Abstract
Introduction:
Field work with bats is an important contribution to many areas of research in environmental biology and ecology, as well as microbiology. Work with bats poses hazards such as bites and scratches, and the potential for exposure to infectious pathogens such as rabies virus. It also exposes researchers to many other potential hazards inherent to field work, such as environmental conditions, delayed emergency responses, or challenging work conditions.
Methods:
This article discusses the considerations for a thorough risk assessment process around field work with bats, pre- and post-occupational health considerations, and delves into specific considerations for areas related to biosafety concerns—training, personal protective equipment, safety consideration in field methods, decontamination, and waste. It also touches on related legal and ethical issues that sit outside the realm of biosafety, but which must be addressed during the planning process.
Discussion:
Although the focal point of this article is bat field work located in northern and central America, the principles and practices discussed here are applicable to bat work elsewhere, as well as to field work with other animal species, and should promote careful considerations of how to safely conduct field work to protect both researchers and animals.
Keywords: bat, field safety, biosafety, rabies, coronaviruses
Introduction
Field research studies have long been an integral part of the biological sciences. Working in the field has been one of the most challenging yet relevant activities for many branches of the biological sciences and is crucial for the epidemiological investigation of emerging and reemerging diseases worldwide. Field research, like laboratory research, poses risks related directly to the study hazards, but frequently includes potential exposure to physical and biological hazards not normally encountered in the laboratory. In addition, safety challenges in research, such as personal protective equipment (PPE) and waste disposal, may be complicated by field work in ways that are not normally addressed in the laboratory.
Safety considerations, and the factors that complicate them in the field, are particularly relevant during studies involving animals. There have been several efforts to promote the development of guidelines for research involving animals in the field, as these types of activities can be very different from those performed in a controlled laboratory setting. However, developed guidelines (i.e., from the American Society of Mammologists) often focus on different purposes; current guidelines do not thoroughly cover aspects related to biological safety when handling animals during research activities.
Bats are an ecologically and economically important taxon that plays important roles in our ecosystems. These mammals have also been reservoirs of a wide variety of pathogens such as Paramyxoviruses, Lyssaviruses, and Coronaviruses (e.g., Severe Acute Respiratory Syndrome, or SARS, associated virus). The aim of this article is to provide guidance and recommendations related to biosafety when working with bats, including many aspects of biological risk assessment. To date, existing guidance focused primarily on the type of pathogens or geographical location, rather than the nature of the activities and risk assessment process. This review will focus on risk assessment considerations for bat research in North and Central America, with discussion points that readers should extrapolate to bat research worldwide, as well as general field research with wild animals.
Focus areas will include risk assessment (general factors to consider for both the research and the environment), medical surveillance and emergency response, special considerations for field research (PPE, training, work practices, decontamination, and waste management), and finally, legal and ethical issues relating to biosafety. On consideration of these factors, biosafety professionals reviewing field research should be able to identify hazards, assess risks, establish prevention strategies, train personnel, and advise on personnel protection strategies as it relates to not only bat field research, but also general field research with animals.
This multidisciplinary group of authors includes biosafety professionals as well as research experts in the fields of biology and conservation of bats. Collectively, we have considered the guidance needs of researchers and institutions conducting bat field research and present the following guidelines to advise biosafety in these research areas. The aim of this review does not intend to replace any local, regional, or site-specific regulations, other than offer guidance in conducting a holistic risk assessment process before the initiation of activities.
Risk Assessment
Risk identification, quantification, and mitigation are critical aspects of planning a successful project and work management. During field operations, veterinarians, biologists, and other research staff need to be aware of the potential risks involved in specific activities (i.e., collecting specimens from wildlife), as well as the possible risks associated with field work. Safety professionals and scientists should work together to identify and mitigate activities that could affect the researchers or the results of a project or field work.
Risk assessment is a key component of biosafety and biosecurity.1,2 The process begins with the identification of biological hazards associated with the work. Characteristics of each agent, such as the route of transmission, infectious dose, environmental stability, etc., will help guide the risk assessment. Consider the epidemiological triad to help address potential risks and their associated activities; this includes the properties of the organism/microorganism (innate/acquired), properties of the host (innate/acquired), and physical factors (properties of the environment/micro-environment, season of year, dose received, etc.).
One must consider both the probability and the consequence of an exposure event occurring to understand the total risk. A risk assessment approach for work with potentially infectious agents should consider not only the agent, but also the broader risk perspective as it relates to the disease, including geographic concerns, communicability of disease (both in the local area and during/after travel), hosts/vector interactions, risk group and biosafety level, and other factors. Appropriate risk mitigation measures should consider all these aspects of the risk assessment, but in the case of field work, additional factors need to be considered, such as naturally occurring biological risks affecting human and animal populations. Therefore, a focus on public health is a sensible way of addressing the full spectrum of biological risks associated with field work.
A risk assessment should be performed before work begins, when there is a change or modification in procedures, environments, or other factors that could impact the risk, and after a near miss or incident. A team approach to risk assessment is needed to understand the full picture and should include someone who is knowledgeable about working in the intended field environment, as there are unique considerations when working outside of the lab. Field work risk assessments need to consider the broader implications of human actions, natural phenomena, and accidents, with each risk assessed effectively to determine the hazards and threats related to the work or research location. Table 1 summarizes risks or conditions that may impact the research or researchers, and which should be considered in addition to the risks of working with biological agents in the field.
Table 1.
Dynamic risk conditions that may be present in the field and should be considered within the risk assessment
| Human actions | Natural phenomena | Accidents |
|---|---|---|
| Altered environment and technology (improvement or destruction such as: deforestation, air pollution) Behavior (deliberate negative actions such as war, crimes, genetic engineering) Detection (surveillance, awareness, diagnostic tools) Prevention (vaccines, prophylactic medication, sanitation) Vector control Medical treatment (safety, efficacy, availability, timeliness) Health system infrastructure Education, training, awareness |
Atmospheric (cyclones, hurricanes, tornadoes, tropical and lightning storms) Heat waves, cold fronts Terrain stability Seismic (fault ruptures, ground shaking, lateral spreading, tsunamis, and seiches) Other geologic and hydrologic debris (avalanches, rock falls, extensive soils, landslides and submarine slides, subsidence [sinking]) Hydrologic (flooding, salinization, drought, desertification, erosion, sedimentation) Volcanic tephra, ash, cinders, lapilli and pyroclastic flows, flows (lava flows, mudflows), projectiles, lateral blasts, gasses Wildfire (brush, forest, grass, savannah) Natural selection (emerging infections, zoonotic, animal and plant diseases) Others related to local/regional organisms/microorganisms such as insect bites (i.e., mosquitoes, ticks, flies, ants), spiders, poisonous snakes, poisonous plants |
Hydrocarbons and oil spills Nuclear and radioactivity facility accidents Laboratory accidents Field accidents (falls, trauma, heat, hypothermia) Travel accidents (vehicle breakdown, crash) Others (dual use research of concern) |
Biohazard Risks and Mitigation Strategies for Researchers
A major hazard of working with bats is the risk of exposure to rabies virus (RABV) or other potential Lyssaviruses. RABV and other pathogenic lyssaviruses have been found in bats in many countries all over the world.3 Each country or region has its own mechanisms for reporting and gathering epidemiological data related to rabies. In the Americas, the Regional Information System for the Epidemiological Surveillance of Rabies (or SIRVERA, for its acronym in Spanish), is a useful resource that gathers epidemiological data about rabies exposures.4 SIRVERA began operating in 1969, and it periodically reports the occurrence of rabies.
Since 2001, SIRVERA has operated in coordination with the Pan American Center for Foot-and-Mouth Disease and Veterinary Public Health of the Pan American Health Organization/World Health Organization (PANAFTOSA/VPH-PAHO/WHO), to provide information on rabies cases in the United States.5 Although a small percentage of the bat population is infected with the RABV, bats are the animal most likely to transmit the virus to humans in the United States, according to the Centers for Disease Control and Prevention (CDC).6,7
Some bats who are infected with the RABV present with aggressive behavior, but this is not always the case. In addition, some bats may also present aggressive or defensive behavior in response to being captured or handled, so this is not a clear sign of infection. Rabies prevalence in bats is likely underreported, as surveillance is primarily passive in nature. It is, therefore, necessary to treat all bats as if they are rabies positive, since the most reliable rabies test can only be performed post-mortem and in specialized laboratories.
RABV can be present in bat saliva and can be transmitted via a bite. In some cases, individuals can be exposed to rabies if infected saliva contacts non-intact skin, such as an open wound, or mucous membranes.8 Rabies can be transmitted via the aerosol route in a laboratory setting, but this route of transmission is rarely seen in the natural environment.9–11 Indirect transmission may present a lower risk. RABV is not particularly stable in the environment and is susceptible to sunlight and desiccation.12,13 Moreover, RABV is inactivated by exposure to different disinfectants, such as 70% ethanol, phenol, formalin, ether, trypsin, and certain detergents. RABV is also susceptible to inactivation by physical means, such as low pH (< 3) or pH above 11, as well as ultraviolet light.12 These factors must be considered when planning for waste and decontamination steps in the field (see the Decontamination and Waste section).
RABV is not the only zoonotic concern when working with bats. Bats can also be carriers of Salmonella, Yersinia, Shigella, Campylobacter, Bartonella and other enteric bacterial pathogens, as well as fungal pathogens (i.e., Cryptococcus), parasites, and vector-borne bacteria.14,15 Bats from outside North and Central America have the potential to harbor many other zoonotic viruses, including, but not limited to, Nipah virus, Hendra virus, Menangle virus, Marburg virus, Ebola virus, SARS-associated virus, and Venezuelan Equine Encephalitis virus.14–16 The list of zoonotic pathogens in bats will likely continue to grow, as they are implicated as a potential origin of one of the newest zoonotic concerns, SARS-CoV-2.17
The zoonotic concerns for a particular field project will be largely dependent on the geographic location and what endemic pathogens circulate or have been detected in that area. A thorough risk assessment must include the likelihood of developing a disease as a result of an exposure, disease severity, and available treatments.
Risk assessments must also consider hazards related to working directly with animals. In the case of field researchers working with bats, it will be important to consider sharps hazards beyond classical sharps such as needles, as these mammals are equipped with claws that allow the bat to grip and hang upside down, as well as sharp teeth that range in size based on species, diet, and age. These naturally occurring sharps can be used by the bat when they feel threatened as a means of self-defense. Bites or scratches by a bat can create wounds and provide a direct route of transmission for pathogens that bats carry, such as RABV, or an opening into the body through which other pathogens in the environment may enter.
These bites and scratches are often small and can go unnoticed, so pre-exposure prophylactic considerations should be part of the risk assessment (see the Pre- and Post-Exposure Prevention and Medical Surveillance, Emergency Response, and the Incident Management section).
One of the biggest challenges when performing a risk assessment for field work is to consider potential known or unknown aspects of the surrounding environment, which presents its own set of biological hazards and is very different from working in a controlled laboratory environment. For example, soil with high levels of bat guano (feces) may contain the fungus Histoplasma capsulatum, the causative agent of histoplasmosis. This fungal disease can be acquired by breathing in dust from bat droppings.14 Coccidioides immitis, the causative agent for Valley Fever, can also be found in bat guano.
Although not specific to bats, spores of tetanus bacteria are ubiquitous in the environment and are a hazard to consider for any field project. These spores can enter the body through broken skin. The presence of other animals not directly associated with the field project could be another source of biological hazards, including RABV, as all mammals are susceptible to this virus. The most common wild reservoirs include raccoons, skunks, and foxes, in addition to bats. If the work is being conducted in an area in which insect vectors are present, the risk assessment should include vector-transmitted diseases as well.
Various vector-borne diseases such as Lyme disease, Zika, Chikungunya, Dengue fever, West Nile virus disease, Rocky Mountain spotted fever, plague, and tularemia may need to be part of the risk assessment, depending on the geographic location and the season in which the field work will take place. One mitigation strategy for vector-borne diseases may be to include bednets as a recommendation when personnel are sleeping in tents or buildings, and this could also be helpful in areas where hematophagous bats can transmit rabies.
Finally, there have been studies in which wildlife vaccines are orally or topically applied to a bat, or administered through aerosols, and the vaccine is subsequently spread between bats through oral contact during grooming.18,19 If researchers will potentially be exposed to a wildlife vaccine, the vaccine safety relative to humans will need to be considered. A complete identification of all biological hazards associated with a field project will dictate which pathogen safety data sheets or other references to consult to help guide researchers and strengthen the biosafety mitigation strategies.
In addition to considerations relative to the infectious agent, the probability of an exposure event should be considered. The type of environment in which the fieldwork is conducted can affect the probability of an exposure event. For example, if bats are being captured while in flight in open air, there is a lower risk for any inhalation hazards compared with working with bats in a cave environment with poor air circulation. Certain environments (caves, mines, rocky areas, cliffs, dense vegetation) may have a higher risk for scrapes and scratches. Work in caves includes risk of collapse, getting lost, or getting trapped in the cave if the exit route becomes blocked (e.g., fire, falling debris).
Work in mines may pose exposure to hazardous substances, such as lead. The types of procedures performed during field work must also be considered as part of the risk assessment. Some of the specialized activities (field work procedures) can include short-term trapping for census, capture for measuring weight or length, blood or tissue sampling, collection of hair, collocation of identification devices (i.e., collars or ear tags), and behavioral observations, among others. Different activities will contribute a variable amount to the probability of an exposure event. The use of certain equipment, such as sharps (scalpels or needles for blood draws, passive integrated transponder [PIT] tagging), should also be considered as factors contributing to the risk of exposure.
Common risk mitigation strategies can be used to reduce the probability of an exposure event. Some laboratory risk mitigation strategies may need to be modified when applied to field work to accommodate environmental challenges such as weather and humidity. Mitigation strategies to reduce the potential of an exposure will be discussed in subsequent sections, including the use of appropriate PPE, training and experience, and the presence of specific activities for sampling, handling animals, transporting specimens, decontamination, and waste management.
Similar to considerations of exposure potential, there are many factors that can impact the other half of the risk equation—the likely consequence of an exposure. For example, the health status of an individual must be considered, as it can dramatically change how their immune system will react to an exposure. Individuals who are immunocompromised or have other medical conditions have the potential to have a more severe reaction to an exposure, resulting in a greater consequence, whereas vaccinated individuals may have a reduced consequence to an exposure, with the reduction dependent on the efficacy of the particular vaccine.
Vaccines are available for some of the biological hazards associated with bat field work, such as rabies and tetanus, but their availability can be country dependent. Additional factors that can change the consequence of an exposure event include the accessibility of prompt first aid, medical care, and post-exposure prophylaxis (PEP; see Pre- and Post-Exposure Prevention and Medical Surveillance, Emergency Response, and Incident Management section). Accessibility will change depending on the country in which the work is being performed, and even from one field site to another in a given region. In some cases, working alone could represent a high-risk activity, especially in those places with difficult access.
Biohazard Risks and Mitigation Strategies for Bats
Much attention is given to the risk of a potential exposure to the researchers performing the field work, but researchers, biosafety professionals, and others, such as the Institutional Animal Care and Use Committee (IACUC), also need to evaluate the risk the research poses to the bats themselves or to the environment. One example is White-Nose Syndrome (WNS), a devastating disease in bats that is caused by the fungus Pseudogymnoascus destructans. Currently, 12 species of bats in North America are impacted by this disease.20 Spores of the fungus are environmentally stable and can stick to a researcher's clothing or equipment.
When the researcher handles members of the bat colony, the fungus can spread and eventually disseminate throughout the colony. Researchers do not need to handle bats directly to infect them; since P. destructans replicates in soil, bringing spores into a cave or other roosting area could be enough to inoculate the environment and subsequently infect the bats. Disinfection of equipment and frequent changes or disinfection of PPE are needed to decrease the probability of exposing a bat to this pathogen. The National WNS Decontamination Protocol provides information about effective disinfectants and procedures to prevent the spread of this fungus.20
In addition to the risk of spreading animal pathogens between animals, researchers also have the potential to expose bats to new pathogens through a reverse zoonotic event. There have been several examples in which a human infected with SARS-CoV-2 has transmitted the virus to another species. Certain bat species have been infected with SARS-CoV-2 experimentally and in some instances, they can spread the infection to other bats of the same species in a laboratory setting.21
A similar potential exists for a researcher to transmit SARS-CoV-2 to a bat in the wild and for that bat to transmit the virus to other bats, creating a viral reservoir.22,23 Consider that SARS-CoV-2 is thought to originate from bats near Wuhan, China, and bats are known to be natural hosts to other coronaviruses.17 In March 2021, researchers from the United States Geological Survey (USGS) published a study indicating that the risk of spreading SARS-CoV-2 to North American bats was 1 in 3333 or less if the proper PPE was worn and scientists tested negative before field work began.24
However, the Delta variant (Pango lineage B.1.617.2), with a more than twofold increase in transmissibility,25 was not identified in the United States until that same month26 and was not a World Health Organization (WHO) designated variant of concern until May 2021.27 With the even greater transmissibility of later variants such as the Omicron variant (Pango lineage B.1.1.529), sending potentially infected researchers out into the field risks spreading a novel virus into an uninfected population of bats. This not only places the animals at risk but also creates a reservoir for the virus to mutate, and it potentially leads to greater consequences for the health of both bats and humans.
The probability of a human-to-bat transmission event can be reduced with the use of respirators that do not have an exhalation valve or vent, gloves, and dedicated protective clothing (see Personal Protective Equipment section). It is recommended that field researchers be up to date with Coronavirus disease 2019 (COVID-19) vaccinations, and they should also assess themselves for COVID-19 symptoms or COVID-19 exposure before interacting with wild bats to further reduce the risk of transmission of SARS-CoV-2. Additional precautions for protecting wildlife can be found on the CDC website.23,28 Although the probability of a reverse zoonotic event is low, the consequence could be devastating, and consideration of the possibilities is an important aspect of risk assessment.
Other Risks and Important Considerations
This section has discussed the risk assessment in the context of biosafety, primarily as it relates to agents and hazards of the work. It is important to consider any additional factors involved in the research, such as the use of chemicals, radiation, or physical hazards, and how these will impact the ability of the researchers to safely perform the work and to keep the animals and environment safe.
The risk assessment process as described in the first part of this section works well when there is enough evidence or available information. However, there could be situations where the information is limited or insufficient to perform a complete risk assessment. In these cases, it could be appropriate to add extra precautions before specimen or animal manipulations, such as:
Mandatory standard or core precautions: primary containment barriers, increased PPE
Heightened precautions: depending on the situation and specific requirements
Transport of biological materials: following national and international regulations.
In the case of unknown outbreaks, local authorities or experts can provide help and advice to researchers to achieve their goal so that every sample or animal can be handled under the best safety and security protocols.
Finally, it is recommended, or in some cases required, that before all field work activities, the researchers consult with the appropriate institutional authorities—the Institutional Biosafety Committee (IBC), IACUC, legal offices, travel offices, etc., and that they comply with local and Federal regulations, as well as guidelines relevant to animal welfare (see the Additional Considerations: Legal and Ethical Issues section).
Pre- and Post-Exposure Prevention and Medical Surveillance, Emergency Response, and Incident Management
Field safety planning includes preparation for many contingencies before traveling, for events that may occur during field work, or that may need to be dealt with after returning from field work. Although bats are the focus of this article, many other hazards can be encountered in the field, including trauma, altitude issues, weather/heat/cold issues, various hazardous plants and animals, as well as infectious agents that are tick-borne, mosquito-borne, water-borne, and much more.
Travel medicine should always be part of the planning to be sure all pre-trip vaccinations, medications, and underlying medical issues are planned for, including any current pandemic-related travel issues.
Bats are reservoirs for numerous infectious agents, and associated zoonotic diseases will vary by geographic location. Researchers should be familiar with the known zoonotic risks from any bats they may encounter (regardless of whether the bats are targeted) in their field sites. Rabies is an infection that should warrant a high degree of consideration after the bite from any mammal.29 Without vaccination, it usually leads to a fatal infection of the brain in both humans and other mammals. Bites from infected dogs cause most rabies deaths to humans worldwide,30 whereas bats account for the majority of human infections in the United States.6,7
Bat bites sustained anywhere in the world should be considered to potentially transmit RABV; bats in other areas can carry related lyssaviruses, including Australian bat lyssavirus, European bat lyssavirus, and Lagos bat virus, among others. Although genetically and antigenically distinct from RABV, infections due to these lyssaviruses also cause human rabies disease. Because current rabies prophylaxis is designed specifically for RABV, it is variously effective against these other viruses. In particular, rabies prophylaxis is unlikely to be protective against Lagos bat virus.31
The risk of rabies infection is related to several factors. These can include the amount of virus transferred through an exposure, often reflected in the amount of saliva transferred through non-intact skin or mucous membranes. Bites through thick clothing may significantly reduce this by removing saliva from the teeth of infected animals. Multiple bite injuries also increase this risk. Bite location is important since bites to the face and neck are riskier than bites to the extremities.32
Another important consideration is that many bats have tiny, sharp teeth that can pierce or scratch skin in a virtually unnoticed way.13,33 Infected saliva contacting and entering pre-existing wounds can also lead to viral transmission. Aerosolized virus that is inhaled into the lungs can be another mode for rabies infection, though realistically the risk of aerosolized virus is highest in laboratory settings.9,10
The incubation period for rabies ranges from a few days to multiple years, as the virus can remain dormant in some cells and become active at a later date. Rabies travels in the body through the nervous system via axonal transport toward the brain. Wounds near the head and neck may, therefore, shorten the incubation time. Overall, about 75% of those infected become symptomatic within the first 90 days of exposure.34
The initial symptoms of rabies are relatively non-specific and include fever, malaise, headache, loss of appetite, nausea, and vomiting. Numbness, tingling, or pain at the wound site may also occur. More advanced symptoms of infection include hallucinations, anxiety, agitation, bizarre behavior, biting, and hydrophobia, which is a heightened irritant reflex of the throat muscles leading to throat spasms, especially involving swallowing fluids. Other manifestations of advanced infections can involve different symptoms focusing more commonly on wounds to the extremities, where paralysis, usually going up the body, is the predominant symptom.
This can be accompanied by headache and neck stiffness, followed by confusion and coma. Once these signs and symptoms occur, treatment is unlikely to be effective, and the disease is almost invariably fatal.8,35 Therefore, the prevention of disease by safety training and exposure risk reduction, pre-exposure prophylaxis with rabies vaccination per Advisory Committee on Immunization Practices (ACIP) guidelines, and effective post-exposure management, is essential.
Medical Surveillance and Pre-Exposure Planning
The U.S. Public Health policy requires that medical surveillance be provided for animal workers,36 and this should include an evaluation of medical history, allergies, immunocompromising conditions, and immunization status. Tetanus/diphtheria, or TdaP, vaccination should be provided per the CDC, generally every 10 years as a routine,37 and individuals should be current on coronavirus vaccination along with other appropriate travel medicine vaccinations determined based on the individual and area of travel. The CDC travel medicine resources38 or other local recommendations should be reviewed.
When planning field work in areas where rabies is endemic or for field work involving the capture of live or dead bats, it is important to implement programs for pre-exposure vaccination, periodic rabies titer checking, and plans for prompt post-exposure rabies prophylaxis. Other bat zoonoses present in the region of study should also be considered. This means a plan for medical service access, and oversight should be in place before the bat-related field work.
Pre-exposure planning is critical for those working with potentially rabid animals. Safety protocols and PPE (such as leather or puncture-resistant bite-proof gloves) should be available to reduce the risk of exposures. A careful review of the animal species and risk for exposures should be assessed in advance to determine the need for pre-exposure prophylaxis with rabies vaccination. Those directly handling potentially infected animals or working with viable virus are among those at highest risk. Fieldwork also entails the risk of exposure to rabid non-study animal species in endemic areas.
In May 2022, the ACIP published modifications to pre-exposure rabies prophylaxis (PrEP) (Table 2).39 The modifications, which were accepted by the Director of the CDC, include:
Table 2.
Updated risk categories for pre-exposure rabies prophylaxis
| ACIP risk category | Description | Notesa |
|---|---|---|
| 1 | Recognizes elevated risk for unrecognized and recognized exposures, including unusual or high-risk exposures, such as with people performing testing for rabies in diagnostic laboratories. | |
| 2 | Recognizes an elevated risk for unrecognized and recognized exposures. Level 2 is applicable to persons who frequently handle bats, have contact with bats, enter high-density bat environments, perform animal necropsies, enter bat roosts, or who collect suspected rabies samples. | Recommend rabies antibody titers checked every 2 years; a booster dose should be administered if titers are <0.5 IU/mL. |
| 3 | Recognizes an elevated risk for recognized exposures and sustained risk and is applicable to people who interact with animals that could be rabid, including occupational activities that typically involve contact with animals, people who handle wildlife reservoir species, and those who explore or study caves. In addition, Level 3 encompasses those who might have difficulty getting prompt access to safe PEP, such as in rural areas or far from the closest PEP clinic. | Recommend either rabies antibody titers checked during years 1–3 after completion of the 2-dose primary series (and a booster dose if the titer is <0.5 IU/mL) or preemptively receive a one-time IM booster dose of rabies vaccine no sooner than day 21 and no later than year 3 after completion of the 2-dose primary series. |
| 4 | Covers the same population as Level 3 except that the risk duration is less than or equal to 3 years, for example, a short-term student project with hands-on animal care less than 3 years after PrEP administration. |
Those who have completed the pre-exposure rabies vaccine series should be informed that if a high-risk exposure occurs (consult with public health on need for post-exposure vaccine after any potential exposure), they will also need PEP with additional doses of a modern cell-culture vaccine administered as soon as possible with a second dose 3 days later (days 0 and 3). Remember that those exposed who have not completed pre-exposure vaccine will need post-exposure vaccine: 4 doses as well as rabies immune globulin.
ACIP, Advisory Committee on Immunization Practices; IM, intramuscular; IU, international unit; PEP, post-exposure prophylaxis; PrEP, pre-exposure rabies prophylaxis.
Adapted from ACIP Guidelines.39
redefined risk categories,
fewer vaccine doses in the primary PrEP schedule (2 doses instead of 3),
flexible options for ensuring long-term protection, or immunogenicity,
less frequent or no antibody titer checks for some risk groups,
a new minimum rabies antibody titer (0.5 international units [IUs]) per mL), and
clinical guidance, including for ensuring effective vaccination of certain special populations.
Biosafety and occupational health professionals, as well as researchers, are encouraged to review the entire ACIP guidance for complete information on determining risk category and associated recommendations.
The ACIP recommendations for risk categories include five groups, with level 1 involving activities with the highest risk and level 5 involving those with the lowest risk. With regard to fieldwork with bats, levels 1, 2, 3, and 4 are pertinent. Level 1 recognizes elevated risk for unrecognized and recognized exposures, including unusual or high-risk exposures, such as with people performing testing for rabies in diagnostic laboratories. Level 2 recognizes an elevated risk for unrecognized and recognized exposures.
Level 2 is applicable to people who frequently handle bats, have contact with bats, enter high-density bat environments, perform animal necropsies, enter bat roosts, or who collect suspected rabies samples. Level 3 recognizes an elevated risk for recognized exposures and sustained risk and is applicable to people who interact with animals that could be rabid, including occupational activities that typically involve contact with animals, people who handle wildlife reservoir species, and those who explore or study caves.
In addition, Level 3 encompasses those who might have difficulty getting prompt access to safe PEP, such as in rural areas or far from the closest PEP clinic. Level 4 covers the same population as Level 3 except that the risk duration is less than or equal to 3 years, for example, a short-term student project with hands-on animal care less than 3 years after PrEP administration.
The minimum acceptable rabies antibody titer level historically recommended by ACIP is one resulting in complete neutralization of RABV at a 1:5 serum dilution by the rapid fluorescent focus inhibition test, which is approximately equal to a titer of 0.1–0.3 IU/mL. The ACIP recognized that most published studies use 0.5 IU/mL as a correlate of protection, and thus this is the level now endorsed by ACIP.
The ACIP recommends that all people for whom rabies PrEP is indicated receive 2 intramuscular (IM) doses of HDCV (human diploid cell vaccine) or PCECV (purified chick embryo cell vaccine) on days 0 and 7. People in the newly defined risk category 2 should have rabies antibody titers checked every 2 years; a booster dose should be administered if titers are <0.5 IU/mL. For those in risk category 3, ACIP recommends they either have rabies antibody titers checked during years 1–3 after completion of the 2-dose primary series (and a booster dose if the titer is <0.5 IU/mL) or preemptively receive a one-time IM booster dose of rabies vaccine no sooner than day 21 and no later than year 3 after completion of the 2-dose primary series. As a reminder, those completing the PrEP series do need post-exposure treatment with 2 doses of rabies vaccine administered on days 0 and 3.
Modern rabies vaccines have been safely administered to people of all ages, including pregnant and immunocompromised people. An adequate immune response is anticipated among all immunocompromised people who receive rabies vaccines in accordance with the ACIP recommendations. However, among people with primary or secondary immunodeficiencies, the immune response to vaccines, including rabies vaccine, can be suboptimal. The ACIP recommendation is to delay vaccination until a temporary immunocompromising condition has resolved or immunosuppressive drugs can be withheld. If an immunocompromising condition cannot be temporarily reversed, the ACIP guidelines indicate that rabies vaccines can be administered but with specific indications and timelines for titer checks and booster doses.
The May 2022 ACIP guidance also details how deviations from the recommendations should be managed. When people change activities that may result in a new risk category, the recommendations of the new risk category should be followed. We note, in addition, that new rabies vaccine products are under development and may result in new recommendations for fieldworkers and others at risk for rabies exposure.40,41
Emergency Response and Incident Management
It is important to plan for any urgencies and emergencies that could develop during field work. Having the appropriate information and supplies, including first aid kits for which individuals are trained, to handle the urgencies and emergencies, as well as the ability to manage the incidents remotely, are key to a safe field work experience.
Rescue planning includes being able to signal distress using two-way satellite messaging devices and planning for needed post-exposure medical care, as well as the potential need for evacuation to higher levels of care via contracted international travel safety organizations. Many travel safety organizations offer subscription services to provide emergency assistance, including medical evacuation services when needed. These resources should be evaluated and put in place before the field work.
As some field work trips may be to locations that do not allow for easy communications with the home base, it is important to plan for emergencies that may occur and determine how such communications can be facilitated, including names and numbers of key personnel, such as occupational health physicians and clinics. Satellite two-way messengers are recommended for emergency communications in remote locations where cell phone coverage is lacking. Emergency response or rescue personnel should be familiar with the conditions of the local environment if researchers need assistance.
For example, when working in a cave system, rescuers should be trained in underground rescue missions. As with all field work, but particularly caves, someone not directly participating in the current activity should be aware of where researchers are working and what time to expect them to return from the location.
Written emergency plans and first aid preparations are essential when organizing travel for field work. The University of California Field Operations Safety Manual includes sections on incident response that can be used as basic reference for developing site-specific plans.42 A field work emergency response kit should be prepared in advance of the trip, and all field personnel should be familiar with the kit, the kit contents, and procedures for using the kit after an exposure. Kit contents should be customized for destination, tasks, hazards, group size, and level of training.
Recommended contents for the kit include, but are not limited to, bandages and skin dressings, trauma shears, antibiotic ointment, gauze pads, rescue mask, Coban wrap, cloth tape, gloves, 12 cc irrigation syringe, elastic wrap, antiseptic towelettes, gloves, and sterile saline, as well as possibly epinephrine injectors for anaphylaxis treatment. Alternatives to some items could be considered—for example, a 12-cc irrigation syringe and sterile saline may be hard to find in some locations, and they could be substituted with a bottle of water, a clean toothbrush, and a small tube of dish soap. Another alternative may be alcohol wipes, which will inactivate many viruses (though not all) and serve as an indicator of a break in skin if a burning sensation occurs.
The kit contents should be in a container that will withstand travel and any environmental conditions, such as moisture and temperature extremes. Kits may be assembled by the researchers or are available commercially from various vendors. The contents should be regularly checked to ensure they are not damaged, missing, expired, or malfunctioning. A tabletop drill is recommended to familiarize field personnel with the types of emergencies that may warrant use of the kit, as well as how and when to use the kit contents. First aid training contributes significantly to overall safety in field work.
In the event of an exposure in the field, it is essential that rapid and thorough wound cleansing with soap and water or saline be performed after an exposure, preferably for a full 15 min, to remove any potential RABV (or other infectious agent) before it can enter the peripheral nervous system. Cleansing should be gentle enough to avoid tissue damage, as this might increase transmission risk. It is also recommended that treatment is sought at a health care facility after initial cleansing.43 Individuals vaccinated pre-exposure will not need rabies immune globulin (RIG), a product not widely available in many areas of the world, particularly in remote areas, though post-exposure rabies vaccination will still be required as detailed by the CDC.44
Often, personnel are unable to return to a sink immediately after an exposure (e.g., a bat bite). In this case, personnel should work as quickly as possible to find a stopping point that is safe for the animal and themselves, and then cleanse the wound with whatever is available (e.g., antiseptic towelette, hand sanitizer) as rabies is sensitive to most detergents and alcohol. Immediately on return to a sink, the researcher should wash the bite location as instructed and seek PEP in the form of two additional rabies vaccines (e.g., PEP regimen) administered on days 0 and 3.
It is recommended that before commencing field work, researchers contact a local pharmacy or hospital and arrange for them to hold enough rabies vaccine to provide PEP for all personnel handling bats. It is important to note, however, that rabies vaccines are often very expensive and a limited resource in developing nations; in addition, the availability of specific rabies vaccines may differ by geographic location. Researchers coming from areas of greater vaccine availability should be mindful of resources around the field site and prepay for any vaccines ordered, regardless of whether they are used.
One unresolved issue in bat fieldwork and medical surveillance is the issue of repeated exposures within a brief period. Laboratory workers have some control over their safety and mechanisms of exposure, and therefore they are unlikely to have repeated exposures in a brief period (provided appropriate safety measures are in place and observed). However, often personnel working with animals, especially researchers who conduct their work in a short, intense field season, are exposed multiple times within a brief time frame.
Most medical doctors agree that after the first exposure, a person with adequate rabies PrEP should start the 2-dose PEP regimen on days 0 and 3. If another exposure (e.g., bat bite) occurs within a brief period after, the general consensus is that more post-exposure prophylaxis is not warranted (Personal communication; G. Balsamo, May 9, 2022). However, to our knowledge, there have been no formal research or recommendations on how long the PEP course should be considered protective, or how to balance the competing goals of (1) preventing rabies, which is almost invariably fatal, (2) preventing adverse reactions from overexposure to the vaccine, and (3) conserving valuable vaccine resources.
In our experience, each researcher or research group is usually left to decide for themselves about this question. A discussion with the Louisiana State Public Health Veterinarian/Assistant State Epidemiologist and his colleagues yielded the suggestion that any bite occurring within 60 days of PEP or PrEP (e.g., the time period during which antibody response to the first PEP course is peaking) should not be treated with additional PEP, but a subsequent exposure beyond 60 days would warrant additional PEP (Personal communication; G. Balsamo, May 9, 2022). We agree with the logic of this, and in the absence of more data, and suggest that researchers consider adopting this schedule if an exposure occurs within 60 days of the completion of a previous PrEP or vaccine series. The question deserves actual focused study and determination.
If an incident occurs during travel or field work, an incident report should be completed and filed with the entity sponsoring the field work, preferably within 24 h of the injury or illness. Individuals experiencing respiratory illness, rash, flu-like, gastrointestinal, or any other illness after field work should discuss the zoonotic potential with a health care professional.
Training
Training is an important component of preparing for any work, including field work with bats. Standard safety training, such as those required for biosafety, bloodborne pathogens, or animal work, should all be completed as applicable. Additional training specific to the work to be performed should also be completed, and competence demonstrated (as well as documented) in practice sessions before performing work in the field. This may include agent-specific training if work will involve bats with known or suspected zoonotic diseases, such as rabies, but also training in how to handle the animals, in the procedures that will be performed, and with the equipment that will be used.
Biosafety and veterinary staff should work together to discuss and design appropriate training. If the institution's veterinary services do not employ personnel with experience in bats, then it may be possible to collaborate with other academic institutions, local zoos or wildlife agencies, or other bat researchers or organizations who can provide the necessary knowledge and overview of the skills to train researchers.
Field work, both the location and general conditions, may also necessitate additional types of training. All researchers who perform field work should have some basic general outdoor training, which may include outdoor safety training, field first aid training, orienteering, map training, etc. If institutions have outdoor clubs or recreational groups, there may already be people who are providing this training. Other options for training may include those offered by local outdoor clubs, retail establishments, or professional outdoor associations.
If field work will occur only during the day, at a location that does not pose specific additional hazards, and within commuting distance of the institution, this may be sufficient. Prolonged field work, particularly if it requires staying at the site, may require other types of specific outdoor training. Consider if training should include camping basics (i.e., planning and packing, tent set-up, cooking, etc.), dealing with weather hazards such as rain, snow, cold or heat, or physical hazards such as carrying equipment and supplies or traveling long distances or over rugged terrain. In this last instance, some physical fitness training may be warranted.
Beyond general outdoor training, work conditions should also be considered. For example, if work entails entering caves to trap or handle bats, researchers may also need training in cave safety and in specific equipment used in caves. If work involves ascending heights, such as when bats roost in the eaves of buildings or within rocky crevices, training in ladder safety or safe climbing techniques will be needed. Evaluation of work conditions such as these should be part of the risk assessment process and recommended or necessary training part of the conclusions of the assessment.
Finally, work at field sites may often be further from emergency response than standard laboratory work. Therefore, researchers should be trained at a minimum in basic first aid, which is often included in basic outdoor training, and in basic emergency responses, such as cardiopulmonary resuscitation, if field sites are not close to emergency medical services. Wilderness first aid training is also advisable. If emergency supplies such as a satellite communication device or a first aid kit are provided, researchers should be trained in the specific use of these items. It is also recommended that tabletop emergency planning and exercises be discussed and practiced, especially if the field work is a significant distance from emergency services, or in a foreign country where evacuation may be needed in a medical or other emergency situation.
Personal Protective Equipment
The PPE is vital for field work, particularly considering the absence of engineering controls and the myriad of potential risks posed by the environment. To determine the specific PPE needed for each unique situation, a risk assessment must be conducted to evaluate all the potential hazards that could be encountered. The hazards directly associated with the research, such as handling subject animals or entering caves, are often straightforward and easier to anticipate and implement appropriate controls.
However, other hazards exist in the field that are not part of the research but are present nonetheless, for example, traffic hazards when performing work near roadways, infectious diseases that may be transmitted from animals, water hazards such as drowning or hypothermia, and hazards posed by animals, plants, or the environment that are not the subject of the research. This article focuses on handling bats in the field, but these other potential hazards should be anticipated and mitigated. When considering types of PPE, be aware that it can itself create hazards to the researchers or the animals that must be carefully considered, and PPE appropriate to the field work selected. Unfortunately, PPE cannot be prescriptive; rather, it must fit the needs of the researchers and project. The PPE that should be considered when handling bats in the field includes, but may not be limited to, gloves, respirators, protective clothing, and appropriate footwear.
Although researchers are frequently aware of the hazards of their work, it is a common struggle in the biosafety profession to ensure that researchers always wear appropriate and adequate PPE. This lack of adherence to certain biosafety practices, especially PPE, may jeopardize both the safety of the bat and the handler.45 The Bat Conservation Trust (BCT) encourages the use of gloves when handling bats.46 Use of gloves is the first line of defense against bat bites and RABV, and it is important to review how much puncture protection different glove materials provide when selecting gloves.47
Gloves should protect not only from bites, but also infectious disease transmission between the human and the animal. When choosing gloves, dexterity should be considered so as to not create additional hazards or possible harm to the animal. Puncture-resistant gloves or needlestick-resistant gloves will offer high levels of protection from bites, but if they impede the wearer's dexterity in a way that increases other risks associated with handling or may result in injuries or improper restraint to the animal, or cannot be decontaminated easily, they may not be suitable for the job.
Risks to both the handler and the animal must be considered. An example of a situation where gloves might need to be briefly removed would be to use fingernails to gently disentangle a particularly trapped bat from a mist net, though gloves should be donned again as soon as feasible. A possible alternative would be to use hand sanitizer between animals, wash hands thoroughly as soon as possible, and use gloves for any further handling.
If any chemicals are used in the research process, consider if the type of glove is appropriate for the chemicals in use. Glove manufacturers should provide chemical compatibility information for their gloves. In addition, personal preference and reactivity to certain types of gloves, such as allergies to latex gloves or powdered gloves, must be considered to ensure the researcher is adequately protected.
Depending on the environment, respiratory protection may need to be considered. Agent transmission and availability of circulating or fresh air at the worksite should be considered when making this determination. A respirator may not be needed in open spaces but could be highly recommended in a dusty cave with large deposits of accumulated dry bat guano. Types of respiratory protection appropriate to the agent and the work conditions should be evaluated, and personnel using required respirators should be enrolled in a respiratory protection program that provides adequate assessment, medical clearance, training, and fit testing as needed on an annual basis and in compliance with the United States Occupational Safety and Health Administration (OSHA).48
In addition, as has been demonstrated by COVID-19, there may also be a need to protect the animals from pathogens exhaled by the researchers. Spillback protection for wildlife has come to the forefront during the COVID-19 pandemic, with several species besides humans contracting the disease, including gorillas, deer, dogs, and cats.49 This is a reminder of the impact that humans have on the environment, and that precautions need to be implemented to protect the subjects of the research and other animals that researchers could encounter during the course of their work. SARS CoV-2 may have originated in bats and therefore could spill back into a naive population.17
In light of these concerns, The National Wildlife Control Operators Association recommends that “a face mask be worn to block or minimize the exchange of respiratory droplets. An N-95 respirator is ideal.”50 This Association also recommends disposal of filters and decontamination of respirators in accordance with WNS decontamination protocols20 to prevent the spread of this disease among bats.
There is evidence that face coverings have protected gorillas in Volcanoes National Park in Rwanda.51 The Park now requires tourists, park personnel, researchers, and other people encountering gorillas to wear face masks, which had not previously been mandated. According to Gorilla Doctors, “Since March 2020, the number of outbreaks of respiratory illness among the park's gorillas has fallen to 1.6 a year, on average, from 5.4.” Thomas Gillespie, a disease ecologist at Emory University, has stated, “The same types of things that can protect wild animals that are susceptible to COVID-19 can also protect them from other human pathogens. Many of these best practices can be applied very successfully to other endangered and threatened species. People need to be doing these things, COVID-19 or not.”52
Therefore, it is prudent to use face coverings or respirators while interacting with bats and other potentially susceptible wildlife. Vaccination, self-monitoring for symptoms, and COVID-19 testing before close interactions are additional measures that can be taken to protect wildlife, as well as prevent future crossover events from occurring.
One aspect of PPE that can be a struggle to ensure researchers adhere to is the use of eye protection, but the eyes are a direct path into the body and offer little immune protection. Something as benign as a bat sneezing on a researcher and resulting in saliva contact with eyes could result in the transmission of rabies, as noted by CDC.9 Eye protection will also protect the researcher if they are working while looking upward toward associated hazards in trees, caves, or mist nets; hazards in these situations include dust, dander, or other falling debris that could drop into the eye. In addition, eye protection can prevent inadvertent contact between contaminated gloved hands and the eyes.
The choice of eye protection will again be dependent on the environment, as fogging can be a concern in humid climates. It could be useful to consider anti-fogging protection on glasses offered by manufacturers, or the application of anti-fog gels such as those sold in the SCUBA industry. Eye protection needs should be considered in the risk assessment to determine whether safety glasses, goggles, or face shields are the most appropriate, given the risks of the work and other factors, such as environmental conditions. Prescription eye protection should be considered as needed.
Protective clothing will be highly dependent on the environment. For example, entering cool caves will require different clothing than sampling in a humid jungle environment. Long pants, long sleeves, and protective footwear are standard in a work environment but may cause heat stress in a field setting if they are too thick or are not made of breathable fabric. Cold weather will require protective layers. Lab coats are not generally feasible in a field environment; researchers and biosafety professionals should work together to assess the most appropriate type of protective clothing for the work and conditions. Footwear should be appropriate to the terrain and hazards of the work.
Clothing should be laundered between sampling events or when contaminated, and when that is not practical, such as in a remote field setting, it can be covered with a liquid-resistant suit or sleeves or similar dedicated protective gear. Be sure to consider whether the material in question is appropriate, as liquid-resistant types of materials in a hot and humid environment may cause heat stress or other issues. When shared facilities, such as a laundromat, are the only option (instead of dedicated PPE laundry machines), clothing used in field work should be laundered independently of other clothing, using the hottest water temperatures appropriate for the clothing and bleach or an appropriate United States Environmental Protection Agency (EPA) registered detergent.53
Aside from gloves, respiratory protection, protective clothing, and appropriate footwear, additional PPE considerations may be warranted based on conditions or the environment. Reflective gear for working near roadways and redlights for seeing at night while not disturbing the animals should be considered when developing a field safety plan and determining necessary supplies. Consider the use of blaze orange if working in an area with an open hunting season, or personal flotation devices if working around water. Caving equipment, climbing equipment, and other PPE, such as helmets, specific to the work will need to be considered as well.
Disposable PPE should be worn, or PPE should be disinfected between handling animals to prevent the spread of pathogens such as P. destructans, the causative agent of WNS. Depending on the situation, disposable gloves should ideally be changed between handling individual animals; if this is not practical, gloves should be disinfected using appropriate methods. However, this may weaken gloves over time, so gloves should also be checked after each use to ensure they are still intact and providing adequate protection.54 Disinfecting PPE, along with all equipment, should occur after field research (see the Decontamination and Waste section). Closed toed shoes should be utilized in any work environment and they should also be disinfected between worksites, as one method of spreading WNS is contaminated human footwear.55
Safety Considerations for Work Practices and Sampling
Obtaining samples from bats can be a challenge to perform safely. Manipulation of bats represents a risk to both the human and the animal. Every time sampling or handling of a wild animal occurs, a potential risk of exposure exists. When working in the field, one of the main biosafety objectives is the manipulation of the animal while preventing contact with potential pathogens. Although there are no established biosafety levels for field work, the approach of the Laboratory Biosafety Manual, 4th edition, from the WHO and other organizations encourages careful consideration of the identification, mitigation, and control of risk factors of the work, such as potential pathogens, routes of exposure, and the presence of any hosts or vectors.56,57
If capture of bats must be performed as part of the work, planning and risk assessment must adequately address, minimize, or mitigate the risk of contact with potential pathogens. Protocols should be planned in such a way that catching seasons can be scheduled based on the minimum needs to accomplish field work objectives.58 Researchers, in conjunction with their IACUC, should also ensure that part of their planning is evaluation of the number of animals to sample so that proper statistical evaluation of their data can be utilized.
Protocols may be planned to maximize the amount of data that can be obtained through a single study. For example, given effort, cost, and risk of fieldwork, epidemiological surveillance in bats could focus on more than one etiologic agent. Other examples include precise record keeping on various data points, such as gender, species, location, health status, etc., to ensure not only complete datasets but also datasets that may be useful for future investigations. Keep in mind that risk assessment and mitigation should address all possible agents to reduce the risk of exposure to animals and specimens.
Planning and Preparing Personnel, Equipment, and Supplies
Preventative safety measures start with good personal hygiene, as well as cleaning and disinfection of all equipment and material used (see the Decontamination and Waste section), to minimize the possibility of transmitting hazards at the field site. Some of these risks have been addressed in the Risk Assessment and Pre- and Post-Exposure Prevention and Medical Surveillance, Emergency Response, and Incident Management sections. In addition, the use of PPE (see Personal Protective Equipment section), including appropriate clothing, and the adoption of specific protocols designed to include safety measures appropriate to mitigate the risks identified in the original assessment are essential.59
As discussed earlier, PPE needs should be determined based on the risks and hazards of each activity. For example, measuring morphological parameters (e.g., length, weight, etc.) may not represent the same risk as collecting blood or other biological fluids. It will be important for the PPE to address the potential routes of exposure in the field (e.g., bites, aerosols) as well as experimental activities, such as the use of sharps. All necessary PPE should be available at the field site, in sizes and fittings appropriate for each individual researcher. Finally, all personnel should be trained in the risks and planned activities for each experiment.60 This should include safety measures for basic tasks, including those discussed here.
Setting and Checking Nets and Traps
Several methods frequently used to catch bats include hand nets, cone traps, harp traps, and mist nets.61 Nets and traps will need to be considered potential sources of fomites, and during and after use, the manipulation of these should always be done with appropriate PPE, including gloves. If capture occurs inside a cave or roost, consider additional PPE needs. The risk of infection by H. capsulatum and some Lyssaviruses may be high in these particular areas due to the increased presence of aerosolizable material containing these agents, such as bat guano.11,62 Goggles or other forms of eye and mucous membrane protection should also be considered, particularly when working in a manner that requires the researcher to look up while working, as this increases the risk of particulate matter falling into the eyes.
Nets and traps must be checked frequently, particularly if there are large numbers of bats in the area and researchers expect traps to fill quickly. Bats should never be left entangled in the net for long periods of time, as they can suffer severe injuries, complicating their release. In addition, predators could be attracted by immobilized animals, and this may represent a risk for the bats and the researchers. Bats can also be prone to hypothermia or catecholamine-induced shock, so capture and sampling must be a delicate process and involve the minimum time necessary to perform the planned experiments. Prolonged capture must be approved by the IACUC.
Restraining Bats During Capture and Sampling
It is important to minimize capture and holding time when working with animals. Multiple methods are available to restrain animals during this process, and these should be reviewed with the IACUC for the one most appropriate to the experiment and animal. Consider methods that will keep animals separated, such as individual cloth sacks, to avoid fighting and stress. Experimental manipulations themselves may be stressful to the animals, so proper training in the procedures should be done before work with live animals to minimize handler error or incident, or unnecessarily prolonged restraint or capture. For example, new personnel should be trained to never hold a bat with the wings fully stretched to avoid injuries to the shoulder or elbow of the animal.
If animals need to be restrained for a significant period, it may be advisable to hydrate animals before collecting samples, particularly if sampling involves collecting blood.63 When administering oral liquids, it is important to hold the bat head-down to prevent the liquid reaching the respiratory system (Figure 1). Oral fluids should be administered through a syringe (no needle) or transfer pipet, in a slow and controlled manner, allowing the bat to swallow ad libitum.
Figure 1.
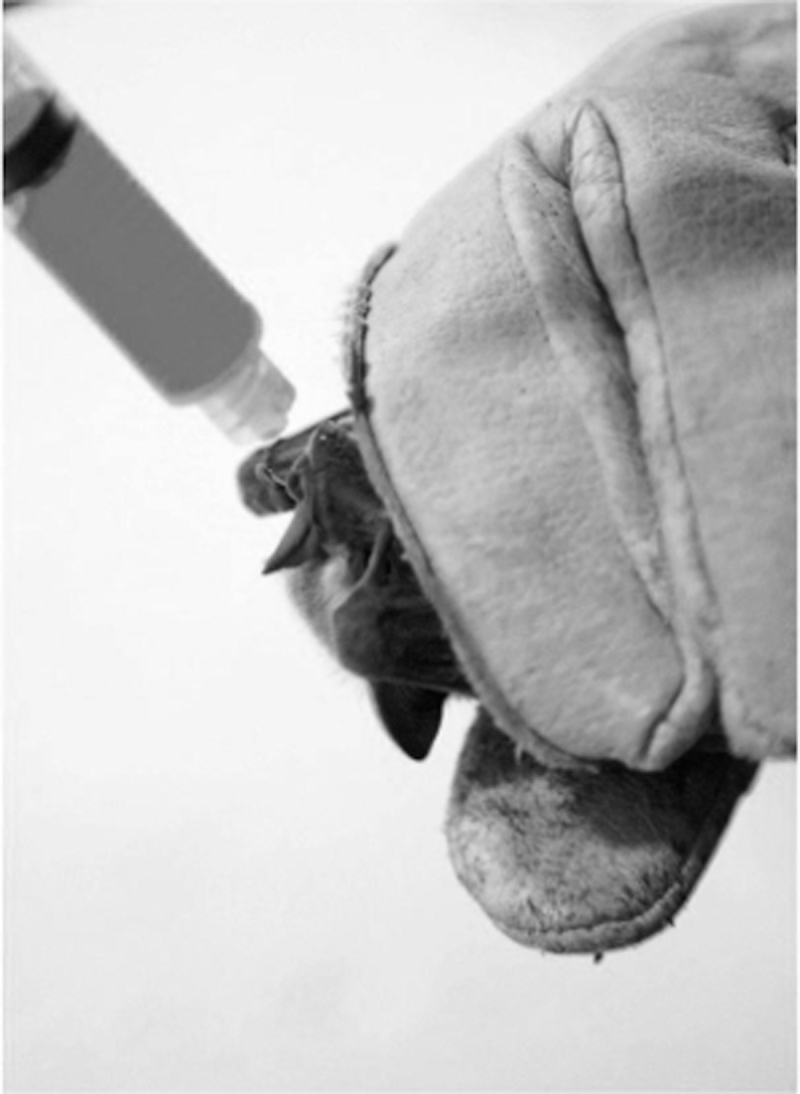
Administering fluids before blood draw is recommended. Hold a bat head-down when administering oral fluids to prevent liquid reaching the respiratory system. (Picture courtesy of Alvaro Aguilar-Setién.)
The authors have observed that hematophagous bats accept Hartmann's or Ringer's solution for oral hydration, whereas large frugivorous species such as Artibeus jamaicensis or Artibeus lituratus accept commercial beverages generally used to help humans recover carbohydrates and electrolytes, and nectarivorous species gladly accept sugar water. Many formulas have been reported for the rehabilitation of lactating or juvenile bats.64 Subcutaneous fluids may also be given, such as lactated Ringer's solution or 0.9% sodium chloride solution.65
Tagging or Marking Bats
The ability to identify individual animals is an important aspect of ecological and epidemiological research. Identification of individuals can provide information on specific animals or the population, including data on migration, territory, survival, or even long-term immune status against various microbial agents. Researchers should consider whether marking will be important in the short-term, such as a few days, or long-term, such as between one season and the next. Many short-term marking methods exist, including the use of non-toxic colored markers, non-toxic adhesives, or fluorescent tape.66
Long-term marking methods include plastic or metal rings fixed over the forearm. Bat bands, or aluminum-magnesium bird rings, between 3 and 5 mm, depending on the size of the bat, can be considered. The authors have observed that certain bird rings are durable, effective, and do not harm animals, as supported by longitudinal studies following hematophagous bats individually for up to 4 years using this method (Figure 2). However, multiple studies have demonstrated that the use of metal bands can result in injury and reduced fitness, and various societies have issued position statements recommending against their use for certain species.67–70 Therefore, the use of metal bands should be carefully considered in conjunction with the IACUC.
Figure 2.
Hematophagous male Desmodus rotundus bat marked with an aluminum-magnesium ring on the forearm; this individual was followed for more than 4 years. (Picture courtesy of Alvaro Aguilar-Setién.)
Another common long-term tagging method is the use of PIT tags, also known as microchips. These consist of small integrated circuit chips enclosed in a biologically inert glass capsule. Appropriate-sized PIT tags can be inserted under the skin using a 12-gauge needle. Alternatively, they can be glued to the dorsal fur of bats. Some PIT tags may be relatively large compared with the bat species being handled, so researchers should be careful to avoid punching through the bat's skin and poking themselves or a helper with the needle. It is advisable to use forceps to tent the skin so that a researcher's hands are further from the needle. This method is very effective and may provide advantages over metal rings in terms of lower injury rates and increased ability to identify and track animals without re-capture but is also more expensive initially.
Other issues with PIT tags may include tag loss, movement within the body, or detection issues, which can be exacerbated in smaller tag sizes.71 However, it is important to consider the body weight of the animal relative to the tag weight; this may be regulated, with some governments setting limits on the maximum chip weight as a function of body weight.72 Therefore, researchers should consider the impacts of long-term PIT tags on bat physiology, such as the potential for reduced fitness due to tag weight or changes in behavior due to tag readers, though both of these issues have been demonstrated to be negligible for certain bat species.73,74
Basic Hazards and Precautions During Sample Collection
Collecting samples represents a risk if it involves potential direct contact with tissues, fluids, or other secretions, or the potential for aerosols. When manipulating bats, important precautions include not only PPE and disinfection of equipment before use, but also minimizing exposure to excreta, avoiding the creation of aerosols, and adequate anesthetizing of animals before handling them, if needed.60 Although RABV is not transmitted by viremia, other infectious agents may be present, so it is important to treat all samples, such as tissues, organs, or fluids, as potentially infectious to prevent exposure.
Good microbial techniques will also prevent contamination of these samples during the collection process. Cross-contamination can occur between the researcher and the animal, or from one animal to the next, so it is important to change PPE such as gloves between each animal, or to use an effective disinfectant (see the Personal Protective Equipment section). Samples should also be collected and stored securely to prevent cross-contamination during later stages of work, such as transport.
Collecting Feces and Urine Samples
Collecting excreta such as feces and urine may be the most successful after bats have fed, such as when they return to a roost. It is important to place the bat in a container that will collect the sample, protect the bat, and prevent any incidents such as bites. A handmade example is shown in Figure 3. A plastic bag to collect the sample is inserted into a hard-sided plastic container. The bat is placed into this bag, and the lid is placed over the container. The lid should have an air hole covered by soft mesh—this provides the animal access to fresh air as well as a place to hang. Excreta can be captured in the plastic bag, and the bat eventually released.
Figure 3.
Handmade container for urine and feces collection. Soft mesh (1) and a lid with an air hole (2) restrain the bat within the plastic collection bag (3) that lines the hard-sided plastic container (4). (Picture courtesy of Alvaro Aguilar-Setién.)
Using Anesthesia
Anesthesia may be recommended where possible when sampling from live animals, as sampling from non-anesthetized bats increases the probability of bites or scratches. Use of anesthesia should be discussed with the IACUC, and methods should be appropriate to the field site and work being performed (time needed for sampling, methods, etc.). Risks to the researchers as well as survival risk to the animal must be considered to determine whether anesthesia is appropriate, or whether sedation protocols where the animal is not fully anesthetized may be more appropriate.
If anesthesia is determined to be necessary, adult bats can be anesthetized using a variety of injectable anesthesia,75,76 and the appropriate type and dose should be evaluated based on bat species, length of restraint time needed, and in conjunction with the IACUC. When choosing a method, consider that animals dosed with injectable anesthetics may have a prolonged recovery period, in which case survival can be compromised. It is important to not release bats until they are fully recovered, so the use of longer acting anesthesia methods should be evaluated for anesthesia duration versus experimental time needs, effects on the bat, and whether time and an adequate process is available to help bats recover before release.
Another anesthesia method involves the use of inhaled isoflurane, which can be performed in the field using a soaked cotton swab and a holding container like that shown for excreta collection in Figure 3. This method, often referred to as “open drop method,” should only be used if the dose of isoflurane can be measured based on the size of the container to ensure adequate induction of anesthesia while minimizing risk of accidental euthanasia. Although it is a fast-acting anesthetic with shorter recovery times than some injectables, researcher exposure to isoflurane during use can lead to various ill-effects on health if not done properly.
Therefore, training and appropriate induction methods must be reviewed before use, and use in confined spaces or where there is limited natural ventilation, such as caves, should be restricted unless absolutely necessary. Also note that transport or use of isoflurane in glass bottles can lead to potential for broken glass and spills of chemicals, so consider plastic-coated bottles if available, proper spill cleanup methods, and training for researchers on methods to prevent overexposure. Overall, it is important to consider the appropriateness of anesthesia, the sensitivity of different bat species and different individuals to the anesthetic, and potential exposure of the researchers (to an incident such as a bite, or to the anesthesia itself) in determining whether anesthesia should be used.
Collecting Blood
Blood collection in bats can be done from a variety of veins, including the cephalic vein, saphenous or interfemoral vein, or brachial vein. The most appropriate site should be determined based on the bat species and size. Regardless of vein choice, proper handling, collection, and safety procedures should be employed. Blood is frequently collected using capillary tubes, and plastic or plastic-coated glass tubes are preferred over glass tubes for sharps safety. In larger bats, a tuberculin syringe with a 25 gauge or similar size needle may be used to collect blood and should be properly handled and discarded after sample collection.
When puncturing a blood vessel with only a thin patagium around it, consider using puncture-resistant gloves, needle stick-proof gloves, or a metal thimble to protect the finger and hand being used to restrain and stabilize the blood vessel. Before beginning blood collection work involving sharps, position a sharps container within easy reach for direct disposal during the process. This article will further discuss some safety particulars relative to the cephalic vein, but these are applicable to bleeding from other appropriate veins as well.
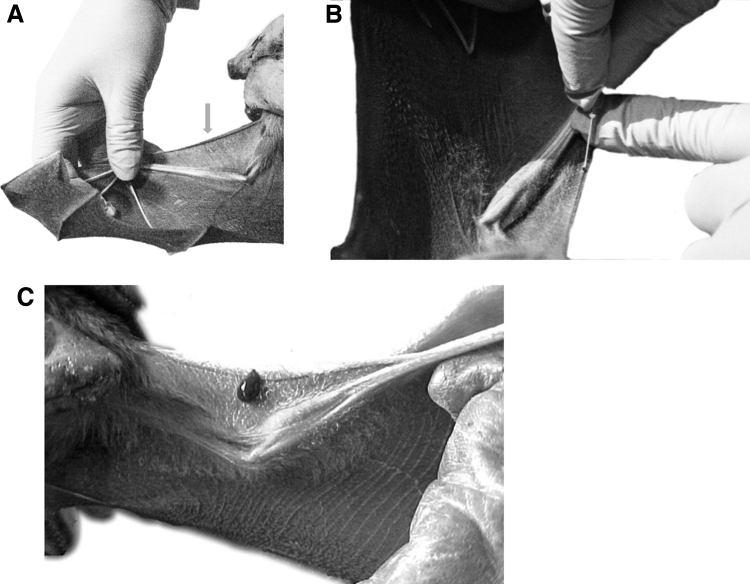
The cephalic vein, or wing marginal vein, is located in the anterior margin of the wing (Figure 4A). A small needle, such as a 25-gauge needle, can be used at a 45° angle to penetrate the vein (Figure 4B). Blood should flow readily from the insertion site (Figure 4C), at which point the needle can be removed (and disposed promptly in a sharps container), and blood collected using plastic capillary tubes, blood filter strips, or other collection methods. Depending on the species and size of the individual, as well as what is approved by the IACUC, 50–300 μL can be collected in this manner (generally <1% of the bat's body mass).77
Figure 4.
(A) Localization of the cephalic, or wing marginal vein, is indicated by the arrow. (B) A 25-gauge needle is inserted at a 45° angle into the cephalic vein. (C) After puncture, blood flows readily from the cephalic vein. (Pictures courtesy of Alvaro Aguilar-Setién.)
Collecting blood may be a process where it is advisable to use chemical restraint (anesthesia) to protect the research and the animal. Physical restraint, either by hand, which may increase risk of bites and scratches to the researcher, or through alternative means, can also be considered. Morgan et al have recently proposed a physical restraint device that could be considered. This consists of a plastic restraining chamber that allows a researcher to handle and collect blood from a bat without assistance, containing the animal and providing easy access to the wing for collection from the cephalic vein as described here, or from the saphenous vein.78
During testing, Morgan et al reported that no needlesticks, bites, or scratches occurred, and they concluded that this device may increase researcher safety while also increasing blood collection efficiency. The current authors of this field work paper caution that the study performed by Morgan et al involved testing and feedback from only five participants, and only in a laboratory, not field setting, and do not specifically endorse this device, but rather encourage researchers to consider the appropriateness of chemical and physical restraint methods, including the use of physical devices rather than restraint by hand.
Collecting Oral Swabs and Saliva
Non-invasive samples such as oral swabs and saliva can be obtained for a variety of experimental purposes, including molecular diagnostics. Oral swabs can be collected using Dacron, Rayon, or nylon swabs (Figure 5). Saliva can be collected with a plastic pipette carefully inserted into the mouth or throat. Saliva stimulators may not be recommended depending on interference with the detection of certain agents. Samples should be placed in appropriate sterile containers, alone or with media. If using chemical methods to preserve DNA or RNA for later collection, be sure researchers have been informed of the hazards and exposure potential of these chemicals, as well as how to clean up spills or decontaminate them. It may be particularly important to know whether bleach can be mixed with certain nucleic acid extraction buffers.
Figure 5.

Oral samples collected using a swab. (Picture courtesy of Nidia Aréchiga-Ceballos.)
Screw-cap tubes, particularly those with an O-ring and that close tightly, are recommended for sample containment, prevention of cross-contamination, and to avoid aerosol creation from “popping” open tops of other types of tubes such as 1.5 mL microcentrifuge tubes. These tubes can further be sealed with parafilm for additional safety. If samples are preserved, such as frozen in liquid nitrogen, be sure researchers are aware of the hazards of cryogens, particularly with respect to skin and eye exposure, burn hazards, and expansion potential of cryogenic liquids. Appropriate transport of cryogens must follow local and Federal guidance. Obtaining and handling oral samples should be considered a high-risk activity with regards to potential RABV exposure.
Collecting Tissues
Tissue collection poses a potential sharps risk, as well as risk of exposure to blood or other bodily fluids. Smaller samples, such as a skin biopsy of the ear pinna tip or patagium, can be obtained with a punch biopsy tool.79,80 Larger tissue samples, including those that result in euthanasia of the animal, should be considered carefully in terms of control methods to mitigate the potential for exposure. If samples are taken in the field, consider how to mitigate sharps risks, collect and preserve samples, allow animals to recover adequately, and clean and decontaminate the work area.
Once animals are captured, it may be preferred to transport them to a laboratory where samples can be processed in a more controlled manner; for example, animal transport to a laboratory may be needed if terminal samples need to be preserved immediately on collection. This may be ideal to allow processing of samples in a biosafety level 2 or higher laboratory. A laboratory will also provide certain engineering controls and safety items, such as eyewashes and safety showers, that are not readily accessible in the field. Any transport or shipping must follow all applicable guidelines and regulations, and planning should include if and how samples need to be pre-processed or inactivated before transport or shipping.
For field processing, choose a site that is relatively secluded and is out of sight from nearby (and unrelated) human activity, and not near livestock or other animals. An outdoor site may be preferred to provide natural ventilation but consider ways to protect researchers and animals from wind, rain, sun, or other elements during the process, such as with a tarp strung between trees. Be sure work surfaces are decontaminated before and after procedures, are clear from clutter, and provide adequate area to perform all necessary steps.
Unnecessary supplies or equipment should be stored out of the immediate area to protect from sprays or splashes, whereas items such as waste or sharps containers should be situated nearby for easy access. The PPE appropriate to the task at hand should be worn, and then removed and collected to prevent cross-contamination during subsequent activities, such as travel to lodgings at the end of a workday.
Decontamination and Waste
Planning for field work does not end with planning the experiments—it must also include how to handle decontamination and waste during and after the experiment has concluded. Plan and be prepared with adequate amounts of appropriate disinfectants for the equipment you plan to use, either to be purchased onsite or brought to the site. This may be simple, such as being able to make freshly prepared 10% final volume/volume bleach or 70% ethanol—actions that will require dilution bottles, clean water, and the relevant disinfectant.
If dilution or spraying is not feasible in the field environment, consider disinfectants that can be applied directly or are purchased ready-to-use, such as quaternary ammonium wipes that are frequently used in hospital settings. Be sure to avoid discarding soap or other disinfectants into the environment, such as any local surface waters. If transporting large quantities of chemicals, consider potential risks related to spills, exposure, and use by the researchers. The IACUC may also have guidance on the disinfection of equipment that is appropriate for field work.
For equipment that will be used in contact with bats, check that the disinfectant will not be harmful to the animals or consider ways in which to remove any chemical residue left by the disinfectant before using the equipment with animals. Be sure to also confirm that the disinfectant is appropriate for the potential pathogens (consider RABV, coronavirus, causative agent of WNS, etc.), and that the contact time is reasonable within a field setting. Consider whether the disinfectant is appropriate for the surface it will be used on; porous surfaces may require different disinfectants or different contact times than hard, non-porous surfaces.
Depending on the situation, materials may need to be disinfected and reused in the field. Reuse of materials should be based on a risk assessment that includes important factors such as the integrity of the materials (i.e., bite-resistant gloves), compatibility with the disinfectant, suitability of use under local environmental conditions (e.g., powered air purifying respirators may be a poor choice for reusable respiratory protection if there is high humidity or dusty conditions which can potentially affect filter cartridge integrity). Field disinfection could include strategies such as flame sterilization of small metal instruments such as forceps with a candle followed by a cooling time before use (proper fire safety must be observed). Larger metal items, such as cages, can be wiped or sprayed with disinfectant (bleach, 70% ethanol, iodine solutions) between uses, then rinsed as needed to remove residue.
Nets, cloth bags, or similar items should have gross contamination removed, placed in a bleach solution for an appropriate length of time, and then thoroughly rinsed in clean water. Other examples may include washing equipment with biodegradable soaps, rinsing with distilled or sterile water if available, and allowing it to air dry. The PPE that cannot be decontaminated easily, such as bite or cut-resistant gloves, can be worn under a disposable pair of gloves to prevent cross-contamination during the field session, then decontaminated on return to the lab (or replaced at the end of each field season).
Some agents, such as rabies, are not stable in the environment,12,13 and therefore an interim field decontamination solution may be to place flat items such as nets in direct sunlight for a prolonged period of time. Other agents, such as P. destructans,20 however, are environmentally stable, and more thorough field disinfection will be needed if these agents are suspected to be present. Items that are difficult to decontaminate in the field, or for which only interim strategies can be used, should be bagged and sealed at the end of use and transported to the lab for further disinfection or disposal.
Check that the personnel using the disinfectant have appropriate PPE to handle the disinfectant (i.e., appropriate gloves for the chemical), and consider that some PPE itself, such as face shields, may need to be disinfected between users. In this circumstance, also consider any personnel sensitivities to cleaning agents. Other PPE that may need to be disinfected between individual animals or sites includes gloves and footwear or waders, particularly to prevent transmission of WNS. The PPE that cannot be decontaminated onsite and is intended for reuse should be bagged for transport to prevent cross-contamination and disinfected appropriately when able.
Hand disinfection for personnel should also be considered. For example, are handwashing facilities available and easily accessible at the research location? If there is access to clean water but no sinks, plan to bring soap and a small dish or travel kitchen sink that could be used for handwashing, or bigger containers for repeated washings or equipment. Be sure to consider whether the water source is clean and appropriate for hand washing.
If immediate handwashing is not feasible, after removing PPE, use hand sanitizer with an alcohol content of at least 60% until a sink with running water and soap is accessible. Hand sanitizer may also be used between handling of individual animals to prevent the transmission of diseases between animals.
Both experimental work and the disinfection process are likely to create waste. Before traveling, be familiar with local waste regulations and what is required. For example, based on local ordinances, what sorts of chemical or disinfected biological waste can be disposed down the drain? If waste cannot be disposed of at the site of generation, determine what regulations apply to transport of waste, such as those from the United States Department of Transportation (DOT),81 the International Air Transportation Association (IATA),82 or the United States EPA,83 and what these agencies require for packaging and movement of waste. If possible, arrange with a local entity or institution to provide information on regulations, as well as access to disposal collection sites so that transport can be limited. Arrange waste disposal options ahead of time if possible.
Consider what waste streams will be generated (biological, sharps, chemical, general trash, etc.) and plan how to collect these individual waste streams in an adequate manner designed to contain the waste while protecting the researchers and the environment. This may include items contaminated with blood, feces, urine, or other bodily fluids from animals, single-use PPE, used sharps, chemically contaminated wipes from disinfection, or other items. If any liquid waste is collected, determine whether the waste can be decontaminated and disposed onsite or locally, or whether it will need to be transported.
Secondary containment of liquid waste may be required. If sharps are used, be sure to have appropriate containers for collection and transport. The authors have used a hard-plastic container with a screw top lid as a temporary sharps container for use in the field and for transport back to the laboratory.
If liquid disinfectant or liquid waste is created, used, or transported, have a spill kit on hand that contains appropriate materials to contain and clean up the spill. This should also be an important consideration for any experimental items containing liquids, and in this case, a spill kit may need to contain appropriate disinfectant—be sure to know whether the disinfectant is compatible with any chemicals used for sample processing, extraction, or preservation.
If research involves obtaining samples that are disinfected, neutralized, fixed, or otherwise made non-infectious, particularly for the purposes of shipping or travel, researchers should keep a field log of the process used in case of audits or inspections from local or regional entities. Field logs should also document any waste treatment, such as decontamination, for the same purposes.
Additional Considerations: Legal and Ethical Issues
In addition to biosafety concerns, proposed field work and work with live animals may lead to additional areas where risk must be addressed. One example of this is the legalities around field work, such as occupational health requirements, research permits, export/import requirements, considerations on who can perform the work (minors may be restricted from handling live animals), and regional impacts, such as on wildlife preserves, to threatened or endangered species, or to the local human population. Although generally outside the scope of biosafety, it may be important to advise researchers to investigate these issues, consult with other institutional entities such as the IACUC, or confirm that they have been adequately addressed with entities beyond the institution. In addition, for issues such as permits, some institutions may have the individual researchers obtain these, whereas at others, an institutional representative may assist or obtain the permit.
The institutional legal group, as well as the risk management group, should be able to advise on any institutional policies that impact the work. One such consideration should include participation of graduate or undergraduate students, and how their status as students versus employees, particularly as it relates to incidents and health care, may impact their ability to travel, receive field medicine, or obtain or pay for emergency services. Another institutional group to consult would be the travel office for rules about booking travel, as well as registering travel insurance to assist with any emergencies that occur. Conversations with these groups should also address institutional responsibilities versus individual responsibilities.
Regarding incidents and potential exposures, researchers should consult with their occupational health representatives around medical surveillance and travel medicine (see the Pre- and Post-Exposure Prevention and Medical Surveillance, Emergency Response, and Incident Management section). This is important not only for research medical clearance, if needed, but also so that occupational health can assist with any reporting that needs to occur, such as potential RABV exposures. Reporting laws vary state to state, and it is important to be aware of local regulations before an incident happens.
Researchers should also be aware of any local regulations requiring the bat to be euthanized and tested for RABV if a bite occurs and ensure that they have methods on hand for appropriate euthanasia and sample preservation, as well as pre-arranged access to entities that can facilitate testing.
A variety of regulations, guidelines, and laws may come into play regarding field work and transport of samples. Researchers should consult Federal, state, and local public health officials to determine what approvals or permits are needed to perform the work, access critical areas for research, import or export samples, or transport samples. For example, permits may be needed from the CDC,84 the United States Department of Agriculture (USDA) Animal and Plant Health Inspection Services (APHIS),85 the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES),86 the United States Fish and Wildlife Services,87 or IATA.82 Local Departments of Public Health or Departments of Fish and Wildlife should also be contacted regarding permits or other applicable regulations.
Finally, the institutional import/export office may also have requirements, such as Material Transfer Agreements (MTA), or be able to assist with permits. If field work involves work in a different country, researchers will need to determine what national or local laws and regulations apply, and obtain the appropriate permits for work, exports, entrance to restricted areas, or transportation, particularly as it pertains to the crossing of international borders. This can be greatly facilitated if there is a local institution with whom to collaborate.
Risk assessment should include any potential risks of a legal nature to the researchers who are preparing to perform the studies. This may include interactions with local law enforcement, potential for socio-economic or political issues to impact travel or work, or, for some researchers, being a member of an at-risk population. Safe field work strategies for all researchers, and at-risk populations in particular, should be considered, discussed, and planned for before conducting field work.88
Beyond the legal concerns expressed lie bioethical concerns. The bioethics of field research for bats and indeed, all research animals, should be considered before departure, particularly the impact of a given research project on the animal population, the local environment, and local human population. The bioethics will likely need to be addressed during permit processes with local, state, or Federal wildlife management agencies. A critical partner in field research ethics is the IACUC.
The IACUCs commonly include at least one member who specializes in ethical concerns, and many universities request additional information from researchers who intend to collect wild animals, including capture and holding procedures, transport procedures, animal health and welfare, emergency euthanasia procedures, and general safety.
Handling of wild animals creates risks for the researcher and the animal. All procedures should be reviewed by the IACUC and adhered to once at the field site. Health and welfare concerns must be addressed and considered for each experimental activity, and potential poor outcomes, such as injury to an animal or the need for emergency euthanasia, must be planned for and approved by the IACUC. Additional consideration must be given to protecting bats from human-borne diseases (see the Risk Assessment section). From the perspective of both risk assessment and bioethics, ensuring field researchers are vaccinated against diseases endemic to the area of research is no different and of no less importance than protecting them from visible hazards of field work.
Finally, field researchers should consider the impact of their research on local environments and populaces. Researchers should be willing to work with local populations to mitigate risks that are faced only intermittently while field work is in progress but on a day-to-day basis by local inhabitants and be aware that their presence or their research may have significant impacts on the interactions of local populations and/or the environment. Local concerns, including those of indigenous communities, should be considered, and it is recommended that researchers contact and collaborate with local institutions.
This promotes not only the safety of the researcher but also equity and inclusion in field research. Researchers should ensure they leave their field sites in good condition, interact in positive ways with local peoples, and work to establish sustained partnerships that can have positive outcomes for all parties.
Summary
Health and safety for researchers is paramount for any institution and is especially important when research involves hazards that may be difficult or unpredictable, such as field work or work with live animals. A careful and considerate risk assessment combined with thoughtful planning and deliberate review with safety experts can identify ways to mitigate the risks that researchers may encounter in the field.
As outlined in this article, field work with bats poses certain risks, but there are both established and creative ways to address these risks and reduce their potential occurrence during work. Although much of the information presented here is specific to bats, the broader considerations presented are applicable to all field work involving animals. Partnership between researchers and biosafety professionals, as well as IACUC or other local, regional, or Federal agencies, actively supports participation in research programs whose outcomes will help protect, preserve, and improve understanding of our planet.
Acknowledgments
The authors thank the following people for initial discussions: Emily Adamson (Boise State University), Rocco Casagrande (Gryphon Scientific), Rob Dettman (Gryphon Scientific), Adam Fleming (Gryphon Scientific), Kelly Kim (Gryphon Scientific), Erin Lauer (Gryphon Scientific), Nicole Marlenee (Colorado State University), Giorgio Scarpellini (Arizona State University), and Jimmy Spencer (University of Arizona). The authors also thank David Gillum (Arizona State University) and Barbara Johnson (Biosafety Biosecurity International) for advice and guidance. Finally, the authors thank Elizabeth Falendysz (USGS) for initial discussions and reviewing the article.
Authors' Contributions
A.A.-S., N.A.-C., H.K.F., G.R.F, E.G.D., T.W.H., S.M.J., C.A.S., and J.T.V.S. contributed to conceptualization and writing of the original draft. G.A.B. and A.J.B. contributed to writing of the original draft. L.A.O.C., G.L.P., and S.E.V. contributed to conceptualization, writing of the original draft, and reviewing and editing the final draft.
Authors' Disclosure Statement
The authors declare that there is no conflict of interest.
Funding Information
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1. Caskey S, Sevilla-Reyes EE. Risk Assessment. In: Laboratory Biorisk Management: Biosafety and Biosecurity. (Salerno R, Gaudioso J. eds.) CRC Press: Boca Raton, FL; 2015; pp. 45–64. [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC), National Institutes of Health (NIH). Biosafety in Microbiological and Biomedical Laboratories, 6th Edition. U.S. Department of Health and Human Services; 2020. [Google Scholar]
- 3. World Health Organization (WHO). Occurrence of Rabies. n.d. Available from: https://www.who-rabies-bulletin.org/site-page/occu
- 4. El Centro Panamericano de Fiebre Aftosa y Salud Pública Veterinaria. New Latin American Rabies Surveillance System (SIRVERA) [in Spanish]. n.d. Available from: https://sirvera.panaftosa.org.br [Last accessed: May 10, 2022].
- 5. Pan American Health Organization. Rabies. n.d. Available from: https://www.paho.org/en/topics/rabies [Last accessed: May 9, 2022].
- 6. Centers for Disease Control and Prevention (CDC). Bats Lead in U.S. Rabies Risk. 2019. Available from: https://www.cdc.gov/media/releases/2019/p0611-bats-rabies.html [Last accessed: May 9, 2022].
- 7. Pieracci EG, Pearson CM, Wallace RM, et al. Vital signs: Trends in human rabies deaths and exposures—United States, 1938–2018. MMWR Morb Mortal Wkly Rep 2019;68(23):524–528; doi: 10.15585/mmwr.mm6823e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayo Clinic. Rabies. n.d. Available from: https://www.mayoclinic.org/diseases-conditions/rabies/symptoms-causes/syc-20351821 [Last accessed: May 9, 2022].
- 9. Centers for Disease Control and Prevention (CDC). How Is Rabies Transmitted? | Transmission. n.d. Available from: https://www.cdc.gov/rabies/transmission/index.html [Last accessed: May 9, 2022].
- 10. Gibbons RV. Cryptogenic rabies, bats, and the question of aerosol transmission. Ann Emerg Med 2002;39(5):528–536; doi: 10.1067/mem.2002.121521. [DOI] [PubMed] [Google Scholar]
- 11. Johnson N, Phillpotts R, Fooks AR. Airborne transmission of lyssaviruses. J Med Microbiol 2006;55(6):785–790; doi: 10.1099/jmm.0.46370-0. [DOI] [PubMed] [Google Scholar]
- 12. Bleck TP. Rabies. In: Tropical Infectious Diseases. (Guerrant RL, Walker DH, Weller PF. eds.) London: Churchill Livingstone; 2006; pp. 839–851. [Google Scholar]
- 13. Rupprecht CE, Gibbons RV. Prophylaxis against Rabies. N Engl J Med 2004;351(25):2626–2635; doi: 10.1056/NEJMcp042140. [DOI] [PubMed] [Google Scholar]
- 14. Chomel BB, Stuckey MJ, Boulouis HJ, et al. Bat-Related Zoonoses. In: Zoonoses - Infections Affecting Humans and Animals. (Sing A. ed.) Springer Dordrecht; 2015;697; doi: 10.1007/978-94-017-9457-2_28. [DOI] [Google Scholar]
- 15. Centers for Disease Control and Prevention (CDC). Wildlife | Healthy Pets, Healthy People. n.d. Available from: https://www.cdc.gov/healthypets/pets/wildlife.html [Last accessed: May 9, 2022].
- 16. Guzmán C, Calderón A, Oviedo T, et al. Molecular and Cellular Evidence of Natural Venezuelan Equine Encephalitis Virus Infection in Frugivorous Bats in Colombia. Vet World 2020;13(3):495–501; doi: 10.14202/vetworld.2020.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau SKP, Luk HKH, Wong ACP, et al. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis 2020;26(7):1542; doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stading B, Ellison JA, Carson WC, et al. Protection of bats (Eptesicus fuscus) against rabies following topical or oronasal exposure to a recombinant raccoon poxvirus vaccine. PLoS Negl Trop Dis 2017;11(10):e0005958; doi: 10.1371/journal.pntd.0005958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aguilar-Setién A, Campos YL, Cruz ET, et al. Vaccination of vampire bats using recombinant vaccinia-rabies virus. J Wildl Dis 2002;38(3):539–544; doi: 10.7589/0090-3558-38.3.539 [DOI] [PubMed] [Google Scholar]
- 20. White-Nose Syndrome Disease Management Working Group. National White-Nose Syndrome Decontamination Protocol. 2020. Available from: www.WhiteNoseSyndrome.org [Last accessed: May 9, 2022].
- 21. Schlottau K, Rissmann M, Graaf A, et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe 2020;1(5):e218–e225; doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Runge MC, Campbell Grant EH, Coleman JTH, et al. Assessing the risks posed by SARS-CoV-2 in and via North American bats—Decision framing and rapid risk assessment: U.S. Geological Survey Open-File Report 2020–1060. 2020; 43p. [Google Scholar]
- 23. Centers for Disease Control and Prevention (CDC). Guidance to Reduce the Risk of SARS-CoV-2 Spreading between People and Wildlife. n.d. Available from: https://www.cdc.gov/healthypets/covid-19/wildlife.html [Last accessed: May 9, 2022].
- 24. Cook JD, Grant EHC, Coleman JTH, et al. Risks posed by SARS-CoV-2 to North American bats during winter fieldwork. Conserv Sci Pract 2021;3(6):e410; doi: 10.1111/csp2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Centers for Disease Control and Prevention (CDC). What You Need to Know About Variants. n.d. Available from: https://www.cdc.gov/coronavirus/2019-ncov/variants/about-variants.html?CDC_AA_refVal=https%3A%2F%2F www.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvariants%2Fdelta-variant.html [Last accessed: May 10, 2022].
- 26. Anthes E. Covid's delta variant: What we know. New York Times. 2021. Available from: www.WhiteNoseSyndrome.org [Last accessed: May 9, 2022.
- 27. World Health Organization (WHO). Tracking SARS-CoV-2 Variants. n.d. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants [Last accessed: May 10, 2022].
- 28. Centers for Disease Control and Prevention (CDC). Rabies Status: Assessment by Country | Resources. n.d. Available from: https://www.cdc.gov/rabies/resources/countries-risk.html [Last accessed: May 9, 2022].
- 29. Centers for Disease Control and Prevention (CDC). What Kind of Animal Did You Come in Contact with? | Exposure | Rabies. n.d. Available from: https://www.cdc.gov/rabies/exposure/animals/index.html [Last accessed: May 9, 2022].
- 30. World Health Organization (WHO). Rabies Epidemiology and Burden. n.d. Available from: https://www.who.int/activities/improving-data-on-rabies/rabies-epidemiology-and-burden [Last accessed: May 9, 2022].
- 31. Coertse J, Geldenhuys M, Le Roux K, et al. Lagos bat virus, an under-reported rabies-related lyssavirus. Viruses 2021;13(4):576–594; doi: 10.3390/v13040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lippi G, Cervellin G. Updates on rabies virus disease: Is evolution toward “zombie virus” a tangible threat? Acta Biomed 2021;92:2021045; doi: 10.23750/abm.v92i1.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Centers for Disease Control and Prevention (CDC). When Should I Seek Medical Attention? | Exposure | Rabies. n.d. Available from: https://www.cdc.gov/rabies/exposure/index.html [Last accessed: May 10, 2022].
- 34. Fishbein DB. Rabies in Humans. In: The Natural History of Rabies. (Baer GM. ed.) Boca Raton, FL: Routledge; 1991; pp. 519–549. [Google Scholar]
- 35. Centers for Disease Control and Prevention (CDC). What Are the Signs and Symptoms of Rabies? | Symptoms. n.d. Available from: https://www.cdc.gov/rabies/symptoms/index.html [Last accessed: May 10, 2022].
- 36. Department of Health and Human Services. Medical Surveillance Program | HHS.Gov. n.d. Available from: https://www.hhs.gov/about/agencies/asa/foh/chs/medical-surveillance/index.html [Last accessed: May 10, 2022].
- 37. Centers for Disease Control and Prevention (CDC). Vaccine Information Statement | Tdap | Tetanus-Diphtheria-Pertussis | VIS. n.d. Available from: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/tdap.html [Last accessed: May 10, 2022].
- 38. Centers for Disease Control and Prevention (CDC). Travelers' Health | CDC. n.d. Available from: https://wwwnc.cdc.gov/travel [Last accessed: May 9, 2022].
- 39. Rao AK, Briggs D, Moore SM, et al. Use of a modified pre-exposure prophylaxis vaccination schedule to prevent human rabies: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep 2022;71(18):619–627; doi: 10.15585/mmwr.mm7118a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fooks AR, Banyard AC, Ertl HCJ. New human rabies vaccines in the pipeline. Vaccine 2019;37(Suppl 1):A140–A145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le T, Sun C, Chang J, et al. MRNA vaccine development for emerging animal and zoonotic diseases. Viruses 2022;14(2):401; doi: 10.3390/v14020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. University of California. Field Operations Safety Manual. n.d. Available from: https://www.ucop.edu/safety-and-loss-prevention/environmental/program-resources/field-research-safety/index.html [Last accessed: May 9, 2022].
- 43. Wallace R, Petersen B, Shlim D. Rabies. In: CDC Yellow Book. (Brunette GW, Nemhauser JB. eds.) Oxford University Press: New York; 2020; pp. 316–324. [Google Scholar]
- 44. Centers for Disease Control and Prevention (CDC). Rabies Post-exposure Prophylaxis (PEP) | Medical Care | Rabies. n.d. Available from: https://www.cdc.gov/rabies/medical_care/index.html [Last accessed: May 11, 2022].
- 45. Van Der Jeucht L, Groom Q, Agosti D, et al. Using iNaturalist to monitor adherence to best practices in bat handling. Biodivers Data J 2021;9:e68052; doi: 10.3897/BDJ.9.e68052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bat Conservation Trust. Wearing Gloves When Handling Bats Contents. n.d. Available from: https://cdn.bats.org.uk/uploads/pdf/Resources/Rabies-documents/Wearing-gloves-when-you-handle-bats_August2020.pdf?v=1596809848 [Last accessed: May 9, 2022].
- 47. Freeman PW, Lemen CA. Puncture-Resistance of Gloves for Handling Bats. J Wildl Manage 2009;73(7):1251–1254. [Google Scholar]
- 48. Occupational Safety and Health Administration (OSHA). Regulations (Standards-29 CFR 1910.134). Available from: https://www.osha.gov [Last accessed: May 9, 2022].
- 49. Centers for Disease Control and Prevention. Animals and COVID-19. n.d. Available from: https://www.cdc.gov/coronavirus/2019-ncov/daily-life-coping/animals.html [Last accessed: May 11, 2022].
- 50. North Carolina Wildlife. Bat Activities During COVID-19. n.d. Available from: https://www.ncwildlife.org/Portals/0/Learning/documents/Profiles/Mammals/COVID-Bats-FINAL.pdf [Last accessed: May 9, 2022].
- 51. Gorilla Doctors Staff. A Possible COVID-19 Silver Lining for Great Ape Conservation—Gorilla Doctors Gorilla Doctors. n.d. Available from: https://www.gorilladoctors.org/a-possible-covid-19-silver-lining-for-great-ape-conservation [Last accessed: May 9, 2022].
- 52. Anthes E. When people take pandemic precautions, gorillas breathe easier. New York Times. 2022. [Google Scholar]
- 53. Centers for Disease Control and Prevention (CDC). Guidelines for Environmental Infection Control in Health-Care Facilities. 2003. Available from: https://www.cdc.gov/infectioncontrol/guidelines/environmental/background/laundry.html [Last accessed: May 11, 2022].
- 54. Garrido-Molina JM, Márquez-Hernández VV, Alcayde-García A, et al. Disinfection of gloved hands during the COVID-19 pandemic. J Hosp Infect 2021;107:5–11; doi: 10.1016/j.jhin.2020.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Forest Service and United States Department of Agriculture. Frequently Asked Questions White-Nose Syndrome (WNS) What Is White-Nose Syndrome? 2012. Available from: https://www.fs.usda.gov/Internet/FSE_DOCUMENTS/stelprdb5383070.pdf [Last accessed: May 11, 2022].
- 56. World Health Organization (WHO). Laboratory Biosafety Manual. 4th ed. Switzerland: World Health Organization; 2020.
- 57. Dudley JP. Global zoonotic disease surveillance: An emerging public health and biosecurity imperative. Bioscience 2004;54(11):982–983; doi: 10.1641/0006-3568(2004)054[0982:GZDSAE]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mitchell-Jones A, McLeish A. (eds.) Bat Workers' Manual. 3rd ed. Peterborough: JNCC; 2004. [Google Scholar]
- 59. Varela-Arias NO. Biosafety in the Management of Wild and Non-Conventional Fauna [in Spanish]. Memorias la Conf interna en Med y Aprovech fauna Silv Exótica 2014;7(1):20–30. [Google Scholar]
- 60. Mills J, Childs JE, Ksiazek TG, et al. Methods for Trapping and Sampling Small Mammals for Virologist Test. U.S. Department of Health and Human Services. 1995. Available from: https://stacks.cdc.gov/view/cdc/11507 [Last accessed: May 10, 2022].
- 61. Finnemore M. Catching Bats. In: Bat Workers' Manual. (Mitchell-Jones A, McLeish A, McOwat T. eds.) The Joint Nature Conservation Committee: Peterborough, UK; 2004; pp. 41–48. [Google Scholar]
- 62. Winkler WG. Airborne rabies transmission in a laboratory worker. JAMA 1973;226(10):1219; doi: 10.1001/jama.1973.03230100043011. [DOI] [PubMed] [Google Scholar]
- 63. Hooper SE, Amelon SK. Handling and blood collection in the little brown bat (Myotis lucifugus). Lab Anim 2014;43(6):197–199; doi: 10.1038/laban.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lollar A. The Rehabilitation and Captive Care of Insectivorous Bats—Bat World Store. Weatherford, TX: Bat World Publication; 2018. [Google Scholar]
- 65. Barnard SM. Bats in Captivity. USA: Wild Ones Animal Books; 1995. [Google Scholar]
- 66. Stebbing R. Ringing and Marking. In: Bat Workers' Manual. (Mitchell-Jones A, McLeish A, McOwat T. eds.) Peterborough: Joint Nature Conservation Committee (JNCC); 2004; pp. 59–61. [Google Scholar]
- 67. Bat World Sanctuary. Position Statement on Banding Insectivorous Bats. 2010. Available from: https://batworld.org/wp-content/uploads/2011/02/BWSposition_statement-banding1.pdf [Last accessed: May 9, 2022]. [Google Scholar]
- 68. Baker G, Lumsden L, Dettmann E, et al. The effect of forearm bands on insectivorous bats (Microchiroptera) in Australia. Wildl Res 2001;28:229–237; doi: 10.1071/WR99068. [DOI] [Google Scholar]
- 69. Ellison LE. Summary and Analysis of the U.S. Government Bat Banding Program. Open-File Report 2008-1363. 2008.
- 70. Herreid CF, Davis RB, Short HL. Injuries due to bat banding. J Mammal 1960;41(3):398–400; doi: 10.2307/1377505. [DOI] [Google Scholar]
- 71. Sandilands A, Morningstar D. Relative Efficacy of 9-Mm and 12-Mm PIT Tags for Studying Little Brown Myotis (Myotis lucifugus): A Cautionary Note. Bat Research News 2001;62:49–50. [Google Scholar]
- 72. Wildlife Ethics Committee. Use of Microchips for Marking Wildlife Policy. 2017. Available from: https://cdn.environment.sa.gov.au/environment/docs/wec-use-of-microchips-marking-wildlife-policy-gen.pdf [Last accessed: May 9, 2022].
- 73. Rigby EL, Aegerter J, Brash M, et al. Impact of PIT tagging on recapture rates, body condition and reproductive success of wild Daubenton's bats (Myotis daubentonii). Vet Rec 2012;170(4):101; doi: 10.1136/vr.100075. [DOI] [PubMed] [Google Scholar]
- 74. Britzke ER, Gumbert MW, Hohmann MG, et al. Behavioral response of bats to passive integrated transponder tag reader arrays placed at cave entrances. J Fish Wildl Manag 2014;5(1):146–150; doi: 10.3996/082012-JFWM-065. [DOI] [Google Scholar]
- 75. Epstein JH, Zambriski JA, Rostal MK, et al. Comparison of intravenous medetomidine and medetomidine/ketamine for immobilization of free-ranging variable flying foxes (Pteropus hypomelanus). PLoS One 2011;6(10):1–4; doi: 10.1371/journal.pone.0025361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tuval A, Las L, Shilo-Benjamini Y. Evaluation of injectable anaesthesia with five medetomidine-midazolam based combinations in Egyptian fruit bats (Rousettus aegyptiacus). Lab Anim 2018;52(5):515–525; doi: 10.1177/0023677218756456. [DOI] [PubMed] [Google Scholar]
- 77. Eshar D, Weinberg M. Venipuncture in bats. Lab Anim (NY) 2010;39(6):175–176; doi: 10.1038/laban0610-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Morgan CN, Mauldin MR, Jones J, et al. A Novel Restraint Device to Improve Safety and Efficacy of Blood Collection During Non-Terminal Sampling of Bats. 2022. Available from: http://biorxiv.org/content/early/2022/03/25/2022.03.22.483499.abstract [Last accessed: May 10, 2022].
- 79. Power ML, Power S, Bertelsen MF, et al. Wing: A suitable nonlethal tissue type for repeatable and rapid telomere length estimates in bats. Mol Ecol Resour 2021;21(2):421–432; doi: 10.1111/1755-0998.13276. [DOI] [PubMed] [Google Scholar]
- 80. Turner GG, Meteyer CU, Barton H, et al. Nonlethal screening of bat-wing skin with the use of ultraviolet fluorescence to detect lesions indicative of white-nose syndrome. J Wildl Dis 2014;50(3):566–573; doi: 10.7589/2014-03-058. [DOI] [PubMed] [Google Scholar]
- 81. United States Department of Transportation. Department of Transportation. n.d. Available from: https://www.transportation.gov [Last accessed: May 10, 2022].
- 82. International Air Transport Association. IATA—Home. n.d. Available from: https://www.iata.org/en [Last accessed: May 10, 2022].
- 83. United States Environmental Protection Agency. Hazardous Waste Transportation. n.d. Available from: https://www.epa.gov/hw/hazardous-waste-transportation [Last accessed: May 10, 2022].
- 84. Centers for Disease Control and Prevention (CDC). Import Permit Program. n.d. Available from: https://www.cdc.gov/cpr/ipp/index.htm [Last accessed: May 10, 2022].
- 85. United States Department of Agriculture. USDA APHIS | APHIS Permits (EPermits and EFile). n.d. Available from: https://www.aphis.usda.gov/aphis/resources/permits [Last accessed: May 10, 2022].
- 86. Convention on International Trade in Endangered Species of Wild Fauna and Flora. CITES. n.d. Available from: https://cites.org/eng [Last accessed: May 11, 2022].
- 87. United States Fish and Wildlife Service. U.S. Fish and Wildlife Service. n.d. Available from: https://www.fws.gov [Last accessed: May 11, 2022].
- 88. Demery AJC, Pipkin MA. Safe fieldwork strategies for at-risk individuals, their supervisors and institutions. Nat Ecol Evol 2020;5(1):5–9; doi: 10.1038/s41559-020-01328-5. [DOI] [PubMed] [Google Scholar]