Abstract
Pharmacologic inhibitors of cyclin-dependent kinases 4 and 6 (CDK4 and 6) are approved for the treatment of subsets of patients with hormone receptor positive (HR+) breast cancer (BC). In metastatic disease, strategies involving endocrine therapy combined with CDK4 and 6 inhibitors (CDK4 and 6i) improve clinical outcomes in HR+ BCs. CDK4 and 6i prevent retinoblastoma tumor suppressor protein phosphorylation, thereby blocking the transcription of E2F target genes, which in turn inhibits both mitogen and estrogen-mediated cell proliferation. In this review, we summarize preclinical data pertaining to the use of CDK4 and 6i in BC, with a particular focus on several of the unique chemical, pharmacologic, and mechanistic properties of abemaciclib. As research efforts elucidate the novel mechanisms underlying abemaciclib activity, potential new applications are being identified. For example, preclinical studies have demonstrated abemaciclib can exert antitumor activity against multiple tumor types and can cross the blood-brain barrier. Abemaciclib has also demonstrated distinct activity as a monotherapeutic in the treatment of BC. Accordingly, we also discuss how a greater understanding of mechanisms related to CDK4 and 6 blockade highlight abemaciclib’s unique in-class properties, and could pave new avenues for enhancing its therapeutic efficacy.
Keywords: abemaciclib, antitumor, breast neoplasms, CDK4 and 6, preclinical
This review provides a summary of recent preclinical data outlining molecular mechanisms by which CDK4 and 6 inhibitors exert their effects in cancer, with a focus on the unique attributes of abemaciclib.
Implications for Practice.
Three cyclin-dependent kinase 4 and 6 inhibitors (CDK4 and 6i) are approved for the treatment of hormone receptor positive (HR+) advanced or metastatic breast cancer. Readers will be presented with a summary of recent preclinical data outlining molecular mechanisms by which these agents exert their effects, with a focus on the unique attributes of abemaciclib. The data discussed provide a strong rationale for exploring further development of these agents in breast and other cancers.
Introduction
Background
Treatment options for advanced breast cancer (ABC) have improved in recent years, although it remains an incurable disease.1 The availability of targeted therapeutics has led to better patient outcomes without the negative effects imparted by chemotherapy. Understanding the molecular processes and aberrations that drive the initiation and growth of breast cancers (BC) has fostered the identification of new targets and the development of related treatments. Of note, therapeutic targeting of the cell cycle machinery has proven to be a highly effective strategy for hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2–) BC, consistent with its frequent dysregulation in these tumors. Recently, three small molecule inhibitors that inhibit cyclin-dependent kinases 4 and 6 (CDK4 and 6)—abemaciclib, palbociclib, and ribociclib—have been approved for the treatment of advanced HR+ BC. Based upon a variety of preclinical and translational insights, it has become increasingly clear that abemaciclib has a several distinct chemical, pharmacologic, and clinical properties. This article is designed to provide a comprehensive overview of the preclinical data pertinent to these agents with a specific focus on abemaciclib.
CDK4 and 6 and Cell Cycle Regulation
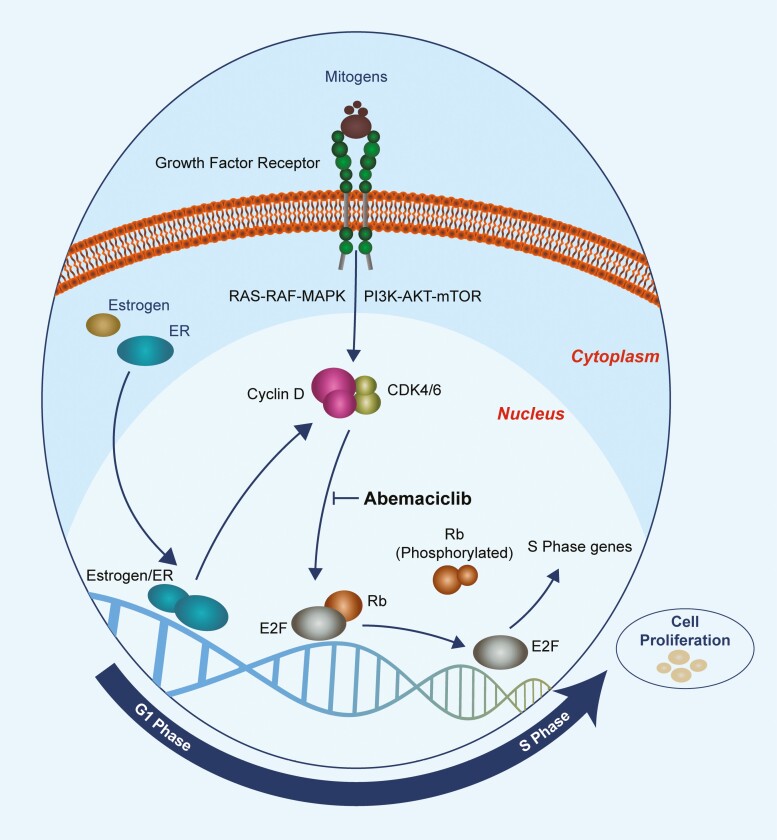
Cellular division requires a well-orchestrated transition through the phases of the cell cycle.2 A family of serine/threonine kinases known as cyclin-dependent kinases (CDKs) plays an important role in regulating cell cycle progression. The catalytic activity of CDKs is controlled by their association with cyclins, which allows CDKs to act upon substrates to trigger coordinated molecular events that drive cellular proliferation. Transient expression of cyclins allows for distinct roles of cyclin-CDK heterodimers during different phases of the cell cycle. CDK4 and CDK6 operate during the G1 to S phase transition,3-5 and their enzymatic activities are governed by the D-type cyclins (Cyclin D1, D2, and D3), which are expressed in response to extracellular signals including stimulatory mitogens, estrogen, cytokines, differentiation inducers, cell-cell contacts, and other spatial cues.6 Once active, the cyclin D-CDK4/6 holoenzyme phosphorylates a wide range of substrates,7 including the retinoblastoma (Rb) tumor suppressor protein.8,9 Rb binds to and represses the E2F group of transcription factors, which control the expression of an array of genes involved in DNA replication, G1 to S phase progression, and mitosis. As a result of phosphorylation of Rb by activated CDK4 and 6-D-cyclin complexes, E2F is released, facilitating the transcription of S phase genes (Figure 1).8,9 While Rb may be the most clinically relevant substrate of CDK4 and 6, Anders et al performed a comprehensive screening of CDK4 and CDK6 in vitro substrates in an effort to investigate the full spectrum of molecules phosphorylated by CDK4 and 6.10 The analysis revealed a considerable number of proteins which may have potential clinical relevance across many tumor types.
Figure 1.
Depiction of the CDK4 and 6 mechanism of action. Upstream signalling promotes the activation of the D-cyclin-CDK4 and 6 complex. This complex phosphorylates Rb, releasing E2F, resulting in the transcription of genes required for transition into S Phase. Abemaciclib inhibits the phosphorylation of Rb, inducing cell cycle arrest. Abbreviations: CDK4/6, cyclin-dependent kinase 4/6; ER, estrogen receptor; E2F, E2 factor; Rb, retinoblastoma; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B; mTOR, mechanistic target of rapamycin protein.
The Importance of the CDK4 and 6-Cyclin D-Rb pathway in BC
The CDK4/6-Rb pathway is a prominent mediator of proliferation induced by estrogen signaling, making it an attractive target in HR+ BC. Signaling from the estrogen receptor (ER) induces expression of the CCND1 gene, which encodes cyclin D1, thereby triggering CDK4 and 6-mediated phosphorylation of Rb and cell cycle progression.9 High levels of cyclin D and phosphorylated Rb have also been observed in endocrine therapy (ET)-resistant, ER+ BC, suggesting that abnormalities in the CDK4/6-cyclin D-Rb pathway may be involved in developing resistance to ET.11-13
CDK4 and 6 Inhibitor Development
Given the critical role CDKs play in cell cycle progression, they are highly attractive targets for therapeutic intervention. At present, 3 selective inhibitors of CDK4 and 6 (palbociclib, abemaciclib, and ribociclib) are approved by the US Food and Drug Administration (FDA) for use in HR+, HER2– ABC.9 Palbociclib (Ibrance, Pfizer) was the first of these agents to be approved. Abemaciclib (Verzenio, Eli Lilly and Company) and ribociclib (Kisqali, Novartis) were approved soon after.
Discovery and Clinical Development of CDK4 and 6 Inhibitors, with a Focus on Abemaciclib
Both palbociclib and ribociclib are fashioned on the pyrido [2,3-d]pyrimidin-7-one scaffold which confers selective inhibitory activity against CDK4 and 6. In contrast, abemaciclib is based upon a distinct 2-anilino-2,4-pyrimidine-[5-benzimidazole] scaffold.14 In a pivotal step, Gelbert et al15 identified the pyrimidine-benzimidazole molecular structure as a promising scaffold to develop a novel selective CDK4 and 6i. Structure-activity relationship studies evaluated properties such as potency, ligand efficiency, and selectivity to optimize the design of the CDK4 and 6i known as LY2835219 (now known as abemaciclib). During this development phase, several modifications were made which retained potency, while providing enhanced selectivity, specificity, and pharmacokinetic properties compared to the original pyrimidine-benzimidazole scaffold.5,15 Preclinical studies using in vitro and in vivo xenograft models demonstrated that abemaciclib inhibited phosphorylation of Rb and induced a G1 cell cycle arrest, resulting in antitumor activity in Rb-proficient malignancies.15-22 Abemaciclib was found to be effective at low doses and suitable for prolonged administration.15,16,20
In the following years, Phase 1 studies investigated the use of palbociclib, ribociclib, and abemaciclib in malignancies including BC.23-27 Dose-escalation and pharmacokinetic studies determined tolerable dosing and an optimal schedule.23,25-28 Palbociclib and ribociclib require intermittent dosing, primarily related to rates of cytopenia (primarily neutropenia).26,27 In contrast, it was established that the safety profile (and in particular lower rates of therapy-induced neutropenia) of abemaciclib permits a continuous dosing schedule. Acceptable toxicity profiles were observed in these early phase trials. Based on findings from the MONARCH 1 (NCT02102490) phase 2 single-arm study of abemaciclib monotherapy,29 the FDA designated abemaciclib as a breakthrough therapy for patients with refractory HR+ ABC or metastatic breast cancer (MBC). Of note, abemaciclib is the only CDK4 and 6i approved for use as a monotherapy, based on the results of this trial.
Pivotal Phase III studies demonstrated clinical efficacy for palbociclib, ribociclib, and abemaciclib in combination with an anti-estrogen in both the first-line and subsequent-line metastatic setting. These clinical results have been extensively reviewed elsewhere.9 MONARCH 2 (abemaciclib plus fulvestrant following progression on ET) and MONARCH 3 (abemaciclib plus aromatase inhibitor as initial therapy) demonstrated that abemaciclib administered with ET is an effective treatment for ABC. To date, palbociclib, abemaciclib, and ribociclib have been granted FDA approval in combination with fulvestrant for adult patients with HR+, HER2– ABC, or MBC with disease progression following ET,30 in combination with an aromatase inhibitor as initial endocrine-based therapy for postmenopausal women, and men, with HR+, HER2– ABC, or MBC.31 As noted above, abemaciclib has also been approved as monotherapy for patients with HR+, HER2– ABC, or MBC with disease progression following ET and prior chemotherapy in the metastatic setting,32 and was recently approved by the FDA as the only CDK4 and 6 inhibitor to be utilized in the adjuvant setting, with ET for patients with HR+, HER2–, node positive, early BC at high risk of recurrence and a Ki67 index ≥20%.33 The clinical application of CDK4 and 6i has also been investigated in solid tumors other than breast, and many of these trials are still ongoing.34,35
Selectivity
Palbociclib, ribociclib, and abemaciclib are all potent inhibitors of CDK4 and 6. However, there are notable differences in their selectivity profiles. Preclinical data have demonstrated that all three agents have high selectivity, albeit with different relative potencies, for both CDK4 and CDK6. While palbociclib displays similar potency against CDK4 and CDK6 with respective binding affinities (KiATP) of 0.26 ± 0.03 nM and 0.26 ± 0.07 nM, and respective half maximal inhibitory concentration (IC50) values of 9 and 15 nmol/L, both ribociclib (KiATP 0.53 ± 0.08 nM and 2.3 ± 0.3 nM, respectively; IC50 = 10 and 39 nmol/L, respectively) and abemaciclib (KiATP 0.6 ± 0.3 nM vs 8.2 ± 1.1 nM, respectively; IC50 = 2 and 10 nmol/L, respectively) are noted to have greater potency against CDK4 than CDK6.14, 36 Biochemical assays show abemaciclib to have 14-fold greater selectivity for CDK4/cyclin D1 compared to CDK6/cyclin D3 .21 Of note, the increased CDK4:CDK6 inhibitory ratio noted for ribociclib and abemaciclib may be an advantage when treating cancers that are primarily CDK4-dependent. The extent to which these targets influence the efficacy and toxicity profile of abemaciclib is a key question for the field, and one which remains largely unanswered, though some speculate that the CDK4:CDK6 inhibitory ratio or other potential targets may contribute to differences in toxicity profiles of CDK4 and 6 inhibitors.37,38
In addition to these primary targets, in vitro kinase screens have identified other potential targets of abemaciclib at higher concentrations. The extent to which these targets influence abemaciclib’s efficacy and toxicity profile is a key question for the field, and one which remains unanswered. Specifically, these targets include CDK9, PIM kinases and GSK3β.15 Subsequent investigation revealed that while CDK9 appeared to be inhibited in enzymatic assays (IC50 57 nM), this did not translate to target inhibition in cell lines or xenograft models, as CDK9 targets (RNA polymerase II C-terminal domain and MCL1) were not affected by abemaciclib treatment.21 Both palbociclib (KiATP = 150 ± 10 nmol/L) and ribociclib (KiATP = 190 ± 20 nmol/L) have measurable affinity against CDK9 though only abemaciclib achieves potent affinity (KiATP = 4.1 ± 1.3 nmol/L) according to biochemical interaction analyses.36
Interestingly, abemaciclib can inhibit the phosphorylation of targets downstream of PIM kinases (including p70S6K, S6, 4EBP1, and BAD) in cell lines.15,39 Another report suggested GSK3β inhibition by abemaciclib resulted in activation of β-catenin-dependent WNT signaling, although these effects were most evident at doses higher than those observed clinically.40 Another study reported inhibition of other kinases such CDK2, at abemaciclib concentrations 0.3-1 µM and above, which associated with cell cycle arrest in Rb-deficient or palbociclib-resistant cell lines.41 These effects are likely occurring at concentrations higher than can be achieved in tumors clinically, based on the observed fraction unbound.42 Of note, it is not yet understood if some of these observations are mediated by additional roles of CDK4 and 6 (part of the CDK4 and 6 mechanism of action), targeting other downstream known or unknown targets. It has also yet to be demonstrated if these targets are meaningfully impacted in vivo. Kinome selectivity analysis of palbociclib has revealed inhibition of MPSK1/STK16, kinases involved in autophagy, such as casein kinase 2 and PIK3R4, and other lipid kinases including PIK3CD and PIP4K2A/B/C.36,43
Markers of Sensitivity
Initially, Finn et al conducted an in vitro study to identify biomarkers of sensitivity to palbociclib and demonstrated HR+ luminal and HER2–amplified BC lines responded to treatment with the CDK4 and 6i, while most basal subtypes were resistant.44 Analysis of differentially expressed genes indicated that elevated expression of RB1 and CCND1, and decreased CDKN2A (p16) expression were associated with drug sensitivity. Interestingly, similar expression patterns of RB1 and p16 reportedly predicted the antiproliferative effect of ribociclib in vitro.45 However, despite the association between these genes and CDK4 and 6i sensitivity in vitro, these alterations are not necessarily an indication of sensitivity in patients, as indicated in the PALOMA-1 and 2 clinical trials of palbociclib plus letrozole as initial therapy for ABC.46-48
To identify other potential CDK4 and 6i-sensitive tumor types, Gong et al49 conducted a comprehensive study of 560 cell lines treated with abemaciclib. Highly sensitive tumor types were found to have “D-cyclin activating features”, including CCND1 t(11; 14) translocation, CCND2 or CCND3 amplification, CCND1-3 3’UTR loss, K-cyclin, or FBXO31 loss. While the work of Finn et al44 pointed to a potential correlation between decreased p16 and sensitivity to CDK4 and 6 inhibition with palbociclib in BC cells, Gong et al49 showed that sensitivity to CDK4 and 6 inhibition via abemaciclib was only transient in certain cell lines of other tumor types with CDKN2A loss, as CDK2 likely compensated for CDK4 and 6 inhibition. Interestingly, while the overall IC50 profile across a panel of cell lines treated with abemaciclib and palbociclib was closely related, abemaciclib displayed increased potency.
O’Brien et al50 conducted exploratory genomic and proteomic analyses of 44 BC cell lines to identify molecular profiles that correspond with sensitivity to abemaciclib. Drug sensitivity was observed in both ER+ and HER2+ cell lines, and of the HER2+ cell lines analyzed, those that were Rb-proficient with elevated levels of ER demonstrated greater response. Consistent with studies of palbociclib described above, these findings suggested HR+, HER2+ BC may be sensitive to CDK4 and 6 inhibition. Elevated levels of androgen receptor (AR) and PIK3CA– activating mutations were also associated with sensitivity, although these markers were detected in cell lines of the luminal BC subtype and therefore may not be independent indicators of response.50 Though, Torres-Guzmán et al51 did also observe antiproliferative activity across a panel of AR-positive prostate cancer cell lines, however while this finding is intriguing, the study was not powered to support the utility of AR as a molecular determinant of response to abemaciclib. Potential biomarkers of sensitivity were also identified in BC xenograft models.50 Response to abemaciclib was characterized by down regulation of 5 genes (RRM2, TOPO2A, MKI67, MCM7, and CDK2) in a dose-dependent manner. These 5 genes are regulated by E2F, indicating on-target inhibition with abemaciclib consistent with other agents in-class. In addition to ER+ and HER2+ cell lines, a subset of triple negative BC cell lines also displayed sensitivity to abemaciclib, particularly those with relatively high levels of Rb (and phosphorylated Rb) and low levels of p16. Combination treatment in sensitive cells with abemaciclib and cytotoxic chemotherapy also inhibited cell proliferation and did not antagonize the apoptotic effect of the cytotoxic chemotherapy.50
Tumor sensitivity to abemaciclib has also been observed in human xenograft models of non-small cell lung cancer (NSCLC) and glioblastoma.23 Although the subset of NSCLCs that benefit the most is not yet known, research is ongoing to identify biomarkers of interest. In a clinical study by Patnaik et al,23KRAS-mutant NSCLCs demonstrated greater degrees of tumor regression in response to abemaciclib when compared to KRAS-wild-type cancers. However, a subsequent trial to confirm improved outcomes did not meet its primary endpoint52: in JUNIPER, a Phase 3, randomized, open-label trial of abemaciclib versus erlotinib in patients with KRAS-mutant advanced NSCLC with progression after prior treatment, abemaciclib did not significantly prolong overall survival (OS) compared with erlotinib (7.4 vs 7.8 months, respectively; hazard ratio [HR], 0.968; 95% confidence interval [CI], 0.768-1.219; P = .77). Notably, analyses of progression-free survival (PFS) and objective response rate (ORR) suggested antitumor activity (median PFS: 3.6 vs 1.9 months; HR, 0.583; 95% CI, 0.470-0.723; P < .000001; ORR: 8.9% vs 2.7%; P = .01; abemaciclib vs erlotinib, respectively).52 Subsequently, an in vitro study by Wu et al found that reduced levels of CDK6 were associated with sensitivity to CDK4 and 6i in NSCLC cell lines.53 A retrospective analysis of tumor RNA expression data from the JUNIPER trial found reduced levels of CDK6 correlated with longer PFS and OS in patients with KRAS-mutant NSCLC.53 Though positive results have not been observed in clinical trials of CDK4 and 6i in patients with NSCLC, combined treatment with biologically synergistic therapies has demonstrated benefit in preclinical models. Combinations with inhibitors of molecules involved in growth factor signaling including EGFR, mTOR, MEK and PI3Ka, have been shown to enhance anticancer effects.54 Moreover, palbociclib has been shown to be an effective combination partner with gefitinib in preclinical studies of lung cancer,55 and results from an in vitro study of palbociclib and osimertinib suggested the combination may overcome acquired resistance to osimertinib.56 A combinatorial benefit was also observed in NSCLC cells treated with ribociclib and nazartinib (not FDA approved).57 Given the preclinical data, it is possible that the development of predictive biomarkers to CDK4 and 6i and biologically synergistic combination therapies may yield greater response rates to these agents in the clinic in new tumor types beyond BC.
It is possible that the discovery and utilization of biomarkers of sensitivity to CDK4 and 6i could allow specific treatment of these tumors and may yield greater response rates to these agents in the clinic. Palbociclib and ribociclib have also been shown to be effective combination partners with tyrosine kinase inhibitors in preclinical models of lung cancer.
Despite the preliminary data outlined above, in BC and other tumor types, specific molecular biomarkers that predict unilateral resistance to a specific CDK inhibitor (eg, palbociclib) while retaining sensitivity to others (eg, ribociclib or abemaciclib), have not been identified. An overview of emerging molecular resistance mediators is included below.
Continuous Inhibition, Senescence, Apoptosis, and ET Combinations
Through measurement of mitotic markers, research efforts have demonstrated that CDK4 and 6i decrease mitotic rate and reduce the proportion of cells in S phase in sensitive cancer cells.15,21,58-60In vitro data for abemaciclib has shown that very short periods of drug exposure (eg, 2 hours) can reduce the percentage of cells in S phase measured 72 hours later, although this may be due to ongoing intracellular retention of drug products rather than a long-lasting drug-independent state.21,61 Cell cycle analysis revealed comparable intracellular effects with palbociclib and ribociclib, though to our knowledge there are no published data pertaining to cell cycle arrest with shorter exposure times.58,59
Consistent with the role of Rb as a mediator of the senescence program, several groups have reported the development of a senescence-like state in cancer cells after CDK4 and 6i treatment, characterized by cellular enlargement, flattening of cells, and increased beta-galactosidase activity.21,60 Of note, abemaciclib has also been shown to induce epigenetic modifications associated with classical senescence. Torres-Guzman et al21 demonstrated that abemaciclib increased total cellular levels of H3K9me3, a repressive chromatin marker associated with in vitro senescence.21,62 Interestingly, this senescence-like state can also be induced by active metabolites of abemaciclib, as reported by Burke et al.63 Also, palbociclib and ribociclib have been found to promote a cytostatic response by inducing cellular senescence after prolonged exposure in a preclinical setting.59,64-66
Whether or not CDK4 and 6i directly induce apoptosis in luminal BC cells at physiologically relevant concentrations remains debated. While only minor increases in apoptosis have been detected after prolonged treatment times with palbociclib and ribociclib, Torres-Guzman et al, reported a time and dose-dependent increase in apoptosis in BC cells treated with abemaciclib.21 Similarly, O’ Brien et al50 demonstrated abemaciclib induced cell death in an in vivo BC model. Using the human epithelial marker protein keratin-19 (CK-19) as an indirect marker to identify loss of human epithelial tumor cells, the authors observed cell death following treatment with abemaciclib combined with ET. Furthermore, cell cycle arrest and cytotoxicity have also been observed with abemaciclib, albeit at high concentrations, even in the absence of Rb; as such, the induction of apoptosis may be attributed to an alternative mechanism of action and/or drug target. Overlapping transcriptional signatures between abemaciclib and the pan-CDK inhibitor alvocidib in Rb-deficient cell lines suggest that this activity may be linked to inhibition of CDKs other than CDK4 and CDK6.41 On a molecular level, the mechanisms leading to apoptosis are not yet known, with some suggesting it might be due to changes in lysosomal integrity.67 Conversely, other studies have shown abemaciclib might reduce apoptosis, evident by reduced apoptotic indices (cleaved PARP and caspase 3), reduced sensitivity to staurosporine, and consistent with the notion that senescent cells are primed toward avoidance of apoptosis.60,62,68
As discussed earlier, the ER and CDK4 and 6 pathways interact synergistically to drive tumorigenesis, making dual inhibition an attractive treatment strategy to further supress E2F target transcription.9,50,69 Initially, efficacy of dual inhibition was demonstrated in several cell lines and xenograft models with numerous CDK4 and 6i.22,44 Palbociclib enhanced sensitivity to tamoxifen in BC cell lines, while the addition of ribociclib to letrozole or fulvestrant increased the inhibition of tumor growth compared to single agent treatment with ET in ER+ BC mouse models and ER+ cell lines.22,44 Consistent with results from other agents in-class, Torres et al found that combinatorial treatment with abemaciclib and antiestrogens is synergistic, leading to decreased phosphorylation of Rb and increased cell cycle arrest in selected HR+ BC cells.70 Additionally, this combination has been shown to induce an effective senescence response as observed by SA-β-Gal. This acquired senescence phenotype is dose and time dependent.50,70 In the study by O’Brien et al,50 abemaciclib in combination with fulvestrant induced a significant and durable arrest of the cell cycle which is maintained for several days after compound removal. However, the optimal and robust effects are observed with continuous exposure of ER+ BC cells to the combination treatment. Increased apoptosis and cell death have also been observed after prolonged treatment with the abemaciclib/fulvestrant combination in BC cell lines.70 The benefit of combining each approved CDK4 and 6i with ET has been demonstrated in MBC in a clinical setting via the MONARCH, PALOMA, and MONALEESA clinical trials.30,31,47,71-75 Exploration of the impact these agents might have in the adjuvant setting is also ongoing. The PALLAS and PENELOPE-B studies explored the utility of palbociclib in patients with early stage BC, and respective findings of these trials did not demonstrate a statistically meaningful impact or improved invasive disease free survival with the additional of palbociclib to adjuvant ET.76,77 Abemaciclib has demonstrated efficacy in combination with ET in node-positive, high-risk, early BC in the monarchE trial,33 and was recently approved by the FDA for use in this setting for patients with high-risk clinical features and an elevated Ki67 score. Additional work remains ongoing to explore the potential utility of ribociclib in this patient population.78
Central Nervous System Activity
Despite improvements in treating extracranial metastatic disease, brain malignancies remain a challenge due to the unique properties of the blood–brain barrier (BBB). By expressing an array of active efflux transporters such as ATP-binding cassette (ABC), P-glycoprotein (P-gp), and BC resistance protein (BCRP), the BBB prevents passive diffusion and actively eliminates drugs from the brain. Although the blood tumor barrier is characteristically leakier to drugs, it tends to increase drug concentration at the tumor core and not the leading margin,79,80 highlighting the need for anticancer drugs that can effectively cross the BBB to treat the whole brain.
To this end, it is critical to understand how the available CDK4 and 6i interact with the BBB, and what clinical impact they may have for patients with CNS metastatic disease. Raub et al79 conducted an exploratory preclinical investigation exploring CNS exposure levels and the antitumor activity of abemaciclib and palbociclib in cell line and xenograft models. In mice, the maximum brain concentration of abemaciclib was observed 2 hours after a single dose, signifying quick absorption and crossing of the BBB. For both agents, unbound drug concentrations in the brain reportedly reached exposure levels expected to produce potent enzyme inhibition in the rat and mice models.15,79 Moreover, abemaciclib significantly increased survival in a rat orthotopic model of glioblastoma and combination treatment with temozolomide had an additive effect, further increasing survival.79
Following the promising preclinical data, a phase I clinical trial demonstrated that abemaciclib can enter the CNS in meaningful concentrations.23 Consequently, a phase II, open-label, multi-cohort study was conducted to evaluate the intracranial objective response rate (iORR) in patients receiving abemaciclib for brain or leptomeningeal metastases secondary to HR+ MBC.81 The authors reported that abemaciclib crossed the BBB with an average ratio between unbound brain metastasis tissue and unbound plasma concentrations (Kp,uu) of 5.6. The unbound brain metastasis tissue concentrations of active analytes were 96- and 19-fold CDK4 and CDK6, respectively, above historic in vitro IC50 values.82 In addition, CSF concentrations were an average of 21- and 4.3-fold CDK4 and CDK6 IC50 values, respectively. Consistent with the preclinical findings, these results indicate that abemaciclib penetrates the BBB in humans and reaches unbound levels in the brain expected to achieve enzyme inhibition. Though limited antitumor activity was observed with only 5.2% of patients reaching iORR, 38% of patients with HR+, HER2– MBC showed some decrease in brain metastatic lesion size. Abemaciclib was also associated with an intracranial clinical benefit rate of 24% in heavily pretreated HR+, HER2– MBC patients with secondary brain metastases. The authors indicated that despite the limitations of small sample size and a heavily pre-treated, heterogeneous patient population, these findings warrant further investigation given the treatment challenges among patients with MBC and CNS involvement.81 For palbociclib, a basket trial gauged the intracranial efficacy of the agent in 15 heavily pre-treated patients with brain metastases and CDK pathway alterations. The study met its primary endpoint with a 53% intracranial benefit rate at 8 weeks, demonstrating that the agent penetrates the CNS and may benefit those with molecularly characterized brain metastases from a range of primary tumor types.83
Immune Modulatory Effects
Another area of growing interest is the reported ability of abemaciclib, and other CDK inhibitors, to induce immunomodulatory effects to augment cytotoxicity against cancer cells. Initially, it was thought that inhibition of proliferation with CDK4 and 6i may disrupt clonal expansion of tumor-specific T cells, causing intra-tumoral immune suppression. However, preclinical studies identified a link between CDKs, their inhibitors, and regulation of T-cell effector function, suggesting that CDK4 and 6 activity itself might play an immunosuppressive role in lymphocytes.84,85 More recently, preclinical studies have shown CDK4 and 6i can promote T-cell activation.86-88 The induced antitumor immunity appears to be driven through enhancement of antigen presentation in tumor cells as well as T-cell-intrinsic mechanisms.68,86 The immunomodulatory mechanisms of CDK4 and 6i, such as the tumor cell- and T-cell-intrinsic mechanisms modulated by CDK4 and 6i are illustrated in a review by Goel et al.89
Tumor Cell-Intrinsic Mechanisms
Abemaciclib affects antigen presentation in tumor cells through mechanisms that have been elucidated using in vitro and in vivo models.68,88,90 The abemaciclib-induced increase in tumor cell interferon (IFN)-signaling is due to an alteration in the DNA methylation profile. The DNA methyltransferase (DNMT) gene DNMT1 is an E2F target gene, and thus is modulated by CDK4 and 6. Abemaciclib-induced inhibition of DNMT reduces methylation of endogenous retroviral genes, triggering “viral mimicry” and a dsRNA response. This, in turn, increases expression of a catalogue of IFN-driven genes, including those in the antigen presentation and processing machinery such as the major histocompatibility complex (MHC) class I molecules.68,87,88 Interestingly, MHC class I expression has been shown to be inversely correlated with CCND1 amplification in BC, suggesting potential CDK4/6-mediated immunosuppression.68,87 Ultimately, enhanced antigen presentation increases the capacity of tumor cells to stimulate antigen-specific cytotoxic T-cells, promoting tumor cell clearance.68 Enhanced immunogenicity through increased expression of MHC class I was also observed in BC cell lines treated with palbociclib. Moreover, this may be due to modified epigenetic processes as there has been a reported correlation between palbociclib treatment and suppressed activity of E2F and DNMT1.91 The epigenetic changes induced by CDK4 and 6i-mediated suppression of the RB-E2F-DNMT1 pathway adds another layer of complexity to how these drugs exert their effects in tumor cells. Collectively, this mechanism is consistent with the enhanced, senescence-induced immune cell clearance of tumor cells associated with the secretory phenotype, which is also linked to activation of IFN-driven genes.92,93
In another study focused on human melanoma samples, CDK4 and 6i was found to sensitize malignant cells to T-cell mediated killing through the reversal of an immunotherapy-resistant cell state.90 The resistance program, mediated by CDK4 and 6, allows the malignant cells to evade immune cells and desensitizes them to immunotherapies. Exposure to abemaciclib augmented antitumor T-cell immunity and increased response to subsequent immunotherapy. Although these findings were observed in melanoma cells, the mechanistic insights expand the potential utility of CDK4 and 6i to a range of tumor types.
T-cell-Intrinsic Mechanisms
CDK4 and 6i-induced antitumor immunity is also driven by the activation of T-cell-intrinsic mechanisms. As demonstrated by Goel et al,68 the effects of CDK4 and 6i on T-cells are in part caused by alterations in cellular epigenetics. As observed in tumor cells, abemaciclib reduces the activity of DNMT1 in regulatory T-cells (Treg cells). DNMT1 inhibition in Treg cells impedes their proliferation through hypomethylation of the CDKN1A promoter resulting in expression of the p21 protein, a potent cell cycle inhibitor.94 Ultimately, this markedly suppresses proliferation in Treg cells, significantly decreasing the Treg:CD8+ T-cell ratio, altering the balance in favour of antitumor immunity.68 The antiproliferative effect on Treg cells appears to be selective compared to naïve CD4+ or CD8+ T-cells, causing cytotoxic T lymphocyte (CTL)-mediated clearance of tumor cells. Notably, this selective impact on Treg proliferation has also been observed in murine tumors treated a trilaciclib, a different CDK4/6 inhibitor.95
Interestingly, in abemaciclib-treated mice, CTLs displayed a significant reduction of inhibitory receptors and markers associated with T-cell exhaustion, such as PD-1, Tim-3, CTLA-4, and LAG3. The study demonstrated the significant role of CTLs in inhibitor-induced antitumor immunity as CTL depletion was shown to significantly attenuate abemaciclib-induced tumor regression.68 Further, levels of the main effector cytokine of CTLs (IFNγ) were 4 times higher in treated tumors. IFNγ modulates immune response and is often associated with increased tumor immunogenicity.87
An in vivo study by Deng et al86 showed an additional T-cell pathway activated by exposure to CDK4 and 6i. The enhanced T-cell response was caused by reduced phosphorylation of members of the NFAT transcription factor family, enhancing their nuclear retention and hence CTL effector function. Importantly, certain NFATs appear to be CDK6 substrates. Moreover, short-term treatment with palbociclib led to elevated secretion of IFNγ from CTLs in the presence of Treg cells. There is also some suggestion this effect is seen in human BC. Through interrogation of a dataset from the neoMONARCH neoadjuvant trial, Hurvitz et al96 indicated an upregulation of IFNγ signaling following combination treatment with abemaciclib plus anastrozole. Similar data have been obtained upon analysis of the NeoPalAna neoadjuvant trial using palbociclib.68 Recently published data suggest CDK4 and 6i might also promote differentiation of CD8+ T-cells towards a memory phenotype, potentially expanding their application as tools to bolster long-term antitumor immunity.97,98
Taken together, these studies imply immune activation may be part of the mechanism of action for abemaciclib and other CDK4 and 6i. In addition, the synergy demonstrated with cancer immunotherapies indicates complementary treatment with abemaciclib may potentiate the efficacy of immune checkpoint inhibitors.68,87,90 Several of these pro-immune phenomena have also been reported for other CDK4 and 6i including palbociclib, ribociclib, and trilaciclib, and it is likely that this is a class effect. To date, there has been little comparison between each of the drugs in this regard. In addition, certain mechanisms that have been outlined with other agents in the class may also be seen with abemaciclib, such as palbociclib-induced activation of the senescence-associated secretory phenotype and the resulting natural killer cell-mediated cytotoxicity in lung cancer models.99
Markers and Mechanisms of Resistance to CDK4 and 6 Inhibitors
As CDK4 and 6i have become the standard of care for treatment of ABC, the need to understand mechanisms of resistance has grown into an important area of active investigation. Potential mechanisms of resistance have been identified through interrogation of clinical samples as well as preclinical studies. Interestingly, to date, no clear mechanisms have been identified that provoke unilateral resistance to specific CDK4 and 6i agents.
A biologically plausible mechanism of resistance is disruption of RB1, as intact Rb is hypothesized to be required for dependence on CDK4 and 6 and, therefore, sensitivity to CDK4 and 6i. The acquisition of RB1 mutations on CDK4 and 6-directed therapy was first noted in a case series describing RB1 mutations in circulating tumor DNA from 3 patients after exposure to CDK4 and 6i (two of these patients received palbociclib, while one received ribociclib).100 Subsequent analysis from the PALOMA-3 study of palbociclib combined with fulvestrant revealed that, while observed, RB1 mutations were relatively rare (4.7%), and were exclusively enriched in the group receiving palbociclib compared to placebo.101 Targeted sequencing of a large cohort of tumors also revealed rare alterations in RB1 (n = 9/348) with a clearly detrimental impact on CDK4 and 6i PFS.102 A separate study, the first to utilize whole-exome sequencing, confirmed rare biallelic loss of RB1 (4/59 samples) exclusively in resistant biopsies103; the majority of these patients received palbociclib, though a small subset received ribociclib or abemaciclib. This study also demonstrated diverse genomic mechanisms of RB1 disruption and included the first example of convergent evolution toward RB1 biallelic disruption in a patient with multiple tumor biopsies. Overall, while genomic disruption of RB1 is rarely associated with resistance to CDK4 and 6 inhibition, it is likely that other mechanisms resulting in Rb loss-of-function may contribute.104
Other cell cycle regulators have also been implicated, including CDK6, which has been shown to be upregulated in cells resistant to CDK4 and 6 inhibition via abemaciclib.105 Knockdown of CDK6 has also been shown to restore CDK4 and 6i sensitivity in these cells. Interestingly, in a large targeted-sequencing cohort, loss-of-function mutations affecting the FAT1 tumor suppressor were linked to CDK4 and 6i resistance; additional exploration in cellular models suggested that FAT1 could regulate CDK6 expression via the Hippo signaling pathway.102 CDK6 expression may also be regulated by microRNAs; specifically, miR-432-5p has been implicated in CDK4 and 6i resistance (both in vitro and in patient samples), and has been linked to CDK6 modulation via TGFβ signaling.106 Despite these lines of evidence implicating CDK6 in vitro and in tumor specimens, direct genomic alterations in CDK6 have not been demonstrated to date in CDK4 and 6i-sensitive or resistant tumor specimens.
Compensatory activation of growth factor signaling pathways has also been observed in CDK4 and 6i-resistant tumors. An in vitro screen identified fibroblast growth factor receptor (FGFR) as a potential mediator of resistance to CDK4 and 6i therapy with ribociclib in combination with ET, which was confirmed through FGFR1 overexpression.107 A second study also implicated FGFR1/2 in CDK4 and 6i resistance through downstream MAPK pathway activation.108FGFR2 alterations, as well as AKT1, ERBB2, and RAS, were detected in metastatic tumor biopsies from patients with HR+, HER2– BC treated with CDK4 and 6i +/– ET, and were associated with intrinsic or acquired resistance.103 In addition to AKT1 alterations, PI3K/mTOR pathway activation (including upregulation of AKT phosphorylation and P70S6K and downregulation of PTEN) was identified in a BC cell line model with acquired palbociclib resistance.109
Preclinical research has also identified a potential correlation between elevated cyclin-E expression and resistance to CDK4 and 6i. Investigations using cell line models have indicated overexpression of cyclin E and consequential activation of CDK2 may impair response to treatment with CDK4 and 6i.110,111 Moreover, gene expression profiles from HR+ BC cell lines with conditioned resistance to palbociclib showed concurrent overexpression of cyclin E1 and downregulation of Rb were common molecular markers associated with resistance.112 Cyclin-E-driven therapy resistance is further supported by clinical evidence, as observed in a study analyzing patient tumor samples from the PALOMA-3 trial.113 Thus, combined inhibition of CDK2 plus CDK4 and 6 may be an effective treatment strategy for a subset of resistant tumors.
Conclusion
CDK4 and 6 inhibition has become an important therapeutic strategy for patients with HR+ BC. Notably, there are important pharmacodynamic and clinical differences within this class of compounds. Understanding these distinctions may have important implications for patient care. Although anticancer activity in Rb-proficient tumors is demonstrable, all of the cellular processes affected by abemaciclib, and the other CDK4 and 6i, have yet to be elucidated. The preclinical and clinical studies discussed in this review reveal the complexity and wide range of cellular processes that are influenced by these agents. As further knowledge of these molecular interactions is elaborated, there is a greater potential to establish novel biomarkers suggesting sensitivity, identify key mechanisms of resistance, and improve treatment strategies across a range of tumor types.
Acknowledgments
Funded by Eli Lilly and Company.
Contributor Information
Seth A Wander, Department of Medical Oncology, Massachusetts General Hospital Cancer Center, Harvard Medical School, Boston, MA, USA.
Neil O’Brien, Department of Medicine, Division of Hematology/Oncology, Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Lacey M Litchfield, Eli Lilly and Company, Indianapolis, IN, USA.
Declan O’Dea, Eli Lilly and Company, Cork, Ireland.
Claudia Morato Guimaraes, Eli Lilly and Company, Indianapolis, IN, USA.
Dennis J Slamon, Department of Medicine, Division of Hematology/Oncology, Geffen School of Medicine at UCLA, Los Angeles, CA, USA.
Shom Goel, Department of Cancer Medicine, Peter MacCallum Cancer Centre, Melbourne, VIC, Australia; The Sir Peter MacCallum Department of Oncology, University of Melbourne, VIC, Australia.
Conflict of interest
Lacey M. Litchfield, Claudia Morato Guimaraes, and Declan O’Dea: Eli Lilly and Company (E, OI); Seth Wander: Genentech (RF), Foundation Medicine, Veracyte, and Eli Lilly and Company (C/A); Shom Goel: Eli Lilly and Company and G1 Therapeutics (RF), Pfizer, Eli Lilly and Company, and G1 Therapeutics (C/A), Eli Lilly and Company (Other—travel, accommodations or expenses), Eli Lilly and Company and Novartis (H), Eli Lilly and Company, Novartis, Pfizer, and G1 Therapeutics (SAB). Dennis J. Slamon: Novartis and Pfizer (RF), Eli Lilly and Company and Novartis (C/A), Novartis (H), BioMarin, Novartis, and Pfizer (Other—travel expenses), BioMarin (Other—leadership), BioMarin, Pfizer, Merck, and Amgen (OI); Neil O’Brien: Novartis and Bristol Myers Squibb (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Author Contributions
Conception/design: S.A.W., L.M.L., C.M.G, S.G. Provision of study material/patients: N.OB., L.M.L., D.J.S. Collection and/or assembly of data: S.A.W., N.OB., L.M.L., D.OD., C.M.G., S.G. Data analysis and interpretation: S.A.W., N.OB., L.M.L., C.M.G., D.J.S., S.G. Manuscript writing: S.A.W., N.OB., L.M.L., D.OD., C.M.G., D.J.S., S.G. Final approval of the manuscript: All authors.
Data Availability
No new data were created in the development of this review article, thus data sharing is not applicable.
References
- 1. Waks AG, Winer EP.. Breast cancer treatment: a review. JAMA 2019;321(3):288-300. [DOI] [PubMed] [Google Scholar]
- 2. Harper JV, Brooks G.. The mammalian cell cycle: an overview. Methods Mol Biol (Clifton, NJ). 2005;296:113-153. [DOI] [PubMed] [Google Scholar]
- 3. Ingham M, Schwartz GK.. Cell-cycle therapeutics come of age. J. Clin. Oncol. 2017;35(25):2949-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matson JP, Cook JG.. Cell cycle proliferation decisions: the impact of single cell analyses. FEBS J. 2017;284(3):362-375. 10.1111/febs.13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sánchez-Martínez C, Gelbert LM, Lallena MJ, de Dios A.. Cyclin dependent kinase (CDK) inhibitors as anticancer drugs. Bioorg. Med. Chem. Lett. 2015;25(17):3420-3435. 10.1016/j.bmcl.2015.05.100. [DOI] [PubMed] [Google Scholar]
- 6. Sherr CJ, Beach D, Shapiro GI.. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov 2016;6(4):353-367. 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi YJ, Anders L.. Signaling through cyclin D-dependent kinases. Oncogene 2014;33(15):1890-1903. 10.1038/onc.2013.137. [DOI] [PubMed] [Google Scholar]
- 8. Macaluso M, Montanari M, Giordano A.. Rb family proteins as modulators of gene expression and new aspects regarding the interaction with chromatin remodeling enzymes. Oncogene 2006;25(38):5263-5267. 10.1038/sj.onc.1209680. [DOI] [PubMed] [Google Scholar]
- 9. Spring LM, Wander SA, Andre F, et al. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet 2020;395(10226):817-827. 10.1016/S0140-6736(20)30165-3. [DOI] [PubMed] [Google Scholar]
- 10. Anders L, Ke N, Hydbring P, et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell 2011;20(5):620-634. 10.1016/j.ccr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Musgrove EA, Sutherland RL.. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9(9):631-643. 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 12. Butt AJ, McNeil CM, Musgrove EA, Sutherland RL.. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr Relat Cancer. 2005;12(Suppl 1):S47-S59. 10.1677/erc.1.00993. [DOI] [PubMed] [Google Scholar]
- 13. Varma H, Skildum AJ, Conrad SE.. Functional ablation of pRb activates Cdk2 and causes antiestrogen resistance in human breast cancer cells. PLoS One. 2007;2(12):e1256e1256. 10.1371/journal.pone.0001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yuan K, Wang X, Dong H, et al. Selective inhibition of CDK4/6: A safe and effective strategy for developing anticancer drugs. Acta Pharm Sin B 2021;11(1):30-54. 10.1016/j.apsb.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gelbert LM, Cai S, Lin X, et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32(5):825-837. 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wright MD, Abraham MJ.. Preclinical discovery and development of abemaciclib used to treat breast cancer. Expert Opin Drug Discov. 2021;16(5):485-496. 10.1080/17460441.2021.1853097. [DOI] [PubMed] [Google Scholar]
- 17. McClendon AK, Dean JL, Rivadeneira DB, et al. CDK4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle 2012;11(14):2747-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dempsey JA, Chan EM, Burke TF, Beckmann RP.. Abstract LB-122: LY2835219, a selective inhibitor of CDK4 and CDK6, inhibits growth in preclinical models of human cancer. Cancer Res. 2013;73(8 Supplement):LB-122. 10.1158/1538-7445.am2013-lb-122. [DOI] [Google Scholar]
- 19. Sanchez-Martinez C, Gelbert LM, Shannon H, et al. Abstract B234: LY2835219, a potent oral inhibitor of the cyclin-dependent kinases 4 and 6 (CDK4/6) that crosses the blood-brain barrier and demonstrates in vivo activity against intracranial human brain tumor xenografts. Mol Cancer Ther. 2011;10(11 Supplement):B234-B234. 10.1158/1535-7163.targ-11-b234. [DOI] [Google Scholar]
- 20. Tate SC, Cai S, Ajamie RT, et al. Semi-mechanistic pharmacokinetic/pharmacodynamic modeling of the antitumor activity of LY2835219, a new cyclin-dependent kinase 4/6 inhibitor, in mice bearing human tumor xenografts. Clin. Cancer Res. 2014;20(14):3763-3774. [DOI] [PubMed] [Google Scholar]
- 21. Torres-Guzmán R, Calsina B, Hermoso A, et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 2017;8(41):69493-69507. 10.18632/oncotarget.17778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O’Brien NA, Tomaso ED, Ayala R, et al. Abstract 4756: in vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER+ breast cancer. Cancer Res. 2014;74(19 Supplement):4756. [Google Scholar]
- 23. Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov 2016;6(7):740-753. 10.1158/2159-8290.CD-16-0095. [DOI] [PubMed] [Google Scholar]
- 24. Beeram M, Tolaney SM, Beck JT, et al. LBA18 - A phase 1b study of abemaciclib, an inhibitor of CDK4 and CDK6, in combination with endocrine and HER2-targeted therapies for patients with metastatic breast cancer. Ann Oncol. 2016;27:vi556. 10.1093/annonc/mdw435.08. [DOI] [Google Scholar]
- 25. Schwartz GK, LoRusso PM, Dickson MA, et al. Phase I study of PD 0332991, a cyclin-dependent kinase inhibitor, administered in 3-week cycles (Schedule 2/1). Br J Cancer. 2011;104(12):1862-1868. 10.1038/bjc.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin. Cancer Res. 2012;18(2):568-576. [DOI] [PubMed] [Google Scholar]
- 27. Infante JR, Shapiro G, Witteveen P, et al. A phase I study of the single-agent CDK4/6 inhibitor LEE011 in pts with advanced solid tumors and lymphomas. J Clin Oncol. 2014;32(15_suppl):2528-2528. 10.1200/jco.2014.32.15_suppl.2528. [DOI] [Google Scholar]
- 28. Fujiwara Y, Tamura K, Kondo S, et al. Phase 1 study of abemaciclib, an inhibitor of CDK 4 and 6, as a single agent for Japanese patients with advanced cancer. Cancer Chemother Pharmacol. 2016;78(2):281-288. 10.1007/s00280-016-3085-8. [DOI] [PubMed] [Google Scholar]
- 29. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(-) metastatic breast cancer. Clin. Cancer Res. 2017;23(17):5218-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2− advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875-2884. 10.1200/jco.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 31. Goetz MP, Toi M, Campone M, et al. MONARCH 3: abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35(32):3638-3646. [DOI] [PubMed] [Google Scholar]
- 32. FDA. FDA approves abemaciclib for HR-positive, HER2-negative breast cancer 2017. Accessed March 24, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer
- 33. Johnston SRD, Harbeck N, Hegg R, et al. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2−, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol. 2020;38(34):3987-3998. doi: 10.1200/jco.20.02514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schettini F, De Santo I, Rea CG, et al. CDK 4/6 inhibitors as single agent in advanced solid tumors. Front Oncol. 2018;8:608. doi: 10.3389/fonc.2018.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Du Q, Guo X, Wang M, et al. The application and prospect of CDK4/6 inhibitors in malignant solid tumors. J Hematol Oncol 2020;13(1):41. doi: 10.1186/s13045-020-00880-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen P, Lee NV, Hu W, et al. Spectrum and degree of CDK drug interactions predicts clinical performance. Mol Cancer Ther. 2016;15(10):22732273.-227322281. doi: 10.1158/1535-7163.mct-16-0300. [DOI] [PubMed] [Google Scholar]
- 37. Marra A, Curigliano G.. Are all cyclin-dependent kinases 4/6 inhibitors created equal?. NPJ Breast Cancer. 2019;5:27. doi: 10.1038/s41523-019-0121-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. George MA, Qureshi S, Omene C, Toppmeyer DL, Ganesan S.. Clinical and pharmacologic differences of CDK4/6 inhibitors in breast cancer. Front Oncol. 2021;11: 693104. 10.3389/fonc.2021.693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Litchfield LM, Boehnke K, Brahmachary M, et al. Combined inhibition of PIM and CDK4/6 suppresses both mTOR signaling and Rb phosphorylation and potentiates PI3K inhibition in cancer cells. Oncotarget 2020;11(17):1478-1492. doi: 10.18632/oncotarget.27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cousins EM, Goldfarb D, Yan F, et al. Competitive kinase enrichment proteomics reveals that abemaciclib inhibits GSK3β and activates WNT signaling. Mol Cancer Res 2018;16(2):333-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hafner M, Mills CE, Subramanian K, et al. Multiomics profiling establishes the polypharmacology of FDA-approved CDK4/6 inhibitors and the potential for differential clinical activity. Cell Chem Biol 2019;26(8):1067-1080.e8. doi: 10.1016/j.chembiol.2019.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Posada MM, Morse BL, Turner PK, et al. Predicting clinical effects of CYP3A4 modulators on abemaciclib and active metabolites exposure using physiologically based pharmacokinetic modeling. J Clin Pharmacol. 2020;60(7):915-930. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sumi NJ, Kuenzi BM, Knezevic CE, Remsing Rix LL, Rix U.. Chemoproteomics reveals novel protein and lipid kinase targets of clinical CDK4/6 inhibitors in lung cancer. ACS Chem Biol. 2015;10(12):2680-2686. doi: 10.1021/acschembio.5b00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Finn RS, Dering J, Conklin D, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reiter FP, Denk G, Ziesch A, et al. Predictors of ribociclib-mediated antitumour effects in native and sorafenib-resistant human hepatocellular carcinoma cells. Cell Oncol. 2019;42(5):705-715. doi: 10.1007/s13402-019-00458-8. [DOI] [PubMed] [Google Scholar]
- 46. Jiang Y, Randolph S, English P, et al. 442O - Cell cycle biomarker analysis from the paloma-1/ trio 18 palbociclib plus letrozole phase II study in Er-positive/Her2-negative advanced breast cancer (Abc). Ann Oncol. 2014;25:iv146. doi: 10.1093/annonc/mdu331.2. [DOI] [Google Scholar]
- 47. Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25-35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 48. Finn RS, Liu Y, Zhu Z, et al. Biomarker analyses of response to cyclin-dependent kinase 4/6 inhibition and endocrine therapy in women with treatment-naïve metastatic breast cancer. Clin Cancer Res. 2020;26(1):110-121. doi: 10.1158/1078-0432.ccr-19-0751. [DOI] [PubMed] [Google Scholar]
- 49. Gong X, Litchfield LM, Webster Y, et al. Genomic aberrations that activate D-type cyclins are associated with enhanced sensitivity to the CDK4 and CDK6 inhibitor abemaciclib. Cancer cell 2017;32(6):761-776.e6. doi: 10.1016/j.ccell.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 50. O’Brien N, Conklin D, Beckmann R, et al. Preclinical activity of abemaciclib alone or in combination with antimitotic and targeted therapies in breast cancer. Mol Cancer Ther. 2018;17(5):897-907. doi: 10.1158/1535-7163.MCT-17-0290. [DOI] [PubMed] [Google Scholar]
- 51. Torres-Guzmán R, Baquero C, Ganado MP, et al. Abstract 4850: targeting prostate cancer with the CDK4 and CDK6 inhibitor abemaciclib. Cancer Res. 2020;80(16 Supplement):4850-4850. doi: 10.1158/1538-7445.am2020-4850. [DOI] [Google Scholar]
- 52. Goldman JW, Mazieres J, Barlesi F, et al. A randomized phase III study of abemaciclib versus erlotinib in patients with stage IV non-small cell lung cancer with a detectable KRAS mutation who failed prior platinum-based therapy: JUNIPER. Front Oncol. 2020;10:578756. doi: 10.3389/fonc.2020.578756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu X, Yang X, Xiong Y, et al. Distinct CDK6 complexes determine tumor cell response to CDK4/6 inhibitors and degraders. Nat Cancer 2021;2(4):429-443. doi: 10.1038/s43018-021-00174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang J, Xu D, Zhou Y, Zhu Z, Yang X.. Mechanisms and implications of CDK4/6 inhibitors for the Treatment of NSCLC. Front Oncol. 2021;11:676041. 10.3389/fonc.2021.676041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu M, Liu H, Chen J.. Mechanisms of the CDK4/6 inhibitor palbociclib (PD 0332991) and its future application in cancer treatment (Review). Oncol Rep. 2018;39(3):901-911. doi: 10.3892/or.2018.6221. [DOI] [PubMed] [Google Scholar]
- 56. Qin Q, Li X, Liang X, et al. CDK4/6 inhibitor palbociclib overcomes acquired resistance to third-generation EGFR inhibitor osimertinib in non-small cell lung cancer (NSCLC). Thorac Cancer 2020;11(9):2389-2397. doi: 10.1111/1759-7714.13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kim S, Tiedt R, Loo A, et al. The potent and selective cyclin-dependent kinases 4 and 6 inhibitor ribociclib (LEE011) is a versatile combination partner in preclinical cancer models. Oncotarget 2018;9(81):35226-35240. doi: 10.18632/oncotarget.26215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3(11):1427-1438. [PubMed] [Google Scholar]
- 59. Tao Y-F, Wang N-N, Xu L-X, et al. Molecular mechanism of G(1) arrest and cellular senescence induced by LEE011, a novel CDK4/CDK6 inhibitor, in leukemia cells. Cancer Cell Int 2017;17:35. doi: 10.1186/s12935-017-0405-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Goel S, Wang Q, Watt AC, et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer cell 2016;29(3):255-269. doi: 10.1016/j.ccell.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Llanos S, Megias D, Blanco-Aparicio C, et al. Lysosomal trapping of palbociclib and its functional implications. Oncogene 2019;38(20):3886-3902. doi: 10.1038/s41388-019-0695-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Watt AC, Cejas P, DeCristo MJ, et al. CDK4/6 inhibition reprograms the breast cancer enhancer landscape by stimulating AP-1 transcriptional activity. Nature Cancer 2021;2(1):34-48. doi: 10.1038/s43018-020-00135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Burke T, Torres R, McNulty A, et al. Abstract 2830: the major human metabolites of abemaciclib are inhibitors of CDK4 and CDK6. Cancer Res. 2016;76(14 Supplement):2830-2830. doi: 10.1158/1538-7445.am2016-2830. [DOI] [Google Scholar]
- 64. Maskey RS, Wang F, Lehman E, et al. Sustained mTORC1 activity during palbociclib-induced growth arrest triggers senescence in ER+ breast cancer cells. Cell Cycle 2021;20(1):65-80. doi: 10.1080/15384101.2020.1859195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jost T, Heinzerling L, Fietkau R, Hecht M, Distel LV.. Palbociclib induces senescence in melanoma and breast cancer cells and leads to additive growth arrest in combination with irradiation. Front Oncol. 2021;11:4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rader J, Russell MR, Hart LS, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res 2013;19(22):6173-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Knudsen ES, Hutcheson J, Vail P, Witkiewicz AK.. Biological specificity of CDK4/6 inhibitors: dose response relationship, in vivo signaling, and composite response signature. Oncotarget 2017;8(27):43678-43691. doi: 10.18632/oncotarget.18435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Goel S, DeCristo MJ, Watt AC, et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017;548(7668):471-475. doi: 10.1038/nature23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McNulty A, Burke T, Dempsey J, et al. Abstract A12: the CDK4/CDK6 inhibitor abemaciclib inhibits transcriptional targets which facilitate growth in ER+ breast cancer cells. Mol Cancer Res. 2016;14:A12-A. [Google Scholar]
- 70. Torres R, Calsina B, Hermoso A, et al. Abstract 2836: characterization of the mechanism of action for abemaciclib with antiestrogen combined therapy in human breast cancer cell lines. Cancer Res. 2016;76(14 Supplement):2836-2836. doi: 10.1158/1538-7445.am2016-2836.26896281 [DOI] [Google Scholar]
- 71. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925-1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 72. Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425-439. doi: 10.1016/S1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 73. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375(18):1738-1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 74. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J. Clin. Oncol 2018;36(24):2465-2472. [DOI] [PubMed] [Google Scholar]
- 75. Tripathy D, Im SA, Colleoni M, et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19(7):904-915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 76. Mayer EL, Dueck AC, Martin M, et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021;22(2):212-222. doi: 10.1016/S1470-2045(20)30642-2. [DOI] [PubMed] [Google Scholar]
- 77. Loibl S, Marmé F, Martin M, et al. Palbociclib for residual high-risk invasive HR-positive and HER2-negative early breast cancer—the penelope-B trial. J Clin Oncol. 2021;39(14):1518-1530. doi: 10.1200/jco.20.03639. [DOI] [PubMed] [Google Scholar]
- 78. Slamon DJ, Fasching PA, Patel R, et al. NATALEE: phase III study of ribociclib (RIBO) + endocrine therapy (ET) as adjuvant treatment in hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) early breast cancer (EBC). J Clin Oncol. 2019;37(15_suppl):TPS597-TPS597. doi: 10.1200/jco.2019.37.15_suppl.tps597. [DOI] [Google Scholar]
- 79. Raub TJ, Wishart GN, Kulanthaivel P, et al. Brain exposure of two selective dual CDK4 and CDK6 inhibitors and the antitumor activity of CDK4 and CDK6 inhibition in combination with temozolomide in an intracranial glioblastoma xenograft. Drug Metab. Dispos. 2015;43(9):1360-1371. doi: 10.1124/dmd.114.062745. [DOI] [PubMed] [Google Scholar]
- 80. Rick JW, Shahin M, Chandra A, et al. Systemic therapy for brain metastases. Crit Rev Oncol Hematol. 2019;142:44-50. doi: 10.1016/j.critrevonc.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tolaney SM, Sahebjam S, Le Rhun E, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin. Cancer Res. 2020;26(20):5310-5319. [DOI] [PubMed] [Google Scholar]
- 82. Tate SC, Sykes AK, Kulanthaivel P, et al. A population pharmacokinetic and pharmacodynamic analysis of abemaciclib in a phase I clinical trial in cancer patients. Clin Pharmacokinet. 2018;57(3):335-344. doi: 10.1007/s40262-017-0559-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Brastianos PK, Kim AE, Wang N, et al. Palbociclib demonstrates intracranial activity in progressive brain metastases harboring cyclin-dependent kinase pathway alterations. Nature Cancer 2021;2(5):498-502. doi: 10.1038/s43018-021-00198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lissy NA, Van Dyk LF, Becker-Hapak M, et al. TCR antigen-induced cell death occurs from a late G1 phase cell cycle check point. Immunity 1998;8(1):57-65. doi: 10.1016/s1074-7613(00)80458-6. [DOI] [PubMed] [Google Scholar]
- 85. Rowell EA, Wang L, Chunder N, Hancock WW, Wells AD.. Regulation of T cell differentiation and alloimmunity by the cyclin-dependent kinase inhibitor p18ink4c. PLoS One. 2014;9(3):e91587. doi: 10.1371/journal.pone.0091587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Deng J, Wang ES, Jenkins RW, et al. CDK4/6 inhibition augments antitumor immunity by enhancing T-cell activation. Cancer Discov 2018;8(2):216-233. doi: 10.1158/2159-8290.CD-17-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Schaer DA, Beckmann RP, Dempsey JA, et al. The CDK4/6 inhibitor abemaciclib induces a T cell inflamed tumor microenvironment and enhances the efficacy of PD-L1 checkpoint blockade. Cell Reports 2018;22(11):2978-2994. doi: 10.1016/j.celrep.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 88. Dowless M, Lowery CD, Shackleford T, et al. Abemaciclib is active in preclinical models of ewing sarcoma via multipronged regulation of cell cycle, DNA methylation, and interferon pathway signaling. Clin. Cancer Res. 2018;24(23):6028-6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Goel S, Bergholz JS, Zhao JJ.. Targeting CDK4 and CDK6 in cancer. Nat Rev Cancer. 2022;22(6):356-372. 10.1038/s41568-022-00456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Jerby-Arnon L, Shah P, Cuoco MS, et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 2018;175(4):984-997.e24. doi: 10.1016/j.cell.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Acevedo M, Vernier M, Mignacca L, et al. A CDK4/6-dependent epigenetic mechanism protects cancer cells from PML-induced senescence. Cancer Res. 2016;76(11):3252-3264. doi: 10.1158/0008-5472.CAN-15-2347. [DOI] [PubMed] [Google Scholar]
- 92. Coppé JP, Desprez PY, Krtolica A, Campisi J.. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99-118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prata LGPL, Ovsyannikova IG, Tchkonia T, Kirkland JL.. Senescent cell clearance by the immune system: emerging therapeutic opportunities. Semin Immunol. 2018;40:101275. doi: 10.1016/j.smim.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Obata Y, Furusawa Y, Endo TA, et al. The epigenetic regulator Uhrf1 facilitates the proliferation and maturation of colonic regulatory T cells. Nat Immunol. 2014;15(6):571-579. doi: 10.1038/ni.2886. [DOI] [PubMed] [Google Scholar]
- 95. Lai AY, Sorrentino JA, Dragnev KH, et al. CDK4/6 inhibition enhances antitumor efficacy of chemotherapy and immune checkpoint inhibitor combinations in preclinical models and enhances T-cell activation in patients with SCLC receiving chemotherapy. J ImmunoTher Cancer. 2020;8(2):e000847. doi: 10.1136/jitc-2020-000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hurvitz SA, Martin M, Press MF, et al. Potent cell-cycle inhibition and upregulation of immune response with abemaciclib and anastrozole in neoMONARCH, phase II neoadjuvant study in HR(+)/HER2(-) breast cancer. Clin. Cancer Res. 2020;26(3):566-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lelliott EJ, Kong IY, Zethoven M, et al. CDK4/6 inhibition promotes anti-tumor immunity through the induction of T cell memory. Cancer Discov. 2021;11(10):2582-2601. 10.1158/2159-8290.CD-20-1554. [DOI] [PubMed] [Google Scholar]
- 98. Heckler M, Ali LR, Clancy-Thompson E, et al. Inhibition of CDK4/6 promotes CD8 T-cell memory formation. Cancer Discov. 2021;11(10):2564-2581. 10.1158/2159-8290.CD-20-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ruscetti M, Leibold J, Bott MJ, et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science (New York, NY). 2018;362(6421):1416-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Condorelli R, Spring L, O’Shaughnessy J, et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 2018;29(3):640-645. [DOI] [PubMed] [Google Scholar]
- 101. O’Leary B, Cutts RJ, Liu Y, et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov 2018;8(11):1390-1403. doi: 10.1158/2159-8290.CD-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li Z, Razavi P, Li Q, et al. Loss of the FAT1 tumor suppressor promotes resistance to CDK4/6 inhibitors via the hippo pathway. Cancer Cell 2018;34(6):893-905.e8. doi: 10.1016/j.ccell.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wander SA, Cohen O, Gong X, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors in patients with hormone receptor-positive metastatic breast cancer. Cancer Discov 2020;10(8):1174-1193. doi: 10.1158/2159-8290.CD-19-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Malorni L, Piazza S, Ciani Y, et al. A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget 2016;7(42):68012-68022. doi: 10.18632/oncotarget.12010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Yang C, Li Z, Bhatt T, et al. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 2017;36(16):2255-2264. doi: 10.1038/onc.2016.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Cornell L, Wander SA, Visal T, Wagle N, Shapiro GI.. MicroRNA-mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Reports 2019;26(10):2667-2680.e7. doi: 10.1016/j.celrep.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Formisano L, Lu Y, Servetto A, et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat Commun. 2019;10(1):1373. doi: 10.1038/s41467-019-09068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mao P, Cohen O, Kowalski KJ, et al. Acquired FGFR and FGF Alterations Confer Resistance to Estrogen Receptor (ER) Targeted Therapy in ER(+) Metastatic Breast Cancer. Clin. Cancer Res. 2020;26(22):5974-5989. [DOI] [PubMed] [Google Scholar]
- 109. O’Brien NA, McDermott MSJ, Conklin D, et al. Targeting activated PI3K/mTOR signaling overcomes acquired resistance to CDK4/6-based therapies in preclinical models of hormone receptor-positive breast cancer. Breast Cancer Res. 2020;22(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Taylor-Harding B, Aspuria PJ, Agadjanian H, et al. Cyclin E1 and RTK/RAS signaling drive CDK inhibitor resistance via activation of E2F and ETS. Oncotarget 2015;6(2):696-714. doi: 10.18632/oncotarget.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Herrera-Abreu MT, Palafox M, Asghar U, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Res. 2016;76(8):2301-2313. doi: 10.1158/0008-5472.CAN-15-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Guarducci C, Bonechi M, Benelli M, et al. Cyclin E1 and Rb modulation as common events at time of resistance to palbociclib in hormone receptor-positive breast cancer. npj Breast Cancer. 2018;4(1):38. doi: 10.1038/s41523-018-0092-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Turner NC, Liu Y, Zhu Z, et al. Cyclin E1 expression and palbociclib efficacy in previously treated hormone receptor–positive metastatic breast cancer. J Clin Oncol. 2019;37(14):1169-1178. doi: 10.1200/jco.18.00925. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created in the development of this review article, thus data sharing is not applicable.