Abstract
BACKGROUND
Since the outbreak of the COVID-19 pandemic, some studies have reported an increased preeclampsia incidence in pregnant women with SARS-CoV-2 infection. Several explanations for this association have been proposed, including a preeclampsia-like syndrome induced by severe COVID-19. This syndrome was described in a small case series and has not been confirmed in larger studies, and its effect on perinatal outcomes has not been studied.
OBJECTIVE
This study aimed to confirm the preeclampsia-like syndrome because of COVID-19 and to investigate its implications on pregnancy outcomes and prognosis.
STUDY DESIGN
This was a prospective, observational study conducted in a tertiary referral hospital. The inclusion criteria were pregnant women admitted to the intensive care unit for severe pneumonia because of COVID-19. They were classified into 3 groups based on clinical and laboratory findings: preeclampsia, preeclampsia-like syndrome, and women without preeclampsia features. The 3 cohorts were analyzed and compared at 3 different times: before, during, and after severe pneumonia. The main outcomes were incidence of adverse perinatal outcomes and signs and symptoms of PE, such as hypertension, proteinuria, thrombocytopenia, elevated liver enzymes, and increased angiogenic factors (soluble fms-like tyrosine kinase 1–to–placental growth factor ratio).
RESULTS
A total of 106 women were admitted to the intensive care unit because of severe pneumonia, and 68 women were included in the study. Of those, 53 (50.0%) did not meet the diagnostic criteria for preeclampsia and remained pregnant after pneumonia (non-preeclampsia); 7 (6.6%) met the diagnostic criteria for preeclampsia, had abnormal (>38) soluble fms-like tyrosine kinase 1–to–placental growth factor ratio (preeclampsia), and delivered during severe pneumonia, and 8 (7.5%) met the diagnostic criteria for preeclampsia, had normal (≤38) soluble fms-like tyrosine kinase 1–to–placental growth factor ratio (preeclampsia like), and did not deliver during pneumonia. Despite not having delivered, most preeclampsia-related features improved after severe pneumonia in women with preeclampsia-like syndrome. Women with preeclampsia had significantly poorer outcomes than women with preeclampsia-like syndrome or without preeclampsia.
CONCLUSION
More than 50% of women with severe COVID-19 and diagnostic criteria for preeclampsia may not be preeclampsia but a preeclampsia-like syndrome, which may affect up to 7.5% of women with severe COVID-19. Preeclampsia-like syndrome might have similar perinatal outcomes to those of normotensive women with severe pneumonia because of COVID-19. For these reasons, preeclampsia-like syndrome should be excluded by using soluble fms-like tyrosine kinase 1–to–placental growth factor ratio in future research and before making clinical decisions.
Key words: angiogenic factors, COVID-19, preeclampsia, preeclampsia-like syndrome, pregnancy, SARS-CoV-2, soluble fms-like tyrosine kinase-1–to–placental growth factor ratio
The etiology and pathogenesis of preeclampsia (PE) are not completely understood; however, deficient trophoblast invasion seems to play a leading role.1 The resulting placental ischemia leads to an angiogenic imbalance in maternal serum, with increased antiangiogenic factors, such as soluble fms-like tyrosine kinase 1 (sFlt-1), and decreased proangiogenic factors, such as placental growth factor (PlGF).2 Some studies have shown that this angiogenic status might induce endothelial cell dysfunction, which contributes to the development of PE with its signs and symptoms (hypertension, proteinuria, thrombocytopenia, elevated liver enzymes, and neurologic disturbances).3
AJOG MFM at a Glance.
Why was this study conducted?
The preeclampsia (PE)-like syndrome has not yet been confirmed in larger studies, and its effect on perinatal outcomes has not been studied.
Key findings
Despite not having delivered, most PE-related features in women with severe COVID-19 improved after severe pneumonia in women with PE-like syndrome. Women with PE had significantly poorer outcomes than women with PE-like syndrome or without PE. Angiogenic factors are useful to discriminate PE-like from true PE.
What does this add to what is known?
PE-like syndrome may be accurately identified by angiogenic factors. Correct identification of cases with PE-like syndrome may improve pregnancy outcomes by reducing iatrogenic complications.
Several disorders have previously been demonstrated to mimic the clinical features of PE, including acute fatty liver, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, acute exacerbation of lupus erythematosus, and COVID-19.4, 5, 6, 7 These conditions share some clinical and laboratory findings with PE, and its diagnostic criteria may overlap; therefore, to provide adequate care, a differential diagnosis, although challenging, is necessary. Some studies have shown that the sFlt-1–to–PlGF ratio is a highly specific marker for placental dysfunction and may help to accurately discriminate PE from some of its imitators.6 , 8 , 9 Since the outbreak of the COVID-19 pandemic, some studies have reported an increased PE incidence in pregnant women with SARS-CoV-2 infection, and several explanations for this association have been proposed.10, 11, 12, 13 On May 2020, a small case series published by Mendoza et al7 described a PE-like syndrome induced by severe COVID-19 during pregnancy. That study showed that up to 62.5% of pregnant women with severe pneumonia because of SARS-CoV-2 infection may go on to develop features of PE. Nevertheless, among the 5 cases with signs and symptoms of PE, only 1 case had abnormal angiogenic status, suggesting that the remaining 4 cases probably did not have PE. Of these 4 cases, only 1 patient was still pregnant after recovering from pneumonia, and all signs and symptoms of PE had disappeared. Given that PE does not resolve spontaneously, and delivery is the only definitive treatment, severe COVID-19-induced PE-like syndrome was the most plausible explanation for this case.
Therefore, only 1 confirmed case of PE-like syndrome was described in that study at the beginning of the COVID-19 pandemic; this syndrome has not been confirmed in larger studies, and its effect on perinatal outcomes has not been studied. Therefore, this study aimed to confirm the existence of a PE-like syndrome in a larger cohort of women with severe COVID-19 and to investigate its implications for pregnancy outcomes and prognosis.
Materials and Methods
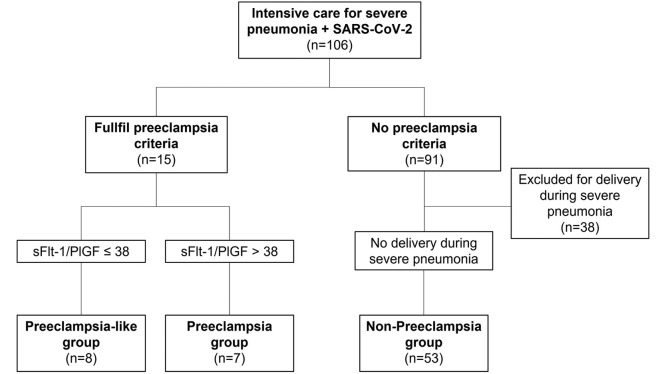
This was a prospective cohort study conducted at Vall d'Hebron Barcelona Hospital Campus, Barcelona, Spain, from February 2020 to September 2021. Our site was a referral hospital for severe COVID-19 pneumonia, especially among pregnant patients. The inclusion criteria were pregnant women admitted to the intensive care unit (ICU) for severe pneumonia because of SARS-CoV-2 infection during pregnancy. SARS-CoV-2 infection was confirmed by a real-time polymerase chain reaction of nasal and pharyngeal swabs. Participants who met the PE diagnostic criteria and had normal sFlt-1–to–PlGF ratio (≤38) were not delivered for PE and were classified as patients with PE-like syndrome. To establish whether the prognosis of women with PE-like syndrome was more similar to that of women with PE or to that of normotensive women, pregnancy outcomes were compared with 2 reference groups: women with true PE (met the PE criteria and had an sFlt-1–to–PlGF ratio of >38) and women who did not meet the PE criteria and did not deliver during the ICU stay (non-PE group). As comparing women with PE-like syndrome with women without PE and electively delivered because of pneumonia during the ICU stay did not provide additional information on the prognosis of patients with PE-like syndrome, these women were excluded from this study (Figure ).
Figure.
Inclusion flowchart
PlGF, placental growth factor ratio; sFlt-1, soluble fms-like tyrosine kinase 1.
Serrano. Preeclampsia-like syndrome in severe COVID-19. Am J Obstet Gynecol MFM 2022.
Gestational age was confirmed by fetal crown-rump length measurement at the first-trimester scan.14 Participants were examined at 3 different times: before severe pneumonia, during severe pneumonia (at the time of ICU stay), and after recovery from severe pneumonia. Demographic characteristics, obstetrical and maternal history, and biophysical and biochemical markers were collected. Biophysical and biochemical data included systolic blood pressure (SBP; mm Hg), diastolic blood pressure (DBP; mm Hg), hypertension (SBP of >140 mm Hg and/or DBP of >90 mm Hg), mean uterine artery pulsatility index (PI) percentile by transabdominal Doppler ultrasound,15 proteinuria (in 24-hour urine samples, spot urine protein-to-creatinine ratio, and/or dipstick urinalysis), platelet count (μL), aspartate aminotransferase (AST; U/L), alanine aminotransferase (ALT; U/L), lactate dehydrogenase (LDH; U/L), and creatinine level (mg/dL). In women with suspected PE, maternal serum PlGF and sFlt-1 (pg/mL) were determined using the fully automated Elecsys assays for sFlt-1 and PlGF on an electrochemiluminescence immunoassay platform (cobas e analyzers; Roche Diagnostics, Rotkreuz, Switzerland). The sFlt-1–to–PlGF ratio was calculated, with ≤38 values considered normal and unlikely to be associated with an underlying placental dysfunction.16
PE was defined as new-onset high blood pressure (SBP of >140 mm Hg and/or DBP of >90 mm Hg), worsening of previous high blood pressure in addition to new-onset proteinuria (protein-to-creatinine ratio of >300), worsening of previous proteinuria, or at least 1 of the following signs and symptoms of severe PE: cerebral or visual symptoms, elevation of liver enzymes to twice the normal concentration, platelet count of <100.000/μL, serum creatinine level of >1.1 mg/dL, or pulmonary edema.17 Here, to diagnose hypertension, at least 5 of 6 consecutive measurements had to be abnormal for a 6-hour period. Hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome) is considered a variant of PE. The diagnostic criteria for the HELLP syndrome were hemolysis with increased LDH (>600 U/L), AST or ALT (≥70 U/L), and platelet counts of <100 000/μL.18 Elective delivery was recommended at ≥37 weeks of gestation in women with PE without severe features and ≥34 weeks of gestation in women with severe PE and/or HELLP syndrome. In COVID-19 cases that met the PE diagnostic criteria and had an sFlt-1–to–PlGF ratio of ≤38, elective delivery because of PE was not recommended; alternatively, these cases were managed according to the clinical evolution of COVID-19. Elective delivery because of severe pneumonia was indicated at ≥36 to 38 weeks of gestation and ≥33 to 35 weeks of gestation if invasive respiratory support was required.
This study was approved by the Vall d'Hebron Barcelona Hospital Campus Ethics Committee (PR[AMI]181/2020) on March 13, 2020. All patients included in the study gave their oral or written consent according to the criteria of our ethics committee.
Statistical analysis
Categorical data were reported as frequency and percentage, and comparisons among the groups were estimated by the chi-square or fisher tests, as appropriate. Continuous variables were reported as median (interquartile range [IQR]). The Mann-Whitney U test was used to assess differences among the groups. The Wilcoxon signed-rank test was used to assess differences of repeated measurements on a single sample. The statistical significance level was set at P<.05, and the Bonferroni correction was applied to compensate for multiple comparisons. The Stata statistical software package was used for data analysis (StataCorp 2019; Stata Statistical Software: Release 16; StataCorp LLC, College Station, TX).
Results
During the study period, 106 patients with severe pneumonia because of COVID-19 were admitted to the ICU. Of those, 38 (35.8%) did not meet the inclusion criteria for needing elective delivery during the ICU stay because of clinical worsening of pneumonia and not having clinical suspicion of PE. Therefore, 68 women (64.2%) were included in the study: 7 (6.6%) with PE, 8 (7.6%) with PE-like syndrome, and 53 (50.0%) without PE features and not needing delivery during severe pneumonia (non-PE group). All 7 cases with PE required elective delivery during severe pneumonia (4 [57.1%] because of PE, 2 [28.6%] because of COVID-19 worsening, and 1 [14.3%] because of nonreassuring cardiotocography). In contrast, none of the cases in the PE-like syndrome group or the non-PE group required elective delivery during severe pneumonia. The median (IQR) gestational age at the time of admission to the ICU was significantly higher in the PE group (35.4 [30.3–36.6]) than in the PE-like syndrome group (25.6 [21.3–30]) and non-PE group (27.7 [24.5–31.3]) (P=.01). No other pregnancy baseline characteristic differed among the groups (Table 1 ).
Table 1.
Demographic characteristics of the study population
| Characteristics | PE like (n=8) | PE (n=7) | Non-PE (n=53) |
|---|---|---|---|
| Age (y) | 31.9 (25.3–33.6) | 33.8 (30–42.4) | 33.6 (31.0–37.2) |
| BMI (kg/m2) | 32.2 (24.8–36.1) | 28.7 (24.3–29.9) | 28.7 (26.4–32.3) |
| Chronic hypertension (%) | 0/8 (0) | 0/7 (0) | 1/52 (1.9) |
| Smoking (%) | 0/8 (0) | 0/7 (0) | 2/52 (3.9) |
| Diabetes mellitus (%) | 0/8 (0) | 0/7 (0) | 1/53 (1.9) |
| Nulliparous | 4/8 (50.0) | 5/7 (71.4) | 11/53 (20.8) |
| ART | 1/8 (12.5) | 1/7 (14.3) | 3/45 (6.7) |
| Race (%) | |||
| White | 6/8 (75.0) | 7/7 (100.0) | 46/53 (86.8) |
| South-Asian | 0/8 (0) | 0/7 (0) | 3/53 (5.7) |
| Asian | 1/8 (12.5) | 0/7 (0) | 1/53 (1.9) |
| Afro-descendant | 1/8 (12.5) | 0/7 (0) | 1/53 (1.9) |
| Mixt | 0/8 (0) | 0/7 (0) | 2/53 (3.8) |
| Gestational age at ICU admission | 25.6 (21.3–30.0)a | 35.4 (30.3–36.6) | 27.7 (24.5–31.3) |
Continuous data are presented as median (interquartile range). Categorical data presented as frequency (percentage).
ART, assisted reproductive treatment; BMI, body mass index; ICU, intensive care unit; PE, preeclampsia.
Statistically significant difference compared to the PE group.
Serrano. Preeclampsia-like syndrome in severe COVID-19. Am J Obstet Gynecol MFM 2022.
We found that some PE-related features worsened similarly during severe pneumonia across all 3 groups; however, most of these features improved significantly after recovery from severe pneumonia, without requiring elective delivery in most cases (Tables 2 and 3 ). Among the 68 women included in this study, there were 15 cases (22.1%) of hypertension, 19 cases (27.9%) of proteinuria, and 43 cases (63.2%) of elevated liver enzymes. There was no case of thrombocytopenia or abnormal LDH or creatinine. Moreover, 15 women (22.1%) met the PE diagnostic criteria during severe pneumonia. When comparing the incidence of PE-related features among groups, hypertension was present in the PE and PE-like syndrome groups (100%) and not in the non-PE group (0%). In addition, the sFlt-1–to–PlGF ratios were higher in the PE group than in the PE-like syndrome and non-PE groups (Table 3). No other significant difference was observed across groups regarding PE-related features. When comparing the PE-like syndrome and PE groups during pneumonia, the sFlt-1–to PlGF ratios (4.6 [2.6–12.8] vs 122.9 [49.4–225.5]; P=.008) and creatinine values (0.32 [0.25–0.41] mg/dL vs 0.49 [0.47–0.64] mg/dL; P=.015) differed significantly, although creatinine values were within the normal range in both groups. In contrast, when comparing the PE-like syndrome and non-PE groups, no significant difference was found, except for hypertension (8/8 (100%) vs 0/53 (0%); P<.001), SBP (153 [147.3–160.8] mm Hg vs 108 [103.5–119] mm Hg; P<.001), and DBP (95.5 [90.8–104.5] mm Hg vs 69 [64.5–76.5] mm Hg; P<.001).
Table 2.
PE-related findings during and after severe pneumonia
| Variable | PE like (n=8) |
PE (n=7) |
Non-PE (n=53) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| During severe pneumonia | After severe pneumonia | P value | During severe pneumonia | After severe pneumonia | P value | During severe pneumonia | After severe pneumonia | P value | |
| SBP (mm Hg) | 153.0 (147.3–160.8) | 115.0 (112.3–125.8) | .012 | 154.0 (145.0–156.0) | 128.0 (123.0–137.0) | .018 | 108.0 (103.5–119.0) | 104.0 (98.0–112.0) | <.001 |
| DBP (mm Hg) | 95.5 (90.8–104.5) | 76.0 (63.8–77.5) | .012 | 100.0 (97.0–109.0) | 83.0 (81.0–90.0) | .018 | 69.0 (64.5–76.5) | 65.0 (60.0–71.0) | .005 |
| Hypertension | 8/8 (100.0) | 0/8 (0) | .005 | 7/7 (100.0) | 2/7 (28.5) | .025 | 0/53 (0) | 1/53 (1.9) | .320 |
| Proteinuria | 6/6 (100.0) | 0/2 (0) | .16 | 5/7 (71.4) | — | — | 8/14 (57.1) | 3/9 (33.3) | .160 |
| Platelet count per μL | 245,000 (191,500–328,300) | 273,500 (224,800–384,500) | .21 | 197,000 (137,000–309,000) | 371,000 (307,000–406,000) | .06 | 259,000 (187,000–323,000) | 290,000 (240,000–352,000) | .045 |
| Thrombopenia<100,000/μL | 0/8 (0) | 0/8 (0) | 1.0 | 0/7 (0) | 0/7 (0) | 1.0 | 0/51 (0) | 0/51 (0) | 1.0 |
| AST (U/L) | 99 (28.3–160.8) | 18 (16–25) | .028 | 104 (42–164) | 29 (24–31) | .022 | 74 (44–144.5) | 23 (17–43) | <.001 |
| ALT (U/L) | 114.5 (32.8–290.3) | 15.0 (9.0–33.0) | .043 | 85.0 (39.0–150.0) | 30.0 (20.0–45.0) | .028 | 58.8 (35.5–136.5) | 25.0 (14.0–37.0) | <.001 |
| Transaminitis more than twice the normal values | 5/8 (62.5) | 1/7 (14.3) | .083 | 6/7 (85.7) | 0/7 (0) | .014 | 32/52 (62.5) | 6/47 (12.8) | <.001 |
| LDH (U/L) | 282.0 (203.8–360.3) | 223.0 (167.0–246.3) | .07 | 497.5 (347.3–538.3) | 328.5 (239.0–367.8) | .68 | 280.5 (234.0–320.5) | 203.0 (173.0–202.5) | .005 |
| LDH>600 U/L | 0/8 (0) | 0/8 (0) | 1.0 | 0/7 (0) | 0/7 (0) | 1.0 | 2/52 (3.9) | 0/53 (0) | .160 |
| Creatinine level (mg/dL) | 0.32 (0.25–0.41) | 0.41 (0.39–0.42) | .036 | 0.49 (0.47–0.64) | 0.43 (0.38–0.49) | .45 | 0.35 (0.31–0.44) | 0.4 (0.34–0.51) | .029 |
| Creatinine level>1 mg/dL | 0/8 (0) | 0/8 (0) | 1.0 | 0/7 (0) | 0/7 (0) | 1.0 | 0/51 (0) | 0/53 (0) | 1.0 |
| Mean uterine artery PI >95th percentile | 1/5 (20.0) | 1/6 (14.3) | 1.0 | — | — | — | 2/13 (15.4) | 4/29 (13.8) | .320 |
| sFlT-1–to–PlGF ratio | 4.60 (2.56–12.80) | 11.30 (7.30–23.30) | .068 | 122.90 (49.40–225.50) | 15.20 (8.50–21.90) | .18 | 6.70 (2.30–10.90) | 5.91 (3.40–10.40) | .790 |
| Fulfill PE criteria | 8/8 (100.0) | 0/8 (0) | .005 | 7/7 (100.0) | 0/7 (0) | .008 | 0/53 (0) | 1/53 (1.9) | 0.310 |
| Fulfill HELLP criteria | 0/8 (0) | 0/8 (0) | 1.0 | 0/7 (0) | 0/7 (0) | 1.0 | 0/53 (0) | 0/53 (0) | 1.0 |
Continuous data are presented as median (interquartile range). Categorical data are presented as frequency (percentage). The Wilcoxon signed-rank test was used to assess differences of repeated measurements on a single sample.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; HELLP, hemolysis, elevated liver enzymes, and low platelet count; LDH, lactate dehydrogenase; PE, preeclampsia; PI, pulsatility index; PlGF, placental growth factor; SBP, systolic blood pressure; sFlt-1, soluble fms-like tyrosine kinase 1.
Serrano. Preeclampsia-like syndrome in severe COVID-19. Am J Obstet Gynecol MFM 2022.
Table 3.
PE-related features during severe pneumonia in the PE-like group vs the PE and non-PE groups
| Variable | PE like (n=8) | PE (n=7) | Non-PE (n=53) |
|---|---|---|---|
| SBP (mm Hg) | 153 (147.3–160.8)a | 154 (145.0–156.0) | 108 (103.5–119.0) |
| DBP (mm Hg) | 95.5 (90.8–104.5)a | 100.0 (97.0–109.0) | 69.0 (64.5–76.5) |
| Hypertension | 8/8 (100.0)a | 7/7 (100.0) | 0/53 (0) |
| Proteinuria | 6/6 (100.0) | 5/7 (71.4) | 8/14 (57.1) |
| Platelet count per μL | 245 (191.5–328.3) | 197 (137.0–309.0) | 259 (187.0–323.0) |
| Thrombopenia<100,000/μL | 0/8 (0) | 0/7 (0) | 0/51 (0) |
| AST (U/L) | 99 (28.3–160.8) | 104 (42.0–164.0) | 74 (44.0–144.5) |
| ALT (U/L) | 114.5 (32.8–290.3) | 85.0 (39.0–150.0) | 58.8 (35.5–136.5) |
| Transaminitis more than twice the normal values | 5/8 (62.5) | 6/7 (85.7) | 32/52 (62.5) |
| LDH (U/L) | 282 (203.8–360.3) | 497.5 (347.3–538.3) | 280.5 (234–320.5) |
| LDH>600 U/L | 0/8 (0) | 0/7 (0) | 2/52 (3.9) |
| Creatinine (mg/dL) | 0.32 (0.25–0.41)b | 0.49 (0.47–0.64) | 0.35 (0.31–0.44) |
| Creatinine level>1 mg/dL | 0/8 (0) | 0/7 (0) | 0/51 (0) |
| Mean uterine artery PI >95th percentile | 1/5 (20.0) | — | 2/13 (15.4) |
| sFlT-1–to–PlGF ratio | 4.6 (2.6–12.8)b | 122.9 (49.4–225.5) | 6.7 (2.3–10.9) |
Continuous data are presented as median (interquartile range). Categorical data presented as frequency (percentage).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; LDH, lactate dehydrogenase; PE, preeclampsia; PI, pulsatility index; PlGF, placental growth factor; SBP, systolic blood pressure; sFlt-1, soluble fms-like tyrosine kinase 1.
Statistically significant difference compared to the non-PE group;
Statistically significant difference compared to the PE group.
Serrano. Preeclampsia-like syndrome in severe COVID-19. Am J Obstet Gynecol MFM 2022.
When we compared the groups after the resolution of severe pneumonia because of COVID-19, only blood pressure was significantly different among groups, being higher in the PE group, although always within the normal range (Table 4 ).
Table 4.
PE-related features after severe pneumonia in the PE-like group vs the PE and non-PE groups
| Variables | PE like (n=8) | PE (n=7) | Non-PE (n=53) |
|---|---|---|---|
| SBP (mm Hg) | 115 (112.3–125.8)a,b | 128 (123.0–137.0) | 104 (98.0–112.0) |
| DBP (mm Hg) | 76 (63.8–77.5)a | 83 (81.0–90.0) | 65 (60.0–71.0) |
| Hypertension | 0/8 (0) | 2/7 (28.5) | 1/53 (1.9) |
| Proteinuria | 0/2 (0) | — | 3/9 (33.3) |
| Platelet count per μL | 273,500 (224,800–384,500) | 371,000 (307,000–406,000) | 290,000 (240,000–352,000) |
| Thrombopenia<100,000/μL | 0/8 (0) | 0/7 (0) | 0/51 (0) |
| AST (U/L) | 18 (16–25) | 29 (24–31) | 23 (17–43) |
| ALT (U/L) | 15 (9–33) | 30 (20–45) | 25 (14–37) |
| Transaminitis more than twice the normal values | 1/7 (14.3) | 0/7 (0) | 6/47 (12.8) |
| LDH (U/L) | 223 (167.0–246.3) | 328.5 (239.0–367.8) | 203 (173.0–202.5) |
| LDH>600 U/L | 0/8 (0) | 0/7 (0) | 0/53 (0) |
| Creatinine (mg/dL) | 0.41 (0.39–0.42) | 0.43 (0.38–0.49) | 0.40 (0.34–0.51) |
| Creatinine level>1 mg/dL | 0/8 (0) | 0/7 (0) | 0/53 (0) |
| Mean uterine artery PI >95th percentile | 1/7 (14.3) | — | 4/29 (13.8) |
| sFLT-1–to–PlGF | 11.3 (7.3–23.3) | 15.2 (8.5–21.9) | 5.91 (3.4–10.4) |
Continuous data are presented as median (interquartile range). Categorical data are presented as frequency (percentage).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; LDH, lactate dehydrogenase; PE, preeclampsia; PI, pulsatility index; PlGF, placental growth factor; SBP, systolic blood pressure; sFlt-1, soluble fms-like tyrosine kinase 1.
Statistically significant difference compared to the PE group;
Statistically significant difference compared to the non-PE group.
Serrano. Preeclampsia-like syndrome in severe COVID-19. Am J Obstet Gynecol MFM 2022.
Regarding perinatal and neonatal outcomes, the PE-like syndrome group had significantly fewer cesarean deliveries (37% vs 100%; P=.015), higher gestational age at delivery (39.1 [38.2–40.7] weeks vs 36.3 [32–36.8] weeks; P=.001), and higher birthweight (2737.5 [2637.3–3037.5] g vs 2412.5 [1683.8–2580] g; P=.004) than the PE group. No difference was found between the PE-like syndrome and non-PE groups. More information can be found in Table 5 .
Table 5.
Neonatal outcomes in the PE-like group vs the PE and non-PE groups
| Variables | PE like (n=8) | PE (n=7+1) | Non-PE (n=53+2) |
|---|---|---|---|
| Delivery | |||
| Vaginal | 3/8 (37.5)a | 0/7 (0) | 34/52 (65.4) |
| Vaginal assisted | 2/8 (25.0)a | 0/7 (0) | 3/52 (5.8) |
| Cesarean delivery | 3/8 (37.5)a | 7/7 (100.0) | 15/52 (28.9) |
| Gestational age at delivery | 39.1 (38.2–40.7)a | 36.3 (32.0–36.8) | 39 (37.9–40.0) |
| Born alive | 8/8 (100.0) | 8/8 (100.0) | 55/55 (100.0) |
| Birthweight (g) | 2737.5 (2637.3–3037.5)a | 2412.5 (1683.8–2580.0) | 3130.0 (2900.0–3540.0) |
| Apgar score of <7 at 5 min | 0/8 (0) | 2/8 (25.0) | 0/50 (0) |
| Arterial pH<7 | 0/7 (0) | 0/8 (0) | 1/21 (4.8) |
| Hypoglycemia | 0/8 (0) | 0/8 (0) | 0/41 (0) |
| Jaundice with phototherapy | 0/8 (0) | 1/8 (12.5) | 1/41 (2.4) |
| Intraventricular hemorrhage | 0/8 (0) | 0/8 (0) | 0/49 (0) |
| Necrotizing enterocolitis | 0/8 (0) | 0/8 (0) | 0/49 (0) |
| Sepsis | 0/8 (0) | 0/8 (0) | 0/49 (0) |
| Seizures | 0/8 (0) | 0/8 (0) | 0/49 (0) |
| Respiratory distress syndrome | 0/8 (0) | 3/8 (37.5) | 1/48 (2.0) |
| Periventricular leukomalacia | 0/8 (0) | 0/8 (0) | 0/49 (0) |
| Composite adverse neonatal outcomes | 0/8 (0) | 3/8 (37.50) | 2/55 (3.64) |
Continuous data are presented as median (interquartile range). Categorical data are presented as frequency (percentage).
PE, preeclampsia.
Statistically significant difference compared to the preeclampsia group.
Serrano. Preeclampsia-like syndrome in severe COVID-19. Am J Obstet Gynecol MFM 2022.
Discussion
Principal findings
During severe pneumonia, up to 14.2% (15/106) of women met the PE diagnostic criteria; however, 8 of 15 cases (53.3%) improved after pneumonia without elective delivery. These 8 cases had normal sFlt-1–to–PlGF ratios, better outcomes than the 7 women with PE, and similar pregnancy outcomes to those of normotensive women with severe COVID-19 who did not deliver during severe pneumonia. This suggests that the sFlt-1–to–PlGF ratio may be a useful marker to identify a subset of patients with PE-like syndrome that otherwise may be misdiagnosed with PE and who would benefit from a more conservative management.
In Table 2, we confirm that in both the PE-like syndrome group and the PE group, most PE features observed during severe pneumonia improved after recovery, especially hypertension and transaminitis. Improvement in the PE group can be attributed to the fact that all women delivered because of COVID-19 and/or PE during ICU stay. In contrast, in the PE-like group, PE-related features improved without delivery. Given that PE does not resolve spontaneously, but only after delivery, the most plausible explanation for this outcome in the PE-like group is a PE-like syndrome induced by severe COVID-19.
In the non-PE group, some PE-related features (such as increased liver enzymes) were observed during severe pneumonia but improved after recovery. This suggests that even in normotensive patients, severe pneumonia because of COVID-19 can induce transient systemic effects that mimic PE and that resolve completely after the resolution of pneumonia.
During severe pneumonia (Table 3), the PE-like and PE groups were identical, except for sFlt-1–to–PlGF and creatinine values that were significantly lower, although within the normal range, in all patients with PE-like syndrome. However, creatinine values were also normal in the PE group, making this finding clinically irrelevant for differential diagnosis. Therefore, the sFlt-1–to–PlGF ratio seems to be the only parameter that allows to accurately discriminate PE from PE-like syndrome.
All women with PE were electively delivered during severe pneumonia. After resolution of severe pneumonia (Table 4), most PE-related features, such as hypertension, transaminitis, and increased sFlt-1–to–PlGF ratio, improved similarly across all groups, with the remarkable difference that all cases in the PE-like syndrome and non-PE groups recovered without elective delivery. This confirms that PE-related features in these 2 groups were due to the systemic effects of COVID-19.
Regarding perinatal outcomes (Table 5), the rate of cesarean deliveries was significantly higher and the birthweight and gestational age at delivery were significantly lower in the PE group than in the PE-like and non-PE groups. No difference was found between the PE-like and non-PE groups for perinatal outcomes.
Results
Several studies have reported an association between PE and COVID-19 that may be explained by several etiopathogenic pathways.13 , 19, 20, 21, 22 PE-like syndrome was described at the beginning of the pandemic in a small case series and could partly explain the higher PE incidence in COVID-19 pregnancies.7 Unfortunately, none of the studies investigating the association between both conditions have examined the sFlt-1–to–PlGF ratio or excluded women with PE-like syndrome from the analyses, making their results difficult to interpret. After that small case series, PE-like syndrome was no further investigated in larger cohorts. For this reason, the exact relationship between PE and COVID-19 remains unknown, as some studies might have misdiagnosed some cases of PE. Here, we provided further evidence that PE-like syndrome is an actual condition that may affect 7.5% (8/106) of women with severe COVID-19 and more than 50% of women meeting the diagnostic criteria for PE. According to the first case series describing the PE-like syndrome, the incidence of this syndrome in women with severe COVID-19 was 50.0%, whereas the incidence was only 7.5% in this study. This difference may be explained by the larger sample size in this study. Another plausible explanation may be the longer study period (19 months) in this study compared with the case series (31 days). Both situations may offer a wider scope of the clinical characteristics of PE-like syndrome through different management protocols and different clinical manifestations because of the increasing vaccine administration rates and the evolving SARS-CoV-2 variants.
Clinical implications
This study has important clinical implications, as it shows that PE-like syndrome is a transient condition that spontaneously resolves after recovery from severe pneumonia and has similar pregnancy outcomes to those of normotensive women with severe COVID-19. Therefore, patients with PE-like syndrome may benefit from a more conservative management. Most women with PE-like syndrome may be erroneously diagnosed with PE with severity features because of the systemic effects of COVID-19 and, therefore, may be electively delivered at ≥34 weeks of gestation. In this context, the sFlt-1–to–PlGF ratio may help to discriminate true PE from PE-like syndrome, thus improving clinical management and pregnancy outcomes for cases with PE-like syndrome by reducing the risk of iatrogenic complications and prematurity.
Research implications
In addition, our results have important research implications, as we show that some cases classified as true PE in previous studies might instead be cases with PE-like syndrome. Therefore, to better assess the association between true PE and COVID-19, in future studies, PE-like syndrome should be excluded using the sFlt-1–to–PlGF ratio.
Strengths and limitations
One of the main strengths of this study was the large number of pregnant women with severe pneumonia because of COVID-19. In addition, this study confirmed the existence of a PE-like syndrome induced by severe COVID-19 by assessing the sFlt-1–to–PlGF in a relatively large cohort of pregnant women with suspicion of PE and severe COVID-19. This study provided novel evidence for the evolution and outcomes of pregnancies with PE-like syndrome induced by severe COVID-19. Another strength of this study was that it further supports the clinical usefulness of the sFlt-1–to–PlGF ratio for discriminating between placental dysfunction and other disorders.4
The main limitation of this study was the reduced number of cases with PE and PE-like syndrome, which makes it harder to identify differences across groups for pregnancy outcomes. For instance, the median birthweight in the PE-like syndrome group tended to be lower than in the non-PE group (2737.5 g vs 3130 g, respectively), despite gestational age at delivery being the same. However, this difference was not statistically significant (P=.09), probably because of the reduced sample size in the PE-like syndrome group. Therefore, it is plausible that patients who are hypertensive with severe COVID-19 and normal sFlt-1–to–PlGF ratio (PE-like group) may have poorer perinatal outcomes than normotensive patients with severe COVID-19 (non-PE group). However, this hypothesis could not be confirmed in this study. Another limitation was that mean uterine artery PI was not recorded in any women in the PE group; for this reason, we could not investigate its role in distinguishing PE from PE-like syndrome.
Conclusions
More than 50% of women with severe COVID-19 that meet the diagnostic criteria for PE have PE-like syndrome. Therefore, future research on COVID-19 and PE should identify the cases with PE-like syndrome to better assess the association of both conditions. PE-like syndrome is a transient condition with similar pregnancy outcomes to those of normotensive women with severe COVID-19. Therefore, correct identification of cases with PE-like syndrome may improve pregnancy outcomes by reducing iatrogenic complications.
Acknowledgments
We thank all physicians who facilitated the recruitment at Hospital Universitari Vall d'Hebron and the participants who agreed to take part in the study.
Footnotes
Nerea Maiz is a member of the European Reference Network on Rare Congenital Malformations and Rare Intellectual Disability (European framework partnership agreement identification: 3HP-HP-FPA ERN-01-2016/739516).
M.M. and I.G.R. received lecture fees from Roche Diagnostics. The remaining authors report no conflict of interest.
This study was approved by the Vall d'Hebron University Hospital Ethics Committee (PR[AMI]181/2020) on March 13, 2020. Written informed consent was waived because of the rapid emergence of this infectious disease. However, verbal informed consent was obtained from all patients, which was included in the patient's medical records.
Cite this article as: Serrano B, Bonacina E, Garcia-Ruiz, et al. Confirmation of preeclampsia-like syndrome induced by severe COVID-19: an observational study. Am J Obstet Gynecol MFM 2022;XX:x.ex–x.ex.
References
- 1.Magee LA, Nicolaides KH, von Dadelszen P. Preeclampsia. N Engl J Med. 2022;386:1817–1832. doi: 10.1056/NEJMra2109523. [DOI] [PubMed] [Google Scholar]
- 2.Erez O, Romero R, Jung E, et al. Preeclampsia and eclampsia: the conceptual evolution of a syndrome. Am J Obstet Gynecol. 2022;226:S786–S803. doi: 10.1016/j.ajog.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turpin CA, Sakyi SA, Owiredu WK, Ephraim RK, Anto EO. Association between adverse pregnancy outcome and imbalance in angiogenic regulators and oxidative stress biomarkers in gestational hypertension and preeclampsia. BMC Pregnancy Childbirth. 2015;15:189. doi: 10.1186/s12884-015-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sibai BM. Imitators of severe pre-eclampsia. Semin Perinatol. 2009;33:196–205. doi: 10.1053/j.semperi.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Inversetti A, Serafini A, Manzoni MF, Dolcetta Capuzzo A, Valsecchi L, Candiani M. Severe hypothyroidism causing pre-eclampsia-like syndrome. Case Rep Endocrinol. 2012;2012 doi: 10.1155/2012/586056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verlohren S, Herraiz I, Lapaire O, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012;206 doi: 10.1016/j.ajog.2011.07.037. 58.e1–8. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza M, Garcia-Ruiz I, Maiz N, et al. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374–1380. doi: 10.1111/1471-0528.16339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirashima C, Ogoyama M, Abe M, et al. Clinical usefulness of serum levels of soluble fms-like tyrosine kinase 1/placental growth factor ratio to rule out preeclampsia in women with new-onset lupus nephritis during pregnancy. CEN Case Rep. 2019;8:95–100. doi: 10.1007/s13730-018-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolfo A, Attini R, Nuzzo AM, et al. Chronic kidney disease may be differentially diagnosed from preeclampsia by serum biomarkers. Kidney Int. 2013;83:177–181. doi: 10.1038/ki.2012.348. [DOI] [PubMed] [Google Scholar]
- 10.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: The INTERCOVID Multinational Cohort Study. JAMA Pediatr. 2021;175:817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med. 2020;382:e100. doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Mascio D, Khalil A, Saccone G, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289. doi: 10.1016/j.ajog.2021.05.014. e1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson HP, Fleming JE. A critical evaluation of sonar “crown-rump length” measurements. Br J Obstet Gynaecol. 1975;82:702–710. doi: 10.1111/j.1471-0528.1975.tb00710.x. [DOI] [PubMed] [Google Scholar]
- 15.Gómez O, Figueras F, Fernández S, et al. Reference ranges for uterine artery mean pulsatility index at 11-41 weeks of gestation. Ultrasound Obstet Gynecol. 2008;32:128–132. doi: 10.1002/uog.5315. [DOI] [PubMed] [Google Scholar]
- 16.Schiettecatte J, Russcher H, Anckaert E, et al. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem. 2010;43:768–770. doi: 10.1016/j.clinbiochem.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Hypertension in pregnancy Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 18.Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A Review. BMC Pregnancy Childbirth. 2009;9:8. doi: 10.1186/1471-2393-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68–89.e3. doi: 10.1016/j.ajog.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conde-Agudelo A, Romero R. Mechanisms that may underlie a causal association between SARS-COV-2 infection and preeclampsia. Am J Obstet Gynecol. 2022;226:280–281. doi: 10.1016/j.ajog.2021.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil A, Samara A, Chowdhury T, O'Brien P. Does COVID-19 cause pre-eclampsia? Ultrasound Obstet Gynecol. 2022;59:146–152. doi: 10.1002/uog.24809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giorgione V, Thilaganathan B. SARS-CoV-2 related myocardial injury might explain the predisposition to preeclampsia with maternal SARS-CoV-2 infection. Am J Obstet Gynecol. 2022;226:279–280. doi: 10.1016/j.ajog.2021.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]