Abstract
Carotid artery stenosis (CAS) develops from atherosclerotic lesions and plaques. Plaque rupture or stenosis may result in occlusion of the carotid artery. Accordingly, the asymptomatic disease becomes symptomatic, characterized by ischemic stroke or transient ischemic attacks, indicating an urgent need for better understanding of the underlying molecular mechanisms and eventually prevent symptomatic CAS. NOX4, a member of the NADPH oxidase family, has anti-atherosclerotic and anti-inflammatory properties in animal models of early atherosclerosis. We hypothesized that NOX4 mRNA expression is linked to protective mechanisms in CAS patients with advanced atherosclerotic lesions as well. Indeed, NOX4 mRNA expression is lower in patients with symptomatic CAS. A low NOX4 mRNA expression is associated with an increased risk of the development of clinical symptoms. In fact, NOX4 appears to be linked to plaque stability, apoptosis and plaque hemorrhage. This is supported by cleaved caspase-3 and glycophorin C and correlates inversely with plaque NOX4 mRNA expression. Even healing of a ruptured plaque appears to be connected to NOX4, as NOX4 mRNA expression correlates to fibrous cap collagen and is reciprocally related to MMP9 activity. In conclusion, low intra-plaque NOX4 mRNA expression is associated with an increased risk for symptomatic outcome and with reduced plaque stabilizing mechanisms suggesting protective effects of NOX4 in human advanced atherosclerosis.
Keywords: Atherosclerosis, Carotid artery stenosis, NADPH oxidase 4, Plaques
Graphical abstract
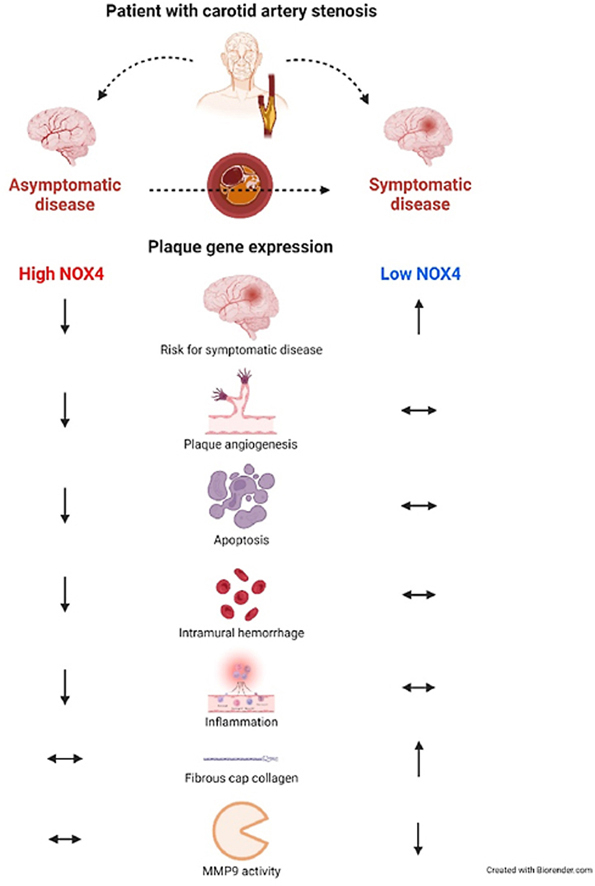
Key question: How is plaque NOX4 mRNA expression regulated and correlated to plaque stabilizing mechanisms in patients with symptomatic and asymptomatic carotid artery stenosis at a clinically relevant stage of disease?Key finding: Plaque NOX4 mRNA expression is lower in symptomatic carotid artery stenosis and low NOX4 is associated with an increased risk for symptomatic disease. Apoptosis and plaque hemorrhage inversely correlate with plaque NOX4 mRNA expression and healing of a ruptured plaque appears to be improved in plaques with high NOX4 mRNA expression.Take-home Message: Our data support a link between NOX4 mRNA expression and plaque stability in humans with late-stage carotid artery stenosis.
Highlights
-
•
Plaque NOX4 mRNA expression is lowered in symptomatic carotid artery disease.
-
•
A low plaque NOX4 mRNA expression is associated with an increased risk for symptomatic outcome.
-
•
High NOX4 mRNA expression was linked to plaque stabilizing mechanisms.
-
•
An increased NOX4 mRNA expression could support healing of a ruptured plaque in symptomatic disease.
-
•
NOX4 mRNA expression is linked to protective mechanisms in patients with advanced atherosclerosis.
Abbreviations
- α-SMA
alpha-smooth muscle actin
- ACE
angiotensin-converting enzyme
- AHA
American Heart Association
- ARBs
angiotensin receptor blockers
- BMI
body mass index
- CAS
coronary artery disease
- CAS
carotid artery stenosis
- CCB
calcium channel blocker
- CEA
carotid endarterectomy
- HDL
high-density lipoprotein
- H2O2
hydrogen peroxide
- LDL
low-density lipoprotein
- MMP
Matrix metalloproteinase
- NASCET
North American Symptomatic Carotid Endarterectomy Trial
- NIHSS
National Institute of Health Stroke Scale
- NOX
NADPH oxidases
- PAD
peripheral artery disease
- ROS
reactive oxygen species
- TIA
transient ischemic attack
- T2D
type 2 diabetes
1. Introduction
Atherosclerosis is a widespread disease, often unrecognized, underestimated and ignored by the patient as it develops slowly and causes no pain. Plaque development occurs in every artery such as aorta, coronary arteries or renal and carotid arteries. Quite often atherosclerotic lesions lead to the formation of a local thrombus [1]. Accordingly, plaque rupture or thromboembolic events result in vessel occlusion or distal embolization in the depending organ, causing e.g. myocardial infarction or stroke. Stroke is one of the leading causes of death globally with increasing incidence [1,2]. Approximately 30% of all strokes result from carotid artery stenosis (CAS) and subsequent plaque rupture [2]. If these events cause retinal or cerebral ischemia [3], it results in symptomatic CAS. In asymptomatic patients, the degree of stenosis correlates with the risk of stroke [4]. 5–10% of individuals older than 65 years suffer from carotid artery occlusion of 50% or more, but most of them live without any symptoms. In these patients, surgical intervention does not reduce the risk of ischemic events [4]. Conservative risk management is the preferred therapy in the majority of patients. This implies an urgent need for a better understanding of how and why vessel occlusion or plaque rupture arise.
Oxidative stress and inflammation are involved in atherosclerosis and plaque rupture [5]. On the other hand, reactive oxygen species are important second messengers [6]. A major source of reactive oxygen species (ROS) in the vasculature is the family of NADPH oxidases (NOX). In humans, seven different NOX homologues are expressed in a tissue- and organ-specific manner. NOX4 is unique in the family of NADPH oxidases, as it is expressed in most differentiated cells and constitutively produces low amounts of hydrogen peroxide (H2O2) for cell homeostasis and metabolism [7]. Notably, NOX4 is considered to be athero-protective in animal models of early atherosclerosis. Deletion or pharmacological inhibition reduces H2O2 formation and promotes atherosclerosis by increased inflammation and macrophage accumulation in vessels of diabetic ApoE−/− and Nox4−/−/Ldlr−/− mice [8,9]. Ligation of the carotid artery reduced NOX4 mainly in endothelial cells in atherosclerosis-prone mice [10]. Endothelial NOX4 overexpression ameliorated atherosclerosis in the carotid artery [10]. A transient loss of NOX4 in smooth muscle cells was unable to interfere with restenosis after wire-induced carotid artery injury [11]. Importantly, NOX4 protein was found to be higher expressed in unstable plaques when compared to stable plaques of the carotid artery [12]. Furthermore, NOX4 expression decreased in complicated atherosclerotic lesions in coronary artery segments from explanted human hearts [13]. In this study, we analyzed NOX4 mRNA expression in symptomatic vs. asymptomatic carotid artery stenosis and hypothesized that NOX4 is linked to protective mechanisms in patients suffering from CAS with advanced atherosclerotic lesions.
2. Materials and methods
For an extended material and methods section, see Supplementary material.
2.1. Patient cohorts, sample acquisition and ethical approval
Patients were classified as asymptomatic or symptomatic. Patients were considered symptomatic after presentation of neurological symptoms (sensory, motoric, visual and speech deficit) within the last six months. The severity of stroke or transient ischemic attack (TIA) was quantified according to the National Institute of Health Stroke Scale (NIHSS). Every patient was assessed by a neurologist pre- and postoperatively. The degree of stenosis was assessed by duplex sonography and additional computed tomography angiography (CTA) and is defined by % NASCET criteria [14]. The % NASCET stenosis was calculated from the ratio of the luminal diameter of the narrowest segment of the diseased portion to the diameter of the carotid artery beyond any poststenotic dilatation [15]. Atherosclerotic plaques were collected intraoperatively during carotid endarterectomy (CEA) (asymptomatic, n = 63 and symptomatic, n = 42). Atherosclerotic plaques were obtained from the distal common or proximal internal carotid artery. Specimens were placed in ice-cold 1xDPBS, dissected and shock frozen in liquid nitrogen or directly proceeded to further analysis. The time between surgical removal and tissue processing was 10–15 min. Blood lipids, glucose, C-reactive protein (CRP), cardiovascular risk factors, comorbidities and medication were evaluated prospectively. Blood was withdrawn pre-operatively in the non-fasted state. Smoking was defined as present smoking or smoking history within the last 10 years. Variations in n numbers result from none available information or insufficient RNA quality, tissue amount or plaque integrity. Supplementary Table 1 summarizes the number of analyzed samples for each parameter and the pairs for correlation with NOX4 mRNA expression.
2.2. Study approval
The study was approved by the ethics committee of the Technische Universität Dresden (EK 151042017). Informed consent was obtained from each patient.
2.3. Statistical analysis
Graph Pad Prism 9.0 (GraphPad Software, Inc., La Jolla, CA, USA) and the R Stats Package (R Core Team and contributors worldwide) software was used for statistical analysis and p ≤ 0.05 was considered as significant. Grubb's test was used to detect significant outliers, which then were excluded from further analysis as indicated in the figure legends. Normality was tested by the D'Agostino and Pearson normality test. Non-Gaussian distributed data were analyzed by Mann-Whitney-U or Kruskal-Wallis and Dunn's multiple comparison test. Unpaired t-tests were applied to data with Gaussian distribution. Correlational analysis in non-Gaussian-distributed data was done using Spearman's correlation coefficient (rS). Differences in distribution of cardiovascular risk factors and medical therapies across the two independent groups were Chi-Square tested. The null hypothesis (H0) postulated that distributions of risk factors and medical therapies has no impact on the outcome of the disease (asymptomatic and symptomatic). Data are presented as scatter dot plots showing the median or mean with range as indicated in the figure legends. The mean was used for normally distributed data, the median for non-normally distributed data. Asymptomatic patients are shown as red circles and symptomatic patients as black squares. Multiple logistic regression was used to test NOX4 mRNA expression, NASCET score and different medical therapies as predictors for asymptomatic stenosis. Furthermore, NOX4 mRNA expression and NASCET were tested as a response to prescription of different medical therapies by multiple logistic regression. NOX4 expression was divided into low (less than mean) and high (more or equal to mean) expression according to the mean of all raised values. NASCET was divided into a medium (60–70%) and a high-grade (80–90%) stenosis. The reference values for NOX4 expression and medical therapies were set as low expression (ref = low) or no intake (ref = none), respectively. The odds ratios (ORs) represent the x-fold chances for being asymptomatic in patients with high NOX4 expression and the prescription of the indicated therapies. When analyzing NOX4 and NASCET as a response, the ORs present the chances for high NOX4 expression and a high NASCET score. Isolated effects of the intake of statins or insulin on high NOX4 expression and persistent asymptomatic disease are presented as odds as obtained from multiple logistic regression and comparison.
3. Results
3.1. Patient characteristics and risk stratification for symptomatic carotid artery stenosis
Baseline comparison of demographics, comorbidities and medical therapies from patients with symptomatic and asymptomatic CAS revealed higher non-fasting glucose (p = 0.03) and low-density lipoprotein (LDL) cholesterol (p = 0.02) in patients with symptomatic disease. The frequency of diagnosed peripheral artery disease (PAD, p = 0.04) and coronary artery disease (CAD, p = 0.01) was found to be higher among asymptomatic patients. Additionally, prescription of statins (p = 0.0002), calcium channel blockers (CCBs, p = 0.03) and β-adrenergic blockers (p = 0.05) was higher in patients with asymptomatic carotid artery stenosis (Table 1). Of importance, prescription of statins, CCBs and β-blockers dramatically increased the chance for having asymptomatic disease by 12-, 2.8- and 2.2-fold, respectively, underlining the importance of secondary prevention (Supplementary Table 6). PAD, coronary artery disease and CAS result from severe atherosclerotic alterations within arteries. Symptomatic disease shows more severe clinical symptoms than asymptomatic disease. Accordingly, the inconsistency in the abundance of PAD and coronary artery disease in asymptomatic disease raised our interest in the applicability of the NASCET score as an independent predictor for asymptomatic and symptomatic CAS. Multivariate logistic regression revealed an equal risk of asymptomatic and symptomatic disease in high (80–90%) and medium (60–70%) NASCET. Even when adjusted for prescription of standard cardio-metabolic medical therapies such as ASA, β-blockers, CCBs, ARBs, statins and insulin, the prediction power of the NASCET remained insignificant. Vice versa, the prescription of ARBs reduces the risk for having a high (80–90%) NASCET score while other standard therapies showed no effects (Supplementary Table 7).
Table 1.
Clinical characteristics in patients with asymptomatic and symptomatic carotid artery stenosis that were analyzed for plaque NOX4 expression. Statistics: All data are presented as median with minimum and maximum values. Comparison of data was done using the Mann-Whitney U test. Comparison of prevalence's for comorbidities and prescribed medical therapies was analyzed by Chi-square (X2) test. In total n = 60 asymptomatic and n = 42 symptomatic patients were included but data on baseline demographics, medical therapies and comorbidities were not available from all patients. Therefore, the number of n varies compared to the initially included patients. Blood was taken in the non-fasted state. Reference values for the corresponding blood parameters are given in the table. Reference values for LDL and total cholesterol (TC) are based on the European Society of Cardiology (ESC) recommendations. Reference values for fasting glucose were excluded due to the withdrawal in the non-fasted state. Stroke was classified as symptoms occurring longer than 24 h, whereas symptoms in a transient ischemic attack (TIA) resolved within the first 24 h. Abbreviations: ACE, angiotensin-converting enzyme; ARBs, angiotensin receptor blocker; BMI, body mass index; ASA, acetylsalicylic acid; CAD, coronary artery disease; CCB, calcium channel blocker; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NASCET, North American Symptomatic Carotid Endarterectomy Trial; NIHSS, National Institute of Health Stroke Scale; PAD, peripheral artery disease; T2D, type 2 diabetes mellitus; TIA, transient ischemic attack.
| Asymptomatic | Symptomatic | p-value | X2 | |
|---|---|---|---|---|
| Age, years, median with minimum and maximum, n | 74.0 | 72.5 | 0.77 | |
| (45.0–86.0) | (57.0–92.0) | |||
| 60 | 42 | |||
| Sex, male:female, % male | 47:13, 78 | 30:12, 71 | 0.43 | 0.64 |
| NASCET, % median with range, n | 80.0 | 80.0 | 0.38 | |
| (70.0–90.0) | (60.0–90.0) | |||
| 57 | 41 | |||
| NIHSS, median with minimum and maximum, n | – | 1 | ||
| (0-18) | ||||
| 39 | ||||
| BMI, kg/m2, median with range, n | 26.3 | 26.9 | 0.49 | |
| (17.0–37.5) | (17.6–40.9) | |||
| 56 | 40 | |||
| Blood glucose, mmol/L, median with range, n | 5.51 | 6.20 | 0.03 | |
| (3.40–17.11) | (4.13–16.04) | |||
| 58 | 39 | |||
| Reference values | Only for fasting glucose possible | |||
| LDL cholesterol, mmol/L, median with range, n | 2.14 | 2.81 | 0.02 | |
| (0.72–6.58) | (1.10–5.59) | |||
| 58 | 35 | |||
| Reference values | <1.40 mmol/L for people with very-high risk [43] | |||
| HDL cholesterol, mmol/L, median with range, n | 1.25 | 1.21 | 0.89 | |
| (0.50–2.71) | (0.73–2.00) | |||
| 58 | 35 | |||
| Reference values | >0.90 mmol/L for men and >1.10 mmol/L for women | |||
| Triglycerides, mmol/L, median with range, n | 1.71 | 1.47 | 0.19 | |
| (0.45–5.81) | (0.52–4.40) | |||
| 57 | 37 | |||
| Reference values | 0.35–1.70 mmol/L | |||
| Total cholesterol, mmol/L, median with range, n | 3.80 | 4.59 | 0.06 | |
| (2.24–9.08) | (2.49–7.38) | |||
| 57 | 35 | |||
| Reference values | <4.00 mmol/L | |||
| CRP, mg/L, median with range, n | 1.90 | 2.65 | 0.45 | |
| (0.30–11.90) | (0.30–36.00) | |||
| 56 | 42 | |||
| Reference values | <5.0 mg/L | |||
| Comorbidities | ||||
| Hypertension, yes:no, % total | 56:2, 97 | 38:3, 93 | 0.39 | 0.75 |
| PAD, yes:no, % total | 19:40, 32 | 6:36, 14 | 0.04 | 4.23 |
| CAD, yes:no, % total | 22:35, 39 | 7:35, 17 | 0.01 | 6.13 |
| HI, yes:no, % total | 15:43, 26 | 7:35, 17 | 0.27 | 1.20 |
| Smoking, yes:no, % total | 22:37, 37 | 13:29, 31 | 0.51 | 0.44 |
| T2D, yes:no, % total | 22:35, 39 | 20:22, 48 | 0.37 | 0.81 |
| Medical therapies | ||||
| ASA, yes:no, % total | 47:11, 81 | 29:13, 69 | 0.17 | 1.92 |
| Anticoagulation, yes:no,% total | 17:40, 30 | 14:28, 33 | 0.71 | 0.14 |
| Statins, yes:no, % total | 55:2, 97 | 29:13, 69 | 0.0002 | 14.17 |
| β-blocker, yes:no, % total | 30:27, 53 | 14:28, 33 | 0.05 | 3.65 |
| ΑRBs, yes:no, % total | 22:34, 39 | 12:30, 29 | 0.27 | 1.22 |
| ACE, yes:no, % total | 22:35, 39 | 16:26, 39 | 0.96 | 0.003 |
| CCBs, yes:no, % total | 25:32, 44 | 9:33, 21 | 0.02 | 5.39 |
| Diuretics, yes:no, % total | 22:35, 39 | 9:35, 21 | 0.07 | 3.31 |
| Insulin, yes:no, % total | 7:50, 13 | 5:37, 12 | 0.96 | 0.003 |
3.2. NOX4 mRNA expression predicts asymptomatic carotid artery disease
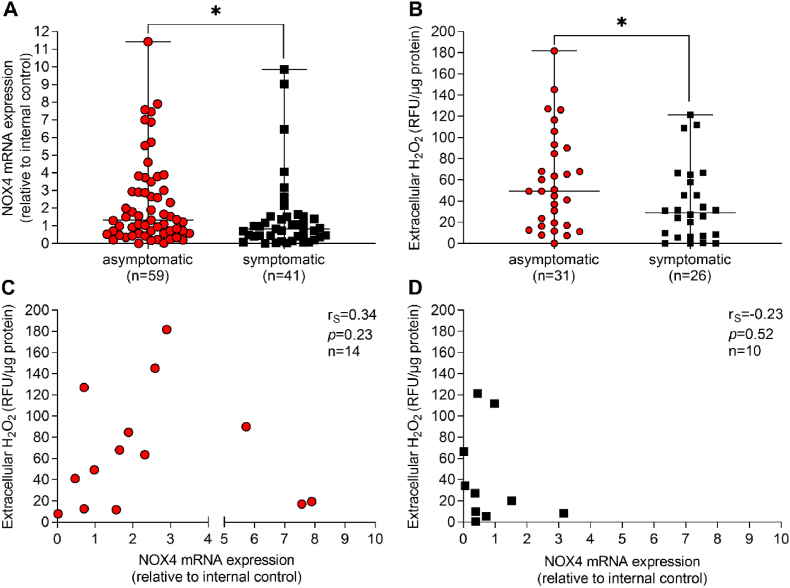
Expression of NOX4 mRNA was 1.6-fold (p = 0.02) and H2O2 release 1.6-fold (p = 0.049) higher in samples of patients with asymptomatic CAS, when compared to symptomatic CAS (Fig. 1A and B). As pointed out above, NOX4 mRNA expression is strongly correlated with NOX4 protein expression and H2O2 release [16]. Surprisingly, here H2O2 release was not directly correlated to NOX4 mRNA expression (Fig. 1D). A possible explanation of this unexpected finding is that other H2O2-generating enzymes in the vasculature [17] contributed to the H2O2 release in the samples collected. Because NOX4 mRNA expression rather than H2O2 release correlates to symptomatic CAS, we did not aim to analyze other H2O2 sources and focused on NOX4 mRNA expression.
Fig. 1.
Plaque NOX4 and H2O2in atherosclerotic lesions from patients with asymptomatic and symptomatic carotid artery stenosis. Atherosclerotic plaques were obtained from patients with asymptomatic and symptomatic carotid artery disease and (A) NOX4 expression was analyzed by qPCR. Data are presented in relation to an internal control (B), Extracellular H2O2 was quantified using the Amplex Red Assay. Relative fluorescence units (RFU) were normalized to protein content (μg) of the plaque specimen. (A, B), Data are presented as scatter dot plots where the horizontal line depicts the median. Samples were compared by Mann-Whitney-U test. *p ≤ 0.05. The number of analyzed samples is given in the figures below. Statistically significant outliers were detected by Grubb's outlier test. One sample in each group (asymptomatic, symptomatic) was detected and was excluded from the analysis. (C–D), Spearman's correlation (rS) analysis between NOX4 and H2O2 release in asymptomatic and symptomatic patients. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
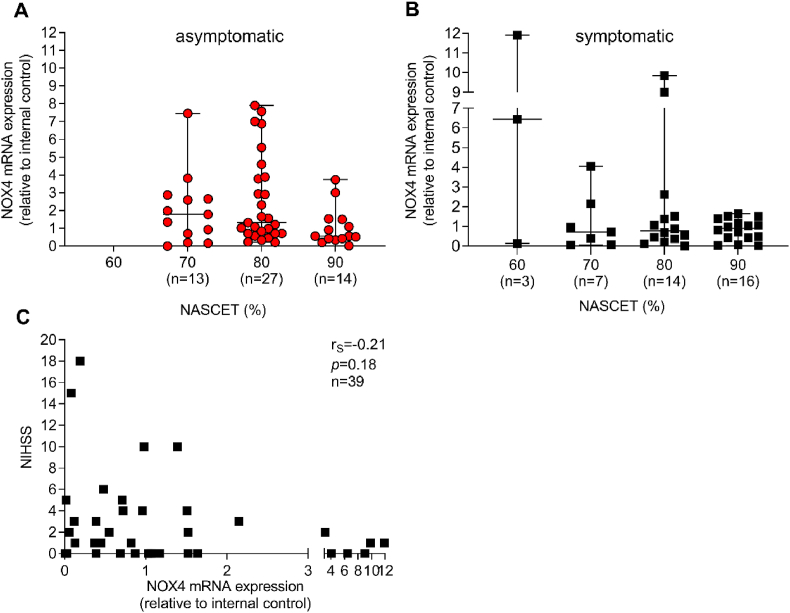
NASCET degree of stenosis is one clinical parameter to estimate the risk of carotid artery stenosis-related stroke. NOX4 mRNA expression was grouped into the different NASCET quantiles. In asymptomatic patients, NOX4 tended (p = 0.09) to be lowered in the highest degree of stenosis (90%) when compared to 80% without reaching significance. In symptomatic patients, NOX4 mRNA was distributed equally among all degrees of stenosis (Fig. 2A and B). The NIHSS score was used to assess the severity of stroke and was not correlated to NOX4 expression (Fig. 2C). This result from different time points of surgical interventions after the initial neurological event and, in cases of thromboembolism, symptoms depend on the occluded cerebral artery. We next analyzed whether NOX4 mRNA expression in plaques is a predictor for an asymptomatic outcome of CAS. High NOX4 mRNA expression was associated with an increased chance for an asymptomatic outcome (OR: 2.87 [1.02, 8.96], p = 0.048). Vice versa, a low NOX4 mRNA expression increased the risk for symptomatic CAS. If adjusted to prescribed medical interventions, the variability of individual ORs is increasing, resulting in a loss of significance (Table 2). We conclude that medical interventions may alter NOX4 mRNA expression. Interestingly, prescribed insulin therapy is associated with a 10.4-fold higher chance of an increased NOX4 mRNA expression (Table 3). It is possible that a high NOX4 expression is accompanied by a reduced insulin release. This will be the subject of further studies, as the role of NOX4 in β-cells is discussed controversially [18]. Because insulin treatment rescues normal insulin and blood glucose levels, it appears unlikely that insulin induces NOX4 mRNA expression. In fact, the chance of high NOX4 expression is not associated with the prescription of insulin, whereas a combination of statins and insulin dramatically increased a high NOX4 expression 4-fold (Table 4). These data support the protective effect of statins, which appear to increase NOX4 mRNA expression in insulin-dependent patients. While it is possible that interventions such as statins directly induce NOX4 mRNA expression, another potential mechanism is the therapeutic stabilization of the plaque, allowing an increased NOX4 expression. Further studies will address this issue.
Fig. 2.
Plaque NOX4 expression, degree of stenosis and stroke severity. (A–B) The degree of stenosis was assessed by duplex sonography and presented in % by the NASCET criteria. NOX4 expression was assessed by qPCR and was grouped according to the degree of stenosis into 60, 70, 80 and 90%. Data of gene expression are presented in relation to an internal control and are presented as scatter dot plots where the horizontal line depicts the median. The number of patients with their plaque NOX4 expression in the corresponding % NASCET criteria is given below each figure. Statistically significant outliers were detected by Grubb's outlier test. One sample in each stenosis group in asymptomatic patients and one in the 90% in symptomatic patients was omitted from the analysis. Data were compared using the Kruskal-Wallis with Dunn's multiple comparison test. (C), Spearman's correlation (rS) between plaque NOX4 and the NIHSS scale that represents the severity of stroke.
Table 2.
NOX4 as a predictor for asymptomatic carotid artery disease. NOX4 expression was analyzed by qPCR and data were normalized to an internal control (=1). Expression was divided into low and high, depending on the mean. Data were analyzed by multivariate logistic regression using NOX4 as a predictor variable due to the assumption that a changed NOX4 expression is prior to the disease outcome (asymptomatic vs. symptomatic). The odds ratio (ORs) refers to the relative increase in risk for asymptomatic disease when comparing patients with high NOX4 expression with those having a low expression (= ref low). The ORs for medical therapies show the chances for asymptomatic disease when the patient received the indicated therapy as compared to those without (ref = none). Unadjusted values were obtained by pairwise comparison of each variable listed in the table with the outcome being asymptomatic was analyzed by holding the effects of the other cardio-metabolic therapies constant and assuming high or low NOX4 expression. Abbreviations: ARBs, angiotensin receptor blockers; ASA, acetylsalicylic acid; CCBs, calcium channel blockers; CI, confidence interval; OR, odds ratio; ref, reference.
| Variable | OR [CI], unadjusted | p-value | OR [CI], adjusted | p-value |
|---|---|---|---|---|
| NOX4 (ref = low) | 2.87 [1.02, 8.96] | 0.048 | 2.93 [0.84, 10.28] | 0.093 |
| ASA (ref = none) | 1.90 [0.68, 5.39] | 0.251 | 0.54 [0.12, 2.35] | 0.408 |
| β-blocker (ref = none) | 2.20 [0.90, 5.55] | 0.088 | 1.78 [0.67, 4.74] | 0.248 |
| CCBs (ref = none) | 2.83 [1.08, 8.02] | 0.035 | 3.04 [1.04, 8.93] | 0.043 |
| ARBs (ref = none) | 1.56 [0.62, 4.09] | 0.410 | 1.51 [0.53, 4.30] | 0.437 |
| Statins (ref = none) | 12.02 | <0.001 | 24.76 | 0.002 |
| [2.47, 117.02] | [3.17, 193.58] | |||
| Insulin (ref = none) | 1.04 [0.26, 4.48] | 1 | 0.57 [0.10, 3.31] | 0.534 |
Table 3.
NOX4 expression and effects of cardio-metabolic therapies. NOX4 expression was analyzed by qPCR and data were normalized to an internal control (=1). Expression was divided into low and high, depending on the mean. Data were analyzed by multivariate logistic regression using a high NOX4 as a variable in response to different prescribed medical therapies. Unadjusted values were obtained by pairwise comparison of each variable listed in the table with the outcome of high or low NOX4 expression. The ORs for the medical therapies refers to the chance for increased NOX4 expression when the patient received the indicated therapy as compared without (ref = none). Adjusted ORs for the chance of high versus low NOX4 account for the effects of the other indicated therapies by holding the effects of the other cardio-metabolic therapies constant and assuming yes or no intake. In the adjusted ORs, a clear reversion of chances was seen for anticoagulation, statins, ARBs and diabetes treatment. This is most likely due to Simpson's paradox by confounding with other medical therapies. Treatment of type 2 diabetes (T2D) includes patients with prescription of biguanides, sodium-glucose co-transporter-2 (SGLT2) inhibitors, Glucagon-like peptide-1 receptor agonists (GLP-1-RA)/glucagon-like-peptide-1 receptor antagonists, dipeptidyl peptidase 4 (DPP-4)-inhibitors and sulfonylureas. Abbreviations: ACE, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blocker; ASA, acetylsalicylic acid; CCBs, calcium channel blockers; CI, confidence interval; OR, odds ratio; ref, reference, T2D, type 2 diabetes mellitus.
| Variable | OR, unadjusted | p-value | OR, adjusted | p-value |
|---|---|---|---|---|
| Anticoagulation (ref = none) | 1.11 [0.38, 3.12] | 1 | 0.67 [0.17, 2.66] | 0.574 |
| ASA (ref = none) | 0.72 [0.24, 2.24] | 0.684 | 0.53 [0.11, 2.68] | 0.445 |
| β-blocker (ref = none) | 1.36 [0.52, 3.60] | 0.636 | 1.46 [0.46, 4.63] | 0.525 |
| CCBs (ref = none) | 2.05 [0.75, 5.56] | 0.175 | 1.89 [0.62, 5.73] | 0.262 |
| ARBs (ref = none) | 1.35 [0.49, 3.64] | 0.678 | 0.73 [0.19, 2.84] | 0.649 |
| ACE (ref = none) | 0.85 [0.31, 2.30] | 0.910 | 0.40 [0.10, 1.64] | 0.201 |
| Diuretics (ref = none) | 2.04 [0.74, 5.61] | 0.189 | 1.17 [0.36, 3.80] | 0.800 |
| Statins (ref = none) | 1.69 [0.41,10.11] | 0.644 | 3.62 [0.50, 26.38] | 0.205 |
| T2D treatment (ref = none) | 3.14 [1.14, 8.78] | 0.023 | 1.13 [0.33, 3.83] | 0.844 |
| Insulin (ref = none) | 10.41 [2.31,65.79] | <0.001 | 13.82 [2.27, 84.15] | 0.004 |
Table 4.
NOX4 expression and prescription of statins and insulin. NOX4 expression was analyzed by qPCR and data were normalized to an internal control (=1). Expression was divided into low and high, depending on the mean. Data were analyzed by multivariate logistic regression and multiple comparison using a high NOX4 as a variable in response to statin and insulin prescription. Adjusted values were obtained by pairwise comparison of each variable listed in the table with the outcome of high NOX4 expression. The odds refers to the chance for increased NOX4 expression if the patient received (1) or did not receive (0) statins or insulin or a combination of both.
| Statin | Insulin | Odds of high NOX4 vs. low NOX4 | p-value |
|---|---|---|---|
| 0 | 1 | 0.11 [0.02, 0.54] | 0.007 |
| 1 | 0 | 0.31 [0.18, 0.53] | <0.001 |
| 0 | 1 | 1.47 [0.27, 8.10] | 0.661 |
| 1 | 1 | 4.04 [0.96, 17.07] | 0.057 |
3.3. NOX4 mRNA expression correlated with indicators of plaque stability
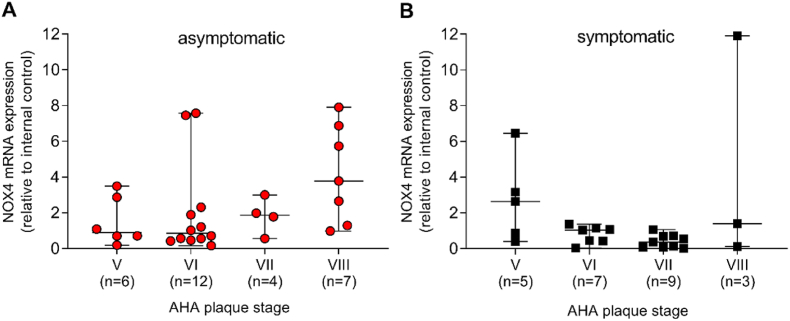
Atherosclerotic lesions were categorized according to the AHA classification [19]. The majority of plaques in asymptomatic disease were scored as type VI, whereas plaques in symptomatic disease were scored as type VII (Supplementary Table 8). Type VI is a complex plaque with possible surface defects, hemorrhage or thrombosis whereas type VII is mostly calcified [19]. Previous reports imply plaque instability as a contributor to symptomatic CAS [20]. Unfortunately, the AHA classification of lesion types does not include plaque stability. Accordingly, we analyzed potential correlations of NOX4 mRNA expression with indicators of plaque stability, such as fibrous phenotype, calcification and intramural bleeding. In asymptomatic disease, the highest level of NOX4 mRNA was found in type VIII plaques (fibrous phenotype), the lowest in type V and VI, both without reaching statistical significance (Fig. 3A). Type VIII plaques showed a slightly lowered erythrocyte and CD68 content and a partly increased collagen positive area in the fibrous cap (Supplementary Figs. 1A–H), giving possible hints towards the involved mechanisms. Additionally, NOX4 mRNA expression was inversely correlated (rS = −0.42, p = 0.02) with intramural bleeding, while no significant correlation was found between NOX4 mRNA expression and calcified areas (rS = −0.31, p = 0.09) (Supplementary Table 9). In symptomatic disease, NOX4 was highest in plaques scored as fibroatheroma type V and lowest in complicated type VII, calcified lesions (Fig. 3B), without any correlation to other score components (Supplementary Table 10). In symptomatic patients, type VI plaques had a slightly lowered collagen content in the fibrous cap and showed increased plaque CD68, cleaved caspase-3 positive areas as well as an increased MMP-9 activity (Supplementary Figs. 2A–H). Therefore, a mechanism involving NOX4, apoptosis, inflammation and MMP activity is likely.
Fig. 3.
Associations between NOX4 and the type of atherosclerotic lesions obtained from asymptomatic and symptomatic patients. (A–B), Atherosclerotic plaques were scored according to the AHA classification of human carotid atherosclerotic lesions. Type V are fibroatheroma; type VI are atheroma with a thrombus and intramural hemorrhage; type VII atheroma with a fibrous cap and calcified nodules; type VIII reflects the fibrous type. NOX4 expression was analyzed by qPCR and normalized to an internal control (=1). Data are presented as scatter dot plots where the horizontal line depicts the median. The number of patients with their plaque NOX4 expression in the corresponding AHA plaque stage is given below each figure. Statistical significant outliers were detected by Grubb's outlier test. In asymptomatic disease, one patient from type V and VI and one from type VI and VII in symptomatic disease, respectively, was omitted from the analysis. Data were compared using Kruskal-Wallis and Dunn's multiple comparison test.
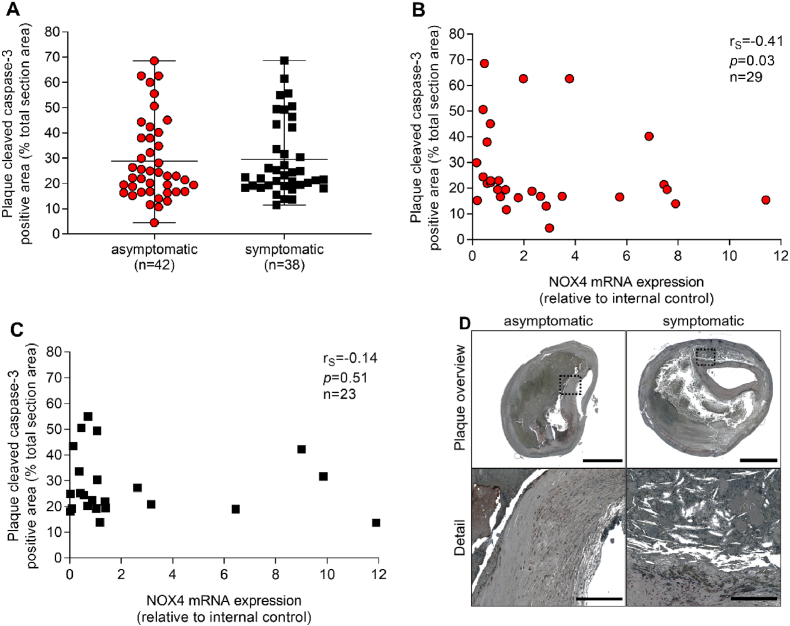
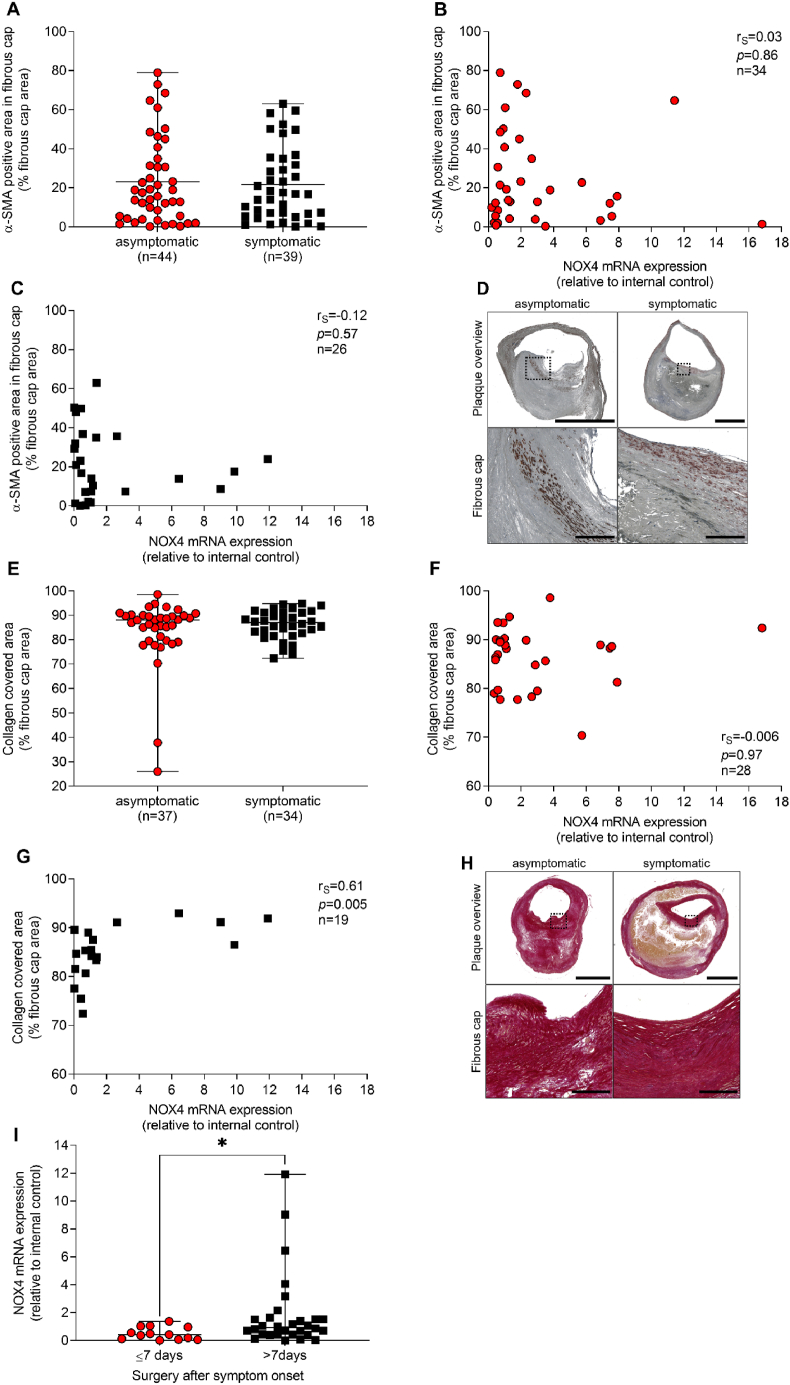
Apoptosis of smooth muscle cells, macrophages and foam cells contribute to growth of the necrotic core [21]. NOX4 can be pro-apoptotic in smooth muscle cells [22] and anti-apoptotic in cells of the renal cortex [23]. Considering that NOX4 determines endothelial cell homeostasis and quiescence, we examined apoptosis as a sign of endothelial cell integrity. Cleaved caspase-3 staining was analyzed as a marker of apoptosis within plaque sections. Cleaved caspase-3 positive areas were similar in plaques of asymptomatic and symptomatic CAS patients. NOX4 mRNA expression correlated inversely with cleaved caspase-3 in asymptomatic disease (rS = −0.41, p = 0.03) (Fig. 4A–C). In order to identify determinants of fibrous cap stability that may differ between plaques from asymptomatic and symptomatic CAS patients, smooth muscle cell-specific α-actin (α-SMA) and collagen positive areas were assessed and turned out to be similar in both groups (Fig. 5A–H). Collagen content positively correlated to NOX4 mRNA expression (rS = 0.61, p = 0.005) in symptomatic disease. Collagen is part of a massive remodeling process during healing after plaque rupture.
Fig. 4.
Correlation between NOX4 and apoptosis in atherosclerotic plaques obtained from patients with asymptomatic and symptomatic carotid artery stenosis. (A) Apoptotic areas were quantified using an antibody against cleaved caspase-3; positive areas were assessed and set in relation to the total plaque area. Data are presented as a scatter dot plot where the horizontal line depicts the median and were compared using the Mann-Whitney-U test. (B–C), Spearman's correlation (rS) between plaque NOX4 and cleaved caspase-3 positive areas in asymptomatic and symptomatic disease. Significant outliers were detected by Grubb's outlier test and one outlier for NOX4 expression within the asymptomatic group was omitted from correlational analysis. (D) Representative slides for cleaved caspase-3 immunohistochemistry in plaques from patients with asymptomatic and symptomatic carotid artery disease. Scale bar upper picture: 2.5 mm. Scale bar lower picture: 300 μm.
Fig. 5.
Correlation between NOX4 and fibrous cap stability in atherosclerotic plaques obtained from patients with asymptomatic and symptomatic carotid artery stenosis. (A) Smooth muscle cells were stained using a specific antibody against alpha-smooth muscle actin (α-SMA). Positive areas were quantified and were set in relation to the total fibrous cap area. (E) Collagen fibers were stained using Picro-sirius Red. Positive areas were quantified and were set in relation to the total fibrous cap area. (I) NOX4 expression in atherosclerotic plaques obtained within the first week (≤7 days) or in the second week (>7 days) after the initial neurological event. NOX4 expression was analyzed by qPCR and data are presented in relation to an internal control (=1). (A, E, I) Data are presented as scatter dot plots where the horizontal line depicts the median. (I) A significant outlier was detected in the data sets ≤7 days for >7 days by Grubb's outlier test and was omitted from the analysis. (A, E, I) Data were compared using the Mann-Whitney-U test. *p ≤ 0.05. (B, C), Spearman's correlation (rS) between plaque NOX4 expression and fibrous cap α-SMA positive areas in asymptomatic and symptomatic disease. (F, H), Spearman's correlation (rS) between plaque NOX4 expression and collagen covered areas in the main fibrous cap in asymptomatic and symptomatic disease. (D) Representative slides for (D) α-SMA immunohistochemistry and (G) Picro-sirius Red staining in plaques from patients with asymptomatic and symptomatic carotid artery disease. Scale bar upper picture: 2.5 mm. Scale bar lower picture: 300 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Based on the German guidelines, that recommend CEA within the first 14 days after symptom onset [24], a cut-off value of 7 days was set and both groups were analyzed for NOX4 mRNA expression. NOX4 mRNA expression was higher (p = 0.02) in plaques obtained in week two compared to week one after symptom onset (Fig. 5I), indicating a positive role of NOX4 in plaque stability and healing after plaque rupture.
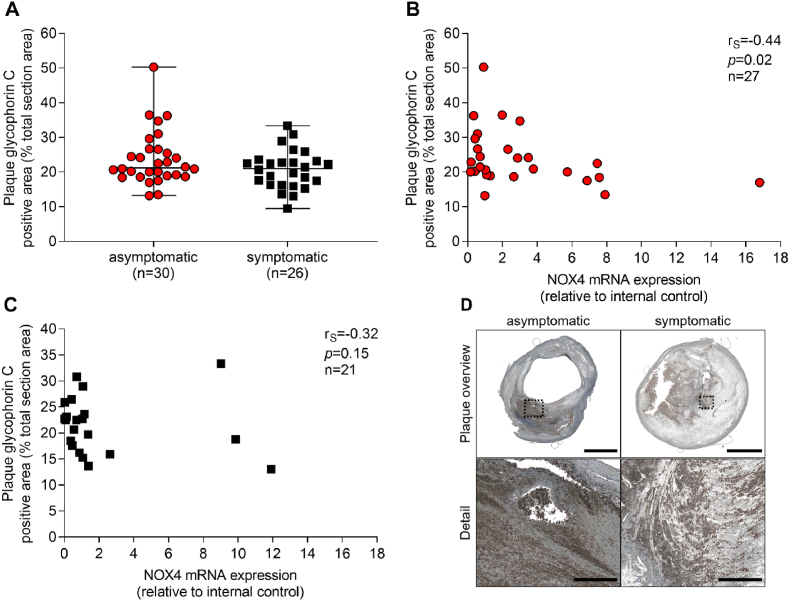
3.4. NOX4 negatively correlated with erythrocytes in plaques of asymptomatic disease
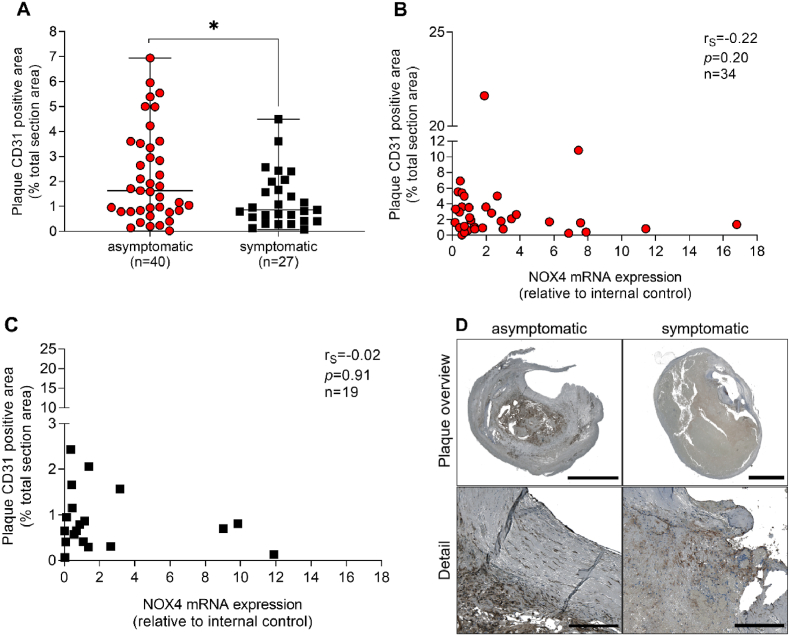
Intramural bleeding was quantified by staining of erythrocytes with glycophorin C. Erythrocyte content was similar in plaques from asymptomatic and symptomatic CAS patients. NOX4 mRNA expression inversely correlated with erythrocytes/glycophorin C staining (rS = −0.44, p = 0.02) in asymptomatic, but not in symptomatic disease (Fig. 6A–C). Plaque hemorrhage can result from extensive plaque angiogenesis when newly formed vessels remain unstable and leaky. Accordingly, we analyzed CD31 as a marker of endothelial cells in plaques from patients suffering from CAS with asymptomatic and symptomatic outcomes. CD31 positive areas were 1.8-fold higher in asymptomatic than in symptomatic plaques (p = 0.04) (Fig. 7A–C). Although NOX4 expression is high in endothelial cells [8], no significant association of CD31 areas with NOX4 mRNA expression was observed (rS = −0.34, p = 0.05). These data may indicate higher NOX4 mRNA expression in CD31 positive cells of plaques of symptomatic when compared to asymptomatic disease. Additionally, other than endothelial cells may contribute to NOX4 expression within the present study. Those however remain unidentified within the present study. A possible reason for this unexpected phenomenon could be an elevated number of macrophages that produce TGF-β [25], which might stimulate NOX4 expression in smooth muscle cells and fibroblasts [26,27].
Fig. 6.
Correlation between NOX4 and erythrocyte content in atherosclerotic plaques obtained from patients with asymptomatic and symptomatic carotid artery disease. (A) Erythrocytes were stained using a specific antibody against glycophorin C and positive areas were quantified. Data were set in relation to the total plaque area. (A), Data are presented as a scatter dot plot where the horizontal line depicts the median and were compared using the Mann-Whitney-U test. (B–C) Spearman's correlation (rS) between plaque NOX4 expression and erythrocytes in asymptomatic and symptomatic patients. (D) Representative slides for glycophorin C immunohistochemistry in plaques from patients with asymptomatic and symptomatic carotid artery disease. Scale bar upper picture: 2.5 mm. Scale bar lower picture: 300 μm.
Fig. 7.
Correlation between NOX4 and endothelial cell marker CD31 in atherosclerotic plaques obtained from patients with asymptomatic and symptomatic carotid artery stenosis. (A) Endothelial cells were stained using a specific antibody against CD31 and positive areas were quantified and set in relation to the total plaque area. (A), Data are presented as a scatter dot plot where the horizontal line depicts the median. Data were compared using the Mann-Whitney-U test. *p ≤ 0.05. (B–C) Spearman's correlation (rS) between plaque NOX4 and CD31 positive areas in asymptomatic and symptomatic disease. (D) Representative slides for CD31 immunohistochemistry in plaques from patients with asymptomatic and symptomatic carotid artery disease. Scale bar upper picture: 2.5 mm. Scale bar lower picture: 300 μm.
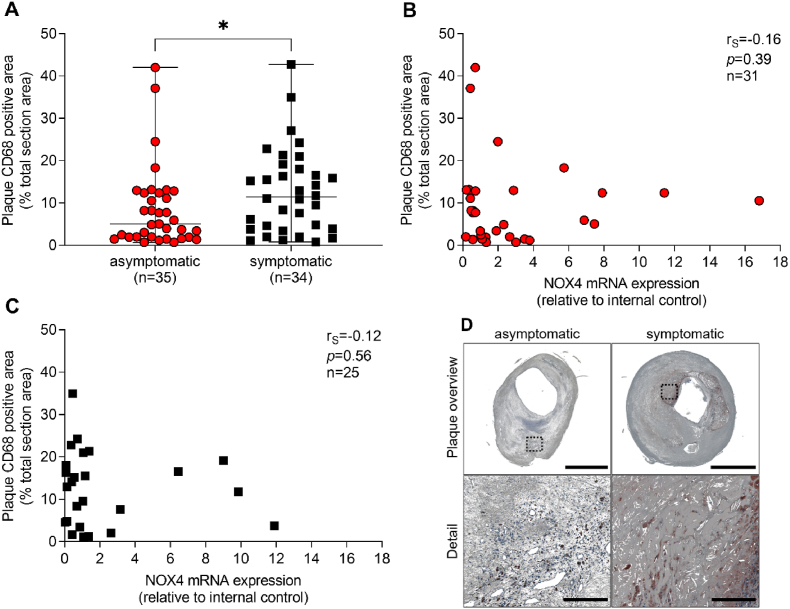
In fact, besides formation of leaky and unstable vessels, activated endothelial cells promote the adhesion of inflammatory cells [28]. We analyzed the macrophage content as measured as CD68 positive cells and the expression of inflammatory cytokines. Plaques from symptomatic patients contained 2.3-fold (p = 0.03) more CD68-positive cells compared to asymptomatic patients (Fig. 8A–C). When grouping the results from a Proteome Profiler Human XL Cytokine Array according to the function of the identified proteins, higher levels of pro-inflammatory factors and cytokines were seen in plaques from symptomatic CAS patients, compared to asymptomatic disease (Supplementary Table 3 and Supplementary Fig. 3). Those factors include urokinase receptor (p = 0.03), MMP9 (p = 0.02), monocyte chemoattractant protein-1 (MCP-1, p = 0.04), angiopoietin-2 (p = 0.04), vascular cell adhesion protein 1 (VCAM-1, p = 0.03) and interleukin-8 (IL-8, p = 0.02). High abundance of inflammatory cells and more pro-inflammatory cytokines may promote the secretion of matrix-degrading enzymes such as MMP9, while MMP2 is mainly found on smooth muscle cells and fibroblasts. Indeed, pro-MMP9 was significantly higher expressed (p = 0.02) in plaques from symptomatic patients when compared to plaques from asymptomatic disease. Activity of MMP9 was slightly different between asymptomatic and symptomatic patients. However, both MMP9 expression (rS = −0.49, p = 0.02) and activity (rS = −0.62, p = 0.002) inversely correlated to NOX4 mRNA expression in plaques from symptomatic patients (Supplementary Figs. 4A–F). Expression of pro-MMP2 and MMP2-activity was similar in plaques from asymptomatic and symptomatic patients (Supplementary Figs. 5A–F).
Fig. 8.
Correlation between NOX4 and macrophage content in atherosclerotic plaques obtained from patients with asymptomatic and symptomatic carotid artery stenosis (A) Macrophages were stained using a specific antibody against CD68 and positive areas were quantified and set in relation to the total plaque area. (A) Data are presented as a scatter dot plot where the horizontal line depicts the median. Data were compared using the Mann-Whitney-U test. *p ≤ 0.05. (B–C), Spearman's correlation (rS) between plaque NOX4 expression and macrophages in asymptomatic and symptomatic disease. (D) Representative slides for CD68 immunohistochemistry in plaques from patients with asymptomatic and symptomatic carotid artery disease. Scale bar upper picture: 2.5 mm. Scale bar lower picture: 300 μm.
In summary, these data indicate that NOX4 mRNA expression is associated with plaque stability, reduced hemorrhage and less inflammation in plaques of patients suffering from carotid artery stenosis.
4. Discussion
This study is the first to analyze a correlation of NOX4 mRNA expression and the risk of stroke or similar events in human carotid artery stenosis. NOX4 mRNA expression was reduced in plaques from patients with carotid artery stenosis who suffered from a symptomatic outcome. Coincidence further allowed correlating an elevated NOX4 mRNA expression with plaque stabilizing mechanisms in human advanced atherosclerotic plaques. NOX4 mRNA expression was inversely correlated with apoptosis, intra-plaque hemorrhage and inflammation, suggesting a protective role in plaques in advanced carotid artery stenosis. Eventually, the data obtained within the present study provide the first evidence that increased NOX4 mRNA expression may be protective after plaque rupture.
We focused on NOX4 and did not analyze other NOX isoforms. It is possible that superoxide anion radical-forming NADPH oxidases such as NOX1, NOX2 or NOX5 [30] will play a role in carotid artery stenosis as well. We hypothesize that their effects may be rather indirect. Furthermore, dismutation of the superoxide anion radicals into H2O2 may have an impact on oxidation of nucleic acids [31] or proteins [17] and signal transduction events [17].
NOX4 has been proven to protect from atherosclerosis in several murine models of the disease. NOX4 especially protects from macrophage accumulation at the endothelium of the vessel wall. It prevents inflammation and extracellular matrix remodeling [8,9,29]. Most studies analyzed the onset and short-term development of atherosclerotic plaques in mice. Late-stage outcomes have not been investigated in NOX4 knockout mice so far. In humans, atherosclerosis is a persistent disease, slowly progressing over decades. Plaque growth over time causes stenosis of the affected vessel that eventually reduces the blood flow below a critical level, thus resulting in perfusion limitations as seen in coronary artery disease, peripheral artery disease, cerebral and renal ischemia and others. In organs with sensitive innervation, including the heart and skeletal muscle, the patient recognizes pain. As a result, most patients visit a doctor due to pain caused by perfusion limitations in late stage atherosclerosis. CAS in contrast, does not cause pain and patients often see the doctor when the disease has progessed for a long time already. Accordingly, this study concentrates on very late stage atherosclerosis.
One major surprising finding of the current study was the relative uncertainty of a high degree of stenosis in NASCET criteria as a predictor for a symptomatic outcome of carotid artery stenosis. It is important to mention that no patients with low NASCET score (40–60%) were included and scoring was based on ultrasound instead of digital subtraction angiography. Besides stenosis, the most important danger of long-lasting atherosclerosis is the development of an unstable plaque and eventually plaque rupture. This event will lead to occlusion of peripheral vessels and subsequent organ failure such as myocardial infarction and stroke. Here we analyzed NOX4 mRNA expression in long-lasting human atherosclerosis in plaques obtained from patients with carotid artery disease. Those patients were separated into two groups: asymptomatic and symptomatic, suffering from stroke and other cerebral perfusion defects. Importantly, the group with symptomatic outcomes of a carotid artery stenosis includes both critically low perfusion and plaque rupture.
The finding that NOX4 mRNA expression was highest in fibrotic plaques without a lipid core and with possible small calcifications (type VIII) of asymptomatic CAS patients supports the hypothesis that NOX4 could be increased in plaques with low rupture tendency. Based on the large degree of stenosis, it is possible that hemodynamic forces [32] contribute to the changes in NOX4 expression. Accordingly, in human coronary arteries NOX4 expression is highest in type IV plaques and lowest in the most complicated lesion (type VI) [13]. In symptomatic CAS, NOX4 mRNA expression gradually decreased with severity of the plaque phenotype being the lowest in calcified plaques (type VII). Changes in NOX4 expression may be due to the distribution of different types of plaques among asymptomatic and symptomatic patients. Whether NOX4 is the cause or consequence of a more stable plaque architecture remains elusive because of the descriptive nature of the present study. Nevertheless, it is likely that plaque stabilization by a higher content of fibrous tissue or smooth muscles [13] is associated with an elevation of NOX4 mRNA expression. In line with this model, plaques from asymptomatic CAS patients express more NOX4 than those from symptomatic patients and high NOX4 mRNA expression correlates with an increased probability to be asymptomatic. Angiogenesis and accumulation of macrophages within a plaque are of major importance with respect to plaque stability [33,34]. Premature, irregular vessels contribute to plaque instability [33] and are highly susceptible to leakage and intraplaque hemorrhage [35] as features of plaque instability [36]. NOX4 promotes angiogenesis and prevents uncontrolled vessel growth and endothelial cell apoptosis [37]. Furthermore, NOX4 stabilizes the resulting vessel structure [[38], [39], [40]]. Simultaneously, NOX4 reduces inflammation [28] and macrophage polarization into the pro-inflammatory M1 phenotype [39]. Accordingly, potential reasons for NOX4-mediated plaque stability as identified herein could be reduced apoptosis and quiescence of endothelial cells. Low NOX4 abundancy may be linked to elevated intraplaque hemorrhage, macrophage infiltration and subsequent production of pro-inflammatory cytokines. In the present study, we did not clearly delineate the cell types that express NOX4. This has to be the subject of a follow-up study but requires specific antibodies, single-cell RNA-sequencing or probes for in situ hybridization. In previous studies using animal models, we found NOX4 expression mainly in the endothelial cells of the vessel wall [8]. NOX4 expression was also shown in other cells of the vessel wall including vascular smooth muscle cells, while macrophages express mainly the isoform NOX2 [8,41,42].
Upon partial plaque rupture, NOX4 could be involved in preventing further symptomatic events as supported by our finding of a negative correlation of fibrous cap collagen content and MMP9 activity with NOX4 mRNA expression in symptomatic patients. Inflammation promotes thinning of the fibrous cap and high amounts of macrophages and foam cells were found in ruptured caps [21]. In the present study, fibrous cap collagen content was analyzed because the fibrous cap mainly contributes to plaque stability. Analysis of the whole plaque content compared to the fibrous cap only [43] might have shown different findings. Macrophage-derived cytokines and matrix-degrading enzymes force inflammation and thinning of the fibrous cap [21]. Furthermore, elevated plasma MMP9 levels increase the risk of stroke [[44], [45], [46]]. Endarterectomized patients suffering from internal carotid artery stenosis who are treated with statins have lower percentage values of MMP9 area than untreated patients [45].
Interestingly, prescription of statins, CCBs and β-adrenergic blockers was higher in patients suffering from asymptomatic carotid artery disease. Especially statin pretreatment is associated with lower odds for a poor outcome after stroke [35,47]. CCBs as well as ACE inhibitors significantly decreased the incidence of stroke in hypertensive subjects [48]. Although our findings underline the importance of secondary prevention in atherosclerotic cardiovascular disease, we did not test here for the patients’ compliance to prescribed medications. Therefore, beneficial effects of statins, β-blockers and CCBs could be even higher.
A major limitation of the present study is its descriptive nature. Accordingly, mechanisms that regulate NOX4 mRNA expression or effects mediated by an altered NOX4 mRNA expression cannot be clearly assigned to protective effects like e.g. plaque stability. Cell-specific repression or overexpression of NOX4 mRNA to assess effects on plaque stability should be the object of further studies involving technologies like single-cell RNA-sequencing or even single-cell proteomics. In fact, the lack of NOX4 protein expression data, due to inappropriate specificity of all tested commercial antibodies for NOX4 in human atherosclerotic plaques, represents another major limitation of the present study.
In conclusion, the present study provides novel evidence for a protective role of an increase in NOX4 mRNA expression in patients with late-stage carotid artery stenosis. Our data support a linkage of NOX4 mRNA with plaque stability in humans. Finally, the study links NOX4 expression to plaque remodeling after an ischemic event; indicating a novel protective role of an increased NOX4 expression after plaque rupture.
Authorship
A.H., C.R. and K.S. designed the study; A.H., F.F., P.S., D.E., I.K. and B.H. conducted the experiments, A.H., F.F., D.E., I.K., D.M.P. and S.W. acquired the data; A.H., F.F., A.K. and K.S. analyzed the data; S.W. A.B. and C.R. collected the samples; A.H. and C.R. wrote the draft manuscript, and S.W., A.B., S.W., D.M.P., S.W. C.B., H.M., K.S. and C.R. edited the draft manuscript. All authors reviewed and approved the final manuscript and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This study was funded by the European Stroke Research Foundation (ESRF) to A.H. and C.R. F.F. received funding by the “Carl Gustav Carus Promotionskolleg” fellowship from the Medical Faculty of the TU Dresden. K.S. was supported by the Cardio-Pulmonary Institute (CPI), EXC 2026, Project ID: 390649896. C.B. and H.M. were funded by the “Deutsche Forschungsgemeinschaft“ (DFG) (Grant 47081312 to C.B., IRTG 2251 to C.B. and H.M.; MO 1695/4-1 and 5-1 to H.M.) and the German Centre for Cardiovascular Research (DZHK) (to K.S. and H.M.).
Declaration of competing interest
All authors do not have any conflict of interest.
Acknowledgment
We would thank Ellen Geibelt from the Light Microscopy Facility, a Core Facility of the CMCB Technology Platform at TU Dresden, for her support at the slide scanner. The authors thank Josef Nees, MD, from the Department of Medicine III for his support in the analysis of medical therapies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2022.102473.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Campbell B.C.V., De Silva D.A., Macleod M.R., Coutts S.B., Schwamm L.H., Davis S.M., Donnan G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019;5:70. doi: 10.1038/s41572-019-0118-8. [DOI] [PubMed] [Google Scholar]
- 2.Stroke-Collaborators Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795–820. doi: 10.1016/S1474-4422(21)00252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goessens B.M.B., Visseren F.L.J., Kappelle L.J., Algra A., Graaf Yvd. Asymptomatic carotid artery stenosis and the risk of new vascular events in patients with manifest arterial disease. Stroke. 2007;38:1470–1475. doi: 10.1161/STROKEAHA.106.477091. [DOI] [PubMed] [Google Scholar]
- 4.Howard D.P.J., Gaziano L., Rothwell P.M. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol. 2021;20:193–202. doi: 10.1016/S1474-4422(20)30484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakhan S.E., Kirchgessner A., Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J. Transl. Med. 2009;7 doi: 10.1186/1479-5876-7-97. 97-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sies H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 2017;11:613–619. doi: 10.1016/j.redox.2016.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brandes R.P., Weissmann N., Schröder K. Nox family NADPH oxidases: molecular mechanisms of activation. Free Radic. Biol. Med. 2014;76:208–226. doi: 10.1016/j.freeradbiomed.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 8.Langbein H., Brunssen C., Hofmann A., Cimalla P., Brux M., Bornstein S.R., Deussen A., Koch E., Morawietz H. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Eur. Heart J. 2016;37:1753–1761. doi: 10.1093/eurheartj/ehv564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schürmann C., Rezende F., Kruse C., Yasar Y., Löwe O., Fork C., van de Sluis B., Bremer R., Weissmann N., Shah A.M., Jo H., Brandes R.P., Schröder K. The NADPH oxidase Nox4 has anti-atherosclerotic functions. Eur. Heart J. 2015;36:3447–3456. doi: 10.1093/eurheartj/ehv460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu P., Wu X., Khandelwal A.R., Yu W., Xu Z., Chen L., Yang J., Weisbrod R.M., Lee K.S.S., Seta F., Hammock B.D., Cohen R.A., Zeng C., Tong X. Endothelial Nox4-based NADPH oxidase regulates atherosclerosis via soluble epoxide hydrolase. Biochim. Biophys. Acta, Mol. Basis Dis. 2017;1863:1382–1391. doi: 10.1016/j.bbadis.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchmann G.K., Schürmann C., Spaeth M., Abplanalp W., Tombor L., John D., Warwick T., Rezende F., Weigert A., Shah A.M., Hansmann M.L., Weissmann N., Dimmeler S., Schröder K., Brandes R.P. The hydrogen-peroxide producing NADPH oxidase 4 does not limit neointima development after vascular injury in mice. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigala F., Efentakis P., Karageorgiadi D., Filis K., Zampas P., Iliodromitis E.K., Zografos G., Papapetropoulos A., Andreadou I. Reciprocal regulation of eNOS, H2S and CO-synthesizing enzymes in human atheroma: correlation with plaque stability and effects of simvastatin. Redox Biol. 2017;12:70–81. doi: 10.1016/j.redox.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sorescu D., Weiss D., Lassègue B., Clempus R.E., Szöcs K., Sorescu G.P., Valppu L., Quinn M.T., Lambeth J.D., Vega J.D., Taylor W.R., Griendling K.K. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 14.Barnett H.J.M., Taylor D.W., Haynes R.B., Sackett D.L., Peerless S.J., Ferguson G.G., Fox A.J., Rankin R.N., Hachinski V.C., Wiebers D.O., Eliasziw M. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N. Engl. J. Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 15.Chang Y.J., Golby A.J., Albers G.W. Detection of carotid stenosis. From NASCET results to clinical practice. Stroke. 1995;26:1325–1328. doi: 10.1161/01.str.26.8.1325. [DOI] [PubMed] [Google Scholar]
- 16.Hakami N., Ranjan A., Hardikar A., Dusting G., Peshavariya H. Role of NADPH oxidase-4 in human endothelial progenitor cells. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bretón-Romero R., Lamas S. Hydrogen peroxide signaling in vascular endothelial cells. Redox Biol. 2014;2:529–534. doi: 10.1016/j.redox.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plecita-Hlavata L., Jaburek M., Holendova B., Tauber J., Pavluch V., Berkova Z., Cahova M., Schroder K., Brandes R.P., Siemen D., Jezek P. Glucose-stimulated insulin secretion fundamentally requires H2O2 signaling by NADPH oxidase 4. Diabetes. 2020;69:1341–1354. doi: 10.2337/db19-1130. [DOI] [PubMed] [Google Scholar]
- 19.Cai J.-M., Hatsukami T.S., Ferguson M.S., Small R., Polissar N.L., Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002;106:1368–1373. doi: 10.1161/01.cir.0000028591.44554.f9. [DOI] [PubMed] [Google Scholar]
- 20.Golledge J., Greenhalgh R.M., Davies A.H. The symptomatic carotid plaque. Stroke. 2000;31:774–781. doi: 10.1161/01.str.31.3.774. [DOI] [PubMed] [Google Scholar]
- 21.Bentzon J.F., Otsuka F., Virmani R., Falk E. Mechanisms of plaque formation and rupture. Circ. Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 22.Xu S., Chamseddine A.H., Carrell S., Miller F.J., Jr. Nox4 NADPH oxidase contributes to smooth muscle cell phenotypes associated with unstable atherosclerotic plaques. Redox Biol. 2014;2:642–650. doi: 10.1016/j.redox.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nlandu-Khodo S., Dissard R., Hasler U., Schäfer M., Pircher H., Jansen-Durr P., Krause K.H., Martin P.-Y., de Seigneux S. NADPH oxidase 4 deficiency increases tubular cell death during acute ischemic reperfusion injury. Sci. Rep. 2016;6 doi: 10.1038/srep38598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eckstein H., Kühnl A., Berkefeld J., Dörfler A., Kopp I., Langhoff R., Lawall H., Ringleb P., Sander D., Storck M. second ed. 2020. [German S3 Guideline for Diagnosis, Therapy and Aftercare of Extracranial Carotid Stenosis] February 3, 2020 AWMF Registration number: 004-028. [Google Scholar]
- 25.Camaré C., Pucelle M., Nègre-Salvayre A., Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017;12:18–34. doi: 10.1016/j.redox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang F., Liu G.S., Dusting G.J., Chan E.C. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol. 2014;2:267–272. doi: 10.1016/j.redox.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturrock A., Cahill B., Norman K., Huecksteadt T.P., Hill K., Sanders K., Karwande S.V., Stringham J.C., Bull D.A., Gleich M., Kennedy T.P., Hoidal J.R. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 28.Schröder K., Zhang M., Benkhoff S., Mieth A., Pliquett R., Kosowski J., Kruse C., Luedike P., Michaelis U.R., Weissmann N., Dimmeler S., Shah A.M., Brandes R.P. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ. Res. 2012;110:1217–1225. doi: 10.1161/CIRCRESAHA.112.267054. [DOI] [PubMed] [Google Scholar]
- 29.Gray S.P., Di Marco E., Kennedy K., Chew P., Okabe J., El-Osta A., Calkin A.C., Biessen E.A., Touyz R.M., Cooper M.E., Schmidt H.H., Jandeleit-Dahm K.A. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling. Arterioscler. Thromb. Vasc. Biol. 2016;36:295–307. doi: 10.1161/ATVBAHA.115.307012. [DOI] [PubMed] [Google Scholar]
- 30.Schröder K. NADPH oxidases: current aspects and tools. Redox Biol. 2020;34 doi: 10.1016/j.redox.2020.101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hofer T., Badouard C., Bajak E., Ravanat J.L., Mattsson A., Cotgreave I.A. Hydrogen peroxide causes greater oxidation in cellular RNA than in DNA. Biol. Chem. 2005;386:333–337. doi: 10.1515/BC.2005.040. [DOI] [PubMed] [Google Scholar]
- 32.Stary H.C., Chandler A.B., Dinsmore R.E., Fuster V., Glagov S., Insull W., Rosenfeld M.E., Schwartz C.J., Wagner W.D., Wissler R.W. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Circulation. 1995;92:1355–1374. doi: 10.1161/01.cir.92.5.1355. [DOI] [PubMed] [Google Scholar]
- 33.McCarthy M.J., Loftus I.M., Thompson M.M., Jones L., London N.J., Bell P.R., Naylor A.R., Brindle N.P. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J. Vasc. Surg. 1999;30:261–268. doi: 10.1016/s0741-5214(99)70136-9. [DOI] [PubMed] [Google Scholar]
- 34.Moulton K.S., Vakili K., Zurakowski D., Soliman M., Butterfield C., Sylvin E., Lo K.M., Gillies S., Javaherian K., Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parma L., Baganha F., Quax P.H.A., de Vries M.R. Plaque angiogenesis and intraplaque hemorrhage in atherosclerosis. Eur. J. Pharmacol. 2017;816:107–115. doi: 10.1016/j.ejphar.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 36.Virmani R., Kolodgie F.D., Burke A.P., Finn A.V., Gold H.K., Tulenko T.N., Wrenn S.P., Narula J. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler. Thromb. Vasc. Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 37.Datla S.R., Peshavariya H., Dusting G.J., Mahadev K., Goldstein B.J., Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler. Thromb. Vasc. Biol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 38.Craige S.M., Chen K., Pei Y., Li C., Huang X., Chen C., Shibata R., Sato K., Walsh K., Keaney J.F. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–740. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helfinger V., Palfi K., Weigert A., Schröder K. The NADPH oxidase Nox4 controls macrophage polarization in an NFκB-dependent manner. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/3264858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vogel J., Kruse C., Zhang M., Schröder K. Nox4 supports proper capillary growth in exercise and retina neo-vascularization. J. Physiol. 2015;593:2145–2154. doi: 10.1113/jphysiol.2014.284901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brendel H., Shahid A., Hofmann A., Mittag J., Bornstein S.R., Morawietz H., Brunssen C. NADPH oxidase 4 mediates the protective effects of physical activity against obesity-induced vascular dysfunction. Cardiovasc. Res. 2020;116:1767–1778. doi: 10.1093/cvr/cvz322. [DOI] [PubMed] [Google Scholar]
- 42.Morawietz H. Cardiovascular protection by Nox4. Cardiovasc. Res. 2018;114:353–355. doi: 10.1093/cvr/cvx252. [DOI] [PubMed] [Google Scholar]
- 43.Dhume A.S., Soundararajan K., Hunter W.J., 3rd, Agrawal D.K. Comparison of vascular smooth muscle cell apoptosis and fibrous cap morphology in symptomatic and asymptomatic carotid artery disease. Ann. Vasc. Surg. 2003;17:1–8. doi: 10.1007/s10016-001-0331-1. [DOI] [PubMed] [Google Scholar]
- 44.Eldrup N., Grønholdt M.-L.M., Sillesen H., Nordestgaard B.G. Elevated matrix metalloproteinase-9 associated with stroke or cardiovascular death in patients with carotid stenosis. Circulation. 2006;114:1847–1854. doi: 10.1161/CIRCULATIONAHA.105.593483. [DOI] [PubMed] [Google Scholar]
- 45.Kunte H., Amberger N., Busch M.A., Rückert R.I., Meiners S., Harms L. Markers of instability in high-risk carotid plaques are reduced by statins. J. Vasc. Surg. 2008;47:513–522. doi: 10.1016/j.jvs.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 46.Jager N.A., Wallis de Vries B.M., Hillebrands J.L., Harlaar N.J., Tio R.A., Slart R.H., van Dam G.M., Boersma H.H., Zeebregts C.J., Westra J. Distribution of matrix metalloproteinases in human atherosclerotic carotid plaques and their production by smooth muscle cells and macrophage subsets. Mol. Imag. Biol. 2016;18:283–291. doi: 10.1007/s11307-015-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reeves M.J., Gargano J.W., Luo Z., Mullard A.J., Jacobs B.S., Majid A. Effect of pretreatment with statins on ischemic stroke outcomes. Stroke. 2008;39:1779–1785. doi: 10.1161/STROKEAHA.107.501700. [DOI] [PubMed] [Google Scholar]
- 48.Chen G.J., Yang M.S. The effects of calcium channel blockers in the prevention of stroke in adults with hypertension: a meta-analysis of data from 273,543 participants in 31 randomized controlled trials. PLoS One. 2013;8 doi: 10.1371/journal.pone.0057854. e57854-e57854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.