Abstract
Histones have a long history of research in a wide range of species, leaving a legacy of complex nomenclature in the literature. Community-led discussions at the EMBO Workshop on Histone Variants in 2011 resulted in agreement amongst experts on a revised systematic protein nomenclature for histones, which is based on a combination of phylogenetic classification and historical symbol usage. Human and mouse histone gene symbols previously followed a genome-centric system that was not applicable across all vertebrate species and did not reflect the systematic histone protein nomenclature. This prompted a collaboration between histone experts, the Human Genome Organization (HUGO) Gene Nomenclature Committee (HGNC) and Mouse Genomic Nomenclature Committee (MGNC) to revise human and mouse histone gene nomenclature aiming, where possible, to follow the new protein nomenclature whilst conforming to the guidelines for vertebrate gene naming. The updated nomenclature has also been applied to orthologous histone genes in chimpanzee, rhesus macaque, dog, cat, pig, horse and cattle, and can serve as a framework for naming other vertebrate histone genes in the future.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13072-022-00467-2.
Introduction
The DNA in all eukaryotic cells is packaged with histones to form chromatin. The basic unit of chromatin in eukaryotes, the nucleosome, consists of 147 base pairs (bp) of DNA wrapped around an octamer of four core histones, comprising an H3–H4 tetramer and two H2A–H2B dimers. In multicellular organisms, there is a histone H1 bound to the linker region between two nucleosomes, which binds to the region where DNA enters and exits the nucleosome. In addition to packaging the DNA into the nucleus, histones play multiple roles in gene expression, DNA replication and DNA damage repair. The core histones can be extensively modified on their N- and C-terminal tails and globular domains, and these modifications may change binding sites for regulatory factors or neutralize the charge of lysine residues via acetylation. These modifications may silence genes or activate them. Not all DNA is packaged into nucleosomes, there are regions of nucleosome-free DNA, particularly at promoters and enhancers of active genes. There are multiple histone H3, H2A and H1 protein variants which replace the canonical histones at specific sites in the genome. Some of these variants are expressed throughout the cell cycle in all cells, while others are expressed predominantly in specific tissues.
Every time a cell divides, it must not only replicate its DNA but also synthesize large amounts of histones to package the newly replicated DNA. In mammalian cells this requires synthesis of about 108 molecules of each of the four core histone proteins. In metazoans, these histone proteins are encoded by the set of replication-dependent histone genes, which encode representatives of all five classes of histone proteins [1]. The replication-dependent histone genes encode messenger RNAs (mRNAs) which differ from all other cellular mRNAs: instead of being polyadenylated, these mRNAs end in a stem-loop structure. These genes do not contain introns, and the only processing event is cleavage of the nascent transcript to form the 3’ end of the histone mRNA. Some of the “replication-dependent” histone genes can also produce polyadenylated mRNAs. For example, analysis of global gene expression in normal non-dividing tissues revealed that a subset of 10 human replication-dependent histone genes produced polyadenylated mRNAs in all non-dividing tissues analyzed [2].
Genomic clustering of replication-dependent histone genes
In mammals, the replication-dependent genes are found at four discrete loci. In the human genome the largest cluster is on chromosome 6 and contains more than 60 genes, and the second cluster on chromosome 1 contains 10–12 genes. There are 4 genes in a third distinct locus on chromosome 1, and a single replication-dependent histone H4 gene on chromosome 12 (with a neighboring H2A gene for which replication dependency is uncertain). This genomic organization is conserved, and all four loci are syntenic, in mammals.
In other vertebrates, the organization of histone genes is variable. In chicken, there is a single large cluster, analogous to the largest mammalian cluster, which contains genes for all five histone types, all of which encode mRNAs ending in a stem loop. Many fish (e.g., zebrafish) and amphibians (e.g., Xenopus), as well as invertebrates like sea urchins and Drosophila, store large amounts of histone mRNA and proteins in the egg and start development with a series of rapid cell cycles in the absence of zygotic transcription. These species contain far more copies of histone genes than there are in mammals, many of which are organized in tandem repeats with one copy of all 5 histone gene types in each repeat. This is an adaptation to fulfill the requirement for synthesis of large amounts of histone mRNA and protein in a short period of time, either in oogenesis (e.g., in zebrafish, Drosophila and Xenopus), or in early embryogenesis (e.g., in sea urchins) depending on the species.
The replication-dependent histone genes in metazoans are present in a nuclear body, the histone locus body (HLB). Transcription and processing of the histone mRNA occurs within the HLB [3]. This structure provides a microenvironment in the nucleus specialized for the biosynthesis of histone mRNAs, and many factors unique to histone mRNA biosynthesis are concentrated there. The critical protein required for formation of the HLB is NPAT, which is only found bound to the replication-dependent histone genes [4]. Phosphorylation of NPAT by Cyclin E/CDK2 is essential for activation of histone gene expression as cells approach S-phase [5]. The need to form the HLB explains why genes for all five histone types involved in replication-dependent histone synthesis are clustered in the genome in metazoans [6].
Replication-independent histones
Replication-independent variant histone genes are usually expressed throughout the cell cycle, typically contain introns, and are transcribed into polyadenylated mRNAs. These genes are positioned throughout the genome, often as single copies, and are not bound by NPAT. There is one histone gene, the H2AX gene, that encodes both a polyadenylated mRNA and an mRNA that ends in a stem loop, and expresses the stem-loop mRNA only in S-phase [7, 8]. Since this gene is not bound by NPAT, it is not considered a replication-dependent gene, although it does express a replication-dependent histone mRNA which is cell-cycle regulated [7]. The variety of histone proteins encoded in the mammalian genome are described in [9] and are presented below along with the corresponding protein nomenclature.
Histone protein nomenclature
Nomenclature for histones has been an evolving topic since their discovery, with standardization of the protein names H1, H2A, H2B, H3, and H4 dating to the Ciba Foundation Symposium of 1975 [10], and revised in 2005 [11] with the addition of suffixes for post-translational modifications. The discovery of the many histone variants in the genomics era resulted in variant names using a plethora of styles, including prefixes, number suffixes, letter suffixes, a variety of punctuation types or no punctuation, synonyms, and near-homographs, resulting in a call for standardization at the EMBO Workshop on Histone Variants in 2011. The outcome of this workshop was summarized in the protein nomenclature proposed by Talbert et al. [12] in 2012, which restricted itself to naming proteins on the basis that gene nomenclature can be organism specific and that paralogous genes can encode an identical histone variant. The resulting protein nomenclature retained the designations H1, H2A, H2B, H3 and H4 for the five classes of histones, and tried to build on existing variant designations while systematizing naming principles. It aimed to balance historical usage with a phylogenetic approach to naming histone variants, using a period (.), already in use to append suffixes to variant names, to designate branch points in the phylogeny. This is referred to hereafter as the “Strasbourg nomenclature” after the location of the EMBO workshop.
Gene nomenclature committees
The HUGO (Human Genome Organization) Nomenclature Committee (HGNC) has been in operation since the late 1970s and is the only group with the authority to approve nomenclature for human genes. The committee has recently published updated guidelines [13] with a new focus on providing stability for the clinical community. The HGNC also has a sister project, the Vertebrate Gene Nomenclature Committee (VGNC) that approves gene symbols and names for selected vertebrate species of community interest with high quality genomes (currently chimpanzee, rhesus macaque, dog, cat, horse, cattle and pig). The HGNC and VGNC both work in close coordination with the other existing nomenclature committees for model organisms, especially the Mouse Genomic Nomenclature Committee (MGNC) of the Mouse Genome Database [14]. HGNC and VGNC gene symbols are in uppercase letters, while rodent gene symbols have an initial uppercase letter followed by lowercase letters. Wherever possible, orthologous genes are assigned equivalent gene symbols across the HGNC, VGNC and MGNC. These committees do not have jurisdiction over protein nomenclature.
Previous histone gene nomenclature
The HGNC and MGNC both previously approved gene nomenclature for the replication-dependent histone genes as described in “The human and mouse replication-dependent histone genes” [15]. This nomenclature system was based on the genomic cluster that the histone genes were located on, with each symbol beginning HIST1/Hist1 for histone cluster 1 (the largest cluster of over 60 genes located on chromosome 6 in human and chromosome 13 in mouse), HIST2/Hist2 for cluster 2 (located on chromosome 1 in human and 3 in mouse; contains 10–12 core histone genes), HIST3/Hist3 for cluster 3 (a small cluster of 4 genes that has not been well studied) and HIST4/Hist4 for “cluster 4” (the smallest cluster containing one replication-dependent H4 gene). The gene symbols then included the histone type encoded by the gene followed by a unique letter identifier, e.g., human symbol HIST1H2AA and mouse symbol Hist1H2aa represented the first (“a”) histone type H2A gene on histone cluster 1. This system had many strengths, such as providing equivalent symbols for most human and mouse orthologs within the clusters. However, those without prior knowledge of histone genes could wrongly assume that the start of the gene symbols represented the histone type. Furthermore, these symbols comprised 9 characters and feedback, including from clinical communities, often requests avoiding the designation of long symbols. Although the organization of histone gene clusters is conserved across mammals, this is not true across all vertebrates—chicken has a single large replication-dependent histone cluster [16]—also meaning that the gene symbols starting with histone cluster identifiers were not transferrable across non-mammalian vertebrate species.
Replication-independent histone variant genes followed a completely separate nomenclature system. These genes were assigned symbols that started with the histone type, then ‘F’ for family, followed by an identifying letter. The letters were usually based on the encoded histone variant as used by the community, e.g., H2AFZ designated the first human gene identified as encoding the H2A.Z variant [17], but the letters could also simply be assigned by the nomenclature committee, e.g., the symbols H2AFY and H2AFY2 were approved for genes encoding the macroH2A subtypes [18]. Having these two separate nomenclature systems for the replication-dependent and -independent genes was arguably problematic, as this large and complex gene family was not united by a common root symbol.
Revised histone gene nomenclature
As mentioned above, the agreed unified nomenclature for histone protein variants was published by 42 experts from the histone field [12]. The HGNC and MGNC were already aware of problems with the existing gene nomenclature, so both committees agreed that they would work towards revising histone gene symbols—with the input of histone researchers—to be as close to the unified protein nomenclature as possible, whilst fulfilling the requirements of standardized mammalian gene nomenclature. For example, one feature of the histone protein nomenclature is the use of periods as separators in symbols to indicate protein variants and/or proteins that represent phylogenetic branch points. Approved gene symbols cannot include periods, primarily as this could cause problems in data processing. It was agreed that where periods exist between a letter and number in protein variant symbols, these are left out of the new gene symbols entirely, e.g., H2AZ1 is the symbol for the gene encoding the H2A.Z.1 variant. Where a separator is needed between two consecutive numbers, a hyphen is used for HGNC and VGNC gene symbols, and the letter ‘f’ in mouse gene symbols, e.g., genes encoding the H1.0 variant are H1-0 in the HGNC and VGNC databases and H1f0 for mouse. Hyphens are avoided in mouse gene symbols because punctuation is reserved for specific usage in mouse allele nomenclature.
The new gene nomenclature uses symbols that begin with the letter H followed by a numeral, or numeral and letter, to indicate which major histone type they encode, e.g., “H2BC3” encodes an H2B type histone. Tissue of expression as a characteristic is not used in the revised histone gene names, due to variability of reporting, discovery of expression in other tissues in new datasets, and possible lack of conservation across species. Efforts have been made to create a nomenclature that makes sense across vertebrates where possible—the revised symbols no longer refer to individual clusters, meaning that the naming scheme can be extended into non-mammalian vertebrates, e.g., human HIST1H2AA, “histone cluster 1 H2A family member a” has been renamed as H2AC1, “H2A clustered histone 1”. Replication-independent genes are named as closely to the agreed upon symbols for the protein variants as possible.
Resolving replication-dependent histone gene nomenclature across mammalian species
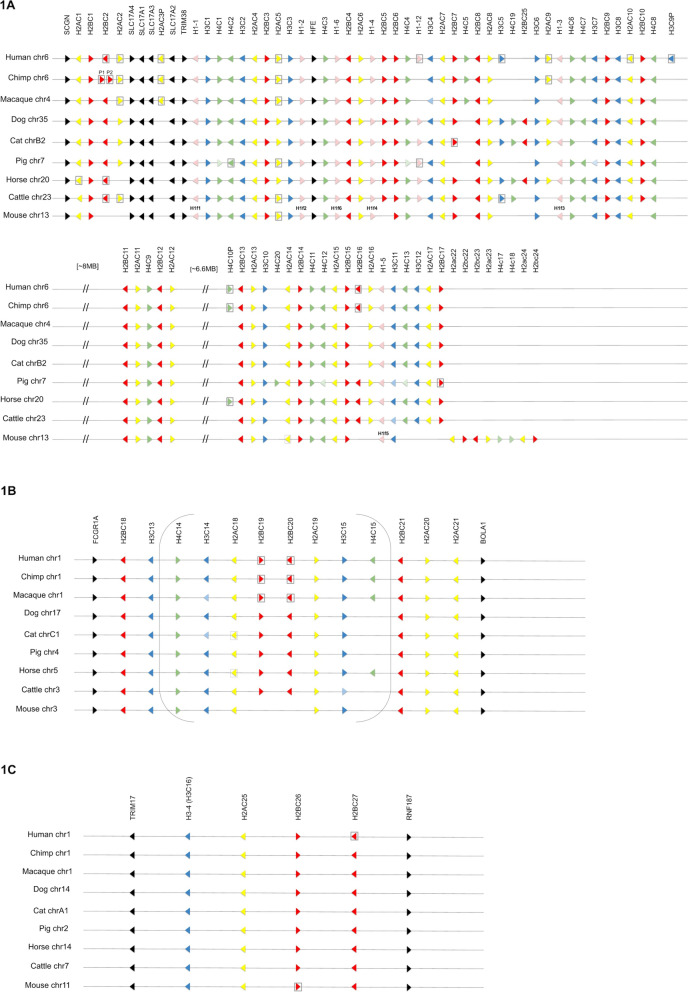
Histone protein nomenclature does not encompass the complexity of histone genes where many paralogs encode identical, or very similar, proteins. The distinct H2A and H2B isoforms encoded by genes within the replication-dependent clusters contain many small variations which have not been characterized as functionally significant and are not well conserved across species. There are two H3 isoforms on the largest two replication-dependent largest clusters, H3.1 and H3.2; all human H3 genes on cluster 1 encode H3.1, while some of the orthologous mouse genes on cluster 1 encode H3.2. For this reason, it is not possible to approve one gene symbol per replication-dependent protein isoform. However, due to the remarkable conservation of gene order within the mammalian replication-dependent clusters, it is possible to identify one-to-one orthologs for most of the genes and name orthologs with equivalent gene symbols (see Fig. 1).
Fig. 1.
The three replication-dependent histone gene clusters in mammals. Gene symbols are shown across the top; species and chromosomal location of each cluster is indicated at the side. Black = non-histone genes, pink = histone H1 genes, yellow = H2A genes, red = H2B genes, blue = H3 genes, green = H4 genes. Pseudogenes are indicated by a gray box around the gene (pseudogenes have the same symbol as their protein-coding orthologs but end with -ps for mouse and P for the other species). A paler shade indicates that the gene is present but currently unannotated and unnamed; a blank space indicates that the gene is missing entirely. Mouse H1 genes contain an ‘f’ in place of the hyphen, so each mouse H1f symbol is shown above each relevant mouse gene. A: The largest replication-dependent cluster, also known as HIST1. There are two large gaps in the cluster in all species. Conservation between species is remarkable, although there are some species-specific duplications, gene losses and in situ pseudogenizations. Mouse has an expansion at the end of the cluster—these genes are shown with the mouse gene symbol format; note that all mouse symbols follow this format but for simplicity only the uppercase format used for other mammalian genes is shown for the conserved genes. B. The second largest replication-dependent cluster, also known as HIST2; each species has at least 10 genes in this cluster that contains genes for the 4 core histones but contains no histone H1 genes. The cluster contains a large inverted repeat, indicated by brackets. C. The third mammalian replication-dependent cluster, also known as HIST3. Note that H3-4 has an exceptional symbol due to the common usage of the H3.4 symbol for the protein encoded by this gene, the systematic H3C16 alias is shown in parentheses
The previous replication-dependent histone gene nomenclature was curated for human and mouse only. It is impossible for automated naming systems to resolve orthology well enough to assign gene symbols to most replication-dependent histone genes in other mammalian species. Therefore, VGNC curators have manually named histone genes in the species chimpanzee, rhesus macaque, dog, cat, pig, horse and cattle to be the same as their identified human orthologs, using a combination of conserved gene order and sequence similarity (Fig. 1 and Additional File 1). Note that numbering of genes in the clusters is not intended to reflect gene order, so that symbols for any additional paralogs identified in new species may be added, e.g., dog, cattle and horse all have the genes H4C19 and H2BC25 which are not present in human or mouse and therefore take higher numbers, so that the nearest 5’ H2B gene to H2BC25 is H2BC8 and the nearest 5’ H4 gene to H4C19 is H4C5 (Fig. 1A).
Comparing the histone clusters across mammalian species has resulted in the resolution of several former gene symbol differences between human and mouse orthologs. In mouse, the genes now named as H2ac18 and H2ac19 were previously named as Hist2h2aa1 and Hist2h2aa2, while the human orthologs that are now named consistently as H2AC18 and H2AC19 were previously named with different symbols—HIST2H2AA3 and HIST2H2AA4. The inverted repeat within histone cluster 2 was missing from the initial human reference genome, meaning that only one copy of each human gene in this repeat was included in the initial round of naming. The primate inverted repeats include two pseudogenes (H2BC19P, previously HIST2H2BD and H2BC20P, previously HIST2H2BC) that are not present in mouse. Aligning these genes with the additional species shows that in cat, pig, horse and cattle these genes encode an H2B protein and the mRNA ends in a stem loop (Fig. 1B), and therefore, these are functional genes and are named as H2BC19 and H2BC20 in cat, pig, horse and cattle (the ‘P’ at the end of the human symbols indicates the locus is a pseudogene). In all species, these H2BC genes are flanked by H2AC18 and H2AC19 (note that H2AC18 appears to be pseudogenized in cat and horse) in a conserved gene order and gene orientation. Therefore, it is clear to see that H2BC19 and H2BC20 orthologs have been lost in mouse but the flanking H2ac18 and H2ac19 genes remain and can be named in concordance with their orthologs in other mammals (Fig. 1A).
Pseudogenization of genes in situ is a feature of replication-dependent histone clusters; studying cluster organization across other mammals has enabled us to rename more mouse and human pseudogenes to be in line with their protein-coding orthologs. Human H2AC10P was not previously named as orthologous to mouse—its previous symbol was HIST1H2APS4, while the previous symbol for the coding mouse ortholog (now H2ac10) was Hist1h2af. All other mammalian species studied here appear to have a coding copy of H2AC10 (Fig. 1A), making it clear that the human gene at this conserved position is a pseudogenized ortholog and can be named accordingly as H2AC10P. As for H2BC19P and H2BC20P mentioned above, there are several other human pseudogenes that have no equivalent mouse ortholog, which have now been named relative to protein-coding orthologs in other species. For example, H1-12 is predicted to be coding in dog, horse and cattle (although the predicted H1.12 protein has not been studied) allowing the naming of the human pseudogenized ortholog as H1-12P (previously HIST1H1PS1); pig also carries a pseudogenized copy which is again named as H1-12P (Fig. 1A). H2BC2 and H2AC2 are predicted to be coding in dog, cat and pig, resulting in the renaming of human HIST1H2BPS1 and HIST1H2APS1 as H2BC2P and H2AC2P (Fig. 1A).
Full description of the revised gene nomenclature by histone type
H1 histone genes
The revised H1 histone gene nomenclature, along with previous symbols and protein variant symbols, is shown in Table 1 for human and Table 2 for mouse. All symbols begin with the root ‘H1’. The H1 genes were relatively simple to fit with the Strasbourg protein nomenclature, where each H1 variant is distinguished by a separate number, because each H1 gene encodes a different histone variant. It has, therefore, been possible to approve gene symbols that are equivalent to the H1 protein symbols, shown in Talbert et al. [12]. As mentioned above, HGNC and VGNC gene symbols include a hyphen in place of a period where two numbers need to be separated. The H1 nomenclature distinguishes replication-dependent from replication-independent genes in the gene names by the presence of the words “cluster member” but “C” for cluster is not used in the gene symbols to preserve parallel H1 protein and gene symbols. The terminology “cluster member” was chosen to feature at the end of replication-dependent histone H1 gene names rather than “clustered”, which is used elsewhere, so that the gene names of all H1 genes can contain the type of H1 followed by the term ‘linker histone’, e.g., “H1.1 linker histone, cluster member”. It is now clear to the non-expert that H1-0 and H1-1 both encode histone H1 genes. Mouse gene symbols include an ‘f’ instead of a hyphen (Table 2) because hyphens are reserved for mouse allele nomenclature where this punctuation has a specific role. For example, in the allele Tg(tetO-H1f0)1Hzo, the hyphen separates the promoter from the expressed gene. Although slightly different, the mouse and human symbols are clearly equivalent, e.g., H1f1 and H1-1.
Table 1.
Revised gene nomenclature for human histone H1 genes
| Variant symbol | HGNC gene symbol | HGNC gene name | Previous symbol | HGNC ID | UniProt ID |
|---|---|---|---|---|---|
| H1.0 | H1-0 | H1.0 linker histone | H1F0 | HGNC:4714 | P07305 |
| H1.1 | H1-1 | H1.1 linker histone, cluster member | HIST1H1A | HGNC:4715 | Q02539 |
| H1.2 | H1-2 | H1.2 linker histone, cluster member | HIST1H1C | HGNC:4716 | P16403 |
| H1.3 | H1-3 | H1.3 linker histone, cluster member | HIST1H1D | HGNC:4717 | P16402 |
| H1.4 | H1-4 | H1.4 linker histone, cluster member | HIST1H1E | HGNC:4718 | P10412 |
| H1.5 | H1-5 | H1.5 linker histone, cluster member | HIST1H1B | HGNC:4719 | P16401 |
| H1.6 | H1-6 | H1.6 linker histone, cluster member | HIST1H1T | HGNC:4720 | P22492 |
| H1.7 | H1-7 | H1.7 linker histone | H1FNT | HGNC:24893 | Q75WM6 |
| H1.8 | H1-8 | H1.8 linker histone | H1FOO | HGNC:18463 | Q8IZA3 |
| NA | H1-9P | H1.9 linker histone, pseudogene | HILS1 | HGNC:30616 | P60008 |
| H1.10 | H1-10 | H1.10 linker histone | H1FX | HGNC:4722 | Q92522 |
| NA | H1-12P | H1.12 linker histone, cluster member pseudogene | HIST1H1PS1 | HGNC:19163 | NA |
Table 2.
Revised gene nomenclature for mouse histone H1 genes
| Variant symbol | Mouse gene symbol | Mouse gene name | Previous symbol | MGI ID | UniProt ID |
|---|---|---|---|---|---|
| H1.0 | H1f0 | H1.0 linker histone | H1f0 | MGI:95893 | P10922 |
| H1.1 | H1f1 | H1.1 linker histone, cluster member | Hist1h1a | MGI:1931523 | P43275 |
| H1.2 | H1f2 | H1.2 linker histone, cluster member | Hist1h1c | MGI:1931526 | P15864 |
| H1.3 | H1f3 | H1.3 linker histone, cluster member | Hist1h1d | MGI:107502 | P43277 |
| H1.4 | H1f4 | H1.4 linker histone, cluster member | Hist1h1e | MGI:1931527 | P43274 |
| H1.5 | H1f5 | H1.5 linker histone, cluster member | Hist1h1b | MGI:1861461 | P43276 |
| H1.6 | H1f6 | H1.6 linker histone, cluster member | Hist1h1t | MGI:1888530 | Q07133 |
| H1.7 | H1f7 | H1.7 linker histone | H1fnt | MGI:117319 | Q8CJI4 |
| H1.8 | H1f8 | H1.8 linker histone | H1foo | MGI:2176207 | Q8VIK3 |
| H1.9 | H1f9 | H1.9 linker histone | Hils1 | MGI:2136691 | Q9QYL0 |
| H1.10 | H1f10 | H1.10 linker histone | H1fx | MGI:2685307 | Q80ZM5 |
| NA | H1f11-ps | H1.11 linker histone, pseudogene | Gm6970 | MGI:3645322 | NA |
Note that replication-dependent histone H1 genes have “cluster member” in the gene name
H2A histone genes
Replication-dependent H2A genes
The complete set of revised H2A histone genes is shown in Table 3 for human and Table 4 for mouse. Two general classes of H2A proteins were first identified by Fred Zweidler using triton-acid urea gel electrophoresis [19] based on a characteristic change at position 51 of the protein sequence where there is a leucine in H2A.1 and a methionine in H2A.2. H2A.1 and H2A.2 are encoded by multiple replication-dependent H2A histone genes and these variant designations were recommended in the unified histone nomenclature [12]. For the most part, H2A genes on the largest replication-dependent cluster, known as HIST1 (Fig. 1A), encode H2A.1 proteins, while those on the second largest cluster, known as HIST2 (Fig. 1B), encode H2A.2 proteins. The H2A protein on the smaller cluster known as HIST3 (Fig. 1C) encodes an H2A.1 protein with more amino acid changes elsewhere in the protein compared to other H2A.1-encoding genes. Human H2AC21 on cluster 2 encodes an H2A.1 protein, while the mouse ortholog, H2ac21, encodes an H2A.2 protein. Therefore, the revised gene nomenclature does not distinguish between H2A.1 or H2A.2 to allow consistent naming of orthologs across vertebrate species. There are alternative histone protein naming systems that do not distinguish between H2A.1 and H2A.2 but refer to replication-dependent H2A as ‘canonical H2A’ [20]. During discussions with the wider histone community, advice was given to avoid use of the term ‘canonical’ as this term can be interpreted in different ways by different researchers. It was during this feedback process that the suggestion was made to use ‘clustered’ to refer to H2A, H2B, H3 and H4 replication-dependent genes. The H2A histone genes on replication-dependent clusters have, therefore, been named with the root symbol ‘H2AC#’ (H2ac# in mouse) for ‘H2A clustered histone’. Although there are multiple proteins encoded by the mammalian replication-dependent H2A genes, with small differences primarily at the C terminus [15], these variations are not conserved between mouse and human orthologs, suggesting they are not functional, and are therefore not reflected at the level of gene nomenclature. The H2AC1 and H2BC1 genes encode the H2A and H2B proteins with the largest number of amino acid changes; they also were initially reported as “sperm specific”, which likely accounts for the variability from the other genes.
Table 3.
Revised gene nomenclature for human histone H2A genes
| Variant symbol | HGNC gene symbol | HGNC gene name | Previous symbol | HGNC ID | UniProt ID |
|---|---|---|---|---|---|
| H2A | H2AC1 | H2A clustered histone 1 | HIST1H2AA | HGNC:18729 | Q96QV6 |
| NA | H2AC2P | H2A clustered histone 2, pseudogene | HIST1H2APS1 | HGNC:18720 | NA |
| NA | H2AC3P | H2A clustered histone 3, pseudogene | HIST1H2APS2 | HGNC:18804 | NA |
| H2A | H2AC4 | H2A clustered histone 4 | HIST1H2AB | HGNC:4734 | P04908 |
| NA | H2AC5P | H2A clustered histone 5, pseudogene | HIST1H2APS5 | HGNC:4728 | NA |
| H2A | H2AC6 | H2A clustered histone 6 | HIST1H2AC | HGNC:4733 | Q93077 |
| H2A | H2AC7 | H2A clustered histone 7 | HIST1H2AD | HGNC:4729 | P20671 |
| H2A | H2AC8 | H2A clustered histone 8 | HIST1H2AE | HGNC:4724 | P04908 |
| NA | H2AC9P | H2A clustered histone 9, pseudogene | HIST1H2APS3 | HGNC:18805 | NA |
| NA | H2AC10P | H2A clustered histone 10, pseudogene | HIST1H2APS4 | HGNC:4732 | NA |
| H2A | H2AC11 | H2A clustered histone 11 | HIST1H2AG | HGNC:4737 | P0C0S8 |
| H2A | H2AC12 | H2A clustered histone 12 | HIST1H2AH | HGNC:13671 | Q96KK5 |
| H2A | H2AC13 | H2A clustered histone 13 | HIST1H2AI | HGNC:4725 | P0C0S8 |
| H2A | H2AC14 | H2A clustered histone 14 | HIST1H2AJ | HGNC:4727 | Q99878 |
| H2A | H2AC15 | H2A clustered histone 15 | HIST1H2AK | HGNC:4726 | P0C0S8 |
| H2A | H2AC16 | H2A clustered histone 16 | HIST1H2AL | HGNC:4730 | P0C0S8 |
| H2A | H2AC17 | H2A clustered histone 17 | HIST1H2AM | HGNC:4735 | P0C0S8 |
| H2A | H2AC18 | H2A clustered histone 18 | HIST2H2AA3 | HGNC:4736 | Q6FI13 |
| H2A | H2AC19 | H2A clustered histone 19 | HIST2H2AA4 | HGNC:29668 | Q6FI13 |
| H2A | H2AC20 | H2A clustered histone 20 | HIST2H2AC | HGNC:4738 | Q16777 |
| H2A | H2AC21 | H2A clustered histone 21 | HIST2H2AB | HGNC:20508 | Q8IUE6 |
| H2A | H2AC25 | H2A clustered histone 25 | HIST3H2A | HGNC:20507 | Q7L7L0 |
| H2A.Z.1 | H2AZ1 | H2A.Z variant histone 1 | H2AFZ | HGNC:4741 | P0C0S5 |
| H2A.Z.2 | H2AZ2 | H2A.Z variant histone 2 | H2AFV | HGNC:20664 | Q71UI9 |
| macroH2A.1 | MACROH2A1 | macroH2A.1 histone | H2AFY | HGNC:4740 | O75367 |
| macroH2A.2 | MACROH2A2 | macroH2A.2 histone | H2AFY2 | HGNC:14453 | Q9P0M6 |
| H2A.X | H2AX | H2A.X variant histone | H2AFX | HGNC:4739 | P16104 |
| H2A.J | H2AJ | H2A.J histone | H2AFJ | HGNC:14456 | Q9BTM1 |
| H2A.B | H2AB1 | H2A.B variant histone 1 | H2AFB1 | HGNC:22516 | P0C5Y9 |
| H2A.B | H2AB2 | H2A.B variant histone 2 | H2AFB2 | HGNC:18298 | P0C5Z0 |
| H2A.B | H2AB3 | H2A.B variant histone 3 | H2AFB3 | HGNC:14455 | P0C5Z0 |
| H2A.P | H2AP | H2A.P histone | HYPM | HGNC:18417 | O75409 |
| NA | H2AQ1P | H2A.Q variant histone 1, pseudogene | NA | HGNC:53962 | NA |
| H2A.L | H2AL1Q | H2A.L variant histone 1Q | NA | HGNC:53959 | NA |
| NA | H2AL1MP | H2A.L variant histone 1 M, pseudogene | NA | HGNC:53961 | NA |
| H2A.L | H2AL3 | H2A.L variant histone 3 | NA | HGNC:53960 | NA |
Table 4.
Revised gene nomenclature for mouse histone H2A genes
| Variant symbol | Mouse gene symbol | Mouse gene name | Previous symbol | MGI ID | UniProt ID |
|---|---|---|---|---|---|
| H2A | H2ac1 | H2A clustered histone 1 | Hist1h2aa | MGI:2448285 | Q8CGP4 |
| H2A | H2ac4 | H2A clustered histone 4 | Hist1h2ab | MGI:2448306 | C0HKE1 |
| NA | H2ac5-ps | H2A clustered histone 5, pseudogene | Gm11336 | MGI:3651860 | NA |
| H2A | H2ac6 | H2A clustered histone 6 | Hist1h2ac | MGI:2448287 | C0HKE2 |
| H2A | H2ac7 | H2A clustered histone 7 | Hist1h2ad | MGI:2448289 | C0HKE3 |
| H2A | H2ac8 | H2A clustered histone 8 | Hist1h2ae | MGI:2448290 | C0HKE4 |
| H2A | H2ac10 | H2A clustered histone 10 | Hist1h2af | MGI:2448309 | Q8CGP5 |
| H2A | H2ac11 | H2A clustered histone 11 | Hist1h2ag | MGI:2448293 | C0HKE5 |
| H2A | H2ac12 | H2A clustered histone 12 | Hist1h2ah | MGI:2448295 | Q8CGP6 |
| H2A | H2ac13 | H2A clustered histone 13 | Hist1h2ai | MGI:248457 | C0HKE6 |
| NA | H2ac14-ps | H2A clustered histone 14, pseudogene | Hist1h2aj | MGI:2448312 | NA |
| H2A | H2ac15 | H2A clustered histone 15 | Hist1h2ak | MGI:2448297 | Q8CGP7 |
| H2A | H2ac18 | H2A clustered histone 18 | Hist2h2aa1 | MGI:96097 | Q6GSS7 |
| H2A | H2ac19 | H2A clustered histone 19 | Hist2h2aa2 | MGI:2448283 | Q6GSS7 |
| H2A | H2ac20 | H2A clustered histone 20 | Hist2h2ac | MGI:2448316 | Q64523 |
| H2A | H2ac21 | H2A clustered histone 21 | Hist2h2ab | MGI:2448314 | Q64522 |
| H2A | H2ac22 | H2A clustered histone 22 | Hist1h2an | MGI:2448300 | C0HKE7 |
| H2A | H2ac23 | H2A clustered histone 23 | Hist1h2ao | MGI:2448302 | C0HKE8 |
| H2A | H2ac24 | H2A clustered histone 24 | Hist1h2ap | MGI:3710573 | C0HKE9 |
| H2A | H2ac25 | H2A clustered histone 25 | Hist3h2a | MGI:2448458 | Q8BFU2 |
| H2A.Z.1 | H2az1 | H2A.Z variant histone 1 | H2afz | MGI:1888388 | P0C0S6 |
| H2A.Z.2 | H2az2 | H2A.Z variant histone 2 | H2afv | MGI:1924855 | Q3THW5 |
| macroH2A.1 | Macroh2a1 | macroH2A.1 histone | H2afy | MGI:1349392 | Q9QZQ8 |
| macroH2A.2 | Macroh2a2 | macroH2A.2 histone | H2afy2 | MGI:3037658 | Q8CCK0 |
| H2A.X | H2ax | H2A.X variant histone | H2afx | MGI:102688 | P27661 |
| H2A.J | H2aj | H2A.J histone | H2afj | MGI:3606192 | Q8R1M2 |
| H2A.B | H2ab1 | H2A.B variant histone 1 | H2ab1 | MGI:3642445 | S4R1E0 |
| H2A.B | H2ab2 | H2A.B variant histone 2 | H2ab2 | MGI:3644980 | S4R1M3 |
| H2A.B | H2ab3 | H2A.B variant histone 3 | H2ab3 | MGI:3644875 | S4R1G7 |
| H2A.P | H2ap | H2A.P histone | Hypm | MGI:1914584 | Q9CR04 |
| H2A.L | H2al1a | H2A histone family member L1A | NA | MGI:3714114 | Q5M8Q2 |
| H2A.L | H2al1b | H2A histone family member L1B | NA | MGI:3650131 | A0A087WP11 |
| H2A.L | H2al1c | H2A histone family member L1C | NA | MGI:3711280 | Q5M8Q2 |
| H2A.L | H2al1d | H2A histone family member L1D | NA | MGI:3710419 | Q5M8Q2 |
| H2A.L | H2al1e | H2A histone family member L1E | NA | MGI:3649617 | Q810S6 |
| H2A.L | H2al1f | H2A histone family member L1F | NA | MGI:3649874 | Q5M8Q2 |
| H2A.L | H2al1g | H2A histone family member L1G | NA | MGI:3710577 | Q5M8Q2 |
| H2A.L | H2al1h | H2A histone family member L1H | NA | MGI:3711282 | Q5M8Q2 |
| H2A.L | H2al1i | H2A histone family member L1I | NA | MGI:3710416 | Q5M8Q2 |
| H2A.L | H2al1j | H2A histone family member L1J | NA | MGI:3643273 | A2BFR3 |
| H2A.L | H2al1k | H2A histone family member L1K | NA | MGI:3710586 | J3QP08 |
| H2A.L | H2al1m | H2A histone family member L1M | NA | MGI:1923633 | Q9DAD9 |
| H2A.L | H2al1n | H2A histone family member L1N | NA | MGI:3643774 | Q497L1 |
| H2A.L | H2al1o | H2A histone family member L1O | NA | MGI:3643069 | L7MU04 |
| NA | H2al1q-ps | H2A histone family member L1Q, pseudogene | NA | MGI:3705686 | NA |
| NA | H2al1r-ps | H2A histone family member L1R, pseudogene | NA | MGI:3705677 | NA |
| H2A.L | H2al2a | H2A histone family member L2A | NA | MGI:1915481 | Q9CQ70 |
| H2A.L | H2al2b | H2A histone family member L2B | NA | MGI:3710623 | A9Z055 |
| H2A.L | H2al2c | H2A histone family member L2C | NA | MGI:3779546 | A9Z055 |
| H2A.L | H2al3 | H2A histone family member L3 | NA | MGI:1922521 | Q9D4U4 |
Replication-independent H2A genes
For replication-independent H2A histones the protein nomenclature has been followed as closely as possible. For example, the human genes encoding the H2A.Z variant that is present in all eukaryotes [21] have been approved as H2AZ1 and H2AZ2 (full gene names “H2A.Z histone 1” and “H2A.Z histone 2”). The macroH2A variant was so named because it is almost three times as large as replication-dependent H2A histones [22]. This variant name is included in the Strasbourg nomenclature and accepted by the histone community. Therefore, an exception has been made and the corresponding gene symbols MACROH2A1 and MACROH2A2 (MacroH2a1 and MacroH2a2 for mouse) approved, even though this means that the symbols do not begin with the root symbol ‘H2A’.
The Strasbourg nomenclature aims to “use letter suffixes for monophyletic clades” [12]. However, the experts that devised this nomenclature recognized that in some cases historical usage and community support for such usage should be taken into consideration. Therefore, they recommended that the histone community continue to use the H2A.X designation even though this histone variant does not appear to be from a separate clade to the replication-dependent H2A histones that have been assigned the root symbol H2AC. This recommendation has been followed and the gene named as H2AX; this gene is not positioned within a replication-dependent cluster and is interesting as it encodes two distinct mRNAs, one ending in a stem loop and the other one polyadenylated [8]. The stem-loop form of the mRNA is expressed in S-phase of the cell cycle and the polyadenylated form expressed outside of S-phase. The H2AX gene does not bind NPAT, distinguishing it from the genes in the clusters.
The same principle has been followed for the gene named as H2AJ, which also does not belong to a separate clade to the replication-dependent H2A histone genes; the encoded variant has been published as H2A.J [23] and has been referred to as replication independent [9]. For the species reported here, the gene produces only polyadenylated mRNA, and does not contain a stem loop; hence, this has been assigned the separate variant-type symbol H2AJ. Note the H2AJ gene is adjacent to the H4C16 gene.
Short H2A replication-independent variants
Short histone H2A variants lack a C-terminal region compared to replication-dependent histones and all appear to be expressed primarily in the testis, although H2A.B is also expressed in brain [24]. These variants all derive from a single gene on the X chromosome of a common ancestor and have since diverged into four distinct clades known as H2A.P, H2A.Q, H2A.B, and H2A.L [25]. The H2A.P-encoding gene had the previously approved symbol HYPM for “huntingtin interacting protein M” and has now been renamed as H2AP for “H2A.P histone” (and from Hypm to H2ap in mouse). H2A.Q is the most recently discovered short H2A variant [25] and a functional protein has been predicted for many non-Euarchontoglires mammals. However, the only VGNC species with a supporting protein-coding gene annotation is dog; this gene has been named as H2AQ1, for “H2A.Q variant histone 1” (Additional File 1). In human the locus is pseudogenized at a conserved position on the X chromosome and has been named H2AQ1P for “H2A.Q variant histone 1, pseudogene”. Although the presence of an orthologous mouse pseudogene is suggested in [25], there is currently no annotated mouse gene.
Human and most other mammals have three paralogs that encode H2A.B histones. In humans, these duplicated paralogs neighbor coagulation factor VIII genes and are numbered consistently with these genes—H2AB1 is next to F8A1; H2AB2 is next to F8A2; H2AB3 is next to F8A3. All three H2AB genes are highly similar in sequence and encode a protein that is identical in the case of H2AB2 and H2AB3, with only one amino acid difference in the protein encoded by H2AB1. In the literature these two proteins have sometimes been referred to as the variants H2A.B.1 (encoded by H2AB2 and H2AB3) and H2A.B.2 (encoded by H2AB1) [25], although many papers do not make this distinction and refer to variant H2A.B only [26–28]. Mouse has three paralogs named H2ab1, H2ab2 and H2ab3; the mouse-encoded H2A.B protein has been referred to as H2A.B.3 [29].
Mouse has an expansion of H2A.L-encoding genes, with fourteen H2al1 protein-coding genes (named H2al1a through to H2al1o), three H2al2 genes (H2al2a, H2al2b and H2al2c) which are the only H2al family members to be found outside of the X chromosome, and one H2al3 gene. Although no H2A.L protein has been detected in human so far [25], human has an ortholog of mouse H2al3 with an intact open reading frame which has therefore been named H2AL3. This gene is conserved in rhesus macaque, cattle, pig, horse and dog (Additional File 1). There is also a human H2AL1 family member, H2AL1Q, which has an intact open reading frame, so has the potential to encode a protein. There is a mouse pseudogene, H2al1q-ps, at a syntenic location and this locus is predicted to be coding in dog, cat and cattle (Additional File 1). Finally, there is a human gene at a conserved genomic position to mouse H2al1m, but this is a pseudogene and has therefore been named H2AL1MP.
Histone H2B genes
Revised human H2B gene nomenclature is shown in Table 5; revised mouse H2B gene nomenclature is shown in Table 6. In accordance with the H2A genes on replication-dependent clusters described above, H2B genes on these clusters have been named with the root symbol H2BC# for ‘H2B clustered histone’ (H2bc# in mouse). Attempts have been made to follow the Strasbourg nomenclature as closely as possible for the H2B replication-independent genes.
Table 5.
Revised gene nomenclature for human histone H2B genes
| Variant symbol | HGNC gene symbol | HGNC gene name | Previous symbol | HGNC ID | UniProt ID |
|---|---|---|---|---|---|
| H2B | H2BC1 | H2B clustered histone 1 | HIST1H2BA | HGNC:18730 | Q96A08 |
| NA | H2BC2P | H2B clustered histone 2, pseudogene | HIST1H2BPS1 | HGNC:18719 | NA |
| H2B | H2BC3 | H2B clustered histone 3 | HIST1H2BB | HGNC:4751 | P33778 |
| H2B | H2BC4 | H2B clustered histone 4 | HIST1H2BC | HGNC:4757 | P62807 |
| H2B | H2BC5 | H2B clustered histone 5 | HIST1H2BD | HGNC:4747 | P58876 |
| H2B | H2BC6 | H2B clustered histone 6 | HIST1H2BE | HGNC:4753 | P62807 |
| H2B | H2BC7 | H2B clustered histone 7 | HIST1H2BF | HGNC:4752 | P62807 |
| H2B | H2BC8 | H2B clustered histone 8 | HIST1H2BG | HGNC:4746 | P62807 |
| H2B | H2BC9 | H2B clustered histone 9 | HIST1H2BH | HGNC:4755 | Q93079 |
| H2B | H2BC10 | H2B clustered histone 10 | HIST1H2BI | HGNC:4756 | P62807 |
| H2B | H2BC11 | H2B clustered histone 11 | HIST1H2BJ | HGNC:4761 | P06899 |
| H2B | H2BC12 | H2B clustered histone 12 | HIST1H2BK | HGNC:13954 | O60814 |
| H2B | H2BC13 | H2B clustered histone 13 | HIST1H2BL | HGNC:4748 | Q99880 |
| H2B | H2BC14 | H2B clustered histone 14 | HIST1H2BM | HGNC:4750 | Q99879 |
| H2B | H2BC15 | H2B clustered histone 15 | HIST1H2BN | HGNC:4749 | Q99877 |
| NA | H2BC16P | H2B clustered histone 16, pseudogene | HIST1H2BPS2 | HGNC:4754 | NA |
| H2B | H2BC17 | H2B clustered histone 17 | HIST1H2BO | HGNC:4758 | P23527 |
| H2B | H2BC18 | H2B clustered histone 18 | HIST2H2BF | HGNC:24700 | Q5QNW6 |
| NA | H2BC19P | H2B clustered histone 19, pseudogene | HIST2H2BD | HGNC:20517 | Q6DRA6 |
| NA | H2BC20P | H2B clustered histone 20, pseudogene | HIST2H2BC | HGNC:20516 | Q6DN03 |
| H2B | H2BC21 | H2B clustered histone 21 | HIST2H2BE | HGNC:4760 | Q16778 |
| H2B | H2BC26 | H2B clustered histone 26 | HIST3H2BB | HGNC:20514 | Q8N257 |
| NA | H2BC27P | H2B clustered histone 27, pseudogene | HIST3H2BA | HGNC:20515 | NA |
| H2B.K | H2BK1 | H2B.K variant histone 1 | H2BE1 | HGNC:53833 | A0A2R8Y619 |
| NA | H2BL1P | H2B.L histone variant 1, pseudogene | H2BP4 | HGNC:54442 | NA |
| H2B.W | H2BW1 | H2B.W histone 1 | H2BFWT | HGNC:27252 | Q7Z2G1 |
| H2B.W | H2BW2 | H2B.W histone 2 | H2BFM | HGNC:27867 | P0C1H6 |
| NA | H2BW3P | H2B.W histone 3, pseudogene | NA | HGNC:44390 | NA |
| NA | H2BW4P | H2B.W histone 4, pseudogene | H2BFXP | HGNC:25757 | NA |
| H2B.N | H2BN1 | H2B.N variant histone 1 | NA | HGNC:56200 | NA |
| H2B | H2BC12L | H2B clustered histone 12 like | H2BFS | HGNC:4762 | P57053 |
Table 6.
Revised gene nomenclature for mouse histone H2B genes
| Variant symbol | Mouse gene symbol | Mouse gene name | Previous symbol | MGI ID | UniProt ID |
|---|---|---|---|---|---|
| H2B | H2bc1 | H2B clustered histone 1 | Hist1h2ba | MGI:2448375 | P70696 |
| H2B | H2bc3 | H2B clustered histone 3 | Hist1h2bb | MGI:2448377 | Q64475 |
| H2B | H2bc4 | H2B clustered histone 4 | Hist1H2bc | MGI:1915274 | Q6ZWY9 |
| H2B | H2bc6 | H2B clustered histone 6 | Hist1h2be | MGI:2448380 | Q6ZWY9 |
| H2B | H2bc7 | H2B clustered histone 7 | Hist1h2bf | MGI:2448383 | P10853 |
| H2B | H2bc8 | H2B clustered histone 8 | Hist1h2bg | MGI:2448386 | Q6ZWY9 |
| H2B | H2bc9 | H2B clustered histone 9 | Hist1h2bh | MGI:2448387 | Q64478 |
| H2B | H2bc11 | H2B clustered histone 11 | Hist1h2bj | MGI:2448388 | P10853 |
| H2B | H2bc12 | H2B clustered histone 12 | Hist1h2bk | MGI:2448399 | Q8CGP1 |
| H2B | H2bc13 | H2B clustered histone 13 | Hist1h2bl | MGI:2448403 | P10853 |
| H2B | H2bc14 | H2B clustered histone 14 | Hist1h2bm | MGI:2448404 | P10854 |
| H2B | H2bc15 | H2B clustered histone 15 | Hist1h2bn | MGI:2448407 | P10853 |
| H2B | H2bc18 | H2B clustered histone 18 | Hist2h2bb | MGI:2448413 | Q64525 |
| H2B* | H2bc21 | H2B clustered histone 21 | Hist2h2be | MGI:2448415 | Q64524 |
| H2B | H2bc22 | H2B clustered histone 22 | Hist1h2bp | MGI:2448409 | Q8CGP2 |
| H2B | H2bc23 | H2B clustered histone 23 | Hist1h2bq | MGI:3702051 | Q8CBB6 |
| H2B | H2bc24 | H2B clustered histone 24 | Hist1h2br | MGI:3710645 | Q8CBB6 |
| NA | H2bc26-ps | H2B clustered histone 26, pseudogene | Hist3h2bb-ps | MGI:1922442 | NA |
| H2B | H2bc27 | H2B clustered histone 27 | Hist3h2ba | MGI:1925553 | Q9D2U9 |
| SubH2Bv/H2B.L** | H2bl1 | H2B.L histone variant 1 | 1700024P04Rik | MGI:1916632 | Q9D9Z7 |
| H2B.W | H2bw2 | H2B.W histone 2 | H2bfm | MGI:1916639 | Q9DAB5 |
*This variant has never been characterized experimentally and so has no Strasbourg variant name
**This variant has been published with two different symbols; following community consultation this gene has been named as encoding the H2B.L variant
H2B.W-encoding histone genes
The H2B.W variant symbol was proposed in the Strasbourg nomenclature [12] for the variant that had been previously known as H2BFWT [30, 31] and TH2B-175 [31]. Human has two H2B.W-encoding paralogs which are now named as H2BW1 and H2BW2 and two pseudogenes (H2BW3P and H2BW4P) all located on the X chromosome between RAB9B and SLC25A3, while mouse has only one H2B.W-encoding gene (H2bw2) found in a syntenic location. Other mammals have between 1 and 4 H2BW paralogs but these are all located at the same conserved location of the X chromosome (Additional File 1).
H2B.L-encoding histone genes
Another mammalian H2B variant was first published as SubH2Bv [32] based on its location in the subacrosomal component of cattle spermatozoa. The homologous mouse variant was published as H2BL1 (originally to denote H2B-like 1) [33]. For the macroH2A variant mentioned above, an exception was made, and the MACROH2A# gene symbols were approved due to the overwhelming usage of macroH2A in the scientific literature. In contrast, the SubH2Bv/H2BL variant has not been well published. Following discussions between the HGNC and groups that have published on this variant, it was agreed to use H2BL# for the genes encoding this variant so that the root symbol H2B# is preserved. Therefore, the cattle gene is now named H2BL1 for “H2B.L histone” (Additional File 1), and the mouse ortholog has the equivalent symbol H2bl1. Human has a pseudogenized version of this gene, which is therefore named as H2BL1P, “H2B.L histone variant 1, pseudogene”.
H2B.K-encoding histone genes
The H2BK1 gene was first discovered via gene annotation [34] and was independently identified in a recent study on H2B variants [35]. There is no mouse ortholog of this gene, but there are one-to-one orthologs in many other mammals (including all curated VGNC species, see Additional File 1), birds and fish. In human there are transcripts overlapping H2BK1 and the upstream gene ABCF2, which, combined with the lack of mouse ortholog, had previously meant this histone gene was not annotated. According to Hidden Markov Model classification using the ‘Analyze sequence’ tool at the HistoneDB 2.0 database [36], the encoded protein does not match a characterized histone variant (67% identity with the most similar protein encoded by the other H2B genes). Therefore, at the initial time of naming, it was decided to name this gene as encoding a new histone variant. This gene was originally approved as H2BE1 for “H2B.E variant histone 1” but as H2B.E has been used in the literature several times for an isoform of mouse H2bc21, the nomenclature has been updated to avoid possible confusion. The variant identifier H2B.K was agreed with the authors of [35] ahead of their publication and the gene has been updated with the corresponding gene symbol H2BK1 and name “H2B.K variant histone 1”.
H2B.N-encoding histone genes
The H2BN1 gene encodes the most recently described H2B variant, H2B.N [35]. Like the H2BK1 gene, the human H2BN1 gene has an exon overlapping a separate gene, in this case the long non-coding RNA gene MYO1D-DT, and has no protein-coding ortholog in mouse. Additionally, as noted in [35], the H2BN1 and H2BK1 genes are both composed of two exons with the same part of the coding sequence encoding the histone fold domain split by the intron in both genes, but phylogenetic analysis in [35] does not support a common origin for the two variants. The H2BK1 gene is present in mammals, fish, birds and reptiles while H2BN1 is only found in mammals. It should be noted that neither the protein variant H2B.K nor H2B.N has been experimentally determined.
H2BC12L histone gene
There is a human-specific duplication of the H2BC12 gene from the chromosome 6 replication-dependent cluster gene on chromosome 21. CAGE tag data [37] supports expression of this gene and as there are no frameshifts or deletions within the open reading frame, it is annotated as coding. Although the gene appears to be expressed there is no direct evidence that a protein is produced. The encoded protein does not represent a new histone variant—it only has one nonsynonymous amino acid difference from the H2B protein encoded by the parent gene H2BC12, and is classified as a “canonical” histone when analyzing the sequence via the Histone DB2.0 database. Therefore, this gene has been named as H2BC12L for “H2B clustered histone 12 like”.
Histone H3 genes
Replication-dependent H3 genes
Nomenclature for histone H3 genes for human is shown in Table 7 and for mouse in Table 8. Histone H3 genes on major replication-dependent clusters are named with the root symbol ‘H3C#’ for ‘H3 clustered histone’ (H3c# in mouse). The Strasbourg nomenclature refers to histone H3.1 and H3.2 for histone proteins encoded on the larger replication-coupled clusters. However, as for H2A.1 and H2A.2 above, it has not been possible to reflect this in the gene nomenclature as it would not allow for consistent naming across orthologs. The H3.1 and H3.2 proteins are identical except that H3.1 has a cysteine at position 96 while H3.2 has a serine at this position. Again, there are examples where an ortholog in one species may encode an H3.1 protein and an H3.2 protein in another, e.g., human H3C2 encodes an H3.1 protein while mouse H3c2 encodes an H3.2 protein. Therefore, the H3.1 vs H3.2 distinction is not reflected in the gene nomenclature.
Table 7.
Revised gene nomenclature for human histone H3 genes
| Variant symbol | HGNC gene symbol | HGNC gene name | Previous symbol | HGNC ID | UniProt ID |
|---|---|---|---|---|---|
| H3.1 | H3C1 | H3 clustered histone 1 | HIST1H3A | HGNC:4766 | P68431 |
| H3.1 | H3C2 | H3 clustered histone 2 | HIST1H3B | HGNC:4776 | P68431 |
| H3.1 | H3C3 | H3 clustered histone 3 | HIST1H3C | HGNC:4768 | P68431 |
| H3.1 | H3C4 | H3 clustered histone 4 | HIST1H3D | HGNC:4767 | P68431 |
| NA | H3C5P | H3 clustered histone 5, pseudogene | NA | HGNC:54427 | NA |
| H3.1 | H3C6 | H3 clustered histone 6 | HIST1H3E | HGNC:4769 | P68431 |
| H3.1 | H3C7 | H3 clustered histone 7 | HIST1H3F | HGNC:4773 | P68431 |
| H3.1 | H3C8 | H3 clustered histone 8 | HIST1H3G | HGNC:4772 | P68431 |
| NA | H3C9P | H3 clustered histone 9, pseudogene | HIST1H3PS1 | HGNC:18982 | NA |
| H3.1 | H3C10 | H3 clustered histone 10 | HIST1H3H | HGNC:4775 | P68431 |
| H3.1 | H3C11 | H3 clustered histone 11 | HIST1H3I | HGNC:4771 | P68431 |
| H3.1 | H3C12 | H3 clustered histone 12 | HIST1H3J | HGNC:4774 | P68431 |
| H3.2 | H3C13 | H3 clustered histone 13 | HIST2H3D | HGNC:25311 | Q71DI3 |
| H3.2 | H3C14 | H3 clustered histone 14 | HIST2H3C | HGNC:20503 | Q71DI3 |
| H3.2 | H3C15 | H3 clustered histone 15 | HIST2H3A | HGNC:20505 | Q71DI3 |
| H3.3 | H3-3A | H3.3 histone A | H3F3, H3F3A | HGNC:4764 | P84243 |
| H3.3 | H3-3B | H3.3 histone B | H3F3B | HGNC:4765 | P84243 |
| H3.4 | H3-4 | H3.4 histone, cluster member | HIST3H3 | HGNC:4778 | Q16695 |
| H3.5 | H3-5 | H3.5 histone | H3F3C | HGNC:33164 | Q6NXT2 |
| NA (H3.6)* | H3P16 | H3 histone pseudogene 16 | H3F3AP6 | HGNC:42982 | NA |
| H3.7** | H3-7 | H3.7 histone (putative) | HIST2H3PS2 | HGNC:32060 | Q5TEC6 |
| NA (H3.8)*** | H3P44 | H3 histone pseudogene 44 | H3F3AP5 | HGNC:42981 | NA |
| H3.Y.1 | H3Y1 | H3.Y histone 1 | NA | HGNC:43735 | P0DPK2 |
| H3.Y.2 | H3Y2 | H3.Y histone 2 | NA | HGNC:43734 | P0DPK5 |
| cenH3 | CENPA | centromere protein A | NA | HGNC:1851 | P49450 |
*The variant symbol is shown as NA (H3.6) because the encoding gene is annotated as a pseudogene and, therefore, named within the H3 pseudogene series as H3P16, but the variant H3.6 has been reported in the literature
**Although this histone variant has been referred to as H3.7 in the literature, its existence is in doubt and it was not included in the Strasbourg variant nomenclature
***The variant symbol is shown as NA (H3.8) because, as for H3P16 above, the encoding gene is annotated as a pseudogene, named as H3P44, but the variant H3.8 has been reported in the literature
Table 8.
Revised gene nomenclature for mouse histone H3 genes
| Variant symbol | Mouse gene symbol | Mouse gene name | Previous symbol | MGI ID | UniProt ID |
|---|---|---|---|---|---|
| H3.1 | H3c1 | H3 clustered histone 1 | Hist1h3a | MGI:2668828 | P68433 |
| H3.2 | H3c2 | H3 clustered histone 2 | Hist1h3b | MGI:2448319 | P84228 |
| H3.2 | H3c3 | H3 clustered histone 3 | Hist1h3c | MGI:2448320 | P84228 |
| H3.2 | H3c4 | H3 clustered histone 4 | Hist1h3d | MGI:2448322 | P84228 |
| H3.2 | H3c6 | H3 clustered histone 6 | Hist1h3e | MGI:2448326 | P84228 |
| H3.2 | H3c7 | H3 clustered histone 7 | Hist1h3f | MGI:2448329 | P84228 |
| H3.1 | H3c8 | H3 clustered histone 8 | Hist1h3g | MGI:2145541 | P68433 |
| H3.1 | H3c10 | H3 clustered histone 10 | Hist1h3h | MGI:2448349 | P68433 |
| H3.1 | H3c11 | H3 clustered histone 11 | Hist1h3i | MGI:2448350 | P68433 |
| H3.2 | H3c13 | H3 clustered histone 13 | Hist2h3b | MGI:2448351 | P84228 |
| H3.2 | H3c14 | H3 clustered histone 14 | Hist2h3c1 | MGI:2448355 | P84228 |
| H3.2 | H3c15 | H3 clustered histone 15 | Hist2h3c2 | MGI:2448357 | P84228 |
| H3.3 | H3f3a | H3.3 histone A | H3f3a | MGI:1097686 | P84244 |
| H3.3 | H3f3b | H3.3 histone B | H3f3b | MGI:1101768 | P84244 |
| H3.4 | H3f4 | H3.4 histone, cluster member | Gm12260 | MGI:3651326 | NA |
| cenH3 | Cenpa | centromere protein A | NA | MGI:88375 | O35216 |
Replication-independent H3 genes
The H3.3 replication-independent variant is found across metazoa [38] while the H3.4 variant is found only in mammals [39]. The H3.3 variant is encoded by two mammalian genes which have been named as H3-3A and H3-3B for “H3.3 histone A” and “H3.3 histone B” (H3f3a and H3f3b in mouse). The H3.4 variant is encoded by a gene previously named HIST3H3 in human and the uninformative gene symbol Gm12260 in mouse. This variant is also commonly referred to as H3.1t [39] or H3t [40] because it was originally thought to be testis specific, but it has since been shown to be expressed at lower levels in other tissues [41]. The Strasbourg nomenclature recommendation was to refer to this as histone H3.4 which supports the symbol first published for this variant [42]. The H3.4 protein has a conserved valine residue at position 25 which has been reported to affect binding of the N-terminal tail by the Tudor domain of PHF1 and PHF19 [41, 43]. Due to the referral in the literature of this as an H3 variant, and the ‘H3.4’ recommendation by the Strasbourg nomenclature, we have named this gene H3-4 in human and other VGNC species (H3f4 in mouse). The gene encodes mRNA with a stem-loop structure and is adjacent to genes named with the H2AC# and H2BC# root symbols (H2AC25 and H2BC26). To reflect its position on a replication-dependent cluster, we have given this gene the full name “H3.4 histone, cluster member” and have added the gene symbol alias “H3C16” (see Fig. 1C).
Following discussions with the histone community, the symbol CENPA has been retained for the H3-like histone encoding gene that is found at the nucleosome core of centromeric chromatin [44], but the symbol alias “cenH3” has been included for this gene.
Primate-specific predicted H3 variants
This section describes symbols and names for a number of primate histone H3 genes. Note that it is not trivial to decide whether histone duplications limited to individual species, or even orders, are protein coding or should be represented as pseudogenes. Other mammals may also have additional predicted protein-coding histone genes that have not yet been named because these species are not currently supported by manual annotation projects.
H3.5-encoding histone gene
The H3.5 variant is a hominid-specific testis expressed gene that is likely a duplication of the H3-3B gene via retrotransposition [45]. While the H3-3A and H3-3B genes encode the same H3.3 protein, the protein predicted from the duplication is distinct. For this reason, common usage in the scientific literature and the variant identifier mentioned in the Strasbourg nomenclature have been followed and the gene named H3-5 for “H3.5 histone”.
H3.7-encoding histone gene
The H3.7 variant identified in [46] is encoded by a duplication of the H3C13 gene, located roughly 6 MB upstream of the “cluster 2” replication-dependent histone genes. The predicted protein is most like an H3.2 variant, but H3.2 variants are characterized by a serine at residue 96 while the H3.7 variant has an arginine at residue 96, which is not seen in any other H3 histone proteins. The nomenclature published in [46] has been followed and the gene assigned as H3-7 with the gene name “H3.7 histone (putative)”. The term “putative” will be removed if there is future experimental evidence that this variant exists. Taguchi et al. [46] also identified two further putative H3 variants which they called H3.6 and H3.8. However, there are insufficient expression data to support annotation of these genes as protein coding, so in the absence of further data, the encoding genes are annotated and named as H3 pseudogenes: H3P16 (H3 histone pseudogene 16) and H3P44 (H3 histone pseudogene 44). These pseudogenes have been given the aliases H3.6 and H3.8.
H3.Y-encoding histone genes
The H3.Y variant is encoded by two genes in human, which were initially referred to as H3.X and H3.Y [47]. As H3.Y forms a clear primate-specific clade incorporating both genes, and the protein referred to as ‘H3.X’ is only predicted from mRNA sequence, the recommendations of the Strasbourg nomenclature have been followed and the two human genes assigned as H3Y1 and H3Y2 for “H3.Y histone 1” and “H3.Y histone 2”. While human and chimpanzee have two paralogs, rhesus macaque appears to only have H3Y1 (Additional File 1). However, in chimp a symbol has only been approved for the H3Y2 ortholog (Additional File 1) because “H3Y1” is currently on an unplaced scaffold; updates to the chimpanzee genome may result in the putative chimp “H3Y1” being assigned.
Histone H4 genes
The genes encoding histone H4 proteins are mostly found within replication-dependent clusters and are, thus, named with the root symbol H4C# for “H4 clustered histone” for human (Table 9) and H4c# for mouse (Table 10). All human H4 genes encode the same protein except for H4C7, which encodes an H4 protein with a truncated C terminus [9], sometimes referred to as H4.7 or H4.G [9]. Interestingly, there is no mouse H4C7 ortholog, although an ortholog is present in chimp, dog, pig, horse and cattle, only the chimp ortholog appears to encode the same H4.7/H4.G variant as human, while the H4C7 gene in dog, pig, horse and cattle encodes the same H4 protein as other H4 genes. A study [48] of the putative protein encoded by human H4C7 found that it is expressed at low levels and in vitro forms unstable nucleosomes. The H4C7 gene is annotated as protein coding because several unique peptides from proteomic projects map to the H4C7 locus on the human genome reference GRCh38; however, as this gene has only been reported to be expressed in tumor cells, its existence as a true histone variant in normal cells is in doubt.
Table 9.
Revised gene nomenclature for human histone H4 genes
| Variant symbol | HGNC gene symbol | HGNC gene name | Previous symbol | HGNC ID | UniProt ID |
|---|---|---|---|---|---|
| H4 | H4C1 | H4 clustered histone 1 | HIST1H4A | HGNC:4781 | P62805 |
| H4 | H4C2 | H4 clustered histone 2 | HIST1H4B | HGNC:4789 | P62805 |
| H4 | H4C3 | H4 clustered histone 3 | HIST1H4C | HGNC:4787 | P62805 |
| H4 | H4C4 | H4 clustered histone 4 | HIST1H4D | HGNC:4782 | P62805 |
| H4 | H4C5 | H4 clustered histone 5 | HIST1H4E | HGNC:4790 | P62805 |
| H4 | H4C6 | H4 clustered histone 6 | HIST1H4F | HGNC:4783 | P62805 |
| H4* | H4C7 | H4 clustered histone 7 | HIST1H4G | HGNC:4792 | Q99525 |
| H4 | H4C8 | H4 clustered histone 8 | HIST1H4H | HGNC:4788 | P62805 |
| H4 | H4C9 | H4 clustered histone 9 | HIST1H4I | HGNC:4793 | P62805 |
| NA | H4C10P | H4 clustered histone 10, pseudogene | HIST1H4PS1 | HGNC:4786 | NA |
| H4 | H4C11 | H4 clustered histone 11 | HIST1H4J | HGNC:4785 | P62805 |
| H4 | H4C12 | H4 clustered histone 12 | HIST1H4K | HGNC:4784 | P62805 |
| H4 | H4C13 | H4 clustered histone 13 | HIST1H4L | HGNC:4791 | P62805 |
| H4 | H4C14 | H4 clustered histone 14 | HIST2H4A | HGNC:4794 | P62805 |
| H4 | H4C15 | H4 clustered histone 15 | HIST2H4B | HGNC:29,607 | P62805 |
| H4 | H4C16 | H4 histone 16 | HIST4H4 | HGNC:20,510 | P62805 |
* This protein predicted to be encoded by the human H4C7 gene has been referred to in the literature as H4.7 or H4.G but its existence is in doubt and there is no separate symbol in the Strasbourg unified nomenclature
Table 10.
Revised gene nomenclature for mouse histone H4 genes
| Variant symbol | Mouse gene symbol | Mouse gene name | Previous symbol | MGI ID | UniProt ID |
|---|---|---|---|---|---|
| H4 | H4c1 | H4 clustered histone 1 | Hist1h4a | MGI:2448419 | P62806 |
| H4 | H4c2 | H4 clustered histone 2 | Hist1h4b | MGI:2448420 | P62806 |
| H4 | H4c3 | H4 clustered histone 3 | Hist1h4c | MGI:2448421 | P62806 |
| H4 | H4c4 | H4 clustered histone 4 | Hist1h4d | MGI:2448423 | P62806 |
| H4 | H4c6 | H4 clustered histone 6 | Hist1h4f | MGI:2448425 | P62806 |
| H4 | H4c8 | H4 clustered histone 8 | Hist1h4h | MGI:2448427 | P62806 |
| H4 | H4c9 | H4 clustered histone 9 | Hist1h4i | MGI:2448432 | P62806 |
| H4 | H4c11 | H4 clustered histone 11 | Hist1h4j | MGI:2448436 | P62806 |
| H4 | H4c12 | H4 clustered histone 12 | Hist1h4k | MGI:2448439 | P62806 |
| H4 | H4c14 | H4 clustered histone 14 | Hist2h4 | MGI:2140113 | P62806 |
| H4 | H4c16 | H4 histone 16 | Hist4h4 | MGI:2448443 | P62806 |
| H4 | H4c17 | H4 clustered histone 17 | Hist1h4m | MGI:2448441 | P62806 |
| H4 | H4c18 | H4 clustered histone 18 | Hist1h4n | MGI:4843992 | P62806 |
H4C16 is the only gene outside of the large mammalian replication-dependent clusters and encodes the same protein as the other H4C genes. In human and other mammals, the H4C16 and H2AJ genes are neighboring and are flanked by the genes WBP11, SMCO3 and ART4 while intriguingly in chicken ART4, SMCO3 and WBP11 are at the 5' end of the single large chicken histone gene cluster. The H4C16 gene is single exon, encodes an mRNA with a stem loop and encodes a protein identical to other H4C genes. It is expressed at high levels in all cells examined, is cell-cycle regulated like the replication-dependent genes in larger clusters, and is bound by the factor NPAT which is only bound to the promoters of replication-dependent histone genes ([7]; [4]). Therefore, this gene has been assigned as H4C16 with the gene name “H4 histone 16”. This is in contrast to the neighboring H2AJ gene, which has been published as a separate variant to other H2A genes, encodes an mRNA with a polyadenylated tail, and has been reported to be expressed in senescent rather than replicating cells [23].
Adoption of the new nomenclature by the Histone Sequence Database
Wherever possible, the HGNC encourages specialist resources to display approved gene symbols to help disseminate nomenclature to the research community. The Histone Sequence Database was first introduced in the mid-1990s with the aim of maintaining a comprehensive collection of all known histone protein sequences in different species [20]. The current version of the database has extended this effort by attributing every histone sequence to a specific histone type (H3, H4, H2A, H2B, H1), histone variant (e.g., H2A.Z, H3.Y, subH2B, etc.), or canonical histone groups including variant-specific annotations [36]. The current version of the database holds 79948 histone sequences with a taxonomic span of 3160 species. The histone database can be used to explore the diversity of histone proteins and their sequence variants in many organisms to better understand how sequence variation may affect functional and structural features of nucleosomes, to browse the histone phylogenetic trees and examine variant-specific features.
Based on the updated list of human histone genes described above a comprehensive list of human histone proteins was compiled, grouped into specific histone types and histone variants. For a few histone variant genes, the attribution to a certain histone variant class has not yet been clearly established in the literature; these include H3P16, H3-7, H3P44 and H4C7. The resulting table consists of 130 entries and is provided as an Additional File (Additional File 2). The list of histone proteins has been integrated into the current version of the Histone Sequence Database (“HistoneDB 2.0: a histone database with variants”) [49] and is available at https://histdb.intbio.org/human/. The interactive table provides links to the pages of HistoneDB 2.0, the HGNC website, the Ensembl database [50], the NCBI RefSeq database [51] and PubMed.
Conclusions
We have approved a standardized nomenclature for the complex multigene histone family that can be applied across vertebrate species. The nomenclature follows agreed upon conventions for histone protein nomenclature as closely as possible. The revised gene symbols are shorter and group histone genes together based on the type of encoded histone protein. The full human and mouse histone gene nomenclature is presented in this paper and data for chimpanzee, rhesus macaque, dog, cat, pig, horse and cattle are shown in the additional files. We encourage the histone community to reference these gene symbols in all future publications on mammalian histone genes.
Supplementary Information
Additional file 1. Gene nomenclature for chimpanzee, rhesus macaque, dog, cat, pig, horse and cattle histone genes. For each gene, the VGNC ID, gene symbol and gene name are provided. Where applicable, the HGNC ID for each orthologous human gene is listed. Full Symbol Reports for each gene can be accessed using these IDs at https://vertebrate.genenames.org/.
Additional file 2. List of human histone proteins from the Histone Sequence Database for every histone gene, the available set of transcript and coding sequence GENCODE annotations were obtained from the Ensembl 105 database. Next, only protein-coding transcripts identical between Ensembl automated annotation and HAVANA manual curation were retained. In those cases where several transcripts of one gene correspond to the same amino acid protein sequence, only one record was retained with preference given to those that match NCBI’s RefSeq annotation. For every histone gene the list includes the HGNC symbol, information about corresponding protein sequences, their length, accession numbers within NCBI and Ensembl resources, as well as a list of relevant literature references in the form of PubMed identifiers. For a few histone variant genes, the attribution to a certain histone variant class has not yet been clearly established in the literature; these are marked in the list by a question mark.
Acknowledgements
We would like to acknowledge all HGNC curators, past and present, for their helpful comments on histone nomenclature during our curator meetings. We would also like to acknowledge the manual gene annotators at the RefSeq and Havana-Ensembl projects for checking and revising histone gene annotation when requested.
Author contributions
WFM, AKS, PBT, PD and RLS wrote sections of the original draft. All other authors provided input during early discussions and drafts, and all authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. The HGNC (RLS, PD and EAB) is currently funded by Wellcome Trust grant 208349/Z/17/Z and the National Human Genome Research Institute (NHGRI) grant U24HG003345. DL was supported by the Intramural Research Program of the National Library of Medicine, National Institutes of Health. WM was supported by NIH grant GM-29832-45. MGNC (MM) is funded by program project grant HG000330 from the National Human Genome Research Institute (NHGRI) of the National Institutes of Health (NIH) as part of the MGD project. ARP was supported by the Department of Pathology and Molecular Medicine, Queen’s University, Canada. ARP is the recipient of a Senior Canada Research Chair in Computational Biology and Biophysics and a Senior Investigator Award from the Ontario Institute of Cancer Research, Canada. HistoneDB 2.0 maintenance (AS and AKG) is supported by Russian Science Foundation grant #18–74-10006. PBT was supported by Howard Hughes Medical Institute. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials
All human gene nomenclature can be fully accessed and downloaded from https://www.genenames.org/. All mouse gene nomenclature can be fully accessed and downloaded from http://www.informatics.jax.org/. All chimpanzee, rhesus macaque, dog, cat, cattle, horse and pig gene nomenclature can be fully accessed and downloaded from https://vertebrate.genenames.org/. HistoneDB 2.0 data can be fully accessed and downloaded from https://histdb.intbio.org.
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marzluff WF, Wagner EJ, Duronio RJ. Metabolism and regulation of canonical histone mRNAs: life without a poly(A) tail. Nat Rev Genet. 2008;9:843–854. doi: 10.1038/nrg2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyons SM, Cunningham CH, Welch JD, Groh B, Guo AY, Wei B, et al. A subset of replication-dependent histone mRNAs are expressed as polyadenylated RNAs in terminally differentiated tissues. Nucleic Acids Res. 2016;44:9190–9205. doi: 10.1093/nar/gkw620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kemp JP, Jr, Yang X-C, Dominski Z, Marzluff WF, Duronio RJ. Superresolution light microscopy of the Drosophila histone locus body reveals a core-shell organization associated with expression of replication-dependent histone genes. Mol Biol Cell. 2021;32:942–955. doi: 10.1091/mbc.E20-10-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaya-Okur HS, Wu SJ, Codomo CA, Pledger ES, Bryson TD, Henikoff JG, et al. CUT&Tag for efficient epigenomic profiling of small samples and single cells. Nat Commun. 2019;10:1930. doi: 10.1038/s41467-019-09982-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye X, Wei Y, Nalepa G, Harper JW. The cyclin E/Cdk2 substrate p220(NPAT) is required for S-phase entry, histone gene expression, and cajal body maintenance in human somatic cells. Mol Cell Biol. 2003;23:8586–8600. doi: 10.1128/MCB.23.23.8586-8600.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzluff WF, Koreski KP. Birth and death of histone mRNAs. Trends Genet. 2017;33:745–759. doi: 10.1016/j.tig.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griesbach E, Schlackow M, Marzluff WF, Proudfoot NJ. Dual RNA 3’-end processing of H2A.X messenger RNA maintains DNA damage repair throughout the cell cycle. Nat Commun. 2021;12:359. doi: 10.1038/s41467-020-20520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mannironi C, Bonner WM, Hatch CL. H2A.X. a histone isoprotein with a conserved C-terminal sequence, is encoded by a novel mRNA with both DNA replication type and polyA 3’ processing signals. Nucleic Acids Res. 1989;17:9113–26. doi: 10.1093/nar/17.22.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Talbert PB, Henikoff S. Histone variants at a glance. J Cell Sci. 2021 doi: 10.1242/jcs.244749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradbury EM. Histone nomenclature. Methods Cell Biol. 1977;16:179–181. doi: 10.1016/S0091-679X(08)60099-0. [DOI] [PubMed] [Google Scholar]
- 11.Turner BM. Reading signals on the nucleosome with a new nomenclature for modified histones. Nat Struct Mol Biol. 2005;12:110–112. doi: 10.1038/nsmb0205-110. [DOI] [PubMed] [Google Scholar]
- 12.Talbert PB, Ahmad K, Almouzni G, Ausió J, Berger F, Bhalla PL, et al. A unified phylogeny-based nomenclature for histone variants. Epigenetics Chromatin. 2012;5:7. doi: 10.1186/1756-8935-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tweedie S, Braschi B, Gray K, Jones TEM, Seal RL, Yates B, et al. Genenames. org: the HGNC and VGNC resources in 2021. Nucleic Acids Res. 2021;49(D1):D939–D946. doi: 10.1093/nar/gkaa980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bult CJ, Blake JA, Smith CL, Kadin JA, Richardson JE, Mouse Genome Database Group Mouse Genome Database (MGD) 2019. Nucleic Acids Res. 2019;47:D801–6. doi: 10.1093/nar/gky1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marzluff WF, Gongidi P, Woods KR, Jin J, Maltais LJ. The human and mouse replication-dependent histone genes. Genomics. 2002;80:487–498. doi: 10.1006/geno.2002.6850. [DOI] [PubMed] [Google Scholar]
- 16.Takami Y, Nishi R, Nakayama T. Histone H1 variants play individual roles in transcription regulation in the DT40 chicken B cell line. Biochem Biophys Res Commun. 2000;268:501–508. doi: 10.1006/bbrc.2000.2172. [DOI] [PubMed] [Google Scholar]
- 17.Hatch CL, Bonner WM. The human histone H2A.Z gene. Sequence and regulation. J Biol Chem. 1990;265:15211–8. doi: 10.1016/S0021-9258(18)77243-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Hong M, Kim JW, Hong YM, Choe YK, Chang SY, et al. Isolation of cDNA clones encoding human histone macroH2A1 subtypes. Biochim Biophys Acta. 1998;1399:73–77. doi: 10.1016/S0167-4781(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 19.Franklin SG, Zweidler A. Non-allelic variants of histones 2a, 2b and 3 in mammals. Nature. 1977;266:273–275. doi: 10.1038/266273a0. [DOI] [PubMed] [Google Scholar]
- 20.Baxevanis AD, Landsman D. Histone sequence database: a compilation of highly-conserved nucleoprotein sequences. Nucleic Acids Res. 1996;24:245–247. doi: 10.1093/nar/24.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson JD, Gorovsky MA. Histone H2A.Z has a conserved function that is distinct from that of the major H2A sequence variants. Nucleic Acids Res. 2000;28:3811–6. doi: 10.1093/nar/28.19.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pehrson JR, Fried VA. MacroH2A, a core histone containing a large nonhistone region. Science. 1992;257:1398–1400. doi: 10.1126/science.1529340. [DOI] [PubMed] [Google Scholar]
- 23.Contrepois K, Coudereau C, Benayoun BA, Schuler N, Roux P-F, Bischof O, et al. Histone variant H2A.J accumulates in senescent cells and promotes inflammatory gene expression. Nat Commun. 2017;8:14995. doi: 10.1038/ncomms14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang X, Soboleva TA, Tremethick DJ. Short histone H2A variants: small in stature but not in function. Cells. 2020 doi: 10.3390/cells9040867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molaro A, Young JM, Malik HS. Evolutionary origins and diversification of testis-specific short histone H2A variants in mammals. Genome Res. 2018;28:460–473. doi: 10.1101/gr.229799.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang X, Wen J, Paver E, Wu Y-H, Sun G, Bullman A, et al. H2A.B is a cancer/testis factor involved in the activation of ribosome biogenesis in Hodgkin lymphoma. EMBO Rep. 2021;22:e52462. doi: 10.15252/embr.202152462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano R, Arimura Y, Kujirai T, Shibata M, Okuda A, Morishima K, et al. Histone variant H2A.B-H2B dimers are spontaneously exchanged with canonical H2A–H2B in the nucleosome. Commun Biol. 2021;4:191. doi: 10.1038/s42003-021-01707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou M, Dai L, Li C, Shi L, Huang Y, Guo Z, et al. Structural basis of nucleosome dynamics modulation by histone variants H2A.B and H2A.Z.2.2. EMBO J. 2021;40:e105907. doi: 10.15252/embj.2020105907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anuar ND, Kurscheid S, Field M, Zhang L, Rebar E, Gregory P, et al. Gene editing of the multi-copy H2A.B gene and its importance for fertility. Genome Biol. 2019;20:23. doi: 10.1186/s13059-019-1633-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boulard M, Gautier T, Mbele GO, Gerson V, Hamiche A, Angelov D, et al. The NH2 tail of the novel histone variant H2BFWT exhibits properties distinct from conventional H2B with respect to the assembly of mitotic chromosomes. Mol Cell Biol. 2006;26:1518–1526. doi: 10.1128/MCB.26.4.1518-1526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J, Park HS, Kim HH, Yun Y-J, Lee DR, Lee S. Functional polymorphism in H2BFWT-5’UTR is associated with susceptibility to male infertility. J Cell Mol Med. 2009;13:1942–1951. doi: 10.1111/j.1582-4934.2009.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aul RB, Oko RJ. The major subacrosomal occupant of bull spermatozoa is a novel histone H2B variant associated with the forming acrosome during spermiogenesis. Dev Biol. 2001;239:376–387. doi: 10.1006/dbio.2001.0427. [DOI] [PubMed] [Google Scholar]
- 33.Govin J, Escoffier E, Rousseaux S, Kuhn L, Ferro M, Thévenon J, et al. Pericentric heterochromatin reprogramming by new histone variants during mouse spermiogenesis. J Cell Biol. 2007;176:283–294. doi: 10.1083/jcb.200604141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mudge JM, Jungreis I, Hunt T, Gonzalez JM, Wright JC, Kay M, et al. Discovery of high-confidence human protein-coding genes and exons by whole-genome PhyloCSF helps elucidate 118 GWAS loci. Genome Res. 2019;29:2073–2087. doi: 10.1101/gr.246462.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raman P, Rominger MC, Young JM, Molaro A, Tsukiyama T, Malik HS. Novel classes and evolutionary turnover of histone H2B variants in the mammalian germline. Mol Biol Evol. 2022 doi: 10.1093/molbev/msac019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Draizen EJ, Shaytan AK, Mariño-Ramírez L, Talbert PB, Landsman D, Panchenko AR. HistoneDB 2.0: a histone database with variants–an integrated resource to explore histones and their variants. Database. 2016 doi: 10.1093/database/baw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Hoon M, Hayashizaki Y. Deep cap analysis gene expression (CAGE): genome-wide identification of promoters, quantification of their expression, and network inference. Biotechniques. 2008;44:627–8. doi: 10.2144/000112802. [DOI] [PubMed] [Google Scholar]
- 38.Szenker E, Ray-Gallet D, Almouzni G. The double face of the histone variant H3.3. Cell Res. 2011;21:421–34. doi: 10.1038/cr.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hake SB, Allis CD. Histone H3 variants and their potential role in indexing mammalian genomes: the “H3 barcode hypothesis”. Proc Natl Acad Sci U S A. 2006;103:6428–6435. doi: 10.1073/pnas.0600803103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witt O, Albig W, Doenecke D. Testis-specific expression of a novel human H3 histone gene. Exp Cell Res. 1996;229:301–306. doi: 10.1006/excr.1996.0375. [DOI] [PubMed] [Google Scholar]
- 41.Kycia I, Kudithipudi S, Tamas R, Kungulovski G, Dhayalan A, Jeltsch A. The Tudor domain of the PHD finger protein 1 is a dual reader of lysine trimethylation at lysine 36 of histone H3 and lysine 27 of histone variant H3t. J Mol Biol. 2014;426:1651–1660. doi: 10.1016/j.jmb.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Albig W, Ebentheuer J, Klobeck G, Kunz J, Doenecke D. A solitary human H3 histone gene on chromosome 1. Hum Genet. 1996;97:486–491. doi: 10.1007/BF02267072. [DOI] [PubMed] [Google Scholar]
- 43.Dong C, Nakagawa R, Oyama K, Yamamoto Y, Zhang W, Dong A, et al. Structural basis for histone variant H3tK27me3 recognition by PHF1 and PHF19. Elife. 2020 doi: 10.7554/eLife.58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Orthaus S, Biskup C, Hoffmann B, Hoischen C, Ohndorf S, Benndorf K, et al. Assembly of the inner kinetochore proteins CENP-A and CENP-B in living human cells. ChemBioChem. 2008;9:77–92. doi: 10.1002/cbic.200700358. [DOI] [PubMed] [Google Scholar]
- 45.Schenk R, Jenke A, Zilbauer M, Wirth S, Postberg J. H3.5 is a novel hominid-specific histone H3 variant that is specifically expressed in the seminiferous tubules of human testes. Chromosoma. 2011;120:275–85. doi: 10.1007/s00412-011-0310-4. [DOI] [PubMed] [Google Scholar]
- 46.Taguchi H, Xie Y, Horikoshi N, Maehara K, Harada A, Nogami J, et al. Crystal structure and characterization of novel human histone H3 variants, H3.6, H3.7, and H3.8. Biochemistry. 2017;56:2184–96. doi: 10.1021/acs.biochem.6b01098. [DOI] [PubMed] [Google Scholar]
- 47.Wiedemann SM, Mildner SN, Bönisch C, Israel L, Maiser A, Matheisl S, et al. Identification and characterization of two novel primate-specific histone H3 variants, H3.X and H3.Y. J Cell Biol. 2010;190:777–91. doi: 10.1083/jcb.201002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang MYH, Sun X, Ausió J, Ishibashi T. Histone H4 variant, H4G, drives ribosomal RNA transcription and breast cancer cell proliferation by loosening nucleolar chromatin structure. J Cell Physiol. 2020;235:9601–9608. doi: 10.1002/jcp.29770. [DOI] [PubMed] [Google Scholar]
- 49.El Kennani S, Adrait A, Shaytan AK, Khochbin S, Bruley C, Panchenko AR, et al. MS_HistoneDB, a manually curated resource for proteomic analysis of human and mouse histones. Epigenetics Chromatin. 2017;10:2. doi: 10.1186/s13072-016-0109-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cunningham F, Allen JE, Allen J, Alvarez-Jarreta J, Amode MR, Armean IM, et al. Ensembl 2022. Nucleic Acids Res. 2022;50:D988–D995. doi: 10.1093/nar/gkab1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Leary NA, Wright MW, Brister JR, Ciufo S, Haddad D, McVeigh R, et al. Reference sequence (RefSeq) database at NCBI: current status, taxonomic expansion, and functional annotation. Nucleic Acids Res. 2016;44:D733–D745. doi: 10.1093/nar/gkv1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Gene nomenclature for chimpanzee, rhesus macaque, dog, cat, pig, horse and cattle histone genes. For each gene, the VGNC ID, gene symbol and gene name are provided. Where applicable, the HGNC ID for each orthologous human gene is listed. Full Symbol Reports for each gene can be accessed using these IDs at https://vertebrate.genenames.org/.
Additional file 2. List of human histone proteins from the Histone Sequence Database for every histone gene, the available set of transcript and coding sequence GENCODE annotations were obtained from the Ensembl 105 database. Next, only protein-coding transcripts identical between Ensembl automated annotation and HAVANA manual curation were retained. In those cases where several transcripts of one gene correspond to the same amino acid protein sequence, only one record was retained with preference given to those that match NCBI’s RefSeq annotation. For every histone gene the list includes the HGNC symbol, information about corresponding protein sequences, their length, accession numbers within NCBI and Ensembl resources, as well as a list of relevant literature references in the form of PubMed identifiers. For a few histone variant genes, the attribution to a certain histone variant class has not yet been clearly established in the literature; these are marked in the list by a question mark.
Data Availability Statement
All human gene nomenclature can be fully accessed and downloaded from https://www.genenames.org/. All mouse gene nomenclature can be fully accessed and downloaded from http://www.informatics.jax.org/. All chimpanzee, rhesus macaque, dog, cat, cattle, horse and pig gene nomenclature can be fully accessed and downloaded from https://vertebrate.genenames.org/. HistoneDB 2.0 data can be fully accessed and downloaded from https://histdb.intbio.org.