Abstract
Nocardia is a genus of Gram-positive, partially acid-fast bacteria consisting of over 120 species, of which 50 are recognized as human pathogens. Nocardia spp. are common colonizers in the environment, particularly in soil and water. Nocardia spp. typically cause opportunistic infections in the immunocompetent host, although cases of nocardiosis have been described in those with a normal immune system. Nocardiosis can be localized, most often in the skin or lung, or be disseminated, with involvement of the brain, bone, and visceral organs. Treatment of nocardiosis is complex, as multiple culture-directed antibacterials with appropriate tissue penetration may need to be used for a prolonged duration. To our knowledge, we describe the first successfully treated case of disseminated Nocardia beijingensis infection in an immunocompetent host with doxycycline and trimethoprim-sulfamethoxazole and hypothesize that his occupational exposure to ubiquitous saprophytes may have led to his infection.
Keywords: Nocardia, Nocardiosis, Disseminated, Doxycycline, Immunocompetent, Occupational health
Case
A previously healthy, 63-year-old white male residing in Western Canada presented to his family physician with a 2-month history of dyspnea, cough and malaise. Physical examination demonstrated decreased entry to the right lower lung zone, concordant with a chest radiograph that demonstrated opacification of the right middle lobe and right lower lobe. Biochemistry tests revealed neutrophilic leukocytosis (15.6 × 109/L, 12.3 × 109/L) and thrombocytosis (615 × 109/L). An empiric 5-day course of amoxicillin 1 g orally thrice daily followed by a 5-day course of azithromycin was prescribed. Given a lack of clinical improvement, computed tomography (CT) of the chest was completed and showed findings suspicious for necrotizing/cavitary pneumonia with a developing lung abscess (Fig. 1). These results prompted an admission to hospital for a diagnostic bronchoscopy, which found thick secretions in the right middle lobe and right lower lobe. Bronchoalveolar lavage did not detect any malignant cells but specimens sent for bacterial and fungal culture grew Nocardia sp., which was identified presumptively based on morphologic and staining characteristics. Using a previously described broad-range 16S rDNA PCR method to sequence the first 400–600 bp of the 16S rDNA gene, the isolate was identified as Nocardia beijingensis complex (Fig. 2) [1].
Fig. 1.
Chest computed tomography demonstrating consolidation within the lateral right middle lobe, inferior right upper lobe and superior right lower lobe, associated with locules of gas and a confluent area of central cavitation.
Fig. 2.
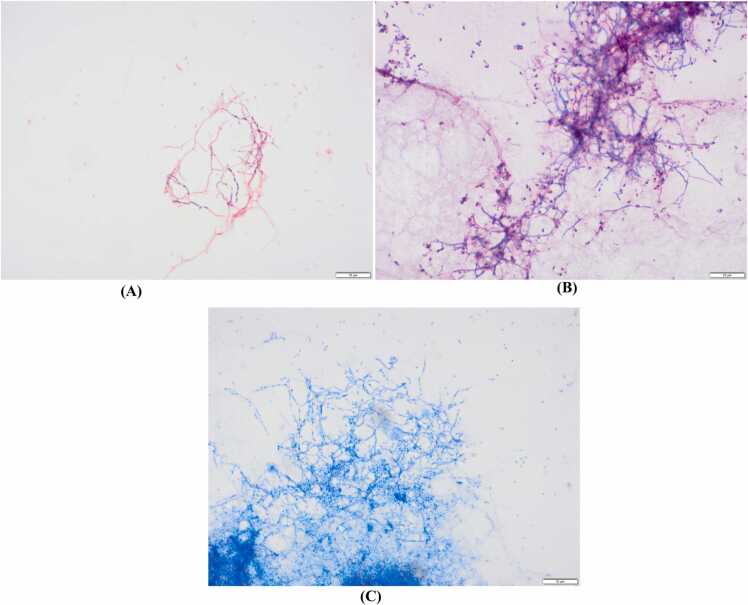
Bronchoalveolar lavage of the right middle lobe, demonstrating branching, filamentous Gram-positive bacilli, partially acid-fast staining on: A) Gram stain, B) modified Kinyoun stain, and C) Kinyoun stain, all at 1000 × magnification.
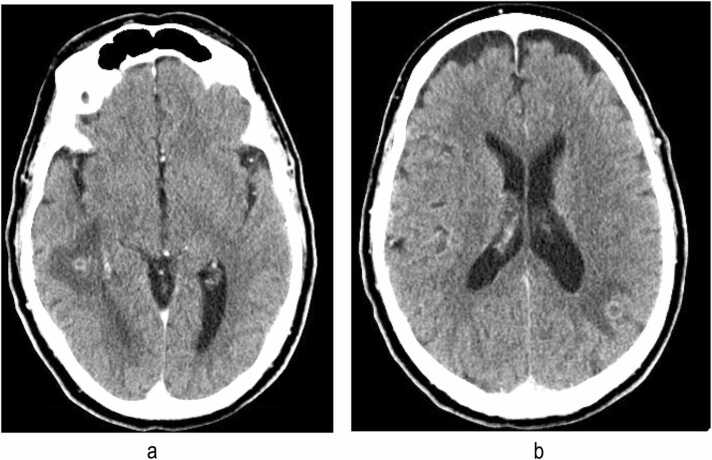
To assess for possible disseminated nocardiosis, a CT scan of the head was performed despite a lack of focal neurologic symptoms (Fig. 3). This imaging demonstrated three 6–10 millimeter intraaxial rim-enhancing masses in the right temporal lobe and bilateral parietal lobes. Significant adjacent vasogenic edema was noted, and the appearance was in keeping with intracranial abscesses. Given a high likelihood of disseminated nocardiosis with brain-lung involvement, a peripherally inserted central catheter was placed to facilitate treatment with imipenem 1 g intravenously every six hours along with high-dose oral trimethoprim-sulfamethoxazole (TMP-SMX) at 15 mg/kg of TMP. Within a week, antimicrobial susceptibilities from the patient’s Nocardia isolate became available, showing susceptibility to ceftriaxone, and his imipenem was changed to ceftriaxone 2 g intravenously every 12 h in addition to TMP-SMX.
Fig. 3.
Head computed tomography demonstrating intra-axial rim-enhancing masses in the bilateral parietal lobes and right temporal lobe, associated with significant adjacent vasogenic edema without distant mass effect.
Ten days into therapy, the patient developed a petechial rash on his lower extremities, and was found to have pancytopenia, acute kidney injury and transaminitis. In consultation with pharmacy, this was felt most likely to be related to his TMP-SMX and his dose was reduced to 800/160 mg twice daily. Within three days of this change, his rash and biochemical abnormalities resolved.
During follow-up visits, further discussions revealed an occupational exposure to landfill leachate (in the absence of personal protective equipment). As it is unusual for disseminated nocardiosis to occur in immunocompetent hosts, a comprehensive workup was conducted to elicit obvious immunodeficiencies. HIV-1 & 2 (by enzyme immunoassay and p24 antigen), hepatitis B and C were negative and serum immunoglobulins (IgG, IgM, IgA, IgE) were within normal limits. He had evidence of a normal immune response, as evidenced by IgG antibodies for measles, mumps, rubella and varicella zoster.
Following a three-month course of ceftriaxone and TMP-SMX, the patient was transitioned to an oral regimen of doxycycline 200 mg twice daily and TMP-SMX 800/160 mg twice daily for a duration of 15 months. The higher daily dose of doxycycline and a prolonged course of therapy was selected owing to central nervous system (CNS) involvement and the patient’s inability to tolerate high dose TMP-SMX. Serial CT-chest and CT-head imaging throughout his 15-month course of TMP-SMX and doxycycline indicated improvement of the involved areas (Fig. 4, Fig. 5).
Fig. 4.
Chest computed tomography demonstrating resolution of opacification in the right middle lobe.
Fig. 5.
Head computed tomography demonstrating resolution of previously identified enhancing foci.
Discussion
Nocardia spp. are branching Gram-positive bacilli, staining weakly acid-fast, found in soil and decaying organic matter. Infections by these bacteria are rare but can be life-threatening. Clinically, nocardiosis presents with three syndromes; pulmonary, cutaneous or disseminated [2].
Infection is thought to arise from direct inhalation or inoculation of Nocardia spp. Subsequent dissemination to secondary sites within the body, including the brain, bone, lymphatics and visceral organs can ensue. Primary cutaneous nocardiosis is associated with skin and soft tissue infection with associated lymphangitic spread. In tropical countries, mycetoma formation occurs, even in immunocompetent hosts. Pulmonary nocardiosis usually occurs only in patients who are immunosuppressed or have chronic lung disease [3].
Nocardia beijingensis was initially isolated in a sewer ditch in China in 2001 [4]. Over the next decade, less than ten additional cases of infections from Nocardia beijingensis have been reported in North America [5], [6], [7], [8], [9], [10]. As per the latest case series of Nocardia beijingensis in the immunocompetent host published by Diioia et al., cases of disseminated disease have been reported with greater regularity since 2020 [11]. As they are ubiquitous in the environment, isolation of Nocardia spp. from non-sterile sites can indicate colonization and contamination rather than invasive infection; therefore, clinical-microbiological correlation is critical for diagnosing infection.
This patient’s occupational duties included leachate management, and its transport to and from landfills. The microbiological milieu within leachate is varied. Workers with leachate and other related substances should always abide by applicable safety protocols and use the recommended personal protective equipment. Further study is needed to determine if there is an occupational risk for nocardiosis. We hypothesize that our patient may have developed pulmonary nocardiosis via his occupational exposure, and ultimately led to dissemination into his brain. Donning of a particulate filtering respirator when working in environments with potential aerosolization of leachate ought to be considered.
The mainstay of treatment for nocardiosis is TMP-SMX, given its activity against most species of Nocardia. While mild pulmonary nocardiosis can be effectively treated with TMP-SMX monotherapy, individuals who are immunocompromised or have disseminated disease require speciation and additional susceptibility testing of their isolates to guide adjunctive therapies that penetrate the site of disseminated infection. Although no evidence exists to guide duration of therapy, immunocompromised individuals with Nocardia infection or those with disseminated nocardiosis are typically treated with at least a 12-month course of therapy. As our patient was intolerant of full dose TMP-SMX, doxycycline was added for its CNS-penetrating properties when ceftriaxone was discontinued.
Conclusion
To our knowledge, this is the fourth case in North America and first case in Canada of disseminated Nocardia beijingensis disease in an immunocompetent host. Our case outlines the utility of doxycycline as an anti-Nocardia medication with CNS penetration and highlights the need to consider occupational protection against infectious diseases encountered in the workplace of select workers.
CRediT authorship contribution statement
John C Lam: Conceptualization, Literature search, Writing – original draft, Writing – review & editing, Submission. Wilson W Chan: Writing – review & editing, resources. Jillian F Walsh: Literature search, Writing – original draft, Writing – review & editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical approval
N/A.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Competing interests
None declared.
References
- 1.Miller R.J., Chow B., Pillai D., Church D. Development and evaluation of a novel fast broad-range 16S ribosomal DNA PCR and sequencing assay for diagnosis of bacterial infective endocarditis: multi-year experience in a large Canadian healthcare zone and a literature review. BMC Infect Dis. 2016;16:146. doi: 10.1186/s12879-016-1476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson J.W. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87(4):403–407. doi: 10.1016/j.mayocp.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinbrink J., Leavens J., Kauffman C.A., Miceli M.H. Manifestations and outcomes of nocardia infections: comparison of immunocompromised and nonimmunocompromised adult patients. Medicine. 2018;97(40) doi: 10.1097/MD.0000000000012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang L., Zhang Y., Lu Z., Shi Y., Liu Z., Maldonado L., et al. Nocardia beijingensis sp. nov., a novel isolate from soil. Int J Syst Evol Microbiol. 2001;51(Pt 5):1783–1788. doi: 10.1099/00207713-51-5-1783. [DOI] [PubMed] [Google Scholar]
- 5.Crozier J.A., Andhavarapu S., Brumble L.M., Sher T. First report of Nocardia beijingensis infection in an immunocompetent host in the United States. J Clin Microbiol. 2014;52(7):2730–2732. doi: 10.1128/JCM.00588-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solano-Varela D.M., Barrios-Vidales E.M., Plaza D.F., Riveros W.M., Guzmán J., Chica C.E., et al. Immunocompetent patient with a brain abscess caused by Nocardia beijingensis in Latin America: a case report. Medicine. 2019;98(11) doi: 10.1097/MD.0000000000014879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keenan J.G., Mohapatra S. Nocardia. IDCases. 2017;9:65–69. doi: 10.1016/j.idcr.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez A., Jennings E., Vaziri S., Yachnis A.T., Kubal A. Second report of a Nocardia beijingensis infection in the United States: nodular scleritis with in vitro imipenem resistance. Digit J Ophthalmol: DJO. 2016;22(3):62–66. doi: 10.5693/djo.02.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aragaki-Nakahodo A., Benzaquen S., Kirschner M. Coinfection by Nocardia beijingensis and Nocardia arthritidis in an immunocompromised patient diagnosed by endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) Respir Med Case Rep. 2014;12:22–23. doi: 10.1016/j.rmcr.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy M., Martial A., Ahmad S. Disseminated Nocardia beijingensis infection in an immunocompetent patient. Eur J Case Rep Intern Med. 2020;7(11) doi: 10.12890/2020_001904. [001904-] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diioia A., Kalra L., Krop L.C. Stroke like presentation of disseminated CNS. IDCases. 2021;25 doi: 10.1016/j.idcr.2021.e01223. [DOI] [PMC free article] [PubMed] [Google Scholar]