Abstract
Introduction:
This study examines the correlation, and clinical meaningfulness, between reachable workspace outcome and reported activities of daily living (ADL) function of individuals with facioscapulohumeral dystrophy (FSHD).
Methods:
Twenty-one FSHD subjects with various disease severity (Clinical Severity Scores 1-4) underwent reachable workspace evaluation and completed the Neurological Disorders Quality of Life (NeuroQoL) upper extremity questionnaire. Spearman and receiver operator curve analyses were performed.
Results:
Moderate correlation was found between NeuroQoL scores and total (ρ = 0.7609; p < 0.01), and upper-quadrants RSAs (ρ = 0.6969; p < 0.01). Five specific items (i.e. shirt on, shirt off, use spoon, pull on pants, pick-up clothes) demonstrated even higher correlations with total (ρ = 0.8397; p < 0.01) and above shoulder RSAs (ρ = 0.8082; p < 0.01). A total RSA cuff-off value of 0.70 would achieve 100% sensitivity and 94% specificity (AUC = 0.975).
Discussion:
Reachable workspace values identify when individuals have difficulties performing ADLs at home. This information improves patient monitoring, and clinical decision making by enabling more timely recommendations for medications, assistive devices or considerations for clinical trial enrollments.
Keywords: Muscular dystrophy, outcome assessment, activities of daily living, correlation, physical function
INTRODUCTION
Facioscapulohumeral dystrophy (FSHD) is characterized by early signs of weakness in facial and shoulder girdle muscles1,2. Although the pattern and severity of weakness are highly variable between individuals, impairments in upper extremity function are seen in most. These upper extremity (UE) limitations eventually produce a gradual decline in reachability that ultimately results in impaired Activities of Daily Living (ADLs). Despite importance of UE assessment, very few outcomes that measure UE limitations exist. Recently, a 3D motion sensor-based outcome assessment was developed by our group that can track UE skeletal motion and reconstruct an individual’s reachable workspace3. The reachable workspace assessment tracks arm motion through standardized movements (that cover range of motion in various directions) using a depth sensor and, with the help of a computer software program, is able to reconstruct a hemispherical area of reachable space for each arm. Preliminary studies thus far in FSHD patients indicate that the reachable workspace measure (relative surface area, RSA) has excellent reliability, internal validity, and sensitivity in tracking changes longitudinally over the course of several years4,5.
Here, we extend our work and investigate the clinical meaningfulness of the reachable workspace outcome in FSHD population (i.e. provide meaning to numeric values beyond simply numeric values or designation of normal vs. abnormal). We will accomplish this using the World Health Organization’s International Classification of Functioning, Disability, and Health (ICF) conceptual model6, which posits that the meaningfulness/interpretability of performance-based outcomes, such as the reachable workspace, can be determined by its association with one’s participation at home (i.e. self-reported activities of daily living; ADL). We investigate the association of our reachable workspace outcome to participation in order to contextualize the ‘true meaning’ of our outcome to an individual’s day-to-day life, and perform receiver operator characteristics (ROC) analyses (sensitivity and specificity) to determine important cutoff values.
The study investigated how both the total and upper quadrant RSA values correlates with an individual’s self-reported upper extremity ADL function.
MATERIALS AND METHODS
Study Participants
A total of 21 subjects with FSHD of varying severity were recruited from August 2018 to March 2019 for this prospective cross-sectional study. Inclusion criteria consisted of genetic confirmation of FSHD1 and clinical severity scores (CSS) within 1.0-4.0. Subjects fitting inclusion criteria but unable to follow study protocols, or those with significant comorbidities precluding participation in the study, were excluded. Demographic and anthropometric information (age, gender, height, and weight) were collected from each subject, in addition to the clinical measures and patient-reported questionnaires listed below. The study protocol was approved by the University Institutional Review Board (IRB) for human protection and privacy for research, and informed consent was obtained from all study subjects prior to study participation.
Clinical Evaluation Scales
The CSS7 and the FSHD evaluation8 scales were collected on all patients. Both scales were performed according to standard published protocols and by an experienced physical therapist (author VC). For this study, we recruited patients using CSS scores between the range of 1.0 – 4.0 (in 0.5 demarcations as published). Scores were determined by the following criteria: (1.0) mild scapular involvement without limitation of arm abduction; (1.5) moderate involvement of scapular and arm muscles or both (arm abduction > 60° and manual muscle strength > 3 in arm muscles), no involvement of pelvic and leg muscles; (2.0) severe scapular involvement (arm abduction < 60° on at least one side); strength <3 in at least one muscle district of the arms; no involvement of the pelvic and leg muscles; (2.5) tibioperoneal weakness; no weakness of pelvic and proximal leg muscles; (3.0) mild weakness of pelvic and proximal leg muscles or both (strength ≥ 4 in all these muscles); able to stand up from wheelchair without support; (3.5) mild weakness of pelvic and proximal leg muscles or both (strength ≥ 4 in all these muscles); able to stand up from a chair with unilateral support; and (4.0) severe weakness of pelvic and proximal leg muscles or both (strength <3 in at least one of these muscles); able to stand from a chair with double support; able to walk unaided. The FSHD evaluation scale ranges from 0 to 15, representing scores from 6 items measuring strength and functionality of 6 muscle groups: (I) facial; (II) scapular girdle (III) upper limb; (IV) leg; (V) pelvic girdle; and (VI) abdominal. Both total FSHD evaluation scores and summated sub-scores (II & III) for upper extremity function were determined as previously published8.
Upper Extremity Reachable Workspace protocol and analysis
The 3D reachable workspace protocol followed previously published literature3–5. Briefly, the subjects were seated in front of the Kinect2 sensor system (Microsoft corp., Redmond, WA, USA) and data was recorded while following video-instructions of the movement protocol under the supervision of a study clinical evaluator (author VC). After completion of the protocol, data from the movement protocol is automatically displayed and expressed via 4 different quadrants (Q1-4) for each arm, with the shoulder joint serving as the origin. The four quadrants represent 4 areas: (Q1)upper medial; (Q2) lower medial; (Q3) upper lateral; and (Q4) lower lateral5. Total reachable workspace (m2) encompassing all quadrants is also calculated. In order to allow comparisons between individuals, data are automatically normalized to each subject’s arm length (per previously published protocol) and relative surface areas (RSAs) are produced. For this study, averaged RSAs (between arms) for each individual were used for analyses. This serves as an appropriate comparison to the NeuroQoL, which is a self-report of overall upper extremity difficulty (and not separated by arm).
Patient Reported Outcome (PRO)
For this study participation at home was determined using the Quality of Life in Neurological Disorders (NeuroQoL)9, a self-reported outcome measure developed by the NIH. There are a total of 13 item banks (in the NeuroQoL) that can be used comprehensively, or individually, used depending on the research question. We utilized the 20-item bank of questions from the “Upper extremity, Fine motor skills, Activities of daily living (ADL)” domain of the NeuroQoL9 to examine the clinical meaning of the reachable workspace measure (relative surface area, RSA) in FSHD patients. The NeuroQOL is a validated research tool that allows clinical researchers to compare the impact from loss of function across various neurological conditions and offers a common metric to express burdens of disease and benefits of treatment. Each question in the item bank addresses a specific task, and respondents are asked to rate, using a 5-point Likert scale, the level of difficulty they have performing tasks. The 5-point Likert scale categories are: 5 = without any difficulty, 4= with a little difficulty, 3= with some difficulty, 2= with much difficulty, and 1=unable to do. Total NeuroQoL scores are a summation of all answers from the 20 questions. A specific question may pertain more to either proximal upper extremity and shoulder activities (i.e. brush teeth, open/close zipper, wash/dry body, shampoo hair, hold plate of food, pull on pants, donning/doffing shirt, picking up clothes and using a spoon), or distal fine motor activities (turn key in lock, pick up coins, write with pen/pencil, open jars, trim finger/toenails, dialing on cell phone, opening medication jars, and removing wrappers from small objects). Sub-NeuroQoL scores refer to a summation of only 5 NeuroQoL questions: use of spoon, picking up clothes, pulling up pants, putting on shirt and taking off shirt. For Box-Plot figures two groups were formed; those marked ‘Independent’ (individuals answering 5’s on all Sub-NeuroQoL questions) vs. ‘Dependent’ (all others not marked as independent).
Statistical Analyses
All statistical analyses were performed using Stata 14.2 software (StataCorp, College Station, TX). Data was checked for normality using Shapiro-Wilk and equality of variances using Levene’s. Analysis of variance (ANOVA) with a Scheffe’s test was used to determine differences in reachable workspace across multiple CSS groups. For this analysis, CSS scores were bucketed into whole numbers (i.e. 1.0 & 1.5 into bucket score 1). Spearman’s correlations were used to determine associations between the reachable workspace (continuous variable between 0 and 1.5) and individual item scores (categorical variable) and total NeuroQoL scores (discrete variable). Receiver operator characteristics (ROC) analyses (sensitivity and specificity) were used to determine the reachable workspace criterion cutoff values that would separate FSHD individuals with ‘independent’ status in ADLs (indicated by 5’s for all questions) from those that are ‘dependent’ (those with all other scores). For ROC curve analyses, NeuroQoL was used as the reference standard for identifying ‘independence’ against either the total RSA or above shoulder RSA reachable workspace (the testing measure(s)). ROC analyses were performed in Stata, using ROC commands with pvc (empirical) and nonparametric parameters. For all statistical analyses, a p-value <0.05 was accepted as the minimum level of statistical significance (p ≤ 0.01 is separately noted when detected during analysis).
RESULTS
Cohort Characteristics
A total of 21 FSHD subjects completed the study. Cohort demographics and body characteristics are summarized in Table 1. Average FSHD total scores for this cohort were 5.8 (range 1-11; Table 1), with an average FSHD upper extremity sub-score of 2.9 (range 1-4; Table 1). Six individuals with CSS 1.0 - 1.5; 4 individuals with CSS 2.0 - 2.5; 6 individuals with CSS 3.0 - 3.5; and 6 individuals with CSS of 4.0 were included for all analyses below.
Table 1:
Cohort characteristics
| Total % (n) |
Average (if applicable) |
Range (if applicable) |
|
|---|---|---|---|
| Demographics | |||
| Male | 57.14 (12) | - | - |
| Age (in years) | 41.6 | 19 – 55 | |
| < 25 | 9.52 (2) | - | - |
| 26 – 40 | 38.10 (8) | - | - |
| 41 – 50 | 19.05 (4) | - | - |
| 50 + | 33.33 (7) | - | - |
| Race | |||
| White | 76.19 (16) | - | - |
| Asian | 14.29 (3) | - | - |
| Hispanic | 9.52 (2) | - | - |
| Disease Characteristics | |||
| CSS | - | 2.7 | 1- 4 |
| FSHD UE subscore | - | 2.9 | 1- 4 |
| FSHD total score | - | 5.8 | 1 - 11 |
| Body Characteristics | |||
| Ht (cm) | - | 1.73 | 1.57 – 1.87 |
| Wt (kg) | - | 79.34 | 56.50 – 106.60 |
| BMI (kg/cm2) | - | 26.50 | 17.92 – 33.27 |
CSS = Clinical Severity Score Ht = Height Wt = Weight BMI = body mass index UE = Upper Extremity
Reachable Workspace Analysis by Clinical Severity Score
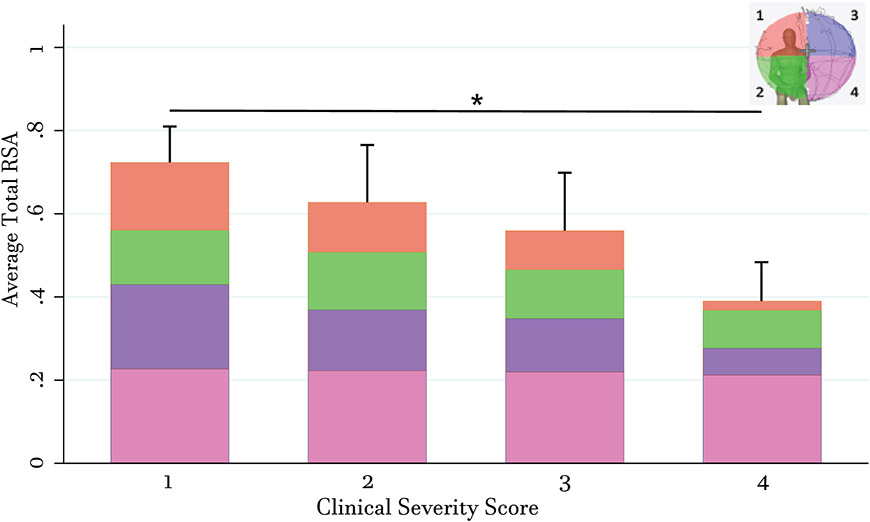
First, the association of mean RSA with levels of impairment using clinical severity scores (CSS) were examined (Figure 1). Mean RSA in the upper quadrants Q1 and Q3 gradually decreased as impairment increased (Table 2). ANOVA analysis revealed a significance between CSS group 1 and 4 (Table 2). Mean RSA for lower quadrant Q2 was consistent between groups CSS 1 and 2 but slightly lower in groups CSS 3 and 4. Mean RSA for lower quadrant Q4 was consistent between all CSS groups. Total RSA gradually decreased as impairment increased. ANOVA analysis again revealed a significant difference in mean total RSA and impairment between CSS 1 and 4 (Table 2).
Figure 1: Reductions in Total and Quadrant Reachable Workspace by Clinical Severity Scores.
Bar graph showing average total and quadrant (Q1, orange; Q2, green; Q3, purple; Q4, pink; shown in representative mannequin in upper right corner) RSAs in FSHD patients, stratified by clinical severity scores upon study entry. Total bar height in each CSS group represents average total RSA (from addition of all 4 quadrants) within that CSS group. Standard errors represent standard deviation of total RSAs per group. Asterisk denotes significance of p < 0.05 between CSS group 1 and CSS group 4.
Table 2:
Mean relative surface areas (RSAs) from reachable workspace surface envelop by clinical severity
| CSS scores (n) | Q1 | Q2 | Q3 | Q4 | Total |
|---|---|---|---|---|---|
| CSS 1.0 – 1.5 (6) | 0.163** ± 0.040 | 0.120 ± 0.018 | 0.203* ± 0.036 | 0.227 ± 0.003 | 0.724**± 0.078 |
| CSS 2.0 – 2.5 (4) | 0.120 ± 0.070 | 0.139 ± 0.007 | 0.146 ± 0.088 | 0.223 ± 0.008 | 0.628 ± 0.149 |
| CSS 3.0 – 3.5 (6) | 0.093 ± 0.080 | 0.118 ± 0.041 | 0.129 ± 0.059 | 0.219 ± 0.004 | 0.559 ± 0.149 |
| CSS 4.0 (6) | 0.022 ± 0.022 | 0.091 ± 0.038 | 0.065 ± 0.062 | 0.212 ± 0.014 | 0.390 ± 0.104 |
Average RSA’s by quadrants and stratified by CSS groups for FSHD cohort. All statistical analyses were performed using ANOVA with Scheffe’s.
Denotes p < 0.05 for CSS group 1.0/1.5 to CSS group 4.0
Denotes p< 0.01 for CSS group 1.0/1.5 to CSS group 4.0 CSS = clinical severity score
Clinical Meaningfulness of Reachable Workspace and Patient-Reported ADLs.
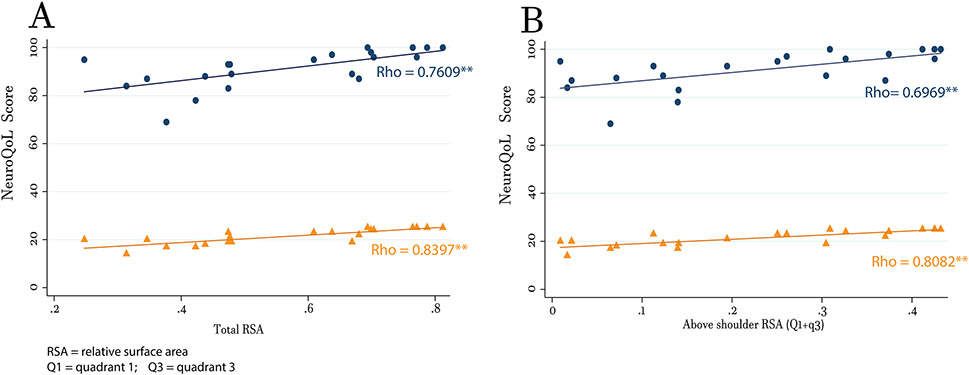
There was a strong association (Figure 2A & Table 4) between total NeuroQoL scores and total RSA, and above shoulder RSAs (Figure 2B & Table 4). To investigate if correlations were higher for proximal and shoulder items only, a scatterplot matrix with total RSA and above shoulder RSA (Q1 & Q3) against each of the 20 NeuroQoL questions were run (data not shown). Visual inspection identified 9 individual questions (Supplemental Table 1) that showed potential for association. Spearman’s correlations against total and above shoulder RSAs identified 5 proximal & shoulder related items with at least a moderate spearman’s coefficient (ρ ≥ 0.60) for both. Spearman correlation analyses using these grouped items (called sub-NeuroQoL) displayed higher coefficients to both total RSA (Figure 2A & Table 3) and above shoulder RSA (Figure 2B & Table 3).
Figure 2: Correlation Between Reachable Workspace and ADLs.
Scatter plots with representative correlation lines to ADLs for (A) total RSA and (B) above shoulder RSAs (Q1 + Q3). For both plots, the blue lines and dots represent summed scores from all 20 NeuroQoL questions and the orange lines and dots represent summed scores from Sub-NeuroQoL questions (5 proximal & shoulder related items). Double asterisks next to representative rho values indicate a p < 0.01.
Table 3:
Spearman Correlation Coefficients for Reachable Workspace to NeuroQoL
| Total RSA | p- value | Above shoulder RSA | p- value | |
|---|---|---|---|---|
| NeuroQoL raw | 0.7609 | 0.0001** | 0.6969 | 0.0004** |
| Sub-NeuroQoL | 0.8397 | <0.000** | 0.8082 | < 0.000** |
denotes significant for Spearman’s correlation where p < 0.01. RSA = relative surface area
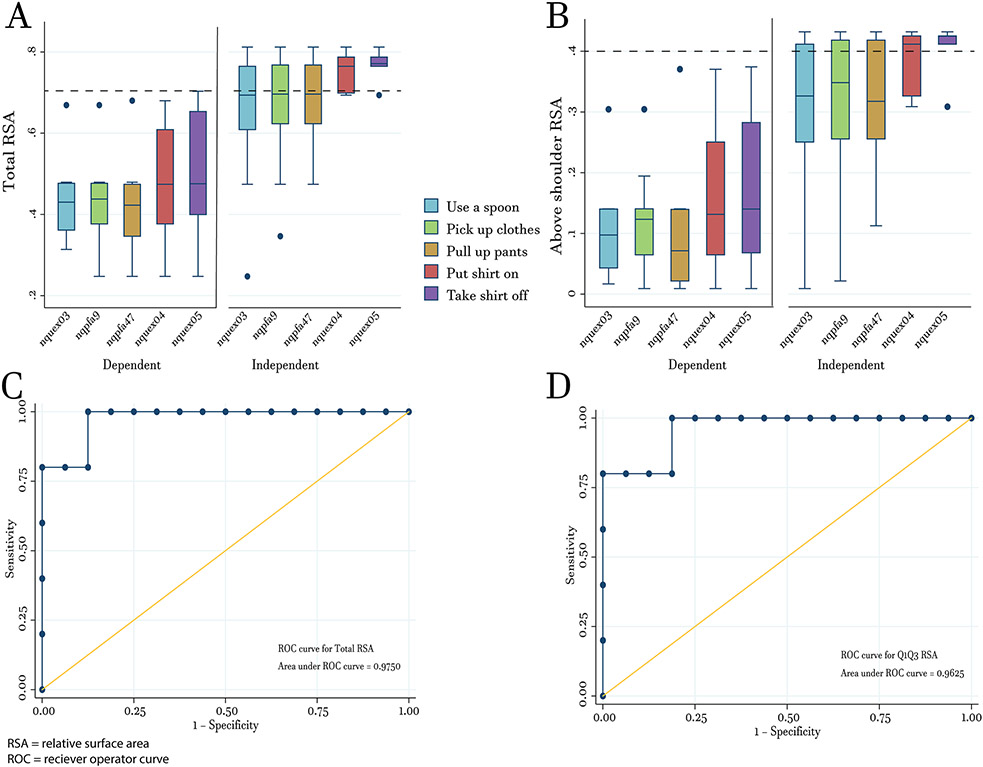
Box-and-Whisker plots show that majority of FSHD individuals with lower total (Figure 3A) and above shoulder RSA (Figure 3B) values are more likely to be dependent in all 5 proximal & shoulder NeuroQoL questions. In contrast, those with higher total and above shoulder RSA values are more likely to be independent in all 5 proximal & shoulder NeuroQoL questions.
Figure 3: Relationship Between Reachable Workspace and ADLs.
Box-and-whisker plots showing the distribution of total RSA (A) and above shoulder RSA (B) in 5 NeuroQoL questions related to proximal & shoulder items separating those that are fully independent (no difficulty) and those that are not (unable/much/some/little difficulty). (C) ROC curve for determining cutoff values for total RSA (cutoff = 0.70) for individuals who are independent (no difficulty) in performing the 5 proximal & shoulder items. (D) Above shoulder RSA (cutoff = 0.40) for individuals who are independent (no difficulty) in performing the 5 proximal & shoulder items. Dashed lines in (A) and (B) represent cutoffs for total and above shoulder RSA values, respectively. Cutoffs were used to determine reported sensitivity/specificity and positive predictive value/negative predictive values.
ROC analysis using Sub-NeuroQoL scores revealed a cuffoff value of 0.70 (Figure 3A) for total RSA to achieve 100% sensitivity and 94% specificity (AUC = 0.975; Figure 3C). If the cutoff value for total RSA was lowered to 0.65, this would still achieve 100% sensitivity but drop specificity to 75%. For above shoulder RSA, ROC analysis revealed an optimal cutoff value of 0.40 (Figure 3B) for above shoulder RSA to achieve 80% sensitivity and 100% specificity (AUC = 0.963; Figure 3D). If the cutoff is lowered to 0.35, 80% sensitivity is retained but specificity would drop to 88%. Clinical decisions concerning a patient are often made based on positive predictive values (PPV) and negative predictive values (NPV) instead of sensitivity and specificity. Using the same data from above, a total RSA cutoff value of 0.70 would produce a PPV of 83% (for those >0.70) and NPV of 100% (for those <0.70). In another example, if the total RSA cutoff was changed to 0.65, this would give PPV and NPV’s of 55% and 100%, respectively. A cutoff value of 0.40 for above shoulder RSA would give PPV and NPV’s of 100% and 94%, respectively. If the cutoff were changed to 0.35, PPV and NPV’s would change to 67% and 93%, respectively.
DISCUSSION
The results presented here show that the reachable workspace is able to predict self-reported, UE activities of daily living using the NeuroQoL; with higher total RSA values indicating greater independence in UE tasks and values above 0.70 marking those that are completely independent. These results are important for a few reasons. Firstly, the majority of our published work to date on the reachable workspace in FSHD has focused on its robustness in terms of reliability, validity and sensitivity over time4,5,10. The current study adds important information regarding the reachable workspace, outside of its psychometric properties. Namely, that the reachable workspace outcome correlates with patient-reported UE activities.
Secondly, our study shows how standard ROC methodology coupled with the WHO’s ICF framework, for characterizing and defining outcome measures, can be utilized for FSHD outcome measures. Several studies have investigated the relationship between performance-based measures and ADLs in various disease populations including multiple sclerosis11, Parkinson’s disease12, and traumatic brain injury13. However, none to date exist in the FSHD field. This increases the novelty of our study, but also makes comparisons difficult. Instead, there are numerous studies comparing the relationship of performance-based measures to ADL disability in elderly individuals that we can use for comparison. For example, gait measures like the Timed-Up-and-Go Test (TUG) have been shown to accurately estimate independence in ADLs14 in elderly populations. Importantly, a recent study by Huang et.al15 was able to add to these studies by determining actual cutoff values for gait including the TUG tests that predict onset of ADL difficulty in elderly individuals using ROC analyses. Indeed, determined cutoff values for all three of these performance-based outcome measures displayed strong predictive abilities with area under the curves ranging from 0.76 to 0.80 while maintaining sensitivity and specificity at 90 percent. These studies show that performance-based outcome measures are capable of predicting ADLs with high levels of sensitivity and specificity similar to our results.
Lastly, and most importantly, our study results show that the reachable workspace outcome provides important, clinically meaningful, information that can help guide clinical decision making. Very few UE functional outcomes exist for the FSHD population that are readily utilized in the clinical setting. Physiological-based assessments, like manual muscle testing, have been the most common functional outcomes to date. These assessments are extremely useful for identifying various proximal and distal muscles involved in UE weakness, and for tracking changes in muscle strength over time16–20. However, the values themselves are not intuitive when assessing one’s ability or perform various ADLs. Therefore, to get a full picture of an individuals’ UE ability, clinicians would need to perform manual muscle testing on a variety of UE muscles, and then also administer a self-reported measure of activity and function. This is often difficult, given the limited amount of time provided in most clinical appointments. We believe this is the added value that the reachable workspace outcome can provide. It characterizes the extent, not just the presence, of UE limitations by tracking how the individual executes common UE motions. This enables two important pieces of clinical information gained from one representative, reachable workspace, outcome. The lower the reachable workspace RSA value, the more dependent the FSHD individual, thus indicating to clinicians when individuals have trouble donning and doffing clothes, eating and/or bathing. It may also indicate when possible therapies or requisition of adaptive equipment may be needed. In addition, the reachable workspace utilizes technology that improves the speed and amount of information gained in one measure. As technology and medical care advance, it is expected that neuromuscular practice, like many other fields in medicine, will see an influx of novel technology- and sensor-based clinical tools that will assist in improved detection and quantitative tracking of patient’s physical function.
Cutoff values for total and above shoulder RSAs can be utilized in clinical settings in a few ways. During interventional evaluation/planning, FSHD individuals with total RSA scores greater than 0.70 (or above shoulder RSA scores greater than 0.40) are in diminished need of interventional strategies because they are less likely to be experiencing significant upper extremity related ADL difficulties. Likewise, these individuals are far less likely to be in need of assistive devices or alterations at home. During routine clinical assessment, these cutoffs may mark the onset of upper extremity difficulties in ADLs at home, and possibly the first phase of significant disease progression. More regular assessments and care may be warranted for FSHD individuals as they approach these RSA cutoff values. It may also mark the first phase when clinicians begin investigation for medications, adaptive equipment orders or other therapeutic evaluation(s). Alternatively, these total and above shoulder RSA cutoffs could also be taken into account appropriately for clinical trial planning purposes to enrich likelihood of enrolling subjects with existing upper extremity impairment.
Limitations to this study include the relatively small sample size, which may impact statistical analyses. The fact that our results are similar to ROC analyses/results from other performance-based outcome measures increases our confidence in our cutoff values. We believe positive and negative predictive values presented will remain fairly consistent if performed on larger cohorts (even if the area under the curve, sensitivity, and specificity are slightly changed). That being said, larger numbers would solidify confidence in the conclusions of the work presented, as well as more granular subgroup analyses. Limitations in local recruitment made it difficult to enroll larger numbers of subjects across the intended spectrum of CSS scores. It also limited our ability to explore additional cutoffs related to differing levels of ADL difficulties. Future studies focused on tracking these data longitudinally in a larger population would be extremely valuable, and enable the determination of minimal clinically important differences.
In summary, results from this study indicate that the reachable workspace demonstrates excellent correlations with patient’s self-reported upper extremity ADL functions in FSHD, making it more useful than physiological-based measures. Results also revealed a total RSA cutoff value of 0.70 identifying individuals with FSHD that have essentially no difficulty completing upper extremity ADL tasks, from those that have at least a little difficulty. This may aid in clinical decision making, enabling more informed recommendations for assistive devices or adaptations at home, or when prescribing interventions or physical therapy. It may also serve as an important clinical indicator when tracking disease progression; marking a point when FSHD individuals’ independent daily activities becomes initially altered.
Supplementary Material
Acronyms/Abbreviations
- ADL
activities of daily living
- CSS
Clinical Severity Score
- FSHD
facioscapulohumeral muscular dystrophy
- NeuroQoL
Neurological Disorders Quality of Life
- NPV
negative predictive value
- PPV
positive predictive value
- Q1
Quadrant 1
- Q2
Quadrant 2
- Q3
Quadrant 3
- Q4
Quadrant 4
- RSA
relative surface area
- ROC
receiver operator curve
- UE
Upper Extremity
- WHO
world health organization
Footnotes
Ethical Publication Statement: We, the authors, confirm that we have read the Journal’s position on issues involved with ethical publication and affirm that this report is consistent with those guidelines.
Disclosure of Conflict: JJH is a consultant for Bioniks and Sanofi/Genzyme. MH, GK, and VC are consultants for Bioniks.
REFERENCES:
- 1.Bergsma A, Cup EHC, Geurts ACH & de Groot IJM Upper extremity function and activity in facioscapulohumeral dystrophy and limb-girdle muscular dystrophies: a systematic review. Disabil Rehabil 37, 1017–1032 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Stübgen J-P & Stipp A Facioscapulohumeral muscular dystrophy: a prospective study of weakness and functional impairment. J. Neurol 257, 1457–1464 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Kurillo G, Chen A, Bajcsy R & Han JJ Evaluation of upper extremity reachable workspace using Kinect camera. Technol Health Care 21, 641–656 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Hatch MN et al. Longitudinal study of upper extremity reachable workspace in fascioscapulohumeral muscular dystrophy. Neuromuscul. Disord 29, 503–513 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han JJ et al. Reachable workspace in facioscapulohumeral muscular dystrophy (FSHD) by Kinect. Muscle Nerve 51, 168–175 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. International Classification of Functioning, Disability and Health (ICF). Geneva: World Health Organization Group; (2011). [Google Scholar]
- 7.Ricci E et al. Progress in the molecular diagnosis of facioscapulohumeral muscular dystrophy and correlation between the number of KpnI repeats at the 4q35 locus and clinical phenotype. Ann. Neurol 45, 751–757 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Lamperti C et al. A standardized clinical evaluation of patients affected by facioscapulohumeral muscular dystrophy: The FSHD clinical score. Muscle Nerve 42, 213–217 (2010). [DOI] [PubMed] [Google Scholar]
- 9.Cella D et al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology 78, 1860–1867 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han JJ, Kurillo G, Abresch RT, Nicorici A & Bajcsy R Validity, Reliability, and Sensitivity of a 3D Vision Sensor-based Upper Extremity Reachable Workspace Evaluation in Neuromuscular Diseases. PLoS Curr 5, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paltamaa J, Sarasoja T, Leskinen E, Wikström J & Mälkiä E Measures of physical functioning predict self-reported performance in self-care, mobility, and domestic life in ambulatory persons with multiple sclerosis. Arch Phys Med Rehabil 88, 1649–1657 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Tanji H et al. A comparative study of physical performance measures in Parkinson’s disease. Mov. Disord 23, 1897–1905 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Feld JA, Rabadi MH, Blau AD & Jordan BD Berg balance scale and outcome measures in acquired brain injury. Neurorehabil Neural Repair 15, 239–244 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Podsiadlo D & Richardson S The timed ‘Up & Go’: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 39, 142–148 (1991). [DOI] [PubMed] [Google Scholar]
- 15.Wennie Huang W-N, Perera S, VanSwearingen J & Studenski S Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J Am Geriatr Soc 58, 844–852 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.FSHD-DY. A prospective, quantitative study of the natural history of facioscapulohumeral muscular dystrophy (FSHD): implications for therapeutic trials. The FSH-DY Group. Neurology 48, 38–46 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Kilmer DD et al. Profiles of neuromuscular diseases. Facioscapulohumeral muscular dystrophy. Am J Phys Med Rehabil 74, S131–139 (1995). [DOI] [PubMed] [Google Scholar]
- 18.Lord JP, Portwood MM, Fowler WM, Lieberman JS & Carson R Upper vs lower extremity functional loss in neuromuscular disease. Arch Phys Med Rehabil 68, 8–9 (1987). [PubMed] [Google Scholar]
- 19.Personius KE, Pandya S, King WM, Tawil R & McDermott MP Facioscapulohumeral dystrophy natural history study: standardization of testing procedures and reliability of measurements. The FSH DY Group. Phys Ther 74, 253–263 (1994). [DOI] [PubMed] [Google Scholar]
- 20.Tawil R, McDermott MP, Mendell JR, Kissel J & Griggs RC Facioscapulohumeral muscular dystrophy (FSHD): design of natural history study and results of baseline testing. FSH-DY Group. Neurology 44, 442–446 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.