Abstract
Background
NC-6300 is a novel epirubicin (EPI) drug conjugated polymeric micelle developed using cutting-edge micellar nanoparticle technology. The nanoparticle epirubicin conjugates EPI to a polymer via a pH-sensitive linker which enables the selective EPI release into tumor. Tumor activity was observed in a monotherapy phase Ib trial, where two of two patients with angiosarcoma achieved a partial response. To further explore the activity of NC-6300 in angiosarcoma, an expansion cohort was undertaken.
Methods
Ten patients with angiosarcoma were enrolled in the expansion cohort. Patients were dosed using the recommended dose of 150 mg/m2 intravenously (IV) once every 3 weeks. The primary endpoint was progression-free survival.
Results
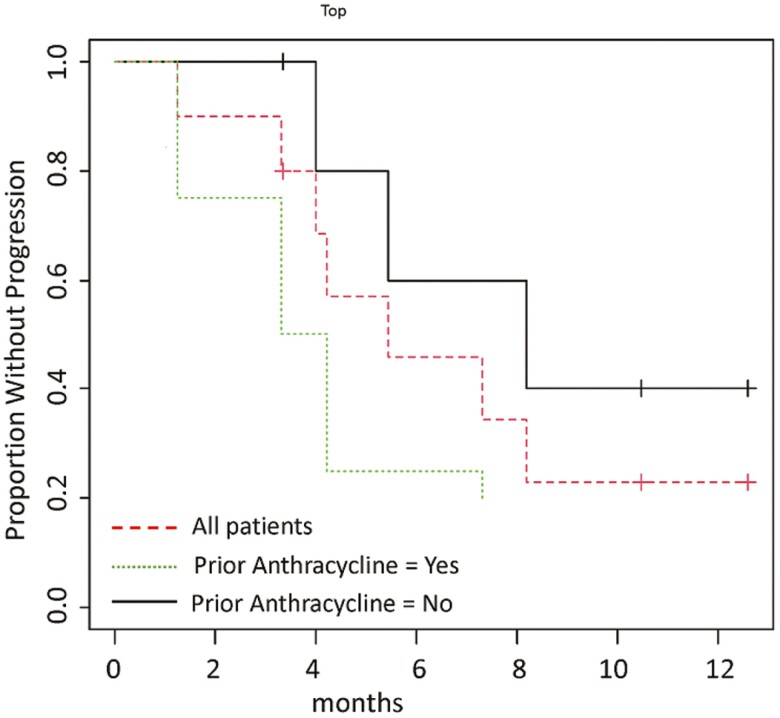
The most common adverse events (AEs) of any grade, regardless of the causal relationship with NC-6300, were neutropenia (90%), fatigue, and thrombocytopenia (60% each) and nausea (50%). The most common grades 3 and 4 AEs were neutropenia (80%), thrombocytopenia (40%), and anemia and leukopenia (20% each). The median progression-free survival (mPFS) for all subjects was 5.4 months. The mPFS was 3.8 months in subjects with prior anthracycline treatment and 8.2 months in subjects without prior anthracycline treatment.
Conclusion
NC-6300 was well tolerated, showing promising activity in angiosarcoma patients without prior anthracycline treatment. NC-6300 warrants further investigation (ClinicalTrials.gov Identifier: NCT03168061).
Keywords: nanoparticle, epirubicin, angiosarcoma, metastatic, soft tissue sarcoma, unresectable, NC-6300
To explore the activity of NC-6300 (nanoparticle epirubicin) in angiosarcoma, an expansion cohort was undertaken. The clinical trial results are reported here.
Lessons Learned.
In this small expansion cohort, NC-6300 at the recommended dose of 150 mg/m2 IV every 3 weeks, was well tolerated and showed promising anti-tumor activity in angiosarcoma patients without prior anthracycline treatment.
Discussion
Angiosarcomas are a subtype of soft-tissue sarcoma that are aggressive, malignant endothelial-cell tumors of vascular or lymphatic origin. Treatment for patients is challenging in many cases and the prognosis is poor. Anthracyclines are widely used in the treatment of many sarcomas, including angiosarcomas, but many patients suffer from rate-limiting side effects. Therefore, a safer treatment remains an area of unmet medical need. EPI, a stereoisomer of doxorubicin, was developed to circumvent the cardiotoxicity associated with the parent compound. Pegylated liposomes and other nanoparticle formulations have been developed to reduce the cardiotoxicity of conventional doxorubicin and other anthracycline derivatives. Although liposomal doxorubicin was designed to alleviate the cardiac toxicity associated with conventional doxorubicin, unique toxicities were reported. NC-6300 was developed to encapsulate EPI into the micellar nanoparticle to selectively deliver the drug into the acidic environment of the endosomal or lysosomal compartments of the target tumor cell.
In the phase Ib trial of NC-6300 in solid tumors and soft-tissue sarcomas, there were 2 patients with angiosarcoma who experienced a partial response by investigator assessment. Originally, a comparative phase II accruing 150 patients in total was planned to investigate NC-6300 + olaratumab vs olaratumab alone. However, the study protocol was amended to pursue an expansion cohort, using NC-6300 alone, due to market withdrawal of olaratumab. The expansion cohort was an open-label trial that enrolled 10 patients designed to further investigate the activity of NC-6300 in angiosarcoma. The primary endpoint was progression-free survival (PFS). Patients were treated with NC-6300 at 150 mg/m2 IV once every three weeks. The baseline characteristics of the patients are listed in Table 1. The results of our trial demonstrated the tolerability and the safety of NC-6300, with the most common adverse events ≥30% in all grades being neutropenia (90%), thrombocytopenia (60%), fatigue (60%), and nausea (50%). Only neutropenia (80%) and thrombocytopenia (40%) had occurrences in grade 3 or 4 ≥30%. The overall mPFS by investigator assessment was 5.4 months. The mPFS was 3.8 months in subjects with prior anthracycline treatment and 8.2 months for subjects without prior anthracycline treatment as shown in Figure 1. Partial response was observed in 3 patients without prior anthracycline treatment, yielding an objective response rate of 30% (3/10).
Table 1.
Patient baseline characteristics (N = 10).
| Characteristics | No. of patients | % |
|---|---|---|
| No. of patients enrolled | 10 | 100 |
| Age, years, median (range) | 63.5 (26-76) | |
| Sex | ||
| Male | 7 | 70 |
| Female | 3 | 30 |
| ECOG PS | ||
| 0 | 1 | 10 |
| 1 | 9 | 90 |
| Angiosarcoma variants | ||
| Cutaneous | 2 | 20 |
| Non-cutaneous | 8 | 80 |
| Previous systemic chemotherapy | ||
| No | 3 | 30 |
| Yes | 70 | 70 |
| Median (range) | 1 (0-5) | |
| Previous anthracycline chemotherapy | ||
| No | 6 | 60 |
| Yes | 4 | 40 |
| Agents used in prior regimens | ||
| Doxorubicin | 4 | 40 |
| Gemcitabine | 4 | 40 |
| Paclitaxel | 4 | 40 |
| Docetaxel | 3 | 30 |
| Pazopanib | 2 | 20 |
| Ifosfamide | 1 | 10 |
| Liposomal doxorubicin | 1 | 10 |
Figure 1.
Progression-free survival (N = 10). The overall mPFS for all subjects included in the trial was 5.4 months. The mPFS was 3.8 months in subjects with prior anthracycline treatment and 8.2 months for subjects without prior anthracycline treatment.
In summary, NC-6300 was safe and tolerable in this expansion cohort, and had promising efficacy especially in angiosarcoma patients without prior anthracycline treatment. Further investigation is warranted.
Trial Information
| Disease | Angiosarcoma |
| Stage of disease/treatment | Recurrent or metastatic setting |
| Prior therapy | Chemotherapy-naïve or no more than two previous systemic therapies for recurrent or metastatic setting |
| Type of study | Interventional |
| Primary endpoint | Median PFS |
| Secondary endpoints | Safety profile (the incidence, severity, seriousness, and relatedness to study drug of TEAEs, laboratory changes or LVEF) |
| ORR, DCR (ORR + stable disease) at 4 months | |
| DOR | |
| Median OS | |
| Investigator’s analysis | Active and should be pursued further |
Drug Information
| Generic/working name | NC-6300 |
| Company name | NanoCarrier Co., Ltd |
| Drug type | Chemotherapy |
| Drug class | Anthracycline |
| Dose | 150 |
| Unit | mg/m2 |
| Route | Intravenous infusion |
| Schedule of administration | Every 3 weeks |
Patient Characteristics
| Number of patients, male | 7 |
| Number of patients, female | 3 |
| Age: median (range) | 63.5 (26-76) years |
| Number of prior systemic therapies: median (range) | 1 (0-5) |
| Performance status: ECOG | 0: 1 |
| 1: 9 | |
| 2: 0 | |
| 3: 0 | |
| 4: 0 | |
| Cancer types or histologic subtypes | Angiosarcoma cutaneous, 2; Angiosarcoma non-cutaneous, 8 |
Primary Assessment Method
| Title | Imaging (CT or MRI) |
| Number of patients screened | 10 |
| Number of patients enrolled | 10 |
| Number of patients evaluable for toxicity | 10 |
| Number of patients evaluated for efficacy | 10 |
| Evaluation method | RECIST 1.1 |
| Response assessment, CR | 0 (0%) |
| Response assessment, PR | 3 (30%) |
| Response assessment, SD | 6 (60%) |
| Response assessment, PD | 1 (10%) |
| Median duration assessments | Every 6 weeks |
Assessment, Analysis, and Discussion
| Completion | Study completed |
| Investigator’s assessment | Active and should be pursued further |
Angiosarcomas are a subtype of soft-tissue sarcoma that are aggressive, malignant endothelial-cell tumors of vascular or lymphatic origin. Anthracyclines, such as doxorubicin, are widely used in the treatment of many tumors, including angiosarcomas, but many patients suffer from significant treatment-related side effects. Treatment for patients is challenging in many cases and the prognosis is poor. As a result, there remains an unmet need for a safer anthracycline agent. Epirubicin, a novel stereoisomer of doxorubicin, was developed to circumvent the cardiotoxicity associated with the parent compound. Pegylated liposomes and other nanoparticle formulations have been developed to reduce the cardiotoxicity of conventional doxorubicin and other anthracycline derivatives. Although liposomal doxorubicin was designed to alleviate the cardiac toxicity associated with conventional doxorubicin, some unique toxicities were observed, including acute infusion-related reaction and Hand-Foot Syndrome, which were not common with conventional doxorubicin. NC-6300 was developed to encapsulate epirubicin into the micellar nanoparticle to selectively deliver the drug into the acidic environment of the endosomal or lysosomal compartments of the target tumor cell. NC-6300 was also designed to stay in the blood circulation for a long period of time and accumulate in the tumor based on the enhanced permeability and retention effects. Superior anti-tumor activity of NC-6300 was expected due to efficient drug release in the tumor.
The phase Ib does-escalation trial of NC-6300 monotherapy (NCT 03168061) enrolled 29 subjects: 17 with soft-tissue sarcoma, 1 with osteosarcoma, and 11 with other solid tumors. Among those patients, there were 2 patients with angiosarcoma who experienced a partial response by investigator assessment. Originally, a comparative phase II study, accruing 150 patients in total, was planned to investigate NC-6300 + olaratumab vs olaratumab alone. However, the study protocol was amended to pursue an expansion cohort, exploring NC-6300 alone in angiosarcoma, due to market withdrawal of olaratumab. The expansion cohort of angiosarcoma was an open-label, non-randomized trial that enrolled 10 patients designed to further investigate the activity of NC-6300. The primary endpoint of the expansion cohort was progression-free survival (PFS). Patient visits took place at screening; on days 1, 8, and 15 of every cycle; and end of treatment. AEs were graded using the NCI Common Terminology Criteria for Adverse Events Version 4.03 (CTCAE v4.03). Patients were treated with NC-6300 at 150 mg/m2 IV once every 3 weeks. The baseline characteristics of the patients are listed in Table 1. All subjects enrolled had a histologically or cytologically confirmed diagnosis of advanced solid tumor, including soft-tissue sarcoma, that had relapsed or was refractory to standard therapy, and an Eastern Cooperative Oncology Group (ECOG) performance status, defined as 0 or 1. Overall, 70% of subjects were men and 30% were women. Median age was 63.5 years (range: 26–76 years). Eighty percent of the enrolled subjects had non-cutaneous angiosarcomas and 20% had cutaneous angiosarcomas. Four subjects (40%) had received prior anthracycline chemotherapy and six subjects (60%) had not received prior anthracycline chemotherapy; 3 subjects had no previous systemic chemotherapy treatment prior to enrolling in the study; 7 subjects had previous systemic chemotherapy treatment. The agents used in the prior treatment included: taxane (70%), doxorubicin (40%), gemcitabine (40%), pazopanib (20%), ifosfamide (10%), and liposomal doxorubicin (10%).
Although immunotherapy has significantly changed the standard of care for many advanced malignancies, neither all patients nor all disease states benefit. As a result, chemotherapy remains an important treatment modality for many individuals with advanced disease. Anthracyclines, including doxorubicin, are some of the most commonly used chemotherapeutic agents, but are limited by hematologic and cardiac toxicities. As a result, novel anthracyclines, such as epirubicin, were developed.
While doxorubicin-based chemotherapy remains a treatment of choice in metastatic soft tissue sarcomas, paclitaxel seems to be an effective treatment, specifically in angosarcoma. In a multicenter phase II trial to assess the efficacy and toxicity of weekly paclitaxel in patients with metastatic or unresectable angiosarcomas, a median time to progression of 4 months was observed.1 Progression-free survival was similar in patients pre-treated with chemotherapy and in chemotherapy-naïve patients.1
Our expansion trial demonstrated the tolerability and safety of NC-6300 with the most common adverse events ≥30% in all grades being neutropenia (90%), thrombocytopenia (60%), fatigue (60%), and nausea (50%). The most common grades 3 and 4 AEs were neutropenia (80%), thrombocytopenia (40%), and anemia and leukopenia (20% each). Adverse events regardless of the causal relationship with NC-6300 are listed in Table 2. These toxicities are commonly observed with conventional anthracycline treatment.2
Table 2.
NC-6300 safety results (N = 10).
| Adverse events* | Grade | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 and 2 | 3 | 4 | All | |||||
| No. | % | No. | % | No. | % | No. | % | |
| Neutropenia | 1 | 10 | 1 | 10 | 7 | 70 | 9 | 90 |
| Fatigue | 6 | 60 | 0 | 0 | 0 | 0 | 6 | 60 |
| Thrombocytopenia | 2 | 20 | 2 | 20 | 2 | 20 | 6 | 60 |
| Nausea | 5 | 50 | 0 | 0 | 0 | 0 | 5 | 50 |
| Anemia | 2 | 20 | 2 | 20 | 0 | 0 | 4 | 40 |
| Decreased appetite | 4 | 40 | 0 | 0 | 0 | 0 | 4 | 40 |
| Stomatitis | 4 | 40 | 0 | 0 | 0 | 0 | 4 | 40 |
| Constipation | 3 | 30 | 0 | 0 | 0 | 0 | 3 | 30 |
| Cough | 3 | 30 | 0 | 0 | 0 | 0 | 3 | 30 |
| Diarrhea | 3 | 30 | 0 | 0 | 0 | 0 | 3 | 30 |
| Leukopenia | 1 | 10 | 1 | 10 | 1 | 10 | 3 | 30 |
| Pyrexia | 3 | 30 | 0 | 0 | 0 | 0 | 3 | 30 |
| Vomiting | 3 | 30 | 0 | 0 | 0 | 0 | 3 | 30 |
| Headache | 2 | 20 | 0 | 0 | 0 | 0 | 2 | 20 |
| Hypomagnesaemia | 2 | 20 | 0 | 0 | 0 | 0 | 2 | 20 |
| Insomnia | 2 | 20 | 0 | 0 | 0 | 0 | 2 | 20 |
*Adverse events regardless of causal relationship with NC-6300
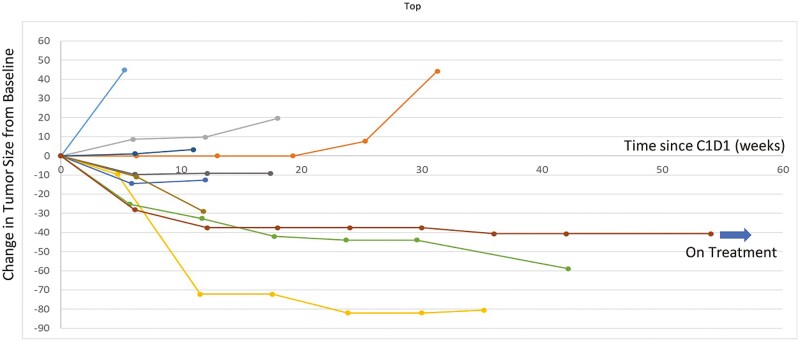
The Kaplan-Meier method was used to calculate the median progression-free survival. The overall mPFS by investigator assessment for all subjects included in the trial was 5.4 months, similar to what was achieved, in prior published studies, with anthracycline (4.9 months) and paclitaxel regimens (4 months) for patients with metastatic or unresectable angiosarcomas.1,3,4 In subjects with prior anthracycline treatment, the mPFS in the study presented here was 3.8 months, similar to the expected mPFS for treatment of angiosarcoma (3.5 months, 3.7 months, and 2.7 months in first-, second-, and third-line setting).5 In contrast, in subjects without prior anthracycline treatment, the mPFS was 8.2 months, as shown in Figure 1. All subjects were evaluated for a radiographic assessment of tumor response using RECIST version 1.1. Partial response was observed in 3 patients without prior anthracycline treatment, yielding an objective response rate of 30% (3/10) (Figure 2). Even in this small sample size, the ORR is similar to what has been previously reported for first-line paclitaxel (23.7%).1
Figure 2.
Spider plot of maximal change in tumor size in angiosarcoma population (N = 10). Partial response was observed in 3 patients without prior anthracycline treatment, yielding an objective response rate (ORR) of 30%.
NC-6300 at the recommended dose of 150 mg/m2 IV every 3 weeks was well tolerated and showed promising anti-tumor activity in patients without prior anthracycline exposure in this expansion cohort. Further investigation of the activity of NC-6300 is warranted.
Acknowledgment
This study was sponsored by NanoCarrier Co., Ltd.
Contributor Information
Richard F Riedel, Duke Cancer Institute, Duke University Medical Center, Durham, NC, USA.
Victoria Chua, Sarcoma Oncology Research Center, Santa Monica, CA, USA.
Ania Moradkhani, Sarcoma Oncology Research Center, Santa Monica, CA, USA.
Natalie Krkyan, Sarcoma Oncology Research Center, Santa Monica, CA, USA.
Amir Ahari, Sarcoma Oncology Research Center, Santa Monica, CA, USA.
Atsushi Osada, NanoCarrier Co., Ltd., Tokyo, Japan.
Sant P Chawla, Sarcoma Oncology Research Center, Santa Monica, CA, USA.
Conflict of Interest
Richard F. Riedel: Bayer, Blueprint, EISAI, EMD Serono, Janssen, Lilly, Ignyta, NanoCarrier, SpringWorks (C/A), AADi, AROG, Ayala, Cogent, Daiichi-Sankyo, Deciphera, Karyopharm, Ignyta, Immune Design, Lilly, NanoCarrier, Novartis, Oncternal, Philogen, Plexxikon, Roche, SpringWorks, Threshold, Tracon, Trillium (RF–institutional), NanoCarrier (SAB), Limbguard, LLC (IP [spouse]); Sant P. Chawla: NanoCarrier, Amgen, Roche, GSK, Ignyta, Tracon, Karyopharm, SARC, Janssen, Inhibrix, Immix, Aadi (C/A, RF, SAB); Atsushi Osada: NanoCarrier Co. Ltd. (E). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Data Availability
This study is still in progress. Once it completes, NanoCarrier will make the data public by entering into ClinicalTrials gov. website.
References
- 1. Penel N, Bui BN, Bay JO, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008;26(32):5269-5274. 10.1200/JCO.2008.17.3146. [DOI] [PubMed] [Google Scholar]
- 2. Nielsen OS, Dombernowsky P, Mouridsen H, et al. High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC soft tissue and bone sarcoma group. Br J Cancer. 1998;78(12):1634-1639. 10.1038/bjc.1998.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park C, Kim M, Kwak Y, et al. Real-world clinical outcomes and prognostic factors for patients with advanced angiosarcoma who received systemic treatment. Cancer Res Treat. 2021;53(4):1195-1203. 10.4143/crt.2020.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young RJ, Natukunda A, Litière S, et al. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: Pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014;50(18):3178-3186. 10.1016/j.ejca.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 5. D’Angelo SP, Munhoz RR, Kuk D, et al. Outcomes of systemic therapy for patients with metastatic angiosarcoma. Oncology 2015;89(4):205-214. 10.1159/000381917. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study is still in progress. Once it completes, NanoCarrier will make the data public by entering into ClinicalTrials gov. website.