Abstract
Inhibition of DPP-4 and stimulation of GLP-1 secretion are therapeutic strategies for controlling glycaemia in type 2 diabetes. The present study assessed the DPP-4 inhibitory activity and GLP-1 secretory action of pigmented and non-pigmented rice (Oryza sativa L.), along with an extruded food product. Cereal-based extruded food products, with or without passion fruit powder, were prepared from red rice using a twin extruder. Optimal extrusion conditions were determined using a CCD of response surface methodology resulting in optimal conditions to be 97.5 °C, a screw speed of 250 rpm, feed moisture of 25.2% and addition of 11.25% passion fruit powder. Samples were sequentially extracted in n-hexane, ethanol (50%) and water. Ethanol/water (50:50) extracts of rice bran significantly inhibited DPP-4 activity by 70.48 ± 1.06%, comparing favourably with RR (42.55 ± 0.84%), PRR (35.91 ± 1.27%) and PA (29.14 ± 1.23%). DPP-4 inhibitory activity was retained in both extruded products albeit at reduced levels. GLP-1 secretion was stimulated mostly by extruded products extracted with n-hexane or ethanol which upregulated basal secretion by 6.1-fold and 4.2-fold, respectively. ICP-MS results showed that extruded food items have a lower arsenic content. In conclusion, there are potential opportunities for the nutraceuticals and functional food products using pigmented red rice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-022-05444-x.
Keywords: Pigmented rice, Rice bran, DPP-4, Antidiabetic, GLP-1
Introduction
Diabetes mellitus (DM) is a heterogeneous metabolic disorder where fasting plasma glucose levels are higher than 7 mmol/l, or if levels exceed 11.1 mmol/l at any time of day (Zendjabil 2016). DPP4 (Dipeptidyl peptidase 4) inhibitors are a novel type of oral glucose-lowering agent that regulate fasting plasma glucose, postprandial glucose, and %HbA1c in the body. They act to halt the physiological breakdown of the incretin hormones glucagon-like peptide (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), decreasing the inactivation of endogenous incretins to trigger the release of insulin in a glucose dependent manner (Giorda, Nada & Tartaglino 2014). There is also a role for GLP-1 mimetics, and potentially GLP-1 secretagogues to effectively manage this disease. Pigmented red rice is an underutilized crop in Northeastern parts of India, despite being rich in various phenolic compounds, e.g., quinic acid, salicylic acid, ferulic acid, gallic acid and many others. Furthermore, it is a rich source of minerals, including aluminium, calcium, copper, chromium, iron, potassium, magnesium, manganese, sodium and zinc (Samyor et al. (2016). Grains and cereals are considered as staple food throughout the Asian population and are consumed as their main source of energy.
Extrusion cooking technology is extensively used to rapidly develop new food products. The raw material undergoes physicochemical changes, including starch gelatinization, protein denaturation, amylose–lipid complex formation, and degradation of heat sensitive components such as vitamins, antioxidants, and pigments (Harper 1989). Various technical parameters such as screw speed and moisture content etc., are important to create acceptable extrudates (Kumar et al. 2008). Diet is a major contributing factor in the development of diabetes, therefore producing a nutritionally rich extrudate with anti-diabetic properties is one of the viable strategies for managing the disease. Various studies reported the association between the consumption of certain foods, their constituents, and the incidence of diabetes.
Arsenic (As) is a category 1 carcinogen (classified by the IARC Monographs). It is a consequence of different contaminants to include industrial production of pesticides, herbicides, wood preservatives and mining, and can severely compromise human health (Mayorga et al. 2013). Several species of “As” are reported in rice and include arsenite (As III), arsenate (As V), dimethyl arsenic acid (DMA) and monomethyl arsonic acid (MMA) (D’Amato et al. 2004). Aresenite and arsenate are inorganic arsenic (i-As) species and are carcinogenic in nature (Mandal and Suzuki 2002), whereas organic “As” species MMA and DMA are less toxic but could be cancer promoters (Heitkemper et al. 2001).
The present study investigated the potential antidiabetic effects of arsenically safe pigmented Oryza sativa L., by examining the ability of extracts to inhibit DPP-4 and augment GLP-1 secretion. Red rice also underwent an extrusion processing to determine the effect of this processing on any detected antidiabetic activity. Rice and extruded samples were extracted with various solvents to understand the basic chemical properties of the active components.
Materials and methods
Rice samples
Red rice (Oryza sativa L.: RR) and white rice locally known as Pungpo ame (WR) were obtained from Manigong Circle, Arunachal Pradesh (India). Rice bran (RB) was obtained from RR by using a rice polisher (Satake, Japan). The remaining grains were also collected and are referred to as polished red rice (PRR).
Extrusion processing
The extrusion process was optimized by using response surface methodology (RSM) for statistical design of experiments (DOE). Central composite design (30 runs) was used to determine the optimal extrusion conditions. The independent variables were temperature (˚C), screw speed (rpm), feed moisture content (%), passion fruit powder (%) and dependent variables were expansion ratio, water absorption index (%), total phenolic acid (mg GAE/100 g) and DPPH (%). A total of 30 different processing conditions were undertaken to reach an optimal condition for the product development. Two extrudates were produced: Control extrudate (CE; non-optimized RR extruded alone) and optimized extrudate (OE; RR combined with 11.25% passion fruit powder) were prepared using a twin extruder (Model FUE-1F, Flytech Engineering, Chennai, India). The processes were optimized to obtain the maximum desirability value. The highest desirability value obtained was 0.906 with extrusion conditions of temperature 97.50˚C, screw speed 250 rpm, feed moisture content 25.20% and passion fruit powder 11.25 The extruded samples were packed into airtight bags.
Extraction
Dried rice samples (moisture content of 12% dry weight) were pulverized to a fine homogenous powder using a planetary ball mill, and stored at 20 ± 5 ˚C. Rice samples (10 g) were chemically partitioned sequentially by immersing the crude powder in 50 ml of solvents of differing polarity including n-hexane, 50% ethanol and water. Each time they were incubated on a roller mixer overnight at room temperature. Supernatants were obtained by filtration using Whatman Grade 1filter paper before drying in a heat block at 50 ˚C. Dried extracts were stored at– 20 ˚C prior to use in assays.
Determination of DPP-4 inhibition activity
DPP-4 inhibition of rice extracts was carried out in a black 96-well plate as previously described by Fujiwara and Tsuru (1978). Sample extracts were dissolved in HEPES buffer (pH 7.4) at a concentration of 2 mg/ml and assessed fluorometrically using Gly-Pro-aminomethylcoumarin (1 mmol/l; BaChem Ltd, Switzerland) and purified porcine DPP-4 (1 mU/ml; Merck Chemicals, UK). Berberine (1 mg/ml; Fluorochem, UK) was used as a positive control as it has already shown potent DPP-4 inhibitory activity (Al-masri et al. 2009). After addition of all reagents (n = 3) plates were incubated at 37˚C for 1 h with gentle agitation. Then, 100 of 5 mM acetic acid was added to stop reactions. Plates were read using a fluorescent microplate reader (Tecan Saffire II; Reading, UK) at excitation and emission wavelengths of 351 and 430 nm, respectively.
STC-1 pGIP/Neo cell culture studies
STC-1 is a heterogeneous plurihormonal cell line producing several prominent gut peptide hormones. pGIP/Neo is a genetically selected sub-clone of STC-1 with augmented levels of glucose-dependent insulinotropic peptide (GIP) (Gillespie et al. 2017) Furthermore, the pGIP/ neo STC-1 cell line is a sub-clone of parental STC-1 cell line. The sub clone is considerably more compliant to work as there is no spontaneous detachment. pGIP/Neo STC-1 cells manifest more consistent monolayer growth as well as a more consistent growth rate overall. STC-1 cells transfected with a plasmid (pGIP/Neo) encoding neomycin phosphotransferase were obtained from Dr. B. Wice (Washington University of St.Louis) with permission from Dr. D. Hanahan (University of California, San Francisco, CA). Cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 4.5 g/l D-glucose with L-glutamine, without sodium pyruvate (Gibco, Paisley, UK) and supplemented with 10% foetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin and 400 µg/ml geneticin (G418; Sigma, UK). Cells were incubated in a 5% CO2 humidified atmosphere at 37 °C and used between passage numbers 20–50 when 70–90% confluence had been reached.
STC-1 pGIP/Neo cells were seeded in 12 well plates at a density of 2 million cells per well with 1 ml DMEM and incubated overnight at 37 °C in a 5% CO2 humidified atmosphere to allow attachment. Media was removed from cells and washed twice with HEPES buffer (pH 7.4) and pre-incubated in HEPES for 1 h. After removal of buffer, samples at 2 mg/ml were reconstituted in HEPES and added to cells in triplicate for 3 h. After the incubation period supernatant was removed, centrifuged at 1000 g for 10 min to remove cellular debris and stored at -20˚C prior to analysis.
Measurement of cell viability
Trypan blue was used to assess cell viability. After trypsinisation, 1 ml of DMEM was added (to inactivate trypsin) and the resulting solution was centrifuged at 1000 g for 5 min. The supernatant was discarded, and cells were resuspended in DMEM. The cell suspension was added to trypan blue (1:1) and cell viability was measured using a Countess Automated Cell Counter (Invitrogen, Life Technologies Ltd, UK).
Determining GLP-1 secretory activity
Cells were seeded in 12 well plates (2 × 106 cells per well) with 1.5 mL DMEM and incubated overnight at 37 °C in a 5% CO2 humidified atmosphere to allow attachment (Hand et al. 2010). Media were removed and cells were washed twice with HEPES buffer (20 mM HEPES, 10 mM glucose, 140 nM NaCl, 4.5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2) and pre-incubated in the same HEPES buffer for 1 h. After removal of buffer, rice samples reconstituted in buffer were added to cells in triplicate for 3 h. After the incubation period the supernatant was removed, centrifuged at 1000 g for 10 min to remove cellular debris and stored at -20 °C prior to analysis. GLP-1 levels were measured by means of ELISA (Millipore, UK) in accordance to manufacturer’s instructions using fluorescence plate reader (Tecan Saffire II; Reading, UK) with excitation and emission wavelengths of 355 nm and 460 nm, respectively.
Inductively coupled plasma mass spectrometry (ICP-MS) of samples
Sample preparation for as speciation
Sample preparation for “As” speciation was described by using Signes-Pastor et al. (2016) method. Samples were dried and milled for 3 min at 500 rpm with a 1 min rotation and a reverse rotation using a Retch PM100 rotary ceramic ball mill. The samples (0.1 g) were weighed accurately to a weight of 0.1 g into 50 ml polypropylene centrifuge tubes to which 10 ml of 1% conc. Aristar nitric acid was added and allowed to sit overnight. Batches were prepared with a blank rice CRM (NIST 1568b Rice flour) which has the “As” species Asi, and dimethylarsinic acid (DMA) concentrations certified. Later, microwave digestion was done in an CEM MARS 6 instrument for 30 min at 95˚C using a 3-stage slow heating program: to 55˚C in 5 min held for 10 min., to 75˚C in 5 min., held for 10 min. to 95˚C in 5 min., held for 30 min. A 1 ml aliquot was transferred to a 2 ml polypropylene vial and then, 10 µl of analytical grade hydrogen peroxide was added to convert any arsenite to arsenate to facilitate subsequent chromatographic detection.
Statistical analysis
Statistical analysis was conducted using Graph Pad Prism version 5 (Graph Pad Software, USA). All experiments were carried out in triplicate. Data are expressed as mean ± SEM and statistical comparisons were assessed using a one-way ANOVA with Tukey’s post-hoc test. A p-value less than 0.05 was deemed statistically significant.
Results
Yield of extracts
Different solvents viz., polar and non-polar were used and resulted in various extraction yields. Differences in the polarity of the extraction solvents, possibly cause a massive change in the level of bioactive compounds in the extract (Truong et al., 2019). Ethanol as a bio-solvent is considered for extraction of products using natural methods because it is completely biodegradable (Chemat et al. 2012). Initially, bioactivity guided fractionation was carried on rice samples (10 g) by immersing the crude powder in 50 ml of solvents of different polarity viz., n-hexane, ethanol and water (50:50) and water alone. In the ethanol and water extract, RB (Rice bran) has highest yield extract (%) i.e., 1.22% when compared with other samples of rice such as RR (Red rice), PRR (Polished red rice) and WR (Pungpo ame). Yields from the sequential extractions varied from 0.21–1.91% (Table 1). Control extrudate (CE) and optimised extrudate (OE) yielded 1.44% and 1.04%, respectively. For ethanol, CE and OE produced yields of 1.02 and 1.49%, respectively, and for water, CE and OE produced yields of 1.18% and 1.91%, respectively.
Table 1.
Percentage yield obtained from each sample when extracted using different solvents
| Solvent used | Sample | Yield extract (%) |
|---|---|---|
| n-hexane | RR (Red rice) | 0.82 |
| PRR (Polished red rice) | 0.82 | |
| WR (Pungpo ame) | 0.99 | |
| RB (Rice bran) | 0.91 | |
| CE (Control extrudate) | 1.44 | |
| OE (Optimized extrudate) | 1.04 | |
| Ethanol: water (50:50) | RR (Red rice) | 0.49 |
| PRR (Polished red rice) | 1.01 | |
| WR (White rice) | 0.37 | |
| RB (Rice bran) | 1.22 | |
| CE (Control extrudate) | 1.02 | |
| OE (Optimized extrudate) | 1.49 | |
| Water | RR (Red rice) | 0.26 |
| PRR (Polished red rice) | 0.21 | |
| WR (White rice) | 0.6 | |
| RB (Rice bran) | 0.8 | |
| CE (Control extrudate) | 1.18 | |
| OE (Optimized extrudate) | 1.91 |
Inhibition of DPP-4 activity
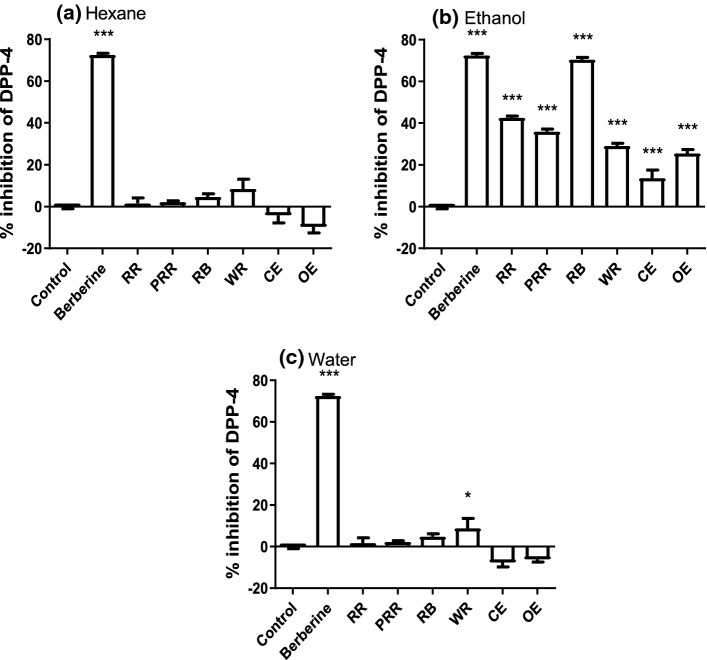
In this study, hexane extracts of red rice and extrudates were unable to inhibit the activity of the enzyme (Fig. 1a). However, all ethanol extracts of rice samples inhibited DPP-4 activity significantly (p < 0.001; Fig. 1b). Specifically, RB was the most potent at inhibiting DPP-4 activity by 70.48 ± 1.06%, followed by RR (42.55 ± 0.84%), PRR (35.91 ± 1.27%), WR (29.14 ± 1.23%), OE (25.49 ± 1.86%) and CE (13.55 ± 3.97%). Furthermore, WR when extracted with water was able to significantly inhibit DPP-4 activity by 8.78 ± 0.84% when compared to a buffer control (p < 0.001; Fig. 1c).
Fig. 1.
DPP-4 inhibition activity. Inhibition of DPP-4 by rice samples extracted by a Hexane, b Ethanol (50%), and c Water at 2 mg/ml. Bars represent mean ± SEM. Data analysed using one way ANOVA followed by Tukey’s Multiple Comparisons Test (* p < 0.05, ** p < 0.01, *** p < 0.001; n = 3). Berberine at 1 mg/ml was used as a positive control. RR = red rice, PRR = red rice polished, RB = red rice bran, WR = white rice, CE = control extrudate and OE = optimized extrudate
GLP-1 secretion
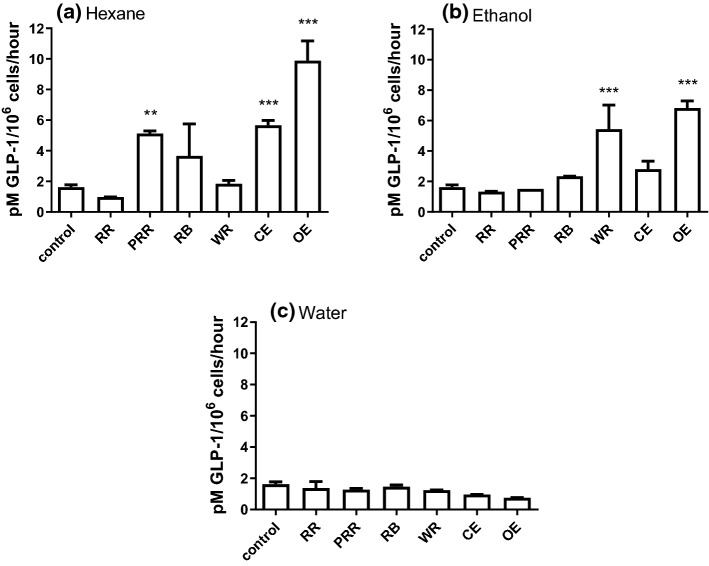
The ability of rice extracts to enhance GLP-1 secretion is shown in Fig. 2. Hexane extracts were able to potently stimulate GLP-1 secretion. In particular, PRR, CE and OE enhanced secretion of GLP-1 3.14-fold (p < 0.01), 3.48-fold (p < 0.001) and 6.06-fold (p < 0.001), respectively compared to buffer control (Fig. 2a). Ethanol extracts of WR and OE also significantly stimulated GLP-1 secretion 3.33-fold and 4.19-fold, respectively (p < 0.001; Fig. 2b). None of the water extracts had any effect on incretin secretion (Fig. 2c).
Fig. 2.
Effect of different rice sample extracts on GLP-1 secretion. GLP-1 secretion stimulated from STC-1 pGIP/Neo cells by rice samples extracted by a hexane, b ethanol (50%), and c water at 2 mg/ml. Bars represent mean ± SEM. Data analysed using one way ANOVA followed by Tukey’s Multiple Comparisons Test (*p < 0.05, ** p < 0.01, *** p < 0.001; n = 3). RR = red rice, PRR = red rice polished, RB = red rice bran, WR = white rice, CE = control extrudate and OE = optimized extrudate
Inductively coupled plasma mass spectrometry (ICP-MS) of rice sample
ICP-MS was conducted in the four rice raw materials (RR, WR, RB and PRR) samples and the two extrudate products (CE and OE) to determine the levels of arsenic. As mentioned, arsenic is highly abundant in rice and is carcinogenic in nature (D’Amato et al., 2003), therefore arsenic was measured to determine whether these rice samples were ‘safe’ for consumption. As shown in Table 2, DMA content was the highest in RR (0.010 mg/kg) > WR (0.005 mg/kg) > RB, PRR, OE (0.003 mg/kg) > CE (0.002 mg/kg). As V (i-As) content was found in significantly higher proportions and was highest in RB > CE > EE > OE > WR > PRR, ranging from 0.026 – 0.176 mg/kg. Australian food standard established the maximum total “As” content for cereals as 1 mg/kg (FSANZ 2006), suggesting that all samples tested here are well below the recommended limits. To date, the Codex Alimentarius Commission (2012) has not formulated any formal guidelines on the safe limits of heavy metals in either white or brown rice, however Asian countries like China, whose staple food crop is rice, has recommended that the safe content for “As” is limited to < 0.2 mg/kg. Choi et al. (2014) reported that grain milling can decrease the content of “As” and distribute the accumulation of “As” closer to the surface rather than in the inner core.
Table 2.
Inductively coupled plasma mass spectrometry (ICP-MS) analysis for arsenic species
| Sample | DMA (Dimethylarsinic acid) | As V | LOD (mg/Kg) |
|---|---|---|---|
| RR (Red rice) | 0.01 | 0.057 | 0.0005 |
| WR (White rice) | 0.005 | 0.055 | 0.0005 |
| RB (Rice bran) | 0.003 | 0.176 | 0.0005 |
| PRR (Polished red rice) | 0.003 | 0.026 | 0.0005 |
| CE (Control extrudate) | 0.002 | 0.062 | 0.0005 |
| OE (Optimized extrudate) | 0.003 | 0.057 | 0.0005 |
Discussion
The extraction technique, as well as the extraction solvent, influenced the extraction yield and biological activity of the resulting extract. For extracting bioactive compounds from plant material, a variety of solvents viz., methanol, ethanol, acetone, and water have been used. Because of the variety of bioactive compounds found in plant materials and their varying solubility properties in different solvents, the best solvent for extraction is determined by the plant materials and the compounds to be isolated (Ajanal et al. 2012 and Mahdi-Pour et al. 2012). Table 1 gives an idea of different solvents used i.e., polar and non-polar and resulted in various extraction yields.
Inhibition of dipeptidyl peptidase-4 (DPP-4) as a novel therapy for type 2 diabetes is based on prevention of the inactivation process of bioactive peptides e.g., glucagon-like peptide-1 (GLP-1). DPP-4 is a widely distributed enzyme that acts on incretin hormones, primarily GLP-1 (glucagon-like peptide-1) and GIP (gastric inhibitory peptide). These incretin hormones keep glucose levels stable by increasing insulin secretion while decreasing glucagon secretion (Capuano et al. 2013) GLP-1 is a hormone produced by the small intestine's enteroendocrine L cells. It aids in blood glucose control by increasing insulin secretion, decreasing glucagon concentrations, and delaying gastric emptying (Pathak and Bridgeman 2010). It has a half-life of less than 2 min whereas, GIP is a hormone secreted by neuroendocrine K-cells in the stomach and proximal small intestine. It has a half-life of about 7 min in healthy people and 5 min in type 2 diabetics (Gupta and Kalra 2011). Due to their short half-life, these incretins are released within minutes of food intake, and DPP-4 degrades them immediately. DPP-4 inhibitors raise GLP-1 and GIP levels by inhibiting the DPP-4 enzyme. As a result, beta-cell insulin secretion in the pancreas increases, lowering postprandial and fasting hyperglycemia (Capuano et al. 2013). Therefore, it is utmost important to do preliminary studies on DPP-4 inhibitory action and GLP-1 secretory activity of the sample. This insight can give a clear idea how DPP-4 inhibitory activity and GLP-1 secretory action work is linked to controlling glycaemia in type 2 diabetes. All the ethanol extracts of rice samples inhibited DPP-4 activity significantly (p < 0.001). Hexane extracts were able to potently stimulate GLP-1 secretion. Ethanol extracts of WR and OE also significantly stimulated GLP-1 secretion 3.33-fold and 4.19-fold, respectively (p < 0.001).
This investigation shows that the bran from pigmented RB contains significant levels of DPP-4 inhibitory activity and this activity is retained after food processing. The reasons for the activity are likely due to the presence of various bioactive compounds found in pigmented rice such as salicylic acid (302.06 ± 0.01 mg/L), ferulic acid (17.21 ± 0.02 mg/L), apigenin (7.03 ± 0.01 mg/L), and gallic acid (0.98 ± 0.04 mg/L) and foam mat dried powder of passion fruit constitutes like a fat-soluble vitamin (Vitamin E), ( ±)-α tocopherol (171.1 mg/100 g) and D-α-tocotrienol (Samyor et al. 2016). Samyor et al. (2020) earlier reported that the foam mat dried powder showed higher amount of β-carotene (13.26 mg/100 g), total phenolic compound (258.12 mg/100 g) and phenolic acids than fruit pulp whereas fruit pulp was contented higher amount of ( ±)-α tocopherol (171.1 mg/100 g) and D- α -tocotrienol (27.19 mg/100 g). Foam mat dried powder of passion fruit was added in the final optimized extruder product to enhance its nutritive value.
Some of these compounds of RB have previously been demonstrated to possess various activities like antidiabetic activity, antioxidant, antiinflammatory, hypocholesterolemic, anticancer, anticolitis, and antidiabetic properties (Sivamaruthi et al. 2018). Hatanaka et al. (2012) stated that rice bran peptides possessed inhibitory activity against DPP-IV.
GLP-1 secretion was relatively potent in the n-hexane and ethanol: water extracts but not in the water extracts. Hexane is not considered a food-grade solvent and so it may be more pragmatic to focus on ethanol-based extractions, or alternatively consider other non-polar solvents to hexane when implementing such an approach. DPP-4 inhibitory is largely confined to the ethanol: water extract, and given that this solvent would be compatible with food productions processes and these extracts should undergo further investigation into their composition, perhaps using a bioactivity-guided fractionation process (Purnomo and Sumitro, 2018). It might be particularly those hexane extracts which potently stimulated GLP-1 secretion (e.g., PRR, CE and OE), in order to discover novel, naturally occurring GLP-1 secretogues. This work also demonstrates that the extrusion of red rice leads to greater extracted yields of material from the grains. For certain applications this could increase the scope of functional food products, better enabling scale-up/production, and making it more cost effective and feasible. However, it is also clear that extrusion does not always improve the bioactivity of the extract, as was the case here for DPP-4 inhibitory activity.
Rice bran (RB) is the pericarp and germ of Oryza sativa seeds making up approximately 10% of rough rice grain by weight. RB is a good source of macromolecules like γ-oryzanol, tocopherols, and tocotrienols, protein, fat, and antioxidants, despite its prospects as a raw material for the preparation of functional foods or nutraceuticals (Bandyopadhyay et al. 2008; Parrado et al., 2006). Several researchers have demonstrated the ability of foods and food products to inhibit DPP-4 (Chia-Ling et al. 2015).
DPP-4 inhibitory activity was found to be retained in the ethanol: water extracts of both extruded products (CE and OE), albeit at reduced levels. Extracts from OE, the optimised extrudate containing passion fruit powder, had greater inhibitory activity than the control extrudate, suggesting that passion fruit might possess its own antidiabetic activities.
Although the activity of passion fruit powder was not tested here, Samyor, Deka & Das (2020) reported that the foam mat dried powder of passion fruit contained higher amounts of β-carotene (13.26 mg/100 g), total phenolic compounds (258.12 mg/100 g) and phenolic acids than fruit pulp whereas fruit pulp contain higher amount of ( ±)—α tocopherol (171.1 mg/100 g) and D-α-tocotrienol (27.19 mg/100 g). Furthermore, phytochemical studies undertaken by RP-HPLC of these samples revealed that β-carotene (1333.1 µg/L) was higher in control but ( ±)-α-tocopherol and D-α-tocopherol content was higher in the optimized sample (Samyor, Deka & Das, 2018). This could be one of the reasons for greater inhibitory activity in optimized product (OE) than control (CE). The most potent promising extract overall was the ethanol: water extract of red rice bran and its concentration-dependent activity was explored, with the IC50 value was calculated as 3.29 mg/ml (Fig. 3).
Fig. 3.
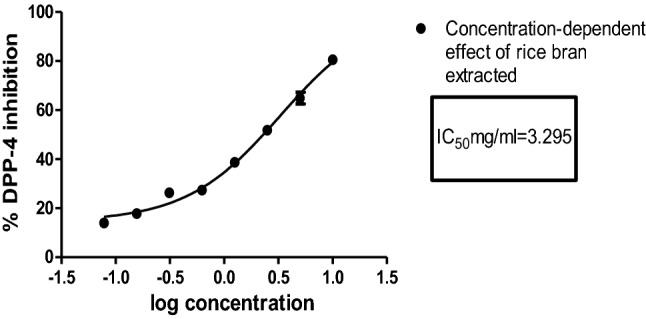
Concentration-dependent effect of rice bran extracted with ethanol (50%) on DPP4 inhibition
These results also revealed that extrusion processing lowers the content of DMA as it was found in lower quantities in CE and OE when compared to the raw rice materials. A more in-depth study on the effect of extrusion on “As” species should be carried out as it may be a promising process which can reduce “As” content in rice and cereals.
Conclusion
This is the maiden investigation where DPP-4 inhibitory activity and GLP-1 secretory action of arsenically safe pigmented and non-pigmented rice (Oryza sativa L.) of Northeast India, along with an extruded food product have been reported. The study also showed different solvents with varying solubility properties. Ethanol extracted red rice bran appears to inhibit DPP-4 effectively and acts in a concentration-dependent manner. Food extrusion processing of red rice mixed with foam mat dried powder also evinced to greater extracted yields of material from the grains. Therefore, pigmented and non-pigmented rice along with its product might be a potentially beneficial source in the management of T2DM via inhibition of DPP-4 enzyme and stimulation of incretin hormones. Further investigation of red rice bran could lead to the development of novel functional food products.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the Newton Bhabha PhD Placement Programme (F.No.BT/IN/UK/DBT-BC/
2015-16) in collaboration with Dept. of Biotechnology, India and British Council, U.K for providing financial support to D.S.
Abbreviations
- DPP-4
Dipeptidyl peptidase 4
- GLP-1
Glucagon-like peptide
- ICP-MS
Inductively coupled plasma-mass-spectrometry
- DMA
Dimethyl arsenic acid
- As III
Arsenite
- As V
Arsenate
- MMA
Monomethyl arsonic acid
- SEM
Standard error of the mean
- STC-1
Secretin tumor cell line
- DMEM
Dulbecco’s Modified Eagle Medium
- HEPES
N-2-Hydroxyethylpiperazine-N'-2-Ethanesulfonic Acid
- ELISA
Enzyme-linked immunoassay.
- RB
Rice bran
- RR
Red rice
- PRR
Polished red rice
- WR
White rice
- OE
Optimized extrudate
- CE
Controlled extrudate
Authors contribution
DS, ABD and I (SCD) designed the experiments for optimization of process parameters using Design Expert 7 Version software. DS, DC, MC and BDG analysed and interpreted the data. DS drafted the manuscript and BDG and I edited the manuscript. All authors approved the final version of the manuscript to be published.
Funding
Newton Bhabha PhD Placement Programme (F.No.BT/IN/UK/DBT-BC/2015–16) in collaboration with Dept. of Biotechnology, India and British Council, U.K for a period of 6 months.
Data availability
All datasets generated for this study are included in the manuscript. Further if any more data are required, it can be provided from the corresponding author on reasonable request.
Code availability
Statistical analysis was conducted using Graph Pad Prism version 5 (Graph Pad Software, USA). All experiments were carried out in triplicate. Data are expressed as mean ± SEM and statistical comparisons were assessed using a one-way ANOVA with Tukey’s post-hoc test. A p-value less than 0.05 was deemed statistically significant.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ajanal M, Gundkalle M, Nayak S. Estimation of total alkaloid in Chitrakadivati by UV-Spectrophotometer”. Anc Sci Life. 2012;31(4):198–201. doi: 10.4103/0257-7941.107361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-masri IM, Mohammad MK, Tahaa MO. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J Enzyme Inhib Med Chem. 2009;24:1061–1106. doi: 10.1080/14756360802610761. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay K, Misra G, Ghosh S. Preparation and characterization of protein hydrolysates from Indian defatted rice bran meal. J Oleo Sci. 2008;57:47–52. doi: 10.5650/jos.57.47. [DOI] [PubMed] [Google Scholar]
- Capuano A, Sportiello L, Maiorino MI, Rossi F, Giugliano D, Esposito K. (2013) Dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapy-focus on alogliptin. Drug Des Devel Ther. 2013;7:989–1001. doi: 10.2147/DDDT.S37647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chemat F, Vian AM, Cravotto G. Green extraction of natural products: concept and principles. Intern J Mol Sci. 2012;13:8615–8627. doi: 10.3390/ijms13078615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia-Ling J, Chuan-Chuan H, Yu-Shan T, Meng-Chun P-Y, Kuo-Chiang CH. The development of bioactive peptides from dietary proteins as a dipeptidyl peptidase IV inhibitor for the management of type 2 diabetes. Bio Med. 2015;5(3):9–15. doi: 10.7603/s40681-015-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Kim JS, Lee JY, Jeon JS, Kim JW, Russo RE, Gonzalez J, Yoo JH, Kim KS, Yang JS, Park KS. Analysis of arsenic in rice grains using ICP-MS and fs LA-ICP-MS. J Anal at Spectrom (JAAS) 2014;29:1233–1237. doi: 10.1039/C4JA00069B. [DOI] [Google Scholar]
- Codex General Standard for Contaminants and Toxins in Food and Feed, Codex Standard, 2012.
- D’Amato M, Forte G, Caroli S. Identification and quantification of major species of arsenic in rice. J AOAC Intern. 2004;87:238–243. doi: 10.1093/jaoac/87.1.238. [DOI] [PubMed] [Google Scholar]
- FSANZ. (2006). Contaminants and natural toxicants. Standard 1.4.1. Available:< http://www.foodstandards.gov.au/_srcfiles/fsc_1_4_1_Contaminants_v78.pdf >. Accessed 2006.
- Fujiwara K, Tsuru D. New chromogenic and fluorogenic substrates for pyrrolidonyl peptidase. J Biochem. 1978;83:1145–1149. [PubMed] [Google Scholar]
- Gillespie AL, Pan X, Marco-Ramell A, Meharg C, Green BD. Detailed characterisation of STC-1 cells and the pGIP/Neo sub-clone suggests the incretin hormones are translationally regulated. Peptides. 2017;96:20–30. doi: 10.1016/j.peptides.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Giorda CB, Nada E, Tartaglino B. Pharmacokinetics, safety, and efficacy of DPP4 inhibitors and GLP-1 receptor agonists in patients with type 2 diabetes mellitus and renal or hepatic impairment. a systematic review of the literature. Endocrine. 2014;46:406–419. doi: 10.1007/s12020-014-0179-0. [DOI] [PubMed] [Google Scholar]
- Gupta V, Kalra S. Choosing a gliptin. Indian J Endocrinol Metab. 2011;15(4):298–308. doi: 10.4103/2230-8210.85583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hand KV, Bruen CM, O'Halloran F, Giblin L. Acute and chronic effects of dietary fatty acids on cholecystokinin expression, storage and secretion in enteroendocrine STC-1 cells. Mol Nutr Food Res. 2010;54:S93–S103. doi: 10.1002/mnfr.200900343. [DOI] [PubMed] [Google Scholar]
- Harper J M (1989). Food Extruders and Their Applications; Mercier C, Linko P, Harper JM, Eds.;American Association of Cereal Chemists: St. Paul, MN, USA,1–15.
- Hatanaka T, Inoue Y, Arima J, Kumagai Y, Usuki K, Kawakami K, Kimura M, Mukaihara T. Production of dipeptidyl peptidase IV inhibitory peptides from defatted rice bran. Food Chem. 2012;134:797–802. doi: 10.1016/j.foodchem.2012.02.183. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Uraji M, Fujita A, Awakami K. Anti-oxidant activities of rice-derived peptides and their inhibitory effects on Dipeptidyl peptidase-IV. Intern J Peptidase Res Ther. 2015;21(4):479–485. doi: 10.1007/s10989-015-9478-4. [DOI] [Google Scholar]
- Heitkemper DT, Vela NP, Stewart KR, Westphal CS. Determination of total and speciated arsenic in rice by ion chromatography and inductively coupled plasma mass spectrometry. J Anal Spectrom. 2001;16(4):299–306. doi: 10.1039/b007241i. [DOI] [Google Scholar]
- Kumar A, Girish GM, Jones DD, Hanna MA. Modelling residence time distribution in a twin-screw extruder as a series of ideal steady-state flow reactors. J Food Eng. 2008;84:441–448. doi: 10.1016/j.jfoodeng.2007.06.017. [DOI] [Google Scholar]
- Mahdi-Pour B, Jothy SL, Latha LY, Chen Y, Sasidharan S. Antioxidant activity of methanol extracts of different parts of Lantana camara. Asian Pac J Trop Biomed. 2012;2(12):960–965. doi: 10.1016/S2221-1691(13)60007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga P, Moyano A, Anawar HM, Garcia-Sanchez A. Uptake and accumulation of arsenic in different organs of carrot irrigated with as-rich water. Clean-Soil, Air, Water. 2013;41(6):587–592. doi: 10.1002/clen.201100697. [DOI] [Google Scholar]
- Nagendra PMN, Sanjay KR, Shravya KM, Vismaya MN, Nanjunda SS. Health benefits of rice bran - a review. J Nutr Food Sci. 2011;1:3. doi: 10.4172/2155-9600.1000108. [DOI] [Google Scholar]
- Nguyen DH, Ta NTA, Bui AV, Do TH, Nguyen HC. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of severinia buxifolia. J Food Quality. 2019 doi: 10.1155/2019/8178294. [DOI] [Google Scholar]
- Parrado J, Miramontes E, Jover M, Gutierrez JF, Terán LC, Bautista J. Preparation of a rice bran enzymatic extract with potential use as functional food. Food Chem. 2006;98:742–748. doi: 10.1016/j.foodchem.2005.07.016. [DOI] [Google Scholar]
- Pathak R, Bridgeman MB. Dipeptidyl peptidase-4 (DPP-4) inhibitors in the management of diabetes. Pharmacy Therapeutics. 2010;35(9):509–513. [PMC free article] [PubMed] [Google Scholar]
- Purnomo Y, Sumitro S. Inhibitory activity of Urena lobata leaf extract on dipeptidyl peptidase-4 (DPP-4): is It different in vitro and in vivo ? Medicinal Plants – Intern. J Phytomed Related Ind. 2018 doi: 10.5958/0975-6892.2018.00016.3. [DOI] [Google Scholar]
- Samyor D, Deka SC, Das AB. Evaluation of physical, thermal, pasting characteristics and mineral profile of pigmented and non -pigmented rice cultivars. J Food Process Preserv. 2016;40(2):174–182. doi: 10.1111/jfpp.12594. [DOI] [Google Scholar]
- Samyor D, Deka SC, Das AB. Effect of extrusion conditions on the physicochemical and phytochemical properties of red rice and passion fruit powder based extrudates. J Food Sci Technol. 2018;55(12):5003–5013. doi: 10.1007/s13197-018-3439-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samyor D, Deka SC, Das AB. Physicochemical and phytochemical properties of foam mat dried passion fruit (Passiflora edulis Sims) powder and comparison with fruit pulp. J Food Sci Technol. 2020;58:787–796. doi: 10.1007/s13197-020-04596-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Y, Bao J. Polyphenols in whole rice: Genetic diversity and health benefits. Food Chem. 2015;180:86–97. doi: 10.1016/j.foodchem.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Signes-Pastor AJ, Meharg CM. Inorganic arsenic in rice-based products for infants and young children. Food Chem. 2016;191:128–134. doi: 10.1016/j.foodchem.2014.11.078. [DOI] [PubMed] [Google Scholar]
- Sivamaruthi BS, Kesika P, Chaiyasut C. A comprehensive review on anti-diabetic property of rice bran. Asian Pacif J Tropic Biomed. 2018;8(1):79–84. doi: 10.4103/2221-1691.221142. [DOI] [Google Scholar]
- Suzuki KT, K T, Arsenic round the world: A review. Talanta. 2002;58(1):201–235. doi: 10.1016/S0039-9140(02)00268-0. [DOI] [PubMed] [Google Scholar]
- Zendjabil M. Biological diagnosis of diabetes mellitus. Curr Res Transl Med. 2016;64:49–52. doi: 10.1016/j.patbio.2015.10.002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the manuscript. Further if any more data are required, it can be provided from the corresponding author on reasonable request.
Statistical analysis was conducted using Graph Pad Prism version 5 (Graph Pad Software, USA). All experiments were carried out in triplicate. Data are expressed as mean ± SEM and statistical comparisons were assessed using a one-way ANOVA with Tukey’s post-hoc test. A p-value less than 0.05 was deemed statistically significant.