Abstract
Background
Previous studies report increasing cholangiocarcinoma (CCA) incidence up to 2015. This contemporary retrospective analysis of CCA incidence and mortality in the US from 2001-2017 assessed whether CCA incidence continued to increase beyond 2015.
Patients and Methods
Patients (≥18 years) with CCA were identified in the National Cancer Institute Surveillance, Epidemiology, and End Results 18 cancer registry (International Classification of Disease for Oncology [ICD-O]-3 codes: intrahepatic [iCCA], C221; extrahepatic [eCCA], C240, C241, C249). Cancer of unknown primary (CUP) cases were identified (ICD-O-3: C809; 8140/2, 8140/3, 8141/3, 8143/3, 8147/3) because of potential misclassification as iCCA.
Results
Forty-thousand-and-thirty CCA cases (iCCA, n=13,174; eCCA, n=26,821; iCCA and eCCA, n=35) and 32,980 CUP cases were analyzed. From 2001-2017, CCA, iCCA, and eCCA incidence (per 100 000 person-years) increased 43.8% (3.08 to 4.43), 148.8% (0.80 to 1.99), and 7.5% (2.28 to 2.45), respectively. In contrast, CUP incidence decreased 54.4% (4.65 to 2.12). CCA incidence increased with age, with greatest increase among younger patients (18-44 years, 81.0%). Median overall survival from diagnosis was 8, 6, 9, and 2 months for CCA, iCCA, eCCA, and CUP. From 2001-2016, annual mortality rate declined for iCCA (57.1% to 41.2%) and generally remained stable for eCCA (40.9% to 37.0%) and for CUP (64.3% to 68.6%).
Conclusions
CCA incidence continued to increase from 2001-2017, with greater increase in iCCA versus eCCA, whereas CUP incidence decreased. The divergent CUP versus iCCA incidence trends, with overall greater absolute change in iCCA incidence, provide evidence for a true increase in iCCA incidence that may not be wholly attributable to CUP reclassification.
Keywords: cholangiocarcinoma, unknown primary tumor, incidence, prevalence, mortality, SEER program
This article analyzes trends in incidence, prevalence, and mortality rates of cholangiocarcinoma to assess whether rates continue to rise in the US and contributing factors that may be influencing current rates, including changing incidence of cancers of unknown primary.
Implications for Practice.
This retrospective SEER registry analysis represents the largest, most current assessment of the cholangiocarcinoma (CCA) incidence, prevalence, and mortality in the US from 2001 to 2017. The study extends previous analyses from 1973 to 2015 to demonstrate a continued increase in CCA incidence beyond 2015, with greatest increases occurring in intrahepatic vs extrahepatic CCA, younger vs older patients, men vs women, and Asian/Pacific Islanders vs other races. The results show this to be a true increase not wholly attributable to reclassification from cancer of unknown primary and indicate a need for improved CCA surveillance and management, particularly for advanced disease.
Introduction
Cholangiocarcinoma (CCA) is a rare cancer representing diverse epithelial tumors originating from cholangiocytes of the biliary tract.1,2 Depending on anatomical location, CCA is classified as intrahepatic (iCCA) or extrahepatic (eCCA), which includes perihilar (pCCA; also called Klatskin tumor) and distal CCA.1,3 Histologically, CCA is also classified as well, moderately, or poorly differentiated adenocarcinomas, or rare variants representing other histology subtypes.1,4 It is not unusual for iCCA to be misclassified as cancer of unknown primary (CUP) because of similarities in clinical presentation and histopathology,5 lack of iCCA-specific diagnostic markers,6 and frequent diagnosis of exclusion.7 The prognosis of patients with CCA is poor owing to a generally aggressive disease course, late-stage diagnosis, and limited treatment options for advanced CCA.8,9
The overall incidence of CCA in the US has increased over the past 5 decades,10-13 and global trends from 1993 to 2012 have shown increases in iCCA and eCCA incidence in most countries.13 A previous analysis of approximately 27 000 CCA cases included in the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) registry from 1973-2012 observed an increase in age-adjusted CCA incidence irrespective of sex and race.11 A SEER analysis of 16 189 CCA cases registered from 2000 to 2015 observed significant annual percentage increases in iCCA and eCCA incidence from 2003 to 2015 (7.043% and 2.069%, respectively).12 Another analysis using data from the US Cancer Statistics (combining the Center for Disease Control and Prevention National Program of Cancer Registries and SEER data)14 for patients with bile duct cancers reported incidences of 1.49 and 0.96 per 100 000 persons for intrahepatic and extrahepatic bile duct cancers, respectively, between 2013 and 2017).15
We performed a contemporary retrospective analysis of the temporal trends in CCA, iCCA, eCCA, and CUP incidence, prevalence, and associated mortality rates in the US for the period from 2001 to 2017, to assess if the previously reported increase in CCA incidence between 1973 and 201511 has continued beyond 2015 and to assess contributions from factors that may influence these rates, including changing CUP incidence.
Methods
Data Sources
Demographic and disease-related data and incidence and mortality data were retrieved from the November 2019 submission of the SEER 18 registry database of patients diagnosed with CCA between 2001 and 2017.16 The SEER program collects cancer incidence and survival data from population-based cancer registries covering approximately 48% of the US population,17 with population data used in calculating cancer rates obtained periodically from the Census Bureau and mortality data obtained from the National Center for Health Statistics.
Analysis Population
Patients with CCA diagnosis were identified using the World Health Organization (WHO) International Classification of Disease for Oncology (ICD-O)-3 coding system from 2001 to 2017. Patients were at least 18 years old at diagnosis and had iCCA (ICD-O-3 topography code C22.1) or eCCA (ICD-O-3 topography codes C24.0, C24.1, and C24.9). A prespecified sensitivity analysis was performed to capture cases of CCA, iCCA, and eCCA, based on both topography codes and linked histology codes that were used in a previous SEER analysis by Saha et al.10 Cases of iCCA were identified using (1) a topography code of C22.0 and histology codes of 8140, 8160, 8161, 8480, 8481, 8500; or (2) a topography code of C22.1 and histology codes of 8000, 8010, 8020, 8140, 8160, 8161, 8260, 8480, 8481, 8490, or 8500. Cases of eCCA were identified using (1) a topography code of C24.1 and histology codes of 8000, 8010, 8020, 8140, 8160, 8161, 8260, 8480, 8481, 8490, 8500; or (2) any diagnoses with topography codes C22.0, C22.1, or C24.0 and a Klatskin tumor histology code of 8162. Patients were excluded if they had cancers with overlapping sites of the biliary tract (as these codes are infrequently used and cannot be reliably mapped to iCCA, eCCA, or other biliary tumor sites), had gallbladder cancers (ICD-O-3 code C23), or had combined hepatocellular carcinoma (HCC) and CCA (ICD-O-3 code 8180).
To assess if misclassification of iCCA as CUP may contribute to iCCA incidence, a separate analysis was performed in patients with unknown primary adenocarcinoma histology (classified by WHO as CUP), or with CCA subtypes collectively categorized as CUP, including unknown primary (ICD-O-3 code C809) and adenocarcinoma histology codes 8140/2 (adenocarcinoma in situ), 8140/3 (adenocarcinoma not otherwise specified), 8141/3 (scirrhous adenocarcinoma), 8143/3 (superficial spreading adenocarcinoma), and 8147/3 (basal cell adenocarcinoma). Patients with squamous and nonepithelial histology codes were excluded because CCA is predominantly of adenocarcinoma histologic type6 and squamous cell carcinoma of the biliary tract is extremely rare.18,19
Outcome Variables
Endpoints included demographic and disease characteristics at diagnosis, incidence of CCA, iCCA, and eCCA, and CUP overall and by age and sex, prevalence of CCA, iCCA, eCCA, and CUP, and 2-year and 5-year overall survival (OS) rates. Incidence rates and limited-duration prevalence (LDP) were calculated for the period from 2001-2017, and for 6-year periods from 2001 to 2006, 2007 to 2012, and 2013 to 2017. Annual mortality rates were calculated for each year from 2001 to 2016 as follows: annual mortality rate = ([incidence – LDP]/incidence) × 100%. Because LDP data for 2017 were not available, the annual mortality rate for 2017 could not be calculated and is not presented. Mortality (5-year and annual) and OS were analyzed retrospectively; OS was defined as survival from the date of diagnosis to the last follow-up or death for the period from 2001 to 2017.
Statistical Analyses
Incidence rates (per 100 000 person-years [p-y]) were age-adjusted to the US Census bureau’s population for the year 2000. Prevalence was calculated as LDP, defined as the proportion of patients alive on a certain day who had a diagnosis of the disease within a specified number of past years. Incidence and LDP were calculated using the SEER*Stat statistical software package (version 8.3.6).20 Estimates of annual percentage changes (APC) per calendar year were calculated for incidence and incidence-based mortality using weighted least squares method (SEER*Stat) together with corresponding 95% confidence intervals (CIs). OS distributions were estimated using the Kaplan-Meier method; mortality risk (hazard ratio with 95% CI) was estimated by multivariate analysis using the Cox proportional hazard model. APC were deemed significant if the lower and upper 95% CIs were of the same sign. For all other tests, P < .05 was considered statistically significant. Statistical analyses of survival were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
Patient Characteristics
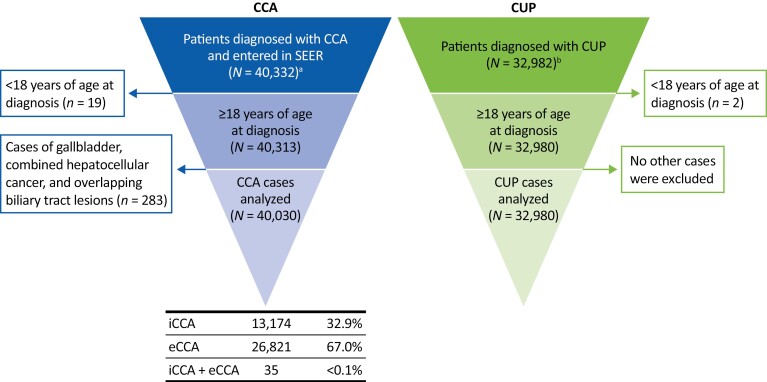
Between 2001 and 2017, 40 332 CCA cases were entered in the SEER registry (Fig. 1), from which 40 030 were included in this analysis (iCCA, n = 13 174 [32.9%]; eCCA, n = 26 821 [67.0%]; iCCA and eCCA, n = 35 [<0.1%]). A total of 32 982 patients were diagnosed with CUP during the same period, 32 980 of whom were ≥18 years old and eligible for inclusion in the analysis (Fig. 1).
Figure 1.
Patient disposition. aPatients diagnosed with iCCA (ICD-O-3 code: C221) or eCCA (ICD-O-3 codes: C240, C241,249). bCancer of unknown primary (ICD-O-3 code C809) and adenocarcinoma histology codes 8140/2 (adenocarcinoma in situ), 8140/3 (adenocarcinoma not otherwise specified), 8141/3 (scirrhous adenocarcinoma), 8143/3 (superficial spreading adenocarcinoma), and 8147/3 (basal cell adenocarcinoma). Abbreviations: CCA, cholangiocarcinoma; CUP, cancer of unknown primary; eCCA, extrahepatic cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma; ICD-O-3, International Classification of Diseases for Oncology.
The demographics and disease characteristics of patents analyzed are shown in Table 1. Among the 40 030 patients diagnosed with CCA in this study, 17.4% were diagnosed during the first 4 years of the study (2001-2004; 13.4% and 19.4% of patients with iCCA and eCCA, respectively) and 31.4% were diagnosed during the last 4 years (2014-2017; 40.1% and 27.1% of patients with iCCA and eCCA respectively). Among the patients diagnosed with CUP, 28.7% were diagnosed during the first 4 years and 20.0% during the last 4 years (Table 1).
Table 1.
Baseline demographics and characteristics.
| CCA | iCCA | eCCA | CUP | |
|---|---|---|---|---|
| (N = 40 030)a | (N = 13 174) | (N = 26 821) | (N = 32 980) | |
| Age at diagnosis, years | ||||
| 18-44 | 1388 (3.5) | 554 (4.2) | 832 (3.1) | 970 (2.9) |
| 45-64 | 11,824 (29.5) | 4384 (33.3) | 7427 (27.7) | 9342 (28.3) |
| 65-84 | 21,582 (53.9) | 6818 (51.8) | 14,746 (55.0) | 18,259 (55.4) |
| ≥85 | 5236 (13.1) | 1418 (10.8) | 3816 (14.2) | 4409 (13.4) |
| Sex | ||||
| Male | 21,114 (52.8) | 6678 (50.7) | 14,417 (53.8) | 14,786 (44.8) |
| Female | 18,916 (47.3) | 6496 (49.3) | 12,404 (46.2) | 18,194 (55.2) |
| Race | ||||
| White | 31,590 (78.9) | 10,382 (78.8) | 21,180 (79.0) | 26,678 (80.9) |
| Black | 3331 (8.3) | 1043 (7.9) | 2287 (8.5) | 3980 (12.1) |
| American Indian/Alaska Native | 327 (0.8) | 121 (0.9) | 206 (0.8) | 217 (0.7) |
| Asian/Pacific Islander | 4686 (11.7) | 1600 (12.1) | 3080 (11.5) | 1978 (6.0) |
| Unknown | 96 (0.2) | 28 (0.2) | 68 (0.3) | 127 (0.4) |
| Diagnosis year | ||||
| 2001-2004 | 6968 (17.4) | 1762 (13.4) | 5199 (19.4) | 9473 (28.7) |
| 2005-2009 | 10,184 (25.4) | 2760 (21.0) | 7414 (27.6) | 9769 (29.6) |
| 2010-2013 | 10,311 (25.8) | 3368 (25.6) | 6933 (25.8) | 7154 (21.7) |
| 2014-2017 | 12,567 (31.4) | 5284 (40.1) | 7275 (27.1) | 6584 (20.0) |
| Grade at presentation | ||||
| Well differentiated (grade I) | 2273 (5.7) | 496 (3.8) | 1777 (6.6) | 130 (0.4) |
| Moderately differentiated (grade II) | 8230 (20.6) | 2082 (15.8) | 6135 (22.9) | 751 (2.3) |
| Poorly differentiated (grade III) | 6635 (16.6) | 1993 (15.1) | 4640 (17.3) | 1844 (5.6) |
| Undifferentiated/anaplastic (grade IV) | 291 (0.7) | 77 (0.6) | 214 (0.8) | 91 (0.3) |
| Unknown | 22,601 (56.5) | 8526 (64.7) | 14,055 (52.4) | 30,164 (91.5) |
| Cancer stageb | ||||
| I | 5513 (13.8) | 1487 (11.3) | 4017 (15.0) | 0 |
| II | 4693 (11.7) | 491 (3.7) | 4198 (15.7) | 0 |
| III | 3872 (9.7) | 1798 (13.6) | 2069 (7.7) | 0 |
| IV | 8117 (20.3) | 3086 (23.4) | 5027 (18.7) | 0 |
| Unknown | 5780 (14.4) | 2129 (16.2) | 3646 (13.6) | 0 |
| Not applicable | 298 (0.7) | 3 (<0.1) | 295 (1.1) | 22,482 (68.2) |
| Missingc | 11,757 (29.4) | 4180 (31.7) | 7569 (28.2) | 10,498 (31.8) |
Includes 35 patients (0.1%) who were classified as having both iCCA and eCCA.
The sixth edition of American Joint Committee on Cancer was used to stratify the cancer stages.
Cancer stage stratification data were only available for 2004-2015 diagnosis years and were missing for patients diagnosed in 2001-2003, 2016, and 2017.
Abbreviations: CCA, cholangiocarcinoma; CUP, cancer of unknown primary; eCCA, extrahepatic cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma.
The disposition, demographics, and disease characteristics of patients included in the sensitivity analysis that included topography and histology codes are presented in Supplementary Results, Table S1, and Fig. S1.
Incidence
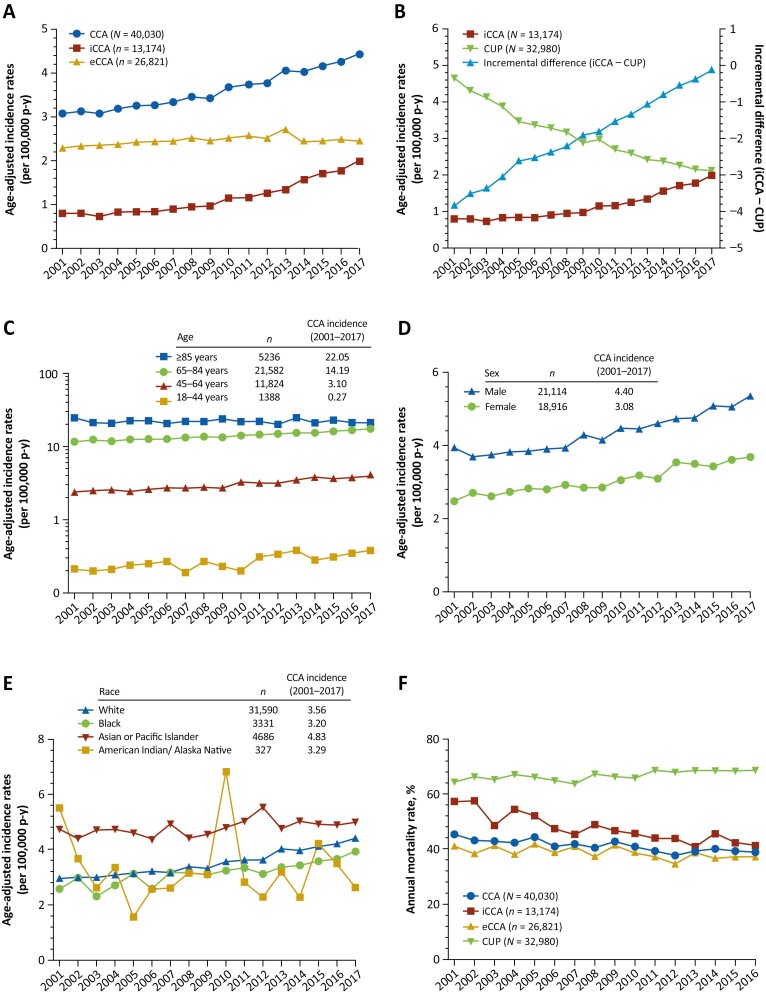
The overall age-adjusted incidence of CCA from 2001 to 2017 was 3.65 per 100 000 p-y (iCCA, 1.19; eCCA, 2.46). The incidence of CCA increased by 43.8% from 3.08 in 2001 to 4.43 in 2017 (Fig. 2A). The APC in incidence of CCA showed a significant increase from 2001 to 2017, and during time periods 2001 to 2006, 2007 to 2012, and 2013 to 2017 (Table 2). The age-adjusted incidence of iCCA increased 148.8% (0.80-1.99) from 2001 to 2017; within this period, iCCA incidence remained relatively constant from 2001 (0.80) to 2006 (0.84), and then increased thereafter by 121.1% from 2007 to 2017 (0.90-1.99; Fig. 2A). Similarly, the APC in incidence of iCCA demonstrated a small, nonsignificant increase from 2001 to 2006 and then significant increases from 2007 to 2012 and 2013 to 2017 (Table 2). The age-adjusted incidence of eCCA increased by 7.5% from 2.28 in 2001 to 2.45 in 2017 (Fig. 2A), and the APC in eCCA incidence only showed a significant increase from 2001-2006, which was followed by a nonsignificant increase between 2007 and 2012 and a nonsignificant decline in incidence between 2013 and 2017 (Table 2). The overall age-adjusted incidence of CUP from 2001 to 2017 was 3.03, declining 54.4% from 4.65 cases in 2001 to 2.12 cases in 2017 (Fig. 2B). The incremental difference between age-adjusted iCCA and CUP incidences increased monotonically from −3.85 in 2001 to −0.13 in 2017 (Fig. 2B). The APC in the incidence of CUP decreased significantly across all 3 time periods assessed (Table 2).
Figure 2.
Age-adjusted incidence of CCA, iCCA, eCCA (A), CUP (B); incidence of CCA by age (C), sex (D), and race (E); annual mortality rates for CCA, iCCA, eCCA, and CUP (F) from 2001 to 2017. Incidence was determined per 100 000 p-y and age-adjusted to the US standard population of 2000. Also shown in panel B is the incremental difference between age-adjusted iCCA and CUP incidences from 2001 to 2017. Note that interpretation of trends in CCA incidence in the American Indian/Alaska Native cohort is limited by the correspondingly small sample size (n = 327).
Abbreviations: CCA, cholangiocarcinoma; CUP, cancer of unknown primary; eCCA, extrahepatic cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma; p-y, person-year.
Table 2.
Annual percentage changes in incidence of CCA, iCCA, eCCA, and CUP.
| Annual percentage change (95% CI), per 100 000 p-y | ||||
|---|---|---|---|---|
| CCA (N = 40 030) | iCCA (N = 13 174) | eCCA (N = 26 821) | CUP (N = 32 980) | |
| 2001-2017 | 2.44 (2.21-2.68)a | 6.77 (5.88-7.67)a | 0.49 (0.14-0.85)a | −4.79 (−5.08 to −4.49)a |
| 2001-2006 | 1.34 (0.49–2.21)a | 1.58 (−1.49 to 4.75) | 1.26 (0.95-1.57)a | −6.44 (−7.58 to −5.29)a |
| 2007-2012 | 2.62 (1.37-3.90)a | 7.33 (4.73-9.99)a | 0.69 (−0.47 to 1.86) | −4.53 (−6.35 to −2.68)a |
| 2013-2017 | 2.38 (0.93-3.86)a | 9.26 (5.62-13.02)a | −1.97 (−5.41 to 1.6) | −3.60 (−4.69 to −2.50)a |
Significant annual percentage changes. Changes were deemed significant if the lower and upper 95% CIs were of same sign, otherwise the change was considered not significant.
Abbreviations: CCA, cholangiocarcinoma; CI, confidence interval; CUP, cancer of unknown primary; eCCA, extrahepatic cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma; p-y, person-years.
The overall age-adjusted incidence of CCA from 2001 to 2017 increased with patient’s age (18-44 years, 0.27; 45-64 years, 3.10; 65-84 years, 14.19; ≥85 years, 22.05) (Fig. 2C). For patients 18-44 years of age, CCA incidence increased by 81.0% from 0.21 in 2001 to 0.38 in 2017; for those ≥85 years of age, incidence decreased by 14.4% from 24.59 in 2001 to 21.04 in 2017. The overall incidence of CCA from 2001 to 2017 was 4.40 in men vs 3.08 in women, reflecting an increase from 3.93 in 2001 to 5.35 in 2017 (36.1%) in men, and from 2.48 in 2001 to 3.68 in 2017 (48.4%) in women (Fig. 2D). The overall incidence of CCA from 2001 to 2017 was 4.83 in Asian/Pacific Islander, 3.56 in White, 3.20 in Black cohorts, and 3.29 in American Indian/Alaska native. The incidence of CCA increased from 4.75 in 2001 to 5.00 in 2017 (5.3%) in Asian/Pacific Islander, 2.96 to 4.42 (49.3%) in White, 2.58 to 3.95 (53.1%) in Black, and decreased from 5.51 to 2.64 (52.1%) in American Indian/Alaska native groups (Fig. 2E).
In the sensitivity analysis, the overall age-adjusted incidence of CCA from 2001-2017 was 2.32 per 100,000 p-y (iCCA, 1.54; eCCA, 0.78). Further information on the incidences and APC in incidences for CCA, iCCA, and eCCA and dependence on patient demographics from 2001 to 2017 derived from the sensitivity analysis are presented in Supplementary Results and Table S2.
Prevalence
From 2001 to 2017, the LDP of CCA was 5.92% (Table 3) and was higher for eCCA vs iCCA (4.23% vs 1.69%). The LDP of iCCA increased from 2001-2006 (0.60%) to 2007-2012 (0.98%) to 2013-2017 (1.37%), whereas the LDP for eCCA remained relatively stable. The LDP of CUP for 2001-2017 was close to that for iCCA (1.40% vs 1.69%) but decreased from 1.69% in 2001-2006 to 0.85% in 2013-2017 (Table 3).
Table 3.
Prevalence of CCA, iCCA, eCCA, and CUP.
| LDP rate,a per 100 000 p-y | ||||
|---|---|---|---|---|
| CCA (N = 40 030)* | iCCA (N = 13 174) | eCCA (N = 26 821) | CUP (N = 32 980) | |
| 2001-2017 | 5.92 | 1.69 | 4.23 | 1.40 |
| 2001-2006 | 3.26 | 0.60 | 2.67 | 1.69 |
| 2007-2012 | 3.93 | 0.98 | 2.96 | 1.22 |
| 2013-2017 | 3.91 | 1.37 | 2.54 | 0.85 |
LDP is defined as the number of people alive on a certain day who had a diagnosis of the disease within the past 16 years; as of January 1, 2017. LDP rate was calculated as LDP normalized with averaging 2016 and 2017 populations, then age-adjusted to the US standard population of 2000.
Abbreviations: CCA, cholangiocarcinoma; CUP, cancer of unknown primary; eCCA, extrahepatic cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma; LDP, limited-duration prevalence; p-y, person-years.
LDP of CCA, iCCA, and eCCA from 2001-2017 derived from the sensitivity analysis are presented in Supplementary Results, Fig. S2, and Table S3.
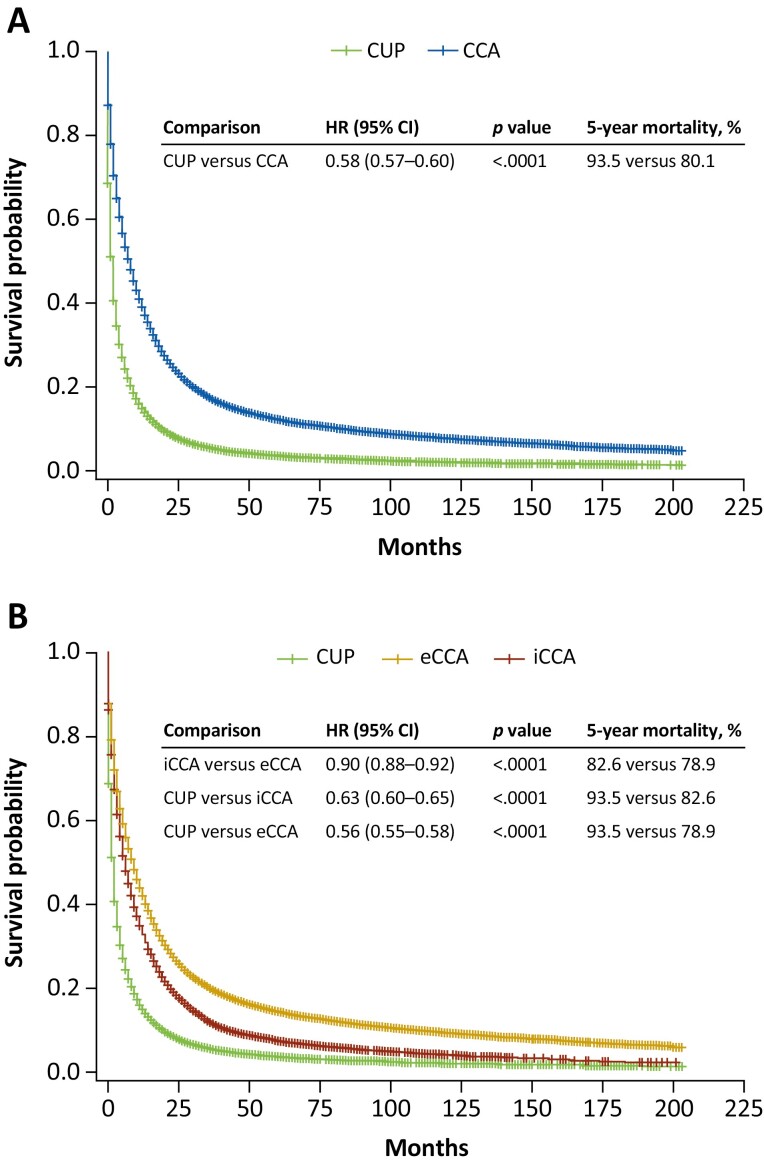
Survival
The median OS from diagnosis was 8, 6, and 9, months, respectively, among patients with CCA, iCCA, eCCA, and 2 months among patients with CUP (Fig. 3). Five-year mortality rates were significantly higher for CUP vs CCA, iCCA vs eCCA, CUP vs iCCA, and CUP vs eCCA (all P < .0001; Fig. 3). The annual mortality rate declined from 45.2% in 2001 to 38.7% in 2016 for CCA, and from 57.1% in 2001 to 41.2% in 2016 for iCCA, but the rates for iCCA remained consistently higher than for eCCA, which generally remained stable (40.9% in 2001 vs 37.0% in 2016; Fig. 2F). Annual mortality for CUP also remained relatively stable (64.3% in 2001; 68.6% in 2016; Fig. 2F). The APC in annual mortality for CCA, iCCA, and eCCA each showed significant increases from 2001 to 2017 (Table 4). The APC in mortality increased significantly in each period (2001-2006, 2007-2012, and 2013-2017) for CCA, increased significantly only in 2007-2012 and 2013-2017 for iCCA, and increased significantly in 2001-2006 while decreasing significantly in 2013-2017 for eCCA (Table 4). The APC in incidence-based mortality due to CCA by age group demonstrated significant increases in patients 18-44, 45-64, and 65-84 years from 2001 to 2017, and a slight nonsignificant decline in mortality among patients ≥85 years of age (Table 4). The APC in mortality due to iCCA showed significant increases in all age groups; APC in mortality due to eCCA showed significant increases in patients 45-64 and 65-84 years of age and a significant decline in those ≥85 years of age (Table 4). APC in mortality due to CUP showed a significant decrease over the 2001-2017 study period (Table 4).
Figure 3.
Overall survival: (A) CCA, CUP; (B) iCCA, eCCA, CUP. Abbreviations: CCA, cholangiocarcinoma; CI, confidence interval; CUP, cancer of unknown primary; eCCA, extrahepatic cholangiocarcinoma; HR, hazard ratio; iCCA, intrahepatic cholangiocarcinoma.
Table 4.
Annual percentage change in incidence-based mortality due to CCA, iCCA, eCCA, and CUP from 2001-2017.
| Annual percentage change (95% CI), per 100 000 p-y | ||||
|---|---|---|---|---|
| CCA (N = 40 030) | iCCA (N = 13 174) | eCCA (N = 26 821) | CUP (N = 32 980) | |
| 2001-2017 | 2.90 (2.57-3.23)a | 6.21 (5.18-7.25)a | 1.33 (0.48-2.19)a | −4.42 (−4.71 to −4.13)a |
| Age, years | ||||
| 18-44 | 3.86 (2.36-5.38)a | 7.90 (5.58–10.27)a | 1.27 (−0.40 to 2.98) | ND |
| 45-64 | 3.42 (2.89-3.96)a | 7.55 (6.70-8.40)a | 1.17 (0.59-1.76)a | ND |
| 65-84 | 2.51 (2.29–2.74)a | 6.81 (5.74-7.89)a | 0.68 (0.29-1.07)a | ND |
| ≥85 | −0.17 (−0.83 to 0.49) | 3.94 (2.49-5.42)a | −1.62 (−2.50 to −0.74)a | ND |
| 2001-2006 | 4.86 (2.28-7.52)a | 0.02 (−5.18 to 5.50) | 6.71 (2.01-11.62)a | −4.78 (−7.19 to −2.3)a |
| 2007-2012 | 2.04 (0.26-3.84)a | 6.92 (4.84-9.05)a | 0.04 (−2.59 to 2.74) | −4.44 (−6.45 to −2.38)a |
| 2013-2017 | 2.38 (1.67-3.09)a | 10.49 (7.57-13.50)a | −2.42 (−3.43 to −0.45)a | −3.81 (−5.44 to −2.16)a |
Significant annual percentage changes. Changes were deemed significant if the lower and upper 95% CIs were of same sign, otherwise the change was considered not significant.
Abbreviations: CCA, cholangiocarcinoma; CI, confidence interval; CUP, cancer of unknown primary; eCCA, extrahepatic cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma; ND, not determined.
APC in incidence-based mortality due to CCA, iCCA, and eCCA from 2001-2017 derived from the sensitivity analysis is presented in Supplementary Results and Table S4.
Discussion
To our knowledge, this retrospective analysis of real-world SEER registry data represents the largest and most current assessment of the incidence, prevalence, and associated mortality rates of CCA and CUP in the US. The study extends the results of recent analyses of CCA and bile duct cancer epidemiology10-12,15 to demonstrate a continued increase in CCA incidence from 2001 to 2017. Whereas these previous studies have also documented a rising CCA incidence, particularly for iCCA,10 data are limited regarding temporal changes in incidence and mortality in various age groups, and it has been unclear if the observed increase in iCCA incidence is solely due to a reclassification from CUP involving the liver.
Whereas iCCA and eCCA incidences increased from 2001-2017, iCCA incidence increased to a much greater extent. The observed increases in iCCA incidence are consistent with previous findings.10-13 However, recent studies have pointed to confounding factors such as evolving updates to the ICD-O coding that may have resulted in misclassification of CCA subtypes; thus impacting on incidence data.6,21 For example, the first edition of ICD-O coding (ICD-O-1) instigated in 1979, did not include a morphology/histology classification for pCCA/Klatskin tumor and thus did not distinguish pCCA/Klatskin tumor from either iCCA or eCCA.21 The second edition (ICD-O-2), which replaced ICD-O-1 for use in the US from 1992, included a specific histology code for pCCA/Klatskin tumor, but this was mapped to iCCA rather than to eCCA. The third edition (ICD-0-3), which superseded ICD-O-2 in the US in 2001, mapped the histology code for pCCA/Klatskin tumor to either iCCA or eCCA. Therefore, misclassification of pCCA/Klatskin tumors as iCCA during the period covered by ICD-O-1 and ICD-O-2 may have resulted in an overestimation of iCCA incidence in the US up to 2001.21 Notably, a recent retrospective study comparing final diagnosis with actual ICD-10 code allocation in UK regional HepatoPancreatoBiliary centers from January 1, 2015 to January 1, 2017 showed that the majority (92%) of pCCA/Klatskin tumors reviewed were misclassified as iCCA.22 Another analysis of SEER data from 1975-1999 hypothesized that the increase in iCCA incidence resulted from earlier or improved detection, resulting from better diagnostic techniques.23 However, arguing against this hypothesis, it was found that the increase in iCCA incidence was not accompanied by any significant changes in the proportion of patients with unstaged or localized disease, histologic diagnosis, or with tumor size <5 cm.23 Taken together, these findings indicate that the observed increase in the incidence of iCCA after 2001 and up to 2017 may not be wholly explained by coding changes or improved diagnostics.23
Other epidemiologic and/or environmental risk factors for iCCA may include, for example, bile duct diseases (eg, Caroli disease, choledochal cysts, primary sclerosing cholangitis), metabolic diseases (eg, type 2 diabetes, obesity), liver cirrhosis, alcohol-related disorders, and hepatitis B virus (HBV) or hepatitis C virus (HCV) infections.24-26 In particular, non-alcoholic fatty liver disease (NAFLD) has been identified as a risk factor for both iCCA and eCCA.26,27 Of note, the results of a recent meta-analysis demonstrated a significant association of NAFLD with CCA, particularly iCCA, but not with eCCA.28 Moreover, the prevalence of NAFLD in the US has increased over the past years in parallel with increases in the prevalence of obesity and type 2 diabetes.29 Taken together, these results suggest that increases in NAFLD may, in part, contribute to the increasing incidence of iCCA observed here. HCV infection is also a risk factor for iCCA and eCCA.26,30 A model-based study of past HCV infection incidence has estimated that incidence increased from the 1960s to 1980s, followed by a decline in incidence in the early 1990s.31 These estimates may, in part, explain the increased CCA incidence in older patients observed in this study, given that liver disease may occur many years after HCV infection.
Intrahepatic CCA can be misclassified as CUP because of similarities in presentation and histopathology,5 lack of iCCA-specific diagnostic markers,6 and frequent diagnosis of exclusion.7 Consistent with this, a molecular profiling study predicted that of 252 cases of CUP examined, 21% may have originated from tumors located in the biliary tract.32 Ferrone and colleagues found that, of 27 intrahepatic adenocarcinomas of unknown origin tested, 22% were positive for albumin, indicating that they should be reclassified as iCCA.33 Another study suggested that an observed long-term decline in the incidence of CUP from 1973-201210 may have arisen from improvements in histopathologic, imaging, and molecular diagnostic techniques (eg, albumin RNA in situ hybridization platform33) for distinguishing CUP from iCCA and from other cancers.10 However, whether the misclassification of iCCA as CUP occurs often enough to have a substantial impact on the reported incidence of iCCA has been questioned.34 The observed divergent and opposing trends in CUP vs iCCA incidence from 2001-2017, along with the observed monotonic increase in the incremental difference between iCCA and CUP incidences across the study period, provide evidence for a true increase in iCCA incidence that may not be wholly explained by a decreased misclassification of iCCA as CUP.
In general, the increase in CCA incidence over the 17-year study period occurred irrespective of patient characteristics. However, asymmetries in sample size distributions across cohorts render interpretation of differences difficult. For example, patients with CCA were predominantly ≥65 years of age at diagnosis (67.0%) and White (78.9%). Importantly, the largest percentage increase in CCA incidence occurred among younger patients (18-44 years, 81.0%); although other factors may confound this observation and further studies are required to assess if this reflects a true shift in patient demographics. As reported elsewhere, the incidence of CCA in the US was the greatest among older patients.12,13,15 The observation that the overall age-adjusted incidence of CCA from 2001 to 2017 was greater in men vs women,11,12,15 and in Asian/Pacific Islanders vs other races12 is also consistent with previous results. Of note, a global study of iCCA and eCCA incidence trends over a 20-year period demonstrated that the highest rates of iCCA and eCCA occurred in Asian countries, particularly, South Korea, Thailand, and Japan.13 This may be associated with the higher risk of parasitic liver-fluke infection in these countries, which have been shown to be strong risk factors for CCA in Thailand35 and Korea.36 In addition, HBV infection has been shown to be associated with increased CCA risk in Asian countries.37
Compared with the analysis based on topography codes alone, use of topography codes linked with histology codes to identify iCCA and eCCA cases in the sensitivity analysis resulted in an observed higher overall incidence of iCCA (1.54 vs 1.19) and a lower incidence of eCCA (0.78 vs 2.46) from 2001 to 2017. Dissection of these observed differences in iCCA and eCCA incidence would require knowledge of the number of tumor diagnoses identified by each histology code, which is beyond the scope of the current analysis. However, the observed increase in iCCA incidence might reflect the use of histology codes that identify adenocarcinomas in addition to iCCA, as well as carcinomas of undifferentiated and unspecified histology, such as “carcinoma, not otherwise specified [NOS]” and “neoplasm, malignant”,10 which might also represent miscoded iCCA cases. Importantly, the finding that the inclusion of histology codes did not affect the temporal trends in iCCA and eCCA incidence from 2001 to 2017 and trends of CCA incidence among demographic cohorts again suggests that these trends may not be wholly explained by differences in coding used to define iCCA and eCCA.
The 5-year mortality rate of patients diagnosed with CCA (80.1%) observed in this study appear to be consistent with those reported previously (patients 18-34 years, 69.9%; 35-49 years, 77.86%; 50-64 years, 83.02%; ≥65 years, 91.41%).11 The observed APC in iCCA, and eCCA mortality are also consistent with incidence-based mortality rates reported previously for the period 2003-2013 (iCCA, 7.5; eCCA, 3.3 per 100 000 p-y).12 Moreover, as found here, mortality rates were reported to decrease significantly from 2013 to 2015, with APC of –18.448 and –23.511 for iCCA and eCCA, respectively.12 In the present study, the mortality rate associated with iCCA was consistently greater than that associated with eCCA (in keeping with the shorter median OS). However, the annual mortality rates for iCCA declined, such that in 2016 the rate for iCCA approaches that of eCCA (41.2% vs 37.0%). Potential reasons for this decline in iCCA mortality may include improvements in surgical techniques, incorporation of standardized systemic and local therapies, as well as enhanced multidisciplinary management. However, information on treatments received by patients with CCA is not included in the SEER registry. In contrast to the observed decrease in annual mortality rates, the APC in incidence-based mortality due to CCA showed a significant increase from 2001-2017 in all age groups (except for a decline in patients ≥85 years of age). The opposite trend likely reflects the fact that APC in incidence-based mortality provides an estimate of new cases of death due to CCA in a specific year relative to the previous year, whereas mortality rate provides an estimate of mortality among incident CCA cases in that specific year. Notably, the magnitude of this increase was greatest for younger patients. This trend was particularly evident in patients with iCCA and is consistent with the observed greater increase in CCA and iCCA incidence in younger versus older patients. Finally, despite the decrease in the incidence of CUP, mortality associated with CUP remained relatively stable from 2001 to 2017 and was associated with shorter median OS and significantly higher mortality risk compared with patients with CCA, iCCA, or eCCA.
Limitations of this study include the retrospective design, absence of clinical and disease-specific parameters in SEER, including chemotherapies, comorbid conditions (eg, diabetes, liver cirrhosis, obesity, alcohol use, and HBV and HCV infections), each of which may have contributed to the observed incidence and mortality rates in patients with CCA. In addition, the analysis of age-adjusted incidence of CCA and CUP by race/ethnicity is limited by the lack of a separate analysis for Hispanic patients, an important segment of the US population. Nevertheless, this study represents the largest analysis of temporal trends in CCA incidence and mortality together with their relationship with changing CUP incidence and mortality, and contributes to information essential for guiding future healthcare policy, including resource allocation.
Conclusions
The results demonstrate that CCA incidence in the US continued to increase from 2001-2017, with the greatest increases occurring in iCCA vs eCCA, in younger vs older patients, in men vs women, and in Asian/Pacific Islanders vs other races. This appears to be a true increase and not wholly attributable to reclassification from CUP.
Supplementary Material
Acknowledgments
Medical writing and editorial assistance were provided by Simon J. Slater, PhD, CMPP, of Envision Pharma Group (Philadelphia, PA), funded by Incyte Corporation.
Contributor Information
Milind Javle, Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Sunyoung Lee, Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Nilofer S Azad, Gastrointestinal Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, MD, USA.
Mitesh J Borad, Mayo Clinic, Phoenix, AZ, USA.
Robin Kate Kelley, University of California at San Francisco, Helen Diller Family Comprehensive Cancer Center, San Francisco, CA, USA.
Smitha Sivaraman, Incyte Corporation, Wilmington, DE, USA.
Anna Teschemaker, Incyte Corporation, Wilmington, DE, USA.
Ishveen Chopra, STATinMED Research, Plano, TX, USA.
Nora Janjan, STATinMED Research, Plano, TX, USA.
Shreekant Parasuraman, Incyte Corporation, Wilmington, DE, USA.
Tanios S Bekaii-Saab, Mayo Clinic, Phoenix, AZ, USA.
Funding
This work was supported by Incyte Corporation (Wilmington, DE).
Conflict of Interest
Mitesh J. Borad: Agios, ArQule, Celgene, Fujifilm, G1 Therapeutics, Halozyme Therapeutics, Inspyr Therapeutics, Novartis, Pieris Pharmaceuticals, Taiho Pharmaceutical, TD2 (C/A), Agios, ARIAD, BioLineRx, Boston Biomedical, Celgene, Dicerna Pharmaceuticals, Eisai, EMD Serono, Halozyme Therapeutics, ImClone Systems, Incyte Corporation, Ionis Pharmaceuticals, MedImmune, miRNA Therapeutics, Puma Biotechnology, QED Therapeutics, Senhwa Biosciences, SillaJen, Sun Biopharma, Taiho Pharmaceutical, Threshold Pharmaceuticals, Toray Industries (RF), AVEO Oncology, Gilead Sciences, GlaxoSmithKline (OI), ArQule, AstraZeneca, Celgene (travel, accommodations, and expenses); Robin Kate Kelley: Exact Sciences, Genentech, Gilead Sciences (C/A), Agios, AstraZeneca, Bayer, Bristol Myers Squibb, Eli Lilly & Company, EMD Serono, Exelixis, Genentech, Merck, Novartis, Partner Therapeutics, QED Therapeutics, Relay Therapeutics, Taiho Pharmaceutical (RF), Agios, AstraZeneca, Bristol Myers Squibb, Merck (SAB), Ipsen (travel expenses), Genentech (participated in an IDMC); Smitha Sivaraman: Gamida Cell (E, OI); Anna Teschemaker: Incyte Corporation (E, OI); Ishveen Chopra: STATinMED Research (E); Nora Janjan: STATinMED Research (E); Shreekant Parasuraman: Incyte Corporation (E, OI); Tanios S. Bekaii-Saab: Array Biopharma, Bayer, Genentech, Incyte Corporation, Ipsen, Merck, Pfizer, Seattle Genetics, AbbVie, AstraZeneca, BeiGene, Boehringer Ingelheim, Celularity, Daichii Sankyo, Eisai, Exact Science, Foundation Medicine, Janssen, Natera, Sobi, Treos Bio Limited, Xilis (C/A); AbGenomics, Agios, Amgen, Array Biopharma, Arys, Bayer, Oston Biomedical, Bristol Myers Squibb, Celgene, Clovis Oncology, Eli Lilly & Company, Genentech, Incyte Corporation, Ipsen, Merck, Merus, Mirati Therapeutics, Novartis, Pfizer, Seattle Genetics (RF), Immuneering, Imugene, Sun Biopharma (SAB), WO/2018/183488 and WO/2019/055687 (IP: inventions/patents), 1Globe Health Institute, AstraZeneca, Eli Lilly & Company, Exelixis, PanCAN (participated in an IDMC/DSMB). Milind Javle and Sunyoung Lee indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: All authors. Collection and/or assembly of data: A.T., S.P., S.S., I.C., N.J. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors.
Data Availability
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase I studies) for which the product and indication have been approved on or after January 1, 2020 in at least one major market (eg, US, European Union, Japan). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960
References
- 1. Blechacz B. Cholangiocarcinoma: current knowledge and new developments. Gut Liver 2017;11(1):13-26. 10.5009/gnl15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khan AS, Dageforde LA.. Cholangiocarcinoma. Surg Clin North Am. 2019;99(2):315-335. 10.1016/j.suc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 3. Huguet JM, Lobo M, Labrador JM, et al. Diagnostic-therapeutic management of bile duct cancer. World J Clin Cases 2019;7(14):1732-1752. 10.12998/wjcc.v7.i14.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Razumilava N, Gores GJ.. Cholangiocarcinoma. Lancet 2014;383(9935):2168-2179. 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varadhachary GR, Raber MN.. Cancer of unknown primary site. N Engl J Med. 2014;371(8):757-765. 10.1056/NEJMra1303917. https://www.nejm.org/doi/10.1056/NEJMra1303917 [DOI] [PubMed] [Google Scholar]
- 6. Bridgewater J, Galle PR, Khan SA, et al. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014;60(6):1268-1289. 10.1016/j.jhep.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 7. Dodson RM, Weiss MJ, Cosgrove D, et al. Intrahepatic cholangiocarcinoma: management options and emerging therapies. J Am Coll Surg. 2013;217(4):736-750.e4. 10.1016/j.jamcollsurg.2013.05.021. https://journals.lww.com/journalacs/Citation/2013/10000/Intrahepatic_Cholangiocarcinoma__Management.21.aspx [DOI] [PubMed] [Google Scholar]
- 8. Rahnemai-Azar AA, Weisbrod A, Dillhoff M, et al. Intrahepatic cholangiocarcinoma: molecular markers for diagnosis and prognosis. Surg Oncol. 2017;26(2):125-137. 10.1016/j.suronc.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 9. Rodrigues PM, Olaizola P, Paiva NA, et al. Pathogenesis of cholangiocarcinoma. Annu Rev Pathol. 2021;16(1):433-463. 10.1146/annurev-pathol-030220-020455. [DOI] [PubMed] [Google Scholar]
- 10. Saha SK, Zhu AX, Fuchs CS, et al. Forty-year trends in cholangiocarcinoma incidence in the U.S.: intrahepatic disease on the rise. Oncologist 2016;21(5):594-599. 10.1634/theoncologist.2015-0446. https://academic.oup.com/oncolo/article/21/5/594/6401575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mukkamalla SKR, Naseri HM, Kim BM, et al. Trends in incidence and factors affecting survival of patients with cholangiocarcinoma in the United States. J Natl Compr Canc Netw. 2018;16(4):370-376. 10.6004/jnccn.2017.7056. https://jnccn.org/view/journals/jnccn/16/4/article-p370.xml [DOI] [PubMed] [Google Scholar]
- 12. Gad MM, Saad AM, Faisaluddin M, et al. Epidemiology of cholangiocarcinoma; United States incidence and mortality trends. Clin Res Hepatol Gastroenterol 2020;44(6):885-893. 10.1016/j.clinre.2020.03.024. [DOI] [PubMed] [Google Scholar]
- 13. Florio AA, Ferlay J, Znaor A, et al. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer 2020;126(11):2666-2678. 10.1002/cncr.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Center for Disease Control and Prevention (CDC). About U.S. Cancer Statistics. U.S. Department of Health & Human Services; 2021. Available at https://www.cdc.gov/cancer/uscs/about/index.htm. Accessed September 18, 2021.
- 15. Ellington TD, Momin B, Wilson RJ, et al. Incidence and mortality of cancers of the biliary tract, gallbladder, and liver by sex, age, race/ethnicity, and stage at diagnosis: United States, 2013 to 2017. Cancer Epidemiol Biomarkers Prev. 2021;30(9):1607-1614. 10.1158/1055-9965.EPI-21-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Cancer Institute Surveillance, Epidemiology, and End Results. SEER*Stat Databases: November 2019 Submission. Incidence - SEER Research Data, 18 Registries, Nov 2019 Sub (2000-2017). 2021. Available at https://seer.cancer.gov/data-software/documentation/seerstat/nov2019/. Accessed September 30, 2021.
- 17. National Cancer Institute Surveillance, Epidemiology, and End Results. SEER Program Overview. 2021. Available at https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf. Accessed September 30, 2021.
- 18. Kang M, Kim NR, Chung DH, et al. Squamous cell carcinoma of the extrahepatic common hepatic duct. J Pathol Transl Med. 2019;53(2):112-118. 10.4132/jptm.2018.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamaoka K, Tanemura M, Furukawa K, et al. Primary intrahepatic squamous cell carcinoma with histological collision of adenocarcinoma and squamous cell carcinoma: a case report. Am J Case Rep. 2018;19:1184-1191. 10.12659/AJCR.910676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. National Cancer Institute Surveillance, Epidemiology, and End Results. SEER*Stat Software. 2021. Available at https://seer.cancer.gov/seerstat/. Accessed July 19, 2021.
- 21. Khan SA, Emadossadaty S, Ladep NG, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us?. J Hepatol. 2012;56(4):848-854. 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 22. Selvadurai S, Mann K, Mithra S, et al. Cholangiocarcinoma miscoding in hepatobiliary centres. Eur J Surg Oncol. 2021;47(3, Part B):635-639. 10.1016/j.ejso.2020.09.039 [DOI] [PubMed] [Google Scholar]
- 23. Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase?. J Hepatol. 2004;40(3):472-477. 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 24. Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010;51(2):660-678. 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 25. Claessen MM, Vleggaar FP, Tytgat KM, et al. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009;50(2):158-164. 10.1016/j.jhep.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 26. Petrick JL, Yang B, Altekruse SF, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based study in SEER-Medicare. PLoS One. 2017;12(10):e0186643. 10.1371/journal.pone.0186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wongjarupong N, Assavapongpaiboon B, Susantitaphong P, et al. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: a systematic review and meta-analysis. BMC Gastroenterol. 2017;17(1):149. 10.1186/s12876-017-0696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corrao S, Natoli G, Argano C.. Nonalcoholic fatty liver disease is associated with intrahepatic cholangiocarcinoma and not with extrahepatic form: definitive evidence from meta-analysis and trial sequential analysis. Eur J Gastroenterol Hepatol. 2021;33(1):62-68. 10.1097/MEG.0000000000001684. [DOI] [PubMed] [Google Scholar]
- 29. Younossi ZM, Stepanova M, Younossi Y, et al. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut 2020;69(3):564-568. 10.1136/gutjnl-2019-318813. [DOI] [PubMed] [Google Scholar]
- 30. Li H, Hu B, Zhou ZQ, et al. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: evidence from a systematic review and meta-analysis of 16 case-control studies. World J Surg Oncol. 2015;13(1):161. 10.1186/s12957-015-0583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Armstrong GL, Alter MJ, McQuillan GM, et al. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology 2000;31(3):777-782. 10.1002/hep.510310332. [DOI] [PubMed] [Google Scholar]
- 32. Hainsworth JD, Rubin MS, Spigel DR, et al. Molecular gene expression profiling to predict the tissue of origin and direct site-specific therapy in patients with carcinoma of unknown primary site: a prospective trial of the Sarah Cannon research institute. J Clin Oncol. 2013;31(2):217-223. 10.1200/JCO.2012.43.3755. [DOI] [PubMed] [Google Scholar]
- 33. Ferrone CR, Ting DT, Shahid M, et al. The ability to diagnose intrahepatic cholangiocarcinoma definitively using novel branched DNA-enhanced albumin RNA in situ hybridization technology. Ann Surg Oncol. 2016;23(1):290-296. 10.1245/s10434-014-4247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bridgewater JA, Goodman KA, Kalyan A, et al. Biliary tract cancer: epidemiology, radiotherapy, and molecular profiling. Am Soc Clin Oncol Educ Book 2016;36:e194-e203. 10.1200/EDBK_160831. [DOI] [PubMed] [Google Scholar]
- 35. Kamsa-ard S, Kamsa-ard S, Luvira V, et al. Risk factors for cholangiocarcinoma in Thailand: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2018;19(3):605-614. 10.22034/APJCP.2018.19.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shin HR, Oh JK, Lim MK, et al. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci. 2010;25(7):1011-1016. 10.3346/jkms.2010.25.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang H, Zhu B, Zhang H, et al. HBV infection status and the risk of cholangiocarcinoma in Asia: a meta-analysis. Biomed Res Int. 2016;2016:3417976. 10.1155/2016/3417976. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Incyte Corporation (Wilmington, DE) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase I studies) for which the product and indication have been approved on or after January 1, 2020 in at least one major market (eg, US, European Union, Japan). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960