Abstract
Background
Plasma protein patterns differ between cancer patients and healthy donors. This study aimed to examine the plasma levels of several cytokines and immunological checkpoint proteins between patients with oral and oropharyngeal cancer and healthy donors.
Materials and methods
Plasma samples from healthy donors, oral cancer patients, and oropharyngeal cancer patients were analyzed using the Human Th Cytokine Panel 13-plex (IL-2, 4, 5, 6, 9, 10, 13, 17A, 17F, 21, 22, IFN-γ, and TNF-α) and Human Immune Checkpoint Panel1 12-plex [sCD25 (IL-2Ra), 4-1BB, sCD27, B7.2 (CD86), Free Active TGF-β1, CTLA-4, PD-L1, PD-L2, PD-1, Tim-3, LAG-3, and Galectin-9]. The plasma 4-1BB levels were verified by Western blot method. In addition, the study of the receive operating curve (ROC) yielded the calculation of a number of diagnostically significant indicators.
Results
Significantly increased levels of IL-6, 4-1BB, PDL-1, PD-1, and CTLA-4 and decreased levels of IL-13 and sCD27 were observed in cancer patients compared with healthy donors. These levels were highly significant, particularly for cancer patients in stage IV. Validation by Western blot revealed that cancer patients had higher plasma levels of 4-1BB than healthy donors (p < 0.05), and ROC curve analysis revealed that plasma 4-1BB had the highest cancer detection capability. Intriguingly, plasma levels of 4-1BB were significantly positively correlated with PDL-1 and PD-1 levels (p < 0.0001).
Conclusion
This data provided descriptive knowledge of oral and oropharyngeal cancer immunity at a fundamental level. Additional research should concentrate on the significantly different factors, especially 4-1BB, PDL-1, and PD-1, which may contribute to the development of novel alternative diagnostic tools or therapies for patients with oral and oropharyngeal cancer.
Keywords: Plasma, Oral cancer, Oropharyngeal cancer, Cytokine, Immune checkpoint
Highlights
-
•
Plasma levels of 4-1BB were increased in head and neck cancer patients.
-
•
The level of plasma 4-1BB correlated with levels of plasma PD-1 and PDL-1.
-
•
Plasma 4-1BB possible to use as head and neck cancer screening.
-
•
Plasma 4-1BB may be developed as an alternative cancer immunotherapeutic drug.
Plasma; Oral cancer; Oropharyngeal cancer; Cytokine; Immune checkpoint.
1. Introduction
The majority of oral and oropharyngeal cancers are squamous cell carcinomas (SCCs), which arise from squamous epithelial cells (Ghantous and Abu Elnaaj, 2017). According to the GLOBOCAN 2020 Thailand estimates, oral cancer accounted for 2.5% of the total cancer cases and 2% of cancer-related deaths worldwide, whereas oropharyngeal cancer accounted for 0.57% of the total cancer cases and 0.42% of cancer-related deaths (Sung et al., 2021). Both cancers are associated with several risk factors, such as alcohol, smoking, and betel quid (Prince et al., 2010). Despite significant improvements in cancer therapies like surgery, radiation, chemotherapy, and immunotherapy, the 5-year survival rate is still dismal (Siegel et al., 2021).
Immune profiles for each patient show how that patient's particular immune response and impact on the treatment. The challenge emphasizes the importance of the immune response in cancer patients. Immune responses are dependent on cytokines. They are molecular messengers that allow immune system cells to coordinate their response to a target antigen through intercellular communication (Arduino et al., 2008). Many researchers have reported the secretion of inflammatory cytokines from cells after the development of cancer. Interleukin-6 (IL-6) plays an important role in regulating differentiation and the levels of growth factors for B and T cells. Some studies found increased levels of IL-6 in patients with different types of cancer, such as lung carcinoma, multiple myeloma, and esophageal SCC (Riedel et al., 2005).
Immunotherapy is now commonly used to treat cancer. Immune checkpoints are a prominent point in immunotherapy that primarily involves T lymphocyte cell function. Two signals control T-cell activation: MHC complexes interacting with T-cell receptors and co-stimulation between T cells and antigen-presenting cells. Immune checkpoint proteins, which are also called co-stimulatory and co-inhibitory factors, work together to keep the immune system in balance. Current anti-cancer treatments target immune checkpoint molecules like programmed death-1 (PD-1), cytotoxic T-cell antigen-4 (CTLA-4), and T-cell immunoglobulin mucin-3 (TIM-3). PD-1 plays a pivotal role in immune tolerance. Two PD1 ligands, PD-L1 and PD-L2, have been identified (Okazaki et al., 2013; Pesce et al., 2017). PD-1 suppresses T-cell activation by inhibiting T-cell proliferation and cytotoxic activity. In addition, CTLA-4, which is also known as CD152, is a member of the immunoglobulin superfamily. It is expressed on activated B cells, activated T cells, and activated monocytes. CTLA-4 binds to both CD80 (B7.1) and CD86 (B7.2), leading to the downregulation of T-cell responses (Thompson and Allison, 1997). In addition, 4-1BB, which is also known as CD137, is temporarily expressed on both CD4 and CD8 T cells (Garni-Wagner et al., 1996). This molecule is able to induce cytokine production, expansion, and functional maturation of T cells, DCs, and monocytes (Wilcox et al., 2002). Moreover, blocking 4-1BB molecules using an antibody can enhance tumor-specific cytotoxicity (Melero et al., 1997).
There is still a scarcity of information on plasma cytokines and immune checkpoint proteins in patients with oral and oropharyngeal cancer. Here, we compared the plasma levels of cytokines and immune checkpoint proteins between Thai patients with oral and oropharyngeal cancer and healthy donors. As well as comparing plasma cytokines and immune checkpoint proteins to clinical data. Different cytokines or immune checkpoint molecules may be useful as biomarkers or in the development of novel cancer immunotherapies.
2. Material and methods
2.1. Study participants and ethical statement
This study included 18 healthy donors, 14 patients with oral cancer, and 11 patients with oropharyngeal cancer. Because there were limits on the total number of samples (such as insufficient protein). During the step of plasma protein screening, the study included 18 healthy donors, 11 patients with oral cancer, and 11 patients with oropharyngeal cancer. While in the step of plasma 4-1BB validation, the study included 12 healthy donors, 12 patients with oral cancer, and 10 patients with oropharyngeal cancer. The particulars of sample selection are shown in Table S1. All samples were obtained from the Department of Otolaryngology, Head and Neck Surgery, Faculty of Medicine, Chulalongkorn University, Thailand. Clinical diagnoses were confirmed by oncology physicians, and clinical staging was recorded using the TNM guideline (PM and NT). Histopathological analysis by a pathologist (NK) confirmed oral and oropharyngeal squamous cell carcinoma. Healthy donors are people who meet the Thai Red Cross Society's blood donation criteria and are in the same age group as cancer patients. Exclusion criteria include participants with systemic diseases, a history of cancer, or a history of immune-related disease. The characteristics of the participants are detailed in Table 1 and Table S1, respectively. This research was approved by the Institutional Review Board of the Faculty of Medicine at Chulalongkorn University (IRB No. 810/62) and conducted in accordance with the Declaration of Helsinki's principles and ethical standards. Prior to the commencement of the study, all participants provided written consent based on their understanding of the research.
Table 1.
Characteristics of participants.
| Healthy donor | Oral cancer | Oropharyngeal cancer | |
|---|---|---|---|
| N | 18 | 14 | 11 |
| AverageAge (Range) | 58 (52–78) | 59 (37–78) | 57 (38–80) |
| Geneder Male:Female | 12:6 | 9:5 | 8:3 |
| ClinicalStage 1 (N) | - | 1 | - |
| ClinicalStage 2 (N) | - | 2 | 4 |
| ClinicalStage 3 (N) | - | 5 | - |
| ClinicalStage 4 (N) | - | 6 | 7 |
2.2. Sample collection
Ethylenediaminetetraacetic acid (EDTA) blood samples were collected from consenting healthy donors and cancer patients and then were centrifuged at 3,000 rpm for 10 min to obtain plasma. All plasma samples were kept at -80 °C before use in the assays described below.
2.3. Multiplex cytokine and immune checkpoint measurements
To measure the levels of several cytokines in plasma samples, the Human Th Cytokine Panel 13-plex (IL-2, 4, 5, 6, 9, 10, 13, 17A, 17F, 21, 22, IFN-γ, and TNF-α; Biolegend, CA, USA) and Human Immune Checkpoint Panel1 12-plex [sCD25 (IL-2Ra), 4-1BB, sCD27, B7.2 (CD86), Free Active TGF-β1, CTLA-4, PD-L1, PD-L2, PD-1, Tim-3, LAG-3, and Galectin-9; Biolegend, USA] were used. Briefly, 25 μl of assay buffer was added into each well. Then, 25 μl of the diluted standard control and plasma samples were added to the wells. The wells were then filled with 25 μl of mixed beads and 25 μl of detection antibodies and incubated for 2 h at room temperature on an orbital plate shaker. Next, 25 μl of streptavidin-PE solution was added, and the plate was shaken orbitally for 30 min at room temperature. Plates were centrifuged at 1,000 rpm for 5 min, liquid was removed, and plates were washed twice with wash buffer. Finally, 150 μl of wash buffer was added to each well, and the plates were shaken for 2–3 min prior to analysis by flow cytometry (BD FACSCalibur™, Becton Dickinson, NJ, USA).
2.4. Western blotting
Protein lysates were analyzed for protein content using the Bicinchoninic Acid (BCA) protein assay kit (ThermoFisher Scientific Waltham, MA, U.S.). Equal amounts of protein samples were loaded onto 12% SDS-PAGE and transferred onto 0.45 μm nitrocellulose membranes (Bio-Rad, Hercules, CA, U.S.). Transferred membranes were blocked for 1 h in 5% nonfat dry milk in Tris-buffered saline with 0.05% Tween 20 (TBST) solution (25 mM Tris-HCl, pH 7.5, 125 mM NaCl, and 0.05% Tween 20) and incubated overnight with anti 4-1BB antibody (abcam, Cambridge, MA, U.S. cat no. ab176606, concentration 1:500) at 4 °C. The membranes were washed three times with TBST and incubated with appropriate horseradish peroxidase (HRP)-labeled secondary antibodies (1:2000) for 2 h at room temperature. Anti- α-tubulin was reprobed as internal control (cell signaling Danvers, MA, U.S. cat no. 2144s concentration 1:1000) at 4 °C. Finally, the expression of proteins was detected using a Chemiluminescent HRP substrate (Millipore Corporation, Darmstadt, Germany). Protein bands were captured by using a Digital imager (Azure Imager c300 Dublin, CA, U.S.) and quantified by using Image J software (NIH).
2.5. Statistical analysis
All statistical analyses and graphs were generated utilizing IBM SPSS Statistics 23.0 (IBM Corporation, Armonk, NY, USA) and Graphpad Prism 6.0 (Graphpad software Inc., La Jolla, CA, USA). The Mann-Whitney U test and the Kruskal-Wallis test were used to compare the cytokine levels and soluble checkpoint protein levels of each group. Spearman's correlation coefficients were applied to all correlations for analysis. All p-values < 0.05 were defined as significant.
3. Results
3.1. Increased IL-6 levels and decreased IL-13 levels in cancer patients
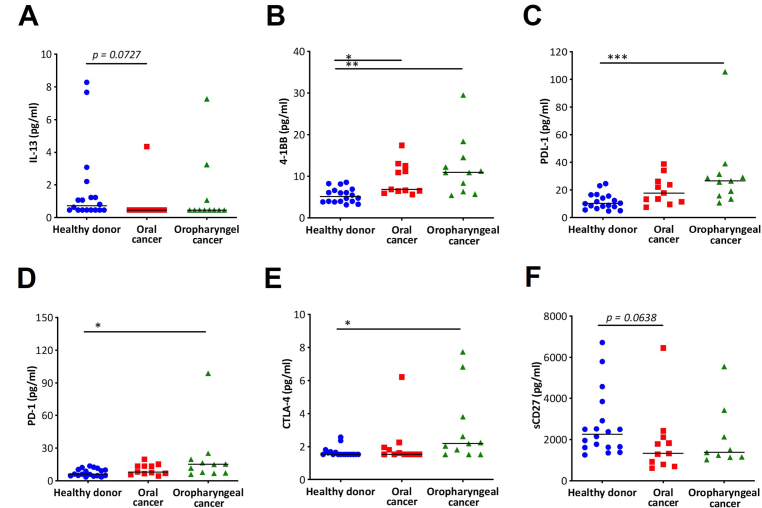
The plasma Th cytokine panel results showed that the plasma levels of 2 out of 13 cytokines were considerably different in cancer patients compared with healthy donors [IL-6 (p = 0.0714) and IL-13 (p = 0.0324), Mann–Whitney U test, Table 2A]. The median levels in healthy donors were 6.12 pg/ml for IL-6 and 0.72 pg/ml for IL-13 whereas those in cancer patients were 10.43 pg/ml for IL-6 and <0.46 pg/ml for IL-13 (Table 2A). In addition, the level of IL-6 in patients with oropharyngeal cancer was slightly higher than that in patients with oral cancer and healthy donors (data not shown). However, the levels of IL-13 in patients with oropharyngeal and oral cancer were comparable but lower than those in healthy donors (Figure 1A). Although numerous cytokines are involved in biological pathways, these findings indicate that only IL-6 and IL-13 differ remarkably between cancer patients and healthy donors.
Table 2.
Median, range and p-value of plasma cytokine levels and soluble checkpoint protein levels (A) Median, range and p value of plasma cytokine levels. (B) Median, range and p value of soluble checkpoint protein levels.
| A | |||
|---|---|---|---|
| Type of Cytokine | Healthy donors (median, range) | Cancer participants (median, range) | p valuea |
| IL-2 | 2.44, 1.05–15.63 | 1.88, 0.90–60.14 | 0.2595 |
| IL-4 | 1.79, <0.58–8.88 | 1.11, <0.58–13.87 | 0.6406 |
| IL-5 | 4.85, 2.19–9.38 | 4.51, 2.04–30.64 | 0.6621 |
| IL-6 | 6.12, 3.33–19.30 | 10.43, <2.59–43.43 | 0.0714 |
| IL-9 | 2.92, <2.07–4.15 | 2.73, <2.07–13.60 | 0.8551 |
| IL-10 | 2.84, 2.31–5.95 | 2.56, 2.13–3.71 | 0.0932 |
| IL-13 | 0.72, <0.46–8.28 | <0.46, <0.46–7.27 | 0.0324 |
| IL-17A | 1.27, <0.61–13.42 | 1.11, <0.61–16.47 | 0.8981 |
| IL17F | <1.04, <1.04–18.14 | <1.04, <1.04–12.19 | 0.8912 |
| IL-21 | <16.54, <16.54–132.25 | <16.54, <16.54–345.51 | 0.2752 |
| IL-22 | 3.73, 1.27–9.86 | 2.38, 1.26–14.40 | 0.1825 |
| IFN-gamma | <2.12, <2.12–346.34 | <2.12, <2.12–65.06 | 0.5876 |
| TNF-alpha |
2.71, <1.68–33.50 |
4.39, <1.68–17.64 |
0.9515 |
| B | |||
| Type of immune checkpoint | Healthy donors (median, range) | Cancer participants (median, range) | p valuea |
| sCD25 (IL-2Ra) | 101.47, 57.72–452.14 | 111.33, 26.62–622.52 | 0.8949 |
| 4-1BB | 5.15, 3.17–8.57 | 10.91, 5.42–29.49 | <0.0001 |
| sCD27 | 2267.12, 1259.27–6716.02 | 1443.61, 612.86->30325.79 | 0.0115 |
| B7.2 (CD86) | 48.30, 18.93–119.48 | 31.98, 16.95–124.82 | 0.0806 |
| Free Active TGF-b1 | <1.70, <1.70–15.86 | <1.70, <1.70–2.78 | 0.7462 |
| CTLA-4 | <1.53, <1.53–33.41 | 1.81, <1.53–7.74 | 0.0596 |
| PD-L1 | 10.17, 4.68–24.51 | 22.80, 7.35–105.44 | 0.0005 |
| PD-L2 | 18447.49, 8497.96–30690.41 | 13991.29, 6406.92–31689.94 | 0.1611 |
| PD-1 | 6.16, 3.53–13.67 | 12.55, 4.31–98.82 | 0.0094 |
| Tim-3 | 3011.90, 1235.55–9094.62 | 2411.11, 907.82–7484.46 | 0.1707 |
| LAG-3 | 60.71, 6.90–294.04 | 79.38, 36.38–412.32 | 0.1272 |
| Galactin-9 | 1293.35, 509.38–3007.67 | 1208.64, 407.27–2068.21 | 0.4241 |
Mann-Whitney test.
Figure 1.
Plasma cytokine levels and soluble immune checkpoint protein levels in oral and oropharyngeal cancer patients and healthy donors (A) IL-13, (B) 4-1BB, (C) PDL-1, (D) PD-1, (E) CTLA-4 and (F) sCD27; Denote: ∗p < 0.01, ∗∗p < 0.001, ∗∗∗p < 0.0001, ∗∗∗∗p < 0.00001.
3.2. Increased 4-1BB, PDL-1, PD-1, and CTLA-4 levels in cancer patients
The immune checkpoint proteins showed that the levels of 4 out of 12 plasma proteins were significantly different, such as 4-1BB, sCD27, PDL-1, and PD-1 (p < 0.0001, 0.0115, 0.0005, and 0.0094, respectively, Mann–Whitney U test, Table 2B). The plasma protein level of 4-1BB, PDL-1, and PD-1 was higher in cancer patients than in healthy donors (4-1BB, 10.91 vs. 5.15 pg/ml; PDL-1, 22.80 vs. 10.17 pg/ml; and PD-1, 12.55 vs. 6.16 pg/ml). However, the plasma protein level of sCD27 was lower in cancer patients than in healthy donors (1443.61 vs. 2267.12 pg/ml). In addition, we found that the plasma protein level of CTLA-4 was higher in cancer patients than in healthy donors, although this result was not statistically significant (p = 0.0596). Next, we separated cancer patients into two groups, and we found that the plasma protein levels of 4-1BB, PDL-1, PD-1, and CTLA-4 were higher in oropharyngeal cancer patients than in oral cancer patients and healthy donors (Figures 1B-1E, Kruskal–Wallis test). In contrast, the plasma protein levels of sCD27 in oropharyngeal and oral cancer patients were not different but were lower than those in healthy donors (Figure 1F, Kruskal–Wallis test).
3.3. Soluble immune checkpoint protein levels in different stages of cancer
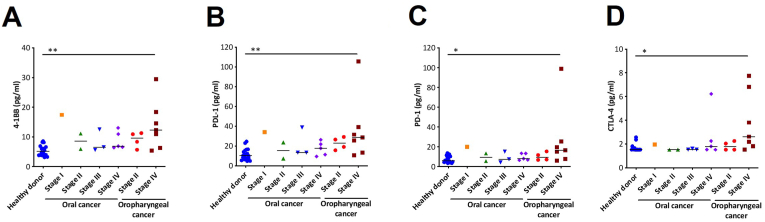
To focus on the progression of cancer, we separated cancer patients by stages I–IV. The results showed that the levels of soluble immune checkpoint proteins, such as 4-1BB, PDL-1, PD-1, and CTLA-4, were higher in stage IV patients than in healthy donors (p < 0.05, Kruskal–Wallis test, data not shown), whereas the plasma protein levels of sCD27 were lower in stage IV patients than in healthy donors (p < 0.05, Kruskal–Wallis test, data not shown). Furthermore, we classified oropharyngeal and oral cancer patients based on the clinical cancer stage. We found that 4-1BB, PDL-1, PD-1, and CTLA-4 levels were significantly higher in oropharyngeal cancer stage IV patients than in stage II and oral cancer patients (p = 0.0049, 0.0054, 0.0115, and 0.0142, respectively, Kruskal–Wallis test, Figures 2A-2D). Our results suggest that these protein levels are increased in more advanced stages of cancer, particularly in oropharyngeal cancer stage IV. Of note, there were no significant correlations between each cytokine and gender or the age of diagnosis.
Figure 2.
Soluble immune checkpoint protein levels in stage I–IV oral and oropharyngeal cancer patients compared with healthy donors (A) 4-1BB, (B) PDL-1, (C) PD-1 and (D) CTLA-4; Denote: ∗p < 0.01, ∗∗p < 0.001, ∗∗∗p < 0.0001.
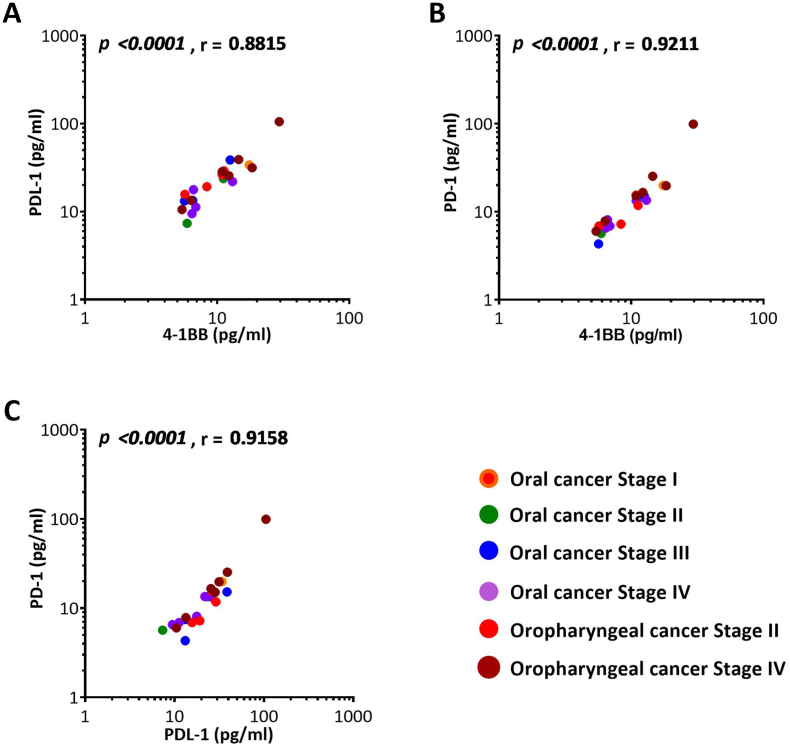
3.4. Correlation among 4-1BB, PDL-1, PD-1and CTLA-4 proteins in cancer patients
The correlation between each pair of plasma protein concentrations was examined. Among the correlations revealed by the results, we discovered that the protein levels of 4-1BB were significantly positively correlated with the level of PDL-1 (p < 0.0001, r = 0.8815, Figure 3A) and PD-1 (p < 0.0001, r = 0.9211, Figure 3B) and PDL-1 protein level was found to be positively correlated with PD-1 protein level (p < 0.0001, r = 0.9158, Figure 3C). No correlations were observed between protein levels of CTLA-4 and 4-1BB (p = 0.4871, r = 0.1564), CTLA-4 and PD-1 (p = 0.3550, r = 0.2071), CTLA-4 and PDL-1 (p = 0.2858, r = 0.2382).
Figure 3.
Correlation between soluble checkpoint protein levels of 4-1BB, PDL-1, and PD-1 in patients with different stages of oral and oropharyngeal cancers (A) 4-1BB and PDL-1, (B) 4-1BB and PD-1, and (C) PDL-1 and PD-1.
3.5. Confirmation of 4-BB by Western blot
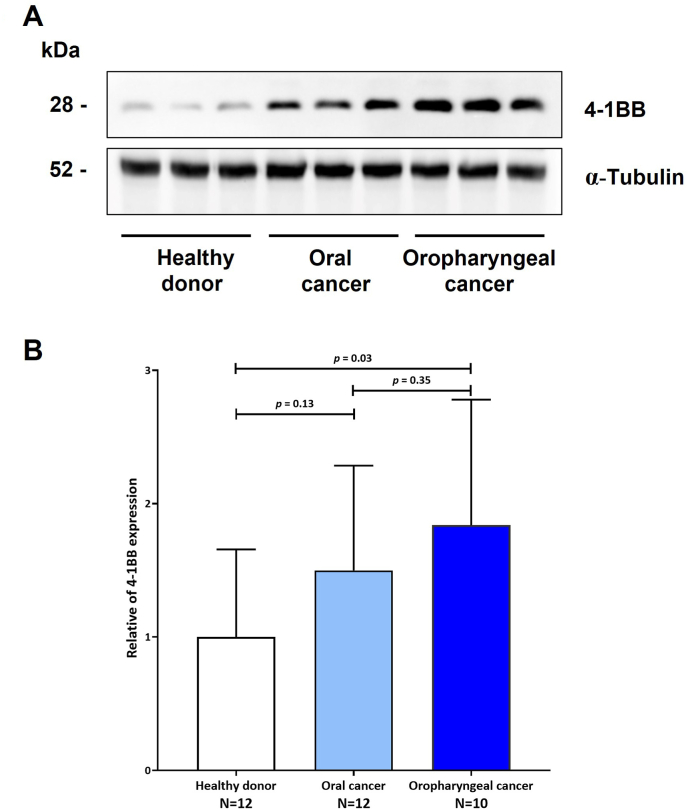
As the 4-1BB results were the most significant. We performed a Western blot to confirm the concentration of 4-1BB in plasma. We discovered that 41BB expression was elevated in oral and oropharyngeal cancer patients compared to healthy donors (Figures 4A, 4B, and S1). Only when the 4-1BB level was compared between healthy donors and oropharyngeal cancer patients was a significant difference discovered (p = 0.03).
Figure 4.
Western blot of plasma proteins probed with anti 4-1BB (4) 4-1BB protein increased in plasma of oral and oropharyneal cancer patients compared with healthy donors. Blots were reprobed with anti α-tubulin to confirm the equal loading of samples. (B) Comparison histograms, experiments were performed in triplicate and data are expressed as the mean. ± standard deviation.
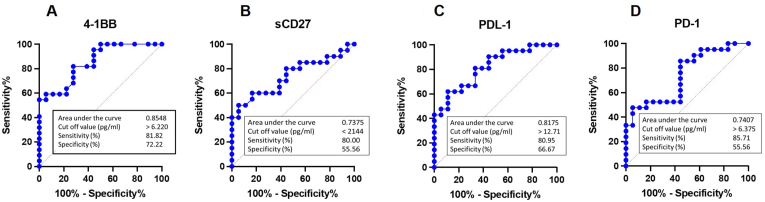
3.6. Utilize the concentration of soluble immune checkpoint proteins as diagnostic indicators
Since our data indicated that 4-1BB, sCD27, PDL-1, and PD-1 have the highest significance, we selected these as possible diagnostic markers for further evaluation. As illustrated in Figure 5, the sensitivity of four markers ranged between 80.00 and 85.71%. In spite of this, the 4-1BB has greater specificity and area under the curve. With a cut-off value of >6.22 pg/ml, plasma 4-1BB has a high percentage of sensitivity (81.82%) and specificity (72.22%) for the detection of patients with oral and/or oropharyngeal cancers in plasma samples. This suggests that this marker might be developed as an alternative screening tool.
Figure 5.
Receiver operating characteristic Curve Analysis of plasma proteins for oral and oropharyngeal cancers screening (A) 4-1BB, (B) sCD27, (C) PDL-1 (D) PD-1.
4. Discussion
In these studies, we determined the levels of cytokines and immune checkpoint proteins in the plasma of oropharyngeal and oral cancer patients. For human cytokines, we observed that the plasma IL-6 level in cancer patients was marginally greater than that in healthy donors (p = 0.07). IL-6 is known to induce tumor progression and metastasis through IL-6 trans-signaling. Previous studies revealed that the plasma level of IL-6 in patients with oral squamous cell carcinoma (OSCC) was significantly elevated and correlated with tumor stage (Chang et al., 2013; Vinícius LA et al., 2019). In contrast, we discovered that the plasma level of IL-13 was significantly lower in cancer patients compared to healthy donors (p < 0.03). IL-13 is an immunosuppressive cytokine secreted by immune and cancer cells and is most well-studied in allergic asthma. Similar to the results of a previous study, which found that the serum level of IL-13 was significantly lower in cancer patients than in healthy donors (Saigusa et al., 2014). Our study discovered a similar trend, but with significant differences. IL-13 therefore requires additional research in larger populations to clarify this matter.
Investigation the Human Immune Checkpoint Panel 12-plex in oropharyngeal and oral cancer patients, we found that the levels of 4-1BB, PD-L1, PD-1, and CTLA-4 were higher in cancer patients than those in healthy donors (p < 0.05). In addition, patients with oropharyngeal cancer had higher levels than those with oral cancer. We observed elevated plasma PD-1 levels comparable to those reported for soluble PD-1 in the blood of melanoma and hepatoma patients (Cheng et al., 2014; Ugurel et al., 2020). PD-1 and PD-L1 plasma levels were elevated, particularly in patients with stage IV oropharyngeal cancer, indicating progression of the disease. These findings are comparable to those indicating a correlation between elevated serum PD-L1 levels and hepatoma stage (Finkelmeier et al., 2016).
CTLA-4, which is also known as CD152, is an immune regulatory receptor, and the soluble form sCTLA-4 is a negative signal of CTLA-4 that downregulates T cells. A number of studies demonstrated a correlation between CTLA-4 and poor cancer patient survival (Zhao et al., 2018). Similar to our findings, plasma CTLA-4 levels were elevated in cancer patients, particularly those with oropharyngeal stage IV.
T-cell activation is linked to CD27, a type I transmembrane protein. We discovered that the plasma sCD27 levels of cancer patients were lower than those of healthy donors (p = 0.0115). Huang et al. discovered that healthy donors have a greater serum CD27 concentration than patients with prostate cancer (Huang et al., 2013), which is consistent with our findings. We believe that diminished CD27 levels are the result of T-cell exhaustion.
4-1BB, also known as CD137, prevents activation-induced cell death in CD8 T cells. Few investigations have evaluated the amounts of the protein 4-1BB in lung tumors, lymphoma cells, and pancreatic cancer cells, among others (Zhang et al., 2007; Ho et al., 2013; Glorieux and Huang, 2019). Our study is the first one to our knowledge to indicate that plasma 4-1BB levels are higher in oral and oropharyngeal cancer patients than in healthy donors and are highest in stage IV oropharyngeal carcinoma. The interaction of 4-1BB with 4-1BB ligand has the potential to restore effector function. 4-1BB inhibitor should be considered as a drug of choice for cancer patients. On the other hand, the 4-1BB antibody known as "urelumab" has been attributed to patients experiencing liver damage (Chester et al., 2018). Besides immunotherapy, the results of ROC curve analysis suggest that high plasma 4-1BB levels in cancer patients may be developed as an alternative screening marker. Due to the small number of people who took part in our study, it is clear that more research is needed with a larger sample size.
Interestingly, we observed a strong positive correlation between PD-L1 and PD-1 levels, PDL-1 and 4-1BB levels, and PD-1 and 4-1BB levels in cancer patients. These immune checkpoint molecules are assumed to share a common origin, as they regulate PD-1, PD-L1, and 4-1BB in people with oral and oropharyngeal cancer. As a toxicity of 4-1BB inhibitor, combining more than one cancer immunotherapy drug, such as PD-1 with CTLA-4 (Shen et al., 2018), or PD-1 with 4-1BB (Shindo et al., 2015; Perez-Ruiz et al., 2017), PDL-1 with 4-1BB (Lin et al., 2018; Song et al., 2018), may improve the safety and efficacy of cancer therapies and is currently being explored.
In conclusion, this research provided descriptive information about the immune system's role in oral and oropharyngeal cancer. Further studies are required to provide a detailed characterization of immune checkpoint molecules, particularly 4-1BB, and the underlying molecular mechanisms, in order to develop it as an alternative screening marker and improve immunotherapies' effectiveness.
Declarations
Author contribution statement
Supranee Buranapraditkun: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Areeya Diloktaweewattana; Narumol Bhummaphan; Chuta Siriwattanakankul; Fardeela Bin-Alee: Performed the experiments.
Patnarin Mahattanasakul; Nakarin Kitkumthorn: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools, or data; Wrote the paper.
Napadol Tangjaturonrasme; Apiwat Mutirangura: Contributed reagents, materials, analysis tools, or data.
Funding statement
This study was supported by the Emerging Infectious Diseases and Vaccines Cluster, Ratchadapisek Sompoch Endowment Fund, Chulalongkorn University (764002-HE04) and the Second Century Fund (C2F), Chulalongkorn University.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Figure S1.
References
- Arduino P.G., Carrozzo M., Chiecchio A., et al. Clinical and histopathologic independent prognostic factors in oral squamous cell carcinoma: a retrospective study of 334 cases. J. Oral Maxillofac. Surg. 2008;66:1570–1579. doi: 10.1016/j.joms.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Chang K.P., Kao H.K., Wu C.C., et al. Pretreatment interleukin-6 serum levels are associated with patient survival for oral cavity squamous cell carcinoma. Otolaryngol. Head Neck Surg. 2013;148:786–791. doi: 10.1177/0194599813478573. [DOI] [PubMed] [Google Scholar]
- Cheng H.Y., Kang P.J., Chuang Y.H., et al. Circulating programmed death-1 as a marker for sustained high hepatitis B viral load and risk of hepatocellular carcinoma. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chester C., Sanmamed M.F., Wang J., et al. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. Blood. 2018;131:49–57. doi: 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- Finkelmeier F., Canli O., Tal A., et al. High levels of the soluble programmed death-ligand (sPD-L1) identify hepatocellular carcinoma patients with a poor prognosis. Eur. J. Cancer. 2016;59:152–159. doi: 10.1016/j.ejca.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Garni-Wagner B.A., Lee Z.H., Kim Y.J., et al. 4-1BB is expressed on CD45RAhiROhi transitional T cell in humans. Cell Immunol. 1996;169:91–98. doi: 10.1006/cimm.1996.0095. [DOI] [PubMed] [Google Scholar]
- Ghantous Y., Abu Elnaaj I. Global incidence and risk factors of oral cancer. Harefuah. 2017;156:645–649. [PubMed] [Google Scholar]
- Glorieux C., Huang P. Regulation of CD137 expression through K-Ras signaling in pancreatic cancer cells. Cancer Commun. 2019;39:41. doi: 10.1186/s40880-019-0386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W.T., Pang W.L., Chong S.M., et al. Expression of CD137 on Hodgkin and Reed-Sternberg cells inhibits T-cell activation by eliminating CD137 ligand expression. Cancer Res. 2013;73:652–661. doi: 10.1158/0008-5472.CAN-12-3849. [DOI] [PubMed] [Google Scholar]
- Huang J., Jochems C., Anderson A.M., et al. Soluble CD27-pool in humans may contribute to T cell activation and tumor immunity. J. Immunol. 2013;190:6250–6258. doi: 10.4049/jimmunol.1300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Chen M., Hong L., et al. Crosstalk between PD-1/PD-L1 blockade and its combinatorial therapies in tumor immune microenvironment: a focus on HNSCC. Front. Oncol. 2018;8:532. doi: 10.3389/fonc.2018.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Chikuma S., Iwai Y., et al. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nature Immunol. 2013;14:1212–1218. doi: 10.1038/ni.2762. [DOI] [PubMed] [Google Scholar]
- Perez-Ruiz E., Etxeberria I., Rodriguez-Ruiz M.E., et al. Anti-CD137 and PD-1/PD-L1 antibodies en route toward clinical synergy. Clin. Cancer Res. 2017;23:5326–5328. doi: 10.1158/1078-0432.CCR-17-1799. [DOI] [PubMed] [Google Scholar]
- Prince A., Aguirre-Ghizo J., Genden E. Head and neck squamous cell carcinoma: new translational therapies. Mt Sinai J. Med. 2010;77:684–699. doi: 10.1002/msj.20216. [DOI] [PubMed] [Google Scholar]
- Saigusa S., Tanaka K., Inoue Y., et al. Low serum interleukin-13 levels correlate with poorer prognoses for colorectal cancer patients. Int. Surg. 2014;99:223–229. doi: 10.9738/INTSURG-D-13-00259.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Fuchs H.E., et al. Cancer Statistics. CA A Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- Shen K., Cui J., Wei Y., et al. Effectiveness and safety of PD-1/PD-L1 or CTLA4 inhibitors combined with chemotherapy as a first-line treatment for lung cancer: a meta-analysis. J. Thorac. Dis. 2018;10:6636–6652. doi: 10.21037/jtd.2018.11.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo Y., Yoshimura K., Kuramasu A., et al. Combination immunotherapy with 4-1BB activation and PD-1 blockade enhances antitumor efficacy in a mouse model of subcutaneous tumor. Anticancer Res. 2015;35:129–136. [PubMed] [Google Scholar]
- Song M., Chen X., Wang L., et al. Future of anti-PD-1/PD-L1 applications: combinations with other therapeutic regimens. Chin. J. Cancer Res. 2018;30:157–172. doi: 10.21147/j.issn.1000-9604.2018.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Thompson C.B., Allison J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- Ugurel S., Schadendorf D., Horny K., et al. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann. Oncol. 2020;31:144–152. doi: 10.1016/j.annonc.2019.09.005. [DOI] [PubMed] [Google Scholar]
- Vinícius L.A., Ingrede T.S.S., José Nilson A.S., et al. Influence of interleukins on prognosis of patients with oral squamous cells carcinoma. J. Bras. Patol. Med. Lab. 2019;55:550–567. [Google Scholar]
- Wilcox R.A., Tamada K., Strome S.E., et al. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J. Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- Zhang G.B., Dong Q.M., Hou J.Q., et al. Characterization and application of three novel monoclonal antibodies against human 4-1BB: distinct epitopes of human 4-1BB on lung tumor cells and immune cells. Tissue Antigens. 2007;70:470–479. doi: 10.1111/j.1399-0039.2007.00943.x. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Yang W., Huang Y., et al. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell. Physiol. Biochem. 2018;47:721–734. doi: 10.1159/000490025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.