Abstract
Aim
Both skin-sparing mastectomy (SSM) and nipple-sparing mastectomy (NSM) have been widely adopted. Although postmastectomy radiation therapy (PMRT) can improve clinical outcomes, it can worsen cosmesis following reconstruction. Therefore, identifying risk factors of ipsilateral breast tumor recurrence (IBTR) could help de-escalate PMRT after NSM/SSM in patients with pT1-2 disease.
Methods
We retrospectively reviewed patients treated with SSM (N = 400) and NSM (N = 156) in patients with pT1-2N0-1 disease between 2009 and 2016. Seventy-four patients received PMRT with 50–50.4 Gy in 25–28 fractions. The Cox proportional hazards model was used to analyze the prognostic factors of IBTR.
Results
With a median follow-up of 66.2 months, 17 IBTR events were observed, with 5-year IBTR-free rate of 97.2%. Although only one IBTR was observed after PMRT, there was no statistical difference in the 5-year IBTR-free rate (PMRT vs. no PMRT, 98.6% vs. 97.0%, p = 0.360). Multivariable analyses demonstrated that age ≤45 years and lymphovascular invasion (LVI) were adverse features of IBTR. The low-risk group (0 risk factor) showed a better 5-year IBTR-free rate than the high-risk group (≥1 risk factor) (100.0% vs. 95.8%, p = 0.003). In the high-risk group, PMRT slightly improved 5-year IBTR-free rate compared with no PMRT (98.6% vs. 95.2%, p = 0.166). In addition, PMRT increased 5-year cumulative incidence of reconstruction failure (10.0% vs. 2.8%, p = 0.001).
Conclusion
We identified risk factors (age and LVI) related to IBTR following upfront SSM/NSM with pT1-2 disease. As a hypothesis-generating study, de-escalation of PMRT by omitting chest wall irradiation in selective patients could improve reconstruction-related complications without compromising oncologic outcomes.
Keywords: Breast cancer, Mastectomy, Skin-sparing mastectomy, Nipple-sparing mastectomy, Radiation therapy, Local recurrence
Highlights
-
•
IBTR-free rate at 5 years after SSM/NSM in pT1-2N0-1 disease was 97.2%.
-
•
Age ≤45 years and tumor with LVI were associated with increased IBTR rate.
-
•
Omitting chest wall RT to selective patients could improve reconstruction outcomes.
1. Introduction
Relatively conservative mastectomies, such as skin-sparing mastectomy (SSM) and nipple-sparing mastectomy (NSM), have been widely adopted recently with increasing interest in improved cosmetic outcomes and immediate breast reconstruction [1,2]. With regard to postmastectomy radiation therapy (PMRT), the indications for PMRT were revisited through recent guidelines and studies, including patients with intermediate-risk factors [[3], [4], [5], [6], [7]]. Therefore, adjuvant radiation therapy (RT) following reconstruction has become a common practice [8]. However, the integration of PMRT and reconstruction contributed to poor patient satisfaction from cosmetic outcomes, increased toxicities of capsular contracture (2.2–51%), and even increased failures of reconstruction (6.4–40.0%) [9,10].
Based on the low rate of local recurrence (<5%) following SSM or NSM in pT1-2 disease, de-escalating PMRT by omitting chest wall irradiation could minimize possible toxicities [11]. However, few data are available to show the possible risk factors for recurrences limited to the ipsilateral chest wall/skin following SSM or NSM [[12], [13], [14], [15]]. Furthermore, there has been no attempt of omitting chest wall RT in the pT1-2N0-1 disease. We hypothesized that identifying the risk factors related to recurrence could categorize potential candidates for omitting chest wall RT. In this context, we aimed to comprehensively analyze the prognostic factors related to local recurrence of T1-2 breast cancer following upfront SSM or NSM.
2. Materials and methods
2.1. Study population
We retrospectively reviewed 816 patients who underwent SSM or NSM between January 2009 and December 2016 at Samsung Medical Center. The exclusion criteria were as follows: (a) diagnosis of ductal carcinoma in situ or phyllodes tumor (N = 107), (b) receiving neoadjuvant chemotherapy (N = 53), (c) pT3Nx stage (N = 31), and (d) positive resection margin (N = 4). We identified and included 556 patients: 482 were treated without PMRT (no PMRT group) and 74 were treated with PMRT (PMRT group). This study was approved by the institutional review board (No. 2020-10-175).
2.2. Surgery
Overall, 400 (71.9%) and 156 (28.1%) patients underwent SSM and NSM, respectively. Most patients (N = 543, 97.7%) underwent reconstruction with subpectoral insertion: immediate reconstruction in 124 patients (22.3%), two-stage reconstruction in 415 patients (74.6%), and delayed reconstruction in four patients (0.7%). At the time of PMRT, tissue expander was irradiated in 59 patients, and autologous tissue was irradiated in 11 patients. Regarding complete reconstruction, 441 patients received implant-based reconstruction, and 102 patients received autologous tissue-based reconstruction. For 393 patients with cN0 disease, sentinel lymph node biopsy was performed with a median number of dissected lymph nodes of 5 (interquartile range [IQR] 4–7); 163 patients underwent upfront axillary node dissection with a median number of dissected lymph nodes of 18 (IQR, 13–22).
2.3. Radiation therapy
Overall, PMRT was performed at median 6.8 months (IQR, 6.4–7.2) following surgery. Based on institutional policy, PMRT was considered in pN1 disease with two or more risk factors such as > 1 positive lymph nodes, lymphovascular invasion (LVI, either focal or extensive), extranodal extension, and involvement of axillary level II or III [16]. Also, patients with pT2N0 disease was treated PMRT due to close margin (≤2 mm) [17,18]. Chest wall and axillar level II-III were covered by PMRT planning. Tissue expander was fully inflated before PMRT. Supraclavicular node irradiation was performed when pN1 disease with 4 or more predictive index scores (Supplementary Table 1) [19]. Internal mammary node irradiation was not performed. All PMRT planning was performed using three-dimensional conformal RT planning with 6 MV photons. A dose of 50–50.4 Gy in 25–28 fractions over 5 weeks was prescribed.
2.4. Follow-up evaluation
Ipsilateral breast tumor recurrence (IBTR) was defined as a local recurrence in the skin, nipple-areola complex, or chest wall muscles. IBTR was confirmed through a needle or excisional biopsy. Reconstruction-related failure was evaluated when implant/expander/flap removal was recommended due to complications.
2.5. Statistical analysis
Differences between the PMRT and no PMRT groups were analyzed using the Pearson chi-square or Fisher's exact test (categorical variables) and the Mann–Whitney U test (continuous variables). The primary endpoint of this study was the IBTR-free rate, and the secondary endpoints were disease-free survival (DFS), overall survival (OS), and reconstruction failure rate. DFS was calculated from the date of surgery to the date of any event (locoregional recurrence or distant metastasis), death, or last follow-up. The Kaplan–Meier method was used to estimate the IBTR-free rate, DFS, and OS using the log-rank test for comparison. The failure rate of reconstruction from surgery was estimated using the cumulative incidence method and compared using Gray's test, which considered death and IBTR as competing risks. The Cox proportional hazards model was used to analyze the significance of prognostic factors that were statistically significant in univariable analyses for IBTR, DFS, and OS. Recursive partitioning analysis (RPA) was performed to stratify patients according to their risk of IBTR using the R-package, “rpart.” Propensity score matching (PSM) analysis was carried out to minimize the effects of selection biases and potential confounders using the R-package, “MatchIt”. Propensity scores were obtained using a multivariate logistic regression model including age, tumor location (lateral vs. central/medial), grade, molecular subtype, number of positive axillar lymph nodes, and LVI. Patients were matched 1:1 nearest matching with a caliper distance of 0.05, standard deviations of the logit of the propensity score. McNemar's test and Wilcoxon signed-rank test were used to compare categorical and continuous variables after PSM. A two-sided P-value of < 0.05 was considered significant. All statistical analyses were performed using the R software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline characteristics
The baseline characteristics of the cohort before and after PSM are summarized in Table 1. Patients in the PMRT group were younger (age ≤45 years, 68.9% vs. 53.7%, p = 0.020), and had frequent intermediate/high-grade tumors (90.6% vs. 73.5%, p = 0.005), a more advanced stage (N1, 90.5% vs. 24.3%, p < 0.001), and frequent LVI (81.1% vs. 24.3%, p < 0.001) compared to those in the no PMRT group. After PSM, there were 31 patients in each group with well-balanced baseline characteristics.
Table 1.
Baseline characteristics.
| Before matching |
After matching |
||||||
|---|---|---|---|---|---|---|---|
| No PMRT |
PMRT |
P-value | No PMRT |
PMRT |
P-value | ||
| N = 482 | N = 74 | N = 31 | N = 31 | ||||
| Age (years) | 45 [[40], [41], [42], [43], [44], [45], [46], [47], [48], [49]] | 42 [[37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] | 0.007 | 41 [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]] | 42 [[38], [39], [40], [41], [42], [43], [44], [45]] | 0.521 | |
| Age ≤45 | 259 (53.7) | 51 (68.9) | 0.020 | 8 (25.8) | 7 (22.6) | 1.000 | |
| Age >45 | 223 (46.3) | 23 (31.1) | 23 (74.2) | 24 (77.4) | |||
| Laterality | Left | 244 (50.6) | 39 (52.7) | 0.835 | 16 (51.6) | 14 (45.2) | 0.799 |
| Right | 238 (49.4) | 35 (47.3) | 15 (48.4) | 17 (54.8) | |||
| Location | Lateral | 167 (34.6) | 25 (33.8) | 0.989 | 16 (51.6) | 12 (38.7) | 0.444 |
| Central/Medial | 315 (65.4) | 49 (66.2) | 15 (48.4) | 19 (61.3) | |||
| Multicentricity | 113 (23.4) | 25 (33.8) | 0.076 | 6 (19.4) | 10 (32.3) | 0.384 | |
| Multifocality | 232 (48.1) | 36 (48.6) | 1.000 | 11 (35.5) | 15 (48.4) | 0.440 | |
| Pathology | IDC | 421 (87.3) | 69 (93.2) | 0.205 | 30 (96.8) | 28 (90.3) | 0.742 |
| ILC | 19 (3.9) | 3 (4.1) | 0 (0.0) | 2 (6.5) | |||
| Others | 42 (8.7) | 2 (2.7) | 1 (3.2) | 1 (3.2) | |||
| Grade | Low | 128 (26.6) | 7 (9.5) | 0.005 | 7 (22.6) | 3 (9.7) | 0.317 |
| Intermediate | 252 (52.3) | 50 (67.6) | 18 (58.1) | 23 (74.2) | |||
| High | 102 (21.2) | 17 (23.0) | 6 (19.4) | 5 (16.1) | |||
| Subtype | HR positive | 392 (81.3) | 68 (91.9) | 0.084 | 29 (93.5) | 29 (93.5) | 1.000 |
| HER2 positive | 64 (13.3) | 5 (6.8) | 2 (6.5) | 2 (6.5) | |||
| TNBC | 26 (5.4) | 1 (1.4) | 0 (0.0) | 0 (0.0) | |||
| High Ki67 (≥20%) | 197 (40.9) | 37 (50.0) | 0.176 | 15 (48.4) | 13 (41.9) | 0.799 | |
| Stage | pT1-2N0 | 365 (75.7) | 7 (9.5) | <.001 | 6 (19.4) | 7 (22.6) | 0.331 |
| pT1N1 | 61 (12.7) | 16 (21.6) | 10 (32.3) | 5 (16.1) | |||
| pT2N1 | 56 (11.6) | 51 (68.9) | 15 (48.4) | 19 (61.3) | |||
| LN metastasis | No | 365 (75.7) | 7 (9.5) | <.001 | 6 (19.4) | 7 (22.6) | 0.324 |
| 1 node | 88 (18.3) | 8 (10.8) | 11 (35.5) | 6 (19.4) | |||
| 2 nodes | 24 (5.0) | 18 (24.3) | 9 (29.0) | 15 (48.4) | |||
| 3 nodes | 5 (1.0) | 41 (55.3) | 5 (16.1) | 3 (9.7) | |||
| LVI | Positive | 117 (24.3) | 60 (81.1) | <.001 | 17 (54.8) | 20 (64.5) | 0.605 |
| SA extension | Invasive/DCIS | 218 (45.2) | 38 (51.4) | 0.391 | 19 (61.3) | 18 (58.1) | 1.000 |
| Resection margin | Negative | 252 (52.3) | 31 (41.9) | 0.124 | 14 (45.2) | 14 (45.2) | 1.000 |
| Close (≤2 mm) | 230 (47.7) | 43 (58.1) | 17 (54.8) | 17 (54.8) | |||
| Surgery | SSM | 347 (72.0) | 53 (71.6) | 1.000 | 28 (90.3) | 22 (71.0) | 0.108 |
| NSM | 135 (28.0) | 21 (28.4) | 3 (9.7) | 9 (29.0) | |||
| LN dissection | SLNB | 381 (79.0) | 12 (16.2) | <.001 | 8 (25.8) | 11 (35.5) | 0.582 |
| ALND | 101 (21.0) | 62 (83.8) | 23 (74.2) | 20 (64.5) | |||
| Adjuvant treatment | |||||||
| Anthracycline | 204 (42.3) | 62 (83.8) | <.001 | 23 (74.2) | 25 (80.6) | 0.761 | |
| Taxane | 97 (20.1) | 62 (83.8) | <.001 | 19 (61.3) | 22 (71.0) | 0.591 | |
| Trastuzumab | 70 (14.5) | 12 (16.2) | 0.836 | 5 (16.1) | 5 (16.1) | 1.000 | |
| Endocrine therapy | 384 (79.7) | 68 (91.9) | 0.019 | 29 (93.5) | 28 (90.3) | 1.000 | |
** Values are presented as patient (%) or median [interquartile range].
Abbreviations: PMRT, postmastectomy radiation therapy; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer; LN, lymph node; LVI, lymphovascular invasion; SA, subareolar; DCIS, ductal carcinoma in situ; SSM, skin-sparing mastectomy; NSM, nipple-sparing mastectomy; SLNB, sentinel lymph node biopsy; ALND, axillary lymph node dissection.
3.2. Clinical outcomes
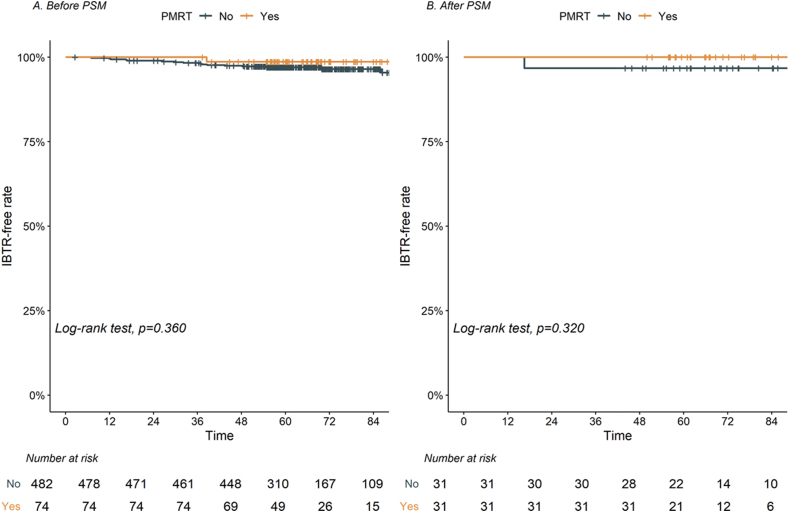
With a median follow-up of 66.2 months (IQR, 58.5–80.4), there were 17 (3.1%) IBTR events: 16 (3.3%) and one (1.4%) in the no PMRT and PMRT groups, respectively. Details regarding patterns of failure are summarized in Supplementary Table 2. Specifically, most IBTR events occurred in the subcutaneous area (N = 12), followed by pectoralis muscle (N = 3), and cutaneous area (N = 2). In addition, 11 IBTR events were located in ventral to implant, followed by the periareolar area (N = 4), medial to implant (N = 1), and dorsal to free flap (N = 1). There was no IBTR in dorsal part of implant. There was no difference in regional recurrence, distant metastasis, and death according to PMRT. The 5-year IBTR-free, DFS, and OS rates for all patients were 97.2%, 92.8%, and 98.5%, respectively. Patients in the PMRT group exhibited a 5-year IBTR-free rate comparable to those in the no PMRT group (98.6% vs. 97.0%, p = 0.360; Fig. 1). After PSM, there was only 1 IBTR (1.6%) in the matched cohort and 5-year IBTR-free rates were comparable between PMRT and no PMRT group (100.0% vs. 96.8%, p = 0.320, Fig. 1B).
Revised Figure 1.
Ipsilateral breast tumor recurrence (IBTR)-free rate according to postmastectomy radiation therapy (PMRT) (A) before propensity score matching (PSM), and (B) after PSM.
Regarding the results of the Cox proportional hazards model, age ≤45 years and LVI were associated with frequent IBTR events (Table 2). Based on this result, RPA resulted in three terminal nodes: group 1 (age >45 years, LVI-negative), group 2 (age >45 years, LVI-positive), and group 3 (age ≤45 years) (Supplementary Fig. 1).
Table 2.
Prognostic factors for ipsilateral breast tumor recurrence.
| Variables | (Ref. vs.) | Univariable analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| PMRT | (No vs. Yes) | 0.40 | 0.05–3.04 | 0.379 | 0.20 | 0.03–1.55 | 0.124 |
| Age (years) | (>45 vs. ≤ 45) | 5.87 | 1.34–25.69 | 0.019 | 5.06 | 1.14–22.40 | 0.033 |
| Location | (Lateral vs. Central/Medial) | 1.74 | 0.57–5.33 | 0.334 | |||
| Multicentricity | (No vs. Yes) | 0.88 | 0.29–2.69 | 0.819 | |||
| Multifocality | (No vs. Yes) | 1.25 | 0.48–3.25 | 0.645 | |||
| Grade | (Low/Intermediate vs. High) | 1.56 | 0.55–4.44 | 0.402 | |||
| Subtype | (HR positive vs. HER2 positive) | 1.58 | 0.45–5.55 | 0.474 | |||
| (HR positive vs. TNBC) | 1.35 | 0.18–10.35 | 0.772 | ||||
| High Ki67 (≥20) | (No vs. Yes) | 1.57 | 0.61–4.07 | 0.353 | |||
| pT stage | (pT1 vs. pT2) | 1.11 | 0.42–2.93 | 0.825 | |||
| pN stage | (N0 vs. N1) | 0.58 | 0.19–1.79 | 0.348 | |||
| LVI | (No vs. Yes) | 3.13 | 1.19–8.23 | 0.021 | 3.37 | 1.25–9.04 | 0.016 |
| Subareolar extension | (No vs. Yes) | 0.36 | 0.12–1.12 | 0.078 | |||
| Resection margin | (Negative vs. Closea) | 1.72 | 0.49–5.98 | 0.395 | |||
| Surgery | (SSM vs. NSM) | 1.03 | 0.36–2.93 | 0.954 | |||
| Taxane | (No vs. Yes) | 0.51 | 0.14–1.76 | 0.284 | |||
Abbreviations: HR, hazard ratio; PMRT, postmastectomy radiation therapy; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; TNBC, triple-negative breast cancer; LVI, lymphovascular invasion; SSM, skin-sparing mastectomy; NSM, nipple-sparing mastectomy; CI, confidence interval.
Close margin refers to margin width ≤2 mm.
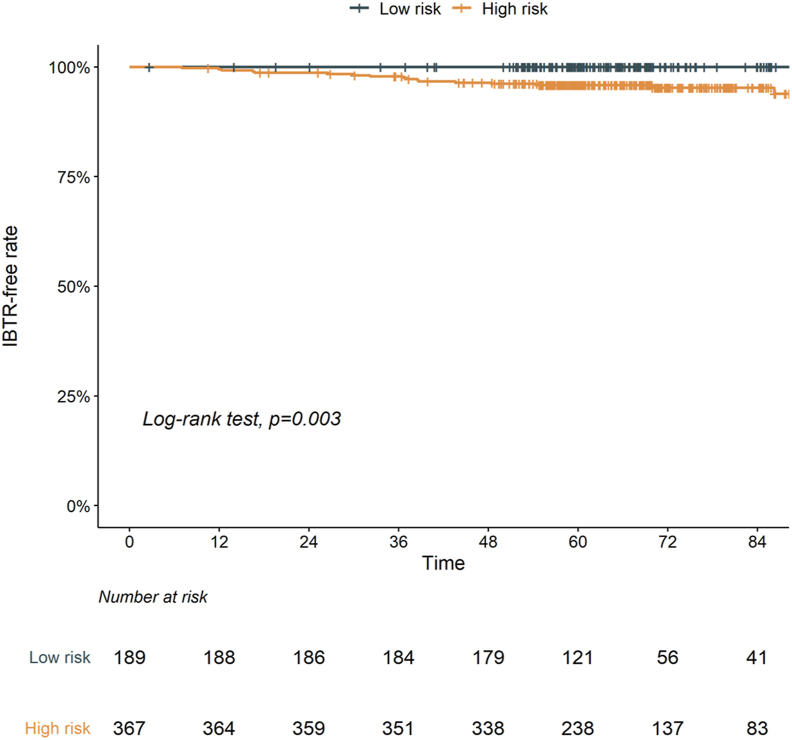
The 5-year IBTR-free rates for groups 1, 2, and 3 were 100.0%, 96.5%, and 95.7%, respectively (p < 0.05, group 1 vs. 2 and group 1 vs. 3; Supplementary Fig. 2). Groups 2 and 3, which had similar IBTR-free rates, were merged and classified as the “high-risk” group (N = 367) and group 1 was classified as the “low-risk” group (N = 189). The patterns of failure according to the risk group are summarized in Supplementary Table 3. The high-risk group showed a significantly lower 5-year IBTR-free rate (95.8% vs. 100.0%, p = 0.003; Fig. 2) and 5-year DFS rate (91.0% vs. 96.3%, p = 0.014; Supplementary Fig. 3A) than the low-risk group, with a comparable 5-year OS rate (99.4% vs. 96.8%, p = 0.120; Supplementary Fig. 3B).
Fig. 2.
Ipsilateral breast tumor recurrence (IBTR)-free rate according to risk group stratified by age (45 years) and lymphovascular invasion. Footnotes: High-risk group, 1 or 2 risk factors; low-risk group, 0 risk factors.
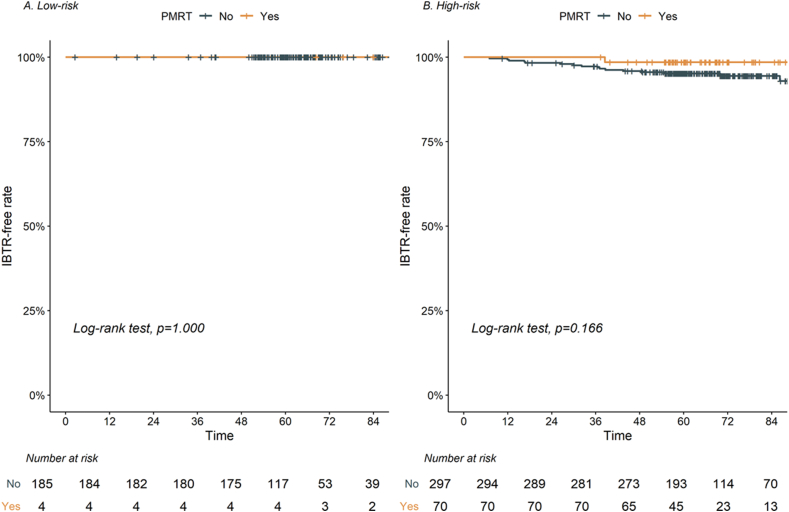
In the subgroup analyses, there was no significant difference in the effects of PMRT according to the risk groups (Fig. 3A–B). However, the 5-year IBTR-free rate in the high-risk group was slightly improved in the PMRT group compared to that in the no PMRT group (98.6% vs. 95.2%, p = 0.166; Fig. 3B).
Fig. 3.
Impact of postmastectomy radiation therapy (PMRT) on ipsilateral breast tumor recurrence (IBTR)-free rate according to subgroups based on risk factors. Footnotes: High-risk group, 1 or 2 risk factors; low-risk group, 0 risk factor.
Additionally, there was no significant difference in DFS and OS according to PMRT (Supplementary Figs. 4A–B). Multivariable analysis demonstrated that PMRT was associated with the outcomes of DFS along with LVI, whereas none was associated with OS outcomes (Supplementary Tables 4 and 5).
3.3. Reconstruction-related complications
Among the 543 patients who underwent reconstruction, 162 (29.8%) experienced any grade of reconstruction-related toxicities after surgery (Table 3): 136/473 (28.8%) and 26/70 (37.1%) in the no PMRT and PMRT groups, respectively (p = 0.196). Wound-related complications (N = 46, 8.5%) were frequently observed, followed by fat necrosis (N = 44, 8.1%), contracture (N = 33, 6.1%), and rippling (N = 32, 5.9%). Among them, 40 patients (7.4%) were surgically treated or hospitalized because of toxicities. Patients in the PMRT group showed a higher rate of reconstruction failure than those in the no PMRT group (11.4% vs. 3.0%, p = 0.004). In addition, the 5-year cumulative incidence of reconstruction failure was higher in the PMRT group than in the no PMRT group (10.0% vs. 2.8%, p = 0.001; Supplementary Fig. 5).
Table 3.
Details of reconstruction-related complications.
| Total |
No PMRT |
PMRT |
P-value |
|
|---|---|---|---|---|
| N = 543 | N = 473 | N = 70 | ||
| Reconstruction-related complication | 162 (29.8) | 136 (28.8) | 26 (37.1) | 0.196 |
| Contracture | 33 (6.1) | 26 (5.5) | 7 (10.0) | |
| Rippling | 32 (5.9) | 28 (5.9) | 4 (5.7) | |
| Wound-related | 46 (8.5) | 34 (7.2) | 12 (17.1) | |
| Fat necrosis | 44 (8.1) | 42 (8.9) | 2 (2.9) | |
| Implant rupture | 7 (1.3) | 6 (1.3) | 1 (1.4) | |
| CTCAE grade | 0.122 | |||
| Grade 1 | 31 (5.7) | 28 (5.9) | 3 (4.3) | |
| Grade 2 | 91 (16.8) | 78 (16.5) | 13 (18.6) | |
| Grade 3 (surgical procedure) | 40 (7.4) | 30 (6.3) | 10 (14.3) | |
| Failure of reconstruction | 22 (4.1) | 14 (3.0) | 8 (11.4) | 0.004 |
** Values are presented as patient (%).
Abbreviations: PMRT, postmastectomy radiation therapy; CTCAE, Common Terminology Criteria for Adverse Events.
4. Discussion
Over the past decades, both SSM and NSM have gained increased acceptance in parallel with an increased interest in quality of life and cosmetic outcomes for patients with breast cancer. However, PMRT involving chest wall RT can lead to poor cosmetic outcomes and increased toxicities [10]. Therefore, identifying the risk factors of IBTR could stratify patients for whom chest wall RT can be omitted. Although previous studies have shown a low IBTR rate of 5%, little data regarding the risk factors of IBTR following SSM/NSM exists [11]. In the current study, we identified a favorable 5-year IBTR-free rate of 97.2% in 556 patients after SSM or NSM in pT1-2N0-1 disease. PMRT did not significantly improved IBTR-free rate both before PSM and after PSM. We also found that age ≤45 years and LVI were independent factors for IBTR. Notably, the 5-year IBTR-free rate was 100% in patients with none of these risk factors and 95.8% in patients with at least one of these risk factors.
A recent meta-analysis including 3365 patients from 19 studies demonstrated 3.5% and 5.2% IBTR after SSM and NSM for mostly early-stage breast cancer, respectively (Table 4) [11]. Despite wide range of adopting PMRT, overall IBTR events after SSM/NSM were infrequent (about 5%, Table 4). Regarding risk factors, several previous series of SSM/NSM suggested high-grade as an adverse feature related to IBTR events [14,15,20,21]. Cont et al. also reported no IBTR events in patients who received PMRT [13]. To our knowledge, the current study was the first to focus specifically on the incidence of and risk factors associated with IBTR with a long-term follow-up period (median follow-up of 66 months) and modern systemic chemotherapy following NSM/SSM. In the current study, we found that both age and LVI were associated with IBTR.
Table 4.
Literature review for rates of ipsilateral breast tumor recurrence (IBTR) stratified by surgery.
| First Author | Yr | No. | FU (months) | PMRT (%) | IBTR, N (%) |
Prognostic factors related to IBTR |
|---|---|---|---|---|---|---|
| SSM/NSM | ||||||
| Franco [15] | 2001 | 173 | 73 | 7.5 | 8 (4.5) | Grade, stage, subtype |
| Vaughan [14] | 2007 | 206 | 59 | 20.0 | 11 (5.3) | Grade |
| Romics [21] | 2012 | 253 | 119 | 47.1 | 6 (3.9) | Grade, stage |
| Petit [20] | 2012 | 934 | 50 | 3.2 | 34 (4.5) | Grade, subtype |
| Liang [22] | 2013 | 249 | 53 | 12.9 | 5 (2.0) | |
| Sakurai [23] | 2013 | 788 | 78 | 0.0 | 65 (8.2) | |
| Stanec [24] | 2014 | 361 | 63 | NA | 15 (4.1) | |
| Agusti [25] | 2016 | 249 | 101 | 28.0 | 11 (4.4) | |
| Frey [26] | 2016 | 319 | 31 | 12.7 | 1 (0.3) | |
| Park [27] | 2016 | 189 | 66 | 10.1 | 7 (3.7) | |
| Cont [13] | 2017 | 518 | 33 | 18.1 | 14 (2.7) | Location |
| Lee [28] | 2018 | 1032 | 94 | 8.5 | 35 (3.4) | |
| Wu [12] | 2019 | 944 | 85 | NA | 39 (4.1) | Multifocality, subtype, grade, EIC |
| Current study | 2022 | 556 | 66 | 13.3 | 17 (3.1) | Age (45 years), LVI |
| Meta-analysis | ||||||
| Joo [11] | 2021 | 4787 | NA | 108 (5.2) - NSM 49 (3.5) - SSM |
||
| Total mastectomy | ||||||
| No PMRT | ||||||
| Sharma [29] | 2010 | 1019 | 90 | 0.0 | 12 (1.2) | Age (40 years) |
| Lai [30] | 2016 | 293 | 83 | 0.0 | 5 (1.7) | Age (40 years), size, EIC |
| Park [31] | 2017 | 1382 | 71 | 0.0 | 39 (2.8) | |
| Chang [32] | 2018 | 3224 | 72 | 0.0 | 70 (2.2) | Age (35 years), LVI, subtype |
| Park [33] | 2018 | 133 | 57 | 0.0 | 3 (3.1) | |
| Zhao [34] | 2020 | 2042 | 63 | 0.0 | 83 (4.1) | Age (45 years), T2, location, subtype |
| PMRT | ||||||
| Yang [35] | 2010 | 544 | 40 | 29.6 | 28 (5.1) | |
| Su [36] | 2014 | 207 | 60 | 39.1 | 12 (5.8) | |
| Kim [37] | 2017 | 714 | 69 | 18.2 | 7 (1.0) | |
| Muhsen [38] | 2018 | 1087 | 132 | 14.9 | 37 (3.4) | |
| Zeiden [39] | 2018 | 684 | 108 | 49.0 | 16 (2.3) | |
| Abi Jaoude [5] | 2020 | 1633 | 132 | 57.6 | 53 (3.2) | |
| Gilmore [40] | 2020 | 379 | 62 | 53.8 | 3 (0.8) | |
| Wang [6] | 2021 | 1474 | 93 | 45.0 | 25 (1.7) | |
Abbreviations: Yr, year; FU, median follow-up; PMRT, postmastectomy radiation therapy; SSM, skin-sparing mastectomy; NSM, nipple-sparing mastectomy; EIC, extensive intraductal component; LVI, lymphovascular invasion; NA, not available.
In contrast, a growing pile of evidence exists regarding IBTR following conventional total mastectomy (Table 4). Similar to the SSM/NSM series, previous studies regarding total mastectomy have described comparably low rates of chest wall recurrence, from 1.2% to 4.1%, in the absence of PMRT for pT1-2N0-1 disease [[29], [30], [31], [32], [33], [34]]. Regarding local recurrence following no PMRT, several risk factors, including age, pT stage, hormonal receptor status, and LVI have been reported [29,30,32,34]. Focusing on IBTR in the chest wall following no PMRT, a multi-institutional study including 3224 patients reported IBTR rates of 1.7% and 2.8% in patients with pT1-2N0 and pT1-2N1 disease [32]. They also found that age (<35 years), LVI, and hormone receptor status were found to be related to IBTR. Given <5% of 10-year IBTR, Chang et al. suggested the necessity of chest wall RT needs to be re-considered balancing between toxicities (to the lung, heart, or contralateral breast) and possible risks from IBTR events [32]. Despite the lack of a definite cutoff value (35–45 years) for young age through previous reports, young age was conceived as an adverse feature of locoregional recurrence [6,[29], [30], [31], [32],34,36,38,41]. Including not only IBTR but also regional recurrence, patient selection based on age or LVI (regardless of molecular subtype) to maximize the beneficial impact of PMRT in pT1-2N1 disease was proposed by previous studies [6,36,38,41,42]. Muhsen et al. reported 10-year rates of locoregional recurrence in patients <40 years with LVI was 28% whereas those in patients ≥40 years without LVI was 2% [38]. If the proportional risk reductions based on EBCTCG meta-analysis were applied to this result, the absolute gain from PMRT would be minimal in patients without risk factors [7]. Consistent with the series of conventional mastectomies, we found that age ≤45 years and LVI (either focal or extensive) were related to increased IBTR possibilities in the setting of SSM/NSM.
Recently, efforts have been made to minimize RT-related toxicities following reconstruction. Muresan et al. reported improved dose homogeneity from the prone positioning technique resulting in fewer complications than supine positioning [43]. Additionally, a positive correlation of RT dose with adverse events related to complication was observed [44,45]. Naoum et al. demonstrated that an increased RT dose through chest wall boost resulted in not only increased toxicities (infection, skin necrosis, and implant exposure) but also implant failure [46]. Chang et al. suggested that the administration of hypofractionated RT with 40–42.56 Gy in 15–16 fractions might play a role in reducing maximum-dose related toxicities [45]. There are several ongoing trials investigating the impact of hypofractionated RT compared to conventional fractionated RT (50–50.4 Gy in 25–28 fractions) (NCT03414970, NCT03422003). In addition, maximal inflation could reduce RT-related complications, considering an inaccurate RT dose calculation from artifacts from partially deflated expanders during a two-stage reconstruction [45,47]. However, the aforementioned efforts mainly focused on RT dose instead of RT field; all of the studies included both the chest wall and axillary area. Regarding omission of PMRT, on-going randomized trial for high-risk N0 and N1 disease (NCT00966888) investigates the oncologic safety of observation compared with PMRT including chest wall and regional nodes. Given the lack of guidelines regarding PMRT following SSM/NSM in patients with stage I-II disease, recent survey from 298 radiation oncologists from Western society suggested omitting PMRT in patients with age ≥50 years, no LVI, unicentric tumor in case of skin flap less than 5 mm thickness [48]. Consistent to our results of most IBTR events located ventral to implant, adopting recent consensus guideline from ESTRO-ACROP could minimize reconstruction-related complications [49]. Recent systematic review found that residual breast tissue after mastectomy could be observed frequently (up to 100%) and this region could be the risk of IBTR [50]. They reported that SSM (vs. NSM), nipple-areolar complex, surgeon's expertise, and outer quadrant of breast could be associated with the amount of residual breast tissue. Therefore, multidisciplinary team approach including surgeons and radiation oncologists is needed to identify the region at risk of IBTR. In this study, we found that for highly selective patients presenting with excellent IBTR-free rates, we could omit chest wall RT in the setting of PMRT. De-escalation of PMRT to the chest wall might improve the cosmetic outcomes and quality of life in these patients.
The interpretation of the current analysis has several limitations, owing to its retrospective nature. First, the small number of IBTR events in relation to the total number of patients could not sufficiently demonstrate the beneficial impact of PMRT in high-risk patients. Although recent advances in surgical and systemic therapies might negate the effect of PMRT in high-risk patients, a small difference in IBTR-free rate should not be a surrogate endpoint to exclude high-risk patients from PMRT. Given the small number of IBTR events, further multi-institutional retrospective analysis based on this hypothesis-generating study could be helpful to validate the current finding and rationalize further randomized clinical trial. Second, disparities in patient and tumor characteristics between the no PMRT and PMRT groups could be a confounding factor in interpreting the current results. Since patients in the PMRT group had more risk factors than those in the no PMRT group, the protective effect of PMRT for IBTR could be underestimated. Although we performed PSM analysis to minimize potential confounders and found no significant benefit of PMRT in these patients, lack of IBTR event could lead to statistical insignificance. In addition, a longer follow-up time (>66 months) might be required to accurately evaluate the true IBTR-free rates and reconstruction-related complications. Cruz et al. reported that weighted average of IBTR after NSM was 11.4% for studies with >5 years of follow-up compared with 5.4% for studies with <3 years of follow-up [51]. Therefore, long-term follow-up should be warranted to verify the oncologic safety of omitting chest wall RT. Finally, prospective randomized studies are warranted to safely omit chest wall irradiation in the setting of PMRT.
In conclusion, a favorable IBTR-free rate of less than 5% was observed in patients who underwent SSM or NSM. Since patients without risk factors, such as age ≤45 years and LVI, showed an excellent IBTR-free rate of 100%, chest wall RT could be omitted in the setting of PMRT. As a hypothesis-generation study, we cautiously suggest that selective omission of chest wall RT in the setting of PMRT could bring promise of fewer reconstruction-related complications and lifelong adverse events.
Funding information
None.
Ethics approval and informed consent
The requirement for informed consent was waived because of the retrospective nature of this study.
Declaration of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.09.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Song W.J., Kang S.G., Kim E.K., Song S.Y., Lee J.S., Lee J.H., et al. Current status of and trends in post-mastectomy breast reconstruction in Korea. Archives of plastic surgery. 2020;47:118–125. doi: 10.5999/aps.2019.01676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong S.M., Chun Y.S., Sagara Y., Golshan M., Erdmann-Sager J. National patterns of breast reconstruction and nipple-sparing mastectomy for breast cancer, 2005-2015. Ann Surg Oncol. 2019;26:3194–3203. doi: 10.1245/s10434-019-07554-x. [DOI] [PubMed] [Google Scholar]
- 3.Recht A., Comen E.A., Fine R.E., Fleming G.F., Hardenbergh P.H., Ho A.Y., et al. Postmastectomy radiotherapy: an American society of clinical oncology, American society for radiation oncology, and society of surgical oncology focused guideline update. Ann Surg Oncol. 2017;24:38–51. doi: 10.1245/s10434-016-5558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou N., Zhang J., Yang L., Wu Y., Wang Z., Zhang M., et al. A prognostic risk stratification model to identify potential population benefiting from postmastectomy radiotherapy in T1-2 breast cancer with 1-3 positive axillary lymph nodes. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.640268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abi Jaoude J., de Azambuja E., Makki M., Tamim H., Tfayli A., Geara F., et al. Post-mastectomy radiation therapy in human epidermal growth factor receptor 2 positive breast cancer patients: analysis of the HERA trial. Int J Radiat Oncol Biol Phys. 2020;106:503–510. doi: 10.1016/j.ijrobp.2019.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Zhang L., Zhang X., Luo J., Wang X., Chen X., et al. Impact of clinical-pathological factors on locoregional recurrence in mastectomy patients with T1-2N1 breast cancer: who can omit adjuvant radiotherapy? Breast Cancer Res Treat. 2021;190:277–286. doi: 10.1007/s10549-021-06378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGale P., Taylor C., Correa C., Cutter D., Duane F., Ewertz M., et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/s0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang G., Chang J.S., Shin K.H., Kim J.H., Park W., Kim H., et al. Post-mastectomy radiation therapy in breast reconstruction: a patterns of care study of the Korean Radiation Oncology Group. Radiat Oncol J. 2020;38:236–243. doi: 10.3857/roj.2020.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jagsi R., Momoh A.O., Qi J., Hamill J.B., Billig J., Kim H.M., et al. Impact of radiotherapy on complications and patient-reported outcomes after breast reconstruction. J Natl Cancer Inst. 2018;110:157–165. doi: 10.1093/jnci/djx148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shumway D.A., Momoh A.O., Sabel M.S., Jagsi R. Integration of breast reconstruction and postmastectomy radiotherapy. J Clin Oncol. 2020;38:2329–2340. doi: 10.1200/jco.19.02850. [DOI] [PubMed] [Google Scholar]
- 11.Joo J.H., Ki Y., Kim W., Nam J., Kim D., Park J., et al. Pattern of local recurrence after mastectomy and reconstruction in breast cancer patients: a systematic review. Gland Surg. 2021;10:2037–2046. doi: 10.21037/gs-21-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Z.Y., Kim H.J., Lee J.W., Chung I.Y., Kim J.S., Lee S.B., et al. Breast cancer recurrence in the nipple-areola complex after nipple-sparing mastectomy with immediate breast reconstruction for invasive breast cancer. JAMA Surg. 2019;154:1030–1037. doi: 10.1001/jamasurg.2019.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cont N.T., Maggiorotto F., Martincich L., Rivolin A., Kubatzki F., Sgandurra P., et al. Primary tumor location predicts the site of local relapse after nipple-areola complex (NAC) sparing mastectomy. Breast Cancer Res Treat. 2017;165:85–95. doi: 10.1007/s10549-017-4312-7. [DOI] [PubMed] [Google Scholar]
- 14.Vaughan A., Dietz J.R., Aft R., Gillanders W.E., Eberlein T.J., Freer P., et al. Scientific Presentation Award. Patterns of local breast cancer recurrence after skin-sparing mastectomy and immediate breast reconstruction. Am J Surg. 2007;194:438–443. doi: 10.1016/j.amjsurg.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Medina-Franco H., Vasconez L.O., Fix R.J., Heslin M.J., Beenken S.W., Bland K.I., et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg. 2002;235:814–819. doi: 10.1097/00000658-200206000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim N., Kim H., Hwang J.H., Park W., Cho W.K., Yeo S.M., et al. Longitudinal impact of postmastectomy radiotherapy on arm lymphedema in patients with breast cancer: an analysis of serial changes in arm volume measured by infrared optoelectronic volumetry. Radiother Oncol. 2021;158:167–174. doi: 10.1016/j.radonc.2021.02.033. [DOI] [PubMed] [Google Scholar]
- 17.Jagsi R., Raad R.A., Goldberg S., Sullivan T., Michaelson J., Powell S.N., et al. Locoregional recurrence rates and prognostic factors for failure in node-negative patients treated with mastectomy: implications for postmastectomy radiation. Int J Radiat Oncol Biol Phys. 2005;62:1035–1039. doi: 10.1016/j.ijrobp.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Abi-Raad R., Boutrus R., Wang R., Niemierko A., Macdonald S., Smith B., et al. Patterns and risk factors of locoregional recurrence in T1-T2 node negative breast cancer patients treated with mastectomy: implications for postmastectomy radiotherapy. Int J Radiat Oncol Biol Phys. 2011;81:e151–e157. doi: 10.1016/j.ijrobp.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J.I., Park W., Huh S.J., Choi D.H., Lim Y.H., Ahn J.S., et al. Determining which patients require irradiation of the supraclavicular nodal area after surgery for N1 breast cancer. Int J Radiat Oncol Biol Phys. 2010;78:1135–1141. doi: 10.1016/j.ijrobp.2009.09.037. [DOI] [PubMed] [Google Scholar]
- 20.Petit J.Y., Veronesi U., Orecchia R., Curigliano G., Rey P.C., Botteri E., et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol. 2012;23:2053–2058. doi: 10.1093/annonc/mdr566. [DOI] [PubMed] [Google Scholar]
- 21.Romics L., Jr., Chew B.K., Weiler-Mithoff E., Doughty J.C., Brown I.M., Stallard S., et al. Ten-year follow-up of skin-sparing mastectomy followed by immediate breast reconstruction. Br J Surg. 2012;99:799–806. doi: 10.1002/bjs.8704. [DOI] [PubMed] [Google Scholar]
- 22.Liang T.J., Wang B.W., Liu S.I., Yeh M.H., Chen Y.C., Chen J.S., et al. Recurrence after skin-sparing mastectomy and immediate transverse rectus abdominis musculocutaneous flap reconstruction for invasive breast cancer. World J Surg Oncol. 2013;11:194. doi: 10.1186/1477-7819-11-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakurai T., Zhang N., Suzuma T., Umemura T., Yoshimura G., Sakurai T., et al. Long-term follow-up of nipple-sparing mastectomy without radiotherapy: a single center study at a Japanese institution. Med Oncol. 2013;30:481. doi: 10.1007/s12032-013-0481-3. [DOI] [PubMed] [Google Scholar]
- 24.Stanec Z., Žic R., Budi S., Stanec S., Milanović R., Vlajčić Z., et al. Skin and nipple-areola complex sparing mastectomy in breast cancer patients: 15-year experience. Ann Plast Surg. 2014;73:485–491. doi: 10.1097/SAP.0b013e31827a30e6. [DOI] [PubMed] [Google Scholar]
- 25.Agusti A., Ward A., Montgomery C., Mohammed K., Gui G.P. Aesthetic and oncologic outcomes after one-stage immediate breast reconstruction using a permanent biodimensional expandable implant. J Plast Reconstr Aesthetic Surg. 2016;69:211–220. doi: 10.1016/j.bjps.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Frey J.D., Alperovich M., Kim J.C., Axelrod D.M., Shapiro R.L., Choi M., et al. Oncologic outcomes after nipple-sparing mastectomy: a single-institution experience. J Surg Oncol. 2016;113:8–11. doi: 10.1002/jso.24097. [DOI] [PubMed] [Google Scholar]
- 27.Park S.H., Han W., Yoo T.K., Lee H.B., Jin U.S., Chang H., et al. Oncologic safety of immediate breast reconstruction for invasive breast cancer patients: a matched case control study. J Breast Cancer. 2016;19:68–75. doi: 10.4048/jbc.2016.19.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee S.B., Lee J.W., Kim H.J., Ko B.S., Son B.H., Eom J.S., et al. Long-term outcomes of patients with breast cancer after nipple-sparing mastectomy/skin-sparing mastectomy followed by immediate transverse rectus abdominis musculocutaneous flap reconstruction: comparison with conventional mastectomy in a single center study. Medicine (Baltim) 2018;97 doi: 10.1097/md.0000000000010680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma R., Bedrosian I., Lucci A., Hwang R.F., Rourke L.L., Qiao W., et al. Present-day locoregional control in patients with t1 or t2 breast cancer with 0 and 1 to 3 positive lymph nodes after mastectomy without radiotherapy. Ann Surg Oncol. 2010;17:2899–2908. doi: 10.1245/s10434-010-1089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai S.F., Chen Y.H., Kuo W.H., Lien H.C., Wang M.Y., Lu Y.S., et al. Locoregional recurrence risk for postmastectomy breast cancer patients with T1-2 and one to three positive lymph nodes receiving modern systemic treatment without radiotherapy. Ann Surg Oncol. 2016;23:3860–3869. doi: 10.1245/s10434-016-5435-5. [DOI] [PubMed] [Google Scholar]
- 31.Park H.J., Shin K.H., Kim J.H., Ahn S.D., Kim J.Y., Park W., et al. Incorporating risk factors to identify the indication of post-mastectomy radiotherapy in N1 breast cancer treated with optimal systemic therapy: a multicenter analysis in korea (krog 14-23) Cancer Res Treat. 2017;49:739–747. doi: 10.4143/crt.2016.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang J.H., Shin K.H., Ahn S.D., Park H.J., Chie E.K., Kim J.H., et al. Chest wall recurrence in pT1-2N0-1 breast cancer patients after mastectomy without radiotherapy. Breast Cancer Res Treat. 2018;169:507–512. doi: 10.1007/s10549-018-4707-0. [DOI] [PubMed] [Google Scholar]
- 33.Park S.H., Lee J., Lee J.E., Kang M.K., Kim M.Y., Park H.Y., et al. Local and regional recurrence following mastectomy in breast cancer patients with 1-3 positive nodes: implications for postmastectomy radiotherapy volume. Radiat Oncol J. 2018;36:285–294. doi: 10.3857/roj.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X., Tang Y., Wang S., Yang Y., Fang H., Wang J., et al. Locoregional recurrence patterns in women with breast cancer who have not undergone post-mastectomy radiotherapy. Radiat Oncol. 2020;15:212. doi: 10.1186/s13014-020-01637-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang P.S., Chen C.M., Liu M.C., Jian J.M., Horng C.F., Liu M.J., et al. Radiotherapy can decrease locoregional recurrence and increase survival in mastectomy patients with T1 to T2 breast cancer and one to three positive nodes with negative estrogen receptor and positive lymphovascular invasion status. Int J Radiat Oncol Biol Phys. 2010;77:516–522. doi: 10.1016/j.ijrobp.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 36.Su Y.L., Li S.H., Chen Y.Y., Chen H.C., Tang Y., Huang C.H., et al. Post-mastectomy radiotherapy benefits subgroups of breast cancer patients with T1-2 tumor and 1-3 axillary lymph node(s) metastasis. Radiol Oncol. 2014;48:314–322. doi: 10.2478/raon-2013-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim Y.J., Park W., Ha B., Park B., Joo J., Kim T.H., et al. Postmastectomy radiotherapy in patients with pT1-2N1 breast cancer treated with taxane-based chemotherapy: a retrospective multicenter analysis (krog 1418) Cancer Res Treat. 2017;49:927–936. doi: 10.4143/crt.2016.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muhsen S., Moo T.-A., Patil S., Stempel M., Powell S., Morrow M., et al. Most breast cancer patients with T1-2 tumors and one to three positive lymph nodes do not need postmastectomy radiotherapy. Ann Surg Oncol. 2018;25:1912–1920. doi: 10.1245/s10434-018-6422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeidan Y.H., Habib J.G., Ameye L., Paesmans M., de Azambuja E., Gelber R.D., et al. Postmastectomy radiation therapy in women with T1-T2 tumors and 1 to 3 positive lymph nodes: analysis of the breast international group 02-98 trial. Int J Radiat Oncol Biol Phys. 2018;101:316–324. doi: 10.1016/j.ijrobp.2018.01.105. [DOI] [PubMed] [Google Scholar]
- 40.Gilmore R.C., Sebai M.E., Psoter K.J., Prasath V., Siotos C., Broderick K.P., et al. Analysis of breast cancer patients with T1-2 tumors and 1-3 positive lymph nodes treated with or without postmastectomy radiation therapy. Sci Rep. 2020;10:9887. doi: 10.1038/s41598-020-66495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moo T.A., McMillan R., Lee M., Stempel M., Patil S., Ho A., et al. Selection criteria for postmastectomy radiotherapy in t1-t2 tumors with 1 to 3 positive lymph nodes. Ann Surg Oncol. 2013;20:3169–3174. doi: 10.1245/s10434-013-3117-0. [DOI] [PubMed] [Google Scholar]
- 42.Moo T.-A., McMillan R., Lee M., Stempel M., Ho A., Patil S., et al. Impact of molecular subtype on locoregional recurrence in mastectomy patients with T1-T2 breast cancer and 1-3 positive lymph nodes. Ann Surg Oncol. 2014;21:1569–1574. doi: 10.1245/s10434-014-3488-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muresan H., Lam G., Cooper B.T., Perez C.A., Hazen A., Levine J.P., et al. Impact of evolving radiation therapy techniques on implant-based breast reconstruction. Plast Reconstr Surg. 2017;139 doi: 10.1097/prs.0000000000003341. [DOI] [PubMed] [Google Scholar]
- 44.Chung S.Y., Chang J.S., Shin K.H., Kim J.H., Park W., Kim H., et al. Impact of radiation dose on complications among women with breast cancer who underwent breast reconstruction and post-mastectomy radiotherapy: a multi-institutional validation study. Breast. 2021;56:7–13. doi: 10.1016/j.breast.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang J.S., Song S.Y., Oh J.H., Lew D.H., Roh T.S., Kim S.Y., et al. Influence of radiation dose to reconstructed breast following mastectomy on complication in breast cancer patients undergoing two-stage prosthetic breast reconstruction. Front Oncol. 2019;9:243. doi: 10.3389/fonc.2019.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naoum G.E., Salama L., Ho A., Horick N.K., Oladeru O., Abouegylah M., et al. The impact of chest wall boost on reconstruction complications and local control in patients treated for breast cancer. Int J Radiat Oncol Biol Phys. 2019;105:155–164. doi: 10.1016/j.ijrobp.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 47.Woo K.J., Paik J.M., Bang S.I., Mun G.H., Pyon J.K. The impact of expander inflation/deflation status during adjuvant radiotherapy on the complications of immediate two-stage breast reconstruction. Aesthetic Plast Surg. 2017;41:551–559. doi: 10.1007/s00266-017-0864-5. [DOI] [PubMed] [Google Scholar]
- 48.Marta G.N., Poortmans P.M., Buchholz T.A., Hijal T. Postoperative radiation therapy after nipple-sparing or skin-sparing mastectomy: a survey of European, north American, and south American practices. Breast J. 2017;23:26–33. doi: 10.1111/tbj.12683. [DOI] [PubMed] [Google Scholar]
- 49.Kaidar-Person O., Vrou Offersen B., Hol S., Arenas M., Aristei C., Bourgier C., et al. ESTRO ACROP consensus guideline for target volume delineation in the setting of postmastectomy radiation therapy after implant-based immediate reconstruction for early stage breast cancer. Radiother Oncol. 2019;137:159–166. doi: 10.1016/j.radonc.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 50.Kaidar-Person O., Boersma L.J., Poortmans P., Sklair-Levy M., Offersen B.V., Cardoso M.J., et al. Residual glandular breast tissue after mastectomy: a systematic review. Ann Surg Oncol. 2020;27:2288–2296. doi: 10.1245/s10434-020-08516-4. [DOI] [PubMed] [Google Scholar]
- 51.De La Cruz L., Moody A.M., Tappy E.E., Blankenship S.A., Hecht E.M. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22:3241–3249. doi: 10.1245/s10434-015-4739-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.