C in a water bath shaker. After termination of GIMs treatment, the digested samples were filtered using a 0.45 µm membrane filter. The resulting filtrates were determined for antioxidative activities. FRAP was assessed following the procedure of Benzie and Strain (1996). ABTS and DPPH radical scavenging activities were determined as detailed by Binsan et al. (2008), and oxygen radical absorbance capacity (ORAC) was examined as described by Sae-leaw and Benjakul (2018). The activities were reported as μmol Trolox equivalents (TE)/L sample.
Microbiological count
Microbiological quality for the pasteurized and sterilized B-nest-Bev samples under different conditions were determined following the Thai Industrial Standards Institute (TISI-TCPS-1083/2009). The sample (10 g) was placed in a stomacher bag filled with 90 mL of saline solution (0.85%). The stomacher blender (Stomacher M400, Seward Ltd., Worthington, England) was used to mix the blend for 1 min. Serial dilutions were done using a 0.85% (w/v) saline solution. Total viable count (TVC) was measured by pour plate method with incubation for 48 h at 37 °C. Yeast and mold counts were enumerated on potato dextrose agar after incubation at 25 °C for 3 days. Staphylococcus aureus, Salmonella spp., Escherichia coli and Coliform, Bacillus cereus and Clostridium perfringens were determined as per the procedures of Bacteriological Analytical Manual (BAM) (FDA 2001). In addition, aerobic and anaerobic counts of mesophilic and thermophilic microorganisms in all B-nest-Bev samples were enumerated following the method of BAM (FDA 2013).
Acceptability
Fifty untrained panelists with ages between 25 and 50, who were familiar with the B-nest-Bev were recruited. Appearance, color, taste, texture, and overall liking of beverage samples were evaluated using the 9-point hedonic scale (Chantakun and Benjakul 2020a). Each sample (10 mL) was placed in a plastic cup with 4.0 cm diameter at room temperature (25 °C). The samples were coded with three random numbers. Panelists rinsed their mouth with water after evaluation of each sample (Chantakun et al. 2020).
Experimental design and statistical analyses
Completely randomized design (CRD, for physical, chemical, and microbiological analyses) and randomized complete block design (RCBD, for sensory analysis) were used. All experiments were run in triplicates. Analysis of variance (ANOVA) was performed and comparison of means was done using Duncan’s multiple range test. For pair comparisons, the t-test was used (Steel and Torrie, 1980). Analyses were carried out using the SPSS software package (SPSS 11.0 for windows, SPSS Inc, Chicago, IL, USA).
Results and discussion
Color
Colors of edible bird’s nest beverage (B-nest-Bev) subjected to alternative thermal treatments are presented in Table 1. All B-nest-Bev samples showed different CIE L* (lightness), CIE a* (redness/greenness) and CIE b* (yellowness/blueness) color parameter. The highest CIE L*-values were found for sterilized beverages from both temperatures, 118 and 121 °C, while those of pasteurized non-acidified and acidified samples were lower (p < 0.05). The high temperature in sterilization might induce coagulation of protein in B-nest, thus increasing light scattering as witnessed by a higher CIE L*. Acidified samples had a higher CIE L* than non-acidified counterparts after similar pasteurization (p < 0.05). However, no difference in CIE L* was noticeable for the same sample type (non-acidified or acidified) between different pasteurization processes. For CIE a*, the sterilized samples had higher value than the pasteurized ones. No difference in CIE a* between pasteurized non-acidified and acidified samples was found, regardless of processing used. Higher CIE b* were noted for B-nest-Bev sample sterilized at 118 °C than at 121 °C. The pasteurized non-acidified sample had higher CIE b* than its pasteurized acidified counterpart (p < 0.05).
Table 1.
Color, soluble solid content, pH, drained weight and sialic acid content of B-nest-Bev produced by various processes
| B-nest-Bev types | Thermal process | CIE L* | CIE a* | CIE b* | ΔE* | Soluble solid content (g/100 g) | pH | Drained weight (g/100 g) | Sialic acid (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| Non-acidified | LTLT | 80.52 ± 0.36aC | 0.62 ± 0.04aC | 9.28 ± 0.62aC | 13.17 ± 0.65aAB | 13.71 ± 0.17aB | 7.52 ± 0.03aAB | 25.48 ± 1.34aB | 423.29 ± 5.42aB |
| HTST | 81.5 ± 40.31aC | 0.60 ± 0.02aC | 9.34 ± 0.36aC | 13.79 ± 1.46aAB | 13.74 ± 0.77aB | 7.54 ± 0.05aAB | 26.65 ± 0.77aB | 462.32 ± 14.23aA | |
| Acidified | LTLT | 83.24 ± 2.00aB | 0.59 ± 0.01aC | 8.69 ± 0.27aCD | 12.56 ± 1.17aB | 14.77 ± 0.06aA | 3.37 ± 0.01aC | 20.79 ± 0.73bC | 186.88 ± 8.53bF |
| HTST | 83.70 ± 0.23aB | 0.58 ± 0.03aC | 8.35 ± 0.10aD | 12.80 ± 0.36aB | 14.89 ± 0.12aA | 3.33 ± 0.01aC | 22.16 ± 0.81aC | 225.18 ± 5.11aE | |
| Sterilization | 118 °C/25 min | 85.89 ± 0.34aA | 1.13 ± 0.03aA | 17.15 ± 0.41aA | 14.63 ± 0.35aA | 14.11 ± 0.04aB | 7.63 ± 0.03aA | 32.39 ± 1.02aA | 348.29 ± 11.14aD |
| 121 °C/11 min | 86.51 ± 1.28aA | 0.95 ± 0.04bB | 15.84 ± 0.17bB | 14.16 ± 0.57aA | 14.13 ± 0.06aB | 7.60 ± 0.21aA | 33.25 ± 1.99aA | 392.39 ± 4.08aBC |
Value are presented as mean ± standard deviation (n = 3)
LTLT: low temperature long time (75 °C, 180 min)
HTST: high temperature short time (90 °C, 15 min)
Different lowercase superscripts in the same column within the same B-nest-Bev type (Non-acidified pasteurized; acidified pasteurized; sterilized) indicate significant differences (p < 0.05)
Different uppercase superscripts in the same column indicate significant differences (p < 0.05)
The total difference in color (∆E*) was lowest in pasteurized acidified samples (p < 0.05) and no difference was found between pasteurized non-acidified and sterilized samples (p > 0.05). Overall, high temperature and pressure used for sterilization could accelerate Maillard reaction, as indicated by increased CIE a* and CIE b*. Simultaneously, CIE L* increased possibly because of the aggregation of proteins. Coagulation of proteins could bring about turbidity and increased lightness (Petruzzi et al. 2017). Acidified samples showed lower CIE b* after pasteurization. This was possibly due to a lesser extent of Maillard reaction in acidic conditions (Dissanayake et al. 2013). Maskan (2006) reported that color is an important quality characteristic and a major factor affecting consumer perceptions of beverages and foods. However, the colors of all B-nest-Bev samples complied with Thai standards (TISI-TCPS-1083/2009). Choice of thermal processing and pH adjustment impacted color of the product. Petruzzi et al. (2017) found that different thermal treatments may induce changes in several chemical and physical properties of fruit (orange, lemon and apple) juices. Thus, the color of B-nest-Bev varied, depending on the thermal process choice and its conditions.
Soluble solid content and pH
Basically, soluble solids are the solutes in the liquid or solvent appearing in the form of solution. Soluble solids contents of B-nest-Bev produced with different thermal processes are shown in Table 1. The soluble solid content of B-nest-Bev was in the range 13.71–14.89 g/100 g sample, and the pasteurized acidified B-nest-Bev samples prepared using either LTLT or HTST processes had higher soluble solid contents than the other samples (p < 0.05). Basically, thermal processing is the main factor causing the degradation of proteins (Chantakun et al. 2020). Chantakun and Benjakul (2020a) reported that the pretreatment of B-nest flakes using sodium alginate in combination with crosslinking divalent cations yielded a gel resistance to retort processing at two tested temperatures (118 and 121 °C). However, the acidity of beverage also induced degradation of protein or peptide (Dissanayake et al. 2013). Andrés-Bello et al. (2013) reported that the pH affects protein properties by inducing denaturing, gelling, or inactivating enzymatic activities, growth, and mortality of microorganisms, etc. In the present study, the acidified beverage underwent more extensive degradation of proteins or peptides, as evidenced by higher soluble solid content. At an acidic pH, heating could induce cleavage of peptide bonds. For non-acidified B-nest-Bev, pasteurization and sterilization had no effects on soluble solids content. Thus, pH was the main factor determining the degradation of proteins in B-nest, as induced by thermal processing.
Drained weight
Table 1 displays the drained weight of B-nest-Bev treated by various thermal processes. Drained weight is the weight of solid portion in the product after draining off the free-flowing liquid syrup. B-nest-Bev from sterilization at 118 and 121 °C showed the highest drained weights of 32.39 and 33.25 g/100 g sample, respectively. On comparing between pasteurized non-acidified and acidified samples, the former had higher drained weight (p < 0.05). This indicated that the former had a higher water holding capacity in the B-nest network. No difference in drained weight was found between samples subjected to pasteurization processes, except for acidified sample in which HTST resulted in a larger drained weight (p < 0.05). Normally, the harsh conditions in sterilizing B-nest-Bev contributed to degradation of proteins. However, B-nest flakes pretreated with sodium alginate solution in conjunction with divalent cations could strengthen protein structure of B-nest in the present study. This might promote the developed complex structures, which could hold water to a higher degree (Chantakun and Benjakul 2020a). On the other hand, acidified B-nest-Bev showed lower drained weight (p < 0.05). This might be associated with the degradation of proteins. Also, the disrupted structure induced by acid and heat could not hold water effectively. Dissanayake et al. (2013) found that whey protein was denatured mainly by disruption of hydrophobic interactions and the extent of whey protein denaturation at pH 3 was more pronounced than at pH 6. Thus, thermal processing conditions directly impact drained weight of B-nest-Bev.
Sialic acid (SLA) content
Table 1 shows SLA contents in B-nest-Bev prepared by different thermal treatments. Among all the samples, pasteurized B-nest-Bev, especially from HTST, showed the highest SLA content (462.32 mg/L sample), followed by LTLT (423.29 mg/L sample). Long heating time might cause the destruction of SAL content. When acidified samples were pasteurized, especially by LTLT, a marked decrease in SLA content was found (186.88 mg/L sample). Therefore, acidic conditions in combination with heat could destroy SAL. For sterilized B-nest-Bev, SLA content ranged from 348.29 to 392.39 mg/L sample. The use of a shorter time (11 min) could preserve SLA more than the longer treatment time (25 min), although the former had a higher temperature. Chen et al. (2014) reported SLA contents in other foods such as crucian eggs, egg yolk, egg-white, cows’ milk, and cheese, which had 0.45, 0.11, 0.03, 0.02, and 0.02 g/100 g (wet weight basis) contents. Quek et al. (2018) documented that N-acetylneuraminic acid (Neu5Ac) was the main SLA found in B-nest. The stability and degradation rate of Neu5Ac is dependent on the storage conditions and pH of the solution. Zhu et al. (2020) found that the degradation rate of Neu5Ac was high under strongly acidic conditions. Decarboxylation and deacetylation of Neu5Ac readily occurred with heating at low pH. On the other hand, at pH 7.0, Neu5Ac showed good thermal stability. In the present study, the acidified B-nest-Bev (pH 3.33–3.37) showed lower SLA content than the others. This confirmed the aforementioned findings. The results reveal that pH is the main factor affecting the stability of SLA, especially after heating for a long time.
Appearance
The appearance of B-nest-Bev in glass bottles is shown in Fig. 1a. All the samples consisted of both a liquid (syrup) and a solid (B-nest) portion. Visual differences in appearances of the samples were observed. The sterilized B-nest-Bev (E and F) contained larger sized B-nest flakes than the pasteurized samples (A-D). However, only the pasteurized non-acidified B-nest-Bev from HTST had a large particle size, similar to the sterilized B-nest-Bev (E and F). This suggests that sterilization at 121 °C and pasteurizing with HTST without acidification are the proper choice for the production of safe B-nest-Bev with high acceptability.
Fig. 1.
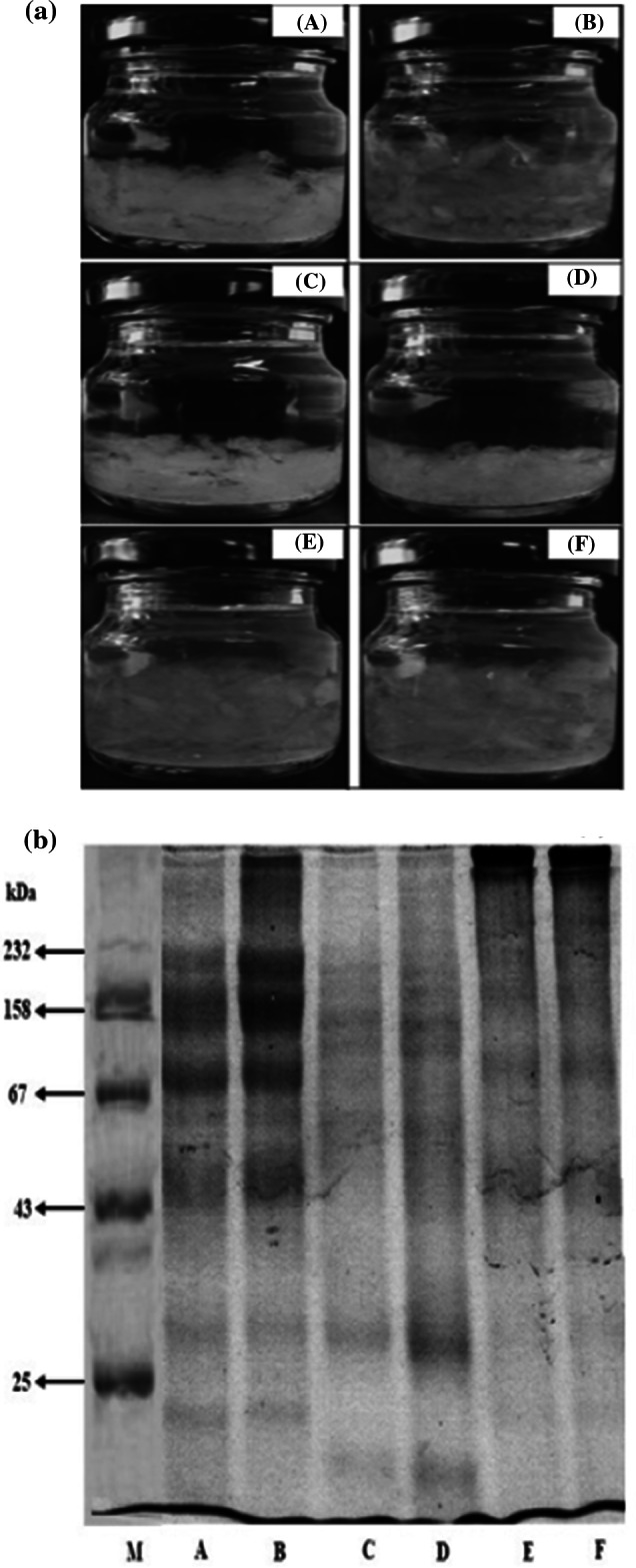
Appearance (a) and protein patterns (b) of B-nest-Bev produced by various processes; M: molecular weight standards; A (pasteurized B-nest-Bev, LTLT); B (pasteurized B-nest-Bev, HTST); C (pasteurized acidified B-nest-Bev, LTLT); D (pasteurized acidified B-nest-Bev, HTST); E (sterilized B-nest-Bev,118 °C) and F (B-nest-Bev using sterilization process at 121 °C)
Protein patterns
Protein patterns in B-nest-Bev produced by various thermal processes are illustrated in Fig. 1b. Different B-nest-Bev samples showed different protein patterns. Pasteurized B-nest-Bev (lanes A and B) had the highest band intensities, in which dominant proteins had molecular weights of 70–235 kiloDalton (kDa). Initial B-nest flakes without thermal processing or treatment had protein bands with molecular weights (MW) of 55–127 kDA (Chantakun and Benjakul 2020b). On comparing between pasteurized B-nest-Bev prepared by LTLT (lane A) and by HTST (lane B), the latter had higher band intensities. This might be due to more degradation with a long time of heating. When the B-nest was modified using alginate and divalent, it could withstand pasteurization temperature to a high extent. On the other hand, most protein bands of pasteurized acidified B-nest-Bev (lanes C and D) disappeared. This might be caused by drastic degradation of proteins, induced by acid and heat (White et al. 2013). Similar protein patterns were found for pasteurized acidified B-nest-Bev samples prepared by LTLT and HTST. However, HTST led to slightly more retained bands with MWs of 17 and 32 kDa. This indicates that different thermal pasteurization processes also influence protein patterns of acidified B-nest-Bev to some degrees. In addition, the proteins of sterilized B-nest-Bev (lanes E and F) were mainly found with MW above 232 kDa, appearing at the top of separation gel, while most of the proteins disappeared. Chantakun et al. (2020) reported that the ionic interactions of sodium alginate induced by divalent cations, followed by drying in an air-conditioned room for a long time, could strengthen the network of B-nest. During sterilization, proteins might undergo polymerization with the aid of strong network stabilized by Na-alginate. As a consequence, larger MW proteins could be formed. No differences in protein patterns were generally observed between samples sterilized at 118 and 121 °C.
Changes in antioxidative activities of B-nest-Bev in GIMs
ABTS radical scavenging activity (ABTS-A)
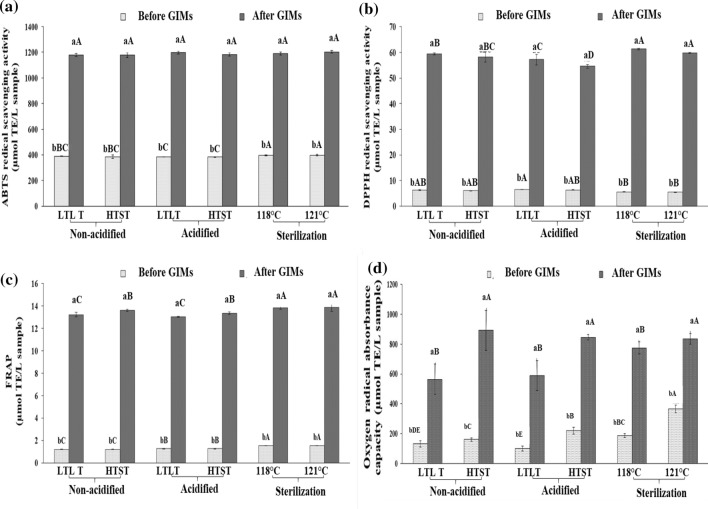
Figure 2A shows ABTS-A of B-nest-Bev before and after GIMs. ABTS-A in all cases was lower before GIMs than after GIMs (p < 0.05). No differences in ABTS-A were observed among all samples after GIMs (p > 0.05). After thermal processing (before GIMs), the B-nest-Bev sterilized at both 118 and 121 °C showed the highest ABTS-A, compared with the pasteurized samples (p < 0.05). Sun et al. (2018) reported that Okara extracts at 230 °C showed higher ABTS-A than those extracted at lower temperatures. Normally, ABTS-A could reflect the hydrogen donating and chain-breaking capacity of the extract or compound. Chantakun et al. (2020) also reported that ABTS-A in B-nest flakes was augmented after thermal processing, since proteins could be cleaved under harsh conditions. The increase in ABTS-A for all samples after digestion in GIMs was likely associated with the release of antioxidative peptides produced by enzymes in the digestion system. Those peptides generated during GIMs increased the ABTS-A.
Fig. 2.
Changes in antioxidative activities before and after gastrointestinal tract model system (GIMs) of B-nest-Bev produced by various processes. Bars represent the standard deviation (n = 3). Different lowercase letters on the bars within the same B-nest-Bev type indicate significant differences (p0.05). Different uppercase letters on the bars within the same GIMs condition (before or after) of each B-nest-Bev type indicate significant differences (p0.05). Caption: See Table 1 footnote
Ferrous reducing antioxidant power (FRAP)
FRAP of B-nest-Bev after the varying treatments (before GIMs) and after GIMs is shown in Fig. 2B. Similarly, the B-nest-Bev produced by sterilization (118 or 121 °C) showed the highest FRAP, followed by pasteurized acidified and pasteurized non-acidified B-nest-Bev, in the descending order. FRAP measures the potential of antioxidants to reduce Fe3+ (ferric ions) to Fe2+ (ferrous ions) (Binsan et al. 2008). Rodríguez-Salinas et al. (2019) reported that an increase in ferric ion reduction occurred as the temperature increased, which was attributed to the synergism of phytochemicals and melanoidins, produced by Maillard reaction. Overall, higher FRAP was attained (p < 0.05) after digestion in the GIMs than before digestion, in all cases. This result confirmed that B-nest-Bev was cleaved by some enzymes in GIMs, resulting in increased antioxidant activities.
DPPH radical scavenging activity (DPPH-A)
B-nest-Bev with different thermal treatments showed increases in DPPH-A after GIMs digestion, as seen in Fig. 2C. After GIMs, the sterilized (118 or 121 °C) B-nest-Bev samples showed the highest (p < 0.05) DPPH-A (61.32 and 60.79 µmol TE/L sample), followed by pasteurized non-acidified and acidified samples. After thermal processing, similar DPPH-A was found in all cases. Chantakun et al. (2020) reported that B-nest-Bev from B-nest flakes pretreated with different divalent cations and sterilized at 121 °C showed different DPPH-A in the range 26.44–57.51 µmol TE/L sample, depending on the type of divalent cation used. In addition, Sae-leaw and Benjakul (2018) found that antioxidant activities of hydrolyzed collagen powder were enhanced after digestion in the GIMs, in which peptides capable of scavenging free radicals with reducing ability were dominant. Generally, the ability of an antioxidant to scavenge DPPH radicals (DPPH-A assay) has been used as an indicator of donating hydrogen to radicals to form a stable diamagnetic molecule (Binsan et al. 2008). Similar trends were observed for FRAP and ABTS-A, in that all cases showed increased antioxidant activity after digestion in the GIMs. Thus, some antioxidative peptides in B-nest-Bev were produced by the digestion in GIMs, leading to increased antioxidant activities (p < 0.05) compared to those before digestion.
Oxygen radical absorbance capacity (ORAC)
ORAC of all the B-nest-Bev cases before and after digestion in the GIMs is shown in Fig. 2D. Sterilizing B-nest-Bev at 121 °C gave the highest ORAC (p < 0.05). The lowest ORAC was found for pasteurized acidified sample after LTLT processing (p < 0.05). After GIMs, ORAC was increased but varied from 773.75 to 892.52 μmol TE/L sample, depending on the case. The results generally had similar trends to ABTS-A, FRAP, and DPPH-A, in which all samples after digestion in GIMs had increased activities. Sae-leaw and Benjakul (2018) reported the increased ORAC after GIMs of hydrolysate collagen from ossein, and also found increased ABTS-A radical scavenging activity and FRAP after GIMs. ORAC assay measures the radical chain breaking ability of antioxidants by monitoring the inhibition of peroxyl radicals (Binsan et al. 2008). Hence, ORAC is considered an index related to the effectiveness of an antioxidant (Sae-leaw and Benjakul 2018). Overall, antioxidant activities of B-nest-Bev were enhanced after GIMs, regardless of the earlier thermal processing used.
Microbiological quality
Both pasteurized and sterilized B-nest-Bev samples had microbial loads under the limit of TISI-TCPS-1083/2009 or of the Food and Drug Administration Thailand Standards: Beverage in a hermetically sealed container (FDA Thailand-std-BHSC 2013) (Table 2). Basically, P-value is an indicator of pasteurization and is used by process operators to considerably reduce the number of microorganisms to a level where they are not able to cause disease (Ghani et al. 2001). On the other hand, the F0-value is an index of sterilization for killing all the microorganisms in the food or beverage (Chantakun and Benjakul 2020b). In addition, Kunitake et al. (2014) reported that the pasteurization conditions HTST (85–95 °C) yielded acidified sugarcane juice beverage free of coliforms and Salmonella sp. Initial raw material had high microbial load (7.8 × 106 CFU/g). However, those thermal processes used in the present study effectively killed those microorganisms present in the raw material. This indicates that various thermal treatments could assure quality and safety for consumption.
Table 2.
Microbial counts of B-nest-Bev produced by various processes
| Microbiological quality | Non-acidified | Acidified | Sterilization | |||
|---|---|---|---|---|---|---|
| LTLT | HTST | LTLT | HTST | 118 °C/25 min | 121 °C/11 min | |
| Coliform (MPN/g) | < 3 | < 3 | < 3 | < 3 | – | – |
| Escherichia coli (MPN/g) | < 3 | < 3 | < 3 | < 3 | – | – |
| Staphylococcus aureus (MPN/g) | < 3 | < 3 | < 3 | < 3 | – | – |
| Salmonella sp. (/25 g) | Negative | Negative | Negative | Negative | – | – |
| Bacillus cereus (CFU/g) | < 3 | < 3 | < 3 | < 3 | – | – |
| Clostridium perfringens (CFU/g) | < 10 | < 10 | < 10 | < 10 | – | – |
| Total viable count (CFU/g) | < 10 | < 10 | < 10 | < 10 | – | – |
| Yeast and mold (CFU/g) | < 10 | < 10 | < 10 | < 10 | – | – |
| Mesophiles, aerobe | –* | – | – | – | Negative | Negative |
| Mesophiles, anaerobe | – | – | – | – | Negative | Negative |
| Thermophiles, aerobe | – | – | – | – | Negative | Negative |
| Thermophiles, anaerobe | – | – | – | – | Negative | Negative |
See Table 1 footnote
* Analysis was not conducted
Acceptability of B-nest Bev
Pasteurized B-nest-Bev samples from HTST and sterilization at 121 °C had higher liking scores for appearance and overall (p < 0.05) than the other cases (Table 3). Regarding liking of color, no differences among the sample were observed (p > 0.05). Pasteurized acidified B-nest-Bev had the lowest liking scores for taste (p < 0.05), mainly because it was sour. A similar texture liking score was found in all cases. Benjakul et al. (2018) found that sterilized herbal soup added with hydrolyzed collagen sterilized at 121 and 115 °C with different retorting times (F0 of 5–11 min) had different acceptability. The higher overall liking score was found in soups sterilized at 121 °C than those using sterilization temperature of 115 °C (p < 0.05). Similarly, Chantakun and Benjakul (2020a) reported that sterilization at 121 °C for 11 min could render the beverage from pretreated B-nest flakes with high acceptability. Thus, pasteurized B-nest-Bev, HTST of non-acidified B-nest-Bev is recommended for production, while sterilization at 121 °C for 11 min could produce B-nest-Bev with a high acceptability.
Table 3.
Acceptability of B-nest-Bev produced by various processes
| B-nest-Bev Types | Thermal Process | Appearance | Color | Taste | Texture | Overall |
|---|---|---|---|---|---|---|
| Non-acidified | LTLT | 6.80 ± 1.32bBC | 7.33 ± 0.90aA | 7.30 ± 1.12bA | 6.87 ± 0.99bAB | 6.07 ± 0.96bC |
| HTST | 7.73 ± 0.88aA | 7.53 ± 0.92aA | 7.57 ± 0.92aA | 7.67 ± 0.49aA | 7.74 ± 0.41aA | |
| Acidified | LTLT | 6.33 ± 1.05bC | 7.33 ± 0.82aA | 6.27 ± 1.53bB | 6.67 ± 1.18bB | 6.20 ± 1.42bC |
| HTST | 6.67 ± 0.90aBC | 7.13 ± 0.92aA | 7.00 ± 0.80aB | 7.09 ± 0.80aAB | 6.90 ± 0.80aB | |
| Sterilization | 118 °C/25 min | 7.00 ± 0.85bBC | 7.19 ± 1.03aA | 7.27 ± 1.10bA | 7.00 ± 1.25bAB | 7.07 ± 1.25bB |
| 121 °C/11 min | 7.33 ± 1.05aA | 7.27 ± 1.16aA | 7.50 ± 1.06aA | 7.40 ± 1.24aA | 7.70 ± 1.10aA |
Different lowercase superscripts in the same column within the same B-nest-Bev type (Non-acidified pasteurized; acidified pasteurized; sterized) indicate significant differences (p < 0.05)
Different uppercase superscripts in the same column indicate significant differences (p < 0.05). Caption: See Table 1 footnote
Conclusions
Beverages based on B-nest flakes pretreated with a solution of sodium alginate and calcium lactate in syrup could be produced using different thermal processes, including pasteurization or sterilization, in which the microbial load was below an acceptable level. Nevertheless, the resulting beverages had varying quality and acceptability, depending on the thermal process used. Antioxidant activities of all B-nest-Bev samples increased during simulated gastrointestinal digestion, thus enhancing their bioactivities. HTST of non-acidified beverage is the recommended process for pasteurization, while sterilization of non-acidified beverage at 121 °C is suggested, in which the B-nest-Bev still had high acceptability.
Authors' contributions
KC: Investigation, data analysis, methodology, validation. writing-original draft. Soottawat B: Conceptualization, funding acquisition and editing of manuscript.
Funding
The authors would like to express their sincere thanks to Prince of Songkla University for the financial support under Prachayacharn program (Grant No. AGR6402088N).
Availability of data and material
Research data are not shared.
Code availability
Not Applicable.
Declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethics approval
The experiment was reviewed and approved by the ethical committee (ethical number FIRIn 2562/023) of Prince of Songkla University, Hat Yai, Thailand.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Amit SK, Uddin MM, Rahman R, Islam SMR, Khan MS. A review on mechanisms and commercial aspects of food preservation and processing. Agric Food Secur. 2017;6:1–22. doi: 10.1186/s40066-017-0130-8. [DOI] [Google Scholar]
- Andrés-Bello A, Barreto-Palacios V, García-Segovia P, Mir-Bel J, Martínez-Monzó J. Effect of pH on color and texture of food products. Food Eng Rev. 2013;5:158–170. doi: 10.1007/s12393-013-9067-2. [DOI] [Google Scholar]
- Benjakul S, Chantakun K, Karnjanapratum S. Impact of retort process on characteristics and bioactivities of herbal soup based on hydrolyzed collagen from seabass skin. J Food Sci Technol. 2018;55:3779–3791. doi: 10.1007/s13197-018-3310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106:185–193. doi: 10.1016/j.foodchem.2007.05.065. [DOI] [Google Scholar]
- Chantakun K, Benjakul S. Effect of pretreatments and retort process on characteristics and sensory quality of edible bird’s nest beverage. Int J Food Sci Technol. 2020;52:2863–2871. doi: 10.1111/ijfs.14542. [DOI] [Google Scholar]
- Chantakun K, Benjakul S. Qualities of dried edible bird’s nest flakes from different drying methods and properties of their beverage. Dry Technol. 2020;2020:1–11. doi: 10.1080/07373937.2020.1783551. [DOI] [Google Scholar]
- Chantakun K, Nuthong P, Benjakul S. Influence of different alginate pretreatments on characteristics of edible bird's nest flakes and their sterilized beverage. LWT-Food Sci Technol. 2020;131:109695. doi: 10.1016/j.lwt.2020.109695. [DOI] [Google Scholar]
- Chen Y, Pan L, Liu N, Troy FA, Wang B. LC-MS/MS quantification of N-acetylneuraminic acid, N-glycolylneuraminic acid and ketodeoxynonulosonic acid levels in the urine and potential relationship with dietary sialic acid intake and disease in 3- to 5-year-old children. Br J Nutr. 2014;111:332–341. doi: 10.1017/S0007114513002468. [DOI] [PubMed] [Google Scholar]
- Dissanayake M, Ramchandran L, Piyadasa C, Vasiljevic T. Influence of heat and pH on structure and conformation of whey proteins. Int Dairy J. 2013;28:56–61. doi: 10.1016/j.idairyj.2012.08.014. [DOI] [Google Scholar]
- Escuder-Vieco D, Espinosa-Martos I, Rodríguez JM, Corzo N, Montilla A, Siegfried P, Pallás-Alonso CR, Fernández L. High-temperature short-time pasteurization system for donor milk in a human milk bank setting. Front Microbiol. 2018;9:1–16. doi: 10.3389/fmicb.2018.00926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration. 2001a. Bacteriological analytical manual. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Food Safety and Applied Nutrition. Retrieved on July 20, 2021 from: https://www.fda.gov/food/laboratory-methods-food/bam-chapter-3-aerobic-plate-count.
- Food and Drug Administration Thailand Standards. (2013). Beverage in a hermetically sealed container. Retrieved on July 20, 2021 from: http://food.fda.moph.go.th.
- Ghani AGA, Farid MM, Chen XD, Richards P. A computational fluid dynamics study on the effect of sterilization temperatures on bacteria deactivation and vitamin destruction. J Process Mech Eng. 2001;215:9–17. doi: 10.1243/0954408011530253. [DOI] [Google Scholar]
- Hobbs JJ. Problems in the Harvest of Edible Birds’ Nests in Sarawak and Sabah, Malaysian Borneo. Biodivers Conserv. 2004;13:2209–2226. doi: 10.1023/B:BIOC.0000047905.79709.7f. [DOI] [Google Scholar]
- Kong F, Tang J, Rasco B, Crapo C. Kinetics of salmon quality changes during thermal processing. J Food Eng. 2007;83:510–520. doi: 10.1016/j.jfoodeng.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Kunitake M, Ditchfield C, Silva C, Petrus R. Effect of pasteurization temperature on stability of an acidified sugarcane juice beverage. Cienc Agrotec. 2014;38:554–561. doi: 10.1590/S1413-70542014000600004. [DOI] [Google Scholar]
- Maskan M. Effect of thermal processing on tristimulus color changes of fruits. Stewart Postharvest Rev. 2006;2:1–8. doi: 10.2212/spr.2006.5.10. [DOI] [Google Scholar]
- Petruzzi L, Campaniello D, Speranza B, Corbo MR, Sinigaglia M, Bevilacqua A. Thermal treatments for fruit and vegetable juices and beverages: a literature overview. Compr Rev Food Sci F. 2017;16:668–691. doi: 10.1111/1541-4337.12270. [DOI] [PubMed] [Google Scholar]
- Quek MC, Chin NL, Yusof YA, Law CL, Tan SW. Characterization of edible bird's nest of different production, species and geographical origins using nutritional composition, physicochemical properties and antioxidant activities. Food Res Int. 2018;109:35–43. doi: 10.1016/j.foodres.2018.03.078. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Salinas P, Muy D, Urías-Orona V, Zavala Garcia F, Suarez-Jacobo A, Heredia J, Rubio-Carrasco W, Niño-Medina G. Thermal processing effects on the microbiological, physicochemical, mineral and nutraceutical properties of a roasted purple maize beverage. Farm. 2019;67:587–595. doi: 10.31925/farmacia.2019.4.5. [DOI] [Google Scholar]
- Sae-leaw T, Benjakul S. Antioxidant activities of hydrolysed collagen from salmon scale ossein prepared with the aid of ultrasound. Int J Food Sci Technol. 2018;53:5786–2795. doi: 10.1111/ijfs.13891. [DOI] [Google Scholar]
- Sinchaipanit P, Kerr WL. Effect of reducing pulpparticles on the physical properties of carrot juice. ASEAN Food J. 2007;14:205–214. [Google Scholar]
- Singh A, Prabowo F, Benjakul S, Pranoto Y, Chantakun K. Combined effect of microbial transglutaminase and ethanolic coconut husk extract on the gel properties and in-vitro digestibility of spotted golden goatfish (Parupeneus heptacanthus) surimi gel. Food Hydrocoll. 2020;109:106107. doi: 10.1016/j.foodhyd.2020.106107. [DOI] [Google Scholar]
- Steel RGD, Torrie JH. Principles and Procedures of Statistics. McGraw-Hill, New York: ABiometrical Approach; 1980. [Google Scholar]
- Sun H, Yuan X, Zhang Z, Su X, Shi M. Thermal processing effects on the chemical constituent and antioxidant activity of Okara extracts using subcritical water extraction. J Chem. 2018;2018:6823789. doi: 10.1155/2018/6823789. [DOI] [Google Scholar]
- Thai Industrial Standards Institute. 2009. Thai Agricultural Standard: Bird’s Nest Beverage (TISI-TCPS-1083/2009). Retrieved on July 20, 2021 from: http://app.tisi.go.th
- White SS, Fox KM, Jervis SM, Drake MA. Influence of heating and acidification on the flavor of whey protein isolate. J Dairy Sci. 2013;96:1366–1379. doi: 10.3168/jds.2012-5935. [DOI] [PubMed] [Google Scholar]
- Zhu W, Chen X, Yuan L, Wu J, Yao J. Degradation kinetics and shelf-life of N-acetylneuraminic acid at different pH values. Molecules. 2020;25:5141–5150. doi: 10.3390/molecules25215141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.
Not Applicable.