Abstract
Background
Drug manufacturers claim that the purpose of financial payments to physicians is to facilitate education about new drugs. This claim suggests 2 testable hypotheses: payments should not be associated with drug revenue and payments for each drug should decline over time as physicians become educated.
Materials and Methods
We used open payments data on industry payments. We included payments for cancer drugs without generic/biosimilar competitors and used federal data sources to measure Medicare spending (a proxy for overall drug revenue) and a number of prescribers. We used generalized estimating equations (GEE) to model the drug-level association between industry payments and Medicare spending. Separately, we used GEE to estimate the change in payments with respect to the duration of time since initial FDA approval.
Results
The sample included 89 drugs and 361 drug-year observations. The total value of industry payments for oncology drugs increased, from $53 333 854 in 2014 to $90 343 731 in 2018. There was no association between log-transformed mean, per-physician industry payments, and per-physician Medicare spending (estimate −0.001, 95%CI, −0.005 to 0.004). Payments for individual drugs decreased over time; estimated payments in the subsequent year for a drug with mean, per-physician payments of $1000 in the index year was: $681* for drugs 0-4 years since approval, $825 for 5-9 years, and $679* for ≥10 years (*P < .05).
Conclusions
Although industry-sponsored education may also serve marketing purposes, the absence of association between industry payments and Medicare spending and the decline in payments subsequent to approval are consistent with claims that industry payments function to facilitate physician education.
Keywords: conflict of interest, drug industry, neoplasms, education, medical
Industry payments to physicians are common and raise concerns about possible influence on physicians’ clinical decision-making. This article examines trends in industry payments related to cancer drugs.
Implications for Practice.
Industry payments to physicians are common and raise concerns due to the potential to influence physicians’ clinical decision-making. However, the industry claims that such payments, commonly occurring in association with events where informational presentations are given, facilitate the beneficial goal of physician education on new drugs. This study examined trends in industry payments related to cancer drugs. There was no association between industry payments and revenue for a particular drug, and payments declined after time passed from initial FDA approval. These observations are consistent with the hypothesis that industry payments function to facilitate physician education.
Introduction
Financial relationships between the pharmaceutical industry and US physicians are common. In 2019, the dollar value of direct monetary transfers and in-kind gifts from the industry to physicians was $2.4 billion.1 These payments have been the subject of concern and criticism regarding the influence of commercial interests on medicine, as research has consistently shown that receipt of industry money affects physicians’ prescribing.2 Industry payments to physicians—which are largely promotional in nature—tend to focus on less-innovative, lower-value drugs.3 As a result, physicians who receive payments prescribe more low-value drugs.4,5
Oncology is no exception. Approximately two-thirds of medical oncologists receive industry payments each year,6 and receive a substantially greater dollar value of industry money than most other specialties.7,8 This amount has increased, and more oncologists now receive greater dollar amounts from industry than in the recent past.8 Research suggests that concerns about the influence on care delivery may be warranted in oncology as well; for example, receipt of industry money is associated with increased prescribing of nilotinib over imatinib for the treatment of chronic myeloid leukemia,9 despite nilotinib’s greater financial cost and higher incidence of serious toxicities.10
Despite the substantial body of research that has evaluated the distribution of industry money across physicians, the distribution of industry money across different drugs remains largely unexamined. An understanding of which drugs industry chooses to promote most heavily through physician payments—and how these payments shift over time—would offer new insights into the changing landscape of oncology practice and the potential for these payments to affect care quality. The industry view on physician payments is that they serve the public interest by fulfilling an otherwise unmet need for physician education about new drugs.11-13 However, whether industry payments to physicians are distributed in a manner to facilitate education has not been evaluated.
We conducted this study to assess the distribution of industry payments across different cancer drugs. In addition, we sought to evaluate whether the distribution of payments for cancer drugs over time is consistent with the goal of physician education, by testing 2 separate hypotheses. First, if payments are for education, then they should be proportional to the number of physician prescribers; after accounting for the number of physician prescribers, payments should not be proportional to other drug characteristics such as the revenue generated. Second, because the educational need for a new drug would be greatest at the time of market entry, payments should decline over time rather than persist.
Methods
Our study sample included all drugs with a primary indication for malignant cancer and without generic or biosimilar competition through the 2014-2018 study period; we focused on drugs without generic or biosimilar competitors because pharmaceutical manufacturers largely stop promotional payments once competition occurs.14 We excluded drugs used for only pediatric cancers, those that were taken off the market during the study period, and those with missing data for Medicare spending or industry payments. We searched the FDA Abbreviated New Drug Application (ANDA) records and Database of Licensed Biological Products (Purple Book) to identify drugs with anticipated market entry of a generic or biosimilar competitor.
For each drug in the sample, we obtained the number of prescribing physicians, the Medicare spending in nominal dollars, and value of industry payments in nominal dollars for each calendar year from 2014 to 2018. For Part D drugs, we obtained physician counts and Medicare spending from the Part D Prescriber Public Use File. For Part B drugs, we obtained physician counts and Medicare spending from the Physician and Other Supplier Public Use File, calculating Medicare spending as the product of the number of services rendered and the average Medicare allowed amount. Open Payments was the source for industry payments for all drugs. We included all “General Payments” (the Open Payments for non-research-associated payments, encompassing types of payments such as meals, travel, consulting, and speaking fees) with individual physician recipients, excluding payments to teaching hospitals. Open Payments includes information on the drug associated with each payment (if any); we summed all eligible payments for each cancer drug with each calendar year. Mean, per-physician payments, and Medicare spending were calculated as the total payments or Medicare spending divided by the number of prescribing physicians within the corresponding calendar year (full dataset available, eSupplement 2).
Our first hypothesis was that if drug industry payments are solely for the purposes of physician education, then they should not be associated with drug revenue. Implicitly, if payments were positively associated with drug revenue (as reflected in health system spending) after accounting for the number of physician prescribers, this would be evidence of factors other than educational needs. We therefore assessed the association between mean, per-physician industry payments (dependent variable) and mean, per-physician Medicare spending (independent variable), including a binary adjustment variable for future generic competition within 3 years out of expectation that payment patterns may change as patent expiration nears.14 Using a 2-sided hypothesis test, a significant, positive association (as opposed to a null or inverse association) would allow us to reject the hypothesis that industry payments to physicians are driven solely by educational need. The unit of analysis was the drug-year pair; we modeled this association using generalized estimating equations (GEE) with repeated observations on the drug level.
Because payment data are highly skewed, the transformation of the independent variable was necessary. We applied 2 different transformations to assess whether model results were sensitive to the transformation method. First, we used a log transformation to maximize interpretability. However, a Box-Cox transformation suggested that a log transformation was not adequate for the data distribution. We therefore also modeled the data with the optimal transformation suggested by Box–Cox (lambda = 0.25) to investigate whether model results were sensitive to the limitations of the log transformation.
Our second hypothesis was that if drug industry payments are solely for physician education, then they should decline over time as the education need is met. Implicitly, if payments stay stable or increase over time, this would be evidence of factors other than educational needs. To analyze payment trends for individual drugs while accounting for the differing levels of baseline payments among drugs, we measured with-drug year-to-year relative changes. For all instances where industry payments for a drug were observed in both a year 0 and a subsequent year +1, the year-to-year relative change was observed as the mean, per-physician industry payments in (year +1)/(year 0), with 1.0 representing no change (eg, if a drug were approved in 2018, it would not have contributed observations in this analysis because, within the 2014-2018 study period, a subsequent year would not have been observed). For each such observation, we also considered the number of years post-approval the drug was during the index year 0. We used generalized estimating equations and to model the aggregate year-to-year change (dependent variable) associated with different time periods since approval (independent variable). We used estimate statements to test the null hypothesis of no change, eg, year-to-year change not being statistically different from 1. A change of <1 would suggest an aggregate decline in payments and be interpreted as consistent with the educational rationale for payments. A Box-Cox transformation was applied to the year-to-year change variable. We again controlled for generic competition within 3 years. For interpretability, we used the point estimate for the aggregate change to predict the mean, per-physician payment in year +1 for a hypothetical drug with $1000 of payments in the index year and no generic competition within 3 years.
Results
The sample included 89 unique drugs and 361 drug-year observations (Table 1). The most common drug class was targeted agents (56%), followed by cytotoxic agents (13%), monoclonal antibodies (10%), immunotherapies (8%), other drug classes (6%), antibody conjugates (2%), and hormonal agents (3%). The median number of unique physician prescribers within a calendar year was 1212 (interquartile range 364, 3358), and the median Medicare spending per physician was $49 650 (interquartile range $34 015, $74 042).
Table 1.
Characteristics of included drugs. Nominal USD.
| Characteristic | Number (percent) |
|---|---|
| Unique drugs | 89 |
| Drug-year observations | 361 |
| Year of approval | |
| 1997 | 2 (2%) |
| 1998 | 2 (2%) |
| 2000 | 1 (1%) |
| 2002 | 2 (2%) |
| 2003 | 2 (2%) |
| 2004 | 3 (3%) |
| 2005 | 3 (3%) |
| 2006 | 5 (6%) |
| 2007 | 3 (3%) |
| 2008 | 2 (2%) |
| 2009 | 2 (2%) |
| 2010 | 3 (3%) |
| 2011 | 7 (8%) |
| 2012 | 11 (12%) |
| 2013 | 6 (7%) |
| 2014 | 8 (9%) |
| 2015 | 13 (15%) |
| 2016 | 5 (6%) |
| 2017 | 9 (10%) |
| Medicare coverage | |
| Part D | 48 (54%) |
| Part B | 40 (45%) |
| Both | 1 (1%) |
| Class | |
| Targeted agent | 50 (56%) |
| Cytotoxic | 12 (13%) |
| Monoclonal antibody | 9 (10%) |
| Immunotherapy | 7 (8%) |
| Other | 5 (6%) |
| Antibody conjugate | 3 (3%) |
| Hormonal agent | 3 (3%) |
| Unique physicians prescribing (median, IQR) | 1212 (364, 3358) |
| Industry payments per unique physician, USD (median, IQR) | 416 (120, 1,030) |
| Medicare spending per unique physician, USD (median, IQR) | 49 650 (34 015, 74 042) |
| Drug spending per drug (millions USD) | |
| 2014 | |
| <1 | 2 (4%) |
| 1-10 | 10 (20%) |
| >10-100 | 20 (39%) |
| >100-1000 | 18 (35%) |
| >1,000 | 1 (2%) |
| 2015 | |
| <1 | 4 (6%) |
| 1-10 | 12 (18%) |
| >10-100 | 27 (42%) |
| >100-1000 | 21 (32%) |
| >1000 | 1 (2%) |
| 2016 | |
| <1 | 1 (1%) |
| 1-10 | 14 (19%) |
| >10-100 | 31 (43%) |
| >100-1000 | 25 (35%) |
| >1000 | 1 (1%) |
| 2017 | |
| <1 | 4 (5%) |
| 1-10 | 16 (19%) |
| >10-100 | 37 (44%) |
| >100-1000 | 25 (29%) |
| >1000 | 3 (4%) |
| 2018 | |
| <1 | 2 (2%) |
| 1-10 | 15 (17%) |
| >10-100 | 38 (43%) |
| >100-1000 | 29 (33%) |
| >1000 | 5 (6%) |
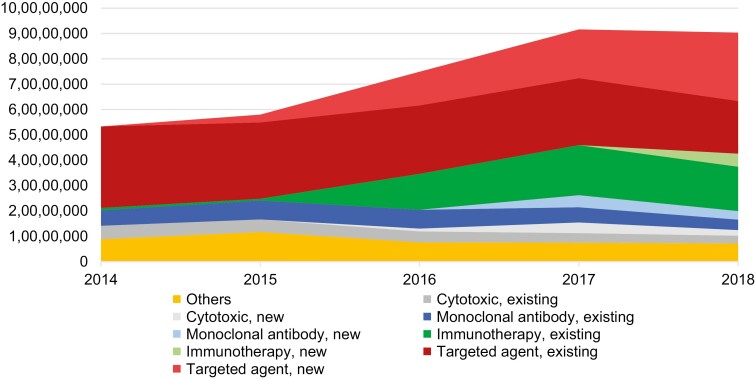
The total amount of industry payment for oncology drugs increased during the study period, from $53 333 854 in 2014 to $90 343 731 in 2018 (Fig. 1). Grouping payments according to drug class and whether the drug was approved in 2014 or before vs. 2015 or later, most of the increase in industry payments from 2014 to 2018 was accounted for by targeted agents approved in 2015 or later and immunotherapies approved in 2014 or before.
Figure 1.
Payments over time, by drug class. Total industry payments to US physicians for cancer drugs in each class are shown in nominal USD. Each class is divided into those agents approved in 2014 or before (“existing”), versus 2015 or later (“new”). “Others” includes hormonal agents, antibody conjugates, and all other classes. Nominal USD.
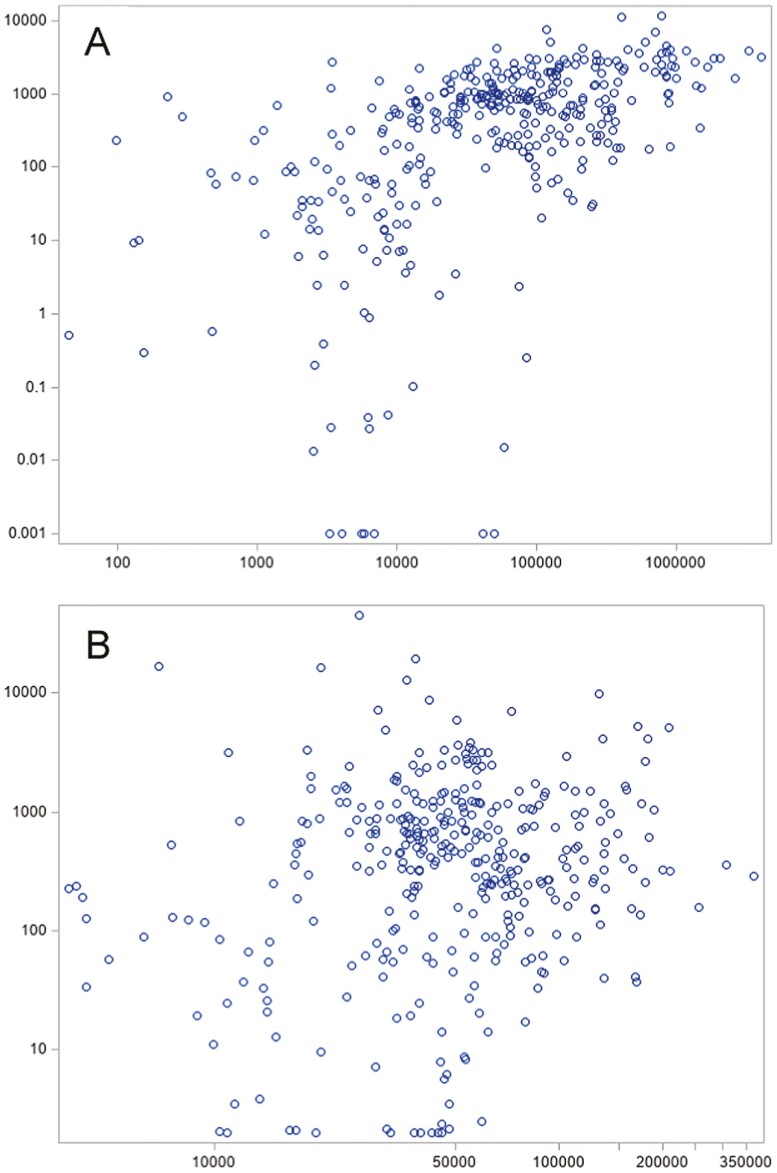
The drugs with the greatest Medicare spending per physician per year were disproportionately those with the greatest overall Medicare spending per year (Supplement Table S1). While the proportion of drugs exceeding $1 billion in Medicare spending within each year never exceeded 6% (Table 1), these drugs constituted 10 of the top 20 drugs with the greatest per-physician Medicare spending. The drugs with the greatest industry payments per physician per year were mostly drugs with a small denominator of prescribing physicians; 19 of the top 20 had a number of physician prescribers below the median of 1212. The only drugs among the top 20 by per-physician Medicare spending and industry payments were Xofigo (radium-223) and Keytruda (pembrolizumab). Overall Medicare spending was correlated with overall industry payments (Pearson correlation 0.39, P < .001); however, Medicare spending and industry payments per prescribing physician were not correlated (Pearson correlation −0.04, P = .47) (Fig. 2).
Figure 2.
Distribution of industry payments and Medicare spending for oncology drugs, 2014-2018. Medicare spending is shown on x-axis and general payments on the y-axis. Each observation represents a drug-calendar year pair; individual drugs are therefore represented multiple times across the 5-year study period. Industry payments and Medicare spending are both standardized to the number of prescribing physicians, representing the mean dollar value per prescribing physician within that calendar year. $2 were added to all y-axis values to allow for the inclusion of observations wherein the mean value of industry payments was <$1 (N = 15). Total Medicare spending vs. total industry payments (A, Pearson correlation 0.39, P < .001) and Medicare spending per prescribing physician vs. industry payments per prescribing physician (B, Pearson correlation −0.04, P = .47) are shown. All values are in nominal USD. A: Medicare spending (1000 USD) on the x-axis and industry payments (1000 USD) on the y-axis. B: Medicare spending per physician (USD) on the x axis and industry payments per physician (USD) on the y-axis.
There were no association between log-transformed mean, per-physician industry payments, and Medicare spending (estimate −0.001, 95%CI, −0.005 to 0.004) (Table 2). This result was unchanged after applying Box-Cox transformation (estimate −0.010, 95%CI, −0.030 to 0.011). The presence of a generic competitor within the subsequent 3 years was associated with lower industry payments (estimate −0.938, 95%CI, −1.384 to −0.492).
Table 2.
Association between industry payments and Medicare spending for cancer drugs, 2014-2018.
| Association | N (%) | Estimate (95% CI) | P value |
|---|---|---|---|
| Log-transformed industry payments | |||
| Medicare spending | n/a | −0.001 (−0.005 to 0.004) | .79 |
| Generic within 3 years | 35 (9.7) | −0.938 (−1.384 to −0.492) | <.0001 |
| Transformed (lambda = 0.25) industry payments | |||
| Medicare spending | n/a | −0.01 (−0.030 to 0.011) | .35 |
| Generic within 3 years | 35 (9.7) | −4.219 (−5.429 to −3.009) | <.0001 |
Individual observations were unique drug-calendar year pairs (N = 361, unique drugs = 89). Generalized estimating equations were used to estimate the outcome of mean industry payments per prescribing physician in that calendar year, with clustering on the level of the unique drug. Independent variables were Medicare spending (modeled as mean spending per prescribing physician in that calendar year, $thousands USD) and whether during the observed calendar year the drug was within 3 years of the market entrance of the first generic competitor. Two modeling approaches were applied: (1) OLS modeling log-transformed industry payments, estimating the log change in the dollar value industry payments associated with a $1000 increase in spending, and (2) OLS modeling transformed (lambda = 0.25) industry payments, estimating the change in transformed industry payments associated with a $1000 increase in spending.
Year-to-year change observations were grouped according to the number of years since approval in the index year: 0-4, 5-9, and ≥10. The majority of year-to-year changes in mean, per-physician payments were reductions: median 0.75 for 0-4 years since approval, 0.81 for 5-9, and 0.80 for ≥10 (Supplementary Table S2). However, the distribution of year-to-year changes was skewed, with a substantial number of observations much greater than 1, indicating a many-fold increase in industry payments (Supplementary Fig. S1).
The estimated year-to-year change among drugs with no generic competitor was downward in each of the 3 groups, and statistically significant for years 0-4 and ≥10 (0-4 years −0.366, P < .001; 5-9 years −0.188, P = .103; ≥10, −0.370, P = .005) (Table 3, Supplementary Fig. 2). Applying the modeled point estimates, a hypothetical drug with $1000 in mean, per-physician payments and which was 0-4 years since approval would fall to $681 the subsequent year; $825 for a drug 5-9 years since approval; and $679 for a drug ≥10 years since approval.
Table 3.
Year-to-year changes in mean industry payments per prescribing physician.
| Years since approval | N (%) | Mean year-to-year change | Median year-to-year change | Generic within 3 years, N (%) | Estimated year-to-year change in ratio, no generic | S | P-value | Estimated payments in year +1, if payments in index year = $1000 |
|---|---|---|---|---|---|---|---|---|
| 0-4 years | 105 (39) | 0.82 | 0.75 | 1 (1%) | −0.366 | 0.072 | <.0001 | $681 |
| 5-9 years | 84 (31) | 1.14 | 0.81 | 5 (6%) | −0.188 | 0.116 | .103 | $825 |
| ≥10 years | 78 (29) | 0.94 | 0.80 | 28 (36%) | −0.370 | 0.131 | .005 | $679 |
Each observation represents the relative change from the index year to the subsequent year, expressed as the ratio of mean, per-prescribing-physician industry payments in the subsequent year to the index year, with 1.0 representing no change. The observation set therefore reflects the subset of drug-calendar year pairs in which the drug was also observed in the subsequent year (N = 267, unique drugs = 85). Observations were grouped according to the number of complete calendar years since approval as of the index year, and generalized estimating equations were used to estimate the year-to-year change associated with each category of years since approval. P-values represent a test for whether year-to-year change was statistically significantly different than the null value of 1. The point estimate for the year-to-year change was used to estimate the dollar value of subsequent-year payments assuming $1000 per prescribing physician in the index year and no generic competition within 3 years.
Discussion
In this study, we aimed to better characterize the landscape of industry payments to physicians to promote cancer drugs and to determine whether the observed payment patterns support claims that these payments have solely educational goals. Our findings were largely consistent with the hypothesized trends if industry payments do have educational goals.
Several prior studies have observed a recent increase in the overall dollar value of industry payments to oncologists.8,15,16 If this increase were driven by payments for older, already-approved drugs, this would suggest that payments were driven by marketing goals rather than educational ones because physicians would have already had sufficient time to become educated. However, we found that the aggregate increase was explained by new drugs, potentially consistent with a new educational need among physicians (Fig. 1). Targeted therapies approved after 2014 and immunotherapies approved in 2014 or before—specifically, nivolumab and pembrolizumab, both approved in 2014—accounted for the increase.
Expectedly, the amount of industry payments per drug was correlated with overall Medicare spending (Fig. 2). We had anticipated that after controlling for the number of physician prescribers (which we did by using mean per-physician measures of industry payments and Medicare spending), industry payments and Medicare spending would still be positively correlated. This would have suggested that the industry engages in greater promotional spending on more highly-profitable drugs—those which have the highest per-physician revenue. However, we did not find evidence of this association. Industry payments did not appear to be driven by per-physician revenue, while a substantial portion of the variation in industry payments was explained by the number of prescribing physicians. This is consistent with the educational explanation for industry payments.
In aggregate, industry payments to physicians declined in the years following drug approval. This is consistent with an educational explanation for these payments; a clinician’s need for education regarding a drug is naturally the greatest soon after approval, and as this educational need is met then the industry decreases its educational efforts and associated payments decline as well. Importantly, while this observation is consistent with an educational explanation, it does not prove it. We hypothesized that if industry payments were for non-educational purposes (eg, solely for marketing and increasing utilization) then payments would remain stable over time because the ability to increase utilization through marketing efforts would persist. However, it is also possible that the effectiveness of marketing-associated payments is highest soon after approval; if this were the case then our observations could potentially be explained by marketing payments as well. It is also possible that the decline in payments following drug approval is physician-driven rather than industry-driven as we have assumed. If physicians are more willing to engage with industry representatives regarding newer drugs than older drugs, then this could explain our findings regardless of the nature of payments.
However, despite this aggregate trend, there were numerous cases of payment increases occurring for older drugs (Supplementary Table S1 and Fig. S1) suggesting that factors other than education do influence industry payments. For example, mean per-physician payments increased more than 5-fold for nilotinib (first approved in 2006) from 2014 to 2017 and more than 2-fold for cabazitaxel (first approved in 2010) from 2014 to 2017, despite the absence of any new indications during these time periods that might have contributed to a new educational need. In the case of nilotinib, this period corresponded to the market entry of generic imatinib, and may reflect the manufacturer’s stated strategy to shift physician prescribing from its older drug (imatinib) to its newer, on-patent drug (nilotinib).17,18 There were also many cases of industry payments remaining at high levels many years after drug approval, which would be inconsistent with an ongoing need to educate providers. For example, even more than a decade past their initial approval, industry payments for nab-paclitaxel (abraxane) and panitumumab (vectibix) remained above the per-physician median for individual drugs.
That industry payments may function primarily to facilitate clinician education regarding new drugs is partially, though not entirely, reassuring. Drug information provided by industry to clinicians often contains false or misleading information.19-21 Industry also focuses promotional efforts on lower-quality and less-innovative drugs,3,22 and receipt of industry drug information has consistently been associated with lower-quality prescribing.23-25 Even if industry payments serve to further clinician education, it is not clear that the education they receive is aligned with patient benefit.
This study has several limitations arising from the underlying data sources. The aggregate measures of industry payments and Medicare spending do not differentiate oncologic from non-oncologic indications, which several of the included drugs have. We used Medicare spending as a proxy for overall drug revenue, making the assumption that the 2 would be relatively proportional across drugs, but this may not be the case for drugs treating cancers that more commonly affect the adult non-elderly population. One of the reasons we analyzed mean per-physician payments was to account for increases in industry payments driven by new drug indications. Insofar as new drug indications increase the number of physicians who may utilize the drug for their patients and hence also become the target of industry payments, mean per-physician payments should account for that expanding denominator; however, it would not account for the possibility that new indications may also increase per-physician prescribing volume. Mean per-physician amounts also do not take into account the distributions of industry payments to individual physicians, hospitals, or geographic regions, which are quite heterogeneous, so we were unable to evaluate payment trends at these levels. It is possible that, in assessing aggregate national trends, we did not observe smaller localized trends that may have provided evidence counter to the educational explanation for payments (eg, increases in payments to individual physicians or cancer centers long after drug approval). Our analysis of the association between industry payments and drug revenue (Table 2) suggests an association between upcoming generic competition and lower industry payments. However, this association may be confounded by calendar time since approval, which is also associated with declining industry payments.
Conclusions
Direct-to-physician payments from the pharmaceutical industry related to oncology drugs are high and increasing. This increase is driven primarily by payments related to new drugs coming onto the market, rather than increasing payments for older drugs. In aggregate, payments were greatest immediately after drug approval and declined thereafter, and they were correlated with the number of prescribing physicians but not with per-physician drug revenue. These trends are consistent with the understanding that the primary goal of industry payments is to facilitate clinician education regarding new drugs.
Supplementary Material
Contributor Information
Aaron P Mitchell, Health Outcomes Research Group, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Akriti Mishra Meza, Health Outcomes Research Group, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Niti U Trivedi, Delfi Diagnostics, Baltimore, MD, USA.
Peter B Bach, Delfi Diagnostics, Baltimore, MD, USA.
Mithat Gönen, Biostatistics Service, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Funding
Funding received from NIH, NCI P30CA008748 and NCI R37CA264563, and the National Institute for Health Care Management Foundation, GC260700. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Conflict of Interest
Akriti Mishra Meza: Johnson & Johnson, DNA, Teladoc Health (OI); Niti U. Trivedi: Delfi Diagnostics (E); Peter B. Bach: EQRx (C/A), Delfi Diagnostics, Oncology Analytics (leadership role), Oncology Analytics (travel expenses), EQRx, Oncology Analytics, Delfi Diagnostics (OI), Kaiser Permanente, Arnold Ventures (RF); Mithat Gönen: Genentech (travel expenses). Aaron P. Mitchell indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board.
Author Contributions
Conception/design: A.P.M., P.B.B., M.G. Obtained funding: A.P.M. Data analysis and interpretation: A.P.M., N.U.T., A.M.M. Statistical analysis: A.P.M., A.M.M., M.G. Manuscript writing: A.P.M. Critical revision and final approval of the manuscript: All authors. Dr Mitchell had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data Availability
The data underlying this article are available in the article and in its Supplementary material.
References
- 1. The facts about open payments data: 2018 totals. CMS. Accessed March 28, 2020. https://openpaymentsdata.cms.gov/summary [Google Scholar]
- 2. Mitchell A, Trivedi N, Gennarelli RL, Chimonas SC, Tabatabai S, Goldberg J, Diaz L Jr., Korenstein D. Are financial payments from the pharmaceutical industry associated with physician prescribing? A systematic review. Ann Intern Med. 2021;174(3):353-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greenway T, Ross JS. US drug marketing: how does promotion correspond with health value? BMJ. 2017;357:j1855. 10.1136/bmj.j1855. [DOI] [PubMed] [Google Scholar]
- 4. Sharma M, Vadhariya A, Johnson ML, Marcum ZA, Holmes HM. Association between industry payments and prescribing costly medications: an observational study using open payments and medicare part D data. BMC Health Serv Res. 2018;18(1):236. 10.1186/s12913-018-3043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hartung DM, Johnston K, Cohen DM, et al. Industry payments to physician specialists who prescribe repository corticotropin. JAMA Netw Open. 2018;1(2):e180482. 10.1001/jamanetworkopen.2018.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marshall DC, Tarras ES, Rosenzweig K, Korenstein D, Chimonas S. Trends in financial relationships between industry and individual medical oncologists in the United States from 2014 to 2017: a cohort study. J Clin Oncol. 2019;37(15_suppl):6520-6520. 10.1200/JCO.2019.37.15_suppl.6520. [DOI] [Google Scholar]
- 7. Inoue K, Blumenthal DM, Elashoff D, Tsugawa Y. Association between physician characteristics and payments from industry in 2015-2017: observational study. BMJ Open. 2019;9(9):e031010. 10.1136/bmjopen-2019-031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell AP, Mishra A, Dey P, Curry MA, Trivedi NU, Haddadin M, Rahman MW, Winn AN, Dusetzina SB, Bach PB. Personal payments from pharmaceutical companies to authors of oncology clinical practice guidelines. Oncologist. Published online May 13, 2021;26(9):771-778. 10.1002/onco.13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mitchell AP, Winn AN, Dusetzina SB. Pharmaceutical industry payments and oncologists’ selection of targeted cancer therapies in medicare beneficiaries. JAMA Intern Med. 2018;178(6):854-856. 10.1001/jamainternmed.2018.0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cole AL, Wood WA, Muluneh B, Lund JL, Elston Lafata J, Dusetzina SB. Comparative safety and health care expenditures among patients with chronic myeloid leukemia initiating first-line imatinib, dasatinib, or nilotinib. JCO Oncol Pract. 2020;16(5):e443-e455. 10.1200/JOP.19.00301 [DOI] [PubMed] [Google Scholar]
- 11. Janssen US Transparency Report 2018. Published online May 2019. Accessed September 30, 2021. https://transparencyreport.janssen.com/_document/2018-janssen-us-transparency-report?id=00000178-7173-da47-a57c-7df731d30000
- 12. Gould M. End of the free lunch? Br Med J. 2008;337:487-488. [DOI] [PubMed] [Google Scholar]
- 13. Physician Engagement, AstraZeneca. Accessed September 30, 2021. https://www.astrazeneca-us.com/sustainability/Corporate-transparency/physician-engagement.html
- 14. Carey C, Lieber EMJ, Miller S. Drug Firms’ payments and physicians’ prescribing behavior in medicare part D. J Public Econ. 2021;197:104402. 10.3386/w26751. [DOI] [Google Scholar]
- 15. Tarras ES, Marshall DC, Rosenzweig K, Korenstein D, Chimonas S. Trends in industry payments to medical oncologists in the united states since the inception of the open payments program, 2014 to 2019. JAMA Oncol. 2021;7(3):440. 10.1001/jamaoncol.2020.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahman MW, Trivedi NU, Bach PB, Mitchell AP. Increasing financial payments from industry to medical oncologists in the United States, 2014-2017. J Natl Compr Cancer Netw. 2021:1-9. 10.6004/jnccn.2021.7024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Palmer E. Novartis wants Tasigna to cannibalize Gleevec to save its sales. Fierce Pharma. https://www.fiercepharma.com/financials/novartis-wants-tasigna-to-cannibalize-gleevec-to-save-its-sales. Published October 26, 2012. Accessed April 25, 2018. [Google Scholar]
- 18. von Schaper E. Novartis cannibalizes gleevec to boost new cancer drug. Bloomberg. http://www.bloomberg.com/news/articles/2012-10-25/novartis-cannibalizes-gleevec-to-boost-new-cancer-drug. October 25, 2012. Accessed July 22, 2016.
- 19. Ziegler MG, Lew P, Singer BC. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273(16):1296-1298. [PubMed] [Google Scholar]
- 20. Lexchin J. What information do physicians receive from pharmaceutical representatives? Can Fam Physician Med Fam Can. 1997;43:941-945. [PMC free article] [PubMed] [Google Scholar]
- 21. Mintzes B, Lexchin J, Sutherland JM, et al. Pharmaceutical sales representatives and patient safety: a comparative prospective study of information quality in Canada, France and the United States. J Gen Intern Med. 2013;28(10):1368-1375. 10.1007/s11606-013-2411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lexchin J. The relation between promotional spending on drugs and their therapeutic gain: a cohort analysis. CMAJ Open. 2017;5(3):E724-E728. 10.9778/cmajo.20170089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lexchin J. Interactions between physicians and the pharmaceutical industry: what does the literature say? Can Med Assoc J. 1993;149(10):1401-1407. [PMC free article] [PubMed] [Google Scholar]
- 24. Lieb K, Scheurich A. Contact between doctors and the pharmaceutical industry, their perceptions, and the effects on prescribing habits. PLoS One. 2014;9(10):e110130. 10.1371/journal.pone.0110130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spurling GK, Mansfield PR, Montgomery BD, et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med. 2010;7(10):e1000352. 10.1371/journal.pmed.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its Supplementary material.