Abstract
Background and Objectives:
Needle-based confocal laser endomicroscopy (nCLE) is a procedure in which an AQ-Flex nCLE mini-probe is passed through an EUS-FNA needle into a pancreatic lesion to enable subsurface in vivo tissue analysis. In this study, we conducted a systematic review and meta-analysis of nCLE for the diagnosis of pancreatic lesions.
Materials and Methods:
We conducted a comprehensive search of several databases and conference proceedings, including PubMed, EMBASE, Google-Scholar, MEDLINE, SCOPUS, and Web of Science databases (earliest inception to March 2020). The primary outcomes assessed the pooled rate of diagnostic accuracy for nCLE and the secondary outcomes assessed the pooled rate of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and adverse events (AE) of nCLE to diagnose premalignant/malignant pancreatic lesions.
Results:
Eleven studies on 443 patients were included in our analysis. The pooled rate of diagnostic accuracy of EUS nCLE was 83% (95 confidence interval [CI] = 79–87; I2 = 0). The pooled rate of sensitivity, specificity, PPV and NPV of EUS nCLE was 85.29% (95% CI = 76.9–93.68; I2 = 85%), 90.49% (95% CI = 82.24–98.74; I2 = 64%), 94.15% (95% CI = 88.55–99.76; I2 = 68%), and 73.44% (95% CI = 60.16–86.72; I2 = 93%), respectively. The total AE rate was 5.41% (±5.92) with postprocedure pancreatitis being the most common AE at 2.28% (±3.73).
Conclusion:
In summary, this study highlights the rate of diagnostic accuracy, sensitivity, specificity, and PPV for distinguishing premalignant/malignant lesions. Pancreatic lesions need to be further defined with more validation studies to characterize CLE diagnosis criteria and to evaluate its use as an adjunct to EUS-FNA.
Keywords: lesions, meta-analysis, needle-based confocal laser endomicroscopy, pancreatic
INTRODUCTION
Pancreatic lesions comprise a broad spectrum of benign and malignant processes.[1] These lesions can further be characterized as cystic, solid, or mixed.[1,2,3] Pancreatic cystic lesions (PCLs) include, but are not limited to, intraductal papillary mucinous neoplasms (IPMN), serous cystic neoplasms (SCN), mucinous cystic neoplasms (MCN), and pancreatic fluid collections.[1,2,3] Most solid lesions are pancreatic ductal adenocarcinomas (PDAC), although chronic pancreatitis can sometimes mimic this.[1,2,3] Pancreatic neuroendocrine tumors (PNET) or solid pseudopapillary neoplasms (SPN) can be solid, cystic, or mixed.[1,2,3] PCLs have an estimated prevalence of 2.4%–13.5% in asymptomatic individuals.[4] The prevalence of solid pancreatic lesions is less well-defined.[5] Compared to solid lesions, the ability of conventional imaging techniques to differentiate between benign and malignant cystic lesions is limited.[6]
The current management for most solid pancreatic lesions (SPL) is surgical resection, whereas management of PCLs typically incorporates the utilization of EUS-FNA or imaging for surveillance depending on the characteristics of the cyst.[3,7,8,9] Differentiating the type of PCL is imperative when the diagnosis is unclear as misdiagnosis may adversely impact the quality of life.[3,10] The sensitivity and specificity of EUS FNA for diagnosing a PCL are variable, ranging from 63%–88% to 88%–92%, respectively.[3,11,12,13,14] Nonetheless, a significant proportion of premalignant PCLs remain undiagnosed, indicating that further investigations are warranted.[15,16]
A needle-based confocal laser endomicroscopy (nCLE) procedure has been developed for the evaluation of pancreatic lesions. In this procedure, an AQ-Flex nCLE mini-probe (Cellvizio; Mauna Kea Technologies, Paris, France) is passed through a 19-G EUS-FNA needle into a pancreatic lesion to enable subsurface imaging of the mucosa for in vivo tissue analysis.[17,18] The technique was first described in 2011 by Konda et al.[20] Several trials have been conducted thereafter with encouraging results.[19,20,21]
The current data regarding nCLE are limited and has varying results. We performed a systematic review and meta-analysis on the diagnostic accuracy of nCLE and its sensitivity and specificity on detecting premalignant/malignant lesions.
METHODS
Search strategy
A comprehensive search from multiple databases and conference proceedings was conducted, including PubMed, EMBASE, MEDLINE, SCOPUS, Cochrane, Web of Science, and Google Scholar from earliest inception to April 2020. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed to identify studies utilizing nCLE for pancreatic lesions[22] Supplementary Figure 1 (387.4KB, tif) ].
Literature search keywords consisted of a combination of “needle-based,” “confocal,” “laser,” “endomicroscopy,” “nCLE,” “pancreatic,” “cysts,” “lesions,” “masses,” “Cellvizio,” “endoscopic” and “ultrasound.” The literature search was isolated to studies on human subjects from peer-reviewed journals. Titles and abstracts from each study were reviewed by two authors (SS, BD) independently and excluded them if our research question was not fulfilled, per prespecified inclusion and exclusion criteria. Further review of the studies was conducted to ascertain relevant information. A third author (SD) reviewed any study that may have had any discrepancies for resolution.
We reviewed the bibliographic sections from the articles of interest for any additional studies.
Study selection
We included studies that evaluated nCLE for pancreatic lesions in our meta-analysis. We included studies regardless of their geographical location, inpatient/outpatient setting, or abstract/manuscript status as long there was relevant information that could be extracted for analysis.
The exclusion criteria included: (1) ages <18 years,(2) sample size <10, and (3) studies published in languages other than English, (4) probe-based confocal laser endomicroscopy (pCLE), (5) pregnant women, and (6) prisoners
In the setting where publications contained either the same or overlapping cohort, data from the most comprehensive or recent study were included in our analysis.
Data abstraction and quality assessment
Information regarding study-related outcomes from each study was abstracted onto a standardized form by three authors (SS, BD, AD) and quality scoring was reported by two authors (SS, BD) independently.
To assess the quality of our studies, we utilized the quality assessment of diagnostic accuracy studies tool (QUADAS-2)[23] [Supplementary Table 1 (514.1KB, tif) ].
Outcomes assessed
Primary outcome
Pooled rate of diagnostic accuracy for nCLE.
Secondary outcomes
Pooled rate of sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of nCLE to diagnose premalignant/malignant pancreatic lesions
Pooled rate of adverse events (AEs) for nCLE
Pooled rate of adverse event subtypes: pancreatitis, intracystic bleeding, abdominal pain, and infection.
Definitions
Definition of outcomes
The pooled rate of diagnostic accuracy of nCLE was defined as the total number of lesions diagnosed by nCLE out of the total number of lesions sampled from the final diagnosis cohort.[20,21,24,25,26,27,28,29,30] Final diagnosis was taken under consideration by the agreement of a multidisciplinary team through a combination of histology from surgical specimens, cross-sectional imaging, observation for 6–12 months, cytology from EUS FNA, and cyst fluid analysis (CEA, amylase).
Premalignant/malignant included MCN, IPMN, PDAC, PNET, SPN, and cystic lymphoma.
Benign lesions included chronic pancreatitis, SCN, PC, retention cyst, epidermoid cyst, lymphoepithelial cyst, and congenital pancreatic cyst.
AEs were defined as complications which were directly related to the nCLE procedure.
Statistical analysis
We utilized the random-effects model, which is a meta-analysis technique suggested by DerSimonian and Laird to assess the pooled outcomes of interest.[31] As adverse event values of zero occurred in our data and we wished to provide an accurate representation of mean events that included zeroes, we constructed syntax to calculate the weighted mean to avoid introducing positive bias to the analysis. The Cochran Q statistical test and I2 statistics were utilized to assess heterogeneity between study-specific estimates.[32,33] In addition to the traditional 95% confidence intervals (CIs) calculated based on the random-effects model, we also provided prediction intervals (PIs) for the estimated total effects as suggested by Riley et al.[34] Heterogeneity was described as low, moderate, substantial, and considerable according to the values of <30%, 30%–60%, 61%–75%, and >75%, respectively.[35] Publication bias was qualitatively assessed visually with a funnel plot and quantitatively via the Luis Furuya-Kanamori (LFK) index and Doi plot.[36] We assessed potential bias after the removal of studies leading to LFK asymmetry and then conducted a sensitivity analysis by recalculating the statistics. The study would be removed from our analysis if the sensitivity analysis impacted the outcomes. All meta-analyses were performed using MetaXL software (v 3.5; EpiGear International Pty Ltd.; Queensland, Australia), and the exact 95% CIs for study accuracy were estimated using the Clopper–Pearson exact method implemented in the <PropCIs> package in R (v 3.6.1; Vienna, Austria).
Author disclosures
Conflicts of interest were disclosed by several authors reporting on nCLE.[20,21,27,28,37] Nakai et al. disclosed competing interests with Mauna Kea Technologies, Cook Medical, and Novartis. Cheesman et al. disclosed competing interests with Olympus, Boston Scientific, Medtronic, Apollo Endosurgery, Gyrus Acmi, Cook Medical, Endogastric Solutions, and US Endoscopy. Giovannini et al., Konda et al., and Napoleon et al. disclosed competing interests with Mauna Kea Technologies.
RESULTS
Search results and population characteristics
From an initial pool of 423 studies, 11 studies reported the use of EUS nCLE. Multiple studies with overlapping cohorts were found in our research, and the most appropriate ones were included in the final analysis.
Our study included 170 males (48%) and 186 females (52%) based on data available from 9 studies. The mean age was 62.86 years, based on data available from six studies. Table 1 describes the characteristics of the included studies.
Table 1.
Demographics of studies included for EUS needle-based confocal laser endomicroscopy
| Study name | Country | Type of study | Single/multi center | Manuscript/abstract | Mean age | Males | Females |
|---|---|---|---|---|---|---|---|
| Keane et al.[24] | UK | Prospective | Multi | Manuscript | - | 35 | 21 |
| Konda et al. (2013)[20] | USA/EU | Prospective | Multi | Manuscript | 63.1 | 36 | 30 |
| Kongkam et al. (2015)[25] | Thailand | Prospective | Single | Manuscript | 62.7 | 14 | 8 |
| Nakai et al. (2015)[21] | USA | Prospective | Single | Manuscript | - | 9 | 21 |
| Krishna et al. (2016)[26] | USA | Retrospective | Single | Manuscript | 54.8 | 10 | 16 |
| Napoleon et al. (2018)[27] | France | Prospective | Multi | Manuscript | - | - | - |
| Cheesman et al. (2020)[28] | USA | Retrospective | Single | Manuscript | 66 | 16 | 28 |
| Haghighi et al. (2019)[38] | USA | Retrospective | Single | Manuscript | 65.6 | 12 | 20 |
| Giovannini et al. (2016)[37] | France | Prospective | Multi | Manuscript | 65 | 18 | 14 |
| Robles-Medranda et al. (2019)[30] | Ecuador | Prospective | Single | Abstract | - | - | - |
| Senturk et al. (2018)[29] | Turkey | Prospective | Single | Abstract | - | 20 | 28 |
Characteristics and quality of included studies
The meta-analysis included 11 independent cohort studies with a total of 443 patients with 443 lesions. Nine studies had patients with single lesions and two studies had patients with multiple lesions in which the largest one was sampled. The majority of the procedures were performed via a transgastric approach. The mean cyst size, as described in 7 studies, was 50.02 mm.
None of the studies were population-based. Four studies were multicenter, and 7 studies were single center. Two studies had more than 50 patients, 7 studies had more than 30 patients, and 2 studies had more than 20 patients. Nine studies were published in manuscript form and 2 were published in abstract form. All of the included studies had clear information reporting on the diagnostic accuracy of EUS nCLE for the diagnosis of pancreatic lesions. Seven out of eleven studies reported outcomes for sensitivity, specificity, NPV, and PPV of EUS nCLE in diagnosing premalignant/malignant lesions. AEs were reported in eight studies.
Meta-analysis outcomes
Primary outcomes
The pooled rate of diagnostic accuracy of EUS nCLE for pancreatic lesions was 83% (95% CI = 79–87; I2 = 0). Figure 1 shows the forest plots for the diagnostic accuracy of nCLE.
Figure 1.
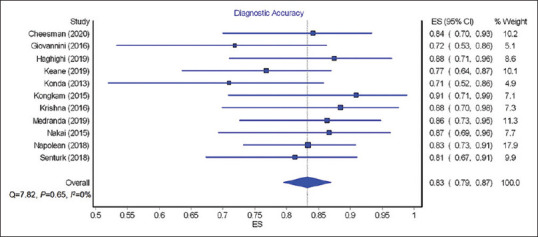
Forest plots showing diagnostic accuracy of EUS needle-based confocal laser endomicroscopy
Secondary outcomes
The calculated pooled rate of sensitivity, specificity, PPV, and NPV of EUS nCLE in diagnosing premalignant/malignant lesions was 85.29% (95% CI = 76.9–93.68; I2 = 85%), 90.49% (95% CI = 82.24–98.74; I2 = 64%), 94.15% (95% CI = 88.55–99.76; I2 = 68%), and 73.44% (95% CI = 60.16–86.72; I2 = 93%), respectively. Figures 2-5 show pooled rate of sensitivity, specificity, PPV, and NPV of EUS nCLE in diagnosing premalignant/malignant lesions. The total adverse event rate was 5.41% (±5.92) with post procedure pancreatitis being the most common adverse event at 2.28% (±3.73). Severity of pancreatitis was only reported in two studies with one case as mild, one case as moderate, and one case as severe.[20,26] AE of EUS nCLE are shown in Table 2.
Figure 2.
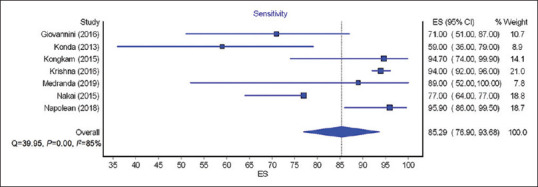
Forest plots showing the sensitivity of EUS needle-based confocal laser endomicroscopy for premalignant/malignant lesions
Figure 5.
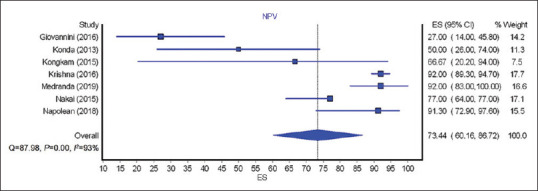
Forest plots showing the negative predictive value of EUS needle-based confocal laser endomicroscopy for premalignant/ malignant lesion
Table 2.
Adverse events of EUS needle-based confocal laser endomicroscopy
| Name of study | Total adverse events | Pancreatitis | Abdominal pain | Intracystic bleeding | Infection | Other |
|---|---|---|---|---|---|---|
| Cheesman et al. (2020)[28] | 4 | 0 | 2 | 1 | 1 | 0 |
| Giovannini et al. (2016)[37] | 0 | 0 | 0 | 0 | 0 | 0 |
| Haghighi et al. (2019)[38] | 0 | 0 | 0 | 0 | 0 | 0 |
| Keane et al.[24] | 2 | 0 | 0 | 0 | 1 | 1 |
| Konda et al. (2013)[20] | 6 | 2 | 1 | 3 | 0 | 0 |
| Kongkam et al. (2015)[25] | 1 | 0 | 0 | 0 | 0 | 1 |
| Krishna et al. (2016)[26] | 3 | 3 | 0 | 0 | 0 | 0 |
| Nakai et al. (2015)[21] | 2 | 2 | 0 | 0 | 0 | 0 |
Figure 3.
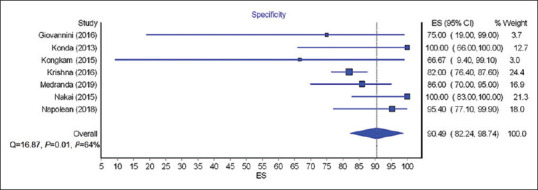
Forest plots showing the specificity of EUS needle-based confocal laser endomicroscopy for premalignant/malignant lesions
Figure 4.
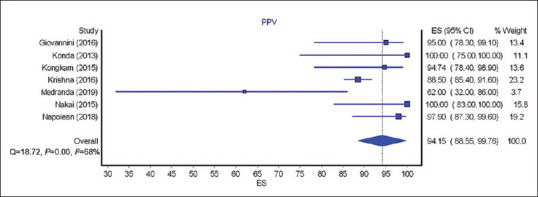
Forest plots showing the positive predictive value of EUS needle-based confocal laser endomicroscopy for premalignant/ malignant lesions
Validation of meta-analysis results
Sensitivity analysis
We excluded one study at a time to analyze its effect on the main estimate. On this analysis, no single study significantly affected the outcome or the heterogeneity.
Heterogeneity
Based on Q statistics, and I 2 analysis for heterogeneity, no heterogeneity was noted in the analysis for diagnostic accuracy of EUS nCLE. Considerable heterogeneity was noted in analysis of sensitivity and NPV and substantial heterogeneity was noted in analysis of specificity and PPV.
Publication bias
Potential publication bias was evident based on the funnel plot, Doi Plot, and LFK index. Sensitivity analysis by removing asymmetric studies revealed the possibility of publication bias, but this did not lead to a statistical change in the calculated estimate or the conclusion of this meta-analysis. Although it should be noted that the ability to detect bias is limited.
DISCUSSION
In this meta-analysis, the pooled diagnostic accuracy rate of nCLE was 83%, which is comparable to EUS-FNA.[39,40,41,42] nCLE provides in vivo visualization of PCLs and acts as an adjunct to conventional diagnostic modalities such as EUS FNA. However, nCLE is limited by prolonged procedure time (the procedure records at 12 frames/s, so 2–5 min of video recording is needed to make certain the lesion undergoes appropriate evaluation) and limited visualization of a pancreatic lesion secondary to significantly restricted maneuverability through a 19G FNA needle, anatomical abnormalities such as duodenal stenosis, or the presence of a solid lesion impeding access to the target lesion.[20,21,24,25,27,28,37,43,44,45]
The pooled sensitivity, specificity, PPV, and NPV rate of nCLE in distinguishing premalignant/malignant versus benign pancreatic lesions were 85.29%, 90.49%, 94.15%, and 73.44%, respectively. There were two studies which evaluated SPLs,[25,37] whereas the other studies evaluated PCLs.[20,21,24,26,27,28,29,30,38] EUS FNA has had high rates of false-negative or inadequate specimens reported in some studies; nCLE can aid in improving sensitivity and specificity for the diagnosis of pancreatic lesions.[46,47,48] However, high cost, the necessity of physician training for nCLE interpretation and variable interobserver agreement limit the use of this technology.[25,37,44,45,49]
The total AE rate was 5.41% with post-procedure pancreatitis being the most common AE reported at 2.28%.[20,21,26] The cause of the pancreatitis was hypothesized to be secondary to extended duration of the nCLE procedure, scope-torque while attempting to access other parts of the lesion, the FNA itself, or a combination of these factors.[20,21,26] The pooled rate of intracystic bleeding in our study was 1.14%, which was self-limited in all cases.[20,28]
There are several limitations in this study. Some studies were retrospective, which is a risk factor for selection bias. This technique may not be generalizable to an unexperienced endoscopist. The population of patients is small, including the number of surgical specimens available for criteria development for nCLE.
Analysis of diagnostic accuracy studies is an area of active research and utilizes many sophisticated models, which could not be accomplished in our study. A more robust sample size with raw data is needed to utilize these models. Our study provides summaries for sensitivity and specificity independently, which emphasizes that our findings are preliminary and hypothesis-generating for future studies.
CONCLUSION
In summary, this study highlights nCLEs high rate of diagnostic accuracy, sensitivity, specificity, and PPV for distinguishing premalignant/malignant lesions. The role of this technique in evaluating pancreatic lesions needs to be further defined. Larger validation studies are needed to further characterize CLE criteria. Future studies should evaluate nCLE as an adjunct with EUS FNA or some other modality such as through the needle microforceps biopsy or cystoscopy as a few studies have demonstrated with promising results.[21,28,30,38]
Supplementary Materials
Supplementary information is linked to the online version of the paper on the Endoscopic Ultrasound website.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Study selection process in accordance with preferred reporting items for systematic reviews and meta-analysis statement
Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2)
REFERENCES
- 1.Olah A. Pancreatic head mass: What can be done.Diagnosis: Surgery? JOP. 2000;1(Suppl 3):127–9. [PubMed] [Google Scholar]
- 2.Nassour I, Choti MA. Types of pancreatic cysts. JAMA. 2016;316:1226. doi: 10.1001/jama.2016.9035. [DOI] [PubMed] [Google Scholar]
- 3.Elta GH, Enestvedt BK, Sauer BG, et al. ACG clinical guideline: Diagnosis and management of pancreatic cysts. Am J Gastroenterol. 2018;113:464–79. doi: 10.1038/ajg.2018.14. [DOI] [PubMed] [Google Scholar]
- 4.de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806–11. doi: 10.1016/j.cgh.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 5.Santo E, Bar-Yishay I. Pancreatic solid incidentalomas. Endosc Ultrasound. 2017;6(Suppl 3):S99–103. doi: 10.4103/eus.eus_72_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brugge WR. Diagnosis and management of cystic lesions of the pancreas. J Gastrointest Oncol. 2015;6:375–88. doi: 10.3978/j.issn.2078-6891.2015.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiarelli M, De Simone M, Terragn S, et al. Asymptomatic pancreatic masses: An overview. Clin Surg. 2018;3:1947. [Google Scholar]
- 8.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 9.European Study Group on Cystic Tumours of the P. European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 2018;67:789–804. doi: 10.1136/gutjnl-2018-316027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang QX, Xiao J, Orange M, et al. EUS-guided FNA for diagnosis of pancreatic cystic lesions: A Meta-analysis. Cell Physiol Biochem. 2015;36:1197–209. doi: 10.1159/000430290. [DOI] [PubMed] [Google Scholar]
- 11.Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 12.Wu QM, Guo YN, Xu YQ. Diagnostic performance of endoscopic ultrasound-guided fine-needle aspiration in pancreatic lesions. Eur Rev Med Pharmacol Sci. 2018;22:1397–401. doi: 10.26355/eurrev_201803_14485. [DOI] [PubMed] [Google Scholar]
- 13.Nigam N, Rastogi A, Bhatia V, et al. EUS-guided FNA in diagnosing pancreatic lesions: Strength and cytological spectrum. J Cytol. 2019;36:189–95. doi: 10.4103/JOC.JOC_5_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ASGE Standards of Practice Committee. Muthusamy VR, Chandrasekhara V, et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest Endosc. 2016;84:1–9. doi: 10.1016/j.gie.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Coban S, Brugge W. EUS-guided confocal laser endomicroscopy: Can we use thick and wide for diagnosis of early cancer? Gastrointest Endosc. 2020;91:564–7. doi: 10.1016/j.gie.2019.10.028. [DOI] [PubMed] [Google Scholar]
- 16.Kohoutova D, Zar S, Repak R, et al. Pancreatic cysts: Diagnostic role of EUS-guided microforceps biopsy and confocal laser endomicroscopy. Gastroenterol Res Pract. 2019;2019:3431048. doi: 10.1155/2019/3431048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004;127:706–13. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 18.Bhutani MS, Koduru P, Joshi V, et al. EUS-guided needle-based confocal laser endomicroscopy: A Novel technique with emerging applications. Gastroenterol Hepatol (N Y) 2015;11:235–40. [PMC free article] [PubMed] [Google Scholar]
- 19.Anand K, Kahaleh M, Tyberg A. Use of needle-based confocal laser endomicroscopy in the diagnosis and management of pancreatic cyst lesions. Endosc Ultrasound. 2018;7:306–9. doi: 10.4103/eus.eus_46_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konda VJ, Meining A, Jamil LH, et al. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006–13. doi: 10.1055/s-0033-1344714. [DOI] [PubMed] [Google Scholar]
- 21.Nakai Y, Iwashita T, Park DH, et al. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204–14. doi: 10.1016/j.gie.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269, W264. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 23.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 24.Keane MG, Wehnert N, Perez-Machado M, et al. A prospective trial of CONfocal endomicroscopy in CYSTic lesions of the pancreas: CONCYST-01. Endosc Int Open. 2019;7:E1117–22. doi: 10.1055/a-0957-2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kongkam P, Pittayanon R, Sampatanukul P, et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for diagnosis of solid pancreatic lesions (ENES): A pilot study. Endosc Int Open. 2016;4:E17–23. doi: 10.1055/s-0034-1393183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishna SG, Swanson B, Hart PA, et al. Validation of diagnostic characteristics of needle based confocal laser endomicroscopy in differentiation of pancreatic cystic lesions. Endosc Int Open. 2016;4:E1124–35. doi: 10.1055/s-0042-116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Napoleon B, Palazzo M, Lemaistre AI, et al. Needle-based confocal laser endomicroscopy of pancreatic cystic lesions: A prospective multicenter validation study in patients with definite diagnosis. Endoscopy. 2019;51:825–35. doi: 10.1055/a-0732-5356. [DOI] [PubMed] [Google Scholar]
- 28.Cheesman AR, Zhu H, Liao X, et al. Impact of EUS-guided microforceps biopsy sampling and needle-based confocal laser endomicroscopy on the diagnostic yield and clinical management of pancreatic cystic lesions. Gastrointest Endosc. 2020;91:1095–104. doi: 10.1016/j.gie.2019.12.022. [DOI] [PubMed] [Google Scholar]
- 29.Senturk H, Köker IH, Keskin EB, et al. Su1345-needle based confocal laser endomicroscopy examination of pancreatic cysts: Single center real life results. Gastroenterology. 2018;154:S–528. [Google Scholar]
- 30.Robles-Medranda C, Olmos JI, Oleas R, et al. Tu1399 eus-through-the-needle technologies in the diagnosis and malignancy detection of pancreatic cysts: A comparative study between different technologies. Gastrointestinal Endosc. 2019;89:AB608–9. [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanwal F, White D. Systematic reviews and meta-analyses in clinical gastroenterology and hepatology. Clinical Gastroenterology Hepatology. 2012;10:1184–6. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. doi: 10.1136/bmj.d549. [DOI] [PubMed] [Google Scholar]
- 35.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence-inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Furuya-Kanamori L, Barendregt JJ, Doi SA. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int J Evid Based Healthc. 2018;16:195–203. doi: 10.1097/XEB.0000000000000141. [DOI] [PubMed] [Google Scholar]
- 37.Giovannini M, Caillol F, Monges G, et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy in solid pancreatic masses. Endoscopy. 2016;48:892–8. doi: 10.1055/s-0042-112573. [DOI] [PubMed] [Google Scholar]
- 38.Haghighi M, Sethi A, Tavassoly I, et al. Diagnosis of pancreatic cystic lesions by virtual slicing: Comparison of diagnostic potential of needle-based confocal laser endomicroscopy versus endoscopic ultrasound-guided fine-needle aspiration. J Pathol Inform. 2019;10:34. doi: 10.4103/jpi.jpi_32_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammad Alizadeh AH, Shahrokh S, et al. Diagnostic potency of EUS-guided FNA for the evaluation of pancreatic mass lesions. Endosc Ultrasound. 2016;5:30–4. doi: 10.4103/2303-9027.175879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oguz D, Oztas E, Kalkan IH, et al. Accuracy of endoscopic ultrasound-guided fine needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions: a single-center experience? J Dig Dis. 2013;14:132–139. doi: 10.1111/1751-2980.12014. PMID: 23167591. DOI: 10.1111/1751-2980.12014. [DOI] [PubMed] [Google Scholar]
- 41.Sugiura R, Kuwatani M, Hirata K, et al. Effect of pancreatic mass size on clinical outcomes of endoscopic ultrasound-guided fine-needle aspiration. Dig Dis Sci. 2019;64:2006–13. doi: 10.1007/s10620-018-5435-3. [DOI] [PubMed] [Google Scholar]
- 42.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 43.Alvarez-Sánchez MV, Napoléon B. New horizons in the endoscopic ultrasonography-based diagnosis of pancreatic cystic lesions. World J Gastroenterol. 2018;24:2853–66. doi: 10.3748/wjg.v24.i26.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karia K, Waxman I, Konda VJ, et al. Needle-based confocal endomicroscopy for pancreatic cysts: The current agreement in interpretation. Gastrointest Endosc. 2016;83:924–7. doi: 10.1016/j.gie.2015.08.080. [DOI] [PubMed] [Google Scholar]
- 45.Krishna SG, Hart PA, DeWitt JM, et al. EUS-guided confocal laser endomicroscopy: Prediction of dysplasia in intraductal papillary mucinous neoplasms (with video) Gastrointest Endosc. 2020;91:551–630. doi: 10.1016/j.gie.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Facciorusso A, Del Prete V, Antonino M, et al. Diagnostic yield of EUS-guided through-the-needle biopsy in pancreatic cysts: A meta-analysis. Gastrointest Endosc. 2020;92:1–8000. doi: 10.1016/j.gie.2020.01.038. [DOI] [PubMed] [Google Scholar]
- 47.Woolf KM, Liang H, Sletten ZJ, et al. False-negative rate of endoscopic ultrasound-guided fine-needle aspiration for pancreatic solid and cystic lesions with matched surgical resections as the gold standard: one institution's experience. Cancer Cytopathol. 2013;121:449–58. doi: 10.1002/cncy.21299. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Iwai T, Kida M, et al. Analysis of the diagnostic yield of endoscopic ultrasonography-guided fine-needle aspiration in patients with a suspected pancreatic malignancy. Rev Esp Enferm Dig. 2018;110:544–50. doi: 10.17235/reed.2018.5455/2017. [DOI] [PubMed] [Google Scholar]
- 49.Krishna SG, Lee JH. Appraisal of needle-based confocal laser endomicroscopy in the diagnosis of pancreatic cysts. World J Gastroenterol. 2016;22:1701–10. doi: 10.3748/wjg.v22.i4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study selection process in accordance with preferred reporting items for systematic reviews and meta-analysis statement
Quality Assessment of Diagnostic Accuracy Studies tool (QUADAS-2)


