Abstract
Purpose
Our previous investigations revealed a significant role of methyltransferase-like 3 (METTL3)-mediated N6-methyladenosine (m6A) modification in the development of corneal inflammation in Fusarium infection, but the exact mechanism is unknown. Therefore, this research aimed to explore how METTL3 affects the inflammatory process of fungal keratitis (FK) in mice.
Methods
We established in vitro and in vivo models by inoculating mice and primary corneal stromal cells with F. solani. METTL3 expression was confirmed by real-time quantitative polymerase chain reaction, immunofluorescence, and western blotting. After that, siRNAMETTL3 and AAV-sh-METTL3 were transfected into cells and mice to explore the role of METTL3 in the PI3K/AKT signaling pathway and inflammation. PI3K, p-PI3K, AKT, and p-AKT expression was analyzed by western blotting. Viability of corneal stromal cells was measured using a Cell Counting Kit-8 (CCK-8). Additionally, we detected interleukin (IL)-6, IL-1β, and tumor necrosis factor alpha (TNF-α) levels in corneal tissues and analyzed the role of METTL3 in inflammation in FK using slit-lamp biomicroscopy and hematoxylin and eosin staining.
Results
Here, our results show that METTL3 increased in mouse FK, and the expression of p-PI3K and p-AKT decreased when METTL3 was downregulated. We also found that knockdown of METTL3 expression attenuated the inflammatory response and decreased TNF-α, IL-1β, and IL-6 expression in corneal-infected mice. Furthermore, inhibition of the PI3K/AKT pathway attenuated the inflammatory response of FK, decreased the expression of the above inflammatory factors, and enhanced the viability of corneal stromal cells.
Conclusions
Based on the study results, METTL3 downregulation attenuates Fusarium-induced corneal inflammation via the PI3K/AKT signaling pathway.
Keywords: fungal keratitis, N6-methyladenosine methylation, METTL3, PI3K/AKT signaling pathway
Fungal keratitis (FK) is an aggressive infectious corneal disease caused by pathogenic fungi that can often result in severe visual impairment or even harm the entire eyeball.1,2 Previous studies have shown that the pathogenic bacteria of FK are mainly Fusarium, Aspergillus, and Candida, among which Fusarium solani (F. solani) is the most reported species in many countries, accounting for 25% to 73.3%.3 Studies have shown that the outcome of FK mainly depends on the ability of the body to eliminate pathogenic fungi and the virulence of pathogenic bacterial species.4 Due to the toxicity, side effects, and development of drug resistance, antifungal therapies using currently available drugs are limited.5 Therefore, in-depth exploration of the pathogenesis of FK can provide a theoretical basis for the development of new therapeutic targets.
N6-methyladenosine (m6A) is the most widely existing posttranscriptional modification of eukaryotic mRNA, which is closely related to a variety of physiological or pathological processes of the body.6,7 As a dynamic reversible process, m6A modification is finely regulated by methyltransferase-like (METTL)3, METTL14, WT1-associated protein, the demethylating enzyme fat mass and obesity-associated protein, and AlkB homolog 5.8–10 More recently, m6A modifications have been identified as key regulators of immune cell function against bacteria and viruses.11 Additionally, METTL3, the most important component of the “writer” complex, plays important roles in the regulation of the immunoinflammatory response. In a recent study, silencing of the METTL3 gene significantly downregulated the m6A modification levels of cluster of differentiation (CD)80, CD40, and TIR domain-containing adaptor protein transcripts in dendritic cells, resulting in reduced protein expression and decreased ability of dendritic cells to secrete the cytokine IL-12 and activate T cells.12 Recent studies have shown elevated METTL3 expression in different acute kidney injury models, as well as in human biopsies and cultured tubular epithelial cells. Moreover, knockdown of METTL3 attenuated renal inflammation and programmed cell death in tubular epithelial cells.13 Notably, our previous research revealed that significantly increased levels of METTL3 in the corneal tissue of F. solani-infected mice.14 However, the molecular pathway and mechanism of the function of METTL3 in FK has not been well elucidated.
The phosphatidylinositol 3-kinase/serine–threonine protein kinase (PI3K/AKT) pathway is the main signaling pathway enriched for differentially m6A-modified genes and is widely involved in performing multiple biological functions.15 The study of this pathway in recent years has shown that it also aids the infection and pathogenic processes of different microbes and plays a role in several corneal diseases.16,17 Bacteria activate the PI3K/AKT signaling pathway via bacterial lipopolysaccharide (LPS) in vitro to induce inflammation in corneal epithelial cells.18 In fungal FK, the extracellular polysaccharide EPS-II partially inhibited the adhesion of Candida albicans to corneal epithelial cells, partially inhibited PI3K/AKT signaling, and reduced the levels of associated inflammatory factors.19 The above findings suggest that the PI3K/AKT signaling pathway plays a crucial role in FK immunoregulation, and further studies on the regulation of the PI3K/AKT pathway are of great significance in understanding the immunoregulation of fungal infection and the mechanism of corneal immunity against fungal infection.
The role of METTL3 in FK was explored by regulating METTL3 expression and observing changes in the PI3K/AKT pathway and inflammatory response. Our study aimed to elucidate the mechanism of the role of METTL3 in the process of corneal fungal infection and to develop new therapeutic strategies by establishing a theoretical foundation.
Materials and Methods
Animals and Corneal Infections
The experiments were conducted on 200 wild-type BALB/C mice, 6 to 8 weeks old, purchased from Jinan Pengyue Experimental Animal Center in Jinan, China, and mice without any corneal lesions were screened by slit-lamp microscopy before the experiments. Mice were housed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Mice were anesthetized with 0.25 to 0.3 mL of 0.6% sodium pentobarbital anesthetic solution, and their whiskers were trimmed after successful anesthesia. Superficial anesthesia was performed using oxybuprocaine hydrochloride eye drops. Under an operating microscope, the eyes were fixed with forceps, and their corneal epithelium was scraped with an electric epithelial scraper so that the area of the epithelial defects was approximately the same. A 5-µL F. solani suspension was topically added to the eye surface. Next, the corneal surface of the anesthetized mice was placed with rat corneal buckles. A single stitch was then placed in the center of the eyelids with 7-0 sutures. The rats were labeled and the eyelids were unrolled after 24 hours.20 The severity of the condition was assessed by removing the filter paper pieces and photographing them for observation on days 1, 3, 5, and 7. A scale from 0 to 4 was used to score cloudiness density, cloudy area, and surface regularity. There were three levels of severity: mild (5 points), moderate (5–9 points), and severe (9–12 points).21 The material was also used for western blotting, polymerase chain reaction (PCR), immunofluorescence, and other experiments when appropriate.
Primary Cell Culture
The corneas of mice were immersed for 12 hours at 4°C in Dispase II (15 mg/mL; Sigma-Aldrich, St. Louis, MO, USA). The next day, the peeled corneal rim epithelium was carefully removed. The posterior elastic and endothelial layers were removed from the cornea, and a portion of the corneal stroma was cut. After cleaning the tissues with sterile phosphate-buffered saline, the tissue was digested in type I collagenase and shaken for 2 hours. Cultured corneal stromal cells were cultivated at 37°C and 5% CO2 in Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12 (DMEM/F-12; Sigma-Aldrich) containing 10% fetal bovine serum. A 48-hour medium change was carried out for the cell culture.
RNA Extraction and Real-Time Quantitative PCR
We cut the corneas of mice into pieces, extracted the total RNA using TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA), and finally obtained the RNA (n = 6 per group).22 A Thermo Scientific RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) was used to synthesize the cDNA. SYBR-Green Master Mix (Highland Biologicals, Takara, Japan) was used for the real-time quantitative PCR, and the internal reaction control was carried out using the β-actin gene.23 The primers are listed in the Table. Circulation thresholds were obtained for each target and endoreactive gene. We calculated and analyzed the relative expression levels of the target genes using the 2−∆∆Ct method.
Table.
Primers Used in This Study
| Gene | Forward | tReverse |
|---|---|---|
| METTL3 | CGCTGCCTCCGATGTTGATCTG | CTGACTGACCTTCTTGCTCTGCTG |
| IL-1β | CTTTCCCGTGGACCTTCCA | CTCGGAGCCTGTAGTGCAGTT |
| IL-6 | ACCACTCCCAACAGACCTGTCT | CAGATTGTTTTCTGCAAGTGCAT |
| TNF-α | ACAAGGCTGCCCCGACTAC | TGGGCTCATACCAGGGTTTG |
| β-actin | GATTACTGCTCTGGCTCCTAGC | GACTCATCGTACTCCTGCTTGC |
Hematoxylin and Eosin Staining
We fixed the eyes of different groups of mice with 4% formaldehyde. A graded ethanol series was used to dehydrate the corneal tissues, which were embedded in paraffin and then cleaned and cut into 5-µm-thick sections. Finally, under the microscope, the pathological changes were observed after staining the sections with hematoxylin and eosin (H&E).
Immunofluorescence
Then, 4% paraformaldehyde was used to fix the corneal stromal cells, and the mouse insula was removed. We then treated the samples with 0.1% Triton X-100 for 5 minutes, followed by closure with 5% bovine serum albumin for 2 hours. The samples were incubated with primary antibody (ab195352; Abcam, Cambridge, UK) at 4°C overnight, and the samples were incubated with Alexa Fluor fluorescently labeled secondary antibodies (Alexa Fluor 488 or Alexa Fluor 594; Proteintech, Wuhan, China) for 2 hours in the dark. Finally, images were taken using a fluorescence microscope.
Western Blotting
Mouse corneal tissues or stromal cells were lysed using radioimmunoprecipitation lysis buffer to extract the proteins, and the total proteins were separated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Following electrophoresis, proteins were transferred to polyvinylidene fluoride membranes through Tris-glycine-SDS buffer and blocked. The main antibodies included Recombinant Anti-METTL3 (ab195352; Abcam), Anti-beta Actin (ab8227; Abcam), Akt Antibody (9272; Cell Signaling Technology, Danvers, MA, USA), Phospho-Akt (Ser473) (D9E) XP Rabbit mAb (4060; Cell Signaling Technology), Recombinant Anti-PI 3 Kinase p85 alpha (ab191606; Abcam), Anti-PI 3 Kinase p85 alpha (phospho Y607) (ab182651; Abcam), Recombinant Anti-TNF alpha (ab183218; Abcam), Anti-IL-1 beta (ab9722; Abcam), and Anti-IL-6 (23431-1-AP; Proteintech). The membranes were incubated with the primary antibodies overnight. The primary antibody was recovered, and the membranes were rinsed three times with Tris-buffered saline with 0.1% Tween 20 and then incubated for 2 hours with a specific secondary antibody. Finally, the proteins were analyzed with enhanced chemiluminescence detection reagents for quantification (WBKLS0100; MilliporeSigma, Burlington, MA, USA).
Short Hairpin RNA Silencing Adeno-Associated Virus and Small interfering RNA Transfection of Pretreated Corneal and Corneal Stromal Cells
Short hairpin RNA silencing adeno-associated virus (AAV-sh-METTL3) and Small interfering RNA (siRNAMETTL3) were used to block the expression of METTL3. AAV-sh-METTL3 and AAV-NC (5 µL/10 µM; RiboBio, Guangzhou, China) were infused subconjunctivally into mice 1 day before infection. Mouse corneas were then collected after day 5 of Fusarium stimulation. Nine 1 × 104 cells/mL corneal stromal cell suspensions were inoculated into 24-well plates and pretreated with siRNAMETTL3 (50 nm/mL) and Small interfering RNA Normal Control (siRNANC) (50 nm/mL) for 36 hours until the cells reached 70% to 80% confluence, followed by stimulation with F. solani for 6 hours. The cells were then analyzed by western blotting and RT-PCR.
Cell Counting Kit-8 Assay for Cell Viability
The cells to be tested were collected, centrifuged, resuspended with fresh solution, and counted. Cells were seeded at 100 µL per well, 2000 cells in total, with three wells for each set of samples; three or more experiments were performed. After 12 hours of cell adhesion, corneal stromal cells were co-incubated with fungal spores (multiplicity of infection ≈ 5) for 6 hours, followed by the addition of Cell Counting Kit-8 (CCK-8) reagent. After adding the reagents, the cells were placed in a cell incubator and incubated for 2 hours. Finally, the plate was placed at 450 nm for measurement.
Cell Wound-Scratch Assay
Cells were divided into three groups: the control group, a group transfected with siRNAMETTL3, and a group transfected with siRNANC. After successful transfection, a 200-µL gun tip was used to cause scratching and co-culture with the fungus. Cells were photographed at 0 hours and at 24 hours to observe the condition of the cells. Cellular changes in each group were analyzed using ImageJ software.
Statistical Analysis
All data are expressed as the mean ± standard deviation. Each experiment was repeated more than three times, and statistical analysis was performed using Student's t-test or one-way analysis of variance, with P < 0.05 being considered statistically significant using Prism 8 (GraphPad, San Diego, CA, USA).
Results
Successful Establishment of a FK Model in Mice
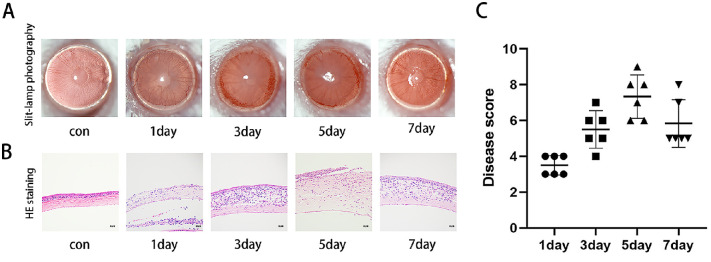
We established an FK mouse model and observed the pathological features of the mice during corneal infection under a slit lamp. As evidenced by the pictures, the corneas of the mice showed the clinical features of FK, including diffuse corneal edema and ulceration after infection with F. solani, and the inflammation peaked on day 5 and decreased on day 7 (Fig. 1A). In addition, H&E staining showed congestion and edema of the corneal rim on postoperative day 1, corneal thickening and neovascularization on postoperative day 3, and severe corneal damage and cellular infiltration on postoperative day 5, with some local ulceration or perforation. On day 7, there was a tendency for inflammation to decrease (Fig. 1B). The clinical scores were consistent with these results (Fig. 1C).
Figure 1.
Clinical features of the FK mouse model. (A) Slit-lamp photographs on days 1, 3, 5 and 7 after fungal infection. (B) Histopathological observation of H&E staining of mouse corneas. Scale bar: 50 µm. (C) Clinical score showing that inflammation reached its highest level on day 5 (n = 6).
METTL3 Expression Is Elevated in FK
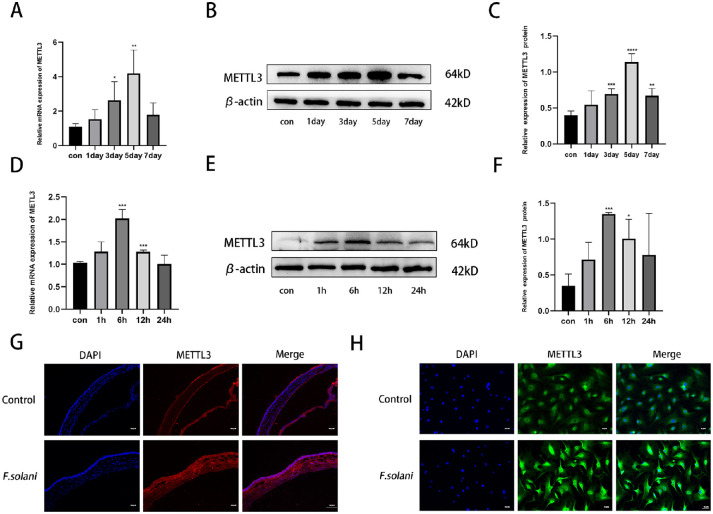
After the FK mouse model was successfully established, the corneas were removed on the first, third, fifth, and seventh days. We found that the expression of METTL3 in corneas infected with F. solani increased significantly on the fifth day and then gradually declined (Fig. 2A). Then, an anti-vimentin antibody was used to identify mouse corneal stromal cells. We examined the changes in METTL3 expression in corneal cells after co-culture with fungi at 1, 6, 12, and 24 hours and found that the expression level of METTL3 peaked at 6 hours of co-culture compared to the control group (Fig. 2D). In addition, we used western blotting to verify the expression of METTL3 protein in the above specimens. The results were consistent with those shown by real-time quantitative PCR (Figs. 2B, 2C, 2E, 2F). Thus, the follow-up experiments were conducted on day 5 for the mouse model and at 6 hours for the cell culture model. According to the immunofluorescence results, METTL3 protein expression was significantly increased at day 5 of corneal infection, whereas the same trend was observed in corneal stromal cells 6 hours after F. solani infection (Figs. 2G, 2H).
Figure 2.
METTL3 is upregulated in FK in mice. (A–C) METTL3 expression in fungus-infected corneal tissues was significantly increased on day 5. (D–F). METTL3 expression was increased in corneal stromal cells after fungal treatment and peaked at 12 hours postinfection. (G) Immunofluorescence analysis of METTL3 in corneal tissue 5 days after fungal infection. Scale bar: 130 µm. (H) Immunofluorescence analysis of corneal stromal cells 12 hours after fungal treatment. Scale bar: 70 µm. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n = 6.
METTL3 May Be Involved in Mediating Activation of the PI3K/AKT Signaling Pathway in FK
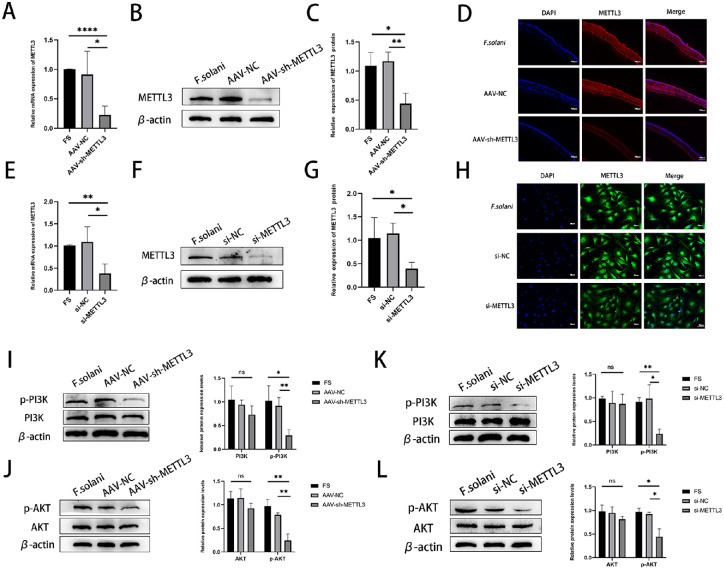
The silencing effect of siRNAMETTL3 and AAV-sh-METTL3 on METTL3 was examined by detecting the expression level of METTL3 at day 5 of infection, which confirmed that siRNAMETTL3 and AAV-sh-METTL3 both effectively downregulated the expression of METTL3 in mouse corneas, as well as in corneal stromal cells. The results of RT-PCR and western blotting showed that METTL3 was decreased in the AAV-sh-METTL3 group after 5 days of infection compared to the normal infection group (Figs. 3A–3C). Immunofluorescence results further supported this conclusion (Fig. 3D). In corneal stromal cells, METTL3 was similarly downregulated in the siRNAMETTL3 group (Figs. 3E–3H). Next, we preregulated METTL3 expression in mouse corneas and corneal stromal cells using AAV-sh-METTL3 and siRNAMETTL3. Compared to the control group, mouse corneas infected with F. solani showed a reduction in the levels of p-PI3K protein and p-AKT protein. However, there was no significant difference in total PI3K and AKT among the groups (Figs. 3I, 3J). This was also demonstrated in corneal stromal cells in the siRNAMETTL3 group (Figs. 3K, 3L). Thus, the observed results demonstrate that METTL3 may have a significant influence on FK by mediating the PI3K/AKT pathway.
Figure 3.
Effect of silencing METTL3 on the PI3K/AKT signaling pathway in Fusarium-stimulated mouse corneas. Effective silencing of METTL3 was confirmed by mRNA (A) and protein (B, C) levels and by immunofluorescence (D). Scale bar: 130 µm. After pretreatment with AAV-sh-METTL3 (n = 6) and after pretreatment with siRNAMETTL3, (E) mRNA (E) and protein (F, G) levels and immunofluorescence (H) confirmed the effective silencing of METTL3. Scale bar: 70 µm. (I, J) Phosphorylation of PI3K/AKT signaling pathway members in Fusarium-stimulated mouse corneas. (K, L) Phosphorylation of PI3K/AKT signaling pathway proteins after Fusarium infection of corneal stromal cells. ns, nonsignificant. *P < 0.05, **P < 0.01, ****P < 0.0001.
METTL3 Mediated the Severity of Corneal Inflammation In Vivo and In Vitro After F. solani Infection
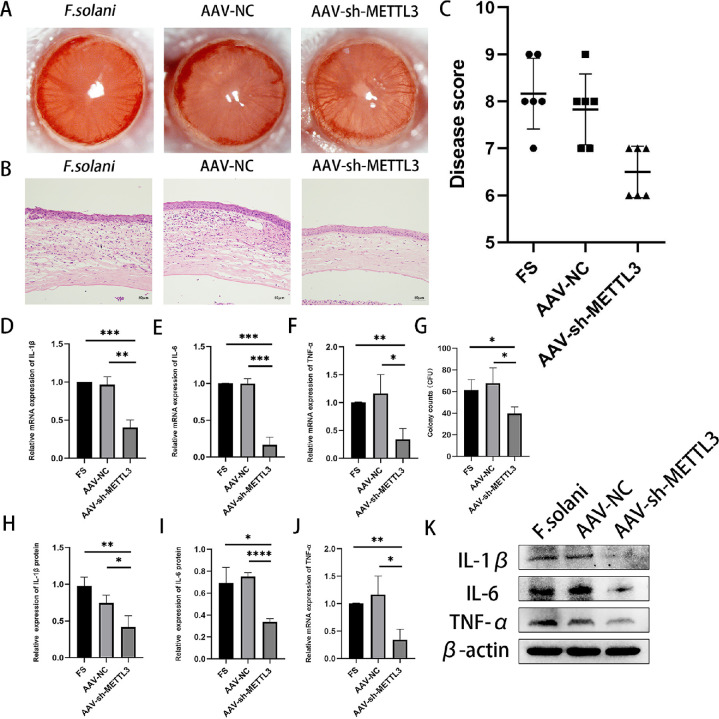
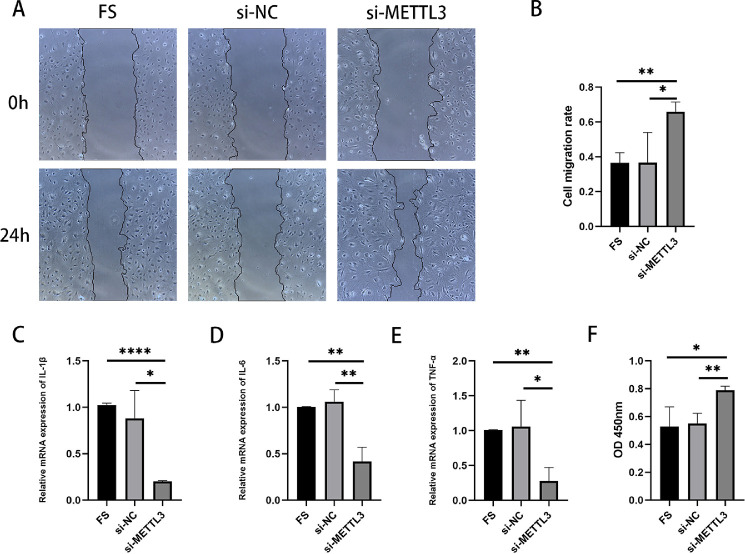
To further understand the role of METTL3 in FK, before infecting the corneas of mice with F. solani we pretreated the mice by subconjunctival injection of AAV-sh-METTL3. Slit-lamp photography was performed 5 days after infection. Compared to the F. solani group, the results demonstrate that corneal edema, cloudiness, and stromal exudation were remarkably alleviated in the AAV-sh-METTL3 group. In comparison, the AAV-NC group and the control group did not differ significantly (Fig. 4A). The degree of inflammatory infiltration in the AAV-sh-METTL3 group appeared to subside, as evident from the H&E staining results (Fig. 4B). The clinical scoring results were consistent with the above results (Fig. 4C). When we silenced METTL3, we found a significant reduction in colonization in the corneas of mice (Fig. 4G). Then quantitative RT-PCR and WB experiments were performed to detect the inflammation-related indicators IL-6, IL-1β, and TNF-α (Figs. 4D–4F, 4H–4J). WB results revealed that the expression of interleukin (IL)-6, IL-1β, and tumor necrosis factor alpha (TNF-α) was apparently downregulated in the AAV-sh-METTL3 group compared to the F. solani group. In the in vitro experiments, after transfection with siRNAMETTL3 and co-culture with F. solani, the scratching experiments showed that the cell migration repair ability was improved in the siRNAMETTL3 group compared with the F. solani group, and the siRNANC group did not significantly differ from the F. solani group (Figs. 5A, 5B). We performed quantitative RT-PCR and found that the expression of related inflammatory factors was also reduced following downregulation of METTL3 (Figs. 5C–5E). According to the CCK-8 assay, mouse corneal stromal cells were significantly more viable after downregulation of METTL3 than the other groups (Fig. 5F). Therefore, we hypothesized that downregulation of METTL3 could reduce the inflammation of FK and thus improve the clinical symptoms.
Figure 4.
Downregulation of METTL3 expression attenuates the inflammatory response and improves FK clinical scores. (A) Slit-lamp biomicrographs of mouse eyes 5 days after infection after pretreatment with AAV-sh-METTL3. (B) Histopathological observation of mouse corneal H&E staining. Scale bar: 50 µm. (C) Clinical scores showed that the inflammatory response was significantly reduced in the AAV-sh-METTL3 group compared with the FS group. (D–F) The mRNA levels of IL-1β, IL-6, and TNF-α were significantly lower in the AAV-sh-METTL3 group than in the FS group. (G) Number of corneal fungal colonies in each group of mice. (H–K) Protein levels of IL-1β, IL-6, and TNF-α were significantly lower in the AAV-sh-METTL3 group than in the FS group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; n = 6.
Figure 5.
Effect of downregulation of METTL3 on corneal stromal cells co-cultured with F. solani. (A) Microscopic images of downregulated METTL3 fungal co-cultured corneal stromal cells were observed by scratch experiments. (B) The gap area percentage and mobility were analyzed by Image J. (C–E) The mRNA levels of IL-1β, IL-6, and TNF-α were significantly lower in the small interfering RNA targeting METTL3 (si-METTL3) group compared with the FS group. (F) The CCK-8 assay showed that the activity of corneal stromal cells in mice with low METTL3 expression was significantly higher than that in controls. *P < 0.05, **P < 0.01, ****P < 0.0001; n = 6.
Inhibition of the PI3K/AKT Signaling Pathway Alleviates Corneal Inflammation After F. solani Infection
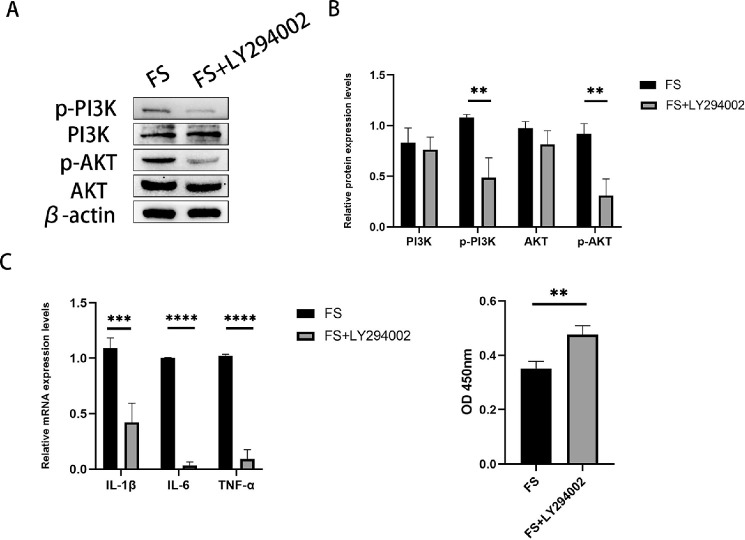
Because METTL3 is correlated with the PI3K/AKT signaling pathway, we hypothesized that the PI3K/AKT pathway might regulate keratitis caused by F. solani. To further investigate this hypothesis, we used a specific PI3K/AKT inhibitor, LY294002, and co-cultured corneal stromal cells pretreated with the inhibitor with Fusarium oxysporum. According to western blot analysis, the degree of pathway activation was decreased in the FS+LY294002 group compared to the control group
(Figs. 6A, 6B). In addition, we detected the associated inflammatory factors IL-6, IL-1β, and TNF-α by quantitative RT-PCR (Fig. 6C). The CCK-8 results indicated greater survival rates of corneal stromal cells in the FS+LY294002 group than in the control group (Fig. 6D).
Figure 6.
Effect of inhibition of the PI3K/AKT signaling pathway on inflammation in experimental FK. (A, B) Western blot analysis confirmed that the PI3K/AKT signaling pathway was inhibited after the addition of LY294002. (C) The mRNA levels of IL-6, IL-1β, and TNF-α were significantly downregulated in the FS+LY294002 group compared to the FS group. (D) The CCK-8 assay showed that the activity of corneal stromal cells was significantly higher in LY294002-treated mice than in the control group. **P < 0.01, ****P < 0.0001; n = 6.
Discussion
Fungal keratitis is a highly aggressive, corneal-damaging emergency that often leads to severe visual impairment because of its rapid progression.24 Fusarium species are among the most common etiologic agents of FK in China.25 The potent virulence and fungal aggressiveness of Fusarium, as well as the lack of clinically effective antimicrobial agents, may lead to poor recovery.26,27 Therefore, it is imperative to find effective therapeutic targets and thus regulate the progression of corneal inflammation. In addition to playing a prominent role in many physiological processes, m6A modification contributes to immune responses following microbial infections.28–30 Several components contribute to the regulation of m6A methylation. These include methyltransferases, demethylases, and binding proteins. METTL3, one of the important methyltransferases in m6A modification, is closely associated with a variety of physiological or pathological processes in the body.31,32 Our study found that METTL3 expression was upregulated in keratitis following F. solani infection in mice. Previously, METTL3 was implicated in inflammatory diseases.14 A recent study found that silencing METTL3 could maintain long-chain fatty acid uptake by blocking TNF receptor associated factor 6–dependent inflammatory responses.33 Recently, in a study on intestinal bacterial infections, it was shown that METTL3 can influence defensin expression through methylation activity and thus protect against bacterial infections.34 However, it is unclear exactly how METTL3 contributes to FK pathogenesis.
The PI3K/AKT signaling pathway, a typical signaling pathway, regulates numerous cell biological processes, including apoptosis, transcription, translation, metabolism, and the cell cycle.35–37 In recent years, it has been shown that this pathway is also implicated in infection and in the pathogenesis of different microorganisms and has a significant impact on various corneal disease.38,39 In our previous research, the PI3K/AKT signaling pathway also appeared to be activated in mice with FK.14 Recently, numerous studies have also demonstrated that the PI3K/AKT signaling pathway is aberrantly activated in inflammatory diseases and that aberrant activation of this pathway is significantly correlated with inflammation onset and inflammation progression. In bacterial keratitis, immunomodulatory effects through modulation of the PI3K/AKT pathway suppress inflammation and delay the progression of keratitis40; in corneal tissue after herpes simplex virus infection, p-PI3K and p-AKT were markedly increased, and this signaling pathway may mediate the process of keratitis.41 In FK, IL-17, which is secreted by Th17 cells, inhibits the development of FK by suppressing the expression of connexin-43 through the AKT signaling pathway.42 Due to its multiple functions in an array of biological processes, such as inflammation, the PI3K/AKT pathway plays a potential role in FK. We used LY294002 to inhibit the PI3K/AKT signaling pathway to observe the changes in corneal stromal cells after fungal infection and found that the levels of proinflammatory cytokines were downregulated after the inhibition of this pathway compared with the control group. The viability of corneal stromal cells was increased after the addition of inhibitors.
METTL3 knockdown resulted in a significant decrease in p-AKT expression in uveal melanoma cells, revealing that m6A methylation may regulate the AKT signaling pathway in uveal melanoma cells.43 METTL3 mediates the PI3K/AKT/mammalian target of rapamycin pathway to promote retinoblastoma progression, and targeting this signaling axis may be a potential therapeutic direction for retinoblastoma.44 In line with this, our study found inactivation of the PI3K/AKT-mediated signaling pathway in F. solani–infected corneal tissues after knockdown of METTL3 expression. Therefore, we speculate that METTL3-mediated m6A modification may also affect the inflammatory process of FK by regulating this signaling pathway.
To further clarify the exact role of METTL3 in FK, we downregulated METTL3 expression in mouse corneas using AAV-sh-METTL3. Our observations indicate that downregulation of METTL3 relieves inflammation and reduces the expression of TNF-α, IL-1β, and IL-6 in corneal-infected mice. As essential proinflammatory cytokines, TNF-α, IL-1β, and IL-6 play important roles in host resistance to pathogenic microorganisms by activating multiple responses.45–47 These data suggest that METTL3 appears to be a proinflammatory protein and contributes significantly to inflammatory responses in the corneas of F. solani–infected mice. The results of our study are concordant with those obtained by others. Silencing of METTL3 significantly inhibited the expression of proinflammatory factors in LPS-treated human dental pulp cells, suggesting that METTL3 regulates the inflammatory response induced by LPS in human dental pulp cells.48 Recently, METTL3-mediated m6A modifications have been found to play a significant role in liver damage in hepatitis B virus–infected mice.49 In addition, METTL3 expression was upregulated in pneumonia patient serum and LPS-treated cells, and LPS-induced inflammatory injury was attenuated by inhibiting METTL3 expression.50 Our findings indicate that METTL3 may contribute to innate immunity in the corneal antifungal process by influencing the inflammatory process. Although METTL3 was shown to mediate the PI3K/AKT pathway and thus significantly affect the FK inflammatory process in this study, the specific mechanism of action of METTL3 has not been fully elucidated. Therefore, finding more specific downstream targets is key for subsequent studies.
In summary, our main findings include the following: (1) upregulation of METTL3 expression occurs in FK caused by F. solani, (2) downregulation of METTL3 attenuated corneal inflammation and inhibited activation of the PI3K/AKT pathway, and (3) PI3K/AKT pathway inhibition reduced the accumulation of inflammatory cytokines in corneal stromal cells. Therefore, we suggest that METTL3 may influence the inflammatory response of fungal-infected corneas by mediating the PI3K/AKT signaling pathway.
Acknowledgments
Supported by a grant from the National Natural Science Foundation of China, Beijing, China (81870636).
Disclosure: L. Huang, None; H. Tang, None; J. Hu, None
References
- 1. Brown L, Leck AK, Gichangi M, Burton MJ, Denning DW.. The global incidence and diagnosis of fungal keratitis. Lancet Infect Dis. 2021; 21: e49–e57. [DOI] [PubMed] [Google Scholar]
- 2. Leal SM Jr, Vareechon C, Cowden S, et al.. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest. 2012; 122: 2482–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Todokoro D, Suzuki T, Tamura T, et al.. Efficacy of luliconazole against broad-range filamentous fungi including Fusarium solani species complex causing fungal keratitis. Cornea. 2019; 38: 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mills B, Radhakrishnan N, Karthikeyan Rajapandian SG, Rameshkumar G, Lalitha P, Prajna NV.. The role of fungi in fungal keratitis. Exp Eye Res. 2021; 202: 108372. [DOI] [PubMed] [Google Scholar]
- 5. Ren Z, Liu Q, Wang Y, Dong Y, Huang Y.. Diagnostic information profiling and evaluation of causative fungi of fungal keratitis using high-throughput internal transcribed spacer sequencing. Sci Rep. 2020; 10: 1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao BS, Roundtree IA, He C.. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017; 18: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang A, Tao W, Tong J, et al.. m6A modifications regulate intestinal immunity and rotavirus infection. eLife. 2022; 11: e73628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frye M, Harada BT, Behm M, He C.. RNA modifications modulate gene expression during development. Science. 2018; 361: 1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du Y, Hou G, Zhang H, et al.. SUMOylation of the m6A-RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res. 2018; 46: 5195–5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schöller E, Weichmann F, Treiber T, et al.. Interactions, localization, and phosphorylation of the m6A generating METTL3-METTL14-WTAP complex. RNA. 2018; 24: 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang C, Fu J, Zhou Y.. A review in research progress concerning m6A methylation and immunoregulation. Front Immunol. 2019; 10: 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Song F, Sun H, Wang Y, et al.. Pannexin3 inhibits TNF-α-induced inflammatory response by suppressing NF-κB signalling pathway in human dental pulp cells. J Cell Mol Med. 2017; 21: 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang JN, Wang F, Ke J, et al.. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci Transl Med. 2022; 14: eabk2709. [DOI] [PubMed] [Google Scholar]
- 14. Hu J, Lin Y.. Fusarium infection alters the m6A-modified transcript landscape in the cornea. Exp Eye Res. 2020; 200: 108216. [DOI] [PubMed] [Google Scholar]
- 15. Di Pilato M, Kim EY, Cadilha BL, et al.. Targeting the CBM complex causes Treg cells to prime tumours for immune checkpoint therapy. Nature. 2019; 570: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yin Y, Dang W, Zhou X, et al.. PI3K-Akt-mTOR axis sustains rotavirus infection via the 4E-BP1 mediated autophagy pathway and represents an antiviral target. Virulence. 2018; 9: 83–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Wu H, Liu C, Tian J, Qu L.. PI3K/Akt/p53 pathway inhibits reovirus infection. Infect Genet Evol. 2015; 34: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Sun M, Long Y.. Cyanidin-3-O-glucoside attenuates lipopolysaccharide-induced inflammation in human corneal epithelial cells by inducing Let-7b-5p-mediated HMGA2/PI3K/Akt pathway. Inflammation. 2020; 43: 1088–1096. [DOI] [PubMed] [Google Scholar]
- 19. Chen H, Zheng Z, Chen P, Wu XG, Zhao G.. Inhibitory effect of extracellular polysaccharide EPS-II from Pseudoalteromonas on Candida adhesion to cornea in vitro. Biomed Environ Sci. 2012; 25: 210–215. [DOI] [PubMed] [Google Scholar]
- 20. Hu J, Hu Y, Chen S, et al.. Role of activated macrophages in experimental Fusarium solani keratitis. Exp Eye Res. 2014; 129: 57–65. [DOI] [PubMed] [Google Scholar]
- 21. Wu TG, Wilhelmus KR, Mitchell BM.. Experimental keratomycosis in a mouse model. Invest Ophthalmol Vis Sci. 2003; 44: 210–216. [DOI] [PubMed] [Google Scholar]
- 22. Song HB, Park SY, Ko JH, et al.. Mesenchymal stromal cells inhibit inflammatory lymphangiogenesis in the cornea by suppressing macrophage in a TSG-6-dependent manner. Mol Ther. 2018; 26: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang Y, Mao J, Wang X, et al.. Genome-wide screening of altered m6A-tagged transcript profiles in the hippocampus after traumatic brain injury in mice. Epigenomics. 2019; 11: 805–819. [DOI] [PubMed] [Google Scholar]
- 24. Yang X, Zhao G, Yan J, et al.. Pannexin 1 channels contribute to IL-1β expression via NLRP3/caspase-1 inflammasome in Aspergillus fumigatus keratitis. Curr Eye Res. 2019; 44: 716–725. [DOI] [PubMed] [Google Scholar]
- 25. Xie L, Zhong W, Shi W, Sun S.. Spectrum of fungal keratitis in north China. Ophthalmology. 2006; 113: 1943–1948. [DOI] [PubMed] [Google Scholar]
- 26. Kuo MT, Chen JL, Hsu SL, Chen A, You HL.. An omics approach to diagnosing or investigating fungal keratitis. Int J Mol Sci. 2019; 20: 3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ortega-Rosales A, Quizhpe-Ocampo Y, Montalvo-Flores M, Burneo-Rosales C, Romero-Ulloa G.. A case of fungal keratitis due to Fusarium solani after an indigenous healing practice. IDCases. 2019; 18: e00618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Feng J, Zhang T, Sorel O, et al.. Global profiling reveals common and distinct N6-methyladenosine (m6A) regulation of innate immune responses during bacterial and viral infections. Cell Death Dis. 2022; 13: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang H, Hu X, Huang M, et al.. Mettl3-mediated mRNA m6A methylation promotes dendritic cell activation. Nat Commun. 2019; 10: 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lichinchi G, Gao S, Saletore Y, et al.. Dynamics of the human and viral m6A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol. 2016; 1: 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gan H, Hong L, Yang F, Liu D, Jin L, Zheng Q. [Progress in epigenetic modification of mRNA and the function of m6A modification]. Sheng Wu Gong Cheng Xue Bao. 2019; 35: 775–783. [DOI] [PubMed] [Google Scholar]
- 32. Li N, Hui H, Bray B, et al.. METTL3 regulates viral m6A RNA modification and host cell innate immune responses during SARS-CoV-2 infection. Cell Rep. 2021; 35: 109091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zong X, Zhao J, Wang H, et al.. Mettl3 deficiency sustains long-chain fatty acid absorption through suppressing Traf6-dependent inflammation response. J Immunol. 2019; 202: 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zong X, Wang H, Xiao X, et al.. Enterotoxigenic Escherichia coli infection promotes enteric defensin expression via FOXO6-METTL3-m6A-GPR161 signalling axis. RNA Biol. 2021; 18: 576–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ecker V, Stumpf M, Brandmeier L, et al.. Targeted PI3K/AKT-hyperactivation induces cell death in chronic lymphocytic leukemia. Nat Commun. 2021; 12: 3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mohseni AH, Casolaro V, Bermúdez-Humarán LG, Keyvani H, Taghinezhad SS.. Modulation of the PI3K/Akt/mTOR signaling pathway by probiotics as a fruitful target for orchestrating the immune response. Gut Microbes. 2021; 13: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu X, Liu W, Ding C, et al.. Taxifolin, extracted from waste Larix olgensis roots, attenuates CCl(4)-induced liver fibrosis by regulating the PI3K/AKT/mTOR and TGF-β1/Smads signaling pathways. Drug Des Devel Ther. 2021; 15: 871–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou Y, Lei W, Su S, et al.. Chlamydia trachomatis plasmid-encoded protein Pgp3 inhibits apoptosis via the PI3K-AKT-mediated MDM2-p53 axis. Mol Cell Biochem. 2019; 452: 167–176. [DOI] [PubMed] [Google Scholar]
- 39. Mohankumar V, Dhanushkodi NR, Raju R.. Sindbis virus replication is insensitive to rapamycin and torin1, and suppresses Akt/mTOR pathway late during infection in HEK cells. Biochem Biophys Res Commun. 2011; 406: 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun M, Zhu M, Chen K, et al.. TREM-2 promotes host resistance against Pseudomonas aeruginosa infection by suppressing corneal inflammation via a PI3K/Akt signaling pathway. Invest Ophthalmol Vis Sci. 2013; 54: 3451–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ke L, Yang Y, Li JW, et al.. Modulation of corneal FAK/PI3K/Akt signaling expression and of metalloproteinase-2 and metalloproteinase-9 during the development of herpes simplex keratitis. Biomed Res Int. 2019; 2019: 4143981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qin XH, Ma X, Fang SF, Zhang ZZ, Lu JM.. IL-17 produced by Th17 cells alleviates the severity of fungal keratitis by suppressing CX43 expression in corneal peripheral vascular endothelial cells. Cell Cycle. 2019; 18: 274–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo G, Xu W, Zhao Y, et al.. RNA m6A methylation regulates uveal melanoma cell proliferation, migration, and invasion by targeting c-Met. Journal of cellular physiology. 2020; 235: 7107–7119. [DOI] [PubMed] [Google Scholar]
- 44. Zhang H, Zhang P, Long C, et al.. m6A methyltransferase METTL3 promotes retinoblastoma progression via PI3K/AKT/mTOR pathway. J Cell Mol Med. 2020; 24: 12368–12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Karki R, Sharma BR, Tuladhar S, et al.. Synergism of TNF-α and IFN-γ triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021; 184: 149–168.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aarreberg LD, Esser-Nobis K, Driscoll C, Shuvarikov A, Roby JA, Gale M Jr. Interleukin-1β induces mtDNA release to activate innate immune signaling via cGAS-STING. Mol Cell. 2019; 74: 801–815.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Martinez AN, Mehra S, Kaushal D.. Role of interleukin 6 in innate immunity to Mycobacterium tuberculosis infection. J Infect Dis. 2013; 207: 1253–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feng Z, Li Q, Meng R, Yi B, Xu Q.. METTL3 regulates alternative splicing of MyD88 upon the lipopolysaccharide-induced inflammatory response in human dental pulp cells. J Cell Mol Med. 2018; 22: 2558–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cheng D, Wu C, Li Y, et al.. METTL3 inhibition ameliorates liver damage in mouse with hepatitis B virus-associated acute-on-chronic liver failure by regulating miR-146a-5p maturation. Biochim Biophys Acta Gene Regul Mech. 2022; 1865: 194782. [DOI] [PubMed] [Google Scholar]
- 50. Li SX, Yan W, Liu JP, Zhao YJ, Chen L.. Long noncoding RNA SNHG4 remits lipopolysaccharide-engendered inflammatory lung damage by inhibiting METTL3-mediated m6A level of STAT2 mRNA. Mol Immunol. 2021; 139: 10–22. [DOI] [PubMed] [Google Scholar]