Abstract
Tendons perform a critical function in the musculoskeletal system by integrating muscle with skeleton and enabling force transmission. Damage or degeneration of these tissues leads to impaired structure and function, which often persist despite surgical intervention. While the immune response and inflammation are important drivers of both tendon healing and disease progression, there have been relatively few studies of the diverse immune cell types that may regulate these processes in the tendon field. To date, most of the studies have focused on macrophages, but emerging research indicate that other immune cell types may also play a role in tendon healing, either by regulation of the immune environment or through direct interactions with resident tenocytes. Here, we synthesize the literature on innate and adaptive immune cells that have been implicated in tendon healing or disease, in the context of animal injury models, human clinical samples, or in vitro experimentation.
Keywords: immune, tendon, innate immune cells, adaptive immune cells, tendinopathy, inflammation
Introduction
Tendons are essential anatomical structures that transmit muscle forces to bones to enable movement and sustain mechanical loads (Franchi et al., 2007). Due to the poor intrinsic regenerative capacity of these tissues, injury frequently results in permanent scar formation and loss of function. Tendon rupture can arise from trauma; however, rupture is more frequently preceded by degeneration, which may be initiated by repetitive over-use, causing micro-damage to the tendon structure (Andarawis-Puri et al., 2012a; Andarawis-Puri et al., 2012b). Tendon dysfunction is broadly categorized under the term tendinopathy. In general, diseased tendons are distinguished by increased cellularity, altered cell phenotype and morphology, disrupted collagenous extracellular matrix (ECM), increased vasculature, increased water content, increased glycosaminoglycans, and neurovascular infiltration (Fenwick et al., 2002; Lozano et al., 2019). Interestingly, tendons that appear pathological by MRI or ultrasound are not always painful (Farnqvist et al., 2020; Rio et al., 2014). Tendinopathies are also not equally distributed between all tendons; tendons of the upper and lower limbs (such as the rotator cuff, patellar, and Achilles tendons) are among the most commonly affected (Figueroa et al., 2016; Longo et al., 2009). For more insight on the etiology, pathophysiology, diagnosis, and management of tendinopathy, please see the excellent and comprehensive review by Millar et al. (2017).
While there are many risk factors that can influence the progression from preclinical tendinopathy to chronic tendinopathy, including environmental factors and genetic predisposition, one critical factor that has been relatively under-appreciated is immune cell dysfunction (Millar et al., 2021). Historically, tendinopathy was sub-divided clinically as inflammatory (termed tendinitis) or non-inflammatory (termed tendinosis). The contribution of inflammation to tendon degeneration was largely ignored prior to 2012, which may be due to an overly narrow definition of inflammation (Mosca et al., 2018). In fact, accumulating evidence now suggest that tendon degeneration and fibrotic tendon healing could be a consequence of failed immune polarization, resulting in prolonged or chronic type I inflammation. For tissue regeneration, a finely tuned balance between inflammation and its resolution is crucial (D’Addona et al., 2017). Indeed, while chronic inflammation is harmful for proper tendon healing, this pro-inflammatory phase is still an essential component of the immune response and early suppression of the acute response also impairs functional tendon healing (Blomgran et al., 2017; D’Addona et al., 2017).
Despite the importance of the immune environment in tendinopathy and poor tendon healing, there is still very little known about the cells that orchestrate the immune response in the context of tendons. Therefore, the purpose of this review is to provide updated information regarding the immune cells involved in tendon inflammation and healing. For an in-depth review of selected type I and type II immune cytokines in tendinopathy and wound healing and advances in immunomodulatory drugs, we direct the reader to our recent review on this topic (Arvind and Huang, 2021). In this review, we will briefly describe tendon structure, function, and resident cell types (tenocytes, epitenon/endotenon cells, progenitor cells, and immune cells) followed by an overview of the known innate and adaptive immune cells that have been implicated in tendon injury and repair.
Tendon structure and function
Healthy tendons are composed of dense, extracellular matrix (ECM) that is primarily composed of highly organized, cross-linked type I collagen (~70% by dry weight) (Kastelic et al., 1978). The main tendon structure is divided into fascicles, which contain collagen fiber bundles. In turn, collagen fibers are composed of fibrils, which are further subdivided into microfibrils. These collagenous components are largely arranged in parallel to the long axis of the tendon, which contributes to the tendon’s mechanical properties (Franchi et al., 2007). Although collagen fibrils are initially homogeneous at birth with small diameter, fibril diameters increase rapidly during postnatal tendon maturation, resulting in a heterogeneous field of small and large collagen fibrils at the end of growth. While collagen fibrils directly contribute to tendon tensile load-bearing, loads are also transferred across discontinuous fibrils through interfibrillar shear and sliding (Szczesny and Elliott, 2014; Szczesny et al., 2017). In addition to type I collagen, tendon ECM also contains other components, including the small leucine rich proteoglycans (for example decorin and biglycan) and minor collagens (for example collagen type II, V, and XII) (Buckley et al., 2013). In general, the arrangement of the different collagen types directly contributes to tendon function by providing resistance, flexibility and elasticity while transmitting forces, dissipating energy, and preventing mechanical failure (Franchi et al., 2007). Recent evidence also suggest tendon extrafibrillar components may not directly contribute to tensile properties after tendon maturation and growth (Szczesny et al., 2017).
Although tendon fascicles form the bulk of tendon structure, individual fascicles are surrounded by specialized tissues called the endotenon while the entire tendon structure is enclosed by a similar tissue called the epitenon. While relatively little is known about endotenon and epitenon tissues, they are thought to play an important role in establishing the organization of the extracellular matrix in the developing tendon via cadherin mediated cell-cell junctions (Richardson et al., 2007). Moreover, the epitenon and endotenon largely contain most of the blood vessels that supply the tendon (Edwards, 1946). However, tendon vascularity can also vary greatly across subjects and tendon types (Cook et al., 2005) (Figure 1).
Figure 1:
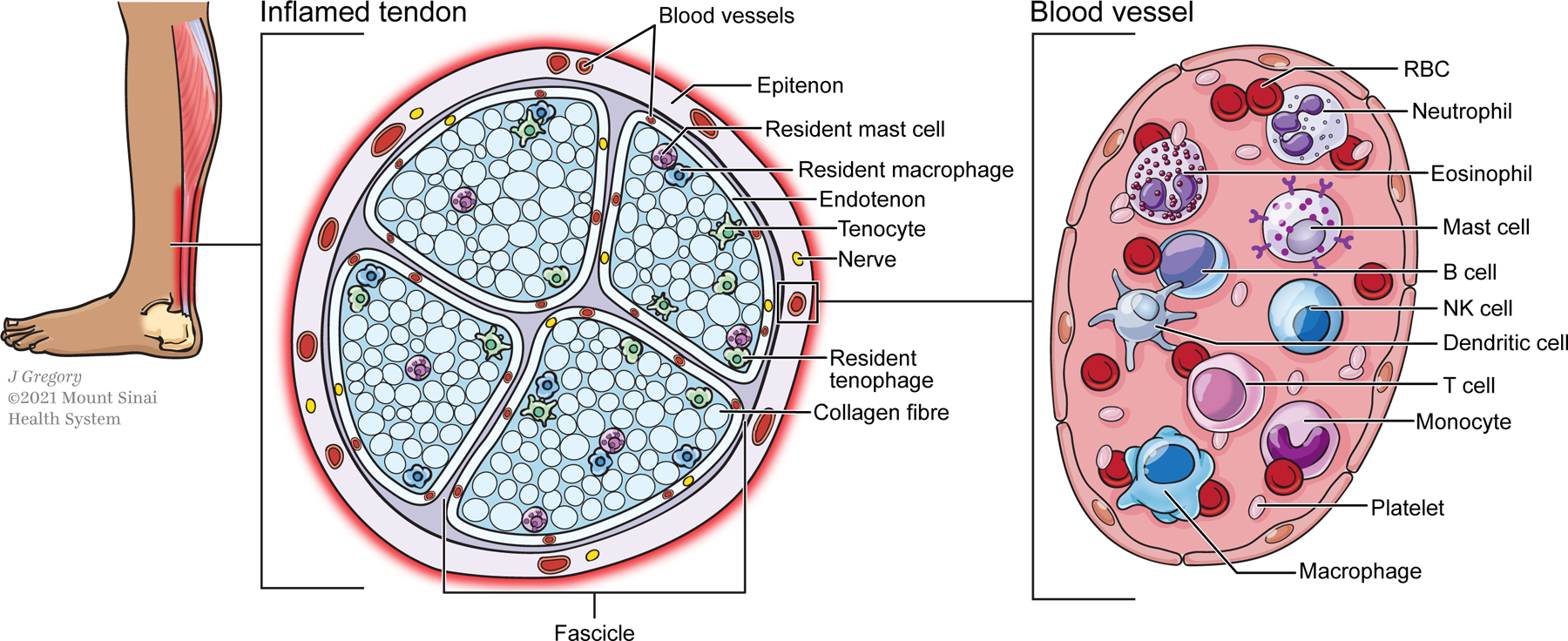
Overview of innate and adaptive immune cells involved in tendon inflammation. A small population of resident immune cells such as mast cells and macrophages can be found in normal tendons and expand with injury or disease. Other immune cells that contribute to tendon inflammation, disease progression, or healing infiltrate the tendon from peripheral sources and interact with resident tendon and epitenon cells. The temporal dynamics of recruited immune cells may vary according to injury model.
Resident tendon cell types
The resident cell type within the tendon fascicle are specialized fibroblastic cells called tenocytes that synthesize and maintain tendon ECM. In the mature tendon, tenocytes are longitudinally oriented and reside in rows. Tenocytes communicate via long protrusions connected by gap junctions composed of proteins such as connexins 32 and 43. To date, only four transcription factors have been identified for tenocytes, including Scx, Mkx, Egr1, and Egr2. While Scx is strongly expressed during embryonic and early postnatal stages, expression levels decrease with tendon maturation and heterogeneity in Scx expression emerges (Best et al., 2021; De Micheli et al., 2020; Howell et al., 2017; Nichols et al., 2019). Although tenocytes were originally considered a relatively homogeneous population, there is growing appreciation that sub-populations of tenocytes express distinctive markers and may have specialized functions (De Micheli et al., 2020; Kendal et al., 2020). While tenocytes are proliferative in the first 1–2 weeks after birth, mitotic capacity is lost with maturation, in parallel with dramatic increases in matrix deposition and mechanical properties (Ansorge et al., 2011; Grinstein et al., 2019). While there is some activation of Scx-expressing tenocytes after injury, the proliferative capacity of adult tenocytes is relatively limited, especially compared to neonatal tenocytes (Best and Loiselle, 2019; Gumucio et al., 2020; Howell et al., 2017).
The cells that reside within the endotenon/epitenon are distinctive from tenocytes in characteristic marker expression. During development, epitenon cells appear after the induction of tenocyte progenitors and express the marker Tppp3 (Staverosky et al., 2009). While this marker is lost in later stages of embryonic development, the marker re-emerges in mature tendons and identifies a sub-population of epitenon cells with stem and regenerative potential (termed tendon stem and progenitor cells) (Harvey et al., 2019). In general, epitenon cells express laminin, aSMA, and PDGFRalpha; these cells are highly activated after tendon injury, proliferate, and contribute to both scar formation and new Scx+ tenocytes (Best et al., 2021; Dyment et al., 2014; Gumucio et al., 2014; Gumucio et al., 2020; Harvey et al., 2019; Taylor et al., 2011).
Resident immune cells
Although tendon was previously thought to be devoid of immune cells, several studies now report the presence of both innate (eg macrophages) and adaptive (eg T cells) immune cell types within normal tendons (Garcia-Melchor et al., 2021; Howell et al., 2021; Kendal et al., 2020). While the function of these immune cells in the context of healthy tendon development and homeostasis has not been explored in detail, tendon mechanical properties are unchanged in Rag2−/− mice devoid of T and B cells (Arvind et al., 2021), indicating a minimal role for these cells in tendon development. The presence of immune cells in tendon suggests that these cells may function as first responders in the event of damage; it is also possible that dysregulated resident immune cells may induce degenerative changes independent of overt mechanical damage. Since immune cells have been shown to be mechanoresponsive (Göhring et al., 2021; Jin et al., 2019; McWhorter et al., 2015), it is intriguing whether resident immune cells may regulate the local immune environment in response to tendon loading.
Although resident immune cells are localized in close proximity to resident tenocytes/epitenon cells, direct interactions between cell types are only beginning to be elucidated. Co-culture and mixed culture experiments suggest there are likely reciprocal interactions between tenocytes and immune cells (Garcia-Melchor et al., 2021; Stolk et al., 2017), however further studies are required to determine the extent of these interactions under healthy and diseased conditions.
Innate Immune Cells in Tendinopathy
Typically, the first cells that trigger acute inflammation in a wounded and/or infected tissue are innate immune cells (Figure 2). These are cells that either circulate in the blood or are resident in tissues. Innate immune cells have a rapid, non-specific response to microbes and injured cells. In fact, most innate immune cells have pattern recognition receptors (PRR) which bind and recognize pathogen associated molecular patterns (PAMP) present on microbes, as well as damage associated molecular patterns (DAMP) (Kumar et al., 2011; Marshall et al., 2018). DAMPs, also known as “alarmins”, are nuclear, mitochondrial, or cytosolic proteins released by cells upon infection, necrosis, or injury (Roh and Sohn, 2018). Once innate immune cells bind proteins that trigger an immune response, they release a great number of cytokines, which in turn stimulate blood flow to recruit more immune cells to the site, thereby increasing inflammation (D’Addona et al., 2017; Marshall et al., 2018). Interestingly, in the case of tendon, tenocytes also release pro-inflammatory cytokines upon injury, contributing to edema and hyperemia (D’Addona et al., 2017; Millar et al., 2017). In this section, we summarize the known research on innate immune cell populations that have been implicated in some way in tendon healing. Unsurprisingly, the vast majority of the research has centered on macrophages, with relatively limited information on other innate immune cells such as neutrophils, mast cells, etc. Characteristic markers that have been used to identify these innate immune cells by flow cytometry are indicated in Table 1.
Figure 2:
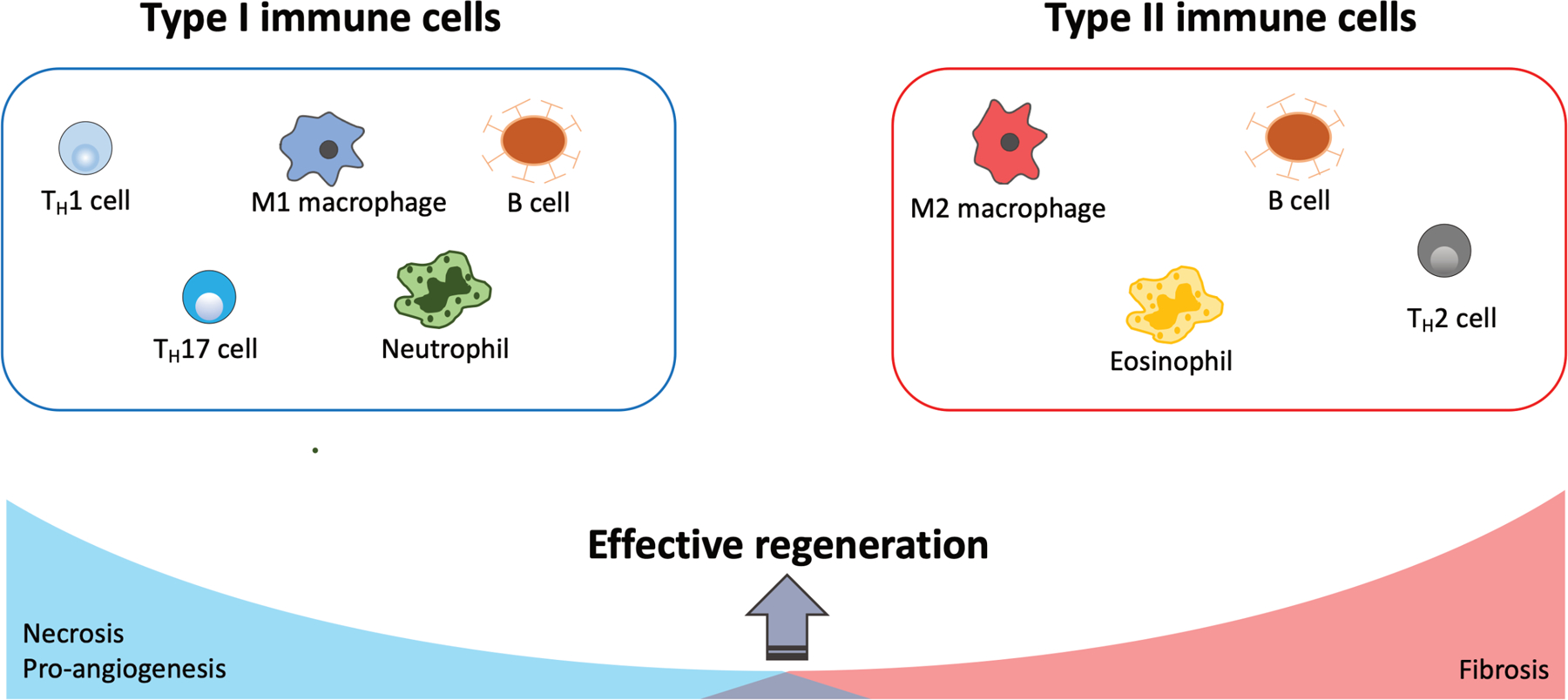
Representative immune cells that regulate type I and type II immune responses. Effective tissue regeneration requires a proper balance of type I and type II immune responses.
Table 1:
Commonly used cell markers to identify murine immune cells. Markers listed are not necessarily unique or exhaustive. Optimal marker choice depends on the cell subtype, the tissue that it is found in and its experimental application.
| Cell type | Common cell surface markers in mice |
|---|---|
| Innate Immune Cells | |
| Macrophage | CD11b, F4/80, CD68 |
| Monocyte | CCR, CX3CR1, LY6C |
| Tenophages | CX3CR1, CX3CL1 |
| Neutrophils | CD11b, GR1, LY6G |
| Mast cells | CD117/C-Kit, IL-3 R alpha/CD123 and Fc epsilon RI |
| Eosinophils | Cd11b, Singlec-F |
| Platelets | CD41, CD62p |
| Dendritic cells | CD11c, MHCII |
| Adaptive Immune Cells | |
| T cells | CD3 |
| Cytotoxic | CD3, CD8 |
| Helper | CD3, CD4 |
| B cells | CD19, CD80, CD73, PD-L2/CD273 |
| Natural killer cells | NK1.1/NKp46, NKG2D |
Macrophages
Macrophages are granulocytic phagocytic innate immune cells whose main function is to engulf pathogens, cell debris, and apoptotic bodies (Marshall et al., 2018). Macrophages either circulate in the bloodstream seeking inflamed areas to penetrate through trans-endothelial migration, or they permanently reside in specific tissues (Weber, 2008). Macrophages, unlike neutrophils (another important phagocytic population), are long-lived cells. For this reason, they play a more prominent role in adaptive immunity as crucial antigen presenting cells (cells that process and present antigens on their surface to activate B and T lymphocytes) (Marshall et al., 2018).
Historically, activated macrophages were thought to exist in two forms, either as M1 or M2 macrophages. M1 macrophages were considered the “typical” pro-inflammatory macrophages that clear pathogens, debris and apoptotic bodies, while releasing cytokines to increase inflammation (Mantovani et al., 2002; Millar et al., 2017; Sunwoo et al., 2020). M2 macrophages, on the other hand, inhibit the inflammatory response, which promotes angiogenesis, tissue remodeling, fibrosis, and healing (D’Addona et al., 2017; Del Buono et al., 2011; Mantovani et al., 2002; Sica and Mantovani, 2012; Zhang et al., 2008). Consensus among immunologists in the past several years now establish that activated macrophages exist along a continuum, from M1-like to M2-like macrophages (Murray et al., 2014). Macrophage polarization can be induced in the presence of specific signals, such as IL-10, IL-4, INF-ɣ, IL-13, glucocorticoid hormones, and vitamin D (Mantovani et al., 2002). Interestingly, M1-like and M2-like macrophages not only differ in their effector function, but also differ in receptor expression, cytokine, and chemokine production (Mantovani et al., 2002; Murray et al., 2014; Sica and Mantovani, 2012). In the context of tendon healing, prolonged activity of M1-like macrophages is thought to be detrimental to healing while M2-like macrophages are generally pro-regenerative. This is supported by recent studies showing that the immune-modulating activities of mesenchymal stem cells in tendon repair are due to their effects on M2-like macrophage polarization (Chamberlain et al., 2019). Mechanistically, this interaction appears driven by extracellular vesicles secreted by mesenchymal stem cells that induce macrophage polarization (Chamberlain et al., 2019). Injection of vesicle-educated macrophages thus promoted improved functional healing after mouse Achilles tendon rupture (Chamberlain et al., 2019).
While the dynamics of M1- to M2-like macrophage polarization is likely critical to tendon healing, the majority of tendon studies generally focus on total macrophage populations. While macrophages are normally scarce in healthy tendons (Best et al., 2019; Howell et al., 2021), macrophage numbers increase with disease. In human supraspinatus tendons, CD68+ tissue-resident macrophages increased in early and intermediate-advanced stages of tendinopathy compared to health tendons (Dakin et al., 2015; Del Buono et al., 2011). The presence of CD206+ macrophages and activation of ALOX15 and CD206 pathways was also associated with resolution of tendon pain after treatment (Dakin et al., 2015). Temporal regulation of macrophage accumulation is variable in the literature however, and depends on the injury model, the subtype of macrophage analyzed (and markers used), and the anatomical tendon analyzed. For example, in tendon grafts for reconstruction of rat anterior cruciate ligaments, recruited macrophages have been identified in the tendon 4 days after surgical reconstruction, while resident macrophages accumulated 11 days after the surgery (Kawamura et al., 2005). In contrast, collagenase-induced Achilles tendon injury in mouse showed an increase in recruited macrophages at 1 day post-injury while resident macrophages increased at 28 days (Marsolais et al., 2001). In general, injury models show consistent upregulation of macrophage numbers with disease or injury (Noah et al., 2020; Wojciak and Crossan, 1993). Regardless of the temporal dynamics post-injury, there is consensus in the field that these cells play an important role in both acute and chronic tendon inflammation (Jomaa et al., 2020). In addition, macrophages have also been shown to directly stimulate tenocyte proliferation and promote extracellular matrix deposition (de la Durantaye et al., 2014; Sunwoo et al., 2020).
Several studies ablating macrophages (either by genetic targeting or clodronate delivery) confirm the important function of macrophages in tendon healing. In adult tendon, depletion of macrophages reduces cell proliferation (de la Durantaye et al., 2014; Godbout et al., 2010) and matrix accumulation after injury (de la Durantaye et al., 2014). Functional outcomes were mixed however, with some studies showing improvement or no change in mechanical properties. Using genetic ablation of macrophages, we recently showed that macrophage ablation in neonatal mice results in failed regeneration, indicated by impaired function, reduced cell proliferation, and reduced neo-tendon formation (Howell et al., 2021). However, one limitation of all of these ablation studies is the inability to precisely target M1-like or M2-like populations, which have distinct functional activities in the healing cascade.
Monocytes
Monocytes are a type of myeloid agranular white blood cell that can differentiate into either macrophages or dendritic (Marshall et al., 2018) Generally, monocytes infiltrate an inflamed area within 24 hours of acute inflammation, together with macrophages and neutrophils (D’Addona et al., 2017). One of the main function of monocytes is to renew tissue-resident macrophages and transport antigens to secondary lymphoid tissues, without differentiating into macrophages (Jakubzick et al., 2017; Kapellos et al., 2019). Inflammatory monocytes typically give rise to M1-like macrophages while anti-inflammatory monocytes give rise to M2-like macrophages (Auffray et al., 2007). Notably, macrophages can also arise from cells other than monocytes, such as embryonic progenitors (Stremmel et al., 2018).
While the role of monocytes in tendon healing has been analyzed to a lesser extent compared to macrophages, resident monocytes have been reported in healthy human tendons and accumulate with injury (Kendal et al., 2020). During the early and intermediate stages of tendinopathy, monocytes are elevated in tendons and contribute to increased macrophage levels (Crowe et al., 2019; Dakin et al., 2015). In general, monocyte accumulation patterns follow macrophage patterns after injury with increased levels observed 3–7 days post-injury depending on injury model (Markworth et al., 2021; Noah et al., 2020).
Chemotactic monocyte factors have been implicated in both pro-inflammatory and anti-inflammatory events in tendons (Crowe et al., 2019). For example, lipoxins produced by both macrophages and monocytes are essential to dampen the inflammatory response and promote tendon healing (Millar et al., 2017). However, monocytes can release alarmins such as S100A8 and S100A9, which thought to participate in a positive feedback mechanism that enhance leukocyte recruitment and the release of more pro-inflammatory cytokines (Crowe et al., 2019).
Tenophages
Recently, the presence of macrophage-like tenocytes in healthy tendons, named “tenophages”, was proposed (Lehner et al., 2019). These tendon-resident cells express the fractalkine receptor CX3CR1, together with its ligand CX3CL1. Moreover, in vitro stimulation of these tenophages induced the production of various pro-inflammatory molecules that are involved in tissue healing and repair (Lehner et al., 2019). Due to the limited evidence, the identity of these cells remains an open question; however, the concept of a specialized sub-population of tenocytes is consistent with the growing consensus in the field that tenocytes are heterogeneous and harbor distinctive functions.
Neutrophils
Neutrophils are among the first granulocytic innate immune cells that respond to macrophage activation (Jomaa et al., 2020). Similar to other white blood cells, neutrophils migrate from the bloodstream to damaged and/or infected tissues through the leukocyte adhesion cascade, following a gradient of chemoattractants (Rosales, 2020). Neutrophils have a variety of anti-microbial functions, which include phagocytosis of invading microorganisms and other mechanisms promoting pathogen death. Toward this end, neutrophils can release cell granule microbicidal contents (termed degranulation), produce reactive oxygen species, and form neutrophil extracellular traps (Chaplin, 2010; Rosales, 2020). In the last decade, additional neutrophil functions have been elucidated. Indeed, these phagocytic cells are important mediators in the immune cell response, as they produce cytokines and chemokines that regulate both the innate and adaptive immune system (Chaplin, 2010; Rosales, 2020). For example, neutrophils are able to recruit and activate T cells at inflamed sites (Rosales, 2020). Neutrophils can also migrate into secondary lymphoid organs and act as antigen presenting cells to directly activate lymphocytes (Rosales, 2020).
In the context of tendon injury, neutrophils have been detected in various tendinopathy models, although peak neutrophil accumulation varies depending on the model. In an ovine model of superficial digital flexor tendon injury, it was found that neutrophils are highly activated as late as 5 months post-injury adults, compared to regenerative fetal counterparts (Ribitsch et al., 2021). With collagenase-induced Achilles tendon injury, the neutrophil population in the tendon peaked one day post-injury before gradually returning to baseline by 7 days (Marsolais et al., 2001). Other models such as tenotomy showed extended temporal dynamics, with persistence of neutrophils from 7–28 days post-injury (Crowe et al., 2019; Millar et al., 2017; Noah et al., 2020). These differences in neutrophil dynamics may be due to differences in injury severity between model systems. Detection of neutrophils at relatively late stages of healing may also suggest their contribution toward chronic inflammation or dysregulated immune cell function. Limited research on neutrophil serine proteases (such as elastase and cathepsin G) found that neutrophil elastase is capable of solubilizing tendon collagen type I, while cathepsin G had little effect (Starkey et al., 1977). Therefore, direct secretion of proteases that disrupt the tendon ECM may be another mechanism by which neutrophils can promote tendon degeneration cascade once induced.
Mast cells
Mast cells are granulocytic phagocytic innate immune cells that reside in most connective tissues and all vascularized areas (Krystel-Whittemore et al., 2016). In innate immunity, they have important anti-viral, anti-parasitic and bacterial responses through degranulation and the release of pro-inflammatory cytokines (Krystel-Whittemore et al., 2016). In healing tendons, an elevated mast cell concentration has been observed in a variety of contexts, including overused rat calcaneal tendons (Pingel et al., 2013), tendinopathic human patellar tendon biopsies (Behzad et al., 2013; Scott et al., 2008), in injured rabbit flexor tendons (Berglund et al., 2010) and in other human tendons (Del Buono et al., 2011; Jomaa et al., 2020).
The role of mast cells in tendon healing has not been fully elucidated. While mast cells can stimulate fibroblast proliferation, collagen deposition, and mediate wound healing (Garbuzenko et al., 2002), other studies using conditioned media suggest that mast cells may stimulate the release of excessive pro-inflammatory proteins (COX-2, PEG2) resulting in reduced type 1 procollagen production by tenocytes (Behzad et al., 2013). Despite these data, it is generally accepted that mast cells do play a role in collagen turnover; however, this has not yet been shown specifically in the context of tendon inflammation and healing (Alim et al., 2020). While the role of mast cells in tendon collagen deposition remains unclear, treatment of injured mouse patellar tendons with sodium cromolyn (a mast cell inhibitor) improved tendon collagen organization and reduced hypercellularity with healing in vivo (Sharma et al., 2011). Furthermore, mast cells are also implicated in neurogenic inflammation and pain associated with tendinopathy, since mast cells produce glutamate receptors and can thus communicate with the peripheral nervous system (Alim et al., 2017; Alim et al., 2020). Indeed, higher numbers of degranulating mast cells and mast cells expressing the glutamate receptor, NMDA-1, have been reported in rat tendon healing (Alim et al., 2017).
Eosinophils
Eosinophils are granulocytic innate immune cells that can be found both circulating in the blood and resident in the lamina propria of the gastrointestinal tract (Rosenberg et al., 2013). Eosinophils have a known role in fighting parasitic, bacterial and viral infections. They are also involved in thrombosis, plaque formation, inflammatory bowel diseases and gastrointestinal diseases (Rosenberg et al., 2013).
To date, there is very limited evidence for eosinophils in the context of tendon healing and disease as these cells are rarely present in chronically inflamed tendons (Jomaa et al., 2020). However, high levels of eosinophils in the blood are associated with eosinophilic fasciitis, which is a connective tissue disorder that is characterized by tendon retraction, subdermal sclerosis and joint contraction (Das et al., 2017). Also, eosinophils can stimulate ECM contraction and may interact with mesenchymal cells to promote ECM remodeling (Zagai et al., 2004). Therefore, data suggests that eosinophils might play a role in tendinopathies that is worth further investigation.
Platelets
Platelets are anuclear, discoidal cells that are derived from megakaryocytes (Thon and Italiano, 2012). These cells function in hemostasis, host defense, tissue repair and resolution of inflammation (van der Meijden and Heemskerk, 2019). In general, the majority of research on platelets for tendon healing focused on the therapeutic potential of platelet rich plasma (PRP) delivery, rather than studies of native platelet function in tendon healing. The beneficial activity of PRP is thought to derive from the high concentration and enrichment of platelets, which harbor growth factors and cytokines that promote regenerative healing responses. PRP delivery was shown to ameliorate tendon inflammation and promote regenerative tendon healing (Andia et al., 2018; Chen et al., 2012; de Almeida et al., 2012; de Vos et al., 2010; Nishio et al., 2020; Solchaga et al., 2014; Virchenko and Aspenberg, 2006). These results have been observed in both Achilles and patellar tendon injuries in mice, rats, and humans (de Almeida et al., 2012).
The specific mechanism of action of PRP in the context of tendon remodeling is still being investigated. So far, it was shown that cell morphology, cellularity, vascularity, and collagen arrangement were improved in injured patellar tendons compared to controls with PRP administration (Nishio et al., 2020). Moreover, PRP increased macrophage infiltration in injured patellar tendons, although different PRPs appeared to recruit different subtypes of macrophages (Nishio et al., 2020). Notably, PRP effects on tendon healing may depend in part on mechanical loading, since tendon unloading by botulinum toxin-induced paralysis led to decreased transverse area and reduced mechanical properties (Virchenko and Aspenberg, 2006). Independent of loading however, tendon stem cells and platelets from PRP treatments appear to work synergistically to promote tendon healing (Chen et al., 2012). One limitation to PRP treatment is the variability in PRP formulations and the undefined nature of PRP itself. It is therefore not surprising that clinical outcomes have been mixed (Bianco et al., 2019; Halpern et al., 2012).
Dendritic cells
Dendritic cells are crucial immune cells that have important functions in both the innate and adaptive immune response. Dendritic cells act as phagocytic innate cells; however, as they mature, they acquire antigen presenting abilities and link the innate immune system to the adaptive immune system by activating T cells (Mellman and Steinman, 2001).
Despite their importance in innate and adaptive immunity, dendritic cells are seldom studied in tendon healing. In Achilles tendons and their associated popliteal lymph nodes, dendritic cells accumulate one week post injury, peaking at two weeks post injury (Noah et al., 2020). Dendritic cells were also found in chronically tendinopathic human samples (Kendal et al., 2020). The functional requirement for dendritic cells in tendon healing and whether dendritic cells promote or resolve inflammation after tendon injury remain open questions.
Adaptive Immune Cells in Tendinopathy
In contrast to the innate immune response, which broadly targets pathogens, the adaptive immune response targets specific antigens (Chaplin, 2010). Adaptive immunity toward unique external molecules depends on the interaction between the antigen and receptors on T and B lymphocytes, which form through somatic rearrangement of genes (Chaplin, 2010). A vast repertoire of T and B cell receptors can therefore be produced, which are highly specific for unique antigens and create immunological memory after exposure to a particular pathogen (Chaplin, 2010).
Although historically less studied in the context of wound healing, there is growing appreciation for the role of adaptive immune cells in regulating inflammation, innate immune cells such as macrophages, and in directly activating resident cells after injury. Studies in muscle for example revealed a requirement for regulatory T cells in muscle regeneration through stimulation of resident satellite cells (Burzyn et al., 2013; Cho et al., 2019). Other T cell subpopulations (such as Th1 and Th2 helper T cells) have also been implicated in poor or regenerative healing, across various musculoskeletal tissues (Bozec et al., 2014; Burzyn et al., 2013; Gyarmati et al., 1983; Horowitz et al., 1984; Li et al., 2007). While this is still an emerging area in tendon research, we highlight the literature suggesting potential roles for adaptive immune cells (T cells, B cells, and natural killer cells) in tendon disease and healing (Figure 2). Characteristic markers identifying T and B cells are indicated in Table 1.
T cells
CD3+ T cells are lymphoid cells with distinctive subtypes, including CD8+ cytotoxic T cells and CD4+ T cells. Cytotoxic T cells act primarily to kill cells infected with intracellular microbes (Chaplin, 2010). Notably, tendon healing has been previously shown to be unaffected by CD8+ depletion in rats, though these cells appear to be important for cancellous bone healing (Bernhardsson et al., 2019). CD4+ T cells include a number of helper T cells (such as Th1, Th2, Th17 and others) as well as regulatory T cells (Tregs). Unlike macrophages, which are defined based on cell surface markers, helper T cell subpopulations are defined by well-established transcription factors (Table 1). Like macrophages, T cell subpopulations can also be classified as pro- or anti-inflammatory. In general, Th1 and Th17 cells are associated with inflammation while Th2 and Treg cells resolve or suppress inflammation (Biton et al., 2016; Rankin et al., 2010).
The majority of studies in tendon research are descriptive characterizations of T cells, their sub-types, and temporal dynamics. After tendon injury, T cell recruitment has been observed as early as 3–7 days (Noah et al., 2020; Wojciak and Crossan, 1993). Analysis of CD4+ T cells showed peak presence in mouse Achilles tendons two weeks after injury and repair, while CD8+ T cells continued to accumulate at four weeks (Noah et al., 2020). In rats, CD4+ T cells were elevated in the flexor tendon synovial sheath and epitenon three days post crush injury (Wojciak and Crossan, 1993). The mechanical loading environment may also be a regulator of T cell recruitment as Botox-induced paralysis after tendon transection resulted in absence of regulatory T cells by 10 days post-injury compared to loaded samples (Blomgran et al., 2016). Similar to animal injury models, T cells are also elevated in human tendinopathic tissues, suggesting a role in disease progression (Kragsnaes et al., 2014; Millar et al., 2010; Schubert et al., 2005). Intriguingly, recent studies using in vitro co-culture systems revealed a positive inflammatory feedback loop between tenocytes and T cells, although T cell subtype was not determined (Garcia-Melchor et al., 2021). On the other hand, other reports surprisingly concluded that T cell numbers are insignificant in injured and control human tendons (Gotoh et al., 1997; Scott et al., 2008).
The accumulation of T cells with tendon injury and disease suggests a role in healing, but there are few mechanistic studies that directly test the requirement of T cells or T cell subpopulations. It was suggested that excessive recruitment of T cells to injured tendons might lead to extracellular matrix damage, which occurs in autoimmune disorders (Jomaa et al., 2020). In contrast, cell culture studies showed that CD4+ T cells and T cell-derived cytokines such as IL2, TGFβ, and IL1 regulate epitenon cell proliferation, adhesion, and extracellular matrix production (Wòjciak and Crossan, 1994). Since CD4+ T cells were not characterized in this study and potentially comprise both pro-inflammatory and anti-inflammatory subpopulations, it is not clear which T cells are driving these responses. While these studies suggest a pathological role for T cells in tendon healing, we recently showed that regulatory T cells (Tregs) are required for tendon regeneration in neonatal mice as depletion of Tregs resulted in poor structural and functional healing. In contrast to adult Tregs, neonatal Tregs facilitated regeneration in part by polarizing macrophages from a pro- to anti-inflammatory profile (Arvind et al., 2021). Adoptive transfer of neonatal Tregs into adult hosts resulted in improved adult macrophage polarization leading to functional recovery. Indeed, different injury models have also shown that IL33, which promotes Treg expansion, has a protective effect in a variety of tissues, though it can be pathological as well (Li et al., 2019; Liew et al., 2016).
B cells
B cells are characterized by the production of immunoglobulins (Ig) either in a transmembrane form (B cell receptors) or secreted form (antibodies), following activation with either a T cell-dependent or independent mechanism. Mature B cells can exist in the form of plasma cells or memory cells. Plasma cells actively produce antibodies when they encounter an antigen, while memory cells are “stored” for future antigen encounters. When this occurs, they convert to plasma cells and quickly start producing antibodies against the foreign molecule.
To date, there are almost no studies on B cells in tendon research. Despite a handful of studies showing B cell accumulation in some animal models of tendon injury and human tendon disease tissues, their function in healing remains completely unknown (Noah et al., 2020; Schubert et al., 2005). In other tissues such as skin, B cell subsets can drive or suppress inflammation and interact with T cells, while application of mature B cells enhances regenerative healing (Debes and McGettigan, 2019). Additional studies will be required to determine temporal dynamics of B cells in tendon healing, as well as mechanistic function (if any).
Natural Killer cells
Though natural killer (NK) cells are part of the lymphoid lineage, they do not have antigen specific receptors. Rather, NK cells have inhibitory receptors whose main function is to mediate killing of cells that have downregulated MHC-I proteins on their surface. This is evolutionarily advantageous since viruses often reduce the production of MHC-I in infected cells (Chaplin, 2010). Though NK cells have been found in chronically inflamed human Achilles tendons, the role of these cells in inflammation and tendon healing has not been determined (Kragsnaes et al., 2014).
Discussion
The immune response is a critical driver of tendon healing and pathology, however, the immune cells that promote and modulate inflammation in these contexts are poorly characterized. While much of the existing research focused on macrophages given their importance in inflammation, there are a vast array of other immune cell types that likely also play important and distinctive roles. One challenge in reconciling different studies is the variability in animal injury models (in terms of injury severity, anatomical tendon targeted, and species) as well as immune cell markers and methodology used (flow cytometry compared to immunohistochemistry for example), which may result in different temporal dynamics reported or conflicting interpretations. In terms of clinical samples, there are additional confounding factors such as painful symptoms that may not be necessarily correlated with structural hallmarks of degeneration. The studies by Dakin et al. clearly show that distinctive immune cells and activated immune pathways can distinguish patients experiencing pain (Dakin et al., 2015).
In addition to immune modulation, specific immune cells may also directly interact with resident tendon cell types such as tenocytes, epitenon cells, or resident stem/progenitor cells). In other tissues, such as muscle, regeneration depends in part on secreted factors from T cells that directly activate muscle satellite cells (Kuswanto et al., 2016). The interactions between immune cells and resident cells have largely focused on immune regulation (such as the pro-inflammatory feedback loop between the cells that may drive a degenerative cascade reported by (Garcia-Melchor et al., 2021), but immune cells may also be the source of tenogenic growth factors such as TGFβ ligands, which have been implicated in both fibrotic and regenerative tendon healing (Kaji et al., 2020; Katzel et al., 2011). Resident cell types may also respond to the same immune signal in different ways. Inflammation for example, may induce proliferation of scar-forming cells while inhibiting resident stem cells or inducing aberrant differentiation.
Finally, while this review focused on individual immune cell types and known findings for each cell type in tendon healing, the immune landscape is likely driven by complex interactions between multiple immune cell populations that change across time. The growing use of sophisticated technologies such as single cell RNA sequencing will allow interrogation of multiple immune cell populations at once.
Acknowledgments
This work was supported by funding from NIH/NIAMS awards R01 AR069537 and R56 AR076984 to AHH.
References
- Alim MA, Ackermann PW, Eliasson P, Blomgran P, Kristiansson P, Pejler G, Peterson M (2017) Increased mast cell degranulation and co-localization of mast cells with the NMDA receptor-1 during healing after Achilles tendon rupture. Cell Tissue Res 370: 451–460. [DOI] [PubMed] [Google Scholar]
- Alim MA, Peterson M, Pejler G (2020) Do Mast Cells Have a Role in Tendon Healing and Inflammation? Cells 9: 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andarawis-Puri N, Sereysky JB, Jepsen KJ, Flatow EL (2012a) The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J Biomech 45: 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andarawis-Puri N, Sereysky JB, Sun HB, Jepsen KJ, Flatow EL (2012b) Molecular response of the patellar tendon to fatigue loading explained in the context of the initial induced damage and number of fatigue loading cycles. J Orthop Res 30: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andia I, Martin JI, Maffulli N (2018) Advances with platelet rich plasma therapies for tendon regeneration. Expert Opin Biol Ther 18: 389–398. [DOI] [PubMed] [Google Scholar]
- Ansorge HL, Adams S, Birk DE, Soslowsky LJ (2011) Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann Biomed Eng 39: 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvind V, Howell K, Huang AH (2021) Reprogramming adult tendon healing using regenerative neonatal regulatory T cells. bioRxiv: 2021.2005.2012.443424 [Google Scholar]
- Arvind V, Huang AH (2021) Reparative and Maladaptive Inflammation in Tendon Healing. Front Bioeng Biotechnol 9: 719047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670. [DOI] [PubMed] [Google Scholar]
- Behzad H, Sharma A, Mousavizadeh R, Lu A, Scott A (2013) Mast cells exert pro-inflammatory effects of relevance to the pathophyisology of tendinopathy. Arthritis Res Ther 15: R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ME, Hildebrand KA, Zhang M, Hart DA, Wiig ME (2010) Neuropeptide, mast cell, and myofibroblast expression after rabbit deep flexor tendon repair. J Hand Surg Am 35: 1842–1849. [DOI] [PubMed] [Google Scholar]
- Bernhardsson M, Dietrich-Zagonel F, Tatting L, Eliasson P, Aspenberg P (2019) Depletion of cytotoxic (CD8+) T cells impairs implant fixation in rat cancellous bone. J Orthop Res 37: 805–811. [DOI] [PubMed] [Google Scholar]
- Best KT, Korcari A, Mora KE, Nichols AE, Muscat SN, Knapp E, Buckley MR, Loiselle AE (2021) Scleraxis-lineage cell depletion improves tendon healing and disrupts adult tendon homeostasis. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best KT, Lee FK, Knapp E, Awad HA, Loiselle AE (2019) Deletion of NFKB1 enhances canonical NF-κB signaling and increases macrophage and myofibroblast content during tendon healing. Scientific Reports 9: 10926-10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best KT, Loiselle AE (2019) Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. FASEB J 33: 8578–8587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco ST, Moser HL, Galatz LM, Huang AH (2019) Biologics and stem cell-based therapies for rotator cuff repair. Ann N Y Acad Sci 1442: 35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biton J, Khaleghparast Athari S, Thiolat A, Santinon F, Lemeiter D, Hervé R, Delavallée L, Levescot A, Roga S, Decker P, Girard JP, Herbelin A, Boissier MC, Bessis N (2016) In Vivo Expansion of Activated Foxp3+ Regulatory T Cells and Establishment of a Type 2 Immune Response upon IL-33 Treatment Protect against Experimental Arthritis. J Immunol 197: 1708–1719. [DOI] [PubMed] [Google Scholar]
- Blomgran P, Blomgran R, Ernerudh J, Aspenberg P (2016) A possible link between loading, inflammation and healing: Immune cell populations during tendon healing in the rat. Sci Rep 6: 29824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgran P, Hammerman M, Aspenberg P (2017) Systemic corticosteroids improve tendon healing when given after the early inflammatory phase. Sci Rep 7: 12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozec A, Zaiss MM, Kagwiria R, Voll R, Rauh M, Chen Z, Mueller-Schmucker S, Kroczek RA, Heinzerling L, Moser M, Mellor AL, David JP, Schett G (2014) T cell costimulation molecules CD80/86 inhibit osteoclast differentiation by inducing the IDO/tryptophan pathway. Sci Transl Med 6: 235ra260. [DOI] [PubMed] [Google Scholar]
- Buckley MR, Evans EB, Matuszewski PE, Chen YL, Satchel LN, Elliott DM, Soslowsky LJ, Dodge GR (2013) Distributions of types I, II and III collagen by region in the human supraspinatus tendon. Connect Tissue Res 54: 374–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, Mathis D (2013) A special population of regulatory T cells potentiates muscle repair. Cell 155: 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain CS, Clements AEB, Kink JA, Choi U, Baer GS, Halanski MA, Hematti P, Vanderby R (2019) Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem Cells 37: 652–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin DD (2010) Overview of the immune response. The Journal of allergy and clinical immunology 125: S3–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Dong SW, Liu JP, Tao X, Tang KL, Xu JZ (2012) Synergy of tendon stem cells and platelet-rich plasma in tendon healing. J Orthop Res 30: 991–997. [DOI] [PubMed] [Google Scholar]
- Cho J, Kuswanto W, Benoist C, Mathis D (2019) T cell receptor specificity drives accumulation of a reparative population of regulatory T cells within acutely injured skeletal muscle. Proceedings of the National Academy of Sciences 116: 26727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JL, Kiss ZS, Ptasznik R, Malliaras P (2005) Is vascularity more evident after exercise? Implications for tendon imaging. AJR Am J Roentgenol 185: 1138–1140. [DOI] [PubMed] [Google Scholar]
- Crowe LAN, McLean M, Kitson SM, Melchor EG, Patommel K, Cao HM, Reilly JH, Leach WJ, Rooney BP, Spencer SJ, Mullen M, Chambers M, Murrell GAC, McInnes IB, Akbar M, Millar NL (2019) S100A8 & S100A9: Alarmin mediated inflammation in tendinopathy. Sci Rep 9: 1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Addona A, Maffulli N, Formisano S, Rosa D (2017) Inflammation in tendinopathy. Surgeon 15: 297–302. [DOI] [PubMed] [Google Scholar]
- Dakin SG, Martinez FO, Yapp C, Wells G, Oppermann U, Dean BJ, Smith RD, Wheway K, Watkins B, Roche L, Carr AJ (2015) Inflammation activation and resolution in human tendon disease. Sci Transl Med 7: 311ra173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Chinoy H, Dick J, Matthews R, Roberts M (2017) A Literature Review of Eosinophilic Fasciitis with an Illustrative Case. Curr Rheumatol Rev 13: 113–120. [DOI] [PubMed] [Google Scholar]
- de Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ (2012) Patellar tendon healing with platelet-rich plasma: a prospective randomized controlled trial. Am J Sports Med 40: 1282–1288. [DOI] [PubMed] [Google Scholar]
- de la Durantaye M, Piette AB, van Rooijen N, Frenette J (2014) Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J Orthop Res 32: 279–285. [DOI] [PubMed] [Google Scholar]
- De Micheli AJ, Swanson JB, Disser NP, Martinez LM, Walker NR, Oliver DJ, Cosgrove BD, Mendias CL (2020) Single-cell transcriptomic analysis identifies extensive heterogeneity in the cellular composition of mouse Achilles tendons. Am J Physiol Cell Physiol 319: C885–C894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos RJ, Weir A, van Schie HT, Bierma-Zeinstra SM, Verhaar JA, Weinans H, Tol JL (2010) Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA 303: 144–149. [DOI] [PubMed] [Google Scholar]
- Debes GF, McGettigan SE (2019) Skin-Associated B Cells in Health and Inflammation. J Immunol 202: 1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Buono A, Battery L, Denaro V, Maccauro G, Maffulli N (2011) Tendinopathy and inflammation: some truths. Int J Immunopathol Pharmacol 24: 45–50. [DOI] [PubMed] [Google Scholar]
- Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW (2014) Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS One 9: e96113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DA (1946) The blood supply and lymphatic drainage of tendons. J Anat 80: 147–152. [PubMed] [Google Scholar]
- Farnqvist K, Pearson S, Malliaras P (2020) Adaptation of Tendon Structure and Function in Tendinopathy With Exercise and Its Relationship to Clinical Outcome. J Sport Rehabil 29: 107–115. [DOI] [PubMed] [Google Scholar]
- Fenwick SA, Hazleman BL, Riley GP (2002) The vasculature and its role in the damaged and healing tendon. Arthritis Res 4: 252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa D, Figueroa F, Calvo R (2016) Patellar Tendinopathy: Diagnosis and Treatment. J Am Acad Orthop Surg 24: e184–e192. [DOI] [PubMed] [Google Scholar]
- Franchi M, Trire A, Quaranta M, Orsini E, Ottani V (2007) Collagen structure of tendon relates to function. ScientificWorldJournal 7: 404–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart FX, Levi-Schaffer F (2002) Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy 32: 237–246. [DOI] [PubMed] [Google Scholar]
- Garcia-Melchor E, Cafaro G, MacDonald L, Crowe LAN, Sood S, McLean M, Fazzi UG, McInnes IB, Akbar M, Millar NL (2021) Novel self-amplificatory loop between T cells and tenocytes as a driver of chronicity in tendon disease. Ann Rheum Dis 80: 1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout C, Bilodeau R, Van Rooijen N, Bouchard P, Frenette J (2010) Transient neutropenia increases macrophage accumulation and cell proliferation but does not improve repair following intratendinous rupture of Achilles tendon. J Orthop Res 28: 1084–1091. [DOI] [PubMed] [Google Scholar]
- Göhring J, Kellner F, Schrangl L, Platzer R, Klotzsch E, Stockinger H, Huppa JB, Schütz GJ (2021) Temporal analysis of T-cell receptor-imposed forces via quantitative single molecule FRET measurements. Nature Communications 12: 2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh M, Hamada K, Yamakawa H, Tomonaga A, Inoue A, Fukuda H (1997) Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J Orthop Res 15: 33–39. [DOI] [PubMed] [Google Scholar]
- Grinstein M, Dingwall HL, O’Connor LD, Zou K, Capellini TD, Galloway JL (2019) A distinct transition from cell growth to physiological homeostasis in the tendon. Elife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio JP, Phan AC, Ruehlmann DG, Noah AC, Mendias CL (2014) Synergist ablation induces rapid tendon growth through the synthesis of a neotendon matrix. J Appl Physiol (1985) 117: 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumucio JP, Schonk MM, Kharaz YA, Comerford E, Mendias CL (2020) Scleraxis is required for the growth of adult tendons in response to mechanical loading. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyarmati J Jr., Mandi B, Fachet J, Varga S, Sikula J (1983) Alterations of the connective tissue in nude mice. Thymus 5: 383–392. [PubMed] [Google Scholar]
- Halpern BC, Chaudhury S, Rodeo SA (2012) The role of platelet-rich plasma in inducing musculoskeletal tissue healing. HSS J 8: 137–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey T, Flamenco S, Fan CM (2019) A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat Cell Biol 21: 1490–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz M, Vignery A, Gershon RK, Baron R (1984) Thymus-derived lymphocytes and their interactions with macrophages are required for the production of osteoclast-activating factor in the mouse. Proc Natl Acad Sci U S A 81: 2181–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, Andarawis-Puri N, Huang AH (2017) Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci Rep 7: 45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell KL, Kaji DA, Li TM, Montero A, Yeoh K, Nasser P, Huang AH (2021) Macrophage depletion impairs neonatal tendon regeneration. FASEB J 35: e21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubzick CV, Randolph GJ, Henson PM (2017) Monocyte differentiation and antigen-presenting functions. Nature Reviews Immunology 17: 349–362. [DOI] [PubMed] [Google Scholar]
- Jin W, Tamzalit F, Chaudhuri PK, Black CT, Huse M, Kam LC (2019) T cell activation and immune synapse organization respond to the microscale mechanics of structured surfaces. Proceedings of the National Academy of Sciences 116: 19835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomaa G, Kwan CK, Fu SC, Ling SK, Chan KM, Yung PS, Rolf C (2020) A systematic review of inflammatory cells and markers in human tendinopathy. BMC Musculoskelet Disord 21: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji DA, Howell KL, Balic Z, Hubmacher D, Huang AH (2020) Tgfβ signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. Elife 9: e51779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, Schultze JL (2019) Human Monocyte Subsets and Phenotypes in Major Chronic Inflammatory Diseases. Front Immunol 10: 2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastelic J, Galeski A, Baer E (1978) The multicomposite structure of tendon. Connect Tissue Res 6: 11–23. [DOI] [PubMed] [Google Scholar]
- Katzel EB, Wolenski M, Loiselle AE, Basile P, Flick LM, Langstein HN, Hilton MJ, Awad HA, Hammert WC, O’Keefe RJ (2011) Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res 29: 684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA (2005) Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res 23: 1425–1432. [DOI] [PubMed] [Google Scholar]
- Kendal AR, Layton T, Al-Mossawi H, Appleton L, Dakin S, Brown R, Loizou C, Rogers M, Sharp R, Carr A (2020) Multi-omic single cell analysis resolves novel stromal cell populations in healthy and diseased human tendon. Sci Rep 10: 13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragsnaes MS, Fredberg U, Stribolt K, Kjaer SG, Bendix K, Ellingsen T (2014) Stereological quantification of immune-competent cells in baseline biopsy specimens from achilles tendons: results from patients with chronic tendinopathy followed for more than 4 years. Am J Sports Med 42: 2435–2445. [DOI] [PubMed] [Google Scholar]
- Krystel-Whittemore M, Dileepan KN, Wood JG (2016) Mast Cell: A Multi-Functional Master Cell. Frontiers in Immunology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kawai T, Akira S (2011) Pathogen recognition by the innate immune system. Int Rev Immunol 30: 16–34. [DOI] [PubMed] [Google Scholar]
- Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, Mathis D (2016) Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 44: 355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C, Spitzer G, Gehwolf R, Wagner A, Weissenbacher N, Deininger C, Emmanuel K, Wichlas F, Tempfer H, Traweger A (2019) Tenophages: a novel macrophage-like tendon cell population expressing CX3CL1 and CX3CR1. Dis Model Mech 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xia N, Wen S, Li D, Lu Y, Gu M, Tang T, Jiao J, Lv B, Nie S, Liao M, Liao Y, Yang X, Hu Y, Shi GP, Cheng X (2019) IL (Interleukin)-33 Suppresses Abdominal Aortic Aneurysm by Enhancing Regulatory T-Cell Expansion and Activity. Arterioscler Thromb Vasc Biol 39: 446–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Toraldo G, Li A, Yang X, Zhang H, Qian WP, Weitzmann MN (2007) B cells and T cells are critical for the preservation of bone homeostasis and attainment of peak bone mass in vivo. Blood 109: 3839–3848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Girard JP, Turnquist HR (2016) Interleukin-33 in health and disease. Nat Rev Immunol 16: 676–689. [DOI] [PubMed] [Google Scholar]
- Longo UG, Ronga M, Maffulli N (2009) Achilles Tendinopathy. Sports Medicine and Arthroscopy Review 17: 112–126. [DOI] [PubMed] [Google Scholar]
- Lozano PF, Scholze M, Babian C, Scheidt H, Vielmuth F, Waschke J, Ondruschka B, Hammer N (2019) Water-content related alterations in macro and micro scale tendon biomechanics. Scientific Reports 9: 7887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology 23: 549–555. [DOI] [PubMed] [Google Scholar]
- Markworth JF, Sugg KB, Sarver DC, Maddipati KR, Brooks SV (2021) Local shifts in inflammatory and resolving lipid mediators in response to tendon overuse. FASEB J 35: e21655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall JS, Warrington R, Watson W, Kim HL (2018) An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol 14: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsolais D, Cote CH, Frenette J (2001) Neutrophils and macrophages accumulate sequentially following Achilles tendon injury. J Orthop Res 19: 1203–1209. [DOI] [PubMed] [Google Scholar]
- McWhorter FY, Davis CT, Liu WF (2015) Physical and mechanical regulation of macrophage phenotype and function. Cellular and molecular life sciences : CMLS 72: 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Steinman RM (2001) Dendritic cells: specialized and regulated antigen processing machines. Cell 106: 255–258. [DOI] [PubMed] [Google Scholar]
- Millar NL, Hueber AJ, Reilly JH, Xu Y, Fazzi UG, Murrell GA, McInnes IB (2010) Inflammation is present in early human tendinopathy. Am J Sports Med 38: 2085–2091. [DOI] [PubMed] [Google Scholar]
- Millar NL, Murrell GA, McInnes IB (2017) Inflammatory mechanisms in tendinopathy - towards translation. Nat Rev Rheumatol 13: 110–122. [DOI] [PubMed] [Google Scholar]
- Millar NL, Silbernagel KG, Thorborg K, Kirwan PD, Galatz LM, Abrams GD, Murrell GAC, McInnes IB, Rodeo SA (2021) Tendinopathy. Nature Reviews Disease Primers 7: 1. [DOI] [PubMed] [Google Scholar]
- Mosca MJ, Rashid MS, Snelling SJ, Kirtley S, Carr AJ, Dakin SG (2018) Trends in the theory that inflammation plays a causal role in tendinopathy: a systematic review and quantitative analysis of published reviews. BMJ Open Sport & Exercise Medicine 4: e000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege J-L, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA (2014) Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41: 14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols AEC, Best KT, Loiselle AE (2019) The cellular basis of fibrotic tendon healing: challenges and opportunities. Transl Res 209: 156–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio H, Saita Y, Kobayashi Y, Takaku T, Fukusato S, Uchino S, Wakayama T, Ikeda H, Kaneko K (2020) Platelet-rich plasma promotes recruitment of macrophages in the process of tendon healing. Regen Ther 14: 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah AC, Li TM, Martinez LM, Wada S, Swanson JB, Disser NP, Sugg KB, Rodeo SA, Lu TT, Mendias CL (2020) Adaptive and innate immune cell responses in tendons and lymph nodes after tendon injury and repair. J Appl Physiol (1985) 128: 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingel J, Wienecke J, Kongsgaard M, Behzdad H, Abraham T, Langberg H, Scott A (2013) Increased mast cell numbers in a calcaneal tendon overuse model. Scand J Med Sci Sports 23: e353–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin AL, Mumm JB, Murphy E, Turner S, Yu N, McClanahan TK, Bourne PA, Pierce RH, Kastelein R, Pflanz S (2010) IL-33 induces IL-13-dependent cutaneous fibrosis. J Immunol 184: 1526–1535. [DOI] [PubMed] [Google Scholar]
- Ribitsch I, Bileck A, Aldoshin AD, Kandula MM, Mayer RL, Egerbacher M, Gabner S, Auer U, Gultekin S, Huber J, Kreil DP, Gerner C, Jenner F (2021) Molecular Mechanisms of Fetal Tendon Regeneration Versus Adult Fibrous Repair. Int J Mol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SH, Starborg T, Lu Y, Humphries SM, Meadows RS, Kadler KE (2007) Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell-cell junctions. Mol Cell Biol 27: 6218–6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio E, Moseley L, Purdam C, Samiric T, Kidgell D, Pearce AJ, Jaberzadeh S, Cook J (2014) The pain of tendinopathy: physiological or pathophysiological? Sports Med 44: 9–23. [DOI] [PubMed] [Google Scholar]
- Roh JS, Sohn DH (2018) Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune network 18: e27–e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales C (2020) Neutrophils at the crossroads of innate and adaptive immunity. J Leukoc Biol 108: 377–396. [DOI] [PubMed] [Google Scholar]
- Rosenberg HF, Dyer KD, Foster PS (2013) Eosinophils: changing perspectives in health and disease. Nature Reviews Immunology 13: 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert TE, Weidler C, Lerch K, Hofstädter F, Straub RH (2005) Achilles tendinosis is associated with sprouting of substance P positive nerve fibres. Ann Rheum Dis 64: 1083–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A, Lian O, Bahr R, Hart DA, Duronio V, Khan KM (2008) Increased mast cell numbers in human patellar tendinosis: correlation with symptom duration and vascular hyperplasia. Br J Sports Med 42: 753–757. [DOI] [PubMed] [Google Scholar]
- Sharma A, Abraham T, Sampaio A, Cowan M, Underhill M, Scott A (2011) Sodium cromolyn reduces expression of CTGF, ADAMTS1, and TIMP3 and modulates post-injury patellar tendon morphology. J Orthop Res 29: 678–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. The Journal of Clinical Investigation 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solchaga LA, Bendele A, Shah V, Snel LB, Kestler HK, Dines JS, Hee CK (2014) Comparison of the effect of intra-tendon applications of recombinant human platelet-derived growth factor-BB, platelet-rich plasma, steroids in a rat achilles tendon collagenase model. J Orthop Res 32: 145–150. [DOI] [PubMed] [Google Scholar]
- Starkey PM, Barrett AJ, Burleigh MC (1977) The degradation of articular collagen by neutrophil proteinases. Biochim Biophys Acta 483: 386–397. [DOI] [PubMed] [Google Scholar]
- Staverosky JA, Pryce BA, Watson SS, Schweitzer R (2009) Tubulin polymerization-promoting protein family member 3, Tppp3, is a specific marker of the differentiating tendon sheath and synovial joints. Dev Dyn 238: 685–692. [DOI] [PubMed] [Google Scholar]
- Stolk M, Klatte-Schulz F, Schmock A, Minkwitz S, Wildemann B, Seifert M (2017) New insights into tenocyte-immune cell interplay in an in vitro model of inflammation. Scientific Reports 7: 9801-9801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremmel C, Schuchert R, Wagner F, Thaler R, Weinberger T, Pick R, Mass E, Ishikawa-Ankerhold HC, Margraf A, Hutter S, Vagnozzi R, Klapproth S, Frampton J, Yona S, Scheiermann C, Molkentin JD, Jeschke U, Moser M, Sperandio M, Massberg S, Geissmann F, Schulz C (2018) Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nature Communications 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunwoo JY, Eliasberg CD, Carballo CB, Rodeo SA (2020) The role of the macrophage in tendinopathy and tendon healing. J Orthop Res 38: 1666–1675. [DOI] [PubMed] [Google Scholar]
- Szczesny SE, Elliott DM (2014) Interfibrillar shear stress is the loading mechanism of collagen fibrils in tendon. Acta Biomater 10: 2582–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczesny SE, Fetchko KL, Dodge GR, Elliott DM (2017) Evidence that interfibrillar load transfer in tendon is supported by small diameter fibrils and not extrafibrillar tissue components. J Orthop Res 35: 2127–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SH, Al-Youha S, Van Agtmael T, Lu Y, Wong J, McGrouther DA, Kadler KE (2011) Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One 6: e16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon JN, Italiano JE (2012) Platelets: production, morphology and ultrastructure. Handb Exp Pharmacol: 3–22. [DOI] [PubMed] [Google Scholar]
- van der Meijden PEJ, Heemskerk JWM (2019) Platelet biology and functions: new concepts and clinical perspectives. Nature Reviews Cardiology 16: 166–179. [DOI] [PubMed] [Google Scholar]
- Virchenko O, Aspenberg P (2006) How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta Orthop 77: 806–812. [DOI] [PubMed] [Google Scholar]
- Weber F (2008) Innate Immunity: Introduction. Encyclopedia of Virology: 111–117. [Google Scholar]
- Wojciak B, Crossan JF (1993) The accumulation of inflammatory cells in synovial sheath and epitenon during adhesion formation in healing rat flexor tendons. Clin Exp Immunol 93: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wòjciak B, Crossan JF (1994) The effects of T cells and their products on in vitro healing of epitenon cell microwounds. Immunology 83: 93–98. [PMC free article] [PubMed] [Google Scholar]
- Zagai U, Skold CM, Trulson A, Venge P, Lundahl J (2004) The effect of eosinophils on collagen gel contraction and implications for tissue remodelling. Clin Exp Immunol 135: 427–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Goncalves R, Mosser DM (2008) The isolation and characterization of murine macrophages. Current protocols in immunology Chapter 14: Unit-14.11. [DOI] [PMC free article] [PubMed]


