Abstract
Hypoglycemic encephalopathy constitutes a critical presentation of severely diminished glucose levels. We present the case of a 53-year-old male patient with a history of diabetes mellitus with hypoglycemic encephalopathy and MRI findings of bilateral middle cerebellar peduncle lesions. Common findings of hypoglycemic encephalopathy described in the literature consist of bilateral compromise of the cerebral cortex, basal ganglia, hippocampus, and long tracts of white matter. The cerebellum and brainstem are usually not affected. This is the ninth report of cerebellar peduncle compromise with hypoglycemia. As increasing evidence regarding prognosis estimation of lesion distribution arises, we consider it important to report the different cases of rare patterns of compromise.
Keywords: Hypoglycemic encephalopathy, Middle cerebellar peduncle, Diagnosis, Prognosis
Introduction
Hypoglycemic encephalopathy is secondary to exceedingly low levels of seric glucose [1]. There's a wide range of pathologies that can cause hypoglycemia. Commonly it is seen in patients receiving treatment for a diagnosed diabetes or in chronic alcoholism. It may appear in stressful metabolic conditions such as sepsis and renal or hepatic failure [2,3]. Clinically these patients present with an acute onset of non-specific symptoms such as altered mental status, seizures or even focal neurological deficits such as hemiplegia [2,4]. These symptoms tend to be reversible but in cases of severe and prolonged hypoglycemia it can lead to catastrophic consequences such as irreversible coma or even death [5].
As most neurological deficits improve after glucose correction, images are reserved for patients with abnormal evolution and reports of MRI findings are limited [2]. Common findings of hypoglycemic encephalopathy described in the literature consist of bilateral compromise of the cerebral cortex, basal ganglia, hippocampus, and long tracts of white matter. The cerebellum and brainstem are usually not affected [6,7]. Herein, we describe an uncommon presentation of hypoglycemic encephalopathy in a patient with bilateral symmetric compromise of the middle cerebellar peduncles and a revision of similar cases reported in the literature.
Case report
A 53-year-old male patient was admitted to our institution due to altered mental status, hypotension, fever, and respiratory distress. He had a history of diabetic nephropathy with requirement of renal replacement therapy (RRT) and diabetic retinopathy with bilateral amaurosis. In the emergency room, he presented a cardiac arrest secondary to acute respiratory failure. He received 3 minutes of cardiopulmonary resuscitation (CPR) with posterior return to spontaneous blood circulation requiring orotracheal intubation (OTI). Paraclinic reports showed normocytic normochromic anemia, mixed hyperbilirubinemia, conserved renal function with no electrolyte disorders and a CT angiography negative for pulmonary embolism. The patient had a positive polymerase chain reaction (rRT-PCR) assay for SARS-CoV-2 which explained the respiratory distress and fever. He hadn't received any vaccination and the COVID-19 variant was unknown.
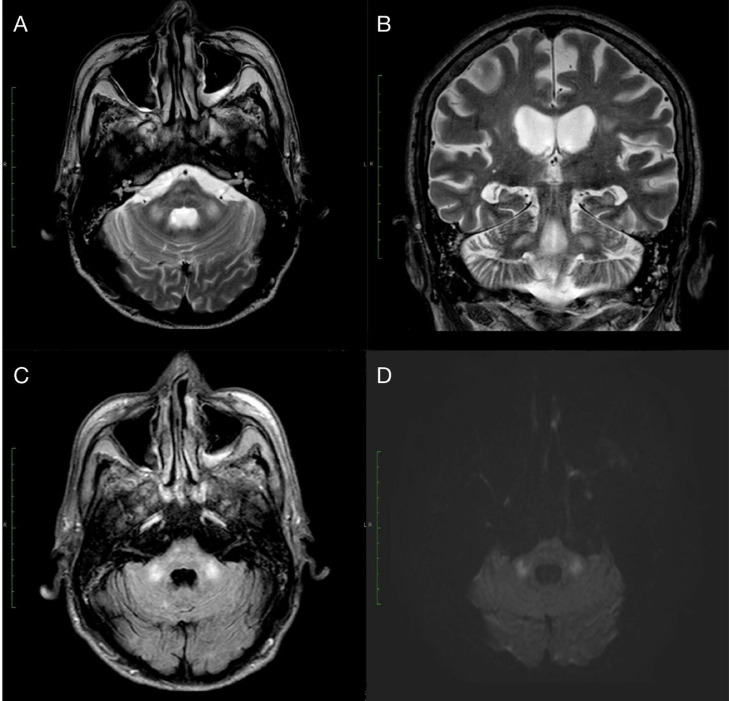
Posterior to the CPR he underwent neurological examination in which he was found somnolent but alertable and obeying simple orders. Verbal response was not assessable due to OTI, he had intact brainstem reflexes and normal motor and sensitive function. Additionally a brain CT was performed showing supratentorial bilateral frontal leukoencephalopathy secondary to small vessel disease and calcified atheromatous plaques in both intracranial internal carotid arteries with no other abnormalities. While in the intensive care unit he presented deterioration of his mental status with stupor even after suspension of sedative agents. The clinical records reported a severe hypoglycemia with glucose levels of 18 mg/dl (74-106). An MRI was performed (Fig. 1) showing symmetric focal high intensity signals in T2-weighted images (T2WI), fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted image (DWI) bilaterally in the middle cerebellar peduncles. This was congruent with the suspected diagnosis of hypoglycemic encephalopathy. After glucose level normalization mental status started to improve. Unfortunately due to his multiple comorbidities and worsen respiratory status, his relatives decided to stop all invasive interventions, and with approval of the ethics committee, end-of-life symptom management order (ESMO) protocol was implemented.
Fig. 1.
MRI images showed symmetrical areas of high signal intensity at the level of the middle cerebellar peduncles on T2WI in both axial (A) and coronal (B) projections, which have the same distribution in FLAIR (C) and maintain a high signal in diffusion-weighted images (D).
Discussion
During severe hypoglycemia the main source of energy for the nervous system is lost. Cerebral energy failure decreases intracellular synthesis of proteins causing malfunctioning of cell membrane ionic pumps which leads to shifts of water from the extracellular to the intracellular compartment. This creates reversible cytotoxic edema specially at the level of the cerebral cortex, basal ganglia, hippocampus, and long tracts of white matter [1,5,8]. The cerebellum and brainstem are mostly unaffected due to increase in local glucose transport mechanisms, which correlates with the usual sparing of the infratentorial compartment during hypoglycemic encephalopathy [9].
Alterations in the homeostasis of neurotransmitters are also important in the pathogenesis of hypoglycemic neuronal damage [10]. As glucose oxidation provides precursors for the synthesis of neurotransmitters such as acetylcholine, GABA, and glutamate. The excitotoxic action of glutamate has been known to cause edema of glial cells and myelin sheaths [10,12]. Alternative energy substrates such as glutamate and glutamine leads to accumulation of ammonia which generate intracellular alkalosis and aspartate release into the extracellular space and may result in selective neuronal necrosis [10,11]. Neuropathological studies initially suggested that gray matter was more vulnerable to hypoglycemic injury than white matter, but recent imagenologic studies suggest that white matter is more sensitive to hypoglycemia than previously thought [6,7,10].
Three typical imagenologic patterns are reported: selective involvement of gray matter, selective involvement of white matter or involvement of both gray and white matter. Most of this with bilateral symmetric alterations [1,2,10,[12], [13], [14], [15]]. A white matter limited compromise has been described with lesions located on the corpus callosum, internal capsule, corona radiata, centrum semiovale and less frequently on the cerebellar peduncles [1,2,10,12,15]. DWI provides key information on areas with restricted diffusion of water within the extracellular space and between intracellular and extracellular spaces secondary to cytotoxic damage.
Lesions related with hypoglycemia are described as hyperintense on T2WI and FLAIR sequences with high signal on the DWI and reduction in the apparent diffusion coefficient (ADC) [1,2,10,[12], [13], [14], [15]]. This radiologic findings may be reversible after correction of hypoglycemia [1,2,10,13]. Studies have found that clinically profound and prolonged hypoglycemia is associated with poor outcome [16]. Multiple studies have looked for a relationship between MRI patterns and prognosis [2,13,14,17]. Results have shown that exclusively focal lesions in the internal capsule correlate with a complete recovery; while diffuse lesions in the hemispheric white matter or the basal ganglia have poor short term prognosis [1], [2], [3],10,[12], [13], [14], [15],17,18].
It is important to take into account differential diagnosis of these imagenological findings. In this specific case COVID-19 related disseminated leukoencephalopathy (CRDL). It's usually described as a pattern of multifocal white matter lesions characterized by reduced diffusivity on DWIMR, T2WI hyperintensity and T1WI central hypointensity. Lesions involving bilateral middle cerebellar peduncles have been described [23,24]. It should be noted that in our patient MRI lesions appeared after an altered mental status related to hypoglycemia and T1W1 central hypointensities were absent.
To the best of our knowledge, there are only 8 cases of bilateral middle cerebellar peduncle hypoglycemic compromise reported in the literature [3,14,[19], [20], [21], [22]]. Comparing the data, we found that 77% of cases occurred in females with a mean age of diagnosis of 53.5 years and DM2 (33.3%) and chronic alcoholism (33.3%) as the most common comorbidities [14,19,20,22]. During hypoglycemic crisis all of the patients reported glucose seric levels of less than 40 mg/dl (74-106) and 88.8% presented with mental status deterioration (coma 44.4%, semicoma 11.1%, stupor 22.2%, and drowsiness 11.1%) [14,[19], [20], [21], [22]]. All patients underwent MR imaging with 33.3% having focal compromise of the middle cerebellum peduncle [3,21]. The remaining 66.6% showed diffuse lesions affecting long tracts of white matter and gray matter [14,19,20,22]. Two out of 6 of the patients with diffuse compromise developed persistent vegetative state secondary to hypoglycemic crisis [14].
Conclusion
Although bilateral middle cerebellar peduncle hypoglycemic lesions are a rare pattern of presentation they can appear alone or with signal alterations in other locations. This is the ninth case of bilateral cerebellar peduncle compromise with hypoglycemic encephalopathy. As increasing evidence regarding prognosis estimation of lesion distribution arises, we consider it important to report the different cases of rare patterns of distribution so further information can be acquired and analyzed.
Patient consent
We confirm that the patient relative has reviewed and agreed to all conditions of the patient consent form provided by the ethics committee of our institution. We confirm that we have obtained the signed consent from the patient close relative and will be able to produce that signed consent form if requested by the journal.
Footnotes
Competing Interests: None.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Ren S, Chen Z, Liu M, Wang Z. The radiological findings of hypoglycemic encephalopathy: a case report with high b value DWI analysis. Medicine (Baltimore) 2017;96(43):e8425. doi: 10.1097/MD.0000000000008425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo L, Tan AC, Umapathi T, Lim CC. Diffusion-weighted MR imaging in early diagnosis and prognosis of hypoglycemia. AJNR Am J Neuroradiol. 2006;27:1222–1224. [PMC free article] [PubMed] [Google Scholar]
- 3.Sugimoto T, Morikawa Y, Ishikawa R, Abe T, Ohno N, Giga M, et al. MRI abnormality of the bilateral middle cerebellar peduncles and long-term follow-up in hypoglycemic encephalopathy: a case report. Neurol Clin Neurosci. 2021;9(1):130–133. [Google Scholar]
- 4.Shirayama H, Ohshiro Y, Kinjo Y, Taira S, Teruya I, Nakachi K, et al. Acute brain injury in hypoglycemia induced hemiplegia. Diabet Med. 2004;21:623–624. doi: 10.1111/j.1464-5491.2004.01185.x. [DOI] [PubMed] [Google Scholar]
- 5.Chuang K, Hsieh KL, Chen C. Hypoglycemic encephalopathy mimicking acute ischemic stroke in clinical presentation and magnetic resonance imaging: a case report. BMC Med Imaging. 2019;19(1):11. doi: 10.1186/s12880-019-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auer RN, Hugh J, Cosgrove E, Curry B. Neuropathologic findings in three cases of profound hypoglycemia. Clin Neuropathol. 1989;8:63–68. [PubMed] [Google Scholar]
- 7.Auer RN, Wieloch T, Olsson Y, Siesjö BK. The distribution of hypoglycemic brain damage. Acta Neuropathol. 1984;64:177–191. doi: 10.1007/BF00688108. [DOI] [PubMed] [Google Scholar]
- 8.Pelligrino D, Almquist LO, Siesjo BK. Effects of insulin induced hypoglycemia on intracellular ph and impedance in the cerebral cortex of the rat. Brain Res. 1981;221:129–147. doi: 10.1016/0006-8993(81)91068-4. [DOI] [PubMed] [Google Scholar]
- 9.Kiessling M, Xie Y, Kleihues P. Regionally selective inhibition of cerebral protein synthesis in the rat during hypoglycemia and recovery. J Neurochem. 1984;43:1507–1514. doi: 10.1111/j.1471-4159.1984.tb06070.x. [DOI] [PubMed] [Google Scholar]
- 10.Kang EG, Jeon SJ, Choi SS, Song CJ, Yu IK. Diffusion MR imaging of hypoglycemic encephalopathy. AJNR Am J Neuroradiol. 2010;31:559–564. doi: 10.3174/ajnr.A1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterworth R.F. In: Glutamine, glutamate and GABA in the central nervous system. Hertz L., Kvamme E., McGeer E.G., Schousboe A., editors. Alan R. Liss; New York: 1983. Metabolism of glutamate and related amino acids in insulin hypoglycemia; pp. 595–608. [Google Scholar]
- 12.Lee C, Liou K, Chen L. Serial magnetic resonance imaging changes in hypoglycemic encephalopathy. Acta Neurol Taiwan. 2013;22(1):22–25. [PubMed] [Google Scholar]
- 13.Yanagawa Y, Isoi N, Tokumaru AM, Sakamoto T, Okada Y. Diffusion-weighted MRI predicts prognosis in severe hypoglycemic encephalopathy. J Clin Neurosci. 2006;13:696–699. doi: 10.1016/j.jocn.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Ma JH, Kim YJ, Yoo WJ, Ihn YK, Kim JY, Song HH, et al. MR imaging of hypoglycemic encephalopathy: lesion distribution and prognosis prediction by diffusion-weighted imaging. Neuroradiology. 2009;51:641–649. doi: 10.1007/s00234-009-0544-5. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Koh SB. Extensive white matter injury in hypoglycemic coma. Neurology. 2007;68:1074. doi: 10.1212/01.wnl.0000258546.83251.36. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda T, Takahashi T, Sato A, Tanaka H, Igarashi S, Fujita N, et al. Predictors of outcome in hypoglycemic encephalopathy. Diabetes Res Clin Pract. 2013;101(2):159–163. doi: 10.1016/j.diabres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Johkura K, Nakae Y, Kudo Y, Tn Y, Kuroiwa Y. Early diffusion MR imaging findings and short-term outcome in comatose patients with hypoglycemia. Am J Neuroradiol AJNR. 2012;33(5):904–909. doi: 10.3174/ajnr.A2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Witsch J, Neugebauer H, Flechsenhar J, Jüttler E. Hypoglycemic encephalopathy: a case series and literature review on outcome determination. J Neurol. 2012;259(10):2172–2181. doi: 10.1007/s00415-012-6480-z. [DOI] [PubMed] [Google Scholar]
- 19.Kim JH, Koh S. Extensive white matter injury in hypoglycemic coma. Neurology. 2007;68(13):1074. doi: 10.1212/01.wnl.0000258546.83251.36. [DOI] [PubMed] [Google Scholar]
- 20.Thakur S, Babu NS, Mokta J, Sharma S. Reversible MRI changes of prolonged hypoglycemia. J Med Nutr Nutraceuticals. 2014;3(2):222. [Google Scholar]
- 21.Okamoto K, Tokiguchi S, Furusawa T, Ishikawa K, Quardery AF, Shinbo S, et al. MR features of diseases involving bilateral middle cerebellar peduncles. Am J Neuroradiol AJNR. 2003;24(10):1946–1954. [PMC free article] [PubMed] [Google Scholar]
- 22.Bin CH, Park MS, Lee S. A case of severe hypoglycemic encephalopathy with extensive brain lesions in non-diabetics and alcoholism. Yŏngnam Ŭidae haksulji. 2010;27(1):37. [Google Scholar]
- 23.Rapalino O, Pourvaziri A, Maher M, Jaramillo-Cardoso A, Edlow BL, Conklin J, et al. Clinical, imaging, and lab correlates of severe COVID-19 leukoencephalopathy. Am J Neuroradiol AJNR. 2021;42(4):632–638. doi: 10.3174/ajnr.A6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman CW, Masur J, Hassankhani A, Wolf RL, Levine JM, Mohan S. Coronavirus disease (COVID-19)-related disseminated leukoencephalopathy: a retrospective study of findings on brain MRI. Am J Roentgenol. 2021;216(4):1046–1047. doi: 10.2214/AJR.20.24364. [DOI] [PMC free article] [PubMed] [Google Scholar]