Abstract
Cs+ was found to induce expression of the kdpFABC operon, encoding a high-affinity K+ uptake system of Escherichia coli. Quantitative expression analyses at the transcriptional and translational levels reveal that CsCl causes much higher induction of kdpFABC than does NaCl. A decrease of the intracellular K+ concentration is found in cells exposed to CsCl. The results indicate that kdpFABC expression is induced when the intracellular K+ concentration is lowered. Moreover, the results imply that the signal transduction cascade mediated by KdpD and KdpE is able to integrate multiple signals.
Escherichia coli uses several K+ transport systems to adjust the intracellular K+ concentration (2). Under physiological conditions the constitutive K+ uptake systems TrkG, TrkH, and Kup are operating. Upon osmotic upshift and under K+-limiting growth conditions ([K+] <2 mM), the high-affinity K+ transport complex KdpFABC is synthesized. Expression of the kdpFABC operon is under control of the regulatory proteins KdpD and KdpE, which constitute a typical sensor kinase/response regulator system (21).
Which stimulus (stimuli) the membrane-bound sensor kinase KdpD is responding to has been puzzling for years. Epstein and coworkers have put forward the hypothesis that KdpD is a turgor sensor (12, 13). The model of Sugiura et al. describes two mechanisms for KdpD activation: K+ limitation and osmotic upshift (18). Other groups argue that the K+ signal is related to the internal K+ level and/or the processes of K+ transport (3, 9) or to the external K+ concentration (16). Based on the results obtained with right-side-out membrane vesicles, a new model has been established, according to which the intracellular K+ concentration and ionic strength directly influence KdpD autophosphorylation activity, whereby K+ has an inhibitory effect and ionic strength has a stimulatory effect (10). Here, we report that extracellular Cs+ significantly induces kdpFABC expression by lowering the intracellular K+ content. The results obtained corroborate our model that the intracellular K+ concentration is sensed by KdpD (10).
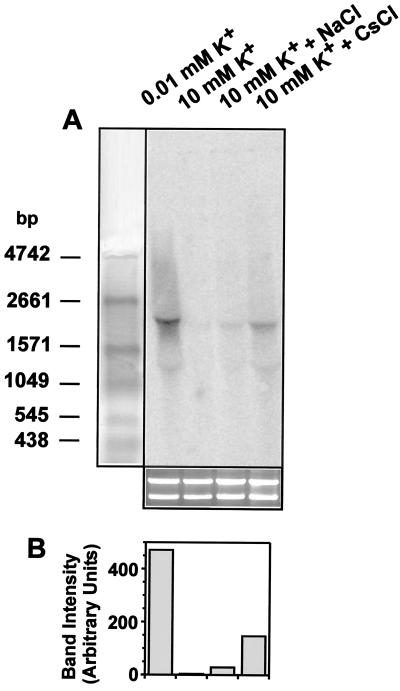
Induction of kdpFABC by ionic osmolytes detected by Northern blot analysis.
The influence of the ionic osmolytes NaCl and CsCl on kdpFABC expression in E. coli K-12 [strain MC4100 (6)] containing all K+ uptake systems (Trk, Kdp, and Kup) was investigated. Cells were grown at 37°C in phosphate-buffered minimal medium (8) containing 10 mM K+ until the mid-logarithmic phase, filtered, and subsequently resuspended in medium of lower K+ concentration (0.01 mM K+) or the same medium as before (10 mM K+) or exposed to an osmotic upshift imposed by NaCl (0.2 M) or CsCl (0.2 M) in medium containing 10 mM K+ for 10 min. RNA was prepared according to Aiba et al. (1). For quantitative Northern blot analysis, 20 μg of RNA from each sample was separated by electrophoresis in 1.2% (wt/vol) agarose–1.1 M formaldehyde gels in MOPS (morpholinepropanesulfonic acid) buffer. Equal loading of samples onto the gel was verified by ethidium bromide staining of the rRNA in a separate gel. RNA was transferred to a Hybond-N nylon membrane (Amersham Pharmacia Biotech) by upward capillary action. Hybridization was performed following a standard protocol (17) using γ-32P-radiolabeled dCTP PCR fragments as specific probes for kdpA (nucleotides 1009 to 1794). Radioactivity was quantified with a PhosphorImager. kdpFABC-specific signals were detected in RNA samples from cells grown under kdpFABC-inducing conditions (K+ limitation and osmotic upshift in response to NaCl) but not in an RNA sample from cells grown at 10 mM K+ (Fig. 1). The expected size of the kdpFABC transcript is 4,296 bp; however, a more diffuse signal with one distinct band around 2,000 bp can be observed. kdpFABC transcripts were also detectable in RNA samples of cells which were exposed to CsCl. Quantitative analysis of the amounts of transcripts revealed an 8-fold-higher transcript level in response to NaCl and a 41-fold-higher level in response to CsCl (Fig. 1B). For comparison, transcription was 369-fold higher in cells exposed to K+ limitation than in unstressed cells (Fig. 1).
FIG. 1.
Detection of kdpFABC transcripts by Northern blot analysis. (A) For Northern blot analysis 20 μg of RNA was loaded in each lane and kdpFABC transcripts were detected using a radiolabeled PCR product complementary to kdpA. Shown also are an RNA standard (left) and ethidium bromide-stained rRNA of the same samples used for the Northern blot (bottom). (B) kdpFABC transcripts quantified by PhosphorImager analysis.
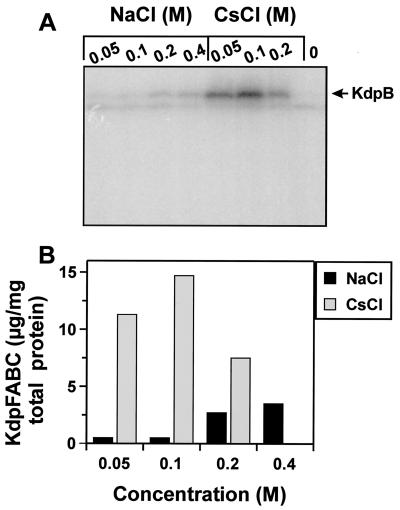
Induction of kdpFABC by ionic osmolytes detected by the amount of synthesized KdpFABC complex.
Expression of kdpFABC was also measured at the translational level by quantitative Western blot analysis (Fig. 2). Cells were grown as described above; however, cells were shifted to media containing 10 mM K+ of various osmolalities and harvested after 30 min. Cells were resuspended in sodium dodecyl sulfate sample buffer and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (11). Quantification of KdpFABC was basically performed following the protocol developed for lactose permease (19). Briefly, proteins were electroblotted to a nitrocellulose membrane. Blots were then blocked with 5% (wt/vol) bovine serum albumin in 10 mM Tris-HCl (pH 7.5)–0.15 M NaCl (buffer A) for 1 h. Anti-KdpB antibody was added at a final dilution of 1:5,000, and incubation was continued for 1 h. After a washing with buffer A, 125I-protein A (Amersham Pharmacia Biotech) was added at a final dilution of 1:5,000, and incubation was continued for 1 h. After being washed thoroughly, the membrane was exposed to a PhosphorImager screen. Known amounts of purified KdpFABC complex were used to obtain a standard curve. The amount of KdpFABC complex was then quantified by comparison to the standard curve.
FIG. 2.
Detection of the KdpFABC complex produced upon exposure of cells to an osmotic upshift imposed by NaCl or CsCl. (A) Autoradiograph of a Western blot of whole-cell extracts developed with anti-KdpB antibodies and 125I-protein A for detection. (B) Graph representing the amounts of KdpFABC synthesized, which were calculated according to a standard curve obtained from known amounts of purified KdpFABC.
The data indicate a correlation between an increase of the osmolality imposed by NaCl and the amount of KdpFABC complex synthesized. Cells exposed to CsCl produced more complex at a concentration of 0.1 M than at 0.2 M CsCl. The decrease in complex formation at 0.2 M CsCl might be related to the toxic effect of Cs+. This approach also indicated that CsCl triggers higher induction of the kdpFABC operon, which was up to 10-fold stronger compared to the effect of NaCl at the same osmolalities (Fig. 2B).
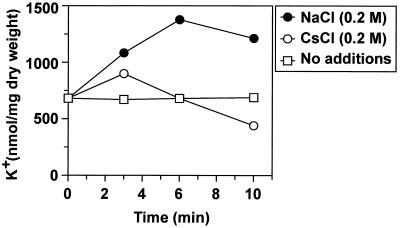
Determination of the intracellular K+ content.
Cells were cultivated as described above. At different time points after the shift to the new medium, samples of 1.0 ml were centrifuged through silicone oil (density = 1.04 g/cm3) and the K+ content of the cell pellets was determined in a flame photometer, model 700 (Eppendorf) (5). We found an increase of the intracellular K+ content 3 min after an osmotic upshift imposed by NaCl or CsCl (Fig. 3). In the case of NaCl the intracellular K+ concentration was further increased at the 6-min time point. In the case of Cs+ the intracellular K+ content decreased over time. Earlier, Bossemeyer et al. (5) found that uptake of Cs+ via the Kup system lowers the intracellular K+ concentration due to K+ release.
FIG. 3.
Determination of intracellular K+ concentrations. The data presented represent average values obtained in at least three independent experiments.
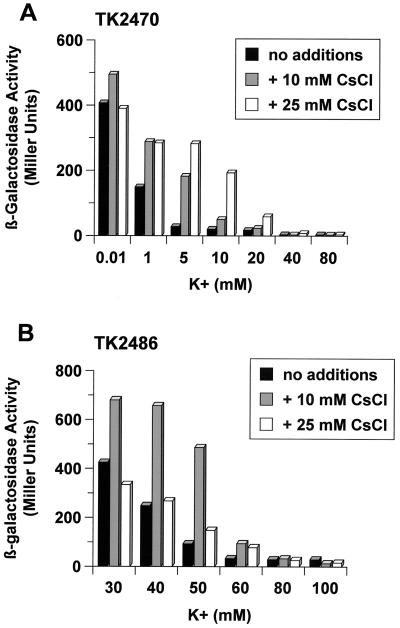
Effect of Cs+ on kdpFABC expression in constitutive K+ uptake system mutants.
Since Cs+ is very similar to K+ (the ionic radii are 165 and 133 pm, respectively), uptake of both ions is mediated through the same transport systems. The effect of CsCl on kdpFABC expression was further tested with two E. coli strains having different K+ uptake systems. E. coli strain TK2486 is Kup+, and strain TK2470 is a Trk+ derivative of strain TK2469 (Trk− Kup− Kdp−) (13), both of which are derivatives of E. coli K-12 kindly provided by W. Epstein, The University of Chicago, Chicago, Ill. Both strains are ΔkdpFABC but carry a stabilized transcriptional kdp::lacZ fusion (15). Cells were grown in minimal medium containing the indicated concentrations of K+ and Cs+, and steady-state expression was determined by measuring β-galactosidase activities as described previously (14). Since high CsCl concentrations inhibit growth, experiments were done under permissive conditions, at concentrations of 10 and 25 mM CsCl. As shown in Fig. 4, kdpFABC expression was significantly induced in both strains when cells were grown in the presence of CsCl but the expression levels were strongly dependent on the availability of K+ for the cells.
FIG. 4.
Influence of CsCl on steady-state expression of kdpFABC. β-Galactosidase activities of strain TK2470 (Trk+ Kup− Kdp− kdp::lacZ) (A) and strain TK2486 (Trk− Kup+ Kdp− kdp::lacZ) (B). The data presented represent average values obtained in at least three independent experiments.
For E. coli strain TK2470 (Trk+ Kup−), β-galactosidase activities were significantly increased in the presence of CsCl when cells were grown in media containing K+ at concentrations which normally prevent kdpFABC expression (5 and 10 mM K+) (Fig. 4A). With a further increase of the K+ concentration (20 mM K+ and higher) kdpFABC expression declined even in the presence of CsCl. These results are in accord with the previously described competitive inhibition of Cs+ on K+ uptake by the Trk system (Ki of 30 mM Cs+) (5).
E. coli strain TK2486 doesn't have the Trk system but has the Kup system. Kup has an approximately 14-fold-higher affinity for K+ than for Cs+ (5). Because of the lack of the Trk system, the onset of kdpFABC induction is shifted to higher K+ concentrations (below 60 mM) (13) (Fig. 4B). This strain exhibited increased β-galactosidase activities in the lower range of K+ in the presence of 10 mM CsCl. Addition of 25 mM CsCl already affected growth (data not shown), which might explain the failure of CsCl to increase kdpFABC expression. Higher K+ concentrations prevented kdpFABC induction. The results obtained reveal that Cs+ is taken up via Kup. Moreover, it is known that Cs+ inhibits K+ uptake via the Kup system much more strongly than via the Trk system (5). These facts explain the greater effects of Cs+ on kdpFABC expression in a Kup+ strain than in a TrkA+ strain.
Implications of the results for the model of kdpFABC regulation.
The results presented here demonstrate that kdpFABC expression is dependent on the intracellular K+ concentration. When E. coli is cultivated in the presence of Cs+, which lowers the intracellular K+ concentration, kdpFABC expression is induced. It is known that Cs+ has an inhibitory effect on K+-uptake systems, and the uptake of Cs+ even leads to K+ release (reference 5 and this work). However, Cs+ cannot substitute for the essential biological functions of K+. Avery (4) confirmed that it is not the presence of Cs+ in cells that is growth inhibitory but rather the resulting decline in intracellular K+. Moreover, it is known, and we confirmed it with these studies, that the external ratio of K+ to Cs+ rather than the absolute Cs+ concentration is the critical factor for the potential toxicity of Cs+.
The data imply that the lowered intracellular K+ concentration is a stimulus for KdpD. Results obtained in an in vitro test system based on right-side-out membrane vesicles indicate an inhibitory effect of K+ on KdpD autophosphorylation activity mediated by the domains of KdpD exposed to the cytoplasmic side of the membrane (10). Based on these findings, it is proposed that the inhibitory effect of K+ on KdpD autophosphorylation activity is suspended in vivo under K+-limiting growth conditions or as shown here when cells were cultivated in the presence of Cs+.
Upon osmotic stress the activities of the constitutive K+ uptake systems are stimulated, the TrkA system at neutral and slightly alkaline pH (7, 15) and the Kup system at low pH (20). These systems mediate rapid uptake of K+, which is the first response of E. coli to restore turgor after an osmotic upshift (22). Induction of the kdpFABC operon is a slow response of the cells but important when the cells are in need of further K+. This seems to be the case when the osmostress is imposed by NaCl. The mechanism of how NaCl activates the KdpD-KdpE signal transduction cascade is clearly different from the effect caused by lowering of the intracellular K+ concentration since under the former conditions the intracellular K+ concentration is increased. Using right-side-out membrane vesicles, we found that an increase of the ionic strength in the lumen of the vesicles stimulated KdpD autophosphorylation activity. In addition, raising of the salt concentration (KCl or NaCl) from the outside also increased autophosphorylation activity of KdpD (10).
In summary, kdpFABC expression is induced by NaCl and CsCl. Cs+ exerts its kdpFABC-inducing effect by lowering the intracellular K+ concentration, which in turn is sensed by KdpD. An increase of the intracellular ionic strength upon osmotic upshift and probably an effect of NaCl on the lipid bilayer stimulate the autophosphorylation activity of the sensor kinase KdpD under these conditions. Thus, the different levels of kdpFABC expression in response to NaCl or CsCl could be explained by the ability of the sensor KdpD to integrate multiple signals.
Acknowledgments
We thank W. Epstein, The University of Chicago, Chicago, Ill., for providing strains and for initial discussions about these studies.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 431, JU 270/3-1) and the Fonds der Chemischen Industrie. Kirsten Jung is the recipient of a fellowship (Heisenberg-Stipendium) from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Aiba H, Adhya S, de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981;256:11905–11910. [PubMed] [Google Scholar]
- 2.Altendorf K, Epstein W. The Kdp-ATPase of Escherichia coli. In: Lee A G, editor. Biomembranes. Vol. 5. London, United Kingdom: JAI Press Inc.; 1996. pp. 403–420. [Google Scholar]
- 3.Asha H, Gowrishankar J. Regulation of kdp operon expression in Escherichia coli: evidence against turgor as signal for transcriptional control. J Bacteriol. 1993;175:4528–4537. doi: 10.1128/jb.175.14.4528-4537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery S V. Caesium accumulation by microorganisms: uptake mechanisms, cation competition, compartmentalization and toxicity. J Ind Microbiol. 1995;14:76–84. doi: 10.1007/BF01569888. [DOI] [PubMed] [Google Scholar]
- 5.Bossemeyer D, Schlosser A, Bakker E P. Specific cesium transport via the Escherichia coli Kup (TrkD) K+ uptake system. J Bacteriol. 1989;171:2219–2221. doi: 10.1128/jb.171.4.2219-2221.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 7.Dosch D C, Helmer G L, Sutton S H, Salvacion F F, Epstein W. Genetic analysis of potassium transport loci in Escherichia coli: evidence for three constitutive systems mediating uptake of potassium. J Bacteriol. 1991;173:687–696. doi: 10.1128/jb.173.2.687-696.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epstein W, Kim B S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frymier J S, Reed T D, Fletcher S A, Csonka L N. Characterization of transcriptional regulation of the kdp operon of Salmonella typhimurium. J Bacteriol. 1997;179:3061–3063. doi: 10.1128/jb.179.9.3061-3063.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung K, Veen M, Altendorf K. K+ and ionic strength directly influence the autophosphorylation activity of the putative turgor sensor KdpD of Escherichia coli. J Biol Chem. 2000;275:40142–40147. doi: 10.1074/jbc.M008917200. [DOI] [PubMed] [Google Scholar]
- 11.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 12.Laimins L A, Rhoads D B, Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malli R, Epstein W. Expression of the Kdp ATPase is consistent with regulation by turgor pressure. J Bacteriol. 1998;180:5102–5108. doi: 10.1128/jb.180.19.5102-5108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller J H, editor. A short course in bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 72–74. [Google Scholar]
- 15.Rhoads D B, Waters F B, Epstein W. Cation transport in Escherichia coli. VIII. Potassium transport mutants. J Gen Physiol. 1976;67:325–341. doi: 10.1085/jgp.67.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roe A J, McLaggan D, O'Byrne C P, Booth I R. Rapid inactivation of the Escherichia coli Kdp K+ uptake system by high potassium concentrations. Mol Microbiol. 2000;35:1235–1243. doi: 10.1046/j.1365-2958.2000.01793.x. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 18.Sugiura A, Hirokawa K, Nakashima K, Mizuno T. Signal-sensing mechanisms of the putative osmosensor KdpD in Escherichia coli. Mol Microbiol. 1994;14:929–938. doi: 10.1111/j.1365-2958.1994.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun J, Wu J, Carrasco N, Kaback H R. Identification of the epitope for monoclonal antibody 4B1 which uncouples lactose and proton translocation in the lactose permease of Escherichia coli. Biochemistry. 1996;35:990–998. doi: 10.1021/bi952166w. [DOI] [PubMed] [Google Scholar]
- 20.Trchounian A, Kobayashi H. Kup is the major K+ uptake system in Escherichia coli upon hyper-osmotic stress at a low pH. FEBS Lett. 1999;447:144–148. doi: 10.1016/s0014-5793(99)00288-4. [DOI] [PubMed] [Google Scholar]
- 21.Walderhaug M O, Polarek J W, Voelkner P, Daniel J M, Hesse J E, Altendorf K, Epstein W. KdpD and KdpE, proteins that control expression of the kdpABC operon, are members of the two-component sensor-effector class of regulators. J Bacteriol. 1992;174:2152–2159. doi: 10.1128/jb.174.7.2152-2159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood J M. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol Mol Biol Rev. 1999;63:230–262. doi: 10.1128/mmbr.63.1.230-262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]