Abstract
In Escherichia coli, the DnaA protein level appears to play a pivotal role in determining the timing of replication initiation. To examine the effects on replication initiation in B. subtilis, we constructed a strain in which a copy of the dnaA gene was integrated at the purA locus on the chromosome under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter. However, increasing the DnaA level resulted in cell elongation and inhibition of cell growth by induction of the SOS response. Transcription of the native dnaA-dnaN operon was greatly reduced at high DnaA levels, but it was increased in a dnaA-null mutant, indicating autoregulation of the operon by DnaA. When a copy of the dnaN gene was added downstream of the additional dnaA gene at purA, the cells grew at high DnaA levels, suggesting that depletion of DnaN (β subunit of DNA polymerase III) within the cell by repression of the native dnaA-dnaN operon at high DnaA levels was the cause of the SOS induction. Flow cytometry of the cells revealed that the cell mass at initiation of replication increased at a lower DnaA level and decreased at DnaA levels higher than those of the wild type. Proper timing of replication initiation was observed at DnaA levels nearly comparable to the wild-type level. These results suggest that if the DnaA level increases with progression of the replication cycle, it could act as a rate-limiting factor of replication initiation in B. subtilis.
Bacterial chromosome replication starts at a fixed site (oriC) and at a specific cell mass (the initiation mass) in the cell division cycle. In early studies, the mass per oriC at initiation was proposed to be constant, irrespective of the growth rate (8). However, a later report suggested that the initiation mass increased by about 50% monotonically with decreasing growth rate (from 2.5 to 0.3 doublings per hour) (45). Nonetheless, in a given culture, initiation occurred within a very narrow range of cell mass, indicating the tight coupling of replication initiation to cell mass (4). Two major regulatory mechanisms are assumed to ensure the fidelity of replication initiation. One is positive in that it triggers initiation at the appropriate initiation mass, while the other is negative in that it suppresses extra initiations until the next round of initiation. The DnaA protein concentration (14) and change in DnaA activity (26) have been proposed to embody such positive factors in Escherichia coli. In contrast, sequestration of newly replicated origins into the cell membrane by SeqA (29) and inactivation of DnaA by DnaN (20) may represent the negative mechanisms present in E. coli. In addition to sequestration, SeqA also serves as a negative modulator of replication initiation because the initiation mass is decreased in the null mutant (4). Thus, another idea was proposed to determine the timing of replication initiation, in which replication initiation is controlled by changes in the balance between positive and negative factors (29). However, the molecular mechanism regulating the balance remains to be uncovered. For Bacillus subtilis, there have been, as yet, no reports about such positive and negative mechanisms. No homologous genes to seqA have been found on the genome (25), suggesting that a mechanism similar to that of SeqA may not be operating in B. subtilis.
The DnaA protein is required for initiation of replication in both E. coli and B. subtilis (31, 33) and is conserved in many bacteria (47). Overproduction of DnaA leads to earlier initiation of replication in the cell division cycle in E. coli, indicating that the DnaA protein level was the primary factor that determines the timing of initiation (27). Later, it was reported that its overproduction also interfered severely with replication elongation, although the reason was not clear (3). In support of the fact that the DnaA level is a primary positive factor, initiation of replication from oriC occurred synchronously at a threshold level of DnaA, albeit under special conditions (11). To explain how the DnaA level controls the timing of initiation, the initiator titration model was proposed (10). According to this model, newly synthesized DnaA molecules first bind to high-affinity DnaA boxes dispersed on the chromosome, and, subsequently, the remaining free DnaA molecules bind to a lower-affinity DnaA box within oriC that triggers initiation. In fact, there are several regions with high-affinity DnaA boxes on the E. coli chromosome (39). Furthermore, when one such region (datA) (21) was deleted from the chromosome, overinitiation and asynchronous initiation were observed (22). Thus, in E. coli, the DnaA protein level appears to play a pivotal role in determining the timing of replication initiation.
In the present studies, a relationship between the timing of initiation and cellular DnaA protein levels was detected, suggesting that such levels could also act as a positive regulator of initiation in B. subtilis. In addition, overproduction of DnaA alone induced the SOS response (46) in this organism. This induction was probably due to depletion of the DnaN protein (β subunit of DNA polymerase III) caused by repression of the native dnaA-dnaN operon at high DnaA levels. Indeed, when DnaN was supplied from another locus on the chromosome, cell elongation caused by the SOS response was not observed at high DnaA levels. The supply of DnaN enabled us to examine the effects of high DnaA levels on replication initiation by flow cytometry.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The B. subtilis strains used in this study are listed in Table 1. Construction of strains NIS2000, NIS2020, and NIS2022 is described below. Transformation of B. subtilis cells was carried out as described previously (24). E. coli strain C600 was used throughout as a cloning host.
TABLE 1.
B. subtilis strains used in this study
| Strain | Relevant genotypea | Reference construction |
|---|---|---|
| CRK6000 | purA16 metB5 hisA3 guaB | 33 |
| NIS2000 | CRK6000 purA::Pspac-lacI-cat | Transformation of CRK6000 with a PCR product (see Materials and Methods) |
| NIS2010 | CRK6000 purA::PrpmH-lacO-lacI-cat | Same as above |
| NIS2020 | CRK6000 purA::PrpmH-lacO-dnaA-lacI-cat | Same as above |
| NIS6500 | CRK6000 amyE::pDLAH(PdinC-bgaB ermC) | 35 |
| NIS2065 | CRK6000 purA::PrpmH-lacO-dnaA-lacI-cat amyE::pDLAH(PdinC-bgaB ermC) | Transformation of NIS2020 with NIS6500 DNA |
| NIS6301 | CRK6000 spoIIIJ::pRK1(oriN cat) ΔoriC | 35 |
| NIS6311 | CRK6000 spoIIIJ::pRK1(oriN cat) dnaA(ochre) | 12, 17 |
| NIS2021 | CRK6000 purA::PrpmH-lacO-dnaA-dnaN-tet-pBR-lacI-cat | Transformation of NIS2020 with pSM5100 |
| NIS2022 | CRK6000 dnaA (ochre) purA::PrpmH-lacO-dnaA-dnaN-tet-pBR-lacI-neo | See Materials and Methods |
P, promoter region; oriN, minimal replication region of low-copy plasmid pLS32 (12).
NIS2000.
Pspac-lacI and cat fragments were amplified from pDH88 DNA (13) by PCR with two sets of primers (Table 2), 1 and 2 and 3 and 4, respectively, and were purified with Centricon 100 (Amicon, Beverly, Mass.). Since a 37-nucleotide sequence of the 5′ end of primer 3 is complementary to the sequence of primer 2, these two PCR products can be combined by PCR as described previously (15). Indeed, by utilizing the overlapping sequence, the two fragments (each 5 to 10 ng) were combined, and the resulting fragment (Pspac-lacI-cat) was further amplified by PCR (25 cycles) with two outer primers, 1 and 4. Next, two fragments of about 7 kb near the purA locus, from yycA to purA and from trnY to yyxA, were amplified from the chromosomal DNA (50 ng) by long PCR (25 cycles of 15 s at 95°C [denaturation] and 6 min at 65°C [annealing and extension]) with rTth DNA polymerase XL (PE Applied Biosystems) and the following primer sets: primers 5 and 6 for yycA∼purA and primers 7 and 8 for trnY∼yyxA. Three PCR products (yycA∼purA, Pspac-lacI-cat, and trnY∼yyxA) were purified with Centricon 100. To combine these fragments by PCR, 22- and 28-nucleotide sequences of primers 6 (7th to 28th bases from the 5′ end) and 7 (1st to 28th bases), which are complementary to those of primers 1 (1st to 22nd bases) and 4 (1st to 28th bases), respectively, were used as shown in Table 2. As a result, a long DNA fragment, yycA∼purA-Pspac-lacI-cat-trnY∼yyxA, was amplified by long PCR (20 cycles of 15 s at 95°C and 12 min at 65°C, followed by a further 12 cycles, increasing the extension time by 15 s per cycle) with the outer two primers 5 and 8 and was used to transform CRK6000 cells after being purified with Centricon 100.
TABLE 2.
Nucleotide sequences of primers used in this study
| Primer no. | Sequencea | Nucleotide no. |
|---|---|---|
| 1 | 5′-TCTACAGTCGACAGTCCAGACTGTTCGGCAC-3′ | |
| 2 | 5′-CAGGAAACAGCTATGACCACATTAATTGCGTTGCGCTC-3′ | |
| 3 | 5′-AGCGCAACGCAATTAATGTGGTCATAGCTGTTTCCTGAGCTTTACCGCAGGCAATAG-3′ | |
| 4 | 5′-ATTAGACTGCAGACTGTAAAAAGTACAGTC-3′ | |
| 5 | 5′-GTTGCTGACCAGTAAACTGGTGTGAG-3′ | 4161896–4161871 |
| 6 | 5′-CCGAATAGTCTGGACTGTCGACTGTAGATCAGACCAACTTATATGCGG-3′ | 4154569–4154588 (20 bases of 3′ end) |
| 7 | 5′-CTGTACTTTTTACAGTCTGCAGTCTAATCGCAGTGTGTACCGTGCGA-3′ | 4154636–4154654 (19 bases of 3′ end) |
| 8 | 5′-GTTCTTCCTCACGTTCGTAATCCACC-3′ | 4147950–4147975 |
| 9 | 5′-CCGCATATAAGTTGGTCTGATCTACAGTCGACTGGTATCCTATTATGGTTGC-3′ | 4214716–4214697 (20 bases of 3′ end) |
| 10 | 5′-GACCTCGTTTCCACCGGATCCGAAATTGTTATCCGCTCACAATTACTTATTATATTTGCGT TACCTATTC-3′ | 4214638–4214665 (28 bases of 3′ end) |
| 11 | 5′-GCAAGCGGATCCGGTGGAAACGAAAACGAGGTCATA-3′ | |
| 12 | 5′-ATGCAACCGGATCCTCGGAAGGAAATGATGACCTCGTTT-3′ | |
| 13 | 5′-AACGGTTGCATTTAAATCTTACATATGTAATACTTTCAAAGACTACATTTGTAAGAATTT GATG-3′ | |
| 14 | 5′-GGAAACGAGGTCATCATTTCCTTCGGATCCAATGTGTACGAATGGTAAGCG-3′ | 315–335 (21 bases of 3′ end) |
| 15 | 5′-GTCTTTGAAAGTATTACATATGTAAGATTTAAATGCAACCGTTCGGTACCTGCTATTTAAG CTGTTCT-3′ | 1754–1735 (20 bases of 3′ end) |
Sequences at http://bacillus.genome.ad.jp/. Boldface letters indicate BamHI sites.
NIS2020.
A promoter region of the rpmH gene (nucleotides 4214716 to 4214638) was amplified from the chromosome by PCR with primers 9 and 10 (Table 2). The lac operator sequence is included in primer 10 (24th to 42nd bases from the 5′ end). Because a 32-nucleotide sequence of primer 9 (1st to 32nd bases) is complementary to that of primer 6 at the 3′ end, this PrpmH-lacO fragment was combined with a long DNA from yycA to purA by long PCR with primers 5 and 10, resulting in yycA∼purA-PrpmH-lacO. In addition, the lacI-cat-trnY∼yyxA fragment was amplified from the NIS2000 chromosome by long PCR with primers 11 and 8. Because two primers, 10 and 11, contain artificial BamHI sites (Table 2), the two PCR fragments were ligated after digestion with BamHI and used to transform CRK6000. Again, yycA∼PrpmH-lacO and lacI-cat∼yyxA fragments were amplified from chromosomal DNA of the transformant (NIS2010) by long PCR with the primer sets 5 and 12 for the former and 13 and 8 for the latter. The coding region of dnaA with the SD sequence (nucleotides 315 to 1754) was also amplified from the CRK6000 chromosome by PCR with primers 14 and 15 (Table 2). By utilizing overlapping sequences between primers 12 and 14 (23 bases) and between primers 13 and 15 (43 bases) (Table 2), three PCR fragments (yycA∼PrpmH-lacO, dnaA, and lacI-cat∼yyxA) were combined and amplified by long PCR with the outer two primers 5 and 8 as described above. The resulting fragment, yycA∼purA-PrpmH-lacO-dnaA-lacI-cat-trnY∼yyxA, was used as a donor DNA for transformation of CRK6000 after being purified with Centricon 100. The nucleotide sequences of PrpmH-lacO-dnaA regions in five chloramphenicol-resistant transformants were determined, and one of them (NIS2020) contained no mutations in the region.
NIS2022.
cat located downstream of lacI in NIS2021 was replaced by neo with pCm::Nm (from BGSC) (44) by transformation, and then the neomycin-resistant purA region containing an additional dnaA-dnaN was transferred to NIS6311 (17) by transformation. Among neomycin-resistant transformants, chloramphenicol-sensitive ones were selected, in which oriN and cat integrated at spoIIIJ of NIS6311 (Table 1) were replaced with the native spoIIIJ of NIS2021 by cotransformation. Finally, the existence of the ochre mutations in dnaA (34) was confirmed by sequencing.
pSM5100.
Both the dnaA and dnaN genes (nucleotides 358 to 1759 and 1913 to 3077, respectively, at http://bacillus.genome.ad.jp/) were amplified by PCR with primer sets by using artificial SpeI-BglII and BglII-BamHI sites, respectively. After digestion of these fragments with the restriction enzymes, they were ligated at the BglII sites and cloned between the XbaI and BglII sites of a pSM5000 derivative that contained a fragment (from EcoRI to BamHI) of pDH88 (13) at the EcoRI site of pSM5000 (34). Both XbaI and BglII sites are located in a multicloning site in the pDH88 portion. Subsequently, approximately the 5′ half of dnaA and a part of the pDH88 portion were removed by digestion with SalI followed by self-circularization. Finally, the other part of the pDH88 portion (from ClaI to ScaI) located downstream of dnaN was replaced with a BamHI fragment conferring tetracycline resistance in pBEST307 (18) after blunting both DNA fragments. Because bla was destroyed by ScaI digestion, E. coli clones were selected by tetracycline resistance (20 μg/ml).
Growth conditions.
B. subtilis cells were grown in antibiotic medium 3 (Difco Laboratories, Detroit, Mich.) supplemented with adenine and guanosine (requirements for growth; 20 μg/ml) at 30°C throughout this study. When necessary, various concentrations of isopropyl-β-d-thiogalactopyranoside (IPTG) and drugs were added (chloramphenicol, erythromycin, and tetracycline at 10, 1, and 5 μg/ml, respectively). Because addition of drugs decreased the growth rate slightly, cells were grown without drugs for flow cytometry.
Immunoblotting.
Preparation of cell lysates from exponentially growing cells and separation of proteins were carried out as described previously (12). Proteins were blotted on a Hybond-P polyvinylidene difluoride membrane (Amersham Pharmacia Biotech, Piscataway, N.J.), and the membrane was incubated with anti-DnaA rabbit polyclonal antibody (33). After the membrane was treated with a second antibody (goat anti-rabbit immunoglobulin G–horseradish peroxidase conjugate), signals were detected by the ECL-Plus enhanced chemiluminescence system (Amersham).
Examination of the SOS response.
The SOS response of cells was examined by monitoring expression from a damage-inducible promoter (dinC) that was fused with a reporter gene, bgaB, encoding Bacillus stearothermophilus β-galactosidase at the amyE locus on the chromosome. To measure the activity of the β-galactosidase expressed, samples were prepared from cells as described previously (48). The β-galactosidase assay was carried out with the fluorogenic substrate MUG (4-methylumbelliferyl-β-d-galactoside) and a Fluoroskan II fluorometer (Labsystems, Helsinki, Finland). One unit of specific activity was defined as 1 pmol of MUG hydrolyzed/ml of culture sample/min, normalized for culture cell density at 600 nm.
Northern hybridization.
Total RNA was extracted from B. subtilis cells basically as described previously (16). Five micrograms of total RNA in each lane was separated in a denaturing agarose (1%) gel containing formaldehyde (2%), followed by blotting on a nylon membrane, Hybond-N+ (Amersham). The presence of nearly equal amounts of RNA in each lane was confirmed by staining with methylene blue. Preparation of RNA probes and the following Northern hybridization method were carried out as described previously (1). Briefly, RNA probes were prepared as follows. First, coding regions of the dnaA and dnaN genes with lengths of 257 bp (nucleotides 829 to 1085 at http://bacillus.genome.ad.jp/) and 266 bp (nucleotides 1958 to 2223), respectively, were amplified by PCR. The promoter sequence for T7 RNA polymerase was added to reverse primers. RNA probes labeled with digoxigenin (DIG) were obtained by in vitro transcription by using these PCR fragments as a template and the DIG RNA labeling kit (SP6/T7) (Roche Molecular Biochemicals, Mannheim, Germany). Hybridization and detection of signals were carried out with the DIG luminescent detection kit (Roche) according to the supplier's manual.
Flow cytometry.
Chloramphenicol was added to cells grown exponentially at a concentration of 200 μg/ml to inhibit new rounds of initiation. Incubation was continued for a further 5 h to complete ongoing replication. A previous paper reported that this drug inhibited replication initiation and cell division simultaneously in B. subtilis cells (40). In fact, the cell length at initiation estimated by flow cytometry corresponded approximately to that obtained previously by an extensive microscopic analysis (41) (data not shown). For flow cytometry, the cells were fixed with ethanol and treated further as described previously (27), and the number of replication origins per cell was measured with a Bryte HS flow cytometer (Bio-Rad Laboratories, Hercules, Calif.).
RESULTS
Overproduction of the DnaA protein induces the SOS response.
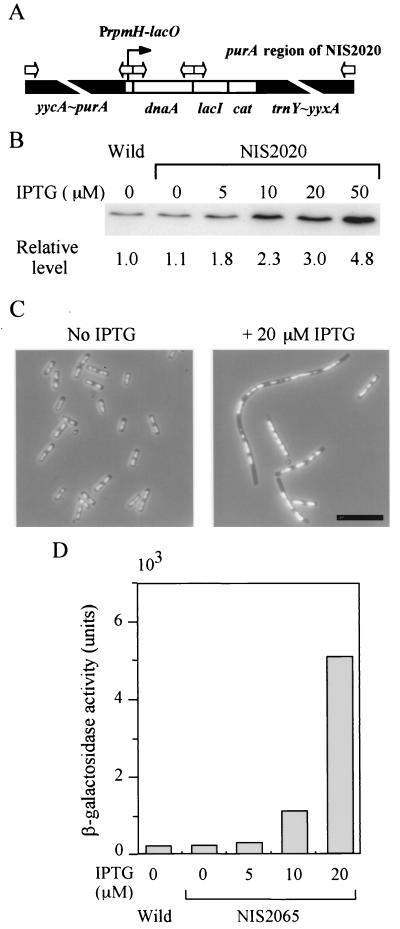
A copy of the dnaA gene was placed under the control of a fusion between the rpmH promoter (gene coding for ribosomal protein L34) and the lac operator. It was then integrated into the purA locus on the chromosome with lacI and cat genes (Fig. 1A). Upon addition of IPTG, the DnaA level increased, as shown by immunoblotting (Fig. 1B). However, cells could not form colonies in the presence of 50 μM IPTG, although they grew in liquid medium for a short period (which permitted us to measure DnaA levels under this condition). At 100 μM or greater concentrations of IPTG, cells could not grow at all in the liquid medium.
FIG. 1.
SOS induction caused by overproduction of the DnaA protein. (A) Schematic presentation of the relevant structure of a DnaA-overproducing strain (NIS2020). Open arrows indicate the location and direction of primers used at the final stage of its construction. From left to right are shown primers 5, 12, 14, 15, 13, and 8, respectively (Table 2). Briefly, yycA∼PrpmH-lacO and lacI-cat∼yyxA DNA fragments were amplified from the NIS2010 chromosome by long PCR with two sets of primers: primers 5 and 12 and 13 and 8, respectively. The dnaA gene was amplified by PCR with primers 14 and 15. After the three PCR products were purified with Centricon 100, they were combined and amplified by long PCR with two outer primers, 5 and 8, utilizing overlapping sequences between primers 12 and 14 and 15 and
To determine why cells could not grow at higher DnaA levels, we examined the morphology and nucleoid distribution of cells grown in the presence of 20 μM IPTG by fluorescence microscopy. Cells were elongated, and nucleoids were irregularly distributed within them (Fig. 1C). In some elongated cells, a small amount of chromosomal DNA was detected, suggesting defects in replication elongation. One possible explanation is that the SOS response was induced in these cells (46). To check this possibility, we measured expression from the promoter of a damage-inducible gene, dinC, by using bgaB, which encodes B. stearothermophilus β-galactosidase as a reporter. As expected, the activity was clearly induced in the presence of 20 μM IPTG (Fig. 1D), indicating that overproduction of DnaA caused the SOS response.
Transcription of the dnaA-dnaN operon is regulated by DnaA.
Because the promoter of the dnaA-dnaN operon is surrounded by DnaA boxes (32), we suspected that overproduction of DnaA might repress the expression of dnaA-dnaN and cause depletion of DnaN (β subunit of DNA polymerase III), which could also explain why replication elongation was inhibited by overproduction of DnaA. To test this possibility, transcription of the operon in the dnaA-null (NIS6311) and DnaA-overproducing (NIS2020) strains was examined by Northern hybridization. In the null mutant, initiation of chromosome replication was dependent on a plasmid (mini-pLS32) integrated into the chromosome (12). Moreover, because two ochre mutations were introduced at the 5th and 10th codons of dnaA, the length of the dnaA transcript would not be affected by these mutations.
When a dnaA probe was used, two transcripts of about 1.7 and 2.9 kb were detected in the wild-type strain (Fig. 2A, lane 1). The two transcripts appeared to cover dnaA alone and the dnaA-dnaN region, respectively (Fig. 2C-a) (assuming that they have the same start site of transcription as that determined earlier for dnaA) (38). When a dnaN probe was used, a 2.9-kb transcript covering the dnaA-dnaN region was mainly detected (Fig. 2B, lane 1). These results indicate that dnaA and dnaN constitute an operon and suggest that transcription from the dnaA promoter often terminates at the noncoding region between dnaA and dnaN. In fact, a ρ-independent terminator-like structure seems to be formed in this region with repeats of the DnaA box (32).
FIG. 2.
Analysis of transcripts from the dnaA-dnaN operon in the dnaA-null mutant and the DnaA-overproducing strains by Northern hybridization. (A) Transcripts detected by a dnaA probe. (B) Transcripts detected by a dnaN probe. Lanes: 1, wild-type strain (CRK6000); 2, dnaA-null mutant (NIS6311) (17); 3 to 6, DnaA-overproducing strain (NIS2020) cultivated with no IPTG or with 5, 10, and 20 μM IPTG, respectively. Arrows indicate positions of size markers. Asterisks indicate the major transcripts detected. Faint bands (1.6 kb) detected in all lanes in panel A are probably generated by cross-hybridization with 16S rRNA. (C) Schematic presentation of structures of the native dnaA-dnaN operon (a) and the additional dnaA region in the DnaA-overproducing strain (b) and length of transcripts predicted in these regions. Bent arrows indicate the positions of promoters and the direction of transcription. Thick bars represent probable regions of transcription. Probes used to detect transcripts are shown as gray boxes.
In the dnaA-null mutant, transcription from the dnaA promoter was enhanced (Fig. 2A and B, lanes 2). A 1.2-kb transcript covering dnaN alone was also detected in this sample (Fig. 2B, lane 2). Since the noncoding region between dnaA and dnaN did not show promoter activity, as examined by fusion with a reporter gene, lacZ (data not shown), the dnaN transcript was probably formed by processing of the transcript from the whole operon. Due to the enhanced transcription, the frequency of the processing may be increased. In a DnaA-overproducing strain, transcription of the dnaA-dnaN operon was reduced with increasing IPTG concentrations as detected by the dnaN probe (Fig. 2B, lanes 3 to 6). Similarly, transcription of dnaA alone was also reduced (Fig. 2A, lanes 3 to 6). However, the amounts of the 2.9-kb transcript detected by the dnaA probe increased with increasing IPTG concentrations. The dnaA probe hybridizes to transcripts produced from a copy of the dnaA gene that is integrated at purA (Fig. 2C-b), in addition to ones from the native dnaA-dnaN operon. They are expected to be about 2.8 or 3.8 kb long, and the transcription should be elevated by addition of IPTG, because the dnaA copy is under the control of an IPTG-inducible promoter (Fig. 2C-b). Thus, the increase in levels of the 2.9-kb transcript observed by addition of IPTG must be due to transcription of the dnaA-lacI region, and transcription from the dnaA-dnaN operon would be hidden by the enhanced transcription of dnaA-lacI due to the similar sizes of both transcripts. These data indicate that the dnaA-dnaN operon is autoregulated by DnaA.
The DnaA-overproducing strain recovers from its growth defects by trans-supply of DnaN from another locus on the chromosome.
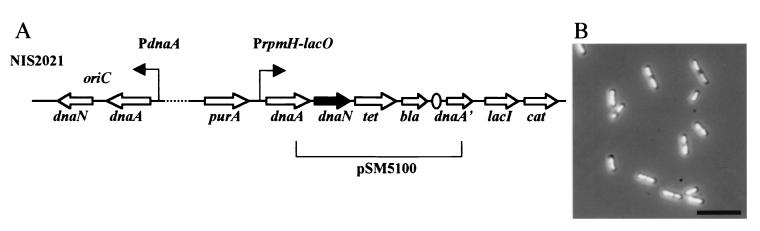
As expected, transcription of dnaN was largely reduced in the DnaA-overproducing strain in the presence of 20 μM IPTG (Fig. 2B, lane 6). This result suggests strongly that the growth defects observed in this strain in the presence of high concentrations of IPTG are due to a deficiency of DnaN. If this is the case, then the supply of DnaN from another locus on the chromosome could be expected to restore growth in the presence of high concentrations of IPTG. To check this possibility, a copy of the dnaN gene was added at the purA locus of the DnaA-overproducing strain (Fig. 3A).
FIG. 3.
4′,6′-Diamidino-2-phenylindole (DAPI)-stained image of NIS2021 cells overproducing DnaA and DnaN. (A) Schematic presentation of relevant structure of NIS2021. Plasmid pSM5100, described in Materials and Methods, was integrated into the NIS2020 chromosome by single crossover with the 3′-half of dnaA as a homologous region for the recombination. The tetracycline-resistant NIS2021 clones were selected in the presence of 50 μM IPTG, which inhibited the cell growth of NIS2020. Proper integration of the plasmid into this purA region, but not into the native dnaA-dnaN operon, was confirmed by PCR. A broken line means that two regions are apart on the chromosome. (B) NIS2021 cells stained with DAPI. Cells grown exponentially in the presence of 20 μM IPTG are shown. The scale bar represents 10 μm.
In contrast to the DnaA-overproducing strain, this newly constructed strain (DnaA-DnaN overproducing) grew even in the presence of 100 μM IPTG. Cell morphology and nucleoid distribution within cells were also normal in the presence of 20 μM IPTG (Fig. 3B), compared to the cell elongation and irregular distribution of nucleoids observed when DnaA alone was overproduced (Fig. 1C). These results indicate that depletion of DnaN is the cause of growth defects and probably of the SOS induction observed when DnaA is overproduced alone.
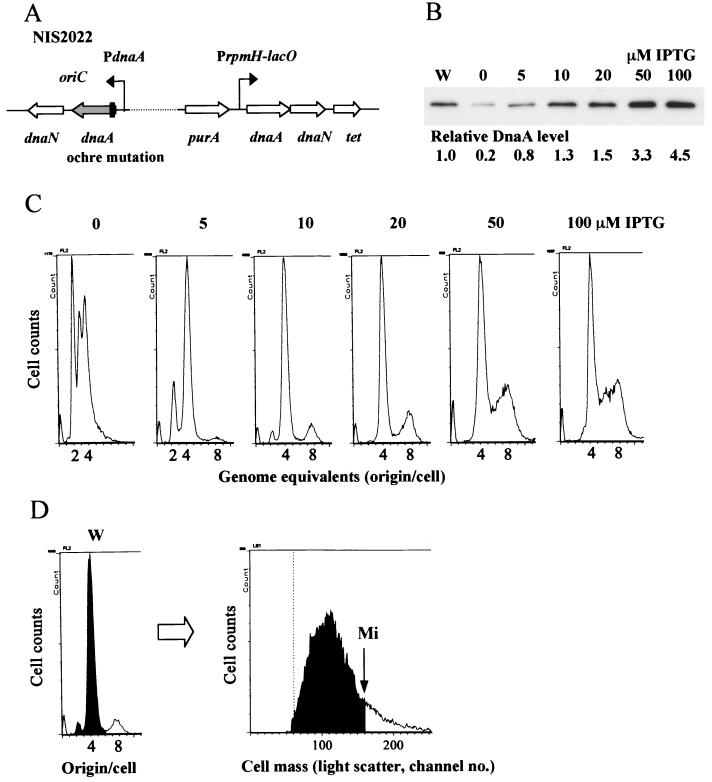
The DnaA protein level influences the initiation mass.
Since the problem of inhibition of cell growth by overproduction of DnaA was solved by adding a copy of dnaN at the purA locus, the effects of various DnaA levels on replication initiation were examined by using the DnaA-DnaN-overproducing strain NIS2021. First, a dnaA-null mutation was introduced into the native dnaA gene of the strain to confirm that the IPTG-inducible dnaA gene at purA is active for replication initiation (Fig. 4A). The NIS2022 cells grew even in the absence of IPTG, probably due to leakiness of the inducible rpmH-lacO promoter. In fact, immunoblotting detected DnaA, but it was reduced to about 20% of the wild-type level (Fig. 4B). The effects of the reduced level on replication initiation were analyzed by flow cytometry. Although B. subtilis cells generally tend to form chains, which can make flow cytometry difficult to interpret, our parent strain (CRK6000) does not form chains under the conditions used for this work. Therefore, flow cytometry was used to determine how many replication origins are present in individual cells (Fig. 4D). The results showed that such cells contained two, four, or eight replication origins under our cultivation conditions. However, most of the cells contained four origins, indicating that initiation occurred at a late stage of the cell division cycle (Fig. 4D). When NIS2022 cells, derivatives of CRK6000, were grown in the absence of IPTG, there were mainly three types of cells having two, three, or four origins detected by flow cytometry (Fig. 4C). Detection of an odd number of origins within the cell indicated that asynchronous initiation was occurring despite the fact that both the growth rate and distribution of the cell size were similar to those of the parent cells (Table 3). Due to the asynchrony of initiation, it was difficult to determine the initiation mass of these cells. Instead, the average mass/origin ratio (proportional to the initiation mass) was calculated from the average number of origins per cell determined by flow cytometry (Fig. 4C), and the average cell mass was also measured by flow cytometry (Fig. 4D and Table 3). As a result, it was increased by about 30% compared to that of wild-type cells (Table 3), suggesting that replication initiation is delayed in the cells containing a low level of DnaA (about 20% of the wild-type level). When 5 or 10 μM IPTG was added, the DnaA levels were almost comparable to wild-type levels (about 80 or 130%, respectively) (Fig. 4B), and DNA histograms obtained by flow cytometry showed patterns similar to those of the wild-type cells (Fig. 4C and D). Since asynchronous initiation was not apparently observed in these cases, the initiation mass (cell mass per replication origin at initiation) could be calculated as described in the legend to Fig. 4D. It was nearly identical in each case to that of the wild-type cells (Table 3). These results indicate not only that DnaA produced from the additional dnaA gene is active for replication initiation, but also that wild-type DnaA levels are required for initiation of replication at the proper cell mass.
FIG. 4.
Effects of overproduction of DnaA protein on initiation of chromosome replication. (A) Schematic presentation of relevant structure in a test strain (NIS2022). A thick vertical bar represents the two ochre mutations near the 5′ end of the native dnaA gene (a thick gray arrow) in NIS2022. (B) Relative DnaA protein levels in NIS2022 cells grown in various concentrations of IPTG as determined by immunoblotting. W, wild type (CRK6000). (C) Flow cytometry of cells grown in the presence of various concentrations of IPTG. DNA histograms of cells after incubation for 5 h in the presence of chloramphenicol are shown. (D) Concept for estimating initiation mass. The wild-type cell culture used in this study is shown as an example. (Left) DNA histogram of cells after a 5-h incubation with the drug. (Right) Light scatter histogram of log-phase cells (at the time of addition of the drug). As determined (42), the genome equivalents in the DNA histogram represent the number of replication origins present at the time of addition of the drug to individual cells. In this case, replication had not initiated in cells having four origins (black area in this figure), but had initiated in cells having eight origins (white area). The cell mass at initiation (Mi) is estimated by dividing the light scatter distribution into two portions (not initiated and initiated) according to the ratio of the number of the two types of cells (black/white). The initiation mass is obtained by dividing this mass by the number of origins present at the time of initiation (in this case, Mi/4).
TABLE 3.
Growth rates and relative values of average cell mass, initiation mass of replication, and mass/origin ratio in various strainsa
| Strain and IPTG concn added | Doubling time (min) | Avg cell mass (relative) | Initiation mass (relative) | Mass/origin ratio (relative) |
|---|---|---|---|---|
| CRK6000 (wild type) | 54 ± 2 | 1.00 | 1.00 | 1.00 |
| NIS2022 | ||||
| No IPTG | 58 ± 2 | 0.98 ± 0.04 | — | 1.32 |
| + 5 μM | 56 ± 2 | 0.94 ± 0.02 | 1.01 ± 0.01 | 1.07 |
| + 10 μM | 56 ± 1 | 1.01 ± 0.06 | 0.98 ± 0.05 | 1.01 |
| + 20 μM | 58 ± 4 | 1.06 ± 0.01 | 0.90 ± 0 | 0.94 |
| + 50 μM | 57 ± 1 | 1.07 ± 0.03 | — | 0.85 |
| + 100 μM | 56 ± 2 | 1.14 ± 0.05 | — | 0.89 |
The doubling time was estimated from cell turbidity at 600 nm. The light scatter distribution of each log-phase cell culture was measured by flow cytometry, and the mean value was divided by that of wild-type cell culture to obtain the relative value of average cell mass. The initiation mass in each culture was calculated as described in the legend to Fig. 4D, and the relative value to that of wild-type cells is shown. In each column, the average and standard deviation from at least two independent experiments are represented. — not calculated because the appearance of cells with three or six origins was not suitable for such calculation. Instead, the average mass/origin ratio was calculated for the samples as follows. The average number of origins per cell was determined from flow cytometry, as shown in Fig. 4C, and the number was then divided by the average cell mass (light scatter) of log-phase cells determined by flow cytometry as described above.
If the DnaA protein level acts as a rate-limiting factor of replication initiation, it could be expected that levels higher than those of the wild type promote initiation and result in earlier initiation in the cell division cycle. To test this possibility, the effects of high DnaA levels on replication initiation were examined by flow cytometry. When the DnaA level was increased by about 1.5 times in the presence of 20 μM IPTG, compared to the wild-type level (Fig. 4B), the ratio of cells having eight origins to those having four origins also increased slightly (Fig. 4C). Corresponding to the changes in DNA histograms, the initiation mass decreased by about 10% (Table 3). In the presence of 50 or 100 μM IPTG (about a 3.3- or 4.5-fold increase in the DnaA level, respectively, as shown in Fig. 4B), cells with between four and eight origins increased (Fig. 4C). These results presumably indicate that asynchronous initiation was occurring, but not inhibition of replication (by blocking progression of replication forks), because the ratio of origin to terminus on the chromosomes was nearly 1 in cells used for flow cytometry (after incubation for 5 h in the presence of chloramphenicol), as examined by Southern blotting (data not shown). Due to the limitation in resolution of the instrument, flow cytometry did not detect obvious peaks between four and eight origins. Since no peaks of cells having more than eight origins were detected (Fig. 4C), this asynchronous initiation was probably not due to excess initiation, but rather was attributable to the fact that earlier initiation occurred for a portion of the origins within the cells. In fact, the mass/origin ratio was decreased by 11 to 15% in these cells compared to that of the wild-type cells (Table 3). It did not decrease further in the presence of 100 μM IPTG compared to that with 50 μM IPTG, although there was a greater number of cells with more than four origins under the former condition (Fig. 4C). This is probably due to a slight elongation of cells grown in the presence of 100 μM IPTG (Table 3). At IPTG concentrations higher than 100 μM, the DnaA level did not increase further (data not shown), probably because expression from the rpmH-lacO promoter was fully saturated at these concentrations. These results suggest that DnaA protein levels may act as a rate-limiting factor for replication initiation in B. subtilis.
DISCUSSION
In this report, we have shown that dnaA and dnaN constitute an operon in B. subtilis and that it is autoregulated by DnaA. Autoregulation of dnaA is also observed in other bacteria. In E. coli, transcription of chromosomal dnaA was repressed by overproduction of DnaA and derepressed by inactivation of the protein in temperature-sensitive dnaA mutants (2, 5, 23). This repression was due to binding of the ATP-bound form of DnaA (not ADP-DnaA) to DnaA boxes in the promoter region (43). Recently, in Streptomyces, deletion of DnaA boxes in the promoter region of dnaA increased its transcription, suggesting autoregulation (19). In B. subtilis, there are eight DnaA boxes, including ones differing in 1 base from the consensus sequence, in the promoter region of dnaA. Binding of DnaA to these boxes was demonstrated by DNase I footprinting (9). Two consensus DnaA boxes facing each other and located 40 to 50 bp downstream of the transcription start site showed the highest binding affinity in the footprinting analysis. Deletion of the two boxes also abrogated the strong incompatibility phenotype that this promoter region exerts when cloned on multicopy plasmids, probably due to titration of DnaA (32). These results suggest that the two consensus DnaA boxes play a major role in autorepression of B. subtilis dnaA.
Although dnaA is autoregulated in both E. coli and B. subtilis, an additional regulation mechanism seems to overlap the promoter region of B. subtilis dnaA. In E. coli, addition of extra DnaA boxes derepresses transcription of dnaA by titrating DnaA (23). However, in B. subtilis, similar treatment did not derepress the dnaA-dnaN operon (36), indicating the existence of a more restrictive control in expression of the B. subtilis dnaA operon. In this context, it should be noted that transcription of the operon was inhibited when replication initiation was blocked by using dnaB mutants at the nonpermissive temperature and that the inhibition was released after initiation resumed (38). These results may suggest that transcription of the operon occurs only after replication initiation and then ceases by autoregulation with newly synthesized DnaA. Once repressed, it stays repressed by an unknown mechanism or mechanisms even in the presence of extra DnaA boxes, as described above, until the next round of replication initiation. This model of dnaA expression also suggests that the DnaA protein required for the next round of replication initiation would be available soon after the present round of initiation is completed. Consistent with this assumption, initiation occurred once even in the absence of protein synthesis during resumption of replication in the temperature-sensitive dnaB27 mutant by temperature downshift (37), despite the fact that transcription from the dnaA promoter was completely blocked at the nonpermissive temperature (38). Because transcription of dnaA is repressed after replication initiation by sequestration in E. coli (6), expression patterns of dnaA in the cell division cycle may be very different between B. subtilis and E. coli.
In E. coli, several models have been proposed concerning regulation of replication initiation. The initiator titration model (10) proposes that the initiator DnaA is titrated by binding to many DnaA boxes dispersed on the chromosome, and after their saturation, free DnaA molecules start to bind to a lower-affinity box within oriC to trigger initiation. In other words, the balance between the DnaA level and the number of DnaA boxes within the cell is the critical factor for timing of replication initiation (14). In fact, overproduction of DnaA leads to excess and earlier initiation of replication (3, 27), and addition of extra DnaA boxes delays initiation (7). Furthermore, deletion of a chromosomal site (datA) that titrates DnaA strongly (21) results in overinitiation (22). These observations support the initiator titration model. The second model is that replication initiation is controlled by changes in the balance between positive and negative factors (29). DnaA and SeqA act as positive and negative regulators, respectively (4). The authors postulate that all factors required for initiation are present at the origin at an early time, but SeqA negatively affects the initiation process at this stage. The third one is based on changes in DnaA activity. Only the ATP-bound form of DnaA is active for initiation, the levels of which increase sharply before replication initiation in the cell division cycle (26, 30), suggesting that the change in nucleotide forms of DnaA plays an important role in timing of replication initiation. Despite these various models, it remains uncertain which of the mechanisms proposed is the primary determinant for timing of replication initiation in E. coli. In B. subtilis, we examined the effects of the DnaA level on replication initiation and observed that cell mass per origin varied with its level. Specifically, the mass per origin increased by 30% at a low DnaA level (about 20% of the wild-type level) and decreased by 10 to 15% at about a three- to fivefold increase in DnaA levels. When intracellular DnaA levels were nearly comparable to the wild-type level, initiation occurred at the proper cell mass. Thus, our results suggest that the DnaA level could act as a positive factor for determining the timing of replication initiation in B. subtilis, if it increases with progression of the replication cycle as in E. coli (14). As described in the section presented above, however, transcription of dnaA seems to occur only after initiation of replication, and it could be autoregulated by newly synthesized DnaA. These events suggest that the DnaA level may reach a constant level at an early stage of the replication cycle in B. subtilis. Thus, it is necessary to examine changes in the DnaA level in the cell division cycle in detail before concluding that the DnaA level acts as the determinant of replication initiation in B. subtilis. Nevertheless, the initiator titration model proposed in E. coli appears unlikely to occur in B. subtilis, because addition of extra DnaA boxes did not derepress transcription of the dnaA operon as described above.
When asynchronous initiation of replication was induced with high DnaA levels, cells with only one origin or more than eight origins were rarely detected, similar to what was observed with wild-type DnaA levels (Fig. 4C). This observation is in significant contrast to that of E. coli dam mutants, in which asynchronous initiation was occurring and a significant fraction (∼15%) of cells contained one origin or a higher number of origins than were observed in wild-type cells (28). Presumably, this asynchrony of the mutants was due to repetitive initiations at the same origin and a loss of discrimination between new and old origins, because newly replicated origins were not sequestered (28). However, in B. subtilis (asynchronous initiation under high DnaA levels), discrimination between new and old origins appears to be maintained. Synchrony of initiation is tightly controlled, because cells with an odd number of origins are hardly detected in log-phase wild-type cells. However, when a synchronized culture prepared from the “baby cell machine” was analyzed, cells with three origins appeared even in wild-type cell cultures soon before the number of cells with four origins reached the peak (28). These results suggest that replication initiation occurs sequentially from one to the other origins present in the cell within a very short time period. Asynchronous initiation observed in cells containing low and high DnaA levels in this study may reflect the nature of sequential initiations, although the effects are magnified under these conditions.
ACKNOWLEDGMENTS
We thank M. Itaya and J. Johnson for providing pBEST307 and for assisting with Northern analysis, respectively. We also thank D. K. Chattoraj and W. Firshein for critical reading of the manuscript.
This work was supported by Grants-in-Aid for Scientific Research (B) (to S.M. and N.O.) from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Asai K, Baik S-H, Kasahara Y, Moriya S, Ogasawara N. Regulation of the transport system for C4-dicarboxylic acids in Bacillus subtilis. Microbiology. 2000;146:263–271. doi: 10.1099/00221287-146-2-263. [DOI] [PubMed] [Google Scholar]
- 2.Atlung T, Clausen E S, Hansen F G. Autoregulation of the dnaA gene of Escherichia coli K12. Mol Gen Genet. 1985;200:442–450. doi: 10.1007/BF00425729. [DOI] [PubMed] [Google Scholar]
- 3.Atlung T, Hansen F G. Three distinct chromosome replication states are induced by increasing concentrations of DnaA protein in Escherichia coli. J Bacteriol. 1993;175:6537–6545. doi: 10.1128/jb.175.20.6537-6545.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boye E, Stokke T, Kleckner N, Skarstad K. Coordinating DNA replication initiation with cell growth: differential roles for DnaA and SeqA proteins. Proc Natl Acad Sci USA. 1996;93:12206–12211. doi: 10.1073/pnas.93.22.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braun R E, O'Day K, Wright A. Autoregulation of the DNA replication gene dnaA in E. coli K-12. Cell. 1985;40:159–169. doi: 10.1016/0092-8674(85)90319-8. [DOI] [PubMed] [Google Scholar]
- 6.Campbell J L, Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990;62:967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- 7.Christensen B B, Atlung T, Hansen F G. DnaA boxes are important elements in setting the initiation mass of Escherichia coli. J Bacteriol. 1999;181:2683–2688. doi: 10.1128/jb.181.9.2683-2688.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donachie W D. Relationship between cell size and time of initiation of DNA replication. Nature. 1968;219:1077–1079. doi: 10.1038/2191077a0. [DOI] [PubMed] [Google Scholar]
- 9.Fukuoka T, Moriya S, Yoshikawa H, Ogasawara N. Purification and characterization of an initiation protein for chromosomal replication, DnaA, in Bacillus subtilis. J Biochem. 1990;107:732–739. doi: 10.1093/oxfordjournals.jbchem.a123117. [DOI] [PubMed] [Google Scholar]
- 10.Hansen F G, Christensen B B, Atlung T. The initiator titration model: computer simulation of chromosome and minichromosome control. Res Microbiol. 1991;142:161–167. doi: 10.1016/0923-2508(91)90025-6. [DOI] [PubMed] [Google Scholar]
- 11.Hansen F G, Atlung T. Initiation of chromosome replication after induction of DnaA protein synthesis in a dnaA(null) rnh mutant of Escherichia coli. Mol Microbiol. 1995;15:149–154. doi: 10.1111/j.1365-2958.1995.tb02229.x. [DOI] [PubMed] [Google Scholar]
- 12.Hassan A K M, Moriya S, Ogura T, Tanaka T, Kawamura F, Ogasawara N. Suppression of initiation defects of chromosome replication in Bacillus subtilis dnaA- and oriC-deleted mutants by integration of a plasmid replicon into the chromosomes. J Bacteriol. 1997;179:2494–2502. doi: 10.1128/jb.179.8.2494-2502.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henner D J. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 1990;185:223–228. doi: 10.1016/0076-6879(90)85022-g. [DOI] [PubMed] [Google Scholar]
- 14.Herrick J, Kohiyama M, Atlung T, Hansen F G. The initiation mess? Mol Microbiol. 1996;19:659–666. doi: 10.1046/j.1365-2958.1996.432956.x. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi R, Krummel B, Saiki R K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Igo M, Losick R. Regulation of a promoter that is utilized by minor forms of RNA polymerase holoenzyme in Bacillus subtilis. J Mol Biol. 1986;191:615–624. doi: 10.1016/0022-2836(86)90449-3. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, Ogasawara N, Ishigo-oka D, Kadoya R, Daito T, Moriya S. Subcellular localization of Dna-initiation proteins of Bacillus subtilis: evidence that chromosome replication begins at either edge of nucleoids. Mol Microbiol. 2000;36:1037–1048. doi: 10.1046/j.1365-2958.2000.01928.x. [DOI] [PubMed] [Google Scholar]
- 18.Itaya M. Construction of a novel tetracycline resistance gene cassette useful as a marker on the Bacillus subtilis chromosome. Biosci Biotechnol Biochem. 1992;56:685–686. doi: 10.1271/bbb.56.685. [DOI] [PubMed] [Google Scholar]
- 19.Jakimowicz D, Majka J, Lis B, Konopa G, Wegrzyn G, Messer W, Schrempf H, Zakrzewska-Czerwinska J. Structure and regulation of the dnaA promoter region in three Streptomyces species. Mol Gen Genet. 2000;262:1093–1102. doi: 10.1007/pl00008652. [DOI] [PubMed] [Google Scholar]
- 20.Katayama T, Kubota T, Kurokawa K, Crooke E, Sekimizu K. The initiator function of DnaA protein is negatively regulated by the sliding clamp of the E. coli chromosomal replicase. Cell. 1998;94:61–71. doi: 10.1016/s0092-8674(00)81222-2. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa R, Mitsuki H, Okazaki T, Ogawa T. A novel DnaA protein-binding site at 94.7 min on the Escherichia coli chromosome. Mol Microbiol. 1996;19:1137–1147. doi: 10.1046/j.1365-2958.1996.453983.x. [DOI] [PubMed] [Google Scholar]
- 22.Kitagawa R, Ozaki T, Moriya S, Ogawa T. Negative control of replication initiation by a novel chromosomal locus exhibiting exceptional affinity for Escherichia coli DnaA protein. Genes Dev. 1998;12:3032–3043. doi: 10.1101/gad.12.19.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kücherer C, Lother H, Kölling R, Schauzu M-A, Messer W. Regulation of transcription of the chromosomal dnaA gene of Escherichia coli. Mol Gen Genet. 1986;205:115–121. doi: 10.1007/BF02428040. [DOI] [PubMed] [Google Scholar]
- 24.Kunst F, Msadek T, Rapoport G. Signal transduction network controlling degradative enzyme synthesis and competence in Bacillus subtilis. In: Piggot P J, Moran C P Jr, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C.: American Society for Microbiology; 1994. pp. 1–20. [Google Scholar]
- 25.Kunst F, Ogasawara N, Moszer I, Albertini A M, Alloni G, Azevedo V, Bertero M G, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 26.Kurokawa K, Nishida S, Emoto A, Sekimizu K, Katayama T. Replication cycle-coordinated change of the adenine nucleotide-bound forms of DnaA protein in Escherichia coli. EMBO J. 1999;18:6642–6652. doi: 10.1093/emboj/18.23.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Løbner-Olesen A, Skarstad K, Hansen F G, von Meyenburg K, Boye E. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell. 1989;57:881–889. doi: 10.1016/0092-8674(89)90802-7. [DOI] [PubMed] [Google Scholar]
- 28.Løbner-Olesen A, Hansen F G, Rasmussen K V, Martin B, Kuempel P L. The initiation cascade for chromosome replication in wild-type and Dam methyltransferase deficient Escherichia coli cells. EMBO J. 1994;13:1856–1862. doi: 10.1002/j.1460-2075.1994.tb06454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu M, Campbell J L, Boye E, Kleckner N. SeqA: a negative modulator of replication initiation in E. coli. Cell. 1994;77:413–426. doi: 10.1016/0092-8674(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 30.Mahaffy J M, Zyskind J W. A model for the initiation of replication in Escherichia coli. J Theor Biol. 1989;140:453–477. doi: 10.1016/s0022-5193(89)80109-2. [DOI] [PubMed] [Google Scholar]
- 31.Messer W, Weigel C. Initiation of chromosome replication. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 2. Washington, D.C.: ASM Press; 1996. pp. 1579–1601. [Google Scholar]
- 32.Moriya S, Fukuoka T, Ogasawara N, Yoshikawa H. Regulation of initiation of the chromosomal replication by the DnaA-boxes in the origin region of the Bacillus subtilis chromosome. EMBO J. 1988;7:2911–2917. doi: 10.1002/j.1460-2075.1988.tb03149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moriya S, Kato K, Yoshikawa H, Ogasawara N. Isolation of a dnaA mutant of Bacillus subtilis defective in initiation of replication: amount of DnaA protein determines cells' initiation potential. EMBO J. 1990;9:2905–2910. doi: 10.1002/j.1460-2075.1990.tb07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moriya S, Atlung T, Hansen F G, Yoshikawa H, Ogasawara N. Cloning of an autonomously replicating sequence (ars) from the Bacillus subtilis chromosome. Mol Microbiol. 1992;6:309–315. doi: 10.1111/j.1365-2958.1992.tb01473.x. [DOI] [PubMed] [Google Scholar]
- 35.Moriya S, Hassan A K M, Kadoya R, Ogasawara N. Mechanism of anucleate cell production in the oriC-deleted mutants of Bacillus subtilis. DNA Res. 1997;4:115–126. doi: 10.1093/dnares/4.2.115. [DOI] [PubMed] [Google Scholar]
- 36.Moriya S, Imai Y, Hassan A K M, Ogasawara N. Regulation of initiation of Bacillus subtilis chromosome replication. Plasmid. 1999;41:17–29. doi: 10.1006/plas.1998.1381. [DOI] [PubMed] [Google Scholar]
- 37.Murakami S, Inuzuka N, Yamaguchi M, Yamaguchi K, Yoshikawa H. Initiation of DNA replication in Bacillus subtilis. III. Analysis of molecular events involved in the initiation using a temperature-sensitive dna mutant. J Mol Biol. 1976;108:683–704. doi: 10.1016/s0022-2836(76)80112-x. [DOI] [PubMed] [Google Scholar]
- 38.Ogasawara N, Moriya S, von Meyenburg K, Hansen F G, Yoshikawa H. Conservation of genes and their organization in the chromosomal replication origin of Bacillus subtilis and Escherichia coli. EMBO J. 1985;4:3345–3350. doi: 10.1002/j.1460-2075.1985.tb04087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roth A, Messer W. High-affinity binding sites for the initiator protein DnaA on the chromosome of Escherichia coli. Mol Microbiol. 1998;28:395–401. doi: 10.1046/j.1365-2958.1998.00813.x. [DOI] [PubMed] [Google Scholar]
- 40.Séror S J, Casarégola S, Vannier F, Zouari N, Dahl M, Boye E. A mutant cysteinyl-tRNA synthetase affecting timing of chromosomal replication initiation in B. subtilis and conferring resistance to a protein kinase C inhibitor. EMBO J. 1994;13:2472–2480. doi: 10.1002/j.1460-2075.1994.tb06532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharpe M E, Hauser P M, Sharpe R G, Errington J. Bacillus subtilis cell cycle as studied by fluorescence microscopy: constancy of cell length at initiation of DNA replication and evidence for active nucleoid partitioning. J Bacteriol. 1998;180:547–555. doi: 10.1128/jb.180.3.547-555.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skarstad K, Bernander R, Wold S, Steen H B, Boye E. Cell cycle analysis of microorganisms. In: Al-Rubeai M, Emery A N, editors. Flow cytometry applications in cell culture. New York, N.Y: Marcel Dekker, Inc; 1996. pp. 241–255. [Google Scholar]
- 43.Speck C, Weigel C, Messer W. ATP- and ADP-DnaA protein, a molecular switch in gene regulation. EMBO J. 1999;18:6169–6176. doi: 10.1093/emboj/18.21.6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steinmetz M, Richter R. Plasmids designed to alter the antibiotic resistance expressed by insertion mutations in Bacillus subtilis, through in vivo recombination. Gene. 1994;142:79–83. doi: 10.1016/0378-1119(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 45.Wold S, Skarstad K, Steen H B, Strokke T, Boye E. The initiation mass for DNA replication in Escherichia coli K-12 is dependent on growth rate. EMBO J. 1994;13:2097–2102. doi: 10.1002/j.1460-2075.1994.tb06485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yasbin R E, Cheo D, Bol D. DNA repair systems. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 529–537. [Google Scholar]
- 47.Yoshikawa H, Ogasawara N. Structure and function of DnaA and the DnaA box in eubacteria: evolutionary relationships of bacterial replication origins. Mol Microbiol. 1991;5:2589–2597. doi: 10.1111/j.1365-2958.1991.tb01967.x. [DOI] [PubMed] [Google Scholar]
- 48.Youngman P. Use of transposons and integrational vectors for mutagenesis and construction of gene fusions in Bacillus species. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1990. pp. 221–266. [Google Scholar]