Abstract
A study was made to expedite ion chromatography method using IonPac analytical column and self-regenerating anion suppressor for phytic acid determination in groundnut seeds and compared with a widely adopted spectrophotometric method based on enzymatic hydrolysis. The Ion Chromatography method equipped with AG11 guard and AS11 analytical columns in isocratic mode using 65 mM NaOH mobile phase at 1 mL min−1 flow rate showed a sharp peak for phytic acid with a retention time of 2.42 ± 0.2 min. The peak area was plotted v/s concentration showed linearity with an R2 value of 0.997, detection limit of 0.028 mg L−1 and recovery of 98% as against R2 value of 0.988 and detection limit of 0.065 mg L−1 in the spectrophotometric method. The study demonstrates that Ion Chromatography method was more accurate with a better detection limit than spectrophotometry. Also, this method provides robust handling with lesser reagent requirements due to combined eluent generation and self-regenerating suppression.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05527-9.
Keywords: Absorption, Phytic acid, Groundnut, Ion chromatography, Spectrophotometric method
Introduction
Groundnut is an important food legume and oilseed crop of the semi-arid and arid part of the world where about 48.8 million tons of pods are produced from about 29.8 million hectares area (Singh et al. 2021). It is consumed worldwide either as raw or after frying, salting, and boiling and is recognised as ready‐to‐use therapeutic food (RUTF) to treat protein-energy malnutrition in children. However, the presence of anti-nutrients like allergens, flatulence producing oligosaccharides (raffinose, stachyose, verbascose), and phytic acid limit groundnut consumption as human food. Phytic acid (myo-inositol-1,2,3,4,5,6-hexakisphosphate or IP6) is the principal storage form of phosphorous (P) in cereals, legumes, oilseeds and nuts, accounting for 50–85% of total P of plants (Phillippy et al. 2003; Singh et al. 2015; Ajay et al. 2017). The amount of phytic acid has been of interest to nutritionists for its ability to cause micronutrient malnutrition by decreasing the bioavailability of essential minerals on one side and on the other to show positive attributes related to the prevention of lead toxicity, lowering of serum cholesterol and triglyceride levels, cancer, and kidney stones (Abdelrahaman et al. 2007). Thus, its detection and quantification in foods become essential. Walnut, groundnut and almond are rich in phytic acid, and their content varies from 0.1–9.4 g 100 g−1 (Venktachalam and Sathe 2006), whereas 0.17–4.47 g 100 g−1 phytic acid has been reported in groundnut. However, reference daily intake (RDI) of phytic acid ranges between 200–800 mg in developed countries and about 2000 mg in developing countries. Under physiological conditions, phytic acid is a highly negatively charged anion with great affinity for binding positively charged cations, especially iron, zinc, calcium and magnesium (Das et al. 2012; Singh et al. 2022). Such ionic conjugates are soluble under the acidic conditions of the stomach but undergo precipitation in the intestine at neutral pH, limiting the bioavailability of minerals and trace elements.
Phytic acid was first detected by measuring the refractive index (Graf and Dintzis 1982), subsequently various methods based on derivatisation were used (Indyk and Woollard 1994). However, all these methods have limitations due to their inability to separate structural isomers, the need for derivatisation, or they were cumbersome. The techniques recently being used to determine phytic acid in foods are colorimetry based on Fe3+–phytate precipitation (Heubner and Stadler 1914) or enzymatic hydrolysis (phytase), Complexometric-Titration (Burgos-Luján and Tong 2015), NMR spectroscopy (Kemme et al. 1999), mass spectroscopy (MS), inductively coupled plasma-MS (Munoz and Valiente 2003), HPLC (Bariya et al. 2015) and anion-exchange chromatography (Talamond et al. 1998). Other methods such as capillary zone electrophoresis (Simonet et al. 2003; Marolt and Kolar 2021) and capillary isotachophoresis are also used but have not been widely applied. Phytic acid being a multivalent anion, IC is an ideal and preferred method due to its specificity, precision, simplicity and relatively low cost per sample. In this study, we present a comparative report of an analytical method utilising IC system with suppressed conductivity detection and enzyme-based spectrophotometric method to quantify phytic acid in groundnut seeds.
Materials and methods
Reagents and materials
The groundnut seeds of cultivar GJG-22 from Rabi-summer 2019 were obtained from Germplasm Resource Section, Directorate of Groundnut Research, Junagadh, Gujarat, India. The chemicals used were of analytical grade and deionised water of specific resistance of 18.2 mµ cm, prepared through Heal Force (NW series) ultra-pure water purification system was used to prepare the reagent solutions. Sodium phytate (C6H6Na12O24P6; HiMedia, India) was used to prepare a standard phytic acid solution (1000 mg L−1) in deionised water. Five working calibration standards of 1, 5, 10, 15 and 20 mg L−1 were prepared by diluting the stock solution with a determined volume of deionised water. One quality control containing 1 mg L−1 of standard sodium phytate was added into the sample before extraction in triplicate to check the per cent recovery in spiked over unspiked samples.
Instrumentation
Phytic acid analysis was performed on Dionex ICS-3000 Ion Chromatography supported by Chromeleon® Chromatography Management software version 6.7. An aliquot of 25 µL of the sample solution was injected after passing through a Thermo scientific Dionex OnGuard II Ag/H cartridge with 65 mM NaOH as an eluant at a flow rate of 1 mL min−1. Separation was achieved at 30 °C temperature by using a guard (Dionex IonPac® AG11 4 × 50 mm) and analytical (Dionex IonPac® AS11 4 × 250 mm) column with a particle diameter of 13 µm (Fig.S1, supplementary material (SM)). The detection of phytic acid in the samples was on the basis of suppressed conductivity. For this, Dionex ASRS 300 anion self-regenerating suppressor (4 mm diameter) was used in recycling mode at 200 mA of current. The total run time was 7 min, where an initial 4 min was used for analyte separation, and 3 min for washing and equilibrating the column with mobile phase between runs.
Methods
Method 1:analysis by ion chromatography
Raw dry seeds of groundnut cultivar GJG-22 were ground and passed through a 60 mesh screen for the analysis of phytic acid. Oil and moisture content was determined using Instalab®700 NIR analyzer (DICKEY-john, Auburn, Illinois, US). For the extraction of phytic acid, 200 mg defatted samples, as obtained by following the method described in one of our laboratory’s earlier publications (Chakraborty et al. 2013), were taken into 50 mL screw cap glass bottles in triplicate, and 10 mL of 3 M HCl was added with continuous shaking. Samples were placed in a boiling water bath for 10 min with constant shaking at 100 rpm, cooled, transferred into a 50 mL volumetric flask and volume was made with deionized water. Each sample was then filtered using Whatman filter paper no. 1. Volume of 2.5 mL of each sample was transferred into a fresh 50 mL volumetric flask and volumes were made with deionized water. The resulting extract (25 µL) was injected into the chromatographic system after passing through the Dionex OnGuard II Ag/H cartridge (Fig. S1).
Method 2: analysis by spectrophotometric method based on enzymatic hydrolysis
Phytic acid as total phosphorus released by phytase and alkaline phosphatase was determined spectrophotometrically by using megazyme kit (Megazyme 2017). One gram of the whole-seed defatted meal (as described by Chakraborty et al. 2013) was digested with 20 mL of 0.66 M HCl in a 100 mL conical flasks, placed on a magnetic stirrer with oscillatory agitation (42 oscillations min−1) for 16H at room temperature. After digestion, 1 mL of the extract was transferred to a 1.5 mL microfuge tube and centrifuged at 13,000 rpm for 10 min at room temperature. Immediately 0.1 mL of the resulting extract supernatant was transferred to a new microfuge tube and neutralized by adding 0.5 mL of 0.75 M NaOH. A control blank sample was maintained with 0.1 mL of 0.66 M HCl. Following the protocol of Megazyme for enzymatic dephosphorylation reaction to measure free and total phosphorus content of the samples, 1.5 mL microfuge tubes were prepared (Table 1) and incubated in a thermomixer at 40 °C for ten minutes. During the first minute of this incubation, the tubes were shaken at 1400 rpm. After incubation, different solutions, as mentioned in Table 1, were added to these tubes for free and total P determination and further incubated at 40 °C for 15 min with shaking during the first minute at 1400 rpm. To this, 0.5 mL of colour reagent, consisting of 5 parts of solution A and 1 part of solution B was added, where solution A is 10% of Ascorbic acid in 1 M Sulphuric acid (w/v) and solution B is 5% of Ammonium molybdate (w/v). This was mixed by a vortex mixer and incubated in a water bath for 1 h at 40 °C. The phosphorus calibration curve was prepared as per the Megazyme protocol (0–7.5 mg L−1). Finally, the absorbance at 655 nm of each sample was read using U-3010 Spectrophotometer (Hitachi High-Technologies Corp., Japan). Finally, the calculation of phosphorus and phytic acid content was carried out following Megazyme instructions.
Table 1.
Preparation of enzymatic dephosphorylation reactions for calculating free and total phosphorus (g P 100 g.−1)
| Stepwise additions for the reactions | Free P (mL) | Total P (mL) |
|---|---|---|
| Distilled water | 0.62 | 0.6 |
| Solution 1 (buffer provided by Megazyme) | 0.2 | 0.2 |
| Neutralized sample extract | 0.05 | 0.05 |
| Suspension 2 (phytase prepared as per Megazyme kit instructions) | – | 0.02 |
| Mixed well and incubated for 10 min at 40 °C | ||
| Distilled water | 0.02 | – |
| Solution 3 (buffer provided by Megazyme) | 0.02 | 0.02 |
| Suspension 4 (alkaline phosphatase provided by Megazyme) | – | 0.02 |
| Solutions were mixed well on a vortex mixer and incubated in a water bath for 15 min at 40 °C | ||
| Trichloroacetic acid (50% w/v) | 0.30 | 0.30 |
Solutions were centrifuged at 13,000 rpm for 10 min and 1.0 mL of supernatant was transferred to a new tube for spectrophotometric estimation
Development and validation of IC method
Linearity
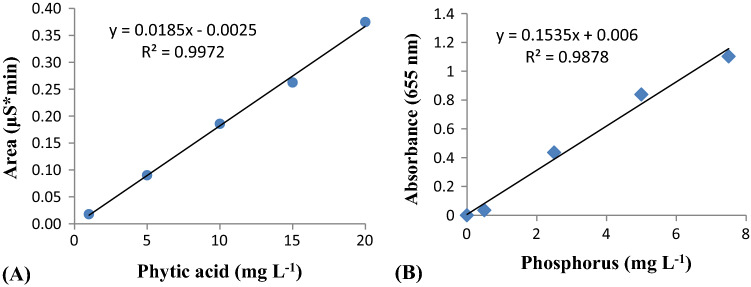
Linearity of phytic acid was evaluated through a graphical representation of concentration in mg L−1 versus peak area in µS*min, depending on total sodium phytate concentration of standard solutions, whereas for linearity of phosphorus graph was plotted between concentration of standard phosphorus in mg L−1 versus absorbance at 655 nm. Table 2 shows the data for obtaining the calibration curve and limit of quantification. Figure 1 shows the regression line in the prediction interval.
Table 2.
Linearity data for analysis of phytic acidand Phosphorus in groundnut seeds
| Sr. No | Concentration of standard solution | Mean Peak Area (µS*min) | Absorbance at 655 nm | % RSDr | R2 | Slop | Intercept | |
|---|---|---|---|---|---|---|---|---|
| Phytic acid (mg L−1) | ||||||||
| 1 | 1 | 0.0175 | – | 0.88 | 0.997 | 0.018 | 0.002 | |
| 2 | 5 | 0.0899 | – | 0.22 |
Linear regression equation: y = 0.018x—0.002 LOD ( mg L−1): 0.028 LOQ ( mg L−1): 0.084 |
|||
| 3 | 10 | 0.1855 | – | 0.08 | ||||
| 4 | 15 | 0.2622 | – | 0.08 | ||||
| 5 | 20 | 0.3745 | – | 0.05 | ||||
| Phosphorus (mg L−1) | ||||||||
| 1 | 0 | – | 0.000 | 0.00 | 0.987 | 0.153 | 0.006 | |
| 2 | 0.5 | – | 0.034 | 8.860 |
Linear regression equation: y = 0.153x + 0.006 LOD ( mg L−1): 0.065 LOQ ( mg L−1): 0.196 |
|||
| 3 | 2.5 | – | 0.435 | 10.08 | ||||
| 4 | 5 | – | 0.838 | 13.09 | ||||
| 5 | 7.5 | – | 1.103 | 17.76 | ||||
Fig. 1.
Regression line and equation of phytic acid (A) and Phosphorus (B) in the prediction interval
Repeatability
Repeatability was evaluated by calculating the relative standard deviation (RSD) of five determinations of standard phytic acid from 1–20 mg L.−1 performed on the same day under the same experimental conditions. The experimental data with% RSDr of less than one (0.05–0.88) showed that the method is precise (Table 1).Relative standard deviation was calculated as:
Recovery (%)
Sample was prepared from GJG-22 variety in three replications and three injections were made of each preparation (Table 3). Solution for spiking was prepared by adding a standard stock solution of phytic acid to the sample before extraction to yield 20 mg L−1 after sample preparation and three injections were made of each spiked sample. The spiked sample solutions were analysed according to the same analytical procedure followed for the samples of GJG-22 variety and the recovery is calculated as: Recovery % = Cu/Cs × 100.Where Cu (g per 100 g) is the concentration of phytic acid measured in non-spiked samples, and Cs (g per 100 g) is the concentration of phytic acid measured in spiked samples.
Table 3.
Amount of phytic acid (g 100 g.−1) in GJG-22 variety of groundnut and Recovery % through IC
| Injection No | Phytic acid (g 100 g−1) | |||||
|---|---|---|---|---|---|---|
| Sample Replicates | Spiked Sample Replicates | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | |
| 1 | 4.381 | 4.002 | 4.197 | 4.384 | 4.1358 | 4.290 |
| 2 | 4.197 | 4.057 | 4.147 | 4.205 | 4.158 | 4.248 |
| 3 | 4.417 | 4.147 | 4.197 | 4.424 | 4.281 | 4.291 |
| Mean | 4.332 | 4.069 | 4.180 | 4.338 | 4.192 | 4.276 |
| RSD% | 2.72 | 1.80 | 0.69 | 2.69 | 1.87 | 0.57 |
| Spiked Concentration | 20 mg L−1 | |||||
| Recovery % | 98.29 | |||||
Statistical analysis
Standard deviation and % RSDr were calculated through Microsoft Excel, version 6.1.7600.16385 (Microsoft, United States). The limit of detection (LOD) and limit of quantification (LOQ) for both methods were calculated from standard solutions using the minimal accepted value of S/N ratio of 3.3:1 and 10:1 respectively (Table 2). The lowest concentration standard (1 ppm) was run thrice and LOD and LOQ were calculated from the given formula using the standard deviation(s) of the area of the peak obtained from injections, and the slope (m) of the calibration according to the formula:
Results and discussion
A challenge, we faced in utilizing the IC method for separating phytic acid was the poor resolution of peaks due to interfering ionic contamination. In this study, the best conditions in terms of concentration of extracting solvent and length of the extraction time to extract phytic acid from groundnut seeds were standardized. Seed sample extraction procedure was firstly standardized for the molarity of HCl (0.5—4 M) used for phytic acid extraction. AOAC’s official method for phytic acid estimation also recommends HCl as an effective medium for extracting phosphates in foods. Kwanyuen and Burton (2005) suggested a 0.5 M concentration of HCl for maximal recovery of phytic acid from soybeans and soy products with an extraction time of 1 h at room temperature. However, based on our experimental results, we found that the optimal molarity of HCl for maximal extraction was 3 M with an extraction time of 10 min in a boiling water bath with constant shaking at 100 rpm (4.69 g 100 g−1). The analytical IC method was optimized to establish a quality separation of phytic acid in groundnut seeds using AG11 guard and AS11 analytical column in isocratic mode i.e. concentration of solvent to elute analyte remained constant. Secondly, potassium hydroxide (KOH) was tested to obtain the separation and resolution with varying flow rates. However, resolution was not obtained even after 20 min of run time. The mobile phase was then changed and 65 mM of NaOH was used at a flow rate of 1 mL min−1 and produced a quite sharp peak of phytic acid at a retention time of 2.42 ± 0.2 min as followed by Talamond et al. (1998). This range of run time was sufficient to produce a sharp peak while ensuring that other compounds with similar conductivity as those of phytic acid may not interfere. The use of suppressor conductivity detection leads to enhanced conductivity of the analyte.
Linearity
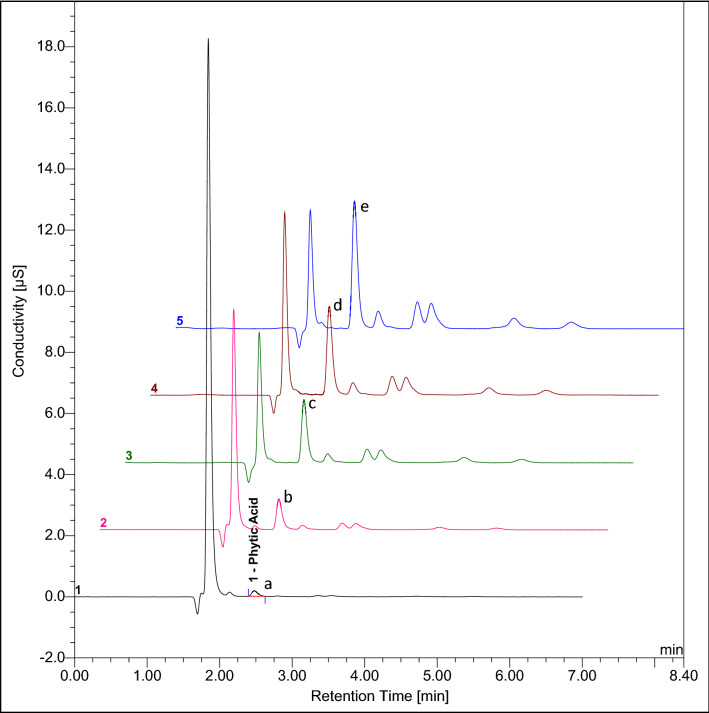
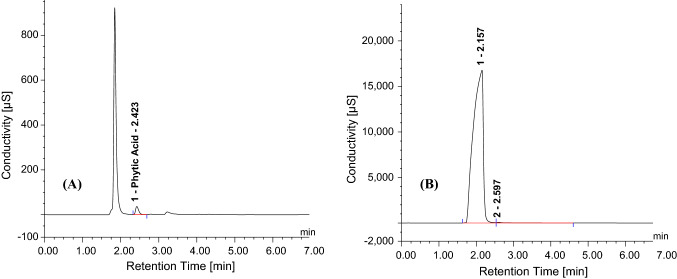
The detector response of IC (peak area, µS*min) and spectrophotometer (A655nm), when plotted against the concentration of standards (Phytic acid and Phosphorus in mg L−1) was found to be linear (Fig. 1) in the concentration range tested. The linearity of the developed IC method was quite evident from the overlay chromatogram (Fig. 2) obtained under the same chromatographic conditions. The peak area was proportional to the phytic acid concentration over the entire range. The observed values of regression coefficient (R2) for Phytic acid (0.997) and Phosphorus (0.987) are in accordance to the ICH Guidelines (2005). It is important to mention here that the Dionex OnGuard II Ag/H cartridge was used for injecting the sample into the IC system so as to remove interfering chloride and carbonate ions without which separation and resolution were not observed (Fig. 3b). This cartridge has layers of resins from Silver (Ag) and Hydrogen (H) which prevents overloading of the column with the chloride in the sample, thus, facilitating better analysis (Fig. 3a). Attempts were also made to optimize and reduce the run time per sample to make the method time efficient. The run time was shortened to 7 min from 20 min and the observed chromatographic data was processed to concentration. Separation and resolution were achieved within an initial 3–4 min which is quite lower than earlier reports (Phillippy et al. 2003; Talamond et al. 1998).
Fig. 2.
Overlay of chromatograms of phytic acidstandard where peak numbers a, b, c, d and e corresponds to 1, 5, 10, 15 and 20 ppm respectively. Separation of phytic acid using guard (Dionex IonPac® AG11 4 × 50 mm) and analytical (Dionex IonPac® AS11 4 × 250 mm) column; eluent: 65 mM NaOH; detection: chemically suppressed conductivity using ASRS300 anion self-regenerating suppressor (4 mm)
Fig. 3.
Chromatogram of GJG-22 (1 mg L.−1) with (A) and without (B) Dionex OnGuard II Ag/H cartridge
Recovery and selectivity
Table 2 indicates the precision and accuracy of the developed chromatographic method over classical spectrophotometric method. Appreciable recovery rates (98.29%) of phytic acid indicated good compatibility of extraction media for groundnut seeds. The minimum standard concentration of phytic acid measured through IC was able to produce LOD of 0.028 mg L−1 and LOQ of 0.084 mg L−1 which was lower than the spectrophotometric method. Per cent RSDr of the IC method was less than 10, showing the stability of developed method (Table 2). Our results are in agreement with Phillippy et al. (2003) and show that the recoveries are good even in the spiked samples, suggesting method accuracy.
Comparison of the two methods
A few methods of phytic acid determination have been developed. Phytic acid is hydrolysed by phytase and alkaline phosphatase during the digestion in the human gut and food processing to form lower inositol phosphates such as inositol di- and monophosphate, inositol triphosphate (IP3), inositol tetraphosphate (IP4) and inositol pentaphosphate (IP5). Out of which IP6 and all forms of IP5 showed to possess a negative effect on the bioavailability of essential minerals, whereas the other hydrolytic products formed have a poor binding capacity to minerals (Sandberg et al. 1989). The IC method is more specific as it gives a combined estimation of all isoforms of phytic acid which restricts the bioavailability of essential minerals and thus gives a correct estimate of phytic acid content in processed foods (Sandberg 1995). As per the megazyme’s kit instructions, defatting of groundnut seed meal was done only in the spectrophotometric method. Being a water-soluble analyte, concentration of phytic acid will not be affected by defatting procedure through n-hexane. The enzymatic method showed a mean value of 1.43 ± 0.41 g 100 g−1 of phytic acid in GJG-22 cultivar which is underestimated when compared with a mean value obtained from the IC method (4.13 ± 0.15 g per 100 g−1). According to Fruhbeck et al. (1995) and Saad et al. (2011), the use of chromatographic purification yielded significantly higher results than those obtained from the spectrophotometric detection method, hence demonstrating that reliable phytic acid estimation in foodstuffs demands the usage of chromatographic methods over spectrophotometric method. Megazyme kit procedure for phytic acid estimation in wheat samples was scaled-down and slightly modified by Magallanes-Lopez et al. (2017) and showed phytic acid in the range of 0.522–0.705% instead of 0.546–0.683%. According to Talamond and coworkers (1998), another spectrophotometric method based on ferric precipitation method cannot be used for the determination of phytic acid in all foods because of the presence of interfering substances and suggested High performance – IC method for routine analysis of phytate in food samples. Moreover, the spectrophotometric method is based on stoichiometric metal replacement reaction from colouring reagent and formation of colourless iron (III)-phytate complex (Dost and Tokul 2006) and the absorbance must be measured within a short defined time as the intensity of colour changes with respect of time (Vaintraub and Lapteva 1988; Marolt and Kolar 2021). Despite the high sensitivity of enzymatic hydrolysis, incomplete or partial hydrolysis constitutes a potential source of error. For this reason, IC method was found to be advantageous over the spectrophotometric method. Recently, IC method was coupled with mass spectrometry to identify IP6 isolated from complex mixtures of soil samples (McIntyre et al. 2020) and the results obtained further proved the robustness of the technique applicable to a wide range of samples.
Conclusion
The advantages of using IC method are twofold. First, the IC separation of phytic acid from the complex matrix provides a purified sample with lesser interfering compounds. Second, the conductivity suppressor in the IC machine removes Cl−1and other ionic contaminants from the eluate via ASRS 300 anion self-regenerating suppressor. Together, the results show that the IC method using IonPac® AG11 guard and IonPac® AS11 analytical column is more rapid, accurate and sensitive than the spectrophotometric method, with a lesser detection limit. The addition of the OnGuard II Ag/H cartridge would further ensure that there will be no interfering peaks (chloride, fluoride etc.) that are often misled with phytic acid determination. The sensitivity, rapidity and less reagent processing of the IC quantification may be exploited further in other food samples.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Authors are grateful to the Director, ICAR-Directorate of Groundnut Research for all the support required to conduct this experiment.
Abbreviations
- IC
Ion chromatography
- LOD
Limit of detection
- LOQ
Limit of quantification
- RSD
Relative standard deviation
- RUTF
Ready‐to‐use therapeutic food
Author contributions
AV: Conducted research, wrote the initial draft of MS, planning and execution of work; S S: Conducted research, analysed data; LKT: Assisted in analysis work, creation of data; MKM: Conceptualize the idea, wrote and edited MS; ALS: Overall supervision, edited MS.
Funding
External was not funding received for this research work. This work was carried out under Institute Projects.
Availability of data and material
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals.
Consent to participate
Informed consent not applicable.
Consent for publication
Not applicable.
Footnotes
Research Highlights Ion chromatography (IC) with suppressed conductivity was used to quantify phytic acid in groundnut kernels. IC facilitated faster quantification of phytic acid in extracts of complex matrices of oilseed groundnut. The study demonstrates that the IC method was more sensitive and accurate with a better detection limit than the spectrophotometric and enzymatic methods.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdelrahaman SM, Elmaki HB, Idris WH, Hassan AB, Babiker EE, El Tinay AH. Antinutritional factor content and hydrochloric acid extractability of minerals in pearl millet cultivars as affected by germination. Int J Food Sci Nutr. 2007;58(1):6–17. doi: 10.1080/09637480601093236. [DOI] [PubMed] [Google Scholar]
- Ajay BC, Meena HN, Singh AL, Bera SK, Dagla MC, Kumar N, Makwana AD. Response of different peanut genotypes to reduced phosphorous availability. Indian J Genet. 2017;77(1):105–111. doi: 10.5958/0975-6906.2017.00014.1. [DOI] [Google Scholar]
- Bariya H, Singh AL, Chaudhari V (2015) Measurement of Fe(II) and Fe(III) in groundnut by in-column and post-column reactions in ion chromatography. Comm Soil Sci Plant Anal 46(3):358–366.10.1080/00103624.2014.981272
- Burgos-Luján I, Tong AZ. Determination of phytic acid in juices and milks by developing a quick complexometric-titration method. Food Anal Methods. 2015;8:1836–1841. doi: 10.1007/s12161-014-0075-5. [DOI] [Google Scholar]
- Chakraborty K, Bishi SK, Singh AL, Kalariya KA, Kumar L. Moisture deficit stress affects yield and quality in groundnut seeds. Ind J Plant Physiol. 2013;18(2):136–141. doi: 10.1007/s40502-013-0020-4. [DOI] [Google Scholar]
- Das A, Raychaudhuri U, Chakraborty R (2012) Cereal based functional food of Indian subcontinent: a review. J Food Sci Tech 49(6):665–672. 10.1007%2Fs13197-011-0474-1 [DOI] [PMC free article] [PubMed]
- Dost K, Tokul O. Determination of phytic acid in wheat and wheat products by reverse phase high performance liquid chromatography. Anal Chim Acta. 2006;558(1–2):22–27. doi: 10.1016/j.aca.2005.11.035. [DOI] [Google Scholar]
- Fruhbeck G, Alonso R, Marzo F, Santidrian SA. Modified method for the indirect quantitative analysis of phytate in foodstuff. Anal Biochem. 1995;225(2):206–212. doi: 10.1006/abio.1995.1145. [DOI] [PubMed] [Google Scholar]
- Graf E, Dintzis FR. Determination of phytic acid in foods by high-performance liquid chromatography. J Agri Food Chem. 1982;30(6):1094–1097. doi: 10.1021/jf00114a022. [DOI] [Google Scholar]
- Heubner W, Stadler H. Uber eineTitrationsmethode zur Bestimmung des Phytins. Biochem. 1914;Z64:422–437. [Google Scholar]
- ICH Guideline (2005) IHT Validation of Analytical Procedures: Text and Methodology. Q2 (R1), Vol. 1.
- Indyk HE, Woollard DC. Determination of free myo-inositol in milk and infant formula by high-performance liquid chromatography. Analyst. 1994;119(3):397–402. doi: 10.1039/an9941900397. [DOI] [PubMed] [Google Scholar]
- Kemme PA, Lommen A, De Jonge LH, Van der Klis JD, Jongbloed AW, Mroz Z, Beynen AC. Quantification of Inositol phosphates using 31P nuclear magnetic resonance spectroscopy in animal nutrition. J Agric Food Chem. 1999;47(12):5116–5121. doi: 10.1021/jf981375v. [DOI] [PubMed] [Google Scholar]
- Kwanyuen P, Burton JW (2005) A simple and rapid procedure for phytate determination in soybeans and soy products. J Am Oil Chem' Soc 82(2):81–84. https://naldc.nal.usda.gov/download/13906/PDF
- Magallanes-Lopez AM, Hernandez-Espinosa N, Velu G, Posadas-Romano G, Ordonez-Villegas VMG, Crossa J, Ammar K, Guzmán C. Variability in iron, zinc and phytic acid content in a worldwide collection of commercial durum wheat cultivars and the effect of reduced irrigation on these traits. Food Chem. 2017;237:499–505. doi: 10.1016/j.foodchem.2017.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marolt G, Kolar M. Analytical Methods for Determination of Phytic Acid and Other Inositol Phosphates: A Review. Molecules. 2021;26(1):174. doi: 10.3390/molecules26010174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CA, Arkell JJL, Arthur CJ, Lawrence PG, Butts CP, Lloyd CEM, Johnes PJ, Evershed RP (2020) Identification and quantification of myo-inositol hexakisphosphate in complex biological and environmental matrices using ion chromatography and high-resolution mass spectrometry in comparison to 31P NMR spectroscopy. Talanta 210:120188. 10.1016/j.talanta.2019.120188 [DOI] [PubMed]
- Megazyme (2017) https://www.megazyme.com/documents/Booklet/K-PHYT_DATA.pdf., Accessed 15.02.2020.
- Munoz JA, Valiente M. Determination of phytic acid in urine by inductively coupled plasma mass spectrometry. Anal Chem. 2003;75(22):6374–6378. doi: 10.1021/ac0345805. [DOI] [PubMed] [Google Scholar]
- Phillippy BQ, Bland JM, Evens TJ (2003) Ion chromatography of phytate in roots and tubers. J Agric Food Chem 51(2):350–353. 10.1021/jf025827m [DOI] [PubMed]
- Saad N, Norhaizan Mohd Esa N, Ithnin H, Shafie NH (2011) Optimization of optimum condition for phytic acid extraction from rice bran. Afr J Plant Sci 5(3):168–176. 10.1007%2Fs13197-011-0521-y
- Sandberg AS. Determination of phytic acid. In recent progress in the analysis of dietary fibre. Luxembourg: European Commission. COST. 1995;92:93–103. [Google Scholar]
- Sandberg AS, Carlsson NG, Svanberg U. Effects of Inositol Tri-, Tetra-, Penta-, and Hexaphosphates on in vitro estimation of iron availability. J Food Sci. 1989;54(1):159–161. doi: 10.1111/j.1365-2621.1989.tb08591.x. [DOI] [Google Scholar]
- Simonet BM, Rios A, Grases F, Valcarcel M. Determination of myo-inositol phosphates in food samples by flow injection-capillary zone electrophoresis. Electrophoresis. 2003;24(12–13):2092–2098. doi: 10.1002/elps.200305404. [DOI] [PubMed] [Google Scholar]
- Singh AL, Chaudhari V, Ajay BC. Screening of groundnut genotypes for phosphorus efficiency under field conditions. Indian J Genet. 2015;75(3):363–371. doi: 10.5958/0975-6906.2015.00057.7. [DOI] [Google Scholar]
- Singh AL, Chaudhari V, Rani K, Kona P, Mahatma MK, Verma A, Reddy KK, Singh S, Kumar LK and Patel CB (2021) Chapter 1. Cultivation, Breeding and Nutritional Uses of Groundnut. In Richard J. Whitworth (Ed.). Book title: Arachis hypogaea: Cultivation, Production and Nutritional Value. Subtitle: Agriculture Issues and Policies. Pp 1–158; Nova Science Publishers, Inc., New York, USA. ISBN: 978–1–53619–386–2.
- Singh S, Singh AL, Gangadhara K, Chaudhari V, Patel CB, Mahatma M, Verma A, Kumar L. High Zn bioavailability in peanut (Arachis hypogaea L.) cultivars: an implication of phytic acid and mineral interactions in seeds. J Plant Nutr. 2022 doi: 10.1080/01904167.2022.2035750. [DOI] [Google Scholar]
- Talamond P, Gallon G, Guyot JP, LapeI M, Treche S (1998) Comparison of high-performance ion chromatography and absorptiometric methods for the determination of phytic acid in food samples. Analusis 26(10):396–399.10.1051/analusis:1998191
- Vaintraub IA, Lapteva NA. Colorimetric determination of phytate in unpurified extracts of seed and the products of their processing. Anal Biochem. 1988;175(1):227–230. doi: 10.1016/0003-2697(88)90382-X. [DOI] [PubMed] [Google Scholar]
- Venktachalam M, Sathe SK (2006) Chemical composition of selected edible nut seeds. J Agric Food Chem 54(13):4705–4714. 10.1021/jf0606959 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].