Abstract
Background and Objectives:
EUS-guided hepaticogastrostomy (EUS-HGS) is in widespread use; however, there are few dedicated devices. The B2 route is technically easier than the B3 route for guidewire insertion, dilation, and stenting but if performed with conventional oblique-viewing (OV) EUS, B2 puncture can cause transesophageal puncture and severe adverse events. The aim of this study was to assess the efficacy of forward-viewing (FV) EUS, which we have developed to improve safety for B2 puncture in EUS-HGS (B2-EUS-HGS).
Patients and Methods:
This single-center retrospective study included 61 consecutive patients who underwent B2-EUS-HGS with FV between February 2020 and March 2021 at Aichi Cancer Center, Japan. The patients were prospectively enrolled, and clinical data were retrospectively collected for these 61 cases.
Results:
The overall technical success rate of EUS-HGS was 98.3% (60/61). The rate of EUS-HGS with FV was 95.0% (58/61) after three cases converted to OV, and that of B2-EUS-HGS with FV was 88.5% (54/61). The early adverse event rate was 6.5% (4/61). There were no instances of transesophageal puncture. Median procedure time was 24 min (range, 8–70), and no patient required cautery dilation.
Conclusions:
B2-EUS-HGS can be performed safely using FV, without transesophageal puncture, and supportability of the device is improved as FV is coaxial with the guidewire. FV was efficacious in B2-EUS-HGS, which shows promise for clinical application in the future.
Keywords: EUS, Interventional EUS, EUS-guided hepaticogastrostomy, EUS-guided biliary drainage, Biliary stricture
INTRODUCTION
EUS-guided hepaticogastrostomy (EUS-HGS) is a commonly used technique that is in widespread use worldwide. Recently, EUS-HGS has been used not only as alternative drainage for failed ERCP cases[1,2] but also as primary drainage.[3] Despite high technical and clinical success rates, there are few dedicated devices for EUS-HGS, and severe adverse events may sometimes occur. Because the occurrence of adverse events is reported to depend on the skill of the endoscopist,[4,5] it is recommended that EUS-guided biliary drainage (EUS-BD) should be performed by expert endoscopists with sufficient clinical experience.[6,7,8] We have previously reported techniques that enable EUS-HGS to be performed more easily and safely.[9] The B2 route is technically easier than the B3 route for guidewire insertion, dilation, and antegrade stenting. However, B2 puncture with conventional oblique-viewing (OV) EUS is prone to transesophageal puncture, which may cause severe adverse events.[10,11] Therefore, the B3 route has been widely recommended.[12] To overcome the problems associated with B2 puncture, we have developed a method for B2 puncture EUS-HGS that employs forward-viewing (FV) EUS. In this retrospective study, we aimed to assess the efficacy of B2-EUS-HGS with FV EUS.
PATIENTS AND METHODS
Patients
A total of 283 consecutive patients underwent EUS-HGS between April 2009 and March 2021 at Aichi Cancer Center, Japan. Of these, B2-EUS-HGS with FV EUS was attempted in 61 consecutive patients following its introduction in February 2020. The patients were enrolled prospectively, and the clinical data of these 61 cases were collected retrospectively. All patients provided informed consent for the procedures, and the local institutional review board approved the study (approval No. 2021-1-032).
METHODS
Details of B2-EUS-hepaticogastrostomy with forward-viewing EUS
Eight endosonographers from our department performed EUS-HGS. All patients were under conscious sedation by intravenous medication during the procedure and received intravenous antibiotics prophylactically. In patients with severe ascites, ascites drainage was performed before EUS-HGS to prevent severe peritonitis[13].
B2-EUS-HGS with FV EUS is performed as follows. Using an FV EUS (TGF-UC260J, Olympus, Tokyo, Japan), a landmark clip is placed at the esophagogastric junction to enable its easy identification and hence prevent puncture of the esophagus.[3] B2 is visualized by FV EUS connected to an ultrasound device (EU-ME2, Olympus). A connector (Rotating hemostatic valve 0.096”; Abbott, Tokyo, Japan) filled with contrast medium and preloaded with a 0.018-inch guidewire (Fielder 18, Olympus) is then attached to a 22-gauge needle (Expect Slimline; Boston Scientific Co., Natick, MA, USA). Guided by color Doppler imaging to avoid vessels, B2 is punctured by the needle, which is stabilized by insertion of the guidewire. By using a connector, we can insert the guidewire first, to stabilize the needle. This technique avoids introducing air into the bile duct and enables easy re-puncture without pneumobilia.
We then inject a small amount of contrast medium. The needle track is gradually enlarged using a dilator catheter (ES dilator; Zeon Medical Co., Ltd., Tokyo, Japan or Uneven Double Lumen Cannula; PIOLAX Medical Devices, Yokohama, Japan), and contrast medium is injected to outline the biliary tree. Finally, a fully covered self-expandable metal stent (FCSEMS) (6 mm × 10 cm or 12 cm HANAROSTENT, Boston Scientific Co.) is placed through the stomach. To prevent internal stent migration, stent length is ≥10 cm.[14] For those with poor intrahepatic bile duct dilation, a plastic stent (7 Fr 14 cm Through and Pass TYPE IT, Gadelius Medical, Tokyo, Japan) is used. The distal end of the HGS stent is positioned at the peripheral side of the confluence of segments 2–3 because this location prevents the risk of B3 obstruction and focal cholangitis. During stent deployment, the scope is pushed toward the gastric wall and the stent is deployed 2 cm inside the endoscopic channel. After slowly pushing the stent delivery system, the stent opens fully in the stomach [Video 1].
If B2 puncture is difficult, we change to B3 puncture; if puncture is still difficult, we switch to an OV EUS scope (GF-UCT260, Olympus).
Computed tomography was performed from the upper abdomen to the pelvis in all patients 24 h after the procedure to confirm the absence of adverse events such as stent migration and bile leakage.
Definitions
Technical success was defined as placement of the stent into the left intrahepatic bile duct through the stomach. Clinical success was defined as the reduction of total serum bilirubin level to less than half of the preoperative level or improvement of liver dysfunction or cholangitis within 2 weeks. Procedural duration was defined as the time interval between insertion of the FV scope and completion of all procedures. We did not perform preprocedural EUS to evaluate the bile duct for puncture.
Adverse events were graded according to the American Society for Gastrointestinal Endoscopy lexicon.[15]
Statistical analysis
The primary outcomes were technical success and procedure-related adverse events of EUS-HGS. All data were analyzed using StatMate V statistical software (ATMS).
RESULTS
Patient characteristics
B2-EUS-HGS with FV EUS was attempted in 61 patients during the study period. Table 1 lists the patients' characteristics. The primary disease was malignant disease (n = 55, 90.1%). The major indications for EUS-HGS were primary BD in 41 (67.2%) and salvage drainage in 20 (32.7%) patients. Salvage cases included conversion from other drainage techniques such as percutaneous transhepatic biliary drainage or ERCP with duodenal stenosis, as well as drainage in hilar bile duct stenosis. There were no cases of cannulation failure of ERCP. Ascites were observed in 14 patients (22.9%), two of whom (3.2%) underwent drainage of ascites to prevent severe peritonitis before the EUS-HGS procedure.
Table 1.
Patient characteristics
| Age (years), median (range) | 68 (38–87) |
| Sex, males/females | 35/26 |
| Primary disease, % (n/N) | |
| Malignant disease | 90.1 (55/61) |
| Pancreatic cancer | 28 |
| Duodenal cancer | 5 |
| Gastric cancer | 4 |
| Gallbladder cancer | 4 |
| Colon cancer | 3 |
| Cholangiocellular carcinoma | 3 |
| Other | 8 |
| Benign disease | 9.8 (6/61) |
| Indication for EUS-HGS*, % (n/N) | |
| Primary drainage | 67.2 (41/61) |
| Salvage drainage | 32.7 (20/61) |
| Ascites**, % (n/N) | 22.9 (14/61) |
| <Mild | 7 |
| ≥Moderate | 7 |
| Ascites drainage before procedure | 3.2 (2/61) |
*Primary drainage was defined as the first drainage, and salvage drainage was defined as conversion from other drainage or additional drainage using another technique; **The grade of ascites was evaluated using CT images and graded as follows: Mild, limited to Morison’s pouch or the pouch of Douglas; moderate, between mild and severe; and severe, covering the abdominal organs. HGS: Hepaticogastrostomy; CT: Computed tomography
Outcomes and adverse events
Table 2 lists the details of the EUS-HGS procedure, and Table 3 lists the clinical outcomes. The technical success rate of EUS-HGS was 98.3% (60/61). The procedure was discontinued and converted to ERCP in one case due to difficult tract dilation. In 4 cases in which B2 dilation was insufficient, we changed to B3 puncture and performed EUS-HGS with FV. In 2 cases, it was difficult to visualize the B2 puncture line with FV (and thus avoid vessels) for the reasons that B2 is often visualized below the portal vein, and B2 dilation was poor in these 2 cases. Therefore, we changed to an OV scope and performed EUS-HGS. The technical success rate of EUS-HGS with FV was 95.0 (58/61) and that of B2-EUS-HGS with FV was 88.5% (54/61). The overall clinical success rate was 88.3% (53/60). Among the clinically unsuccessful cases, the general condition had worsened due to rapidly progressive advanced cancer in 4 cases, internal stent migration occurred the day after the procedure in 1 case, and there was early stent dysfunction due to kinking in 2 cases.
Table 2.
Details of EUS - hepaticogastrostomy procedure
| Procedure time (min), median (range) | 24 (8–70) |
| Puncture site, % (n/N) | |
| B2 | 93.4 (57/61) |
| B3 | 6.5 (4/61) |
| Bile duct diameter (mm), median (range) | 3 (1.1–6.9) |
| EUS scope, % (n/N) | |
| Forward-viewing | 96.7 (59/61) |
| Oblique-viewing (changed from FV) | 3.2 (2/61) |
| Needle, % (n/N) | |
| 22 G | 68.8 (42/61) |
| 19 G | 31.1 (19/61) |
| Dilator, % (n/N) | |
| Bougie | 70.4 (43/61) |
| Balloon | 29.5 (18/61) |
| Cautery | 0 (0/61) |
| Stent, % (n/N) | |
| 6 mm FCSEMS | 72.1 (44/61) |
| Plastic stent | 26.2 (16/61) |
| Discontinued before stent deployment | 1.6 (1/61) |
| Antegrade stent, % (n/N) | 11.4 (7/61) |
FCSEMS: Fully covered self-expandable metal stent; FV: Forward-viewing
Table 3.
Clinical outcomes of EUS - hepaticogastrostomy
| Technical success rate, % (n/N) | 98.3 (60/61) |
| EUS-HGS with FV | 95.0 (58/61) |
| B2-EUS-HGS with FV | 88.5 (54/61) |
| Overall clinical success rate, % (n/N) | 88.3 (53/60) |
| Early (≤30 days) adverse event rate, % (n/N) | 6.5 (4/61) |
| Early adverse events, grade | |
| Fever | 1, moderate |
| Peritonitis | 1, moderate |
| Bile leakage | 1, moderate |
| Internal stent migration | 1, moderate |
| Bleeding | 0 |
| Transesophageal puncture | 0 |
| Late (>30 days) adverse event rate, % (n/N) | 0.0 (0/61) |
HGS: Hepaticogastrostomy; FV: Forward-viewing
The rate of early adverse events was 6.5% (4/61), all of which were moderate. Of these 4 patients, one with fever was treated conservatively with intravenous antibiotics. One patient with peritonitis had ascites that required drainage and intravenous antibiotics. Although moderate ascites had been observed in this patient before EUS-HGS, it was not enough to drain. However, fever and CRP elevation occurred after the EUS-HGS procedure, and imaging examinations (computed tomography and abdominal ultrasound) revealed severe ascites with septa. Bile leakage was observed in one patient with plastic stent, and EUS-guided drainage was performed. In the fourth early adverse event, internal stent migration occurred in a patient with a 6 mm × 10 cm FCSEMS (HANAROSTENT® Benefit™, Boston Scientific Co.). The stent was inside the stomach when EUS-HGS was performed; however, CT obtained the next day revealed internal stent migration. We immediately performed transpapillary removal of the EUS-HGS stent followed by transpapillary biliary stenting. The patient was able to resume oral intake the next day and was discharged home on day 5 after EUS-HGS. As the HANAROSTENT® Benefit™ is very soft, it can easily bend between the stomach and liver, and internal stent migration can easily occur. After this experience, we stopped using HANAROSTENT® Benefit™ for EUS-HGS. Transesophageal puncture was not observed, and there were no fatalities.
Cases of early stent dysfunction are shown in Table 4. Early stent dysfunction was seen in two patients, both of whom had stent kinking. In the first patient, kinking of the plastic stent (TYPE IT™, Gadelius Medical Co, Ltd., Tokyo, Japan) occurred in the stomach at 3 days after the EUS-HGS procedure. Because the fistula had not yet matured, we performed additional transpapillary biliary stenting. In the second patient, the onset of early stent dysfunction was on day 17, after the fistula had matured, and so we exchanged the EUS-HGS stent.
Table 4.
Early stent dysfunction
| Early (≤30 days) stent dysfunction rate, % (n/N) | 3.3 (2/60) |
| Kinking | 2 |
| Obstruction | 0 |
| Dislocation | 0 |
| Stent deviation into the esophagus | 0 |
DISCUSSION
In this study, we evaluated the technical success and clinical outcomes for B2-EUS-HGS with FV. Giovannini et al.[16] first reported the feasibility of EUS-BD in 2001, and Burmester et al.[17] first described intrahepatic bile duct puncture (i.e., EUS-HGS) through the gastric approach in 2003. Numerous studies since then had reported the efficacy of EUS-HGS, which is now in widespread use. Dedicated stents have been developed to simplify the procedure and prevent adverse events;[18,19,20] however, there are still few dedicated devices for EUS-HGS. Many devices designed for ERCP are also used for EUS-BD, and the endoscope manipulation and puncturing techniques are similar to EUS-guided fine needle aspiration (EUS-FNA); therefore, it is recommended that EUS-BD should be performed by expert endoscopists with sufficient clinical experience in both ERCP and EUS-FNA.[7] One of the reasons for the difficulty of EUS-HGS is B3 puncture. B3 anatomically crosses the portal vein radicle, where it has a shoulder that increases the difficulty of guidewire negotiation, dilation of the route, and stent insertion. B2 puncture is recommended in EUS-guided rendezvous technique (EUS-RV) because it is easier to advance the guidewire toward the hepatic hilum with this method compared with B3 puncture.[21] However, it has been reported that transesophageal puncture can occur unexpectedly in EUS-RV.[22] Due to the importance of avoiding transesophageal puncture during dilation and stent placement in EUS-HGS, the B3 route has been widely recommended. In the present study, we used FV to avoid the risks associated with B2 puncture and found that B2-EUS-HGS with FV was possible in 54/61 patients (88.5%) with no cases of transesophageal puncture. As the range of up-angulation is wider with FV than with other scopes, B2 can be visualized and punctured by up-angulation with the FV scope inside the stomach [Figure 1]. In addition, the shorter rigid portion of the FV eliminates the gap between the position of the instrument channel and the puncture position. These two features of FV greatly reduce the risk of transesophageal puncture during B2 puncture. We have reported the usefulness of FV previously for application to EUS-guided choledochoduodenostomy (EUS-CDS)[23,24] but not EUS-HGS.
Figure 1.
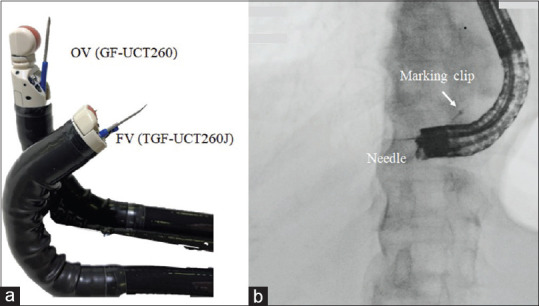
(a) Photograph showing OV (GF-UCT260) and FV (TGF-UC260J) EUS scopes. The range of up-angulation is 130° for GF-UCT260, whereas that for TGF-UC260J is 180°; (b) Fluoroscopic image of B2-EUS-HGS with FV shows that B2 puncture is possible from inside the stomach using up-angulation with FV
Another advantage of FV is the straight channel port. Reduced resistance provides greater puncture force and increased control of devices. This feature of FV has been reported in its application to diagnostic EUS-FNA.[25,26] FV assists in efficient transfer power because it has the same axis as the guidewire.
We have previously reported an association of the use of cautery dilation with bleeding in EUS-BD.[27] It is noteworthy that none of the present patients required cautery dilation. Another study that compared cautery and mechanical dilation found no significant difference in terms of technical success rates: procedure-related bleeding developed in 6 (18%) patients with cautery dilation and in none during mechanical dilation (P = 0.04).[28] Because FV EUS is coaxial with the guidewire, device supportability is improved and the opportunities for cautery dilation in EUS-HGS are reduced; therefore, FV may facilitate the HGS procedure and reduce adverse events such as bleeding.
FV also has some disadvantages. The scanning range is 90°, which is half that of OV. In the present study, we changed the scope from FV to OV for two patients. Normally, B3 is detected above the portal vein, but B2 is often detected below the portal vein. Therefore, in cases of poor B2 bile duct dilation, with FV, it may be difficult to detect the puncture line that can avoid the portal vein. Despite these disadvantages, the use of FV is highly advantageous in EUS-HGS.
The limitations of our study are its retrospective design and that it was performed at a single center with a small number of patients. In addition, we did not include a control group.
CONCLUSIONS
We demonstrated the efficacy of B2-EUS-HGS with FV. FV has the advantages of enabling B2 puncture to be performed be done safely and simply without transesophageal puncture and of improved device supportability because FV is coaxial with the guidewire. B2-EUS-HGS with FV shows promise for clinical application in the future.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Video Available on: www.eusjournal.com
REFERENCES
- 1.Park DH, Jang JW, Lee SS, et al. EUS-guided biliary drainage with transluminal stenting after failed ERCP: Predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276–84. doi: 10.1016/j.gie.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 2.De Cassan C, Bories E, Pesenti C, et al. Use of partially covered and uncovered metallic prosthesis for endoscopic ultrasound-guided hepaticogastrostomy: Results of a retrospective monocentric study. Endosc Ultrasound. 2017;6:329–35. doi: 10.4103/2303-9027.209869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okuno N, Hara K, Mizuno N, et al. Efficacy of the 6-mm fully covered self-expandable metal stent during endoscopic ultrasound-guided hepaticogastrostomy as a primary biliary drainage for the cases estimated difficult endoscopic retrograde cholangiopancreatography: A prospective clinical study. J Gastroenterol Hepatol. 2018;33:1413–21. doi: 10.1111/jgh.14112. [DOI] [PubMed] [Google Scholar]
- 4.Vila JJ, Pérez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: A Spanish national survey. Gastrointest Endosc. 2012;76:1133–41. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Oh D, Park DH, Song TJ, et al. Optimal biliary access point and learning curve for endoscopic ultrasound-guided hepaticogastrostomy with transmural stenting. Therap Adv Gastroenterol. 2017;10:42–53. doi: 10.1177/1756283X16671671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hara K, Yamao K, Mizuno N, et al. Endoscopic ultrasonography-guided biliary drainage: Who, when, which, and how? World J Gastroenterol. 2016;22:1297–303. doi: 10.3748/wjg.v22.i3.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isayama H, Nakai Y, Itoi T, et al. Clinical practice guidelines for safe performance of endoscopic ultrasound/ultrasonography-guided biliary drainage: 2018. J Hepatobiliary Pancreat Sci. 2019;26:249–69. doi: 10.1002/jhbp.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paik WH, Park DH. Outcomes and limitations: EUS-guided hepaticogastrostomy. Endosc Ultrasound. 2019;8:S44–9. doi: 10.4103/eus.eus_51_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hara K, Okuno N, Haba S, et al. How to perform EUS-guided hepaticogastrostomy easier and safer. J Hepatobiliary Pancreat Sci. 2020;27:563–4. doi: 10.1002/jhbp.774. [DOI] [PubMed] [Google Scholar]
- 10.Okuno N, Hara K, Mizuno N, et al. Risks of transesophageal endoscopic ultrasonography-guided biliary drainage. Gastrointest Interv. 2017;6:82–4. [Google Scholar]
- 11.Ogura T, Higuchi K. Technical tips for endoscopic ultrasound-guided hepaticogastrostomy. World J Gastroenterol. 2016;22:3945–51. doi: 10.3748/wjg.v22.i15.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogura T, Higuchi K. Endoscopic ultrasound-guided hepaticogastrostomy: Technical review and tips to prevent adverse events. Gut Liver. 2021;15:196–205. doi: 10.5009/gnl20096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okuno N, Hara K, Mizuno N, et al. Infectious peritonitis after endoscopic ultrasound-guided biliary drainage in a patient with ascites. Gastrointest Interv. 2018;7:40–3. [Google Scholar]
- 14.Okuno N, Hara K, Mizuno N, et al. Stent migration into the peritoneal cavity following endoscopic ultrasound-guided hepaticogastrostomy. Endoscopy. 2015;47(Suppl 1 UCTN):E311. doi: 10.1055/s-0034-1392314. [DOI] [PubMed] [Google Scholar]
- 15.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 16.Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: A new technique for biliary drainage. Endoscopy. 2001;33:898–900. doi: 10.1055/s-2001-17324. [DOI] [PubMed] [Google Scholar]
- 17.Burmester E, Niehaus J, Leineweber T, et al. EUS-cholangio-drainage of the bile duct: Report of 4 cases. Gastrointest Endosc. 2003;57:246–51. doi: 10.1067/mge.2003.85. [DOI] [PubMed] [Google Scholar]
- 18.Park DH, Koo JE, Oh J, et al. EUS-guided biliary drainage with one-step placement of a fully covered metal stent for malignant biliary obstruction: A prospective feasibility study. Am J Gastroenterol. 2009;104:2168–74. doi: 10.1038/ajg.2009.254. [DOI] [PubMed] [Google Scholar]
- 19.Umeda J, Itoi T, Tsuchiya T, et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos) Gastrointest Endosc. 2015;82:390–6.e2. doi: 10.1016/j.gie.2015.02.041. [DOI] [PubMed] [Google Scholar]
- 20.Park DH, Lee TH, Paik WH, et al. Feasibility and safety of a novel dedicated device for one-step EUS-guided biliary drainage: A randomized trial. J Gastroenterol Hepatol. 2015;30:1461–6. doi: 10.1111/jgh.13027. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchiya T, Itoi T, Sofuni A, et al. Endoscopic ultrasonography-guided rendezvous technique. Dig Endosc. 2016;28(Suppl 1):96–101. doi: 10.1111/den.12611. [DOI] [PubMed] [Google Scholar]
- 22.Isayama H, Nakai Y, Kawakubo K, et al. The endoscopic ultrasonography-guided rendezvous technique for biliary cannulation: A technical review. J Hepatobiliary Pancreat Sci. 2013;20:413–20. doi: 10.1007/s00534-012-0577-8. [DOI] [PubMed] [Google Scholar]
- 23.Hara K, Yamao K, Hijioka S, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013;45:392–6. doi: 10.1055/s-0032-1326076. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto S, Hara K, Mizuno N, et al. Risk factor analysis for adverse events and stent dysfunction of endoscopic ultrasound-guided choledochoduodenostomy. Dig Endosc. 2020;32:957–66. doi: 10.1111/den.13620. [DOI] [PubMed] [Google Scholar]
- 25.Kida M, Araki M, Miyazawa S, et al. Fine needle aspiration using forward-viewing endoscopic ultrasonography. Endoscopy. 2011;43:796–801. doi: 10.1055/s-0030-1256508. [DOI] [PubMed] [Google Scholar]
- 26.Matsuzaki I, Miyahara R, Hirooka Y, et al. Forward-viewing versus oblique-viewing echoendoscopes in the diagnosis of upper GI subepithelial lesions with EUS-guided FNA: A prospective, randomized, crossover study. Gastrointest Endosc. 2015;82:287–95. doi: 10.1016/j.gie.2014.12.051. [DOI] [PubMed] [Google Scholar]
- 27.Okuno N, Hara K, Mizuno N, et al. Outcomes of endoscopic ultrasound-guided biliary drainage in patients undergoing antithrombotic therapy. Clin Endosc. 2021;54:596–602. doi: 10.5946/ce.2020.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honjo M, Itoi T, Tsuchiya T, et al. Safety and efficacy of ultra-tapered mechanical dilator for EUS-guided hepaticogastrostomy and pancreatic duct drainage compared with electrocautery dilator (with video) Endosc Ultrasound. 2018;7:376–82. doi: 10.4103/eus.eus_2_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


