Summary
The oral cavity is a highly regenerative epithelial tissue that results in minimal scarring after injury. This protocol describes the preparation of a mouse palate wound model. The protocol includes steps to place an excisional wound on the mouse palate, followed by harvesting of wound tissue and bone decalcification. We detail how to overcome the technical challenge of limited anatomical space, avoid damaging the nasal cavity, manage bleeding, and collect tissue for downstream genomic or immunohistochemical analysis.
Subject areas: Health sciences, Model organisms, Tissue engineering
Graphical abstract

Highlights
-
•
Protocol for the preparation of oral palate wounds in mice
-
•
Steps of palate wound placement and subsequent would tissue harvesting
-
•
Guidance on how to avoid tissue damage and manage bleeding
-
•
Suitable for direct comparisons of healing in skin and oral mucosa
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The oral cavity is a highly regenerative epithelial tissue that results in minimal scarring after injury. This protocol describes the preparation of a mouse palate wound model. The protocol includes steps to place an excisional wound on the mouse palate, followed by harvesting of wound tissue and bone decalcification. We detail how to overcome the technical challenge of limited anatomical space, avoid damaging the nasal cavity, manage bleeding, and collect tissue for downstream genomic or immunohistochemical analysis.
Before you begin
The protocol below describes the specific steps to prepare a mouse palate wound followed by tissue collection at 24 h post injury. This tissue is suitable for downstream DNA or RNA analysis or fixation and decalcification for downstream immunohistochemical studies. This protocol expands upon an earlier model (Graves et al., 2001), providing technical advances that improve success rates and reproducibility.
Note: Use sterilized surgical instruments for all procedures.
Note: Become familiar with your surgical area and the small space of the mouse oral cavity. Practicing the placement of the oral spacer and palate wound on cadavers is extremely helpful when initially learning this procedure.
Note: This protocol was performed on 8–12 week old C57BL/6J female mice. Mice should be 8 weeks of age at a minimum. Mice that are 10–12 weeks old are recommended, as the mice are larger and the surgery is easier to perform. This protocol is also suitable for male mice.
Note: All materials should be prepared before starting the procedure. This includes making and sterilizing the oral spacers, sterilization of surgical instruments, cotton tip applicators and gauze, and formulation of anesthetic and post-surgical pain medications (if required by your institution).
Institutional permissions
All procedures involving animals were approved by The Animal Care Committee at the University of Illinois Chicago and conducted in accordance with institutional guidelines (ACC 20-209).
Preparation of surgical tools and oral spacers
Timing: 30 min (not including autoclave time)
-
1.To prepare oral spacers, grab a new single edge razor blade, a P1000 pipette tip, and use a clean lab bench surface.
-
a.Make a cut perpendicular to the pipette tip length approximately 2/3 of the way up from the dispensing side (Figure 1A).
-
b.Then, make a second cut approximately 2–3 mm from your original cut to yield a cylindrical spacer (Figure 1B).
-
i.Repeat these steps 2 to 3 times for each pipette tip (Figure 1C).
-
ii.Make sure to examine for sharp edges on any of the cut spacers.
-
iii.If there are any sharp edges, use the razor blade to smooth them.
-
iv.Discard any spacers with sharp edges that cannot be smoothed.
-
i.
-
a.
-
2.Repeat step 1 until you have enough spacers of various sizes for your experimental needs.
-
a.Place these spacers into a sterilization pouch and set to the side.
-
a.
-
3.Make sure that all instruments have been cleaned with soap and water and have dried completely.
-
a.Place surgical instruments, cotton tip applicators, and gauze into separate sterilization pouches and seal.
-
a.
-
4.
Place all of your filled and ready to autoclave sterilization pouches into the autoclave. Use a gravity autoclave setting at 121°C for a minimum of 20 min or according to the sterilization pouch instructions.
-
5.
Remove sterilized material from the autoclave, cool, and store in a dry area for later use.
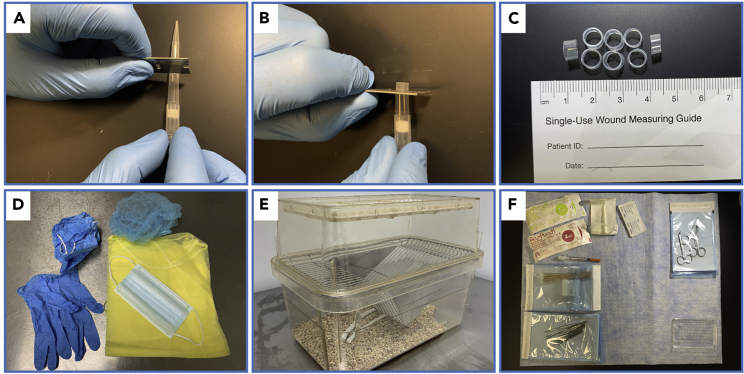
Figure 1.
Preparation for surgery
(A) Image showing where to make the first cut on the pipette tip with a razor blade.
(B) Image showing where to make the second cut.
(C) Representative oral spacers after cutting pipette tips with the razor blade.
(D) Personal protective equipment including: bonnet, gown, gloves, surgical mask, and foot covers.
(E) Recovery cage.
(F) Example surgical area with tools placed in easy to reach areas.
Preparation for surgery
Timing: 15 min
- 6.
-
7.Prepare the surgical area.
-
a.Remove any extraneous equipment from the surgical area.
-
b.Sanitize the surgical area with a suitable disinfectant.
-
c.Plug in and turn on a germinator bead sterilizer.
-
d.Place the heating pad on the cleaned surface, plug in, and turn on.
-
e.Open sterilized surgical tools and place them so they are easily accessible, including scissors, forceps, oral spacers, and 1 mm biopsy punches (Figure 1F).
-
a.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Ketamine hydrochloride (100 mg/mL) | Covetrus | NDC: 11695-0703-1 |
| Xylazine injection sterile solution (20 mg/mL) | Akorn Family Health | NDC: 59399-110-20 NADA# 139-236 |
| Dulbecco’s phosphate-buffered salt solution 1× | Corning | Mfr# 21-031-CV |
| EDTA disodium salt | Research Products International | CAS# 6381-92-6 |
| Sodium hydroxide (pellets) | Fisher Chemical | Cat# S318-500 |
| Neutral buffered formalin solution 10% | Sigma-Aldrich | HT50-1-128 |
| Paraformaldehyde 4% w/v aqueous solution | Thermo Scientific | Mfr# 473929M |
| RNAlater Stabilization Solution | Thermo Scientific | Cat# AM7020 |
| TRIzol Reagent | Thermo Scientific | Cat# 15596026 |
| Chloroform | Thermo Scientific | Cat# J67241-AP |
| Hematoxylin solution, Mayer’s | MilliporeSigma | SKU# MHS16-500ML PubChem Substance ID: 24897407 |
| Eosin Y solution, alcoholic | MilliporeSigma | SKU# HT110116-500ML |
| Reagent alcohol | MilliporeSigma | SKU# 362808-1L Pubchem Substance ID: 329755001 |
| Xylenes | MilliporeSigma | CAS# 1330-20-7 Pubchem Substance ID: 329752157 |
| Critical commercial assays | ||
| Quick-DNA Miniprep Plus Kit | Zymo Research | Cat# D4068 |
| DNA Clean & Concentrator-5 | Zymo Research | Cat# D4003 |
| RNA Clean & Concentrator-5 (DNase included) | Zymo Research | Cat# R1013 |
| Experimental models: Organisms/strains | ||
| C57BL/6J female mice, 8–12 weeks old | The Jackson Laboratory | Strain #: 000664 RRID: IMSR_JAX:000664 |
| Other | ||
| 1cc Insulin syringe U-100 29 Gauge | Becton Dickinson | 309311 |
| Instant sealing sterilization pouches | Fisher Scientific | Cat# 01-812-51 |
| Curved scissors | Fine Science Tools | Cat# 14085-08 |
| Dissector scissors | Fine Science Tools | Cat# 14082-09 |
| Short fine blade straight scissors | Excelta | Mfr# 288 |
| Forceps Dumont #5 Fine Tips | Fine Science Tools | Cat# 11254-20 |
| Straight broad strong tip general application forceps | Fisher Scientific | Cat# 11-100-107 |
| General purpose pinning forceps | Fisher Scientific | Cat# 10-270 |
| 1 mm biopsy punch (25/pack) | Acuderm Inc. | Cat# P150 |
| 2 mm biopsy punch (25/pack) | Acuderm Inc. | Cat# P250 |
| Cotton tipped applicators, 6″ wood handle | Fisherbrand | Cat# 14-960-3Q |
| Gauze sponges | Fisher Scientific | Cat# 13-761-52 |
| Sterile alcohol prep pads | Fisherbrand | Cat# 22-363-750 |
| Absorbant underpads, mats | Fisherbrand | Cat# 1420662 |
| Animal warming pad | N/A | N/A |
| Warming lamp | N/A | N/A |
| Puralube vet ophthalmic (eye) ointment | MWI Veterinary | Mfr# 027505 |
| P1000 1,000 μL pipette tips | N/A | N/A |
| Razor blade 0.0009″ | Personna | Mfr# 94-0491-0000 |
| Richard-Allan paraffin wax – Type 9 | Thermo cientific | Cat# 8337 |
| Glass 20 mL scintillation vials and Urea caps | DKW Life Sciences | Mfr# 986562 |
| Accu-Edge low profile microtome blades | Sakura | Mfr# 4689 |
| Tissue path IV tissue cassettes | Fisher Scientific | Cat# 22-272416 |
| Fisherbrand Superfrost plus microscope slides | Fisher Scientific | Cat# 12-550-15 |
| Tissue-Tek stainless steel base mold | Sakura | Cat# 4162 |
| Round toothpicks | N/A | N/A |
| Pencil | N/A | N/A |
| pH meter | N/A | N/A |
| Roboz surgical germinator 500 bead sterilizer | Roboz Surgical | Mfr# DS401 |
| Light microscope | N/A | N/A |
| Microtome | N/A | N/A |
| Floatation bath for tissueprep | Fisher | N/A |
| Paraffin embedding station | N/A | N/A |
| General purpose compact oven | Thermo Scientific | Mfr# PR305220G |
| Microscope slide drying rack | N/A | N/A |
| Autoclave machine | N/A | N/A |
| Paraffin wax melting pot | N/A | N/A |
Materials and equipment
EDTA decalcification working solution
| Reagent | Final concentration | Amount |
|---|---|---|
| EDTA | 4.5% | 45 grams |
| NaOH | 0.45% | 4.5 grams |
| 10% Formalin | 0.1% | 10 mL |
| dH2O | N/A | 990 mL |
| Total | N/A | 1,000 mL |
Store at room temperature for up to 2 years.
Note: Bring pH of the EDTA decalcification solution to 7.0 using a 1 N NaOH solution and a calibrated pH meter.
Ketamine Xylazine anesthetic solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Ketamine | 100 mg/Kg | 1 mL |
| Xylazine | 5 mg/Kg | 0.5 mL |
| PBS sterile | N/A | 8.5 mL |
| Total | N/A | 10 mL |
Store at room temperature for up to one week.
Step-by-step method details
Anesthetizing the mouse
Timing: 10–30 min
Before performing the palate wound it is essential to ensure the mouse is fully anesthetized. The hard palate is well innervated and can cause the mouse pain if anesthesia is not adequate. The following intraperitoneal injection regimen will produce a surgical level of anesthesia for 15–30 min and sedation for 1–2 h.
-
1.Prepare the anesthetic syringe.
-
a.Take a new sterile 1cc syringe, slowly draw in approximately 200–250 μL of anesthetic solution into the syringe trying not to draw in any excess air.
-
b.Point the syringe upward to bring any air bubbles to the top of the needle.
-
c.Plunge the syringe gently to expel any air until no air can be seen in the syringe chamber and only liquid expels from the syringe, and double check volume.
-
d.Set syringe on a clean surface and do not let the needle touch anything.
-
a.
-
2.Administer the anesthetic.
-
a.Carefully remove the lid of the cage housing the mice.
-
b.Grasp the tail of a mouse with your thumb and index finger to restrain it.
-
c.Place the mouse on a surface such as the metal cage bars and pull back gently to allow them to grip the bars.
-
d.With your free hand, use your thumb and index finger to scruff the mouse on the dorsal surface of the neck/shoulder area, grabbing and pulling back a large amount of skin until their head is properly restrained to prevent movement.
-
e.Then, lift the mouse and turn it over, keeping the mouse restrained against the palm of your hand.
-
f.Use your pinky finger from your hand, scruffing the mouse and placing it against the tail of the mouse so that it is against the pinky and your palm.
-
g.The mouse should be fully restrained, unable to freely move the head.
-
h.While restraining the mouse, grab the syringe with your free hand so that you can clearly see and read the numbers on the syringe.
-
i.Insert the needle into the lower right quadrant of the abdomen at an angle that allows it to penetrate the skin, approximately 15–30 degrees from the surface.
-
j.Inject the anesthetic slowly, and then withdraw the needle.
-
k.Place the mouse into the anesthetization cage and wait.
-
l.When the heartrate has visibly slowed and the mouse has stopped moving, place the mouse on the heating pad.
-
m.Perform a toe pinch to test the anesthetic depth.
-
n.If the mouse does not respond to the toe pinch, you can proceed to the next step. If the mouse reflex occurs, wait a few minutes and try again.
 CRITICAL: Do not begin the mouse surgery until the mouse is properly anesthetized and does not respond to the toe pinch test.
CRITICAL: Do not begin the mouse surgery until the mouse is properly anesthetized and does not respond to the toe pinch test. -
o.Apply ophthalmic ointment to both eyes to prevent corneal drying.
 CRITICAL: It is important to make sure they ophthalmic ointment is applied to prevent any dryness that may otherwise occur during the surgical procedure.
CRITICAL: It is important to make sure they ophthalmic ointment is applied to prevent any dryness that may otherwise occur during the surgical procedure.
-
a.
Palate wound placement
Timing: 10–20 min
This step will successfully place a circular 1 mm diameter excisional wound on the palate of the mouse. If done properly, there will be minimal bleeding and the entire epithelium will be removed.
-
3.Placement of an oral spacer.
-
a.Carefully place a pair of closed forceps between the upper and lower incisors, and release to open (Figure 2A).
-
b.With your other hand, use a pair of forceps to grab an oral spacer.
-
c.Push the spacer between forceps holding the mouth open. Brace spacer against one side of the buccal pouch to wedge into place (Figure 2B).
-
d.Use forceps to adjust the spacer placement (Figure 2C).
-
e.The palate should be clearly visible if spacer is placed appropriately (Figure 2D).
-
a.
-
4.Placement of the palate wound.
-
a.Remove a new sterile 1 mm biopsy punch from its bag and place in the hand you are most comfortable using to place the wound.
-
b.With your other hand, use your thumb and index finger to support the nasal bridge of the mouse (Figure 2E).
-
c.Place the biopsy punch below the first rugae and center it medially on the palate (Figure 2E).
-
d.Ensuring contact of the punch against the palate, rotate the punch in a circular motion to make the excision until you feel friction (Figure 2E).
-
e.Remove the punch and inspect the wound, being sure to remove any excess blood with a cotton tipped applicator or gauze (Figure 2F).
-
f.Use forceps to grab the outer edge of the tissue and remove (Figure 2G).
-
g.Inspect the wound and forceps to ensure removal of tissue (Figure 2H).
-
h.Hold pressure against the wound with a cotton tipped applicator until hemostasis has been achieved (Figure 2I).
-
i.Place the mouse in the recovery cage directly under a warming lamp and monitor until the mouse has recovered from anesthesia.
-
j.Check for any signs of bleeding, then return the mouse to its original cage.
-
k.If the experimental duration before subsequent wound collection is greater than 24 h, observe the mice on a daily basis to ensure no unusual behavior, paying special attention to eating and drinking behavior.
-
a.
Figure 2.
Palatal wound placement
(A) Placement of forceps between incisors to hold the mouth open.
(B) Wedging of spacer into place using forceps and buccal pouch as support.
(C) Use of forceps to adjust spacer within buccal pouch.
(D) A properly placed oral spacer.
(E) 1 mm biopsy punch placement, with thumb and index finger of other hand supporting mouse head.
(F) A properly placed 1 mm wound along the medial line and below first rugae.
(G) Use forceps to physically remove excised tissue.
(H) Appearance of palate after wound tissue is removed.
(I) Use of cotton tipped applicator to apply pressure to the wound to achieve hemostasis.
Harvesting wound tissue for analysis
Timing: 20–30 min
This step will lead to the removal of the palate and underlying bone tissue at the time point of interest followed by an overnight fixation using paraformaldehyde. An optional step is included for those who wish to use the tissue for genomic analyses. For our studies, we routinely collected samples at six hours, one day, three days, and seven days after the initial wound placement. It takes approximately five to seven days for re-epithelialization to occur, and by day seven a majority of the tissue is replaced.
-
5.Euthanize the mouse.
-
a.Euthanize the mouse through the use of carbon dioxide (CO2) inhalation followed by cervical dislocation or according to the protocol approved by your institution.
-
b.Place the mouse in a supine position, and use curved scissors to make two cuts, one along each side of the buccal tissues (Figure 3A).
-
c.Retract the upper and lower mandibles using two pairs of forceps, taking care not to damage the wound area.
-
a.
Optional: If the samples are going to be used for genomic analyses, a 2 mm punch biopsy and a pair of forceps can be used to collect the soft tissue at the wound site. For RNA, it is recommended to place these samples immediately into 400 μL of RNAlater in a 1.5 mL nuclease free Eppendorf tube and placed at 4°C for 24 h, then processed or frozen at −20°C or −80°C. For DNA, samples can be snap frozen in a 1.5 mL nuclease free Eppendorf tube using dry ice. Then proceed to isolate the respective nucleic acid based on user preferences. For immunohistochemical studies, continue to the next step.
-
6.For histologic analysis, remove the palate with underlying bone and soft tissue intact.
-
a.While holding the mouse in place with forceps in one hand, use dissector scissors to make a horizontal incision inferior to the palate (Figure 3B).
-
b.Then with either dissector scissors or short fine blade straight scissors (user preference), cut along the outer edge of the each side of the palate, lateral from the molar teeth (with respect to the midline). Again, forceps are helpful to keep the mouse positioned properly (Figure 3C).
-
c.Using forceps in one hand and dissector scissors in the other, carefully cut along the anterior most region of the palate (Figure 3D).
-
d.Now the palate should be freed from the surrounding structures. Use forceps to remove the palate (Figure 3E). If you experience any resistance, use scissors to cut palate from any remaining structures (Figure 3F).
 CRITICAL: Be careful to not damage the wound tissue when cutting the anterior region of the palate. This area cuts through the incisors and requires a significant amount of force.
CRITICAL: Be careful to not damage the wound tissue when cutting the anterior region of the palate. This area cuts through the incisors and requires a significant amount of force. -
e.Rinse the palate briefly in 1× PBS, dry with gauze, place in a 4% paraformaldehyde solution in a glass scintillation vial under a fume hood, and keep at room temperature for 24 h.
-
a.
Figure 3.
Palate removal
(A) Curved scissor placement to separate upper and lower mandible.
(B) Initial incision inferior to palate with dissector scissors.
(C) Lateral incision parallel and lateral to teeth.
(D) Final incision using dissector scissors as this cuts through the incisors.
(E) Removal of palate using forceps.
(F) Fully removed palate after 1× PBS rinse.
Bone decalcification
Timing: 30 days
This step will produce a decalcified tissue structure that is suitable for downstream immunohistochemistry procedures such as paraffin embedding and tissue sectioning. This process is necessary as the underlying palatal bone is removed with the tissue to maintain tissue integrity of the wound site. In order to section the tissue samples, bone must be completely decalcified before beginning the embedding process.
-
7.Decalcify the bone.
-
a.After the palate sample has been in 4% paraformaldehyde under a fume hood for 24 h, remove the tissue using forceps and wash in 1× PBS for one minute.
-
b.Then, place the tissue in a glass scintillation vial containing the EDTA decalcification solution, cap the vial, and leave at room temperature for 5–7 days.
-
c.Every 5–7 days, move the palate sample to a new glass scintillation vial containing fresh (unused) EDTA decalcification solution.
-
d.Use forceps to monitor the strength of calcified structures (such as the teeth) each week.
 CRITICAL: If the calcified structures are not pliable upon manipulation with metal forceps, the tissue sample needs to remain in the EDTA decalcification solution. Failure to do so will lead to inability to acquire tissue sections using the microtome.
CRITICAL: If the calcified structures are not pliable upon manipulation with metal forceps, the tissue sample needs to remain in the EDTA decalcification solution. Failure to do so will lead to inability to acquire tissue sections using the microtome. -
e.The tissue is ready for histologic processing (e.g., paraffin embedding) when the bone is pliable when grabbed with forceps or can be cut with a blade. This takes approximately 30 days.
-
a.
Expected outcomes
It is expected that with this protocol, one should be able to generate high quality palate wounds that can be used for downstream genomic analyses or histology studies.
Limitations
This protocol requires a high level of dexterity and can be difficult to perform if not comfortable working in the oral cavity. Due to the size of the oral cavity, only one wound per mouse can be made. Further, for genomic analyses the tissue quantity is limited and therefore the amount of RNA or DNA from each sample may be insufficient for standard downstream genomic analyses.
Troubleshooting
Problem 1
Failure to place the oral spacer (step 3).
Potential solution
It is highly recommended that the person performing this procedure practice placement of the oral spacer on cadavers (Figures 2A–2D). Test different types of forceps if necessary until placement of the spacer becomes routine and does not dislodge easily. If one spacer is not fitting, use a different sterile spacer. It is important to make and sterilize a large number spacers of different sizes as the size of the oral cavity will vary from mouse to mouse. This step also requires use of both hands, so try switching the hands you use to hold the oral cavity open and to place the spacer in order to identify which orientation is most comfortable.
Problem 2
Failure to place the palate wound (step 4).
Potential solution
Practicing on cadavers is highly recommended. It is important to identify how much resistance is required to maintain the mouse head in the appropriate position with one hand together with how much pressure is required from the hand holding 1 mm punch biopsy to make a full thickness wound (Figure 2E). If one uses too little pressure, an incomplete excisional biopsy will occur and the wound will likely only remove the outer epithelial layer. If one applies too much pressure the biopsy punch can break the vomer bone and potentially penetrate into the vomeronasal organ. When enough practice attempts are performed, it is easier to tell when a full thickness wound has been achieved. Finally, it is important to use a new biopsy punch for each mouse for sterility reasons and due to the fact that the punch will dull upon one use.
Problem 3
Mouse is bleeding after initial wound placement prior to wound tissue removal (step 4).
Potential solution
Use a cotton tipped applicator to apply pressure for a minimum of 30 s in order to stop the bleeding and to enable visualization of the wound. If the mouse continues to bleed, reapply firm pressure until the bleeding has stopped.
Problem 4
Mouse palate wounds provide inadequate amounts of DNA or RNA (step 5, optional).
Potential solution
If this occurs, it may be necessary to collect wounds from multiple mice and pool the samples before isolating RNA or DNA. If one individual wound per mouse is desired, using methods that allow for concentration of nucleic acids may be necessary. For example, DNA can be initially isolated using a column based kit (Zymo Research Quick-DNA Miniprep Plus Kit) followed by concentrating the eluant into a low volume (≥6 μL) using a concentrator kit (Zymo Research DNA Clean & Concentrator-5). For RNA assays, one can isolate the RNA using the traditional TRIzol®/chloroform extraction followed by a column based cleanup kit (Zymo Research RNA Clean & Concentrator-5) that allows for low elution volumes (≥6 μL). Use of this kit with TRIzol®/chloroform is explained in the appendix section of the Zymogen protocol PDF. If RNA sequencing is desired, this approach together with downstream library preparation methods that can take low inputs of RNA that are generally used for single cell sequencing is advised.
Problem 5
Wound is difficult to identify during tissue sectioning (step 7).
Potential solution
If unable to identify the wound during the tissue sectioning process, it may be necessary to label the wound site with a dye applied to the biopsy punch (Byrd et al., 2019).
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Luisa A. DiPietro (Ldipiet@uic.edu).
Materials availability
This study did not generate new unique reagents.
Acknowledgments
This work was supported by National Institutes of Health grants: R01-GM50875 (L.A.D.), R35-GM139603 (L.A.D.), and F31-DE028747 (T.R.L.). The graphical abstract was created with BioRender.com.
Author contributions
T.R.L. devised the protocol; L.A.D. and L.C. assisted in protocol refinement. T.R.L. drafted the manuscript, L.C. reviewed the manuscript, and L.A.D. edited and finalized the manuscript.
Declaration of interests
The authors declare no competing interests.
Data and code availability
This protocol did not generate datasets or code.
References
- Byrd K.M., Piehl N.C., Patel J.H., Huh W.J., Sequeira I., Lough K.J., Wagner B.L., Marangoni P., Watt F.M., Klein O.D., et al. Heterogeneity within stratified epithelial stem cell populations maintains the oral mucosa in response to physiological stress. Cell Stem Cell. 2019;25:814–829.e6. doi: 10.1016/j.stem.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves D.T., Nooh N., Gillen T., Davey M., Patel S., Cottrell D., Amar S. IL-1 plays a critical role in oral, but not dermal, wound healing. J. Immunol. 2001;167:5316–5320. doi: 10.4049/jimmunol.167.9.5316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This protocol did not generate datasets or code.