Abstract
Myeloid leukemia is one of the major causes of deaths among elderly with very poor prognosis. Due to the adverse effects of existing chemotherapeutic agents, plant-derived components are being screened for their anti-leukemic potential. Momordica charantia (bitter gourd) possesses a variety of therapeutic activities. We have earlier demonstrated anti-leukemic activity of acetone extract of M. charantia seeds. The present study reports purification of differentiation inducing principle(s) from further fractionated seed extract (hexane fraction of the acetone extract, Mc2-Ac-hex) using HL-60 cells. Out of the 5 peak fractions (P1–P5) obtained from normal phase HPLC of the Mc2-Ac-hex, only peak fraction 3 (P3) induced differentiation of HL-60 cells as evident from an increase in NBT-positive cells and increased expression of cell surface marker CD11b. The P3 differentiated the HL-60 cells to granulocytic lineage, established by increased CD15 (granulocytic cell surface marker) expression in the treated cells. Further, possible molecular mechanism and the signalling pathway involved in the differentiation of HL-60 cells were also investigated. Use of specific signalling pathway inhibitors in the differentiation study, and proteome array analysis of the treated cells collectively revealed the involvement the of ERK/MAPK mediated pathway. Partial characterization of the P3 by GC–MS analysis revealed the presence of dibutyl phthalate, and derivatives of 2,5-dihydrofuran to be the highest among the 5 identified compounds. This study thus demonstrated that purified differentiation-inducing principle(s) from M. charantia seed extract induce HL-60 cells to granulocytic lineage through ERK/MAPK signalling pathway.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10616-022-00547-x.
Keywords: Momordica charantia, Acute myeloid leukemia, HL-60 cells, Differentiation, Granulocyte, Antibody array
Introduction
Acute myeloid leukemia (AML), at an 80% of occurrence rate in the adult population, is the most common subtype of leukemia. AML incidence rate has increased globally from 1997 to 2017, mostly in male and elder people (Yi et al. 2020). The median survival rate for AML patients is less than a year and only 32% and 24% of patients have an overall survival time of 2-year and 5 years, respectively (Shallis et al. 2019; Song et al. 2018). Chemotherapy is the most reliable therapy for treating AML. However, severe toxicity, high relapse rate, and drug resistance are major concerns with this approach. Since leukemia arises due to a defect in the differentiation pathway of the precursor cells, differentiation therapy has gained interest in the past few decades and has shown tremendous potential for treating AML patients. Differentiation therapy involves the use of external agents that trigger differentiation of defective leukemic cells, irreversibly alter the cancer cell phenotype into normal cells which subsequently undergo apoptosis. ATRA (all-trans-retinoic acid), a vitamin A derivative has been used clinically in differentiation therapy for treating AML patients (Breitman et al. 1980; Brown and Hughes 2012; Lo-coco et al. 2013). However, its adverse side effects such as differentiation syndrome, leucocytosis, and resistance limit its use against AML. Therefore, the search for new molecules capable of inducing differentiation of leukemic cells with minimal side effects and better efficacy continues. Few of these molecules include DMSO, arsenic trioxide, securinine, luteolin, homoharringtonine, etc., many of which are plant-derived. These potential molecules could be effective by themselves or can be used in combination with the existing therapies.
The present study was aimed to identify the differentiation-inducing potential of Momordica charantia seed extract. Momordica charantia (Mc), commonly known as bitter melon belongs to the Cucurbitaceous family with great nutritional and medicinal value (Lucas et al. 2010). It is widely distributed in the tropical area of Asia, Africa, South America, part of the Amazon Basin, and the Caribbean. Different parts of M. charantia plant contain several biologically active phytochemicals, which have been reported to confer a variety of biological activities including anti-cancer, anti-tumor, anti-oxidant, anti-inflammatory (Budrat and Shotipruk 2008). M. charantia extracts (pulp, peel, seed, and whole fruit) reduced tumor burden, incidence rate, the cumulative number of papillomas in mice with skin papilloma genesis (For review, please see Shetty et al. 2005). The M. charantia extract or its active constituents have also been reported to possess anti-leukemic activity, as well as protective effects against lymphoma, Hodgkin’s disease, and a variety of cancer (reviewed by Raina et al. 2016). The anti-cancer activity of M. charantia or its components is exerted through a variety of mechanisms viz. regulation of cell signaling, activation of reactive oxygen species (ROS), regulation of glucose and lipid metabolism, hypoxia, inhibition of invasion and angiogenesis, induction of apoptosis, and augmentation of immune defense system (Sur and Ray 2020). The anti-leukemic potential of M. charantia was first established in 1971 by West and colleagues (West et al. 1971). These investigators reported prolonged survival (over 2 years) of a patient suffering from lymphatic leukemia, owing to the consumption of an aqueous extract of bitter melon (200 ml, twice daily). Earlier studies from our laboratory have demonstrated that the seed extract of M. charantia (Pusa Vishesh variety) induced differentiation of HL-60 cells toward granulocytic lineage, which was characterized by increased CD11b expression, specific esterase activity, and decreased c-Myc transcription (Soundararajan et al. 2012).However, the active principle(s) responsible for the induction of differentiation has not been purified, and also the mechanism of induction of differentiation is not elucidated. Therefore, the present study was undertaken to purify and identify the major differentiation-inducing bioactive principle(s) of M. charantia seed extract, as well as to decipher the signalling pathways involved in the induction of differentiation in human’s promyelocytic leukemic cells (HL-60 cells).
Materials and methods
Reagents
MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide], NBT (nitroblue tetrazolium), DMSO (dimethyl sulfoxide), doxorubicin, TPA (12-O-tetradecanoylphorbol-13-acetate), antibiotic antimycotic solution (100X) were purchased from Sigma-Aldrich Chemical Company, USA. RPMI 1640, trypan blue, fetal bovine serum (FBS), and penicillin–streptomycin were purchased from Biological Industries, Israel. HPLC grade solvents namely methanol, ethanol, isopropanol, acetone, hexane, dimethylformamide (DMF), chloroform, dimethyl sulfoxide (DMSO)-NMR grade, were procured from Merck, Germany.
Cell surface marker anti-human PE-cy5 anti-human CD11b antibody, FITC-labeled anti-human CD15, and FITC-labeled anti-human CD14 antibody were from Biolegend, USA. Pharmacological kinase inhibitors, PD98059 (MEK1/2 inhibitor), SP600125 (JNK1/2/3 inhibitor), wortmannin (PI3 kinase inhibitor), SB202190 (P38 inhibitor), and sunitinib (receptor tyrosine kinase inhibitor) were purchased from Sigma-Aldrich Chemical Company, USA.A protein kinase A inhibitor (H89) was procured from Tocris Bioscience, UK.
Rabbit monoclonal antibody against p44/42 MAPK (ERK1/2) was obtained from Cell Signaling Technology, USA (Cat. No. 4695S). Mouse monoclonal antibody against phosphorylated p44/42 MAPK (p-ERK1/2) was obtained from Santa Cruz Biotechnology, Inc. USA (sc-7383). Mouse monoclonal anti-GAPDH antibody was obtained from Thermo Fisher Scientific, USA (Cat. # AM4300). Secondary antibodies i.e. HRP-conjugated goat anti-rabbit IgG (Cat. No. AP106P) and goat anti-mouse IgG (Cat. No. A9917) were from Sigma-Aldrich, USA. Clarity™ ECL Western blot substrate kit was obtained from Bio-Rad, USA.
Plant material
M. charantia (F1 hybrid sadabahar variety) seeds were purchased from the Indian Agricultural Research Institute (IARI) Pusa, New Delhi, India.
Cell culture
HL-60 cells obtained from National Center for Cell Science, Pune, India were grown and maintained in a complete 1 × RPMI 1640 culture medium supplemented with 10% FBS and 1 × antibiotic antimycotic solution under sterile conditions in humidified 5% CO2 atmosphere at 37 °C in a CO2 incubator.
Preparation of fractionated M. charantia seed extract
M. charantia seeds rinsed in 0.1 N HCl, were air-dried, decorticated, and ground into a fine powder using liquid nitrogen. Acetone extract of M. charantia seeds was prepared essentially as described by Soundararajan et al. (2012). Briefly, the seed powder (200 g) was resuspended in 10 volumes of acid ethanol buffer (0.2 N HCl in 75% ethanol, 1 mM PMSF) followed by centrifugation at 20,000×g for 1 h. The soluble fraction (Mc-1) was concentrated to 1/4th of its original volume under reduced pressure followed by neutralization with 0.05 M (NH4)2CO3 and liquid ammonia to adjust the pH to 7.4. The resultant solution was centrifuged at 20,000×g for 45 min. The pellet and the supernatant fractions were designated as Mc-2 and Mc-3 respectively. The Mc-2 pellet fraction was suspended in acetone and incubated overnight (O/N) at -70 °C. After centrifugation at 20,000×g at 4 °C for 45 min, the pellet was discarded and the acetone extractable fraction (Mc2-Ac) was obtained by evaporation. The Mc2-Ac was resuspended in water and sequentially extracted with 5 volumes of organic solvents in decreasing order of hydrophobicity i.e. hexane, chloroform, diethyl ether and ethyl acetate, with continuous stirring for 3 h, followed by centrifugation (20,000×g) at 4 °C for 1 h. After each extraction, the organic solvents were carefully harvested, and dried under vacuum. The resultant powder thus obtained was referred to as Mc2-Ac-hex, Mc2-Ac-CHCl3, Mc2-Ac-DE and Mc2-Ac-EA, respectively. Yields of different fractions obtained from 200 g decorticated seed powder are mentioned in Supplementary Table 1. Each of these fractions were assessed for differentiation-inducing potential together with parent fractions Mc2-Ac and Mc2-Ac-hex.
Analytical HPLC
Firstly, analytical normal phase-HPLC (NP-HPLC) was performed to optimize conditions for the separation of components (peaks) present in Mc2-Ac-hex for further purification, using a silica column (L × ID − 250 mm × 4.6 mm with 5 µM particle size) fitted with a guard column (L × ID − 30 mm × 10 mm) in Varian 920 (Agilent, USA) HPLC system. The peaks identified by absorbance at 270 nm were separated using a linear gradient of hexane (65–61%): chloroform (35–39%) containing 0.1% TFA at a flow rate of 0.5 ml/min for 15 min.
Semi-preparative HPLC
Agilent 1260 infinity series HPLC system was used for analytical and semi-preparative NP-HPLC. For purification of different peak fractions, a semi-preparative silica column (L × ID − 250 mm × 10 mm with 5 µM particle size) protected with 5 µM (L × ID − 30 mm × 10 mm) guard column was used. A linear gradient of hexane and chloroform containing 0.1% TFA was run for 45 min at a flow rate of 1 ml/min. The absorption was measured at 270 nm.
NBT reduction assay
Nitroblue tetrazolium assay to assess the generation of superoxide radicals in differentiated HL-60 cells was carried out as described by Breitman et al. (1980). HL-60 cells were cultured in a 12 well plate at a density of 0.2 × 106 cells/well. The cells were treated with the test fractions for different time points, as mentioned in the legends. The cells treated with 1. 2% DMSO were taken as positive control whereas the cells treated with 0.18% DMSO were included as vehicle control (VC). Post-treatment, the cells were harvested by centrifugation (200×g, 5 min) and resuspended in 100 µl (2 × 105 cells) of complete RPMI medium containing TPA (10 µg/ml) and NBT (at a final concentration of 0.1% v/v prepared in 30% DMF), and incubated at 37 °C for 30 min. The cells were then centrifuged again (200×g, 5 min). The cell pellet was washed once with 1 × PBS, and finally resuspended in 100 µl 1 × PBS. Approximately 200 cells were scored for NBT staining (in triplicates) using a light microscope. The extent of differentiation was expressed as percent NBT positive cells.
Minimum time required to trigger differentiation
HL-60 cells were seeded in 12 well-plate at a density of 0.2 × 106 cells/ml/well. The next day, the cells were treated with Mc2-Ac-hex fraction (20 µg/ml) for different time intervals (24 h, 48 h, 72 h, and 96 h). At each time point (24 h, 48 h, 72 h, and 96 h), the cell culture medium in the plate was replaced with the normal complete RPMI medium without any Mc2-Ac-hex. On 96 h, the treated cells were scored for NBT positive cells (as described in the above section) from day 1 to day 4.
Assessment of cell viability
To assess the cytotoxicity of HPLC-purified active peak 3 fraction (P3) of M. charantia Mc2-Ac-hex extract, the HL-60 cells were seeded in 96 well plates at a density of 5 × 103cells/well/100 µl and incubated O/N in a humidified 5% CO2 atmosphere at 37 °C. After incubation, the cells were treated with different concentrations of P3. DMSO (0.18%) treated cells were included as vehicle control and Doxorubicin (10 μM)-treated cells were included as a positive control. Cell viability was measured using MTT assay at 96 h post-treatment as described by Yedjou et al. (2006). Absorbance was measured at 570 nm using a microplate reader (Tecan-Sunrise). Percent cytotoxicity was calculated with respect to untreated cells.
Immunophenotyping of differentiated HL-60 cells for lineage determination
HL-60 cells were seeded (1 × 106 cells/3 ml/well) in a 6 well plate. After 12 h, the cells were treated with Mc2-Ac-hex (20 µg/ml), P3 fraction (20 µg/ml), and vehicle control (0.18% DMSO). DMSO (1.2%) treated cells were taken as a positive control. Cells were harvested at 96 h by centrifugation (200×g, 5 min) and washed with ice-cold 1 × PBS containing 10% FBS and 1% sodium azide. The cell samples (in 400 µl of cold 1 × PBS containing 10% FBS and 1% sodium azide) were stained individually with PE-cy5-labeled anti-human CD11b antibody, FITC-labeled anti-human CD15, or FITC-labeled anti-human CD14 antibody (0.3 µg/ml each) at 4 °C for 45 min in dark. The cells were washed with 1 × PBS and resuspended in 400 µl of ice-cold PBS containing 10% FBS and 1% sodium azide. Finally, the cells were assessed for CD11b/CD15/CD14 expression (excitation at 488 nm; emission at 667 nm for PE-Cy5 CD11b, at 578 nm for FITC-labelled CD14 and CD15) using FACS-Aria flow cytometer, and results were analyzed by FACS-Diva software.
Identification of signalling pathway
To delineate the signalling pathway underlying the P3- induced differentiation of HL-60 cells, specific inhibitors namely PD98059 (Ajenjo et al. 2000), SP600125 (Jiang et al. 2003), Wortmannin (Ito et al. 2016), SB202190 (Jacquel et al. 2006), Sunitinib (Ocadlikova et al. 2021), and H89 (Chijiwa et al. 1990) targeting specific pathways at the optimized concentrations were used to block the targeted pathway. For this, HL-60 cells (0.3 × 106 cells/ml) were seeded in a 12 well plate and incubated for 12 h in a CO2 incubator. After 12 h, the cells were treated with reported effective concentrations of different inhibitors (references cited above). The cells were treated with 5 µM each of PD98059, SP600125, Wortmannin, SB202190, and H89 and 15 µM of Sunitinib, for 45 min prior to the addition of the P3 fraction. The cells were further cultured for 96 hand finally subjected to NBT staining and CD11b expression analysis as described above.
Western blot analysis
In order to confirm the inhibitory effect of PD98059, a specific inhibitor of activation of ERK1/2, on P3-induced differentiation is through ERK pathway, effect of different concentrations of PD98059 on the expression levels of phosphorylated ERK1/2 were analyzed by Western blotting. For this, HL-60 cells were seeded (1 × 106 cells/3 ml/well) in a 6 well plate. After 12 h, the cells were treated with different concentrations of PD98059 for 45 min prior to the addition of P3 fraction (20 µg/ml). Vehicle (0.18% DMSO)-treated and P3-treated cells were included as negative and positive controls, respectively. The cells were cultured in a humidified CO2 atmosphere (5%) for 96 h. The cells were harvested, washed with 1 × PBS and lysed in 150 µl mammalian cell lysis buffer (50 mM Tris–HCl, pH 7.5,150 mM NaCl, 1% triton-X-100, 10 mM iodoacetamide, 1 mM EDTA supplemented with 1 × protease inhibitor cocktail (ProteaseArrest, Cat. # 786-108, G-Biosciences, USA). Protein concentration was estimated using Bicinchoninic acid assay kit (G-Biosciences, USA).
Western blot analysis was performed by the method of Karan et al. (2021). Equal amounts of proteins (~ 50 µg) from each sample electrophoresed on SDS-PAGE gels (12%) were electro-transferred onto nitrocellulose membrane. After blocking the membrane with 2% BSA in 1 × PBST (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4, and 0.05% Tween 20) overnight at 4 °C, the membrane was washed three times with 1 × PBST, and finally incubated with primary antibody diluted in 1 × PBST [anti-ERK1/2, anti-p-ERK1/2 (1:500); anti-GAPDH (1:1000)] for 1 h at room temperature. The membrane was then incubated with the corresponding secondary antibody (1:5000) for 1 h at room temperature followed by PBST washes. The blot was developed using Clarity™ enhanced chemiluminescence (ECL) Western blot substrate (Bio-Rad, USA). The images were acquired using ChemiDoc XR S + Gel imaging system (Bio-Rad, USA).
Proteome profiling
Proteome Profiler Human Phospho-Kinase Array Kit (ARY003B, R&D Systems, USA) was used according to the manufacturer’s protocol to analyze the relative level of phosphorylation of different proteins. The cell lysates of the P3-treated and vehicle-treated cells were prepared at 96 h post-treatment and protein content was estimated using BCA protein estimation kit (G Biosciences, USA) as per manufacturer’s instructions. The analytes-coated membrane was incubated with array buffer 1 for blocking, followed by incubation with 600 µg of the cell lysates (4 °C, overnight). The membrane was then washed 3 × with wash buffer followed by incubation at room temperature for 2 h with detection antibody. The membrane was washed with wash buffer to remove the unbound antibodies, followed by incubation with diluted streptavidin horseradish peroxidase for 30 min with shaking. Finally, chemiluminescence reagent (1 ml) was added to the membrane and the reactive spots were detected using ChemiDoc (Syngene, USA). The ImageJ software was used for densitometry analysis (imagj.nih.gov/ij) of different spots to determine relative intensities.
GC–MS analysis
Hexane extract of M. charantia and peak fraction P3 were subjected to gas chromatography interfaced to the mass spectrometer, GC–MS–QP2010 (Shimadzu, Japan). GC–MS was performed on a Rxi-5 Sil silica capillary column (0.25 mm i.d. × 0.25 µm df), composed of 5% diphenyl- 95% dimethylpolysiloxane. The carrier gas helium (99.99%) was administered in the column at a constant flow rate of 1.21 ml/min and a linear velocity of 40.9 cm/sec with pressure at 90.5 kPa. The ion source temperature and interface temperature were kept at 220 °C and 270 °C, respectively with an ionization voltage of 70 eV. The sample injection mode was set at a split ratio of 10:1 inflow control mode. Mass spectra were recorded at an event time of 0.20 s with a scan speed of 3333 over a range set at m/z of 40 Da to 650 Da. The column oven temperature was initially kept at 100 °C, which was gradually increased till it reached the injection temperature of 260 °C. The GC–MS was run for 39.98 min with a solvent delay time of 4 min.
Compounds were identified by comparing the mass spectra of the putative components present in the analyzed extract with the established mass spectra of known references available online in the NIST chemical library (National Institute of Standards and Technology Standard Reference Data Program Gaithersburg, MD 20899).
Statistical analysis
Data represent mean ± standard deviation (SD) from a minimum of 3 independent experiments, performed in triplicates. Statistical analysis was performed using one-way ANOVA and a p value ≤ 0.05 was considered statistically significant. Prism 10 (GraphPad, USA) was used for the data analysis.
Results
It has been established earlier in our laboratory that the differentiation-inducing potential of M. charantia is present in the acetone extractable fraction of its seeds. Therefore, to further purify and characterize the active components responsible for the induction of differentiation of HL-60 cells, the acetone extractable fraction (Mc2-Ac) was further extracted using organic solvents with decreasing hydrophobicity and test of their differentiation inducing ability.
Differentiation inducing ability of different organic fractions of Mc2-Ac
Analysis of the differentiation-inducing ability of different organic fractions revealed that the differentiation-inducing principle(s) was predominantly present in hexane extract (Mc2-Ac-hex) (Table 1). As evident from NBT staining at 96 h, the Mc2-Ac-hex resulted in ~ 60% NBT positive cells at a concentration of 20 µg/ml which is higher than the number of NBT positive cells observed with the parent fraction Mc2-Ac treatment (~ 37%), thus showing enrichment of differentiation-inducing principle(s) in Mc2-Ac-hex. On the other hand, chloroform, diethyl ether, and diethyl acetate extractable fractions showed no significant difference in the % NBT positive cells when compared to vehicle-treated cells. The yield of the Mc2-Ac-hex fraction was determined to be 74.2 mg from 100 g seed powder.
Table 1.
Differentiation inducing potential of different organic fractions of K2A extract
| Treatment | % NBT positive cells at 72 h (mean ± SD) |
% NBT positive cells at 96 h (mean ± SD) |
|---|---|---|
| Vehicle control | 18.67 ± 1.26 | 20.56 ± 2.08 |
| DMSO (1.2%) | 60.25 ± 1.71** | 80.00 ± 2.94** |
| Mc2-Ac | 36.50 ± 2.08** | 57.25 ± 3.40* |
| Mc2-Ac-hex | 42.23 ± 2.06** | 64.22 ± 2.22* |
| Mc2-Ac-CHCl3 | 17.05 ± 3.46 | 21.75 ± 2.50 |
| Mc2-Ac -DE | 22.00 ± 1.83 | 26.73 ± 3.59 |
| Mc2-Ac -EA | 16.43 ± 2.08 | 21.50 ± 2.06 |
The HL-60 cells (1 × 105 cells/ml/well) were treated with equal amount of different fractions (20 µg/ml). The cells treated with the corresponding volume of vehicle (% DMSO) were included as negative control whereas the cells treated with 1.2% DMSO taken as a positive control. Cells were analysed for cell differentiation by NBT staining at 72 h and 96 h post induction. Data represent mean ± SD of three independent experiments, performed in triplicates. The Mc2-Ac represents acetone extractable fraction of M. charantia Mc-2 extract (Soundarajan et al. 2012). The Mc2-Ac-hex, Mc2-Ac-CHCl3, Mc2-Ac-DE, and Mc2-Ac-EA represent hexane fraction, chloroform fraction, diethyl ether fraction and ethyl acetate of fraction Mc-2-Ac, resepectively. Statistical significance (p value) was calculated using Student’s t-test
*p ≤ 0.01; **p ≤ 0.001
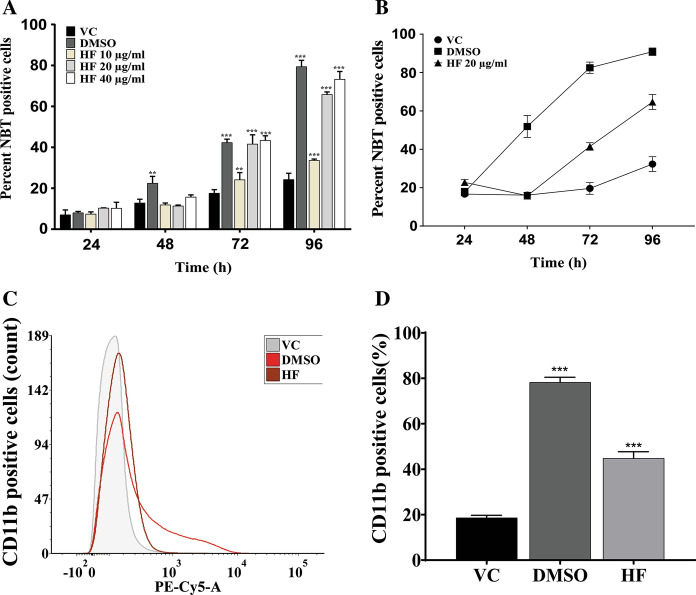
The differentiation-inducing potential of different concentrations of Mc2-Ac-hex fraction (HF) was assessed using NBT reduction assay (Fig. 1A) and confirmed by flow cytometric analysis using antibodies against differentiation marker (Fig. 1C, D). As shown in Fig. 1A, treatment of HL-60 cells with the fraction Mc2-Ac-hex at 10 µg/ml did not show any significant increase in the number of NBT positive cells in comparison to vehicle-treated control. However, treatment of HL-60 cells with Mc2-Ac-hex at 20 µg/ml and 40 µg/ml resulted in a significant increase (p ≤ 0.001) in NBT positive cells (41.64 ± 4.46%, 43.40 ± 2.23%, respectively), when compared with the vehicle-treated cells (17.65 ± 1.67%) at 72 h post-treatment. The number of NBT positive cells further increased at 96 h (65.84 ± 1.21% and 73.28 ± 3.74% with 20 µg/ml and 40 µg/ml, respectively). As expected, DMSO (1.2%) included as positive control also resulted in a significantly enhanced population of NBT positive cells (79.43 ± 3.06%; p ≤ 0.001) at 96 h.
Fig. 1.
Analysis of differentiation-inducing potential of Mc2-Ac-hex (HF). A Time and concentration dependence analysis of differentiation by Mc2-Ac-hex. NBT reduction assay was performed in a time-dependent manner at three different concentrations (10 µg/ml, 20 µg/ml, 40 µg/ml) of Mc2-Ac-hex (HF). B Minimum time required to induce differentiation of HL-60 cells by Mc2-Ac-hex (HF). The HL-60 cells were cultured in the presence of Mc2-Ac-hex (20 µg/ml) for the indicated time periods, followed by culturing in fresh medium without Mc2-Ac-hex (HF). The cells were scored for differentiation by NBT reduction assay at 96 h. C Flow cytometric analysis of CD11b positive HL-60 cells treated with Mc2-Ac-hex (HF) fraction, DMSO (1.2%, positive control) and VC (DMSO, 0.18%). The panel depicts a representative figure showing the overlay of the fluorescent cell population using PE-cy5 conjugated anti-CD11b antibodies at 96 h post-treatment. D The bar diagram shows the quantitative analysis of cells expressing CD11b, shown in panel (C). The data in all the panels represent the mean ± SD of three independent experiments, performed in triplicate. The significant difference between groups with respect to vehicle (0.18% DMSO) treated control cells (VC) were analyzed by ordinary One-way ANOVA (Dunnett’s multiple comparisons). **p ≤ 0.01; ***p ≤ 0.001
As the maximum number of NBT-positive cells was noted at 96 h post-treatment, before proceeding further, induction of differentiation of HL-60 cells by Mc2-Ac-hex fraction (20 µg/ml) was confirmed by FACS analysis at 96 h using fluorochrome-conjugated anti-CD11b antibody, a myeloid differentiation marker, (Fig. 1C, D). Representative dot plot corresponding to Fig. 1C is shown in Fig. S1. A significantly (p ≤ 0.001) enhanced population (44.83 ± 2.86%) of the CD11b-positive cells was observed in the Mc2-Ac-hex treated cells when compared to the cells treated with the vehicle (VC, 18.66 ± 1.04%).
To determine the minimum time required for induction of differentiation of HL-60 cells, the cells were cultured in the presence of Mc2-Ac-hex (20 µg/ml) for different time periods (24 h, 48 h, 72 h, and 96 h), followed by culturing in a normal medium (without Mc2-Ac-hex) till 96 h. Analysis of the NBT positive cell population at 96 h (Fig. 1B) showed a negligible increase in the NBT positive cells when treated with Mc2-Ac-hex for 48 h. However, the presence of Mc2-Ac-hex in the medium for 72 h significantly (p ≤ 0.001) increased the number of NBT positive cells (41.47 ± 1.96%), which subsequently peaked at 96 h post-treatment (64.64 ± 3.96%). These results indicate that the presence of Mc2-Ac-hex for a minimum period of 72 h was necessary to induce differentiation.
HPLC purification of bioactive principle(s) from Mc2-Ac-hex fraction of M. charantia seeds extract
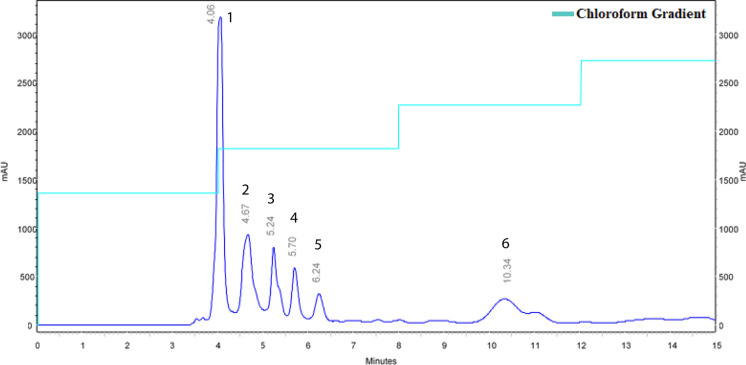
Chromatographic separation of Mc2-Ac-hex fraction using a normal phase silica analytical column resulted in six-well resolved and separated peaks (Fig. 2). During, semi preparatory HPLC, a very thin peak collected that eluted before P1 (Fig. S2) could not be analyzed further, due to very small amounts and poor separation. The retention time and total peak yield of the 5 major peaks obtained with large-scale semi preparatory HPLC are given in Supplementary Table 2. Purity check analysis of each collected peak fraction using an analytical NP-HPLC revealed significant purification with the negligible presence of some minor peaks (Fig. S3).
Fig. 2.
Analytical normal phase HPLC of the Mc2-Ac-hex. Different components (peaks) of the Mc2-Ac-hex fraction were resolved using a silica column at a gradient of chloroform (35–39%) against hexane (65–61%) at a flow rate of 0.5 ml/min with a runtime of 15 min. The chromatogram depicts the elution profile with absorbance measured at 270 nm. Injection volume-15 µl (1 mg/ml dissolved in hexane)
Determination of differentiation-inducing potential of different peak fractions of Mc2-Ac-hex
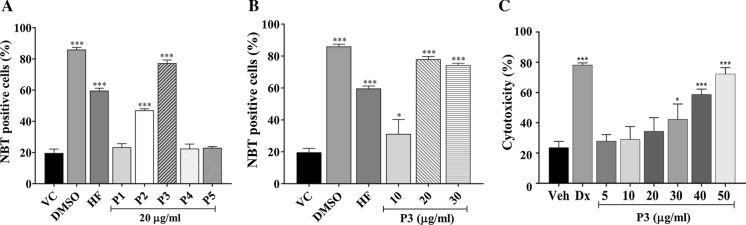
The five peak fractions of Mc2-Ac-hex (P1–P5) were assessed for their differentiation-inducing potential to identify the active peak fraction (Fig. 3A). Analysis of NBT-positive cells at 96 h post-treatment showed that the treatment with P2 and P3 resulted in a significant increase (p ≤ 0.001) in NBT positive cells (47 ± 1.0% and 77.25 ± 2.062%, respectively) when compared with the vehicle-treated control cells. The number of NBT-positive cells in the parent Mc2-Ac-hex (HF) fraction was 64.33 ± 0.57%. Peaks 1, 4, and 5 did not show any significant increase in the population of NBT positive cells when compared to vehicle-treated control.
Fig. 3.
Analysis of differentiation-inducing potential of HPLC purified peak fractions. A Different peak fractions (P1–P5) and parent fraction Mc2-Ac-hex (HF) (20 µg/ml each) were assessed for their differentiation-inducing potential by NBT reduction assay at 96 h. B HL-60 cells were treated with different concentrations of P3 and were scored for NBT positive cells at 96 h. DMSO at 0.18% and 1.2% was included as vehicle control (VC) and Positive control, respectively. C Cytotoxicity analysis of P3. HL-60 cells were treated with different concentrations of P3 and cell viability was measured using MTT reduction assay. Doxorubicin (Dx) at 10 µM was included as a positive control. Percent cytotoxicity is calculated with respect to untreated cells. Data represent the mean ± SD of three independent experiments, performed in triplicates. Ordinary One-way ANOVA (Dunnett’s multiple comparisons) was performed to calculate the significant difference with respect to VC. *p ≤ 0.05; ***p ≤ 0.001
Since P3 showed the highest percentage of NBT-positive cells (greater than the parent Mc2-Ac-hex fraction), further analysis was done with P3. To determine the optimum concentration required for induction of differentiation, HL-60 cells were treated with different concentrations of P3 (10 µg/ml, 20 µg/ml, and 30 µg/ml) and scored for NBT-positive cells at 96 h post-treatment (Fig. 3B). Parent hexane fraction (Mc2-Ac-hex, 20 µg/ml) was included for comparative evaluation of induction of differentiation. A significantly increased NBT positive cell population was observed at all the concentrations when compared to vehicle control. At 10 µg/ml of P3, 31.20 ± 8.98% of the cells were found to be positive (p ≤ 0.05). The percentage of NBT positive cells more than doubled to 78.0 ± 1.73% upon treatment with 20 µg/ml and significantly higher (p ≤ 0.001) in comparison to vehicle-treated cells. There was no further increase in the % NBT positive cells upon treatment with the higher concentration of P3 (30 µg/ml).
Assessment of P3 cytotoxicity
As the differentiation-inducing agent shouldn’t be cytotoxic to the cells, the cytotoxic effect of P3 on HL-60 cells was assessed at 96 h post-treatment. As shown in Fig. 3C, no significant cytotoxicity was observed at any of the tested concentrations of P3 (5 µg/ml to 20 µg/ml). However, treatment of HL-60 cells with P3 at higher concentrations (30 µg/ml to 50 µg/ml) lead to significant decrease in cell’s viability (p ≤ 0.05 at 30 µg/ml; p ≤ 0.001 at 40 µg/ml and 50 µg/ml). Therefore, P3 at a concentration of 20 µg/ml (which was capable of inducing significant differentiation with no apparent cytotoxicity) was used for further studies.
Mc2-Ac-hex and P3 induce differentiation of HL-60 cells towards granulocytic lineage
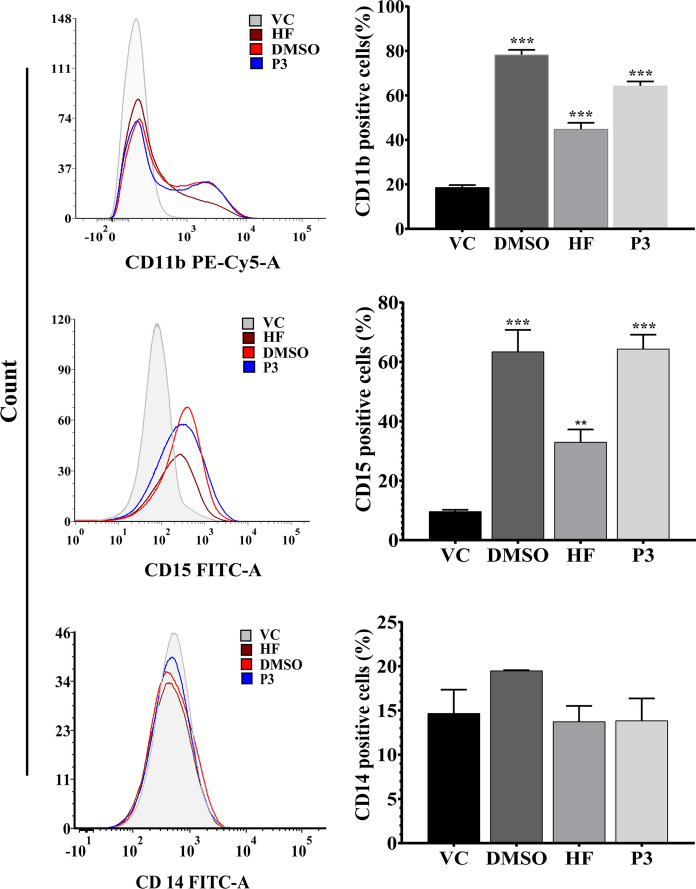
To further confirm that the P3-induced differentiation of the treated cells, and to determine the lineage of differentiated cells, expression analysis of differentiation-specific cell surface markers (CD11b, a general differentiation marker; CD14, monocytic cell surface marker, and CD15, a granulocytic cell surface marker) was carried out in and Mc2-Ac-hex (HF) and P3-treated HL-60 cells (Fig. 4, Fig. S4). Expression of CD11b was significantly higher (p ≤ 0.001) in Mc2-Ac-hex- P3-treated HL-60 cells (~ 45% and ~ 65%) at 96 h with respect to vehicle-treated cells (~ 19%), thus confirming induction of differentiation. A significant increase in the CD15 positive cells upon treatment with Mc2-Ac-hex (33.01 ± 4.02%, p ≤ 0.01) and P3 (64.36 ± 4.79%, p ≤ 0.001) was observed when compared to vehicle-treated cells. The percentage of CD14 expressing cells was minimal in the Mc2-Ac-hex- or P3-treated HL-60 cells (13.75 ± 1.77% and 13.86 ± 2.50%, respectively). An enhanced population of CD15-positive cells with a negligible population of CD14-positive cells upon Mc2-Ac-hex and P3- treatment confirmed the commitment of HL-60 cells to differentiate towards granulocytic lineage. A significantly higher population of CD11b (78.28 ± 2.17%) and CD15(63.4 ± 37.33%) positive cells in the DMSO-treated cells in comparison to vehicle-treated cells, also confirmed granulocytic differentiation by DMSO, included as a positive control.
Fig. 4.
Analysis of CD11b+, CD15+, and CD14+ expression. To confirm the differentiation and determination of lineage of differentiated cells, expression analysis of surface expression markers was performed using flow cytometry in the HL-60 cells treated with Mc2-Ac-hex and P3 (20 µg/ml each) at 96 h. Cells treated with 0.18% DMSO and 1.2% DMSO were taken as VC and positive control, respectively. Three different sets were separately labelled with fluorescently tagged antibodies against CD11b (PE-cy5 conjugated anti-CD11b), CD14 and CD15 (FITC-conjugated anti CD14 and FITC conjugated anti-CD15). Representative figure showing the overlay of the fluorescent HL-60 cells population in different treatment groups is shown in the left panel. The bar diagrams on the right show the quantitative analysis of HL-60 cells expressing CD11b+ (top right), CD15+ (middle), and CD14+ (bottom right). Data represent the mean ± SD of three independent experiments. Ordinary One-way ANOVA (Dunnett’s multiple comparisons) was performed to calculate the significant difference with respect to vehicle control. **p ≤ 0.01; ***p ≤ 0.001
P3-induces differentiation of HL-60 cells through ERK/MAPKpathway
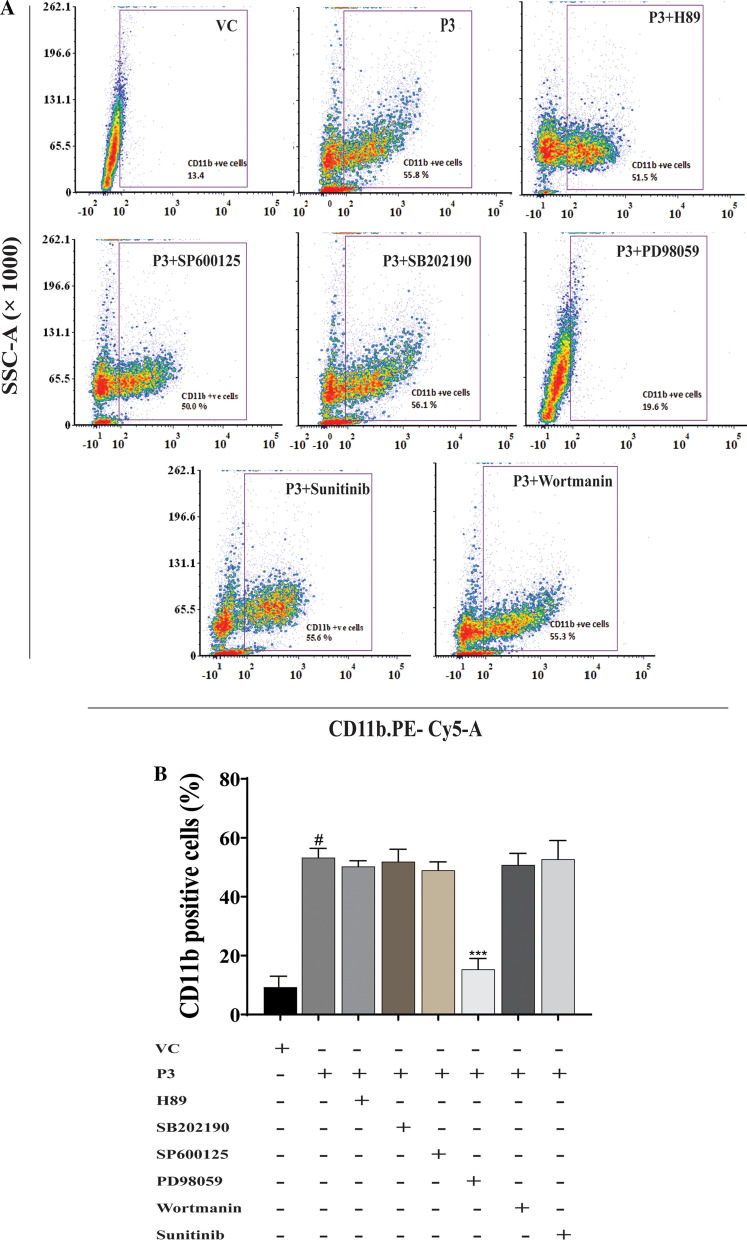
To delineate the molecular mechanism of the differentiation of HL-60 cells with P3, HL-60 cells were treated with different inhibitors to block the specific signalling pathway, before the addition of P3, and the extent of differentiation was compared with the P3-treated cells alone using NBT reduction assay (Fig. 5) and CD11b expression analysis (Fig. 6). As shown in Fig. 5, the cells treated with P3 in the presence of PD98059, a selective inhibitor for MEK1/2 kinase showed a significantly declined (p ≤ 0.001) population of NBT positive cells (23.00 ± 0.81%) when compared to the cells treated with P3 alone (72.00 ± 4.00%). No significant decrease in the NBT positive cell population was noted in the presence of any other kinase inhibitor when compared to P3-treated cells.
Fig. 5.
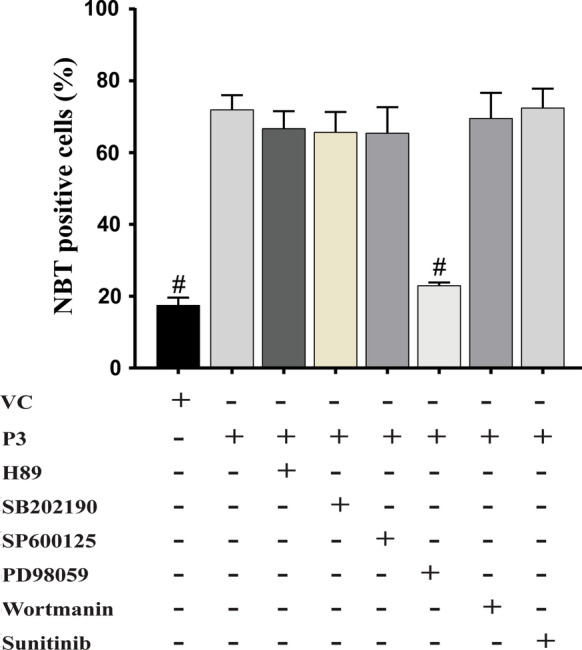
Effect of different kinase inhibitors on P3-induced differentiation. Cells treated with different kinase inhibitors 45 min before the addition of P3 fraction (20 µg/ml) were scored for NBT positive cells at 96 h post-treatment. Data represent the mean ± SD of three independent experiments. Statistical significance (p value) was calculated with respect to P3-treated HL-60 cells using Ordinary One-way ANOVA (Dunnett’s multiple comparisons test). #p ≤ 0.001
Fig. 6.
Confirmation of inhibition of differentiation by different kinase inhibitors by surface expression marker (CD11b) analysis. The cells treated with different kinase inhibitors prior to addition of P3 as mentioned in Fig. 5, were labelled with (PE-cy5 conjugated anti-CD11b) at 96 h post-treatment and fluorescent cell population was analyzed by FACS. A Dot plot showing fluorescent cell population treated with P3 in the presence of different inhibitors. B The bar diagram shows the percentage of CD11b positive cells in P3-treated HL-60 cells in the presence of different kinase inhibitors. Data represent the mean ± SD of three independent experiments. Ordinary One-way ANOVA (Dunnett’s multiple comparisons) was performed to calculate the statistical significant difference with respect to VC in the case of P3-treated cells, whereas the P3-treated cells in the presence of inhibitor were compared with the cells treated with P3 alone. ***p ≤ 0.001; #p ≤ 0.0001
Flow cytometric analysis of CD11b of another set of cells treated with different inhibitors prior to P3 addition also confirmed the results of NBT reduction assay. A significantly lower population of CD11b expressing cells (15.32 ± 3.70%) was noted in the HL-60 cells treated with P3 in the presence of PD98059 (p ≤ 0.0001) when compared with the only P3-treated cells (53.24 ± 3.16%). Other inhibitors like SP600125 (a selective inhibitor for JNK), SB202190 (selective inhibitor for P38) H89 (protein kinase A inhibitor), wortmannin (PI3 kinase inhibitor) did not affect the population of CD11b positive cells in P3-treated HL-60 cells (Fig. 6). Thus, both the NBT reduction assay and CD11b expression analysis collectively indicated the involvement of ERK/MAPK signalling pathway in P3-induced HL-60 cells’ differentiation.
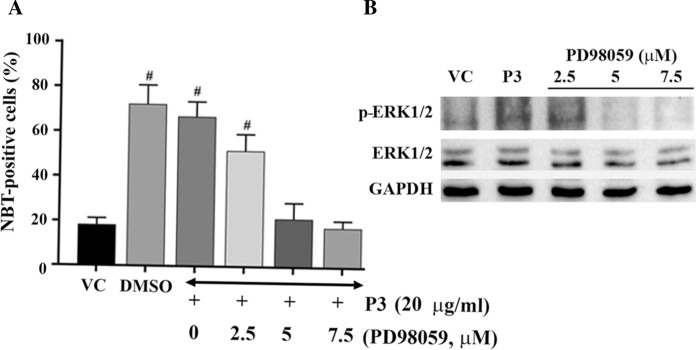
Effect of different concentrations of PD98059 on P3-induced differentiation was carried out to study its concentration dependence. As shown in Fig. 7A, exposure of HL-60 cells to 2.5 µM PD98059 prior to P3-treatment resulted in decrease in NBT-positive cells when compared to HL-60 cells treated with P3 only. PD98059 at 5 µM resulted in significant decline in NBT-positive cells in P3-treated cells and the number of NBT-positive cells was comparable to that observed in vehicle treated cells. Further increase in PD98059 concentration to 7.5 µM and 10 µM did not result in any further decrease in percentage NBT-positive cells when compared to the cells exposed to 5 µM. Morphological examination of the cells under light microscope also showed significant cell death at 10 µM PD98059, therefore NBT-positive cells were not scored (data not shown). Since PD98059 is a highly specific inhibitor of ERK1/2 activation through phosphorylation, changes in expression levels of phosphorylated ERK1/2 were examined in HL-60 cells treated with only 2.5 µM, 5 µM and 7.5 µM PD98059 using Western blot analysis (Fig. 7B). P3-treatment resulted in activation of ERK1/2 evident from increased levels of phosphorylated ERK1/2 (p-ERK1/2) compared to that of vehicle treated cells. A concentration dependent decrease in p-ERK1/2 levels was noted in P3-treated HL-60 cells, till 5 µM PD98059, beyond which no further decrease was noted in p-ERK1/2 levels, when compared to the cells treated with P3 only.
Fig. 7.
Effect of different concentratins of PD98059 on HL-60 cells’ differentiation and activation of ERK1/2. A Cells treated with 2.5 µM, 5 µM and 7.5 µM of PD98059, 45 min before the addition of P3 fraction (20 µg/ml) were scored for NBT positive cells at 96 h post-treatment. Data represent the mean ± SD of three independent experiments. Statistical significance (p value) was calculated with respect to vehicle-reated HL-60 cells using Ordinary One-way ANOVA (Dunnett’s multiple comparisons test). #p ≤ 0.001. B Western blot analysis of ERK1/2, p-ERK1/2 and GAPDH levels in HL-60 cells treated with 0.18% DMSO (VC), peak 3 fraction (P3) alone and P3-treated cells, pre-exposed to different concentrations of PD98059
Analysis of phosphorylation status of phosphokinases
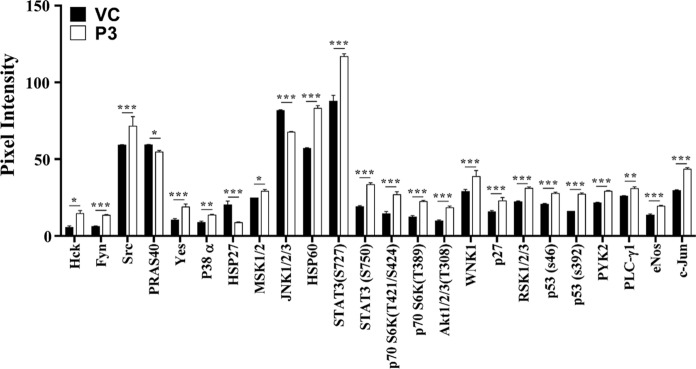
Human phosphokinases array was used to assess the signalling pathway(s) involved in the P3-induced differentiation of HL-60 cells (Fig. 8). At 96 h post-treatment, a total of 24 protein kinases’ phosphorylation was upregulated in P3-treated HL-60 cells in comparison to vehicle-treated cells. Phosphorylation of the receptor tyrosine kinases Fyn and Src, was upregulated at the significance level of p ≤ 0.001, whereas that of Hck and Yes was increased at significance level of p ≤ 0.05. Besides these, a significant increase in phosphorylation of kinases involved in MAPK pathways such as p38 α (p ≤ 0.01), MSK1/2 (p ≤ 0.05), and c-Jun (p ≤ 0.001) was also noted. Phosphorylation of some other kinases like p53, p27, RSK1/2/3, STATs was also significantly upregulated (p ≤ 0.001). In comparison to vehicle-treated cells, the kinases with decreased phosphorylation in P3-treated cells included JNK1/2/3 and HSP27 (p ≤ 0.001) and PRAS40 (p ≤ 0.05),. The protein array and inhibitor assay suggested the involvement of ERK/MSK signaling modules in the differentiation of P3-treated HL-60 cells.
Fig. 8.
Human phosphokinase array analysis. The HL-60 cells were treated with the test samples i.e. P3 (20 μg/ml) and 0.18% DMSO (Vehicle control, VC) for 96 h at 37 °C in a 5% CO2 humidified atmosphere. The cell lysates prepared at 96 h post-treatment were analyzed for phosphorylation status of different kinases using a human phospho-kinase antibody array kit (ARY003B, R&D systems). Only the kinases that showed significant change in their phosphorylation status are shown. Data represent the mean ± SD of two independent experiments, with each antibody spotted in duplicate. Student’s t-test was employed to determine statistical significance (p value) with respect to vehicle-treated control cells. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001
Identification of components in active peak fraction, P3 by GC–MS
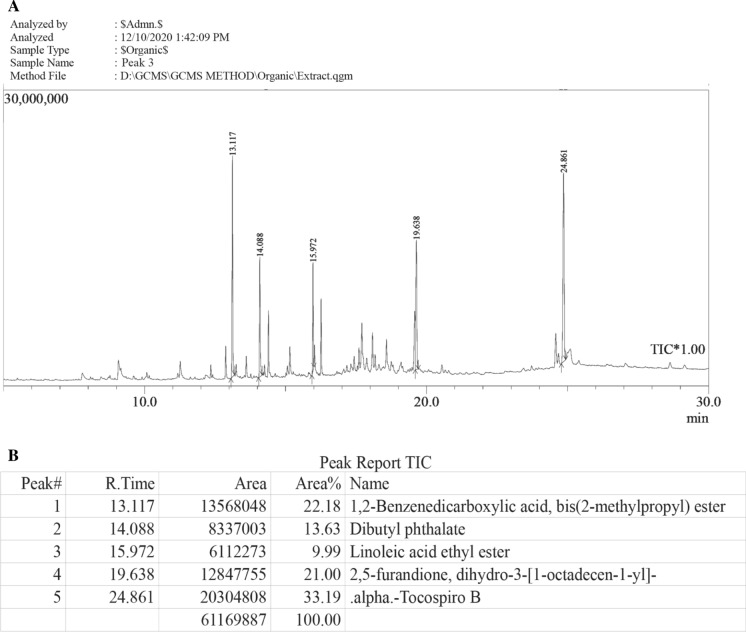
The phytochemical screening using GC–MS revealed presence of 65 compounds in the Mc2-Ac-hex fraction. The total ion chromatogram (TIC) report of Mc2-Ac-hex seed extract is shown in Fig. S5A. The 13 predominant components present in Mc2-Ac-hex are given in Fig. S5B. GC–MS analysis of active peak fraction P3 showed the presence of 27 compounds in all, (TIC report shown in Fig. 9A) with 5 predominant peaks. The peak report of the TIC of these 5 major components is shown in Fig. 9B. P3 showed enrichment of compounds such as 2,5-furanone and dibutyl phthalate with an increased percentage from Mc2-AC-hex fraction (compare with Fig. S5), as these showed less than 1% area in Mc2-Ac-hex fraction.
Fig. 9.
GC–MS analysis of P3. A The gas chromatogram represents the presence of various compounds present in P3 in the total ion chromatogram (TIC) report. NSIT compound library was used for peak matching. B The panel lists the most predominant compounds (in terms of area percent) present in P3 fraction, with their retention time (min)
Discussion
Leukemia, a hematopoietic disorder, occurs when precursor cells fail to maintain a balance between proliferation and differentiation. The conventional treatment strategies for the treatment of leukemia possess undesirable and life-threatening side effects, especially for aged leukemic patients (Marchal et al. 2006). Therefore, instead of targeting cancer cells’ death, another approach, differentiation therapy, is being seriously considered as an alternative therapeutic strategy, Differentiation therapy involves use of external agents (chemical or physical) to direct the immature cells to differentiate naturally by modulating a certain signalling pathway. All trans-retinoic acid (ATRA), dimethyl sulfoxide, d,l-α-difluoro methyl ornithine (DFMO), vitamin D3, and methyl ornithine, are a few of the known inducers of differentiation of leukemic cells of both erythroid and myeloid origin. Of these, ATRA has attained the most success, of which ATRA has been approved for clinical use to treat leukemic patients. However, many patients develop resistance against ATRA and also suffer from severe side effects (Gallagher 2002). Therefore, the search for novel differentiation inducers with minimal side effects continues.
Many plant derived molecules such as vinca alkaloids from Catharanthus roseus and paclitaxel from Taxus batata are known to exhibit anticancer properties. Recently, pinostrobin, a naturally occurring alkaloid, has exhibited cytotoxic and apoptotic activity, mediated through ROS and DNA damage, against cervical and cancer stem cells (Jadaun et al. 2018, 2019). Another plant-derived alkaloid, securinine has been shown to induce differentiation of myeloid leukemic cells through JNK/MAPK pathway (Gupta et al. 2011; Sharma et al. 2021). Studies from our laboratory have established the differentiation inducing potential of acetone extract of M. charantia seeds in HL-60 cells (Soundararajan et al. 2012). However, identification of the active principle(s) and the mechanism of action of these principles is yet known.
Present study was therefore envisaged to further purify the bioactive principle(s) involved in induction of differentiation, and elucidate possible mechanism of its action. Fractionation of the acetone extract of M. charantia’s seeds with different organic solvent based on their decreasing hydrophobicity and assessment of their differentiation inducing ability using NBT staining showed that only hexane fraction was able to induce differentiation of HL-60 cells, indicating that the compounds of non-polar nature are involved in inducing differentiation.
The P3 induced significant differentiation at the concentration of 20 µg/ml, with no apparent cytotoxicity. Therefore, for further analysis, this concentration was used. Earlier studies have demonstrated that different compounds trigger differentiation in cells at different time points. For example, DMSO and vitamin D3 require a minimum of ~ 48 h to induce differentiation (Radhika et al. 1995), whereas ATRA takes only 4 h to induce differentiation (Choudhary et al. 1999). Presence of l-α Difluoromethylornithine is required only for 24 h to induce differentiation of murine erythroleukemic cells (Choudhary et al. 1999). On the other hand, fractionated M. charantia seed extract takes 72 h to induce differentiation of HL60 cells. Therefore, we carried out a similar analysis with Mc2-Ac-hex to determine the minimum time required for active principle(s) present in fraction Mc2-Ac-hex to reprogram the cells toward differentiation and we have found that the presence of Mc2-Ac-hex in the medium for 72 h significantly (p ≤ 0.001) increased the number of NBT positive cells, which continued to increase at 96 h post-treatment (76.2 + 6%). So though the phenotypic confirmation of differentiation occurs at 72 h, the cells could have become committed to differentiation much earlier, somewhere between 48 and 72 h. Although Mc2-Ac-hex required more time to trigger differentiation, its presence is not required once the differentiation started.
Since the hexane (being more hydrophobic than other solvents) fraction is expected to contain hydrophobic compounds, we performed Normal Phase-HPLC (NP-HPLC) using different gradients of hexane and chloroform for further purification and separation of biologically active component(s) of the Mc2-Ac-hex fraction. These solvents have been extensively used for the purification of the non-polar component of extracts prepared from several medicinal plants (Lee et al. 2009; Jiang et al. 2016). Hexane has been used for the extraction of highly nonpolar terpenes and terpenoids (Jiang et al. 2016). Likewise, chloroform has been used for the extraction of the phenolic compound from olives (Lee et al. 2009). Purification of Mc2-Ac-hex fraction using normal phase-HPLC resulted in six peaks, out of which peak 3 (P3) induced significant differentiation (~ 60%) of HL-60 cells. Like the parent Mc2-Ac-hex fraction, differentiation was confirmed by CD11b expression. Though the yield for Peak 2 (P2) fraction was higher than the P3 fraction, its differentiation potential was only 50% of the P3. Therefore, further analysis was performed using P3. As evident from the extent of differentiation, P3 showed enrichment of differentiation-inducing principle when compared to Mc2-Ac-hex which contained other compounds. This enrichment is achieved due to the removal of other molecules which could have exerted an inhibitory effect on the active molecule(s). This assumption was supported by a study in which the antioxidant activity of R. caffra was reduced due to the antagonist effect of two phytochemicals present in the crude extract (Milugo et al. 2013). Our results are in line with earlier report put forth by Suberu and co-workers (2013) who demonstrated the tea extract of Artemisia annua L. to contain several compounds, out of which 2 compounds (9-epi-artemisinin and artemisitene) antagonized artemisinin’s (antimalarial lactone present in the tea of Artemesia annua L.) activity against both chloroquine-sensitive and chloroquine-resistant strains of P. falciparum. This effect was attributed to minor structural differences of antagonizing agents competing for a common target and reducing the overall efficacy (Naeim et al 2018). This indicates enrichment of differentiation-inducing principle(s) in P3. P2 could possibly contain other compounds of the same nature eluting together with the active component which could inhibit the activity of differentiation-inducing principle. On the other hand, the presence of some other components in P3 and Mc2-Ac-hex fraction could promote the differentiation-inducing activity of the active principle(s).
After establishing the differentiation-inducing ability of P3, it was necessary if it retained the same active principle present in Mc2-Ac-hex and directed the HL-60 cells’ differentiation to the same lineage. Use of fluorophore-conjugated antibodies against the monocytic (CD14) or granulocytic (CD15) cell surface markers ((Naeim et al. 2018; Baxter et al. 2009) in the differentiation studies confirmed differentiation of HL-60 cells (established by augmented CD11b expression) and commitment to granulocytic lineage (established by augmented CD15 expression) upon P3 treatment.
After confirming the differentiation-inducing ability of P3 of M. charantia seed extract in HL-60 cells, it was necessary to understand the molecular mechanism involved in differentiation induction. A number of signalling pathways are known to participate in HL-60 cells’ differentiation. These include the PI3-K (Komada et al. 1991), PKC (Marcinkowska et al. 1997), and MAPK pathways (Miranda et al. 2002). ATRA induces differentiation by activating closely related retinoic acid receptors (RARα, RARβ, and RARγ) heterodimerizes with retinoid X receptors (RXRs) which then binds to retinoid responsive response element at the promoter region of the retinoid target gene (Brown and Hughes 2012). The MEK/ERK MAPK pathway is involved in PMA-, ATRA- and G-CSF-induced myeloid differentiation, whereas PKC and ERK have been reported to be involved in monocytic differentiation of HL-60 cells induced by combined treatment of either combination of 1,25-(OH)2D3 with costunolide or with parthenolide (Kim et al. 2009). DMSO in the presence of TNF-α induces differentiation by activating the ERK/MAPK pathway (Yu et al. 2008). Thus, different inducers activate different signalling pathways/cascades to induce differentiation. To screen and analyze the protein kinases involved in the P3-dependent differentiation of HL-60 cells we have uses a phosphokinase antibody array, and predict the major signalling pathway.
Proteome phosphokinase array results revealed that P3 treatment led to increased phosphorylation of proteins involved in MAPK pathways such as stress-activated kinases (MSK1/2), ribosomal s6 kinases (RSK1/2/3), and p38α, and decreased the phosphorylation of JNK1/2/3. MSK1/2 and RSK 1/2/3, located downstream of ERK1/2 (Duda and Frödin 2013). MSK is one of the most crucial kinases present in the eukaryotic cells that facilitate signal transduction through the MAPK cascade which is activated by ERK1/2 and p38 families of MAP kinases. KMT2A, also known as MLL (Mixed lineage leukemia gene), is expressed abundantly in blood and bone marrow and plays an important role in the development of hematopoietic stem cells differentiation by interacting with multiple target genes for transcriptional regulation. MSK1 is also known to physically associate with the KMT2A/MLL (Chen and Ernst 2019) complex which is necessary to regulate KMT2A-regulated genes for differentiation of hematopoietic stem cells (Wiersma et al. 2016). Increased levels of p53 in the proteome array of P3-treated cells are in agreement with the elevated MSK levels as MSK1/2 is also known to enhance p53 expression (Ahn et al. 2018). The P3-treated cells also showed increased phosphorylation of Src family kinases (Src, Hck, Fyn, and Yes). Although Src, Fyn, and Yes are present ubiquitously, Hck is restricted to hematopoietic cells and is involved in myeloid differentiation (Johnson 2008). Hck phosphorylation at Y411 is associated with activation of the Ras/MAPK pathway. Cells with inactive Hck mutant showed inhibition of growth and phosphorylation of AKT and ERK1/2 (Pecquet et al. 2007). We have also observed increased phosphorylation of other proteins like c-Jun, eNOS, PLC γ1, and AKT1/2/3 and downregulation of HSP27 phosphorylation level. Although c-Jun is known to be activated by JNK, an earlier report on microglial cells showed the activation of c-Jun by phosphorylating at S63 and S73 does not require JNK; instead ERK1/2 signalling cascade is involved in the same (Deng et al. 2012). This could thus explain the reason for no change in c-Jun phosphorylation with decreased phosphorylation of JNK in P3-treated HL-60 cells. Heat shock proteins (HSPs), are synthesized in the cell in response to different stress conditions. HSPs are known to interact with p53 (Hsu and Hsu 1998). HSP60 belongs to high molecular weight HSPs which are ATP-dependent chaperons whereas HSP27 belongs to low molecular weight HSPs which are ATP independent. HSP60 has been reported to suppress tumors in hepatocellular carcinoma by inducing differentiation (Zhang et al. 2016). Thus, increased phosphorylation of HSP60 in the P3-treated cells is in agreement with the earlier reports.
The presence of various kinase inhibitors in the P3-treated HL-60 cells was employed to further confirm the involvement of the pathway involved in P3-induced differentiation. Significantly reduced differentiation of P3-treated HL-60 cells, established by reduced number of NBT- and CD11b-positive cells, only in the presence of PD98059, a specific inhibitor of ERK/MAPK pathway further confirmed the array results and established that P3-induced differentiation is mediated through ERK/MAPK pathway. Effect of different concentrations of PD98059 showed a dose-dependent increase in inhibition of P3-induced differentiation till 5 µM, beyond which no significant increase in inhibition was noted. P3-treated cells showed relatively higher levels of p-ERK1/2, indicating activation of MAPK/ERK pathway, in comparison to vehicle treated cells. A concomitant decrease in the p-ERK1/2 levels and percentage of NBT-positive cells in the presence of PD98059 further confirm that the P3-induced differentiation is indeed mediated through ERK/MAPK pathway. Our results are in agreement with earlier reports on specific inhibition of MAPK activation by PD98059 at (Ajenjo et al. 2000; Ansari et al. 2001; Chen et al. 2015; Hou et al. 2017). Earlier studies on concentration dependence of PD98059 on inhibition of ERK phosphorylation have also established it to be effective at 5.5 µM in HL60 cells (Milella et al. 2001). Many other compounds such as vitamin D3 (), bortezomib, helanlin (pseudo guaianolide sesquiterpene lactone), and genistein (a flavonoid), methanolic extract of Antrodia cinnamomea have also been shown to induce differentiation through ERK/MAPK pathways (Ji et al. 2002; Kim et al. 2005; Marcinkowska et al. 2006; Sánchez et al. 2009; Zhou et al. 2013), signifying its importance in the differentiation of leukemic cells.
GC–MS analysis of both the parent fraction (Mc2-Ac-hex) to identify the major components revealed predominant (more than 4% in terms of area percent in the TIC report) presence of only 4 compounds (Linoleic acid ethyl ester, ledenAlkohol, simiarenol, ethyl hexadecanoate) in the Mc2-Ac-hex. GC–MS analysis of P3 showed the presence of only 5 predominant compounds with area > 5%. Out of these 5 compounds, two (derivatives of 2,5-dihydrofuran (succinic anhydride) and dibutyl phthalate) have been reported to possess anticancer activity (Wen et al. 2012; Zhang et al. 2012; Grytting et al. 2019). Cytotoxic activity of a derivative of 2, 5 dihydrofurans against a number of cancer cell lines such as SW480 (human colon carcinoma cells), A-549 (human lung adenocarcinoma cells), QGY-7701 (human hepatoma cells), and HeLa (human cervical carcinoma cell line) cells has already been reported (Zhang et al. 2012).. Earlier studies on the methanolic extract of mycelia of Antrodia cinnamomea (MEMAC) that induced differentiation of HL-60 cells to contain terpenoids, sterols, triterpenes, and derivatives of malic and succinic acid (Wen et al. 2012). Thus, presence of a succinic anhydride in the P3 fraction is not surprising. Recently, the other major compound present in P3, dibutyl phthalate, has been reported to boost PMI-induced differentiation of THP-1 cells by interacting with PPARγ (Grytting et al. 2019), suggesting its potential in differentiation. Comparative analysis of TIC report of the Mc2-Ac-hex and P3 revelaed an enrichment in 2,5-dihydrofuran (21%) and dibutyl phthalate (13%) when compared to Mc2-Ac-hex.
Thus, our results, in agreement with their earlier reported activities, suggest that these compounds could therefore be, in part being responsible for the differentiation-inducing ability of P3. However, further studies need to be conducted with the purified compounds to validate these observations. Peak fraction P2 also induced differentiation in HL-60 cells to some extent. Therefore, it was necessary to look for similarity in the GC–MS profile of both the peaks i.e. P2 and P3. Linoleic acid ethyl ester, hexadecanoic acid ethyl ester, and octadecanoic acid ethyl ester were the compounds present in adequate quantity in both the peaks. However, there is no documented report demonstrating their role in differentiation. Thus, differentiation of HL-60 cells by peak 2 could be due to some other compound, not yet reported, and requires further investigations.
Conclusion
The present study thus demonstrates that the hexane extractable fraction of M. charantia seeds and the HPLC-purified active principle(s) extracted from the same (P3) induce differentiation in HL-60 cells towards granulocytic lineage (as evidenced by differentiation markers CD11b and CD15. Further, the study gives a deep insight into the molecular/signalling pathway by which the P3-induced differentiation. Inhibition of differentiation of HL-60 cells in the presence of PD98059 (a MEK1/2 kinase inhibitor) established involvement of the ERK/MAPK signalling pathway in P3-induced differentiation, along with the involvement of non-receptor tyrosine kinases. Schematic summary of the possible mechanism/pathway by which M. charantia P3 fraction induces differentiation of HL-60 cells to granulocytes is shown in Fig. 10. GC–MS data suggested the presence of 5 major compounds in P3 which may or may not be acting together for induction of HL-60 cells’ differentiation. Further studies with the purified compounds present in the P3, alone or in combination, would throw light on the possible combinatorial additive or synergistic action of these compounds. With the immense potential of inducing differentiation of the leukemic cells, the M. charantia seed extracts could possibly be considered to be part of complementary therapeutic strategy, in combination with the existing anti-leukemic treatments.
Fig. 10.
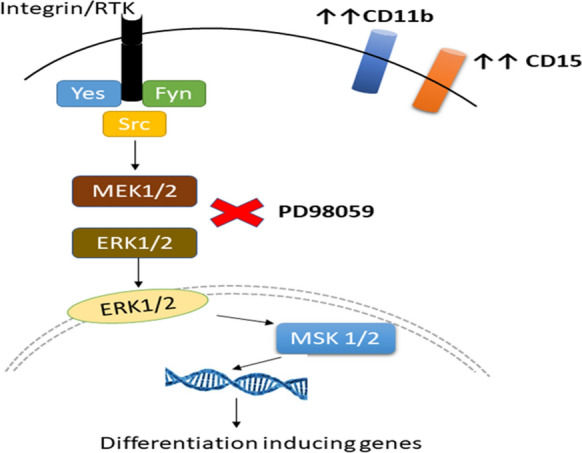
Possible mechanism(s) of P3-induced granulocytic differentiation of HL-60 cells. P3 activates MEK1/2-ERK1/2 mediated differentiation through activation of cytoplasmic non-receptor tyrosine kinase (Yes, Fyn, and Src). Increased levels of CD11b and CD15 confirmed the granulocytic lineage commitment of differentiated HL-60 cells. The presence of PD98059, an ERK1/2 specific inhibitor, inhibited differentiation of HL-60 cells by P3, suggesting involvement of ERK/MAPK pathway in P3-induced differentiation
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
JS and PP thank the Jawaharlal Nehru University, New Delhi, for the research fellowship. RS and SG acknowledge the University Grants Commission, New Delhi for the research fellowship. AD acknowledges intramural support from the Jawaharlal Nehru University, New Delhi. JS thanks Dr. Ankita Pandey and Dr. Sapna Sharma for their inputs. Authors thank Dr. Swati Tiwari, School of Biotechnology, Jawaharlal Nehru University, New Delhi for fruitful discussions, valuable inputs and access to her lab facility for immunoblotting analysis.
Author contributions
JS and PP: formal execution, analysis of experiments, data curation, writing—original draft preparation. RS: experimentation and manuscript draft preparation. SG: experimentation and data curation. AD: conceptualization, project administration, and supervision, writing—review, and editing.
Funding
No funds, Grants, or other support were received for conducting this study.
Data availability
All the data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human or animal participants performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jeetesh Sharma and Punit Prabha have contributed equally to this work.
References
- Ahn J, Lee JG, Chin C, et al. MSK1 functions as a transcriptional coactivator of p53 in the regulation of p21 gene expression. Exp Mol Med. 2018;50:1–12. doi: 10.1038/s12276-018-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajenjo N, Aaronson DS, Ceballos E, et al. Myeloid leukemia cell growth and differentiation are independent of mitogen-activated protein kinase ERK1/2 activation. J Biol Chem. 2000;27:7189–7197. doi: 10.1074/jbc.275.10.7189. [DOI] [PubMed] [Google Scholar]
- Ansari HR, Husain S, Abdel-Latif AA. Activation of p42/p44 mitogen-activated protein kinase and contraction by prostaglandin F2alpha, ionomycin, and thapsigargin in cat iris sphincter smooth muscle: inhibition by PD98059, KN-93, and isoproterenol. J Pharmacol Exp Ther. 2001;299:178–186. [PubMed] [Google Scholar]
- Baxter SS, Carlson LA, Mayer AM, et al. Granulocytic differentiation of HL-60 promyelocytic leukemia cells is associated with increased expression of Cul5. In Vitro Cell Dev Biol Anim. 2009;45:264–274. doi: 10.1007/s11626-008-9163-4. [DOI] [PubMed] [Google Scholar]
- Breitman TR, Selonick SE, Collins SJ. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci USA. 1980;77:2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G, Hughes P. Retinoid differentiation therapy for common types of acute myeloid leukemia. Leuk Res Treat. 2012;2012:939021. doi: 10.1155/2012/939021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budrat P, Shotipruk A. extraction of phenolic compounds from fruits of bitter melon (Momordica charantia ) with subcritical water extraction and antioxidant activities of these extracts. Chiang Mai J Sci. 2008;35:123–130. [Google Scholar]
- Chen Y, Ernst P. Hematopoietic transformation in the absence of MLL1/KMT2A: distinctions in target gene reactivation. Cell Cycle. 2019;18:1525–1531. doi: 10.1080/15384101.2019.1618642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XY, Cai HZ, Wang XY et al (2015) Application of the ERK signaling pathway inhibitor PD98059 in long-term in vivo experiments. Genet Mol Res 14:18325–18333. 10.4238/2015.December.23.20 [DOI] [PubMed]
- Chijiwa T, Mishima A, Hagiwara M, et al. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. doi: 10.1016/S0021-9258(19)34116-X. [DOI] [PubMed] [Google Scholar]
- Choudhary SK, Sharma D, Dixit A. L-α Difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase induces differentiation in MEL cells. Cell Biol Int. 1999;23:489–495. doi: 10.1006/cbir.1999.0398. [DOI] [PubMed] [Google Scholar]
- Deng Z, Sui G, Rosa PM, et al. Radiation-induced c-Jun activation depends on MEK1-ERK1/2 signaling pathway in microglial cells. PLoS ONE. 2012;7:e36739. doi: 10.1371/journal.pone.0036739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda K, Frödin M (2013) Stimuli that activate MSK in cells and the molecular mechanism of activation. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000–2013. https://www.ncbi.nlm.nih.gov/books/NBK91435. Accessed 12 Oct 2021
- Gallagher RE. Retinoic acid resistance in acute promyelocytic leukemia. Leukemia. 2002;16:1940–1958. doi: 10.1038/sj.leu.2402719. [DOI] [PubMed] [Google Scholar]
- Grytting VS, Olderbø BP, Holme JA, et al. Di-n-butyl phthalate modifies PMA-induced macrophage differentiation of THP-1 monocytes via PPARγ. Toxicol in Vitro. 2019;54:168–177. doi: 10.1016/j.tiv.2018.09.004. [DOI] [PubMed] [Google Scholar]
- Gupta K, Chakrabarti A, Rana S, et al. Securinine, a myeloid differentiation agent with therapeutic potential for AML. PLoS ONE. 2011;6:e21203. doi: 10.1371/journal.pone.0021203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Hou X, Wang L, et al. PD98059 impairs the cisplatin-resistance of ovarian cancer cells by suppressing ERK pathway and epithelial mesenchymal transition process. Cancer Biomark: Sect A Dis Mark. 2017;21:187–194. doi: 10.3233/CBM-170644. [DOI] [PubMed] [Google Scholar]
- Hsu PL, Hsu SM. Abundance of heat shock proteins (hsp89, hsp60, and hsp27) in malignant cells of Hodgkin’s disease. Cancer Res. 1998;58:5507–5513. [PubMed] [Google Scholar]
- Ito M, Karasawa Y, Takeuchi M. Downstream signal pathway of epidermal growth factor receptor on the in vitro corneal wound healing model. Investig Ophthalmol vis Sci. 2016;257:1277. [Google Scholar]
- Jacquel A, Herrant M, Defamie V, et al. A survey of the signaling pathways involved in megakaryocytic differentiation of the human K562 leukemia cell line by molecular and c-DNA array analysis. Oncogene. 2006;25:781–794. doi: 10.1038/sj.onc.1209119. [DOI] [PubMed] [Google Scholar]
- Jadaun A, Sharma S, Malek SNA, et al. Induction of apoptosis by pinostrobin in human cervical cancer cells: possible mechanism of action. PLoS ONE. 2018;13:e0191523. doi: 10.1371/journal.pone.0191523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadaun A, Verma R, Dixit A. Pinostrobin inhibit proliferation and induces apoptosis in cancer stem-like cells through a reactive oxygen species like mechanism. RSC Adv. 2019;9:12097–12109. doi: 10.1039/C8RA08380K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Y, Kutner A, Verstuyf A, et al. Derivatives of vitamins D2 and D3 activate three MAPK pathways and upregulate pRb expression in differentiating HL60 cells. Cell Cycle. 2002;1:410–415. doi: 10.4161/cc.1.6.269. [DOI] [PubMed] [Google Scholar]
- Jiang G, Dallas-Yang Q, Liu F. Salicylic acid reverses phorbol 12-myristate-13-acetate (PMA)- and tumor necrosis factor alpha (TNF alpha)-induced insulin receptor substrate 1 (IRS1) serine 307 phosphorylation and insulin resistance in human embryonic kidney 293 (HEK293) cells. J Biol Chem. 2003;278:180–186. doi: 10.1074/jbc.M205565200. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Kempinski C, Chappell J. Extraction and analysis of terpenes/terpenoids. Curr Protoc Plant Biol. 2016;1:345–358. doi: 10.1002/cppb.20024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE. Src family kinases and the MEK/ERK pathway in the regulation of myeloid differentiation and myeloid leukemogenesis. Adv Enzyme Regul. 2008;48:98–112. doi: 10.1016/j.advenzreg.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan S, Choudhury D, Dixit A. Immunogenic characterization and protective efficacy of recombinant CsgA, major subunit of curli fibers, against Vibrio parahaemolyticus. Appl Microbiol Biotechnol. 2021;105:599–616. doi: 10.1007/s00253-020-11038-4. [DOI] [PubMed] [Google Scholar]
- Kim SH, Oh SM, Kim TS. Induction of human leukemia HL-60 cell differentiation via a PKC/ERK pathway by helenalin, a pseudoguainolidesesquiterpene lactone. Eur J Pharmacol. 2005;511:89–97. doi: 10.1016/j.ejphar.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Kim SH, Cho SS, Simkhada JR, et al. Enhancement of 1,25-dihydroxyvitamin D3- and all-trans retinoic acid-induced HL-60 leukemia cell differentiation by Panax ginseng. Biosci Biotechnol Biochem. 2009;73:1048–1053. doi: 10.1271/bbb.80823. [DOI] [PubMed] [Google Scholar]
- Komada F, Nishikawa M, Uemura Y, et al. Expression of three major protein kinase C isozymes in various types of human leukemic cells. Cancer Res. 1991;51:4271–4278. [PubMed] [Google Scholar]
- Lee OH, Lee BY, Lee J, et al. Assessment of phenolics-enriched extract and fractions of olive leaves and their antioxidant activities. Bioresour Technol. 2009;100:6107–6113. doi: 10.1016/j.biortech.2009.06.059. [DOI] [PubMed] [Google Scholar]
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- Lucas EA, Dumancas GG, Smith BJ, et al. Health benefits of bitter melon (Momordica charantia) Bioact Foods Promot Health. 2010;35:525–549. doi: 10.1016/B978-0-12-374628-3.00035-9. [DOI] [Google Scholar]
- Marchal JA, Rodriguez-Serrano F, Campos J, et al. Differentiation: an encouraging approach to anticancer therapy. Ital J Anat Embryol. 2006;111:45–64. [PubMed] [Google Scholar]
- Marcinkowska E, Wiedłocha A, Radzikowski C. 1,25-Dihydroxyvitamin D3 induced activation and subsequent nuclear translocation of MAPK is upstream regulated by PKC in HL-60 cells. Biochem Biophys Res Commun. 1997;241:419–426. doi: 10.1006/bbrc.1997.7832. [DOI] [PubMed] [Google Scholar]
- Marcinkowska E, Garay E, Gocek E, et al. Regulation of C/EBP beta isoforms by MAPK pathways in HL60 cells induced to differentiate by 1,25-dihydroxyvitamin D3. Exp Cell Res. 2006;312:2054–2065. doi: 10.1016/j.yexcr.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milella M, Kornblau SM, Estrov Z, et al. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Investig. 2001;108:851–859. doi: 10.1172/JCI12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milugo TK, Omosa LK, Ochanda JO, et al. Antagonistic effect of alkaloids and saponins on bioactivity in the quinine tree (Rauvolfiacaffra sond.): further evidence to support biotechnology in traditional medicinal plants. BMC Complement Altern Med. 2013;13:285. doi: 10.1186/1472-6882-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda MB, McGuire TF, Johnson DE. Importance of MEK-1/-2 signaling in monocytic and granulocytic differentiation of myeloid cell lines. Leukemia. 2002;16:683–692. doi: 10.1038/sj.leu.2402400. [DOI] [PubMed] [Google Scholar]
- Naeim F, Rao PN, Song SX. Chapter 2—principles of immunophenotyping. Atlas of hematopathology. 2. Cambridge: Academic Press; 2018. pp. 29–56. [Google Scholar]
- Ocadlikova D, Lecciso M, Broto JM. Sunitinib exerts in vitro immunomodulatory activity on sarcomas via dendritic cells and synergizes with PD-1 blockade. Front Immunol. 2021;12:577766. doi: 10.3389/fimmu.2021.577766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecquet C, Nyga R, Penard-Lacronique V, et al. The Src tyrosine kinase Hck is required for Tel-Abl- but not for Tel-Jak2-induced cell transformation. Oncogene. 2007;26:1577–1585. doi: 10.1038/sj.onc.1209949. [DOI] [PubMed] [Google Scholar]
- Radhika S, Choudhary SK, Garg LC, et al. Induction of differentiation in murine erythroleukemia cells by lα,25-dihydroxy vitamin D3. Cancer Lett. 1995;90:225–230. doi: 10.1016/0304-3835(95)03707-4. [DOI] [PubMed] [Google Scholar]
- Raina K, Kumar D, Agarwal R. Promise of bitter melon (Momordica charantia) bioactives in cancer prevention and therapy. Semin Cancer Biol. 2016;40:116–129. doi: 10.1016/j.semcancer.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Y, Amrán D, de Blas E, et al. Regulation of genistein-induced differentiation in human acute myeloid leukaemia cells (HL60, NB4) Protein kinase modulation and reactive oxygen species generation. Biochem Pharmacol. 2009;77:384–396. doi: 10.1016/j.bcp.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Shallis RM, Wang R, Davidoff A, et al. Epidemiology of acute myeloid leukemia: recent progress and enduring challenges. Blood Rev. 2019;36:70–87. doi: 10.1016/j.blre.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Sharma J, Pandey A, Sharma S, et al. Securinine induces differentiation of human promyelocytic leukemic HL-60 cells through JNK mediated signaling pathway. Nutr Cancer. 2021;2021:1–16. doi: 10.1080/01635581.2021.1925710. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Kumar GS, Sambaiah K, et al. Effect of bitter gourd (Momordica charantia) on glycaemic status in streptozotocin induced diabetic rats. Plant Foods Hum Nutr. 2005;60:109–112. doi: 10.1007/s11130-005-6837-x. [DOI] [PubMed] [Google Scholar]
- Song X, Peng Y, Wang X, et al. Incidence, survival, and risk factors for adults with acute myeloid leukemia not otherwise specified and acute myeloid leukemia with recurrent genetic abnormalities: analysis of the surveillance, epidemiology, and end results (SEER) database, 2001–2013. Acta Haematol. 2018;139:115–127. doi: 10.1159/000486228. [DOI] [PubMed] [Google Scholar]
- Soundararajan R, Prabha P, Rai U, et al. Antileukemic potential of Momordica charantia seed extract on human myeloid leukemic HL60 cells. Evid Based Complement Alternat Med. 2012;2012:732404. doi: 10.1155/2012/732404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suberu JO, Gorka AP, Jacobs L, et al. Anti-plasmodial polyvalent interactions in Artemisia annua L. aqueous extract—possible synergistic and resistance mechanisms. PLoS ONE. 2013;8:e80790. doi: 10.1371/journal.pone.0080790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S, Ray RB. Bitter melon (Momordica charantia), a nutraceutical approach for cancer prevention and therapy. Cancers. 2020;12:2064. doi: 10.3390/cancers12082064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen CL, Teng CL, Chiang CH, et al. Methanol extract of Antrodia cinnamomea mycelia induces phenotypic and functional differentiation of HL60 into monocyte-like cells via an ERK/CEBP-β signaling pathway. Phytomedicine. 2012;19:424–435. doi: 10.1016/j.phymed.2011.11.003. [DOI] [PubMed] [Google Scholar]
- West ME, Sidrak GH, Street SP. The anti-growth properties of extracts from Momordica charantia L. West Ind Med J. 1971;20:25–34. [PubMed] [Google Scholar]
- Wiersma M, Bussiere M, Halsall JA, et al. Protein kinase Msk1 physically and functionally interacts with the KMT2A/MLL1 methyltransferase complex and contributes to the regulation of multiple target genes. Epigenet Chromatin. 2016;9:52. doi: 10.1186/s13072-016-0103-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yedjou CG, Moore P, Tchounwou PB. Dose- and time-dependent response of human leukemia (HL-60) cells to arsenic trioxide treatment. Int J Environ Res Public Health. 2006;3:136–140. doi: 10.3390/ijerph2006030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Li A, Zhou L. The global burden and attributable risk factor analysis of acute myeloid leukemia in 195 countries and territories from 1990 to 2017: estimates based on the global burden of disease study 2017. J Hematol Oncol. 2020;13:72. doi: 10.1186/s13045-020-00908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HN, Lee YR, Noh EM, et al. Tumor necrosis factor-alpha enhances DMSO-induced differentiation of HL-60 cells through the activation of ERK/MAPK pathway. Int J Hematol. 2008;87:189–194. doi: 10.1007/s12185-008-0037-z. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhong H, Wang T, et al. Synthesis of novel 2, 5-dihydrofuran derivatives and evaluation of their anticancer activity. Eur J Med Chem. 2012;48:69–80. doi: 10.1016/j.ejmech.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhou X, Chang H, et al. Hsp60 exerts a tumor suppressor function by inducing cell differentiation and inhibiting invasion in hepatocellular carcinoma. Oncotarget. 2016;7:68976–68989. doi: 10.18632/oncotarget.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Fang Y, Jing H, et al. Involvement of mitogen-activated protein kinase in signal transducer and activator of transcription-1 mediated differentiation induced by bortezomib in acute myeloid leukemia cells. Mol Carcinog. 2013;52:18–28. doi: 10.1002/mc.20873. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data used to support the findings of this study are available from the corresponding author upon request.