Abstract
As the sister group to bilaterians, cnidarians stand in a unique phylogenetic position that provides insight into evolutionary aspects of animal development, physiology, and behavior. While cnidarians are classified into two types, sessile polyps and free-swimming medusae, most studies at the cellular and molecular levels have been conducted on representative polyp-type cnidarians and have focused on establishing techniques of genetic manipulation. Recently, gene knockdown by delivery of short hairpin RNAs into eggs via electroporation has been introduced in two polyp-type cnidarians, Nematostella vectensis and Hydractinia symbiolongicarpus, enabling systematic loss-of-function experiments. By contrast, current methods of genetic manipulation for most medusa-type cnidarians, or jellyfish, are quite limited, except for Clytia hemisphaerica, and reliable techniques are required to interrogate function of specific genes in different jellyfish species. Here, we present a method to knock down target genes by delivering small interfering RNA (siRNA) into fertilized eggs via electroporation, using the hydrozoan jellyfish, Clytia hemisphaerica and Cladonema paciificum. We show that siRNAs targeting endogenous GFP1 and Wnt3 in Clytia efficiently knock down gene expression and result in known planula phenotypes: loss of green fluorescence and defects in axial patterning, respectively. We also successfully knock down endogenous Wnt3 in Cladonema by siRNA electroporation, which circumvents the technical difficulty of microinjecting small eggs. Wnt3 knockdown in Cladonema causes gene expression changes in axial markers, suggesting a conserved Wnt/β-catenin-mediated pathway that controls axial polarity during embryogenesis. Our gene-targeting siRNA electroporation method is applicable to other animals, including and beyond jellyfish species, and will facilitate the investigation and understanding of myriad aspects of animal development.
Subject terms: Biological techniques, Developmental biology
Introduction
The phylum Cnidaria is the sister group to Bilateria, having separated from their common ancestor over 500 million years ago. Cnidarians have diversified their morphologies across an array forms, including corals, sea anemones, hydroids, and jellyfish, all of which are divided into two clades: Anthozoa and Medusozoa. The two differ in that Anthozoa includes only sessile polyp-type animals, while Medusozoa (Hydrozoa, Staurozoa, Scyphozoa, and Cubozoa) contains two forms: polyp and medusa, commonly known as jellyfish (Fig. 1A)1,2. The life cycle of most, though not all, jellyfish consists of five forms: gametes, fertilized eggs, planulae, polyps, and medusae3. Fertilized eggs undergo embryogenesis to become planula larvae, which metamorphose into sessile polyps. While vegetatively-growing polyps give rise to free-swimming medusae through the process of budding or strobilization, medusae sexually reproduce by releasing gametes. Due to their unique phylogenetic position, studies using cnidarians have provided evolutionary insight into development, regeneration, and behaviors in multicellular animals1,4,5. Despite divergent morphologies and lifestyles among cnidarians, to date, the molecular and cellular understanding of cnidarians has been acquired primarily using polyp-type animals such as the anthozoan Nematostella vectensis and the hydrozoan Hydra and Hydractinia. By contrast, jellyfish biology remains largely unestablished.
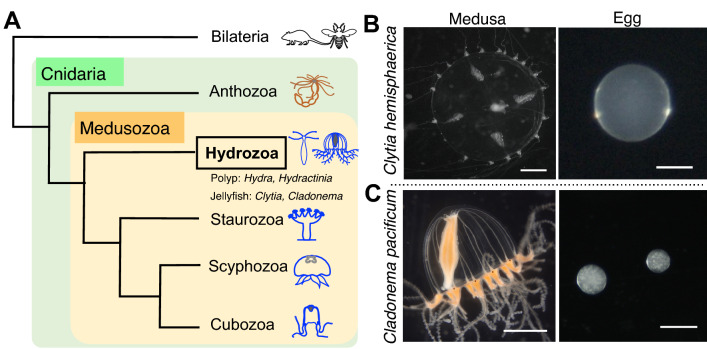
Figure 1.
The hydrozoan jellyfish Clytia hemisphaerica and Cladonema pacificum. (A) Cladogram depicting the phylogenetic position of cnidarian jellyfish. As the sister group of Bilateria, the phylum Cnidaria is divided into two clades, Anthozoa and Medusozoa, which consists of four classes: Hydrozoa, Staurozoa, Scyphozoa, and Cubozoa. Hydrozoa includes polyp type animals without a medusa stage (e.g. Hydra and Hydractinia) and jellyfish that have both polyp and medusa stages (e.g. Clytia and Cladonema). (B, C) Photos of an adult medusa and the eggs of Clytia hemisphaerica (B) and Cladonema pacificum (C). Scale bars: 1 mm for medusae; 100 µm for eggs.
Among jellyfish species, the hydrozoan Clytia hemisphaerica is the best-studied laboratory model6,7. The well-established methodology for maintaining and propagating animals ensures a reliable source for daily experiments8. Transparent and small-sized medusae make whole-mount visualization achievable, and relatively large-sized eggs (~ 180 µm diameter) enable different manipulations via microinjection (Fig. 1B). Indeed, gene silencing by morpholino oligos and mRNA microinjection as well as gene knockout via CRISPR/Cas9 allow for the investigation of functions of genes of interest9–13. Furthermore, the Clytia genome assembly is complete, and transcriptome profiles are available for various stages and tissues, even at the single-cell level, which creates the foundation for research at the molecular level12,14–18. These resources and techniques, together with recently-established transgenesis19, have accelerated research into numerous facets of distinct Clytia life stages. While initial studies focused on embryogenesis, current research topics using Clytia include gametogenesis, regeneration, and behavior exhibited by adult medusa 9,10,12,17,19,20.
Compared to the established model jellyfish Clytia, the hydrozoan Cladonema pacificum is an emerging model that has recently been utilized for various studies in development, regeneration, and physiology21. The ease of rearing all stages of Cladonema without a filtration system or large water tank, along with their high spawning rate, enable easy lab maintenance. Small-sized medusae with branched tentacles allow for investigations of body size control and tentacle morphogenesis (Fig. 1C)22–24. Cladonema gametogenesis is regulated by a light–dark cycle as in Clytia, and recent work has identified the neuropeptides involved in oocyte maturation25. Furthermore, Cladonema medusae possess eyes with a complex structure that includes lenses (ocelli) in their tentacle bulbs, and studies using the closely related species Cladonema radiatum have identified conserved light-sensitive opsins and regulators of eye formation (Pax, Six, and Eya)25–29, providing a model for the evolutionary developmental biology of photoreceptor organs. Despite these attractive features of Cladonema as a laboratory animal, no genome assembly or transcriptome is currently available, and genetic manipulation techniques are only just being developed. One major technical issue in manipulating Cladonema is their small eggs—with a diameter of approximately 60 µm (Fig. 1C), regular microinjection is quite difficult. Establishing genetic manipulations is required to facilitate the in-depth investigations needed to further understand the biology of Cladonema, and an alternative method that eliminates the need for microinjection would greatly facilitate that objective.
RNA interference (RNAi), the phenomenon of double stranded RNA (dsRNA) mediated silencing of target genes, has been widely exploited in living organisms to analyze gene function30, primarily using chemically- or in vitro-synthesized double-stranded small interfering RNAs (siRNAs) or vector-based short hairpin RNAs (shRNAs)31. In cnidarians, siRNA-mediated gene silencing was initially applied to Nematostella and Hydra polyps via soaking or electroporation32–34. More recently, gene knockdown via shRNA microinjection or electroporation into eggs has been utilized in studies with Nematostella and Hydractinia to show efficient reduction in gene expression and associated phenotypes in early developmental stages35–38. In particular, shRNA delivery via electroporation, which does not require the rigors of microinjection, allows for the experimental gene knockdown of large numbers of individuals simultaneously36,38, opening up the possibility of manipulating genes in different aquatic animals, even those that produce very small eggs like Cladonema.
The mechanism of RNAi-mediated gene repression after introducing foreign dsRNA into cells involves the microRNA (miRNA) pathway, an endogenous gene repression machinery conserved in both animals and plants39. In cnidarians, the presence of the miRNA pathway has been demonstrated in Nematostella and Hydra32,40,41. However, little is known about the endogenous RNAi pathway, especially the presence and roles of miRNAs and the miRNA-related genes, in jellyfish such as Clytia and Cladonema. Furthermore, in mammalian cultured cells, siRNAs can induce gene repression independent of endogenous dsRNA processing factors, while shRNAs cannot42,43. On this basis, we selected siRNA instead of shRNA for gene knockdown to avoid the potential pitfall of dsRNA processing in jellyfish.
Here we report a gene knockdown method for jellyfish embryos with siRNA via electroporation. Using two hydrozoan species, Clytia hemisphaerica and Cladonema pacificum, we demonstrate that siRNA delivery into fertilized eggs effectively reduces the expression of endogenous genes. We also confirm the known loss-of-function phenotypes in Clytia after knocking down GFP1 or Wnt3, which are induced in a dose-dependent manner with siRNA. We further show that knockdown of Wnt3 in Cladonema embryos results in the reduction of gene expression of the oral marker Brachyury in planula, implicating the Wnt/β-catenin pathway in the control of oral-aboral patterning. Overall, our siRNA-mediated knockdown approach allows for the manipulation of a large number of embryos through electroporation and enables functional analysis of early development, providing a new experimental platform applicable to different jellyfish species and other marine invertebrates.
Materials and methods
Animal culture and spawning induction
Clytia hemisphaerica (Z11, Z4B as females and Z4C2, Z23 as males) and Cladonema pacificum (6 W as females and UN2 as males) were used for this research.
Clytia hemisphaerica were cultured using a previously reported method8 with a few modifications. Artificial sea water (ASW) was prepared using 220 g SEA LIFE (Marin Tech) per 5L MilliQ water (Merk Millipore) with antibiotics (40 units/ml of penicillin and 40 μg/ml of streptomycin). Medusae, embryos, and planula larvae were maintained at 20 °C. Medusae were fed daily with Vietnamese brine shrimp (A&A Marine LLC, Elk Rapids, MI, USA). Spawning timing was controlled by a 13 h dark /11 h light cycle, and spawning was induced by light. Male and female medusae were transferred into V-7 cups (AS ONE) before spawning (60 min for male and 90 min for female after light stimulation).
Cladonema pacificum were cultured as previously described23. Cladonema medusae were maintained at 22 °C in ASW, which was prepared using SEA LIFE (Marin Tech) dissolved in tap water with chlorine neutralizer (Coroline off, GEX Co. ltd) (24 p.p.t) and antibiotics (40 units/ml penicillin and 40 μg/ml of streptomycin). Spawning timing was controlled by a 30 min dark/23.5 h light cycle, and spawning of male and female gametes was induced by dark stimulation. Before dark stimulation, adult females and males were separately transferred into V7 cups (AS ONE) and 60 mm dishes (Corning), respectively.
Nematostella vectensis were cultured as previously described44, with a few modifications. Briefly, adult animals were maintained in brackish water at a salinity one-third of artificial seawater (35 g/l, pH 7.5–8.0, SEA LIFE, Marin Tech) and fed with freshly hatched artemia twice per week. Spawning induction was performed at 26 °C under light for at least 11 h.
siRNA and shRNA
siRNA sequences (19 mer RNA + 2 mer DNA) for CheGFP1, CheWnt3, and CpWnt3 were designed based on their CDS sequences by Nippon Gene Co., Ltd, and siRNA duplexes were synthesized by manufacturers (Nippon Gene Co., Ltd and Sigma-Aldrich, Merk). Lyophilized siRNA was resuspended in RNase free water to a final concentration of 6 μg/μl as stock solution. The siRNA stock solution was diluted with RNase free water to a total volume of 10 μl and then added to 90 μl of fertilized eggs in 15% Ficoll (Nacalai tesque, Japan) in ASW just prior to electroporation.
shRNAs were synthesized as described in previous reports36,38. Briefly, shRNAs were synthesized by in vitro transcription (IVT) from double stranded DNA templates using the AmpliScribeTM T7-flashTM transcription kit (Lucigen, Inc.) and were purified using Direct-Zol TM RNA Miniprep kits (Zymo Research, R2070). Concentrations of shRNA was measured with a NanoDrop One (Thermo fisher).
Collection of gametes and electroporation for Clytia and Cladonema fertilized eggs
For the preparation of gametes, sexually-mature Clytia medusae were treated following a previously reported method8, partially modified to fit our experimental equipment. Adult male and female medusae were maintained on a 13 h dark/11 h light cycle, and light stimuli induced gametogenesis in 60 min for sperm and 90 min for eggs (Fig. 2A). For Cladonema pacificum, sexually-mature medusae were maintained on a 30 min dark/23.5 h light cycle. Dark stimulation induced gametogenesis in 25 min for both sexes.
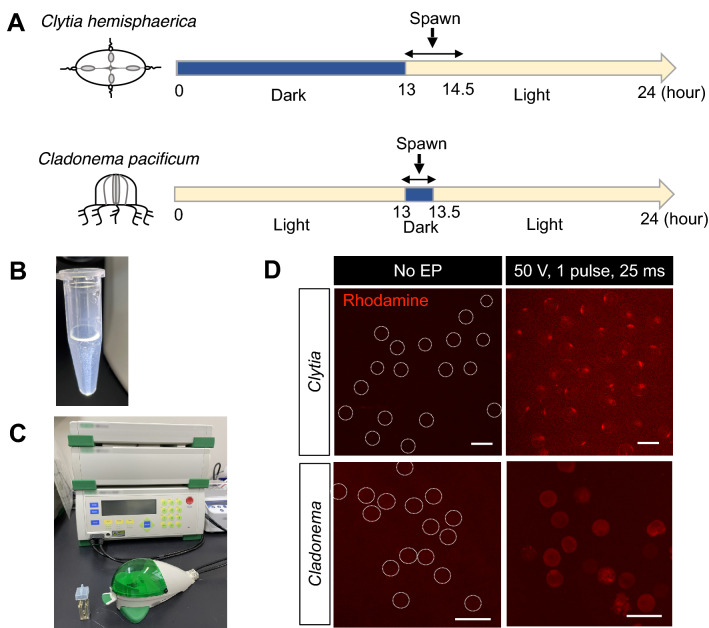
Figure 2.
The electroporation procedure for jellyfish eggs. (A) Schematic of spawning under the 24 h light/dark cycle. Clytia hemisphaerica are maintained on a 13 h dark/11 h light cycle. After light stimuli, sperm spawning occurs within 60–90 min and egg spawning occurs within 90–120 min. Cladonema pacificum are maintained on a 23.5 h light/0.5 h dark cycle. After dark stimuli, sperm and egg spawning occurs within 25 min. (B) Fertilized eggs are resuspended in 15% Ficoll/artificial sea water to prevent precipitation of eggs during electroporation. (C) Electroporation is performed with the Bio-Rad Gene Pulser Xcell electroporation system and a cuvette with a 4 mm gap. (D) Visualization of the Rhodamine-Dextran delivery into eggs by electroporation. Rhodamine is shown in red. While little or no fluorescence was observed in eggs without electroporation (No EP), under the condition of a single 50 V pulse for 25 ms, rhodamine signals were detected in both Clytia and Cladonema eggs without cell damage. For clarity, egg outlines are indicated with dashed lines for eggs without electroporation. Scale bars: 200 µm.
Unfertilized Clytia and Cladonema eggs were collected and resuspended in 15% Ficoll (Nacalai tesque, Japan) in ASW to prevent the eggs settling from at the bottom of the microtube or sticking to the microtube surface, keeping the egg solution homogeneous (Fig. 2B)36,38. Sperm water was added into the egg solution, and the eggs and sperm were incubated for 5 min at room temperature (20–22 °C) until fertilization occurred. The sperm water was then removed from the egg solution, and fertilized eggs were resuspended in 15% Ficoll in ASW to adjust the total volume needed for the number of electroporations. For each electroporation, 200–400 of Clytia fertilized eggs or 200–700 of Cladonema fertilized eggs were prepared in 90 μl of 15% Ficoll in ASW. 10 μl of siRNA/shRNA solution that was adjusted to the proper concentration in RNase free water was added to the fertilized egg solution.
The total 100 μl of fertilized egg and RNA mixture was carefully transferred into a 4 mm cuvette using a 200 μl pipet tip and placed into the shockpod cuvette chamber connected to the Gene Pulser Xcell (Bio-Rad). In most experiments, the Gene Pulser Xcell was used for pulsing with a square wave voltage of 50 V and a single pulse duration of 25 ms, except when optimizing electroporation conditions (Supplementary Figs. 1–2). After electroporation, fertilized eggs were transferred to a dish, and incubated for 10 min. The 15% Ficoll solution was removed and replaced with new ASW, and the samples were incubated at 20–22 °C.
Collection of egg sacs, dejellying, and electroporation of Nematostella fertilized eggs
The collection of egg sacs, dejellying, and electroporation steps were performed as previously described36 with several modifications. Briefly, unfertilized egg sacs were incubated in culture media with sperm at 20–25 °C for 30 min. The fertilized egg sacs were then dejellied in freshly prepared L-Cysteine solution (0.04 g/ml, Nacalai tesque, in brackish water; pH adjusted by 5 N NaOH at 7.5–8.0) on a shaker for 10 min. The dejellied eggs were rinsed with brackish water before they were suspended in Ficoll solution (Sigma-Aldrich, in brackish water) at a final concentration of 15%. The suspended eggs and siRNA (5 μg/μl stock solution, in MilliQ) were transferred to a 4 mm cuvette (Bio-Rad) with a final volume of 200 μl and were gently mixed. The Bio-Rad Gene Pulser Xcell was used for pulsing, with a square wave voltage of 50 V and a pulse duration of 25 ms. The samples were transferred to a 100 mm dish and maintained in brackish water for 3 days.
Imaging
GFP and Rhodamine fluorescent imaging as well as bright field imaging, including in situ hybridization imaging, were taken with an Axio Zoom V16 (ZEISS), and image processing was done with ZEN 3.4 blue edition (ZEISS) and Fiji/ImageJ software45. Intensity of mean values of green fluorescence was measured using the average brightness (pixel value) of the pixels. To prevent planula movement, we added 0.5 M sodium azide (final concentration was less than 0.25 M).
Morphological quantification of planula larvae
After Wnt3 knockdown by siRNA electroporation of Clytia or Cladonema, we approximated the planula body shape into a two dimensional ellipse, measured both the long and short axes (Figs. 4C, 5D), and calculated the aspect ratio of those values using Fiji/ImageJ software (Figs. 4D, 5E).
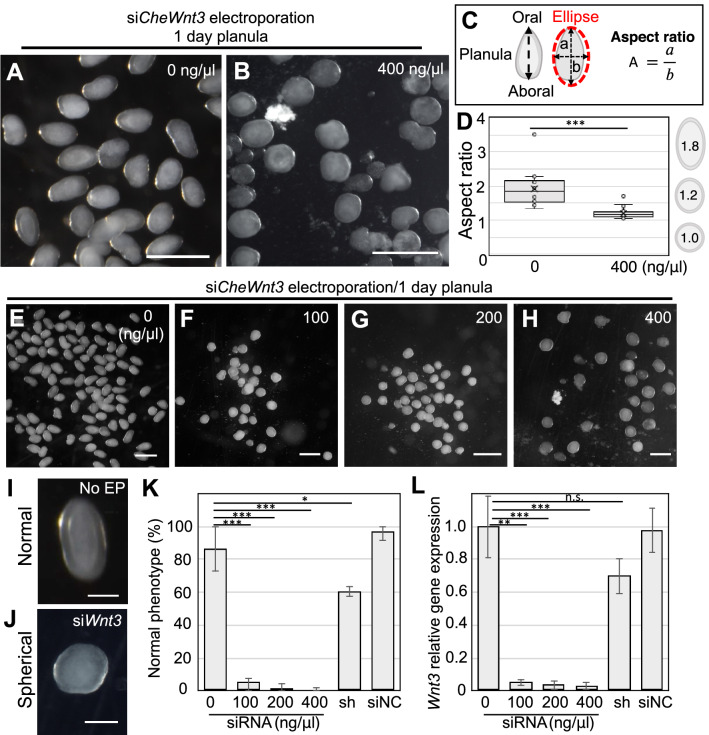
Figure 4.
Phenotypes of Wnt3 knockdown with siRNA in Clytia fertilized eggs. (A, B) Typical phenotypes of Clytia 1-day planula. While morphologies of control planula (0 ng/µl) have an elongated oval-shape (A), those of CheWnt3 siRNA (400 ng/µl) are spherical in shape (B). (C) Schematic of ellipse approximation for planula morphology and calculation of the aspect ratio. The aspect ratio was calculated by dividing the long axis (a) by short axis (b). (D) Boxplots showing the aspect ratio of the 1-day planula. Center lines show the medians; x’s denote the mean values; box limits indicate the 25th and 75th percentiles; whiskers show maximum and minimum values; inner points and outliers are represented by circles. Number of examined planula: 0 ng/µl, n = 127; 400 ng/µl. n = 25, ***p < 0.001. (E–H) Phenotypes of 1-day planula after siRNA targeting CheWnt3 electroporation (0, 100, 200, and 400 ng/µl siCheWnt3). (I and J) For morphology quantification, we classified planula phenotypes into two categories: elongated oval shape as “normal” and spherical (circle) shape as “spherical”. (K) Bar plots show the percentage of normal phenotypes across four different siRNA doses as well as planulae treated with shRNA and siNC, a siRNA universal negative control. Percentages are the mean value and error bars indicate standard deviation. Numbers of examined planula: 0 ng/µl, n = 127; 100 ng/µl, n = 75; 200 ng/µl, n = 86; 400 ng/µl, n = 95. shRNA 400 ng/μl, n = 288; siNC 400 ng/μl, n = 45. Experiments were repeated three times. Error bars: maximum and minimum values. p-values: 100 ng/μl, p = 0.000599; 200 ng/μl, p = 0.000438; 400 ng/μl, p = 0.000402; 400 ng/μl of shRNA, p = 0.031928; 400 ng/μl of siNC, p = 0.283359. *p < 0.05, ***p < 0.001. (L) Quantification of CheWnt3 mRNA levels of 1-day planula by RT-qPCR. CheEF1alpha was used as an internal control. Bar heights represent the mean value. CheWnt3 expression levels are standardized relative to the control (0 ng/µl). Error bars: standard deviation. p-values: 100 ng/μl, p = 0.00799; 200 ng/μl, p = 0.000754; 400 ng/μl, p = 0.000733; 400 ng/μl of shRNA, p = 0.095403; 400 ng/μl of siNC, p = 0.900264. **p < 0.01, ***p < 0.001. n.s., not significant. Experiments were performed in triplicate and repeated at least three times. Scale bars: (A, B, E–H) 400 µm, (I, J) 100 µm.
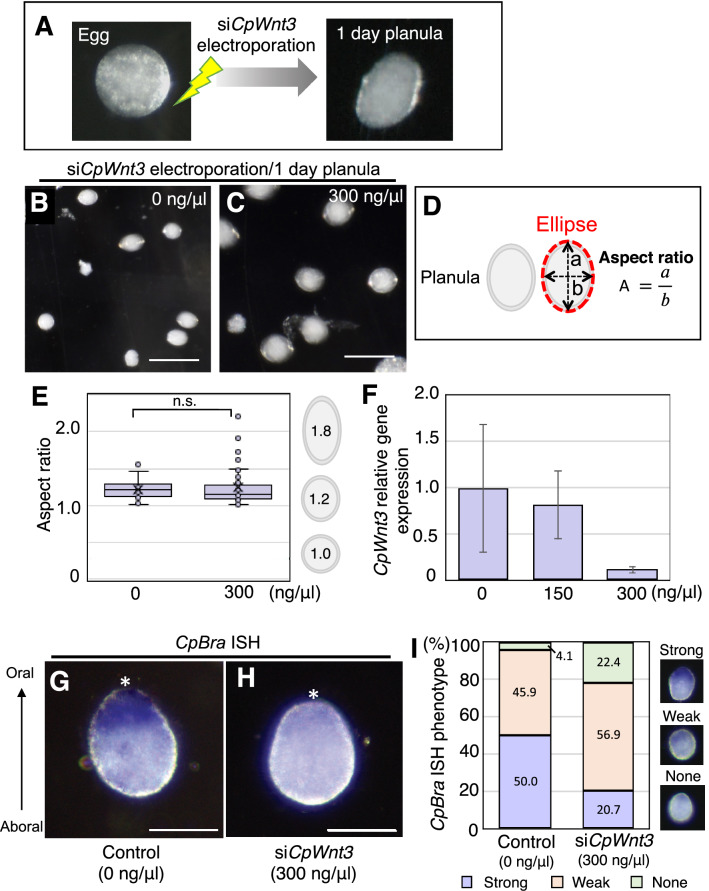
Figure 5.
Phenotypes of Wnt3 knockdown with siRNA in Cladonema fertilized eggs. (A) Typical morphology of Cladonema egg and planula. The wild-type Cladonema planula larvae exhibit a slight oval shape. To deliver siRNAs targeting CpWnt3, electroporation was performed in fertilized eggs (1-cell stage), and phenotypes were confirmed at planula in one day. (B, C) Phenotypes of 1-day planula after CpWnt3 siRNA knockdown. (D) Schematic of ellipse approximation for planula morphology and calculation of the aspect ratio. The aspect ratio was calculated by dividing the long axis (a) by short axis (b). (E) Boxplots showing the aspect ratio of the 1-day planula after siCpWnt3 electroporation (0 and 300 ng/µl). Center lines show the medians; x’s denote the mean values; box limits indicate the 25th and 75th percentiles; whiskers show maximum and minimum values; inner points and outliers are represented by circles. Number of examined planulae: 0 ng/µl, n = 22; 300 ng/µl, n = 50. p = 0.658303. n.s., not significant. (F) Quantification of CpWnt3 mRNA levels in 1-day planula by RT-qPCR. CpEF1alpha was used as an internal control. Bar heights represent the mean value. Error bars indicate standard deviation. CpWnt3 expression levels are standardized relative to the control (0 ng/µl). Experiments were performed in triplicate and repeated three times. p-values: 150 ng/μl, p = 0.376498; 300 ng/μl, p = 0.073956. (G, H) Representative in situ hybridization (ISH) image of oral CpBra expression in 1-day planula for control (G: 0 ng/µl siCpWnt3) and CpWnt3 siRNA (H: 300 ng/µl siCpWnt3). The oral pole is indicated by an asterisk. (I) Quantification of CpBra expression phenotypes based on ISH images. Stacked bar plots show the percentage of 1-day planulae in each phenotypic class. CpBra expression patterns are categorized into three phenotypes (strong, weak and none). 0 ng/µl, n = 74; 300 ng/µl, n = 58. Scale bars: (B, C) 200 µm, (G, H) 50 µm.
RT-qPCR
For Clytia and Cladonema samples, total RNA was extracted with RNeasy Mini or Micro kits (Qiagen). Lysate was treated with DNaseI (Qiagen) for 15 min at room temperature (RT). cDNA was synthesized with the PrimeScript II 1st strand cDNA synthesis kit (Takara). RT-qPCR was performed with CFX connect (Bio-Rad) using iTaqTM universal SYBER Green Supermix (Bio-Rad) or the QuantStudio 6 Flex Real-Time PCR System (Thermo Fisher) using TB Green Premix Ex TaqII (Tli RNaseH Plus) (Takara, RR820). Gene expression was normalized to the housekeeping gene, EF1alpha or F-actin capping protein subunit beta, and the delta-delta-ct method was used for quantification (CFX maestro software, Bio-Rad or QuantStudio 6 Flex Real-Time PCR System software, Thermo Fisher).
For Nematostella samples, total RNA was extracted using the RNeasy Mini Kit and RNase-Free DNase Set (Qiagen). cDNA synthesis was conducted using the SuperScript IV First-Strand Synthesis System (Thermo Fisher). RT-qPCR was performed with the StepOnePlusTM Real-Time PCR System (Applied Biosystems, Thermo Fisher) using PowerUP SYBR Green Master Mix (Thermo Fisher). Gene expression was normalized to the housekeeping gene, NvEf1alpha, and the delta-delta-ct method was used for quantification (StepOne™ and StepOnePlus™ Software v2.3).
In situ hybridization
Purified total RNA was reverse transcribed into cDNA by PrimeScript II 1st strand cDNA Synthesis Kit (Takara). The target gene fragments (Brachyury, CpBra) were amplified from a cDNA library. The primer sets used for PCR cloning are as follows: CpBra: 5′-GCTCCCATAAGATCCGGTCG-3′ (forward) and 5′-TTTGTCGCAGTCGAAGACCA-3′ (reverse), or 5′-GAACGGTGATGGACAGGTCA-3′ (forward) and 5′-GGATTCCAAGGATTGGGCGT-3′ (reverse). PCR products were sub-cloned into the TAK101 vector (TOYOBO). The resulting plasmids were used for RNA probe synthesis with digoxigenin (DIG) labeling mix (Roche), and T7 or T3 RNA polymerase (Roche) was used, according to the insert direction. The detail for the probe synthesis was referred to the published protocol46.
Planula larvae were anesthetized in 50% 0.5 M NaN3 in ASW for 5 min and fixed overnight at 4 °C with 4% paraformaldehyde (PFA) in ASW. The following whole mount in situ hybridization was performed following the protocol in Hou et al.24 with slight modifications. Briefly, fixed samples were washed three times with PBS containing 0.1% Tween-20 (PBST), followed by pre-hybridization in hybridization buffer (HB buffer: 5 × SSC, 50% formamide, 0.1% Tween-20, 50 μg/ml tRNA, 50 μg/ml heparin) at 55 °C for 2 h. Samples were hybridized with HB Buffer containing the antisense probes (final probe concentration: 0.5–1 ng/µL in HB Buffer) at 55 °C overnight. The samples were washed each twice with wash buffer 1 (5 × SSC, 50% formamide, 0.1% Tween-20), wash buffer 2 (2 × SSC, 50% formamide, 0.1% Tween-20) and 2 × SSC. The samples were then washed with 0.1% PBST and incubated in 1% blocking buffer (1% blocking reagent [Roche, 11175041910] in Maleic acid) for 1 h. The samples were incubated with alkaline phosphatase (AP)-conjugated anti-DIG antibodies (1:2000, Roche, 11175041910) in 1% blocking buffer for 4 h at room temperature. Colorimetric reactions were performed by NBT/BCIP (Roche, 11175041910) in alkaline phosphatase buffer (100 mM NaCl, 100 mM Tris–HCl (pH 9.5), 50 mM MgCl2, 0.1% Tween-20) until the signals were detected.
Sequence alignment and phylogenetic analysis
Amino acid sequence alignment of Wnt3 and Wnt family proteins were performed by ClustalW47. GenBank accession numbers and protein names are listed in Supplementary Table 3 and 4. The alignment data was visualized on Jalview software48. Maximum likelihood (ML) tree calculation was inferred on ClustalW with the PhyML bootstrap method. Branch supports were computed out of 100 bootstrapped trees. The tree visualization was created using MEGA-X software49.
qPCR primers
| Name | Sequence (5′ → 3′) | |
|---|---|---|
| CpWnt3 qP FW | CAAATGTGGTCGATCAAACG | This study |
| CpWnt3 qP RV | TACGCCTTCTGCAACACTTG | This study |
| CpEF1-a_qPCR_F7 | GGTCAATCTCGTTCCCTCCA | This study |
| CpEF1-a_qPCR_R7 | TTTCCACCAGAGGTATCGGC | This study |
| CpBrachyury F | AAGGCGTATGTTTCCGGTCC | This study |
| CpBrachyury R | CAACGATGGTCTTCGACTGC | This study |
| CpFoxQ2a F | AGTTGGAGAAACAGCGTTCG | This study |
| CpFoxQ2a R | CCGTTCGCAAAGTCGTCAAA | This study |
| CpCapZbeta_qPCR_F | AAAGAAAGCTGGAGACGGTTCA | This study |
| CpCapZbeta_qPCR_R | GTAGTGGGCATTTCTTCCGC | This study |
| CheGFP1 qP F | TTGCTGTCCGAATAGTGCAG | Fourrage et al., 2014 Open Biol |
| CheGFP1 qP RV | GACAACTCCTCCTCCGAGTG | Fourrage et al., 2014 Open Biol |
| CheGFP1_2 qP F | ACAATCGCGTCACACTTAAAGG | This study |
| CheGFP1_2 qP RV | CGTTGTTTTCTTTGTCCGGC | This study |
| CheWnt3 qP F | ATCATGGCAGGTGGAAACTC | Leclère et al., 2012 Dev Biol |
| CheWnt3 qP RV | CCCCATTTCCAACCTTCTTC | Leclère et al., 2012 Dev Biol |
| CheEF1a qP F | TGCTGTTGTCCCAATCTCTG | Leclère et al., 2012 Dev Biol / Fourrage et al., 2014 Open biol |
| CheEF1a qP RV | AAGACGGAGTGGTTTGGATG | Leclère et al., 2012 Dev Biol / Fourrage et al., 2014 Open biol |
| NvEf1alpha FW | GGTTGCCTCTTCGCTTACCACT | This strudy |
| NvEf1alpha RV | CGTTCCTGGCTTTAGGACAC | This study |
| NvBra1_Fw_1032 | CGGGCTCACACTCTCACTTA | This study |
| NvBra1_Rv1174 | CTTGCGGTATGGTGTTCCAG | This study |
siRNA design
| Name | Sequence (5′ → 3′) | ORF | |
|---|---|---|---|
| siCheGFP1_1 sense | CCAUCAGCUUCGAAAAUGAdTdT | This study | HQ397706.1 |
| antisense | UCAUUUUCGAAGCUGAUGGdTdT | This study | |
| siCheGFP1_2 sense | UGAUGGCGCUUAUAAAGUUdTdT | This study | HQ397706.1 |
| antisense | AACUUUAUAAGCGCCAUCAdTdT | This study | |
| siCheGFP1_3 sense | CGGACUCAUUCUUCAAAAAdTdT | This study | HQ397706.1 |
| antisense | UUUUUGAAGAAUGAGUCCGdTdT | This study | |
| siCheWnt3_1 sense | GCAUGUGACUGUAAAUUUAdTdT | This study | EU374721.1 |
| antisense | UAAAUUUACAGUCACAUGCdTdT | This study | |
| siCheWnt3_2 sense | ACAGCUUGGUAAAAUGUUAdTdT | This study | EU374721.1 |
| antisense | UAACAUUUUACCAAGCUGUdTdT | This study | |
| siCpWnt3_1 sense | GUGGAACUGUAGUGUUUCAdTdT | This study | EU374721.1 |
| antisense | UGAAACACUACAGUUCCACdTdT | This study | |
| siCpWnt3_2 sense | AGCGUGUGCUGAAGGUAAAdTdT | This study | EU374721.1 |
| antisense | UUUACCUUCAGCACACGCUdTdT | This study | |
| siNvBra1_1 sense | GAAGAGAUCACGAGUCUAAdTdT | This study | AF540387.2 |
| antisense | UUAGACUCGUGAUCUCUUCdTdT | This study | |
| siNegative_Control-1 for Nv sense | GCAACACGCAGAGTCGTAAdT | Same sequence as Karabulut et al., 2019 Dev Biol | |
| antisense | TTACGACTCTGCGTGTTGCdT | Same sequence as Karabulut et al., 2019 Dev Biol |
shRNA template
| Name | Sequence (5′ → 3′) | |
|---|---|---|
| CheWnt3_SI1_F | TAATACGACTCACTATA GCATGTGACTGTAAATTTA TTCAAGAGA TAAATTTACAGTCACATGC TT | This study |
| CheWnt3_SI1_R | AAGCATGTGACTGTAAATTTATCTCTTGAATAAATTTACAGTCACATGCTATAGTGAGTCGTATTA | This study |
| CheWnt3_SI2_F | TAATACGACTCACTATA ACAGCTTGGTAAAATGTTA TTCAAGAGA TAACATTTTACCAAGCTGT TT | This study |
| CheWnt3_SI2_R | AAACAGCTTGGTAAAATGTTATCTCTTGAATAACATTTTACCAAGCTGTTATAGTGAGTCGTATTA | This study |
Graphs and statistical analysis
All graphs were prepared in Excel. To assess phenotypes and RT-qPCR statistical significance, we used the percentage values and delta-delta-Ct values to perform two-tailed Student’s t tests, except in Fig. 5F, where a one-tailed Student’s t test was used. For statistics related to fluorescence intensity mean value and aspect ratio, Kolmogorov–Smirnov normality tests were used. All statistical tests were performed at https://www.socscistatistics.com.
Results
Optimization of electroporation conditions
In both hydrozoan jellyfish Clytia hemisphaerica and Cladonema pacificum, gametogenesis is regulated by light–dark transitions6,50. A previous report showed that Clytia medusae release eggs within 110–120 min and sperm within 60–90 min upon light illumination after 3–8 h of darkness12. Cladonema pacificum, which live along coastal areas in Japan, exhibit two types of gametogenesis depending on habitat: the dark–light transition (light stimulation type) and the light–dark transition (dark stimulation type), respectively. Dark stimulation Cladonema medusae, which we used in this study, release eggs or sperm within 30 min of dark stimulus after 20–24 h of constant exposure to light25,50. By utilizing the characteristic gametogenesis of these jellyfish species, we can control egg/sperm release to induce fertilization at any time of day. Indeed, we routinely obtained Clytia sperm within 60 min and Clytia eggs within 90 min of light on a 13 h dark/11 h light cycle (Fig. 2A). Similarly, we collected gametes from Cladonema within 25 min of dark stimulation on a 23.5 h light/30 min dark cycle (Fig. 2A).
To determine optimal electroporation parameters, we first tested different electroporation conditions and monitored the delivery efficiency of the red fluorescent dye Rhodamine-Dextran into unfertilized Clytia and Cladonema eggs (Supplementary Figs. 1–2). Given that the average molecular weight of siRNA (21-mer, ~ 13,300 Da) is similar to the molecular size of Rhodamine-Dextran (10,000 Da), electroporation trials with Rhodamine-Dextran can visually confirm that small molecules are incorporated into jellyfish eggs, as previously shown in Hydractinia eggs38. The collected eggs were suspended in 15% Ficoll artificial seawater to prevent precipitation and to make a homogeneous solution (Fig. 2B)36,38. We performed electroporation trails for Rhodamine-Dextran using a cuvette with a 4 mm gap and tested several conditions of voltage (V) and pulse length time (milliseconds, ms) using a conventional electroporation system (Fig. 2C). As previous work showed that increasing the number of pulses does not increase the Dextran delivery rate but decreases the survival rate of embryos38, we fixed the number of pulses to one and incubated unfertilized eggs with 1 mg/ml Dextrane-Rhodamine solution. Clytia eggs have little autofluorescence in the red channel, and without electroporation (No EP), red fluorescence was rarely detected in unfertilized eggs (Fig. 2D and Supplementary Fig. 1A). We compared the eight different electroporation conditions and found that the rhodamine fluorescence was detected in Clytia eggs and correlated with the strength of electric voltage, as long as the voltage was in the range of 50–100 V (Supplementary Figs. 1B–D). By contrast, when the voltage exceeded 200 V, the eggs ruptured from physical damage, and cellular debris was observed (Supplementary Figs. 1E–G).
We performed similar electroporation trials on unfertilized Cladonema eggs under eight different conditions. While red autofluorescence was negligible in the Cladonema eggs in the absence of electroporation (Fig. 2D and Supplementary Fig. 2A), Rhodamine fluorescence was frequently detected with 50 V (Fig. 2D and Supplementary Fig. 2D). In the more intense conditions beyond 100 V (Supplementary Figs. 2E and 2F), the eggs were severely damaged. Based on these results, we established the optimal electroporation conditions (50 V, 1 pulse, 25 ms) that provide high frequency of Dextran-positive eggs and low levels of cell damage for both jellyfish eggs (Fig. 2D). Of note, during the initial trials, we used unfertilized eggs, but we found that the electroporation process severely affected the survival rate of subsequent embryos. In the case of Cladonema, electroporation before fertilization severely decreased the survival rate of fertilized eggs (7.04% at 1 h post fertilization) while electroporation after fertilization did not influence the survival rate (No EP: 59.67%, With EP: 60.15%) (Supplementary Table 1). This dissimilarity is likely due to the decrease of fertilization rate after electroporation, and both elapsed time after spawning and physical damage by electroporation can affect fertilization efficiency. Therefore, we decided to conduct further electroporation experiments with fertilized eggs.
Gene knockdown with siRNA in Nematostella vectensis
Before attempting gene knockdown via siRNAs electroporation in fertilized jellyfish eggs, we first used Nematostella vectensis, where egg electroporation methodology has been established36. We selected Nematostella Brachyury (NvBra), a gene expressed in the blastopore margin that functions in the early embryo51,52, as a target. After electroporation of siRNA targeting NvBra in fertilized eggs, we examined NvBra expression in planula larvae at 3 days-post-fertilization (dpf) by RT-qPCR and found that NvBra expression was drastically suppressed compared to no electroporation controls and negative control siRNAs (Supplementary Fig. 3). We further evaluated survival rates after electroporation and confirmed that siRNA electroporation does not severely affect survival rates of 3 dpf planula in any of the tested conditions (Supplementary Table 2). These results suggest that siRNA delivery into fertilized eggs via electroporation is an effective gene knockdown method that is comparable to the effect of shRNA delivery into unfertilized eggs via electroporation36, supporting our attempt to perform gene knockdown via siRNA electroporation using fertilized jellyfish eggs.
Endogenous GFP1 knockdown with siRNA in Clytia hemisphaerica
The hydrozoan jellyfish Clytia endogenously expresses green fluorescent proteins, which are encoded by 14 genes grouped into 4 clusters (CheGFP1-4)16. The expression pattern for each GFP changes by stage in the life cycle: for instance, Clytia eggs express maternal CheGFP2 mRNA, and planula larvae show typical ring-like CheGFP1 fluorescence in the lateral ectoderm at 3 dpf11. While CheGFP1 gene expression becomes dominant in the planula stage; CheGFP2, CheGFP3, and CheGFP4 are expressed in different tissues and organs in the medusa53. Momose et al. succeeded in genome editing by injecting CRISPR/Cas9 targeting the endogenous CheGFP1 into fertilized eggs, and confirmed the knockout effect by loss of green fluorescence in 3 dpf planula larvae11. In addition, CheGFP1 is not essential for animal survival, which allows for the monitoring of knockdown effects after embryogenesis. For these reasons, we chose endogenous CheGFP1 for the first target gene of siRNA knockdown.
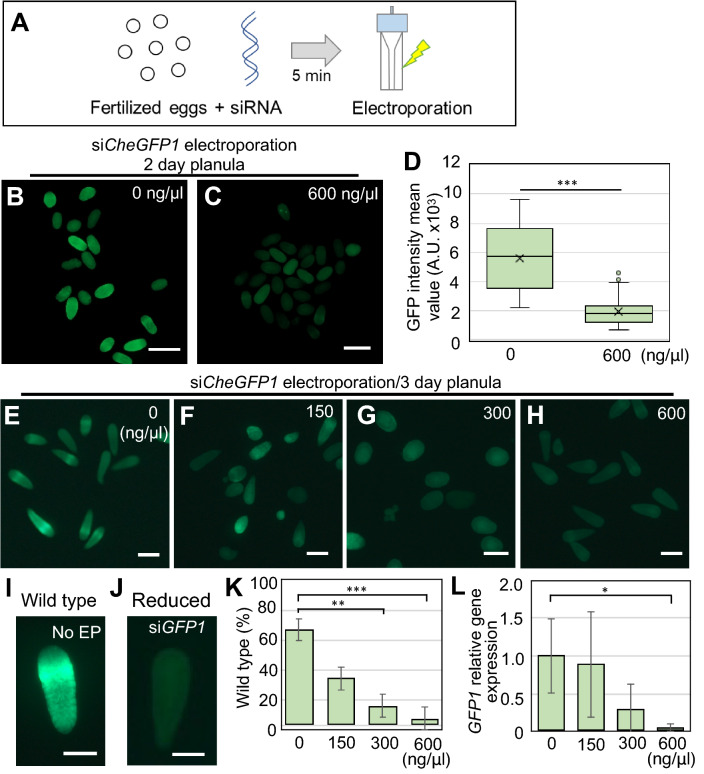
In order to maximize the effect of gene knockdown, we used a combination of three different siRNAs against CheGFP1 (Accession No. HQ397706.1; Supplementary Fig. 4A) in a mixture with a 1:1:1 ratio, and electroporated 600 ng/μl of siRNA mix (200 ng/μl per each siRNA) into fertilized eggs (Fig. 3A). To verify the effect of gene knockdown, we measured green fluorescence intensity in 2 dpf planula and found that siRNA (600 ng/μl siRNA mix) electroporated embryos showed dramatically reduced fluorescence intensity compared to the no-siRNA controls (Fig. 3B–D; mean intensity value: 5.7 × 103 for 0 ng/μl control, 1.8 × 103 for 600 ng/μl). To evaluate the knockdown effects at varying doses of siRNA, we next electroporated different concentrations of the CheGFP1 siRNA mix (0, 150, 300, and 600 ng/μl) into fertilized eggs. While control 3 dpf planulae with no-electroporation (No EP) as well as those with no-siRNA showed typical ring-shape GFP fluorescence in the ectoderm, many planulae with CheGFP1 siRNA electroporation lost green fluorescence (Fig. 3E–H). To evaluate the GFP knockdown effects, we classified planulae with typical GFP1 fluorescence as “wild type” and planulae with reduced fluorescence as “reduced” for further quantification (Fig. 3I, J). The percentage of wild type phenotype dramatically reduced in an siRNA dose-dependent manner (Fig. 3K). To further quantify the above result on a molecular level, we examined relative CheGFP1 mRNA levels by RT-qPCR using the primer set that was evaluated in a previously study53. Consistent with the image analysis results, we confirmed a marked reduction of CheGFP1 gene expression at different concentrations of CheGFP1 siRNA (Fig. 3L). These results demonstrate that siRNAs targeting CheGFP1 repress its expression upon electroporation and that the knockdown effects are dose-dependent.
Figure 3.
Phenotypes of GFP1 knockdown with siRNA in Clytia fertilized eggs. (A) Schematic of the siRNA electroporation procedure using fertilized eggs. Fertilized eggs in 15% Ficoll/artificial sea water mixed with siRNA are incubated for 5 min, and electroporation is conducted after transferring into a cuvette. (B, C) Phenotypes of Clytia 2-day planula after CheGFP1 siRNA knockdown. (D) Boxplots showing the GFP fluorescence intensity mean values in 2-day planula after electroporation of 0 and 600 ng/µl siRNAs targeting CheGFP1. Center lines indicate the medians; x’s denote the means; box limits represent the 25th and 75th percentiles; whiskers show the maximum and minimum values. 0 ng/µl, n = 24; 600 ng/µl, n = 81. ***p < 0.001. (E–H) Phenotypes of 3-day planula after siRNA targeting CheGFP1 electroporation (0, 150, 300, and 600 ng/µl siCheGFP1). (I, J) For quantification of green fluorescence, we classified phenotypes into two categories: planula with ring-like green fluorescence as “wild-type,” and planula with reduced green fluorescence as “reduced”. (K) Quantification of planula larvae positive for CheGFP1 (wild-type). Bar plots show the percentage of wild-type phenotype after CheGFP1 knockdown. Number of examined planula: 0 ng/µl, n = 82; 150 ng/µl, n = 64; 300 ng/µl, n = 58; 600 ng/µl, n = 114. Error bars: maximum and minimum values. Experiments were repeated three times. p-values: 150 ng/μl, p = 0.064505; 300 ng/μl, p = 0.001437; 600 ng/μl, p = 0.00066. **p < 0.01, ***p < 0.001. (L) Quantification of CheGFP1 mRNA expression levels in 3-day planula by RT-qPCR. CheEF1alpha was used as an internal control. Bar heights represent mean values of at least three independent experiments. CheGFP1 expression levels are standardized relative to the control (0 ng/µl) condition. Error bars: standard deviation. Experiments were performed in triplicated and repeated at least three times. p-values: 150 ng/μl, p = 0.829361; 300 ng/μl, p = 0.08588; 600 ng/μl, p = 0.037359. *p < 0.05. Scale bars: 200 µm.
Given the presence of multiple CheGFP1 loci (seven nearly identical CheGFP1 genes) in the Clytia genome16, it was not clear whether three siRNAs against GFP1 sufficiently suppress gene expression in different versions of CheGFP1, particularly those expressed in the planula stage (Supplementary Fig. 4A). We thus performed RT-qPCR using the newly designed primer set that amplifies all seven CheGFP1 genes and found a dramatic reduction of gene expression upon electroporation of CheGFP1 siRNAs (600 ng/μl) (Supplementary Fig. 4B). Altogether, these results indicate that our combination of CheGFP1 siRNAs suppresses gene expression of CheGFP1 in the Clytia planula.
Wnt3 knockdown with siRNA in Clytia hemisphaerica
Since electroporation of siRNAs targeting endogenous CheGFP1 was shown to be effective, we next decided to investigate the effects of knocking down other genes that play important roles in early development. Wnt3 is a secreted signaling protein in the Wnt/β-catenin pathway that is involved in diverse developmental processes including cell fate decisions and patterning54,55. In Clytia, Wnt3 RNA is maternally localized to the animal cortex, the future oral side of the embryo, and Wnt3 organizes axial patterning as the main ligand for the Wnt/β-catenin pathway in the early embryonic stage10. After embryogenesis, the Clytia planula normally elongates along the oral-aboral axis and becomes oval in shape (Fig. 4A). By contrast, after CheWnt3 morpholino injection, the morphant loses oral-aboral axis polarity and exhibits a spherical shape10. This raises the possibility that knockdown of CheWnt3 via siRNA may result in a similar spherical morphology for Clytia larvae as is observed in CheWnt3 morphants. Therefore, to evaluate siRNA knockdown effects on Clytia, we chose CheWnt3 as our second target.
We used a mixture of two different siRNAs (1:1 ratio) targeting CheWnt3, and carried out electroporation of the siRNA mix into Clytia fertilized eggs. After electroporation of CheWnt3 siRNAs, 1 dpf planula larvae showed nearly complete spherical morphology (Fig. 4B), which is reminiscent of CheWnt3 morphants10. To quantify the effect of siRNA knockdown on Clytia larval morphology, we calculated the aspect ratio in two dimensions, where values greater than 1.0 shows the tendency to be oval (ellipsoid-like) while a value of 1.0 indicates a perfect circle (sphere) (Fig. 4C). The control larvae with 0 ng/μl siRNA showed a median aspect ratio of 1.69, indicating a typical ellipsoid-like morphology. By contrast, larvae that underwent electroporation with CheWnt3 siRNAs (400 ng/μl) showed a median aspect ratio of 1.16, suggesting a much greater tendency toward spherical morphology (Fig. 4D). These results quantitively confirmed the effect of CheWnt3 knockdown on larval morphology, which phenocopies CheWnt3 morphants10. To analyze the level of gene-specific knockdown at varying doses of siRNAs, we next electroporated mixtures of 0, 100, 200, and 400 ng/μl of CheWnt3 siRNAs into Clytia fertilized eggs (Fig. 4E–H). To evaluate CheWnt3 siRNA effects on morphology, we classified 1 dpf planulae with elongated ellipsoid-like shapes as “normal” and planulae with spherical shapes as “spherical” (Fig. 4I, J). The percentage of normal phenotype dramatically reduced after CheWnt3 siRNA-electroporation (< 1.0% in 400 ng/μl), even at the lowest dose (5.2% in 100 ng/μl) (Fig. 4K). To further evaluate the result at a molecular level, we analyzed relative CheWnt3 mRNA levels by RT-qPCR and confirmed a significant reduction in CheWnt3 gene expression at all concentrations (Fig. 4L). Of note, after electroporation with universal negative control siRNA (Nippon Gene Co., Ltd.), both morphological phenotypes and relative gene expression were comparable to no-siRNA controls (Fig. 4K, L), further confirming that siRNA-mediated knockdown by electroporation is target-specific. These results together indicate that siRNAs targeting endogenous genes effectively knock down their expression in Clytia embryos.
Effective gene knockdown via shRNA electroporation has previously been reported using two cnidarian polyps, Nematostella vectensis and Hydractinia symbiolongicarpus36,38. To test whether shRNAs are also effective in the hydrozoan jellyfish Clytia, we designed shRNAs targeting CheWnt3 using the same 19 bp target sequence that was used for siRNAs, and performed electroporation on fertilized eggs with a mix of two different Wnt3 shRNAs. After electroporation of Wnt3 shRNAs, relative Wnt3 mRNA expression in 1 dpf planula was weakly down-regulated (0.83), and was not significant compared to the striking effects of Wnt3 siRNA electroporation (0.039) at the same concentration (400 ng/µl) (Fig. 4L). In addition, electroporation of Wnt3 siRNAs resulted in an extensive morphological change toward spherical shaped planulae, showing almost no "normal" phenotype (0.71%), whereas electroporation of shRNA had a limited effect (60.4%) (Fig. 4K). These results suggest that the effect of siRNA is much stronger than that of shRNA in Clytia when treated with the same target sequence and concentration.
Identification of Wnt3 in Cladonema pacificum
In contrast to Clytia, the sole genetic model jellyfish, previous studies have neither established genetic manipulation nor performed genome sequencing of Cladonema pacificum, despite its unique biological features. This is partly because the extremely small size of the Cladonema egg makes microinjection difficult. By testing whether siRNA-mediated knockdown via electroporation can work in Cladonema, we can start to manipulate genes in Cladonema while also demonstrating that siRNA electroporation can be applicable to other jellyfish species. While green fluorescent protein (GFP) is often an easy early target for gene manipulation in emerging organisms, Cladonema does not exhibit apparent endogenous green fluorescence expression at any stage, unlike Clytia. We instead turned our focus to Wnt3 as the target for gene knockdown in Cladonema since Wnt3 plays an important role in early embryonic development in several cnidarian species56,57.
To identify the Wnt3 ortholog in Cladonema, we utilized RNA-seq results from the Cladonema polyp, stolon, and medusa manubrium (data not shown). We performed Cladonema Wnt3 CDS annotation using the Clytia CDS database (MARIMBA, Marine models database: http://marimba.obs-vlfr.fr) and found one contig annotated with Wnt3 (CpWnt3) and multiple Wnt genes (Supplementary Figs. 5 and 6). We then performed phylogenic analysis using the neighbor-joining method, and confirmed CpWnt3 (LC720435) is grouped with medusozoan Hydra vulgaris (HvWnt3) and Clytia Wnt3 (CheWnt3) rather than Anthozoa Nematostella and Acropora digitifera (Supplementary Fig. 5A). Multiple sequence alignment further showed highly conserved amino acids sequences among different species including bilaterians and cnidarians (Supplementary Fig. 5B). These findings raise the possibility that CpWnt3 has a similar function in controlling the Wnt/β-catenin pathway in Cladonema.
Wnt3 knockdown with siRNA in Cladonema pacificum
Does Wnt3 function during Cladonema embryogenesis, particularly during axial patterning? Interestingly, Cladonema planula larvae do not show a clear elongated shape (Fig. 5A), as observed in Clytia. It is thus possible that morphogenesis, including axial patterning and/or Wnt/β-catenin pathway function, differs between these two jellyfish species. It is also possible that the limited elongation in Cladonema planulae might hamper phenotypical appearance upon inhibition of Wnt/β-catenin signaling.
In order to verify that siRNA electroporation works in Cladonema and that Wnt3 affects axial patterning, we performed electroporation of siRNAs targeting CpWnt3. We prepared fertilized Cladonema eggs as we did in Clytia, and used a mixture of two different siRNAs against CpWnt3 CDS with a 1:1 ratio (300 ng/μl) and performed electroporation with the previously established parameters (50 V, 1 pulse, 25 ms) in fertilized Cladonema eggs. After electroporation of siRNAs for CpWnt3, planula larvae did not exhibit morphological differences compared to controls (Fig. 5A–C; median aspect ratio: 1.21 for 0 ng/μl control, 1.16 for 300 ng/μl for siCpWnt3). We then quantified the morphological phenotypes by calculating the aspect ratio of 1 dpf planulae and confirmed that CpWnt3 knockdown does not cause a significant morphological change (Fig. 5D, E), which is consistent with the possibility that Cladonema planulae simply exhibit limited elongation.
Another possibility that would explain the above result is a defect in the siRNA knockdown itself. To test this potential explanation, we carried out RT-qPCR using mRNA samples from 1 dpf planula after electroporating different concentrations of CpWnt3 siRNA mix (0, 150, and 300 ng/µl) into fertilized eggs. We confirmed a reduction of Wnt3 gene expression in an siRNA dose-dependent manner (Fig. 5F), eliminating siRNA knockdown defects as the cause of our initial result.
To further confirm whether the Wnt/β-catenin pathway is affected by Wnt3 knockdown, we examined the gene expression of axial markers Brachyury (CpBra) and FoxQ2a (CpFoxQ2a), whose expression are influenced by the Wnt/β-catenin pathway in Clytia10. From RT-qPCR, we found that CpWnt3 knockdown causes a decrease in CpBra gene expression and an increase in CpFoxQ2a gene expression (Supplementary Fig. 7). We also examined CpBra expression by in situ hybridization and confirmed the reduction of CpBra expression on the oral side of planula larvae (Fig. 5G–I), which is similar to the phenotype exhibited by CheWnt3 morphants in Clytia10. Notably, after CpWnt3 siRNAs electroporation, the rate of metamorphosis from planula to primary polyp decreased dramatically (Supplementary Table 5), implying potential developmental defects upon abrogation of the Wnt/β-catenin pathway. These results suggest that, although overall oral-aboral polarity is less prominent in Cladonema compared to Clytia at the morphological level, the axial patterning mechanism mediated by Wnt/β-catenin signaling may be conserved between these two jellyfish species.
Discussion
In this study, we have established a method to knock down endogenous genes in two hydrozoan jellyfish, Clytia hemisphaerica and Cladonema pacificum, via the siRNA electroporation of fertilized eggs. We showed that knockdown of endogenous GFP1 in Clytia causes the loss of GFP fluorescence in the planula stage (Fig. 3), as previously achieved by CRISPR/Cas9-mediated GFP1 knockout11. We also confirmed that knockdown of Wnt3 in Clytia induces spherical morphology of the planula (Fig. 4), mirroring the results of injections of Wnt3 morpholino antisense oligo10. We further succeeded in efficient repression of Wnt3 gene expression in Cladonema after Wnt3 siRNA electroporation and found that expression of axial patterning genes is controlled by the Wnt/β-catenin pathway (Fig. 5), suggesting that the conserved mechanism is involved in embryogenesis and planula morphogenesis across hydrozoan jellyfish species.
Our results show that the knockdown efficiency of siRNA is much greater than that of shRNA for electroporation of Clytia fertilized eggs with the same Wnt3 target sequence in the same concentration (Fig. 4K–L). One possibility that would explain such distinct effects between siRNA and shRNA electroporation is the existence of differences in the RNAi machinery or RNA processing efficiency among cnidarians. During RNAi in mammals, the RNase III Dicer protein processes shRNA in collaboration with cofactors TRBP (Transactivation response element RNA-binding protein) and PACT (protein activator of the interferon-induced protein kinase) to produce a mature form of siRNA39. Mature siRNA in the Dicer and TRBP/PACT complex is associated with the Argonaute protein and cleaves endogenous complementary mRNAs as the RNA-induced silencing complex 30,58. These small RNA biogenesis factors, Dicers and cofactors (TRBP in bilaterians, HYL1 in plants and cnidarians), are found in Nematostella vectensis, Acropora digitifera, and Hydra vulgaris41. Although Dicers (TCONS_00004571 and TCONS_00004525) and Hyl1 (TCONS_00010722) are predicted to exist in Clytia based on MARIMBA transcriptome data, the expression level of Dicers in oocytes and early gastrulation stages is lower than that in other stages, suggesting the possibility that insufficient shRNA to siRNA processing efficiency is responsible for the difference in knockdown efficiency between siRNA and shRNA electroporation. It will be interesting to elucidate the detailed molecular mechanism of RNAi in different cnidarian species and address RNA processing efficiency in distinct developmental stages. The lower efficiency of knockdown by shRNA compared to siRNA in Clytia could also be simply explained by lower shRNA electroporation efficiency. This possibility could be tested by inserting shRNA or siRNA into fertilized eggs by microinjection, instead of electroporation, which would be followed by assessing gene expression and phenotypes.
During Clytia early embryogenesis, Wnt3 RNA is localized to the animal cortex of the egg and contributes to the formation of the oral-aboral axis10. Accordingly, knockdown of Wnt3 in Clytia fertilized eggs induces suppression of axis formation and planula elongation (Fig. 4). In contrast, despite the fact that no morphological phenotype was observed after Wnt3 knockdown in Cladonema, gene expression of the conserved axial patterning genes, Brachyury and FoxQ2a, was disrupted (Fig. 5G–I and Supplementary Fig. 7). In addition to Clytia, the Wnt/β-catenin pathway is involved in axis formation during cnidarian embryogenesis including Nematostella44,56,59, Acropora60, and Hydractinia57. Furthermore, given that the Wnt/β-catenin pathway is also associated with axis formation during metamorphosis in Hydractinia57,61 and Clytia62, the reduced metamorphosis rate observed in CpWnt3 knockdown Cladonema may be attributed to disrupted axis formation (Supplementary Table 5). Taken together, our data support the pivotal role of Wnt/β-catenin signaling in cnidarian development.
An interesting feature of jellyfish is that their morphology dramatically changes across their life cycle from planula to polyp and then to medusa. In particular, polyps and medusae, different adult stages of the same animal, exhibit distinct regenerative ability, lifespan, and behaviors. Although regeneration mechanisms have been extensively studied in polyp-only animals such as Hydra and Hydractinia, classical studies have used the medusa stage of several jellyfish species and demonstrated their regenerative potential21. Recent work using Clytia medusae has further shown a remarkable remodeling and repatterning mechanism orchestrated by muscle systems upon organ loss17. At the behavior level, the medusae of the upside-down jellyfish Cassiopea exhibit a sleep-like state63, which is similarly observed in Hydra polyps64. More recent work using transgenic Clytia has characterized feeding behaviors in medusae at a neural-network resolution19. To understand how these diverse biological processes in jellyfish are regulated at the molecular level, the functions of specific genes at various stages must be analyzed. Although gene knockdown by dsRNA electroporation into polyps in the scyphozoan jellyfish Aurelia has been reported65, gene knockdown at the medusa stage has not yet been achieved. Given that siRNA electroporation is applicable to Hydra polyps33,66 in addition to cnidarian fertilized eggs, we now have a better chance to apply this technique to the medusa stage, although, because they are susceptible to electric shock, electroporation parameters must be optimized in order to achieve gene knockdown in medusae. Collectively, our gene knockdown via siRNA electroporation method will complement the existing shRNA electroporation approach in cnidarian polyps and will enable molecular-level analysis of the vast biological phenomena exhibited across the different life stages in jellyfish.
Supplementary Information
Acknowledgements
We thank R. Deguchi (Miyagi Univ. Education, Japan) for sharing Cladonema pacificum and EMBRC France for sharing Clytia hemisphaerica. We thank T. Momose (Sorbonne University, CNRS, France) for helpful discussion. We thank I. Nagai, H. Nakatani, and A. Sasaki for technical assistance; and A. Dahal, A. Tanimoto, and J. Higuchi for Nematostella culture. This work was supported by JST Grant Number JPMJCR1852 to E.K., AMED under Grant Number JP21gm6110025 to Y.N., and the JSPS KAKENHI grant numbers JP21H05255 to E.K., and JP17H06332, JP19K22550, JP22H02762 to Y.N.
Author contributions
T.M.-O. conceptualized and designed the project, performed experiments using jellyfish, analyzed data, prepared figures, and wrote the manuscript. S.F. performed experiments and analyzed data. R.N. performed experiments using Nematostella. H.W. conceptualized and designed the project. E.K. contributed reagents. Y.N. conceptualized and designed the project, prepared figures, and wrote the manuscript. All authors approved the final manuscript.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request and nucleotide sequences are available in the GenBank/EMBL/DDBJ under the accession numbers (CpWnt1, LC720432; CpWnt1b, LC720433; CpWnt2, LC720434; CpWnt3, LC720435; CpWnt5, LC720436; CpWnt6, LC720437; CpWnt8, LC720438; CpWntA, LC720439; CpBrachyury, LC720440; CpFoxQ2a, LC720441; F-actin capping protein subunit beta, LC720442; CpEF1alpha, LC720443).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-20476-1.
References
- 1.Technau U, Steele RE. Evolutionary crossroads in developmental biology: Cnidaria. Development. 2011;138:1447–1458. doi: 10.1242/dev.048959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zapata F, et al. Phylogenomic analyses support traditional relationships within Cnidaria. PLoS ONE. 2015;10:e0139068. doi: 10.1371/journal.pone.0139068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leclere L, Rottinger E. Diversity of cnidarian muscles: function, anatomy, development and regeneration. Front. Cell Dev. Biol. 2016;4:157. doi: 10.3389/fcell.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holstein TW, Hobmayer E, Technau U. Cnidarians: an evolutionarily conserved model system for regeneration? Dev. Dyn. 2003;226:257–267. doi: 10.1002/dvdy.10227. [DOI] [PubMed] [Google Scholar]
- 5.Bosch TCG, et al. Back to the basics: Cnidarians start to fire. Trends Neurosci. 2017;40:92–105. doi: 10.1016/j.tins.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houliston E, Momose T, Manuel M. Clytia hemisphaerica: A jellyfish cousin joins the laboratory. Trends Genet. 2010;26:159–167. doi: 10.1016/j.tig.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Houliston E, Leclere L, Munro C, Copley RR, Momose T. Past, present and future of Clytia hemisphaerica as a laboratory jellyfish. Curr. Top. Dev. Biol. 2022 doi: 10.1016/bs.ctdb.2021.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Lechable M, et al. An improved whole life cycle culture protocol for the hydrozoan genetic model Clytia hemisphaerica. Biol. Open. 2020;9:10. doi: 10.1242/bio.051268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Momose T, Houliston E. Two oppositely localised frizzled RNAs as axis determinants in a cnidarian embryo. PLoS Biol. 2007;5:e70. doi: 10.1371/journal.pbio.0050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Momose T, Derelle R, Houliston E. A maternally localised Wnt ligand required for axial patterning in the cnidarian Clytia hemisphaerica. Development. 2008;135:2105–2113. doi: 10.1242/dev.021543. [DOI] [PubMed] [Google Scholar]
- 11.Momose T, et al. High doses of CRISPR/Cas9 ribonucleoprotein efficiently induce gene knockout with low mosaicism in the hydrozoan Clytia hemisphaerica through microhomology-mediated deletion. Sci. Rep. 2018;8:11734. doi: 10.1038/s41598-018-30188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quiroga Artigas G, et al. A gonad-expressed opsin mediates light-induced spawning in the jellyfish Clytia. Elife. 2018 doi: 10.7554/eLife.29555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quiroga Artigas G, et al. A G protein-coupled receptor mediates neuropeptide-induced oocyte maturation in the jellyfish Clytia. PLoS Biol. 2020;18:e3000614. doi: 10.1371/journal.pbio.3000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lapebie P, et al. Differential responses to Wnt and PCP disruption predict expression and developmental function of conserved and novel genes in a cnidarian. PLoS Genet. 2014;10:e1004590. doi: 10.1371/journal.pgen.1004590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condamine T, et al. Molecular characterisation of a cellular conveyor belt in Clytia medusae. Dev. Biol. 2019;456:212–225. doi: 10.1016/j.ydbio.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Leclere L, et al. The genome of the jellyfish Clytia hemisphaerica and the evolution of the cnidarian life-cycle. Nat. Ecol. Evol. 2019;3:801–810. doi: 10.1038/s41559-019-0833-2. [DOI] [PubMed] [Google Scholar]
- 17.Sinigaglia C, et al. Pattern regulation in a regenerating jellyfish. Elife. 2020 doi: 10.7554/eLife.54868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chari T, et al. Whole-animal multiplexed single-cell RNA-seq reveals transcriptional shifts across Clytia medusa cell types. Sci. Adv. 2021;7:eabh1683. doi: 10.1126/sciadv.abh1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weissbourd B, et al. A genetically tractable jellyfish model for systems and evolutionary neuroscience. Cell. 2021;184:5854–5868 e5820. doi: 10.1016/j.cell.2021.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclere L, et al. Maternally localized germ plasm mRNAs and germ cell/stem cell formation in the cnidarian Clytia. Dev. Biol. 2012;364:236–248. doi: 10.1016/j.ydbio.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Fujita S, Kuranaga E, Nakajima YI. Regeneration potential of jellyfish: Cellular mechanisms and molecular insights. Genes (Basel) 2021 doi: 10.3390/genes12050758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujiki A, Hou S, Nakamoto A, Kumano G. Branching pattern and morphogenesis of medusa tentacles in the jellyfish Cladonema pacificum (Hydrozoa, Cnidaria) Zool. Lett. 2019;5:12. doi: 10.1186/s40851-019-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita S, Kuranaga E, Nakajima YI. Cell proliferation controls body size growth, tentacle morphogenesis, and regeneration in hydrozoan jellyfish Cladonema pacificum. PeerJ. 2019;7:e7579. doi: 10.7717/peerj.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou S, Zhu J, Shibata S, Nakamoto A, Kumano G. Repetitive accumulation of interstitial cells generates the branched structure of Cladonema medusa tentacles. Development. 2021 doi: 10.1242/dev.199544. [DOI] [PubMed] [Google Scholar]
- 25.Takeda N, et al. Identification of jellyfish neuropeptides that act directly as oocyte maturation-inducing hormones. Development. 2018 doi: 10.1242/dev.156786. [DOI] [PubMed] [Google Scholar]
- 26.Stierwald M, Yanze N, Bamert RP, Kammermeier L, Schmid V. The sine oculis/six class family of homeobox genes in jellyfish with and without eyes: Development and eye regeneration. Dev. Biol. 2004;274:70–81. doi: 10.1016/j.ydbio.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Suga H, Schmid V, Gehring WJ. Evolution and functional diversity of jellyfish opsins. Curr. Biol. 2008;18:51–55. doi: 10.1016/j.cub.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 28.Suga H, et al. Flexibly deployed Pax genes in eye development at the early evolution of animals demonstrated by studies on a hydrozoan jellyfish. Proc. Natl. Acad. Sci. USA. 2010;107:14263–14268. doi: 10.1073/pnas.1008389107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graziussi DF, Suga H, Schmid V, Gehring WJ. The, “eyes absent” (eya) gene in the eye-bearing hydrozoan jellyfish Cladonema radiatum: Conservation of the retinal determination network. J. Exp. Zool. B Mol. Dev. Evol. 2012;318:257–267. doi: 10.1002/jez.b.22442. [DOI] [PubMed] [Google Scholar]
- 30.Meister G, Tuschl T. Mechanisms of gene silencing by double-stranded RNA. Nature. 2004;431:343–349. doi: 10.1038/nature02873. [DOI] [PubMed] [Google Scholar]
- 31.Rao DD, Vorhies JS, Senzer N, Nemunaitis J. siRNA vs. shRNA: similarities and differences. Adv. Drug Deliv. Rev. 2009;61:746–759. doi: 10.1016/j.addr.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Moran Y, et al. Cnidarian microRNAs frequently regulate targets by cleavage. Genome Res. 2014;24:651–663. doi: 10.1101/gr.162503.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watanabe H, et al. Nodal signalling determines biradial asymmetry in Hydra. Nature. 2014;515:112–115. doi: 10.1038/nature13666. [DOI] [PubMed] [Google Scholar]
- 34.Pankow S, Bamberger C. The p53 tumor suppressor-like protein nvp63 mediates selective germ cell death in the sea anemone Nematostella vectensis. PLoS ONE. 2007;2:e782. doi: 10.1371/journal.pone.0000782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He S, et al. An axial Hox code controls tissue segmentation and body patterning in Nematostella vectensis. Science. 2018;361:1377–1380. doi: 10.1126/science.aar8384. [DOI] [PubMed] [Google Scholar]
- 36.Karabulut A, He S, Chen CY, McKinney SA, Gibson MC. Electroporation of short hairpin RNAs for rapid and efficient gene knockdown in the starlet sea anemone, Nematostella vectensis. Dev. Biol. 2019;448:7–15. doi: 10.1016/j.ydbio.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 37.DuBuc TQ, et al. Transcription factor AP2 controls cnidarian germ cell induction. Science. 2020;367:757–762. doi: 10.1126/science.aay6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quiroga-Artigas G, Duscher A, Lundquist K, Waletich J, Schnitzler CE. Gene knockdown via electroporation of short hairpin RNAs in embryos of the marine hydroid Hydractinia symbiolongicarpus. Sci. Rep. 2020;10:12806. doi: 10.1038/s41598-020-69489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 40.Krishna S, et al. Deep sequencing reveals unique small RNA repertoire that is regulated during head regeneration in Hydra magnipapillata. Nucleic Acids Res. 2013;41:599–616. doi: 10.1093/nar/gks1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moran Y, Praher D, Fredman D, Technau U. The evolution of microRNA pathway protein components in Cnidaria. Mol. Biol. Evol. 2013;30:2541–2552. doi: 10.1093/molbev/mst159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Murchison EP, Partridge JF, Tam OH, Cheloufi S, Hannon GJ. Characterization of Dicer-deficient murine embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2005;102:12135–12140. doi: 10.1073/pnas.0505479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kok KH, Ng MH, Ching YP, Jin DY. Human TRBP and PACT directly interact with each other and associate with dicer to facilitate the production of small interfering RNA. J. Biol. Chem. 2007;282:17649–17657. doi: 10.1074/jbc.M611768200. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe H, et al. Sequential actions of beta-catenin and Bmp pattern the oral nerve net in Nematostella vectensis. Nat. Commun. 2014;5:5536. doi: 10.1038/ncomms6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fujita S, Kuranaga E, Miura M, Nakajima YI. Fluorescent in situ hybridization and 5-ethynyl-2'-deoxyuridine labeling for stem-like cells in the hydrozoan jellyfish Cladonema pacificum. J. Vis. Exp. 2022 doi: 10.3791/64285. [DOI] [PubMed] [Google Scholar]
- 47.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deguchi R, Kondoh E, Itoh J. Spatiotemporal characteristics and mechanisms of intracellular Ca(2+) increases at fertilization in eggs of jellyfish (Phylum Cnidaria, Class Hydrozoa) Dev. Biol. 2005;279:291–307. doi: 10.1016/j.ydbio.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 51.Scholz CB, Technau U. The ancestral role of Brachyury: Expression of NemBra1 in the basal cnidarian Nematostella vectensis (Anthozoa) Dev. Genes Evol. 2003;212:563–570. doi: 10.1007/s00427-002-0272-x. [DOI] [PubMed] [Google Scholar]
- 52.Servetnick MD, et al. Cas9-mediated excision of Nematostella brachyury disrupts endoderm development, pharynx formation and oral-aboral patterning. Development. 2017;144:2951–2960. doi: 10.1242/dev.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fourrage C, Swann K, Gonzalez Garcia JR, Campbell AK, Houliston E. An endogenous green fluorescent protein-photoprotein pair in Clytia hemisphaerica eggs shows co-targeting to mitochondria and efficient bioluminescence energy transfer. Open Biol. 2014;4:130206. doi: 10.1098/rsob.130206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guder C, et al. The Wnt code: Cnidarians signal the way. Oncogene. 2006;25:7450–7460. doi: 10.1038/sj.onc.1210052. [DOI] [PubMed] [Google Scholar]
- 55.Lee PN, Pang K, Matus DQ, Martindale MQ. A WNT of things to come: Evolution of Wnt signaling and polarity in cnidarians. Semin. Cell Dev. Biol. 2006;17:157–167. doi: 10.1016/j.semcdb.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Kraus Y, Aman A, Technau U, Genikhovich G. Pre-bilaterian origin of the blastoporal axial organizer. Nat. Commun. 2016;7:11694. doi: 10.1038/ncomms11694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plickert G, Jacoby V, Frank U, Muller WA, Mokady O. Wnt signaling in hydroid development: Formation of the primary body axis in embryogenesis and its subsequent patterning. Dev. Biol. 2006;298:368–378. doi: 10.1016/j.ydbio.2006.06.043. [DOI] [PubMed] [Google Scholar]
- 58.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 59.DuBuc TQ, Stephenson TB, Rock AQ, Martindale MQ. Hox and Wnt pattern the primary body axis of an anthozoan cnidarian before gastrulation. Nat. Commun. 2018;9:2007. doi: 10.1038/s41467-018-04184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yasuoka Y, Shinzato C, Satoh N. The mesoderm-forming gene brachyury regulates ectoderm-endoderm demarcation in the coral Acropora digitifera. Curr. Biol. 2016;26:2885–2892. doi: 10.1016/j.cub.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 61.Duffy DJ, Plickert G, Kuenzel T, Tilmann W, Frank U. Wnt signaling promotes oral but suppresses aboral structures in Hydractinia metamorphosis and regeneration. Development. 2010;137:3057–3066. doi: 10.1242/dev.046631. [DOI] [PubMed] [Google Scholar]
- 62.Krasovec G, Pottin K, Rosello M, Queinnec E, Chambon JP. Apoptosis and cell proliferation during metamorphosis of the planula larva of Clytia hemisphaerica (Hydrozoa, Cnidaria) Dev. Dyn. 2021;250:1739–1758. doi: 10.1002/dvdy.376. [DOI] [PubMed] [Google Scholar]
- 63.Nath RD, et al. The jellyfish Cassiopea exhibits a sleep-like state. Curr. Biol. 2017;27:2984–2990 e2983. doi: 10.1016/j.cub.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kanaya HJ, et al. A sleep-like state in Hydra unravels conserved sleep mechanisms during the evolutionary development of the central nervous system. Sci. Adv. 2020 doi: 10.1126/sciadv.abb9415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuchs B, et al. Regulation of polyp-to-jellyfish transition in Aurelia aurita. Curr. Biol. 2014;24:263–273. doi: 10.1016/j.cub.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Lommel M, et al. Hydra mesoglea proteome identifies thrombospondin as a conserved component active in head organizer restriction. Sci. Rep. 2018;8:11753. doi: 10.1038/s41598-018-30035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request and nucleotide sequences are available in the GenBank/EMBL/DDBJ under the accession numbers (CpWnt1, LC720432; CpWnt1b, LC720433; CpWnt2, LC720434; CpWnt3, LC720435; CpWnt5, LC720436; CpWnt6, LC720437; CpWnt8, LC720438; CpWntA, LC720439; CpBrachyury, LC720440; CpFoxQ2a, LC720441; F-actin capping protein subunit beta, LC720442; CpEF1alpha, LC720443).