Abstract
Background:
Fetal brain development in the first half of pregnancy is dependent on maternal thyroid hormone (TH), highlighting the importance of trans-placental TH transport. It is yet unclear which transporters are involved in this process. We aimed to identify the major TH transporters in a human placental cell model (BeWo cells).
Methods:
Messenger RNA expression of the known TH transporters (the monocarboxylate transporter [MCT]8, MCT10, the L-type amino acid transporter [LAT]1, LAT2, the organic anion transporting peptide [OATP]1A2 and OATP4A1) in BeWo cells and human placenta were determined by quantitative PCR. To determine the specificity and efficacy of transporter inhibitors, we first determined TH uptake at different inhibitor concentrations in African green monkey kidney fibroblast-like cells (COS1 cells) overexpressing TH transporters. We then tested TH uptake in BeWo cells in the presence or absence of the optimal inhibitor concentrations.
Results:
All tested TH transporters were expressed in human term placentas, whereas MCT8 was absent in BeWo cells. Both 2-amino-2-norbornanecarboxylic acid (BCH) and L-tryptophan at 1 mM inhibited LATs, whereas at the highest concentration (10 mM) L-tryptophan also inhibited MCT10. Verapamil inhibited OATP1A2 and less efficiently both MCTs, but not LATs. Both rifampicin and naringin reduced OATP1A2 activity. Finally, silychristin inhibited MCT8 at submicromolar concentrations and OATP1A2 partially only at the highest concentration tested (10 μM). In BeWo cells, verapamil reduced triiodothyronine (T3) uptake by 24%, BCH by 31%, and 1 mM L-tryptophan by 41%. The combination of BCH and verapamil additively decreased T3 uptake by 53% and the combination of BCH and 10 mM L-tryptophan by 60%, suggesting a major role for MCT10 and LATs in placental T3 uptake. Indeed, transfection of BeWo cells with MCT10-specific small interfering RNA significantly reduced T3 uptake. Only the combination of BCH and verapamil significantly reduced thyroxine (T4) uptake in BeWo cells, by 32%.
Conclusions:
Using pharmacological inhibitors, we show that MCT10 and LATs play a major role in T3 uptake in BeWo cells. T4 uptake appears independent of known TH transporters, suggesting the presence of, currently unknown, alternative transporter(s).
Keywords: placenta, pregnancy, BeWo, pharmacological inhibitor, thyroid hormone transporter
Introduction
Thyroid hormone (TH), the collective name for the prohormone thyroxine (T4) and biologically active hormone 3,5,3′-triiodothyronine (T3), is crucial for development. Because the fetal thyroid gland is not fully functional until gestational weeks 18–20 (1,2), fetal development in the first half of pregnancy largely depends on maternal TH (3). Even mild alterations in maternal TH levels are associated with a worse neuropsychological performance of the child (4,5). Levothyroxine therapy is commonly used in clinical practice for pregnant women with overt and subclinical hypothyroidism. However, we recently showed that not only low, but also high maternal free T4 level during early pregnancy are associated with a lower child intelligence quotient, as well as a lower gray matter and cortex volume, even within the reference ranges (1,5). This underscores the importance of tight regulation of trans-placental TH transport. However, current knowledge of the TH transport across human placenta and its regulation is still very limited.
The major route by which TH is transferred across the placenta is likely through cell membrane TH transporters (6). Of the known TH transporters, the monocarboxylate transporters (MCT8 and MCT10), the L-type amino acid transporters (LAT1 and LAT2), and the organic anion transporters (OATP1A2 and OATP4A1) are expressed in human placenta (7–10).
The placenta consists of chorionic villi that are built up of various types of cells, including the syncytiotrophoblasts and the cytotrophoblasts (11). In the first trimester of pregnancy, when the fetus is fully dependent on maternal TH concentrations, the cytotrophoblasts form a layer beneath the syncytiotrophoblast layer in chorionic villi (6) and both the cytotrophoblasts and the syncytiotrophoblasts participate in maternofetal exchange (11). Studies in isolated microvillous plasma membrane of the syncytiotrophoblasts of human term placenta suggested that the majority (67%) of saturable T4 uptake was mediated by LATs and MCT10, whereas most (87%) of saturable T3 uptake by MCT8 and MCT10 (12). The single cell RNA-seq study on the placentas from early pregnancy (8 weeks of gestation) by Liu et al. showed that these six known TH transporters are also expressed in the cytotrophoblasts (13). However, it is currently unknown if, and to what extent, these transporters functionally contribute to the transport of TH in this cell type.
BeWo cells are choriocarcinoma-derived trophoblasts and have been widely used as a cell model to investigate the trans-placental transport of drugs. The transport kinetics of multiple compounds in the BeWo cell model correlate well with those found in ex vivo placental perfusion models (14). In this study, we used BeWo cells as a proxy for placental cytotrophoblasts. By using pharmacological inhibitors against known TH transporters, we aimed to identify the major TH transporters that contribute to T3 and T4 uptake.
Materials and Methods
Reagents
2-Amino-2-norbornanecarboxylic acid (BCH), naringin, rifampicin, silychristin, and verapamil were purchased from Sigma-Aldrich (Zwijndrecht, The Netherlands) and L-tryptophan from Fluka (Landsmeer, The Netherlands). BCH and verapamil were dissolved in MilliQ water, and naringin and silychristin in dimethyl sulfoxide. L-tryptophan and rifampicin were dissolved in Dulbecco's phosphate-buffered saline (DPBS)/0.1% glucose.
[125I]-3,3′-T2, [125I]-T3 and [125I]-T4 were prepared as previously described (15).
Cell culture
The BeWo cell line clone 30 was kindly provided by Dr. L. Mathiesen from the Department of Public Health, University of Copenhagen, Denmark with permission from Dr. A.L. Schwartz from the Department of Biochemistry, Washington University School of Medicine (St. Louis, MO). African green monkey kidney fibroblast-like cells (COS1 cells) were purchased from the European Collection of Authenticated Cell Cultures (Sigma-Aldrich). BeWo and COS1 cells were cultured as previously described (16).
Constructs
Human (h) OATP1A2 in pSport1 was kindly provided by Prof. Dr. Peter J. Meier (Institute of Clinical Pharmacology and Toxicology, University Hospital Zurich, Zurich, Switzerland), and subcloned into the pSG5 vector (Stratagene, La Jolla, CA) using NotI and Acc65I restriction sites. hSLC3A2 (4F2/CD98), hSLC7A5 (LAT1) and hSLC7A8 (LAT2) in pcDNA3 (17), hCRYM (μ-crystallin) in pSG5 (18), hMCT8 in pcDNA3 (19), and hMCT10 in pcDNA3.1 (20), were generated in previous studies.
Transfection
COS1 cells were seeded and transfected as described previously (16) with 100 ng transporter expression vector (50 ng for MCT8) or the matching empty vector as control, together with 50 ng hCRYM. For hSLC7A5 and hSLC7A8, cells were cotransfected with 100 ng hSLC3A2 that encodes the glycosylated 4F2 heavy chain (CD98/SLC3A2) that is required for LAT1 and LAT2 activity (21).
Knockdown of MCT10 in BeWo cells
BeWo cells were transfected with 20 nM small interfering RNA (siRNA) of MCT10 (No. 4392420; Thermo Fisher [s42149], Breda, The Netherlands) or negative control siRNA (No. 4390844; Thermo Fisher) at 30–50% cell confluency using Lipofectamine RNAiMAX transfection reagent (Thermo Fisher) according to the manufacturer's protocol.
RNA isolation and real-time quantitative PCR
The study received exemption for approval from the local institutional Medical Ethics Committee according to the Dutch Medical Research with Human Subjects Law (MEC-2017-418).
Term placentas of uncomplicated singleton pregnancies were collected immediately after delivery through cesarean section at Erasmus University Medical Center (Rotterdam, The Netherlands). All patients gave written consent before donating their placentas. About 0.5 × 0.5 × 0.5 cm tissues were taken from 3 different locations from maternal and fetal sides of the placentas, respectively. Tissue biopsies were snap frozen in liquid nitrogen and stored in −80°C freezer. Total RNA of homogenized placental tissue and BeWo cells were isolated using TRI reagent (Sigma Aldrich). One microgram of RNA was converted to complementary DNA (cDNA) using the Transcriptor High Fidelity cDNA Synthesis Kit (Roche) according to the manufacturer's instructions. Real-time quantitative PCR was performed using 0.2 μM of each primer (primer sequences are listed in Supplementary Table S1; Supplementary Data), and SYBR green PCR master mix (Eurogentec, Maastricht, The Netherlands) or FastStar universal probe master mix (Roche) for primer-probe assays. Results were normalized to the house-keeping gene glyceraldehyde 3-phosphate dehydrogenase.
TH uptake
BeWo cells were seeded at a density of 5 × 104/cm2 in poly-d-lysine (Sigma-Aldrich) precoated 24-well plates. For COS1 cells, uptake assays were performed at 48 hours after transfection in 24-well plates. Upon confluency, cells were washed with assay buffer (DPBS+Ca2+/Mg2++0.1% glucose, without bovine serum albumin [BSA]) and incubated for the indicated times with 375 μL assay buffer containing 1 nM 3, 3′-T2 (for LAT1 and LAT2), T3 (for MCT8 and MCT10), or 1 μM T3 (for OATP1A2) with the corresponding 125I-labeled iodothyronines (5 × 104 counts per minute) in the presence of inhibitor(s) or vehicle in a 37°C incubator. After incubation, cells were quickly rinsed with DPBS/0.1% glucose/0.1% BSA and lysed in 500 μL 0.1 N NaOH. The radioactivity in the lysates was measured in a RIASTAR gamma counter (Packard).
Statistical analysis
GraphPad Prism 5 (GraphPad, La Jolla, CA) was used for statistical analysis. A value of p < 0.05 was considered as significant.
Results
Messenger RNA expression of TH transporters in BeWo cells and human term placenta
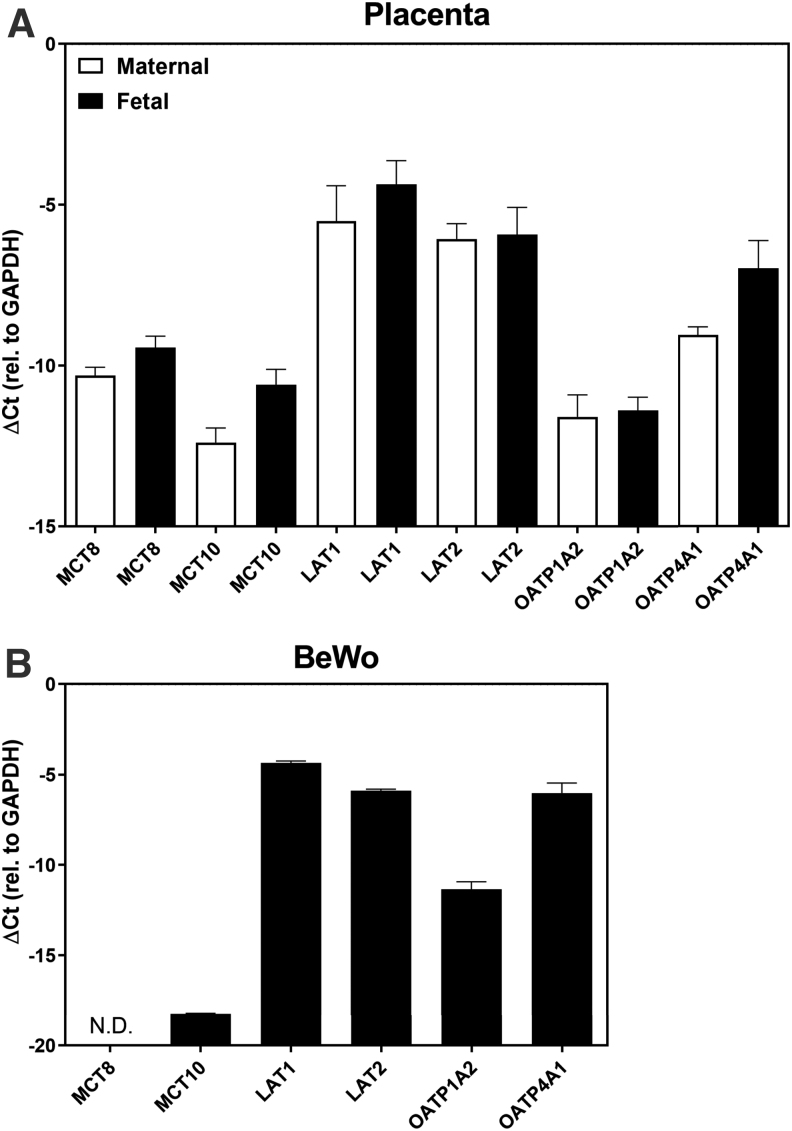
To verify whether BeWo cells might be a representative cell model for human cytotrophoblasts from term placenta, we first compared the messenger RNA (mRNA) expression pattern of the known TH transporters. All tested 6 transporters were expressed in human term placenta. The mRNA levels of the TH transporters were not significantly different between the maternal and the fetal sides (Fig. 1A). In BeWo cells, MCT8 is not expressed and MCT10 is relatively lowly expressed, whereas other TH transporters are expressed with a similar pattern as in human term placenta (Fig. 1B).
FIG. 1.
Relative mRNA expression of the TH transporters in human term placenta (A) and BeWo cells (B). The mRNA expression level of the TH transporters was normalized to GAPDH. Data are represented as mean ± SEM of three experiments in duplicates or three placentas. Unpaired two-tailed t-test was used for the statistical analysis to compare the mRNA expression level of the known TH transporters in placental tissues collected from the maternal and fetal sides and they are not significant. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LAT, L-type amino acid transporter; MCT, monocarboxylate transporter; mRNA, messenger RNA; N.D., not detected; OATP, organic anion transporting peptide; SEM, standard error of the mean; TH, thyroid hormone.
Specificity and efficacy of TH transporter inhibitors
We first determined the specificity and efficacy of pharmacological inhibitors of the 6 TH transporters that are expressed in human term placenta in an overexpression model, using COS1 cells transfected with constructs for the human forms of the individual TH transporters and with μ-crystallin (CRYM), a high affinity cytosolic TH binding protein (20), to minimize TH efflux. Transport activity was determined in the presence of increasing concentrations of the inhibitor. To ensure that uptake levels were sufficiently robust to reliably test the effects of inhibitors, uptake inhibition studies were performed using the preferred iodothyronine for each transporter, namely T3 for MCT8, MCT10, and OATP1A2, and 3,3′-T2 for LAT1 and LAT2 (17) (Supplementary Fig. S1).
In the absence of inhibitors, LAT1 and LAT2 induced uptake of 1 nM 3,3′-T2 (29.4% and 26.2%, respectively vs. 14.4% by empty vector [EV]), MCT8 and MCT10 induced uptake of 1 nM T3 uptake (42.5% and 43.4%, respectively vs. 15.8% by EV), and OATP1A2 induced uptake of 1 μM T3 (10.8% vs. 6.7% by EV) (Supplementary Fig. S1). In our hands, mouse Oatp4a1 displayed cell membrane expression and induced T3 uptake (15.7% vs. positive control MCT8 35.5%) and T4 uptake (13.6% vs. MCT8 27.5%) (Supplementary Figs. S2 and S3) after 30-minute incubation; however, no reliable uptake with either 1 nM or 1 μM T3 or T4 was observed for human OATP4A1 (data not shown), despite the presence of the OATP4A1 protein at the plasma membrane was confirmed by cell surface biotinylation assay (Supplementary Fig. S2).
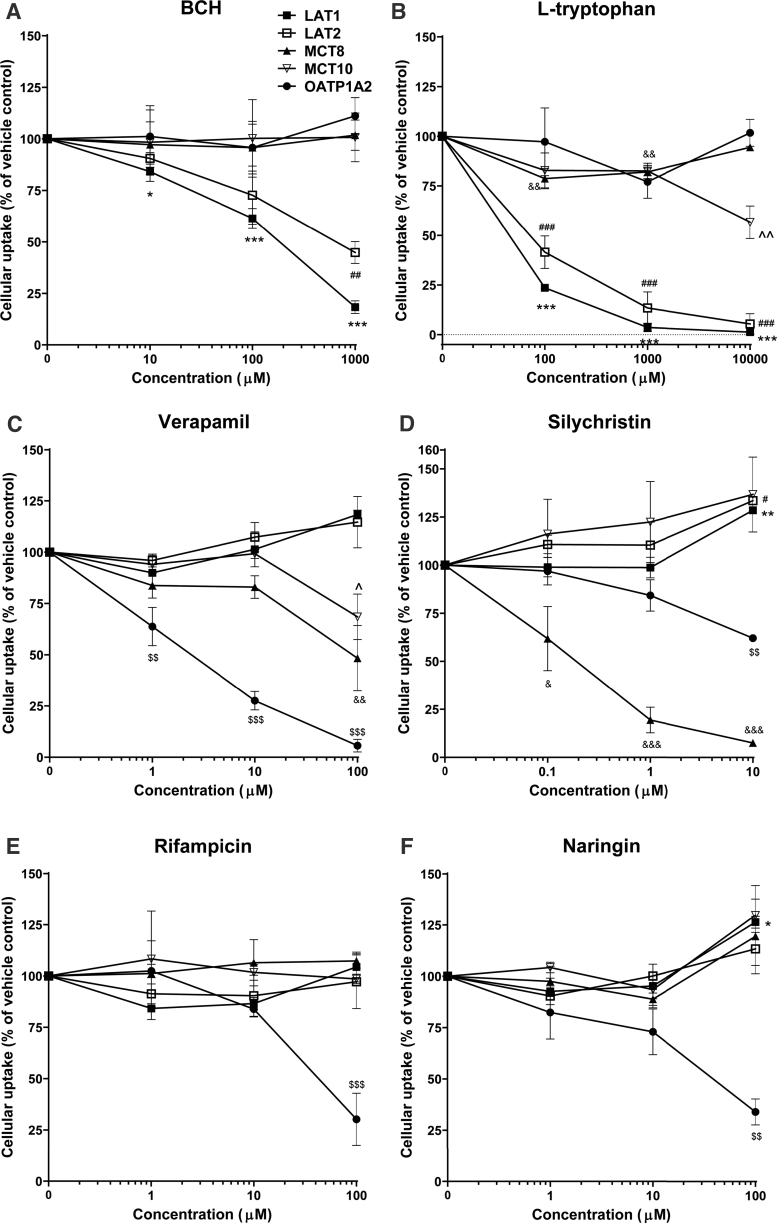
BCH robustly inhibited LAT1 and LAT2 activity in a concentration-dependent manner (Fig. 2A; Table 1). L-tryptophan reduced 3,3′-T2 uptake by LAT1 and LAT2 by ∼50% at the lowest concentration (0.1 mM), with complete inhibition at the highest concentration tested (10 mM), whereas the highest concentration was required to partly block T3 uptake by MCT10 (Fig. 2B). Verapamil effectively blocked T3 transport by OATP1A2, with full inhibition at the highest concentration tested (100 μM), whereas this concentration only partially inhibited both MCTs (Fig. 2C). Verapamil did not affect LAT activity (Fig. 2C). Silychristin already blocked MCT8 activity by ∼40% activity at the lowest concentration tested (0.1 μM) and fully inhibited MCT8 activity at higher concentrations, whereas MCT10 activity was not inhibited at any concentration, confirming previous studies that identified silychristin as a potent and selective MCT8 inhibitor with a half maximal inhibitory concentration ∼100 nM (Fig. 2D) (22). Only at the highest concentration (10 μM) did silychristin also reduce OATP1A2 activity by 38% (Fig. 2D). The broad OATP-family inhibitor rifampicin and the more OATP1A2-selective inhibitor naringin only inhibited OATP1A2, with near complete transport blockade at the highest concentration (100 μM) (Fig. 2E, F).
FIG. 2.
The specificity and efficacy of the TH transporter inhibitors. The cellular uptake of iodothyronines was determined in COS1 cells overexpressing the TH transporters and CRYM by uptake assays in the presence or absence of various concentrations of the inhibitors (BCH [A], L-tryptophan [B], verapamil [C]. silychristin [D], rifampicin [E] and naringin [F]). Substrates were used at a concentration of 1 nM (3, 3′-T2 for LAT1 and LAT2, T3 for MCT8 and MCT10), except for OATP1A2 where significant uptake was only achieved with 1 μM 125I-T3. Uptake levels were corrected for background uptake in EV transfected control cells, incubated under the same condition and presented as a percentage. Resulting uptake levels were presented relatively to the uptake levels observed in the presence of vehicle control. Data are represented as mean ± SEM of three experiments performed in duplicate. One-way ANOVA followed by the Dunnett's post-test was used for the statistical analysis. Statistical significance for TH transporters: *LAT1; #LAT2; &MCT8; ^MCT10; $OATP1A2, *, #, &, ^, $: 0.01 < p ≤ 0.05; **, ##, &&, ^^, $$: 0.001 < p ≤ 0.01; ***, ###, &&&, $$$: 0.0001 < p ≤ 0.001. ANOVA, analysis of variance; BCH, 2-Amino-2-norbornanecarboxylic acid; COS1, African green monkey kidney fibroblast-like cells.
Table 1.
Overview of the Specificity and Efficacy of the Pharmacological Inhibitors
| Inhibitors | LAT1 | LAT2 | MCT8 | MCT10 | OATP1A2 |
|---|---|---|---|---|---|
| BCH | ✓(146 μM) | ✓(∼100 μM) | X | X | X |
| L-tryptophan | ✓(17 μM) | ✓(45 μM) | X | ✓(>10 mM) | X |
| Verapamil | X | X | ✓(∼100 μM) | ✓(>100 μM) | ✓(1.5 μM) |
| Silychristin | X | X | ✓(0.12 μM) | X | ✓(>10 μM) |
| Rifampicin | X | X | X | X | ✓(50 μM) |
| Naringin | X | X | X | X | ✓(23 μM) |
The half maximal inhibitory concentration values of the inhibitors for the TH transporters were calculated using nonlinear curve fitting by GraphPad Prism 5 and depicted between brackets.
✓, inhibition; X, no inhibition; BCH, 2-amino-2-norbornanecarboxylic acid; LAT, L-type amino acid transporter; MCT, monocarboxylate transporter; OATP, organic anion transporting peptide; TH, thyroid hormone.
Identification of the major TH transporters in BeWo cells using pharmacological inhibitors
We first determined the time dependence of T3 and T4 uptake in BeWo cells. T3 and T4 uptake showed similar time curves, with intracellular concentrations increasing linearly at least in the first 60 minutes of incubation (Supplementary Fig. S4). Therefore a 60-minute incubation time was chosen to test the effect of individual inhibitors, or a combination of inhibitors, which does not impose cell cytotoxicity (Supplementary Fig. S5) on TH uptake to identify the main TH transporters in BeWo cells.
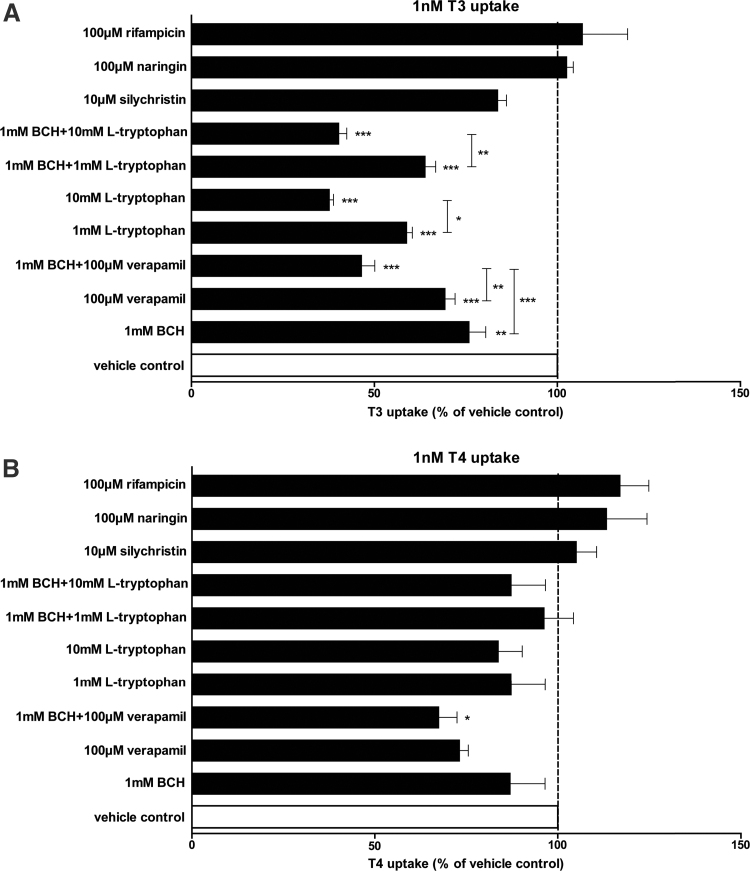
The LAT inhibitors BCH and L-tryptophan, both at 1 mM, significantly decreased uptake of 1 nM T3 (24% and 41%, respectively), suggesting that LAT1 and/or LAT2 contribute to a substantial amount of T3 uptake (Fig. 3A). The combination of 1 mM BCH and 1 mM L-tryptophan did not further reduce T3 uptake (36%), indicating that both inhibitors targeted LATs. A higher concentration of 10 mM L-tryptophan, which also reduces MCT10 activity, or the combination of 1 mM BCH and 10 mM L-tryptophan further decreased T3 uptake (62% and 60%, respectively) (Fig. 3A), suggesting an additive role of MCT10 next to LATs in T3 uptake (Fig. 3B). A combination of 100 μM verapamil and 1 mM BCH reduced T3 uptake by 53%, which exceeded the inhibitory effects of 100 μM verapamil or 1 mM BCH alone (31% and 24%, respectively) (Fig. 3A). This suggests again an additive effect of the blockade of LATs and another, verapamil-sensitive, TH transporter that based on the inhibitor studies in COS1 cells could either be OATP1A2, MCT8, or MCT10. However, the OATP1A2 inhibitors naringin and rifampicin, both at the high concentration of 100 μM, did not affect T3 uptake in BeWo cells, and neither did the MCT8 inhibitor silychristin (Fig. 3A). This rules out a significant role of OATP1A2 in T3 uptake and is in line with the absent expression of MCT8, and may again point to MCT10 as one of the transporters contributing to T3 transport in BeWo cells. Taken together, these studies indicate that MCT10 and LATs are the major known TH transporters involved in T3 uptake in BeWo cells.
FIG. 3.
One nanomolar T3 (A) and T4 (B) uptake in BeWo cells in the presence of the inhibitors or the combination of the inhibitors. Data are represented as mean ± SEM of three experiments performed in duplicate. One-way ANOVA followed by the Dunnett's post-test (comparing each group with the vehicle control) or the Bonferroni's post-test (comparing two indicated groups) was used for the statistical analysis. *0.01 < p ≤ 0.05; **0.001 < p ≤ 0.01; ***0.0001 < p ≤ 0.001.
Even at the highest concentration, BCH and L-tryptophan did not affect T4 uptake in BeWo cells, indicating that both LATs and MCT10 play no major role in T4 uptake (Fig. 3B). A total of 100 μM verapamil reduced T4 uptake by 27% and in combination with 1 mM BCH by 32%, although only the latter reached statistical significance (Fig. 3B). This suggests that part of T4 uptake is verapamil sensitive. Because neither silychristin nor naringin reduced T4 uptake, T4 uptake cannot be attributed to MCT8 or OATP1A2. Taken together, the results show that T4 uptake in BeWo cells is partly verapamil sensitive, but not facilitated by any of the known TH transporters.
Knockdown of MCT10 confirms T3 uptake by MCT10 in BeWo cells
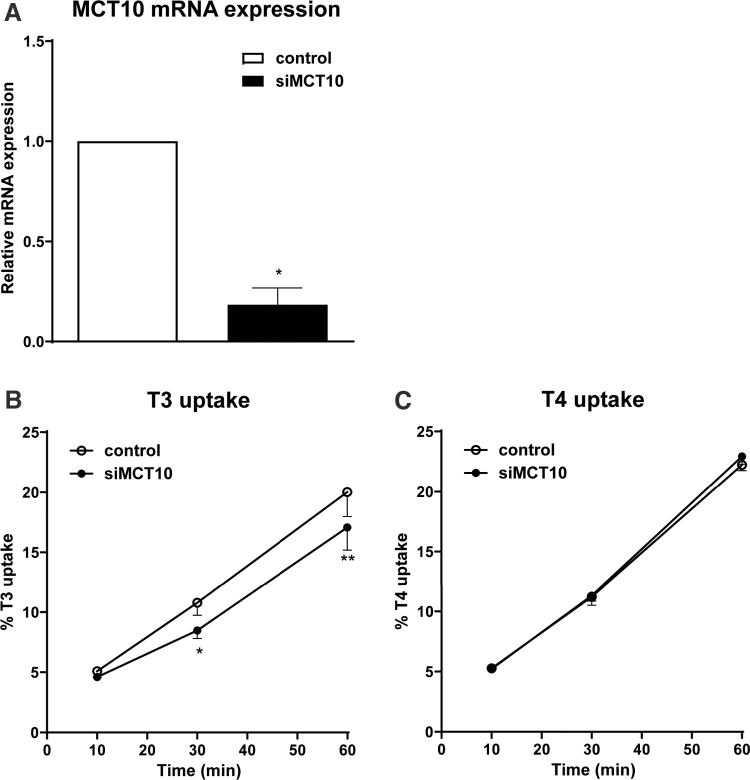
The inhibition of T3 uptake in BeWo cells by the specific LAT inhibitor, as well as 1 mM L-tryptophan, supports a role for LATs (23). Although the inhibitory effects of 10 mM L-tryptophan and verapamil suggest a role for MCT10 in T3 uptake as well, these inhibitors are less specific and could therefore also block TH transport by other unknown T3 transporters. To verify the role for MCT10 in T3 uptake, we knocked down MCT10 expression by transfecting BeWo cells with a specific siRNA against MCT10. After 48-hour transfection, MCT10 mRNA expression was decreased by 82% in MCT10 siRNA transfected cells compared with negative control siRNA transfected cells (Fig. 4A), whereas expression of the other 4 TH transporters were not affected (Supplementary Fig. S6). Unfortunately we were not able to reliably detect endogenous MCT10 protein expression probably owing to its low expression level.
FIG. 4.
TH uptake in MCT10 knockdown BeWo cells. (A) MCT10 mRNA expression in BeWo cells transfected with 20 nM MCT10 siRNA for 48 hours (data are represented as mean ± SEM of three experiments). (B, C) Time-dependent uptake of 1 nM T3 (B) and T4 (C) in BeWo cells transfected with 20 nM MCT10 siRNA or negative control siRNA for 48 hours (data are represented as mean ± SEM of three experiments performed in duplicate). Paired two-tailed t-test was used and statistical significance was compared to negative control. *0.01 < p ≤ 0.05; **0.001 < p ≤ 0.01. siRNA, small interfering RNA; T3, triiodothyronine; T4, thyroxine.
One nanomolar T3 uptake was reduced by 21% after 30-minute incubation and 19% after 60 minutes in MCT10 siRNA transfected BeWo cells, which is in agreement with the contribution of MCT10 based on the above inhibitor study (Fig. 4B). On the contrary, uptake of 1 nM T4 was not reduced by the MCT10 knockdown (Fig. 4C). To allow for further reduction of MCT10, we also checked the mRNA expression of MCT10 and T3 uptake after knockdown for 72 hours. MCT10 mRNA expression was even further reduced compared with negative control, whereas the reduction in T3 uptake was comparable with 48-hour knockdown (Supplementary Fig. S6). These results confirm that MCT10 is important for T3 but not for T4 uptake in BeWo cells.
Discussion
In this study, we used the BeWo cell model to model TH transport in human cytotrophoblasts. Using pharmacological inhibitors, we identified LATs and MCT10 as important transporters for T3 uptake in BeWo cells, whereas the major T4 transporters are still unknown. The contribution of MCT10 to T3 transport was further confirmed by siRNA knockdown.
We first determined the specificity and efficacy of pharmacological inhibitors against individual TH transporters that are expressed in human placenta in an overexpression model, to provide a pharmacological tool box to study the contribution of these transporters to TH transport in BeWo cells. Our findings regarding the inhibitory effect of the inhibitors against the TH transporters are in agreement with previous studies (12,22,24–27). We also showed that most inhibitors are specific for their targets and have little effect on nontargeted TH transporters. These findings facilitate future application of these inhibitors to explore the physiological role of the TH transporters in cultured cells or tissues.
We observed significant induction of T4 uptake in mouse Oatp4a1 transfected COS1 cells but not in human OATP4A1 transfected cells, although we confirmed the correct sequence of hOATP4A1 gene insert and presence of the protein at the cell membrane. This is in disagreement with a study of Fujiwara et al. who found modest induction of TH uptake (1.6-fold for T4 and 2.9-fold for T3) in Xenopus laevis oocytes injected with hOATP4A1 cRNA (28). This discrepancy may be owing to the different expression systems, applied buffers, and/or differential expression of endogenous TH transporters in COS-1 cells and oocytes that may influence TH influx and efflux.
Our results using combinations of inhibitors indicate a major role for LATs and MCT10 in T3 uptake and, later specifically, knockdown of MCT10 confirmed its role in BeWo cells. The combination of 1 mM BCH and 100 μM verapamil reduced T3 uptake by 53% and with 10 mM L-tryptophan alone or in combination with BCH by ∼60%. However, because 10 mM L-tryptophan only inhibits ∼40% and 100 μM verapamil inhibits ∼30% of the transport activity for MCT10, we expect that the residual T3 uptake in BeWo cells would further decrease to <40% when LATs and MCT10 are fully inhibited, which means that their activity accounts for the majority of T3 uptake. In agreement with an important role for MCT10, Loubière et al. reported that 87% of saturable T3 uptake is mediated by MCT8 and MCT10 in microvillous plasma membrane-isolated human term syncytiotrophoblasts (12).
The contribution of MCT8 to T3 uptake could not be compared in the BeWo model, owing to the lack MCT8 expression in the BeWo cells. Because MCT8 is a specific TH transporter and it is expressed in the syncytiotrophoblasts and the cytotrophoblasts of the human placenta throughout gestation (7), it constitutes an important limitation of the BeWo cell model. Furthermore, MCT10 expression in BeWo cells is relatively low compared with that in human term placenta; however, its expression in human placenta has a trend to increase with gestation age (8). The relatively low MCT10 expression in BeWo cells may represent early stage of pregnancy when cytotrophoblasts are indispensable for TH trans-placental transport. In addition, the relative contribution of MCT10 and LATs to TH trans-placental transport in vivo may differ as we tested TH transport in DPBS/0.1% glucose in vitro, whereas extensive amino acid transport mediated by these transporters may occur in human placenta.
The T4 transport unaccounted for may be owing to either a novel T4 transporter or multiple low-affinity transporters or a combination of both. Our results show that some transport is attributable to (a) verapamil-sensitive transporter(s), including but not limited to MCT10, OATP1A2, p-glycoprotein (ABCB1), ABCG2, and OCTN2 (29–31). Given the absence of any inhibitory effects in the presence of alternative inhibitors or siRNA, MCT10 and OATP1A2 appear to have no role in T4 transport. This aligns with the presumed substrate specificity of MCT10. Furthermore, p-glycoprotein is a T3 transporter (29) but it is not expressed in BeWo cells according to the Human Protein Atlas. Therefore p-glycoprotein is unlikely responsible for this verapamil-sensitive T4 uptake.
With this wide range of potential targets of verapamil-sensitive transporters we are not able to pinpoint a specific T4 transporter. If the majority of T4 transport is facilitated by multiple low-affinity transporters in the BeWo model, finding these transporters would be a cumbersome approach with possibly limited relevance for physiology. If T4 transport is facilitated by one or two unknown transporters, finding these transporters would likely require a large screen of candidates, also because such transporter may not reside in one of the known families of TH transporters. [As an example, SLC17A4 was recently identified as a novel TH transporter (32,33), although the SLC17 family was only known as phosphate and uric acid transporters].
In summary, our study shows that LATs and MCT10 are important for T3 transport in the BeWo cytotrophoblast cell model, a proxy for placental TH transport in cytotrophoblasts that form a cell layer for maternofetal exchange during the first trimester of pregnancy, the stage during which maternal to fetal TH transfer is critical for fetal development. The transporters for T4 uptake are currently unidentified. Our study may provide relevant information for any future studies using the BeWo cell line as model for human placenta, particularly toxicology studies in which interference with TH transport is tested.
Supplementary Material
Acknowledgments
The authors thank Stefania Farina for the generation of the OATP1A2 construct. This work was presented at the 41st Annual Meeting of the European Thyroid Association in 2018 and the meeting abstract was published (DOI: 10.1159/000491542).
Authors' Contributions
Z.C., M.E.M., W.E.V., and R.P.P. designed the study. Z.C., A.S.E.v.d.S., S.G., and L.J.d.R. performed the experiments and processed the experimental data. Z.C. analyzed the data. Z.C., M.E.M., W.E.V., and R.P.P. wrote the article. All authors critically reviewed, revised, and approved the article.
Author Disclosure Statement
The authors have nothing to disclose.
Funding Information
This study is funded by a Vidi grant (016.176.331) from the Netherlands Organization for Scientific Research to R.P.P. This research is funded by the EU Horizon 2020 program, ATHENA project, Grant No. 825161, which is gratefully acknowledged. This publication reflects only the authors' view, and the European Commission is not responsible for any use that may be made of the information it contains.
Supplementary Material
References
- 1. Korevaar TI, Muetzel R, Medici M, et al. 2016. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol 4:35–43. [DOI] [PubMed] [Google Scholar]
- 2. Korevaar TIM, Medici M, Visser TJ, et al. 2017. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol 13:610–622. [DOI] [PubMed] [Google Scholar]
- 3. Vulsma T, Gons MH, de Vijlder JJ. 1989. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med 321:13–16. [DOI] [PubMed] [Google Scholar]
- 4. Hollowell JG Jr., Garbe PL, Miller DT. 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med 341:2016–2017. [PubMed] [Google Scholar]
- 5. Jansen TA, Korevaar TIM, Mulder TA, et al. 2019. Maternal thyroid function during pregnancy and child brain morphology: a time window-specific analysis of a prospective cohort. Lancet Diabetes Endocrinol 7:629–637. [DOI] [PubMed] [Google Scholar]
- 6. Landers K, Richard K. 2017. Traversing barriers—how thyroid hormones pass placental, blood-brain and blood-cerebrospinal fluid barriers. Mol Cell Endocrinol 458:22–28. [DOI] [PubMed] [Google Scholar]
- 7. Chan SY, Franklyn JA, Pemberton HN, et al. 2006. Monocarboxylate transporter 8 expression in the human placenta: the effects of severe intrauterine growth restriction. J Endocrinol 189:465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Loubiere LS, Vasilopoulou E, Bulmer JN, et al. 2010. Expression of thyroid hormone transporters in the human placenta and changes associated with intrauterine growth restriction. Placenta 31:295–304. [DOI] [PubMed] [Google Scholar]
- 9. Ritchie JW, Taylor PM. 2001. Role of the System L permease LAT1 in amino acid and iodothyronine transport in placenta. Biochem J 356:719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel P, Weerasekera N, Hitchins M, et al. 2003. Semi quantitative expression analysis of MDR3, FIC1, BSEP, OATP-A, OATP-C, OATP-D, OATP-E and NTCP gene transcripts in 1st and 3rd trimester human placenta. Placenta 24:39–44. [DOI] [PubMed] [Google Scholar]
- 11. Patel J, Landers K, Li H, et al. 2011. Delivery of maternal thyroid hormones to the fetus. Trends Endocrinol Metab 22:164–170. [DOI] [PubMed] [Google Scholar]
- 12. Loubiere LS, Vasilopoulou E, Glazier JD, et al. 2012. Expression and function of thyroid hormone transporters in the microvillous plasma membrane of human term placental syncytiotrophoblast. Endocrinology 153:6126–6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu Y, Fan X, Wang R, et al. 2018. Single-cell RNA-seq reveals the diversity of trophoblast subtypes and patterns of differentiation in the human placenta. Cell Res 28:819–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li H, van Ravenzwaay B, Rietjens IM, et al. 2013. Assessment of an in vitro transport model using BeWo b30 cells to predict placental transfer of compounds. Arch Toxicol 87:1661–1669. [DOI] [PubMed] [Google Scholar]
- 15. Mol JA, Visser TJ. 1985. Synthesis and some properties of sulfate esters and sulfamates of iodothyronines. Endocrinology 117:1–7. [DOI] [PubMed] [Google Scholar]
- 16. Groeneweg S, Friesema EC, Kersseboom S, et al. 2014. The role of Arg445 and Asp498 in the human thyroid hormone transporter MCT8. Endocrinology 155:618–626. [DOI] [PubMed] [Google Scholar]
- 17. Zevenbergen C, Meima ME, Lima de Souza EC, et al. 2015. Transport of iodothyronines by human L-type amino acid transporters. Endocrinology 156:4345–4355. [DOI] [PubMed] [Google Scholar]
- 18. Friesema EC, Ganguly S, Abdalla A, et al. 2003. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135. [DOI] [PubMed] [Google Scholar]
- 19. Friesema EC, Kuiper GG, Jansen J, et al. 2006. Thyroid hormone transport by the human monocarboxylate transporter 8 and its rate-limiting role in intracellular metabolism. Mol Endocrinol 20:2761–2772. [DOI] [PubMed] [Google Scholar]
- 20. Friesema EC, Jansen J, Jachtenberg JW, et al. 2008. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol 22:1357–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanai Y, Segawa H, Miyamoto K, et al. 1998. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J Biol Chem 273:23629–23632. [DOI] [PubMed] [Google Scholar]
- 22. Johannes J, Jayarama-Naidu R, Meyer F, et al. 2016. Silychristin, a flavonolignan derived from the milk thistle, is a potent inhibitor of the thyroid hormone transporter MCT8. Endocrinology 157:1694–1701. [DOI] [PubMed] [Google Scholar]
- 23. Kinne A, Wittner M, Wirth EK, et al. 2015. Involvement of the L-type amino acid transporter LAT2 in the transport of 3,3'-diiodothyronine across the plasma membrane. Eur Thyroid J 4(Suppl. 1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Friesema EC, Docter R, Moerings EP, et al. 2001. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology 142:4339–4348. [DOI] [PubMed] [Google Scholar]
- 25. Morimoto E, Kanai Y, Kim DK, et al. 2008. Establishment and characterization of mammalian cell lines stably expressing human L-type amino acid transporters. J Pharmacol Sci 108:505–516. [DOI] [PubMed] [Google Scholar]
- 26. Bailey DG, Dresser GK, Leake BF, et al. 2007. Naringin is a major and selective clinical inhibitor of organic anion-transporting polypeptide 1A2 (OATP1A2) in grapefruit juice. Clin Pharmacol Ther 81:495–502. [DOI] [PubMed] [Google Scholar]
- 27. Kim DK, Kanai Y, Matsuo H, et al. 2002. The human T-type amino acid transporter-1: characterization, gene organization, and chromosomal location. Genomics 79:95–103. [DOI] [PubMed] [Google Scholar]
- 28. Fujiwara K, Adachi H, Nishio T, et al. 2001. Identification of thyroid hormone transporters in humans: different molecules are involved in a tissue-specific manner. Endocrinology 142:2005–2012. [DOI] [PubMed] [Google Scholar]
- 29. Mitchell AM, Tom M, Mortimer RH. 2005. Thyroid hormone export from cells: contribution of P-glycoprotein. J Endocrinol 185:93–98. [DOI] [PubMed] [Google Scholar]
- 30. Salomon JJ, Gausterer JC, Selo MA, et al. 2019. OCTN2-mediated acetyl-l-carnitine transport in human pulmonary epithelial cells in vitro. Pharmaceutics 11:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takara K, Matsubara M, Yamamoto K, et al. 2012. Differential effects of calcium antagonists on ABCG2/BCRP-mediated drug resistance and transport in SN-38-resistant HeLa cells. Mol Med Rep 5:603–609. [DOI] [PubMed] [Google Scholar]
- 32. Teumer A, Chaker L, Groeneweg S, et al. 2018. Genome-wide analyses identify a role for SLC17A4 and AADAT in thyroid hormone regulation. Nat Commun 9:4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Groeneweg S, van Geest FS, Chen Z, et al. 2021. Functional characterization of the novel thyroid hormone transporter SLC17A4. Thyroid 32:326–335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.