Abstract
Portulaca oleracea Linn. (P. oleracea L.) has recently gained attention as a functional food due to the chemical composition of this plant regarding bioactive compounds. The special attention to the use of P. oleracea as an ingredient in functional food products is also due to the promotion of sustainable food. It is an unconventional food plant, and its consumption may contribute to preserving biodiversity due to its cultivation in a polyculture system. Food sovereignty may be achieved, among other strategies, with the consumption of unconventional food plants that are more resistant in nature and easily cultivated in small places. P. oleracea grows spontaneously and may be found in streets and sidewalks, or it may be cultivated with seeds and cuttings propagation. The culinary versatility of P. oleracea opens up opportunities to explore the development of sustainable, functional food products. This mini-review shows that functional food products developed from P. oleracea are already available at the research level, but it is expected that more scientific literature focusing on the development of P. oleracea functional products with proven anticancer activities may be released in the near future. Polysaccharides, some phenolic compounds, alkaloids, and cerebrosides are associated with the inhibition and prevention of carcinogenesis through in vitro and in vivo investigations. The anticancer activities of P. oleracea, its bioactive compounds, and the involved molecular mechanisms have been reported in the literature. The importance of further elucidating the cancer inhibition mechanisms is in the interest of forthcoming applications in the development of food products with anticancer properties for implementation in the human diet.
1. Introduction
The common purslane (P. oleracea L) is a herbaceous succulent annual plant from the Portulacaceae family, native to the Middle East and India [1, 2]. It may be found on roadsides, gardens, and cultivated areas in the tropical and subtropical regions [3, 4]. There are various cultivars of P. oleracea distributed worldwide, mainly with morphological differences, with the common purslane having green-red stems, obovate leaves, yellow flowers, and single-layered petals, while the ornamental purslane produces flowers of different colors [1]. The stems and leaves have a slightly acid and salty taste and are usually consumed in salads, soups, and stews [5, 6]. It is an edible plant in regions of European, Mediterranean, African, and Asia countries and Australia [6]. In Brazil, P. oleracea is known as an “unconventional food plant”, a term referring to plants that are not part of the usual consumption of most of the population in a particular region, country, or even the planet because basic food is very homogeneous, with the use of few food species [7].
P. oleracea has a high nutritional value and many antioxidant properties due to its phenolic compound and omega-3 fatty acid abundance, particularly α-linolenic acid. It is well-known in traditional Chinese medicine [2]. for its use in diuretic, febrifuge, antiseptic, antispasmodic, and vermifuge treatments [8]. Among its various pharmacological properties are its anti-inflammatory [9], antioxidative [10], renoprotective [11], neuroprotective [12], hepatoprotective [13], and muscle-relaxing effects [14].
Anticarcinogenic activities have been reported for P. oleracea. Investigations were carried out to screen the activities for antihepatocellular carcinoma [15, 16], colon cancer [17], glioblastoma multiforme [18], ovarian cancer [19] sarcoma [20], lung cancer [16], anti-cervical [21], gastric cancer [22], and pancreatic cancer [23]. P. oleracea contains bioactive compounds with antioxidant properties, act on metastasis and invasion, modulate the immune system, and inhibit tumor formation [19, 24, 25, 4].
Thus, this mini-review aimed to assemble the anti-cancer effects of bioactive compounds of P. oleracea, demonstrating the molecular mechanisms and the potential for the development of functional food products with anticancer properties.
2. The Nutritional Value and Bioactive Compounds of P. Oleracea
Proximate analyses of P. oleracea components including leaves, seeds, stems, buds, and flowers, have been performed. Ash, fiber, protein, and fat approximate contents of P. oleracea leaves as 20.56%, 36.27%, 12.82%, and 3.75%, respectively, are found on a dry matter basis [26]. P. oleracea also contains minerals in its leaves with concentration values approximate such as potassium (3710 mg/100 g of dry matter), calcium (2390 mg/100 g), nitrogen (2170 mg/100 g), magnesium (580 mg/100 g), phosphorus (350 mg/100 g), sulfur (200 mg/100 g), iron (32.4 mg/100 g), manganese (5.8 mg/100 g), boron (2.8 mg/100 g), zinc (2 mg/100 g), and copper (1.1 mg/100 g) [26]. This study showed higher levels of potassium, calcium, magnesium, phosphorus, and iron when compared to those of spinach (336 mg/100 g of dry matter, 98 mg/100 g, 82 mg/100 g, 25 mg/100 g, and 0.4 mg/100 g, respectively) [27].
P. oleracea contains high amounts of Omega-3 fatty acids, as discussed by Siriamornpun and Suttajit [28] that found higher levels of Omega-3 fatty acids in fresh leaves, with 523.146 ± 2.29 mg/100 g, while, for stems and flowers, the authors reported 148.87 ± 3.30 mg/100 g and 216.17 ± 1.16 mg/100 g, respectively. Other plants (analysis of leaves in dry matter) contain lower levels of Omega-3 fatty acids than P. oleracea, such as mint (194.9 mg/100 g), watercress (179.6 mg/100 g), spinach (129.2 mg/100 g), parsley (124.8 mg/100 g), and broccoli (110.3 mg/100 g) (analysis of leaves in dry matter) [29]. Omega-3 fatty acids may have pharmacological effects such as anti-hyperlipidemic, antimicrobial, anti-inflamatory, neuroprotective and nephroprotective activities [3, 30, 31, 32, 11]. P. oleracea also contains high levels of tocopherols, vitamin A, β-carotene and ascorbic acid [3, 32–34]. Antimicrobial and antioxidant activities were related to these compounds [3, 33].
High concentrations of oxalic acid have also been detected in P. oleracea. The intake of oxalic acid provided by the diet with P. oleracea may form complexes with minerals such as calcium and iron (insoluble salts) or sodium, magnesium, and potassium (soluble salts), reducing their bioavailability and possibly leading to the development of kidney stones through the formation of calcium oxalate crystals [35]. Thus, consumption of P. oleracea should be moderated by individuals with a propensity to develop kidney stones. Amounts of 23.45 ± 0.45 g, 5.58 ± 0.18 g, and 9.09 ± 0.12 g of total oxalates per kilogram of fresh weight oxalates were obtained in fresh leaves, stems, and buds, respectively, with 75.0% being soluble oxalates in the stems and buds, and only 27.5% in the leaves [36]. The authors reported a 66.7% reduction (p < 0.001) of soluble oxalates after cooking the leaves for a short time, discarding the water, and pickling them with white vinegar [36]. Some other bioactive compounds from secondary metabolism of P. oleracea such as flavonoids, alkaloids, terpenoids and their pharmacological activity can be seen in Table 1.
Table 1.
Some classes of bioactive compounds from secondary metabolism of P. oleracea and their pharmacological activity.
| Compounds | Plant structure | Form (fresh or dry) | Pharmacological activity | References |
|---|---|---|---|---|
| Flavonoids | Aerial part | Dry | Antifertility | [37] |
| Aerial part | Dry | Antimicrobial | [38] | |
| Leaves | Fresh | Antioxidant | [39] | |
| Seeds | Dry | Antidiabetic | [40] | |
|
| ||||
| Polyphenols | Leaf, steam and flower | Dry | Antioxidant | [41] |
| Whole plant | Fresh | Antimutagenic | [42] | |
|
| ||||
| Phenolic acids | Aerial parts | Dry | Antioxidant | [43] |
| Alkaloids | Aerial part | Dry | Anticancer | [44] |
| Whole plant | Fresh | Anti-inflamatory | [45] | |
| Whole plant | Dry | Antioxidant | [46] | |
|
| ||||
| Terpenes | Whole plant | Dry | Hepatoprotective, antibacterial and antifungal | [47] |
| Aerial part | Dry | Anti-hypoxia | [48] | |
Flavonoids (a class of phenolic compounds) in P. oleracea were associated with anti-fertility, antimicrobial, antioxidant and antidiabetic effects [37–40]. Combined effects of polyunsaturated fatty acids, flavonoids and polysaccharides on hypoglycaemic, hypolipidaemic and insulin resistance reducer effects through ingestion of P. oleracea seeds in clinical test with humans were observed [40]. Other phenolic compounds (Polyphenols and phenolic acids) in P. oleracea have antioxidant and antimutagenic effects [41–43].
Other bioactive compounds with pharmacological importance in P. oleracea are alkaloids and terpenes. Anticancer, anti-inflamatory and antioxidant effects were described for alkaloids found in this plant while hepatoprotective, antibacterial, antifungal and anti-hypoxia effects were described for terpenes of P. oleracea [44–48].
3. Potential Antioxidant of the P. Oleracea
This plant is rich in antioxidants such as vitamin A, tocopherols, ascorbic acid, beta-carotene, and phenolic compounds [33, 49]. Beta-carotene was found in P. oleracea with content ranging from 21 μg/g to 30 μg/g of fresh mass in leaves and 3.6 μg/g to 6.5 μg/g of fresh mass in stems [50]. The antioxidant potential was measured at different growth stages (15, 30, 45, and 60 days) of aerial parts of P. oleracea [49]. The total phenolic content (TPC) for the young shoots at 15 days was significantly lower than at 30, 45, and 60 days, while the ascorbic acid content (AAC) did not show a significant decrease from the developing to the mature stage. According to the study, the IC50 value of 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity ranged from 1.30 ± 0.04 mg/ml (60 days) to 1.71 ± 0.04 mg/ml (15 days), while the ascorbic acid equivalent antioxidant content (AEAC) values ranged from 229.5 ± 7.9 mg AA/100 g (15 days) to 319.3 ± 8.7 mg AA/100 g (60 days), the TPC varied from 174.5 ± 8.5 mg GAE/100 g (15 days) to 348.5 ± 7.9 mg GAE/100 g (60 days), the AAC varied from 60.5 ± 2.1 mg/100 g (60 days) to 86.5 ± 3.9 mg/100 g (15 days), and the ferric reducing antioxidant power (FRAP) ranged from 1.8 ± 0.1 mg GAE/g (15 days) to 4.3 ± 0.1 mg GAE/g (60 days). Thus, mature plants (60 days) of P. oleracea had higher TPC and antioxidant activities than immature plants.
The dry weights of the samples (leaves, flowers, and stems) from two different locations were investigated for potential antioxidant activity by Silva and Carvalho [41], who found that stems had a higher total phenolic content and total antioxidant activity than the flowers and leaves. The oil from seeds, leaves, and stems of P. oleracea were analyzed and found that the peroxide value was significantly higher for seed oil and the lowest for stem oil [51]. Furthermore, the highest ascorbic acid content was found for P. oleracea seed oil (41.67%), followed by leaf oil (32.29%), and the highest DPPH was obtained for leaf oil (12.55%), followed by seed oil (2.05%). Values for lettuce (IC50 = 17.07 mg/ml), artichoke (IC50 = 18.14 mg/ml), turmeric (IC50 = 21.14 mg/ml), spinach (IC50 = 22.87 mg/ml), and escarole (IC50 = 32.2 mg/ml) were reported by Tiveron et al. [52], showing that P. oleracea presents the lowest IC50 necessary to reduce 50% of DPPH free radicals.
4. Functional Food Products and P. Oleracea
The P. oleracea plant may be used as an ingredient in functional food products due to its nutritional value and bioactive compounds that will be incorporated into the formulations.
The use of the P. oleracea plant as food may not only enhance the nutrients and bioactive composition of functional products but also influence their sensory and technological characteristics. Although it is well-known that sensory acceptance by consumers is essential for a product's commercial success on the market, few studies in the literature have reported the application of P. oleracea in products and its performance or the sensory profile of such products.
Regarding the technological aspect, the incorporation of the durum wheat flour with 5% of P. oleracea to bread resulted in the improvement of the rheological characteristics, an increase in antioxidant properties, and a decrease in the Omega-6-to-Omega-3 ratio, which is beneficial for human health, in addition to improving the sensorial quality [53].
The durum wheat spaghetti fortified with 10% of P. oleracea, a potential functional food, was appreciated by consumers. It showed a high concentration of α-linolenic acids (Omega-3), total phenolic compounds, and antioxidant properties, so that, considering 100 g of pasta per day, it is possible to obtain 75 mg of essential linoleic acid and 9 mg of linolenic acid, along with a four-fold increase in total phenolic compounds [54]. The Omega-3 fatty acids can also inhibit carcinogenesis and slow tumor growth, as demonstrated by in vitro, in vivo, and clinical investigations [55].
The analysis of bread incorporated with four different concentrations of P. oleracea powder (0%, 5%, 10%, and 15%) showed increasing water absorption capacity, stability under the mixer, and softening levels as the P. oleracea powder concentration in the samples increased. The protein, fat, total ash, moisture, and fiber contents also increased along with the P. oleracea concentrations [56]. However, the bread with 15% of P. oleracea powder showed a decreased farinograph quality number and presented the lowest scores for sensory properties and color, taste, texture, and overall liking. The optimized formulation containing 10% of P. oleracea powder had the highest acceptance.
P. oleracea has also been used to produce powder mixtures with two other plant species, Amaranthus hybridus L. and Chenopodium berlandieri L. The powder mixtures containing P. oleracea showed more significant contents of phenolic compounds, with an increase in the antioxidant activity [57].
Another innovative functional product assessed was a fermented P. oleracea juice added with a selected lactic acid bacteria. Results demonstrated an increase in total antioxidants, preserved vitamin C, A, and E levels, and increased contents of vitamin B2 and phenolic compounds. In addition, decreased levels of pro-inflammatory mediators and reactive oxygen species were observed, with a consequent increase in the restorative characteristics of the use of P. oleracea juice for intestinal inflammation and epithelial injury [58].
The combination of yogurt or coconut plant extract or coconut cream with fresh leaves of P. oleracea reduced the overall oxalate content by simple dilution. The soluble oxalate content decreased from 53.0% to 10.7% when P. oleracea leaves were added to yogurt. However, the coconut plant extract and coconut cream had no effect on the percentage of soluble oxalate content but provided the mixture with an acceptable flavor [59].
The addition of fresh purslane leaves (ranging from 1% to 10%, w/w) to tomato sauces resulted in a decrease of total soluble solids from 9.57°Bx to 9.20°Bx, beneficially impacting sugar reduction. On the other hand, the amount of protein significantly increased from 0.12% to 1.83% from the lowest to the highest concentrations, respectively [60].
5. Bioactive Compounds of P. Oleracea on Anticancer Activity
P. oleracea presents phytochemicals and nutrients associated with anticarcinogenic properties. The 12% reduction in the activity of the mutagenic nitrosation mixture may be attributed to the ascorbic acid (vitamin C), α and β-carotene, chlorophyll, and polyphenols of the P. oleracea extract obtained through a standard juice extractor [42].
Phenolic compounds such as kaempferol and apigenin from a hydroethanolic extract of P. oleracea have effects in vitro against human glioma cells, and homoisoflavonoids showed in vitro selective cytotoxic activity for SF-268, NCI-H460, and SGC-7901 cell lines, as shown Table 2 [18, 61].
Table 2.
Bioactive compounds of P. oleracea, types of extracts, and molecular mechanisms for cancer inhibition.
| Experimental model | Compounds | Types of extract | Types of cancer inhibited | Mechanisms and results | References | |
|---|---|---|---|---|---|---|
| In vitro | In vivo | |||||
| Rats | Polysaccharides | Aqueous extract | Ovarian | Scavenge superoxide anion, (DPPH-), nitric oxide, and hydroxyl radicals Inhibit RBC hemolysis Spleen, thymocyte, T and B lymphocyte proliferation |
[19] | |
| Human cancer cell lines SF-268, NCI-H460, K-562, SGC-7901, and SMMC-7721 | Homoisoflavonoids | Hydroalcoholic extract | Homoisoflavonoids showed in vitro cytotoxic activities towards four human cancer cell lines | [61] | ||
| Treatment of HeLa cell | Mice | Polysaccharides | Aqueous extract | Cervical | Sub-G1 phase cell cycle arrest, triggering DNA damage Inducing apoptosis |
[21] |
| Mice | Polysaccharides | Aqueous extract | Inhibit the growth of transplantable sarcoma 180 Increase in the number of white blood cells (WBC) and CD4+ T-lymphocytes Increase in the CD4+/CD8+ ratio |
[20] | ||
| Rats | Polysaccharides | Aqueous extract | Gastric | Interleukin-2 (IL-2), interleukin-4 (IL-4), and tumor necrosis factor-alpha (TNF-α) was enhanced Provide dose-dependent protection against MNNG-induced oxidative injury by enhancing SOD, CAT, GSH-Px |
[22] | |
|
| ||||||
| Human lung (K562 and A549) and breast (MCF-7 and MDA-MB-435) cancer cell lines | Alkaloids | Hydroalcoholic extract | Lung Breast |
Moderate cytotoxic activities against A549 and weak cytotoxic activities against K562. The compounds showed low cytotoxic activity against MCF-7 and MDA-MB-435 cells. | [54] | |
| Human hepatocellular carcinoma cells | Seed alcoholic extract | Hepatocellular | Significantly reduced the cell viability of HepG2. | [15] | ||
| The uterine cervical carcinoma (U14) cell line | Polysaccharides | Aqueous extract | Cervical | Upregulated the expression of CD80, CD86, CD83 Increase in IL-12, TLR-4, Decrease in IL-10 |
[29] | |
| Human HL60 cell line | Portulacerebroside A | Aqueous extract | Leukemia | Mitochondrial membrane potential ROS accumulated Increase in RNA expressions and protein levels of Bax/Bcl-2, caspase-3, and caspase-9 ERK1/2, JNK1/2 and p38 MAPK pathway were blocked |
[49] | |
| HepG2 and A-549 cell lines | Seed oil | Liver Lung |
Significant cytotoxicity and inhibition of growth of the liver cancer (HepG2) and lung cancer (A-549) cell lines | [20] | ||
| Human liver cancer HCCLM3 cells | Portulacerebroside A | Aqueous extract | Liver | Increase in RNA and protein expression levels of TIMP-2 and nm23-H1 Inhibition of the mRNA expression of MTA1, MMP-2, and MMP-9 Suppression of the protein expression of MTA1, RhoA, Rac1/Cdc42, MMP-2, but not RhoC and MMP-9 |
[23] | |
| Cervical cancer HeLa cells, esophageal cancer Eca-109 cells and breast cancer MCF-7 cells | Seed oil | Cervical Esophageal Breast |
Stronger inhibitory effect on the proliferation of MCF-7 cells and significantly inhibited the proliferation of HeLa cells and Eca-109 cells | [51] | ||
| PANC-1 cancer cell line | Aqueous extract | Pancreatic | Significant effect on apoptosis in pancreatic cell line and high expression of P53 and reduction of CDK gene expression | [23] | ||
| Human colon adenocarcinoma (HCT-15) and normal (Vero) cell line | Chloroform extract | Colon adenocarcinoma | Chloroform extract does not have cytotoxic activity and was not safe to normal Vero cell line. | [67] | ||
| Colon cancer cells (HT-29) and HT-29 cancer stem cells | Ethyl alcohol extract | Colon Stem cells |
Inhibited the proliferation of both HT-29 cancer cells and HT-29 cancer stem cells Significantly decreased the expression of the Notch1 and β-catenin genes in both cell types |
[52] | ||
| The human cervical cancer HeLa cells. | Polysaccharides | Aqueous extract | Cervical | Decrease HeLa cell proliferation Upregulate Bax level and downregulate Bcl-2 level in a concentration-dependent manner Inhibit the protein expression levels of TLR4, MyD88, TRAF6, AP-1 and NF-κB subunit P65 Reduce the production of cytokine/chemokine |
[50] | |
| The mouse cervical carcinoma U14 cells | Polysaccharides | Aqueous extract | Intestinal | Dendritic cell (DC) apoptosis in U14-bearing mice Increase intestinal DC survival Stimulate the TLR4-PI3K/AKT-NF-κB signaling pathway |
[48] | |
| Human glioblastoma cancer cell line (U-87) | Hydroethanolic extract | Cytotoxicity and apoptogenic effects Anti-NF-κB activity along with two upstream ROS and NO mechanisms |
[17] | |||
Polysaccharides from P. oleracea act on free radicals through the antioxidant mechanism, modulating the immune system, which may be preventive and therapeutic in rat ovarian and gastric cancer and mouse cervical cancer and sarcomas, as shown Table 2 [19–22, 62].
Another bioactivity from P. oleracea is portulacacerebrosie A, a cerebroside compound that suppresses the invasion and metastasis of liver cancer HCCLM3 cells and acts in leucocythemia treatment are show in Table 2 [24, 63].
Polysaccharides showed activity against ovarian cancer by inhibiting the red blood cell (RBC) hemolysis in the spleen, thymocyte, and T and B lymphocyte proliferation [19]. These compounds also act against cervical cancer through Sub-G1 phase cell cycle arrest triggering DNA damage, inhibit the growth of transplantable sarcoma 180, increase the number of white blood cells (WBC), CD4+ T-, the CD4+/CD8+ ratio, IL-12, and TLR-4, decrease IL-10 and HeLa cell proliferation, reduce the production of cytokine/chemokine and the expression levels of CD80, CD86, CD83, Bax, and downregulate the Bcl-2 level in a concentration-dependent manner. In addition, polysaccharides inhibit the protein expression levels of TLR4, myeloid differentiation primary response 88 (MyD88), TNF receptor associated Factor 6 (TRAF6), activator protein-1 (AP-1), and factor nuclear kappa B (NF-κB) subunit P65 [20, 21, 63, 64].
In gastric cancer, interleukins (IL-2 and IL-4) and TNF-α were enhanced by polysaccharides that also provide dose-dependent protection against N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) induced oxidative injury by enhancing Superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH-Px) [22]. In addition to acting against ovarian, gastric, and cervical cancer, polysaccharides also work against intestinal cancer by stimulating the TLR4-PI3K/AKT-NF-κB signaling pathway and Anti-NF-κB activity along with two upstream ROS and NO mechanisms [18, 62], showing the importance of studying these molecules in P. oleracea matrices.
The cerebroside compound, Portulacerebroside A, affects leukemia and cervical, liver, esophageal, breast, and colon cancer and cancer stem cells [16, 24, 63, 65, 66]. Some mechanisms involved with Portulacerebroside A have increased RNA expressions and protein levels of Bax/Bcl-2, caspase-3, and caspase-9, protein expression levels of TIMP-2 and nm23-H1, inhibition of the mRNA expression of MTA1, MMP-2, and MMP-9, RhoA, Rac1/Cdc42, MMP-2, and downregulation of the expression of the Notch1 and β-catenin genes.
Alkaloids inhibited lung and breast cancer through moderate cytotoxic activities against A549, weak cytotoxic activities against K562, and low cytotoxic activity against MCF-7 and MDA-MB-435 cells.
Some possible mechanisms of P. oleracea for anticancer activity are represented in Figure 1. The bioactivity of P. oleracea and the potential to develop new products from this underused plant in some regions deserve attention regarding its valorization as a functional food and its pharmacological properties. Different anticancer mechanisms of P. oleracea were explored and reported in this review. Aqueous extracts, seed oil, and hydroethanolic extracts present cytotoxicity to cancer cell lines while chloroform extract does not have cytotoxic activity [67]. Further studies will be needed to determine anticancer activity in particular food matrices and beverages.
Figure 1.
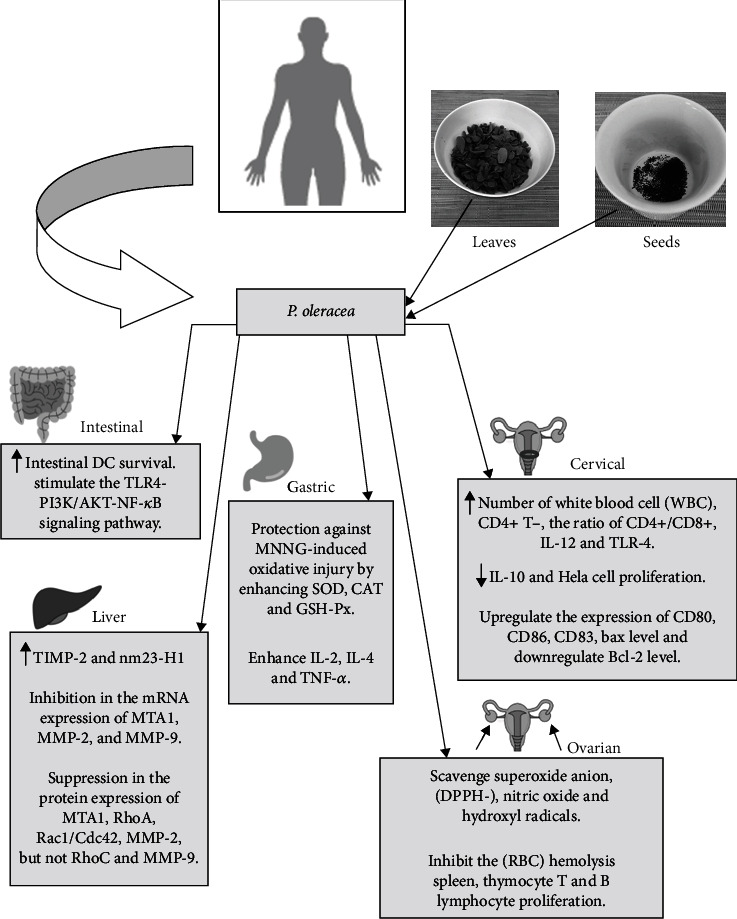
Some possible mechanisms of P. oleracea for anticancer activity.
6. Conclusion
The P. oleracea plant may be promising for developing and innovating potential functional food products. The high levels of antioxidants such as phenolic compounds, carotenoids, and other nutrients such as minerals and Omega-3 fatty acids are supported by functional food studies. Research has indicated the anticancer activity of P. oleracea extracts. Polysaccharides, some phenolic compounds, alkaloids, and cerebrosides detected in P. oleracea and contained in aqueous extracts, seed oil, and hydroethanolic extracts are associated with inhibition and prevention of carcinogenesis. However, more studies are needed to prove the anticancer activity of food products containing P. oleracea as an ingredient to promote health benefits to the consumers.
Acknowledgments
This project was funded by the Brazilian National Council for Scientific and Technological Development (142514/2020-9) and the Coordination for the Improvement of Higher Education Personnel.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- 1.Alam M., Juraimi A. S., Rafii M. Y., et al. Genetic improvement of purslane (Portulaca oleracea L.) and its future prospects. Molecular Biology Reports . 2014;41(11):7395–7411. doi: 10.1007/s11033-014-3628-1. [DOI] [PubMed] [Google Scholar]
- 2.Iranshahy M., Javadi M., Iranshahi M., et al. A review of traditional uses, phytochemistry and pharmacology of _Portulaca oleracea_ L. Journal of Ethnopharmacology . 2017;205:158–172. doi: 10.1016/j.jep.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Uddin M. K., Juraimi A. S., Hossain M. S., Nahar M. A. U., Ali M. E., Rahman M. M. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. The Scientific World Journal . 2014;2014:6. doi: 10.1155/2014/951019.951019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahimi V. B., Ajam F., Rakhshandeh H., Askari V. R. A pharmacological review onPortulaca oleraceaL.: focusing on anti-inflammatory, anti- oxidant, immuno-modulatory and antitumor activities. Korean Pharmacopuncture Institute . 2019;22(1):7–15. doi: 10.3831/KPI.2019.22.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim Y. Y., Quah E. P. L. Antioxidant properties of different cultivars of _Portulaca oleracea_. Food Chemistry . 2007;103(3):734–740. doi: 10.1016/j.foodchem.2006.09.025. [DOI] [Google Scholar]
- 6.Nemzer B., Al-Taher F., Abshiru N. Phytochemical composition and nutritional value of different plant parts in two cultivated and wild purslane (_Portulaca oleracea_ L.) genotypes. Food Chemistry . 2020;320, article 126621 doi: 10.1016/j.foodchem.2020.126621. [DOI] [PubMed] [Google Scholar]
- 7.Kinnup V. F., Lorenzi H. Plantas Alimentícias Não-Convencionais (PANC) no Brasil . São Paulo: Instituto Plantarum de Estudos da Flora; 2014. [Google Scholar]
- 8.Xiang L., Dongming X., Wang W., Wang R., Ding Y., Du L. Alkaloids from _Portulaca oleracea_ L. Phytochemistry . 2005;66(21):2595–2601. doi: 10.1016/j.phytochem.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Chan K., Islam M. W., Kamil M., et al. The analgesic and anti-inflammatory effects of _Portulaca oleracea_ L. subsp. _sativa_ (Haw.) Celak. Journal of Ethnopharmacology . 2000;73(3):445–451. doi: 10.1016/S0378-8741(00)00318-4. [DOI] [PubMed] [Google Scholar]
- 10.Dkhil M. A., Moniem A. E. A., Al-Quraishy S., Saleh R. A. Antioxidant effect of purslane (Portulaca oleracea) and its mechanism of action. Journal of Medicinal Plants Research . 2011;5(9):1589–1593. [Google Scholar]
- 11.Hozayen W., Bastawy M., Elshafeey H. Effects of aqueous purslane (Portulaca Oleracea) extract and fish oil on gentamicin nephrotoxicity in albino rats. Nature and Science . 2011;9(2):47–62. [Google Scholar]
- 12.Wang C. Q., Yang G. Q. Betacyanins from _Portulaca oleracea_ L. ameliorate cognition deficits and attenuate oxidative damage induced by D-galactose in the brains of senescent mice. Phytomedicine . 2010;17(7):527–532. doi: 10.1016/j.phymed.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Eidi A., Mortazavi P., Moghadam J. Z., Mardani M. Hepatoprotective effects ofPortulaca oleraceaextract against CCl4-induced damage in rats. Pharmaceutical Biology . 2015;53(7):1042–1051. doi: 10.3109/13880209.2014.957783. [DOI] [PubMed] [Google Scholar]
- 14.Gonnella M., Charfeddine M., Conversa G., Santamaria P. Purslane: a review of its potential for health and agricultural aspects. European Journal of Plant Science and Biotechnology . 2010;4(1):131–136. [Google Scholar]
- 15.Farshori N. N., Al-Sheddi E. S. S., Al-Oqail M. M., Musarrat J., Al-Khedhairy A. A., Siddiqui M. A. Cytotoxicity assessments of Portulaca oleracea and Petroselinum sativum seed extracts on human hepatocellular carcinoma cells (HepG2) Asian Pacific Journal of Cancer Prevention . 2014;15(16):6633–6638. doi: 10.7314/APJCP.2014.15.16.6633. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sheddi E. S., Farshori N. N., Al-Oqail M. M., Musarrat J., Al-Khedhairy A. A., Siddiqui M. A. Portulaca oleracea seed oil exerts cytotoxic effects on human liver cancer (HepG2) and human lung cancer (A-549) cell lines. Asian Pacific Journal of Cancer Prevention . 2015;16(8):3383–3387. doi: 10.7314/APJCP.2015.16.8.3383. [DOI] [PubMed] [Google Scholar]
- 17.Asnani G. P., Kokare C. R. In vitro and in vivo evaluation of colon cancer targeted epichlorohydrin crosslinked Portulaca-alginate beads. Biomolecular Concepts . 2018;9(1):190–199. doi: 10.1515/bmc-2018-0019. [DOI] [PubMed] [Google Scholar]
- 18.Rahimi V. B., Mousavi S. H., Haghghi S., Soheili-Far S., Askari V. R. Cytotoxicity and apoptogenic properties of the standardized extract of Portulaca oleracea on glioblastoma multiforme cancer cell line (U-87): a mechanistic study. EXCLI Journal . 2019;18:165–186. doi: 10.17179/excli2019-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youguo C., Zongji S., XiaoPing C. Evaluation of free radicals scavenging and immunity-modulatory activities of Purslane polysaccharides. International Journal of Biological Macromolecules . 2009;45(5):448–452. doi: 10.1016/j.ijbiomac.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Shen H., Tangg G., Zeng G., et al. Purification and characterization of an antitumor polysaccharide from Portulaca oleracea L. Carbohydrate Polymers . 2013;93(2):395–400. doi: 10.1016/j.carbpol.2012.11.107. [DOI] [PubMed] [Google Scholar]
- 21.Zhao R., Gao X., Cai Y., et al. Antitumor activity of _Portulaca oleracea_ L. polysaccharides against cervical carcinoma in vitro and in vivo. Carbohydrate Polymers . 2013;96(2):376–383. doi: 10.1016/j.carbpol.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Hu Y., Shi S., Jiang L. Evaluation of antioxidant and immuno-enhancing activities of Purslane polysaccharides in gastric cancer rats. International Journal of Biological Macromolecules . 2014;68:113–116. doi: 10.1016/j.ijbiomac.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 23.Alipour S., Pishkar L., Chaleshi V. Cytotoxic effect of Portulaca Oleracea extract on the regulation of CDK1 and P53 gene expression in pancreatic cancer cell line. Nutrition and Cancer . 2022;74(5):1792–1801. doi: 10.1080/01635581.2021.1960386. [DOI] [PubMed] [Google Scholar]
- 24.Ji Q., Zheng G. Y., Xia W., et al. Inhibition of invasion and metastasis of human liver cancer HCCLM3 cells by portulacerebroside a. Pharmaceutical Biology . 2015;53(5):773–780. doi: 10.3109/13880209.2014.941505. [DOI] [PubMed] [Google Scholar]
- 25.Askari V. R., Rezaee S. A. R., Abnous K., Iranshahi M., Boskabady M. H. The influence of hydro-ethanolic extract of _Portulaca oleracea_ L. on Th1/Th2 balance in isolated human lymphocytes. Journal of Ethnopharmacology . 2016;194:1112–1121. doi: 10.1016/j.jep.2016.10.082. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira D. C. S., Wobeto C., Zanuzo M. R., Severgnini C. Composição mineral e teor de ácido ascórbico nas folhas de quatro espécies olerícolas não-convencionais. Horticultura Brasileira . 2013;31(3):472–475. doi: 10.1590/S0102-05362013000300021. [DOI] [Google Scholar]
- 27.NEPA/UNICAMP. Tabela Brasileira de Composição de Alimentos . Campinas: NEPA/UNICAMP; 2011. [Google Scholar]
- 28.Siriamornpun S., Suttajit M. Microchemical components and antioxidant activity of different morphological parts of Thai wild purslane (Portulaca oleracea) Weed Science . 2010;58(3):182–188. doi: 10.1614/WS-D-09-00073.1. [DOI] [Google Scholar]
- 29.Pereira C., Li D., Sinclair A. J. The alpha-linolenic acid content of green vegetables commonly available in Australia. International Journal for Vitamin and Nutrition Research . 2001;71(4):223–228. doi: 10.1024/0300-9831.71.4.223. [DOI] [PubMed] [Google Scholar]
- 30.Simpopoulos A. P. Omega-3 fatty acids and antioxidants in edible wild plants. Biological Research . 2004;37(2):263–277. doi: 10.4067/S0716-97602004000200013. [DOI] [PubMed] [Google Scholar]
- 31.Naem F., Khan S. H. Purslane (Portulaca oleracea L.) as phytogenic substance—a review. Journal of Herbs, Spices and Medicinal Plants . 2013;19(3):216–232. doi: 10.1080/10496475.2013.782381. [DOI] [Google Scholar]
- 32.Zhao R., Zhang T., Zhao H., Cai Y. Effects ofPortulaca oleracea L.Polysaccharides on phenotypic and functional maturation of murine bone marrow derived dendritic cells. Nutrition and Cancer . 2015;67(6):987–993. doi: 10.1080/01635581.2015.1060352. [DOI] [PubMed] [Google Scholar]
- 33.Petropoulos S., Karkanis A., Martins N., Ferreira I. C. F. R. Phytochemical composition and bioactive compounds of common purslane (_Portulaca oleracea_ L.) as affected by crop management practices. Trends in Food Science & Technology . 2016;55:1–10. doi: 10.1016/j.tifs.2016.06.010. [DOI] [Google Scholar]
- 34.Viana M. M. S., Carlos L. A., Silva E. C., Pereira S. M. F., Oliveira D. B., Assis M. L. V. Composição fitoquímica e potencial antioxidante de hortaliças não convencionais. Horticultura Brasileira . 2015;33(4):504–509. doi: 10.1590/S0102-053620150000400016. [DOI] [Google Scholar]
- 35.Palaniswamy U. R., Bible B., McAvoy R. J. OXALIC acid concentrations in purslane (Portulaca oleraceae L.) is altered by the stage of harvest and the nitrate to ammonium ratios in hydroponics. Acta Horticulturae . 2004;629(629):299–305. doi: 10.17660/ActaHortic.2004.629.38. [DOI] [Google Scholar]
- 36.Poeydomenge G. Y., Savage G. P. Oxalate content of raw and cooked purslane. Journal of Food, Agriculture and Environment . 2007;5(1):124–128. [Google Scholar]
- 37.Nayaka H. B., Londonkar R. L., Umesh M. K., Tukappa A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. International Journal of Bacteriology . 2014;2014:8. doi: 10.1155/2014/175851.175851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Du Y.-K., Jing L., Li X.-M., et al. Flavonoids extract from Portulaca oleracea L. induce Staphylococcus aureus death by apoptosislike pathway. International Journal of Food Properties . 2017;20(1):534–542. [Google Scholar]
- 39.Scari V., Loizzo M. R., Tundis R., Mincione A., Pellicano T. M. Portulaca oleracea L. (purslane) extracts display antioxidant and hypoglycaemic effects. Journal of Applied Botany and Food Quality . 2018;91:39–46. [Google Scholar]
- 40.El-Sayed M.-I. K. Effects of _Portulaca oleracea_ L. seeds in treatment of type-2 diabetes mellitus patients as adjunctive and alternative therapy. Journal of Ethnopharmacology . 2011;137(1):643–651. doi: 10.1016/j.jep.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 41.Silva R., Carvalho I. S. In vitro antioxidant activity, phenolic compounds and protective effect against DNA damage provided by leaves, stems and flowers of Portulaca oleracea (purslane) Natural Product Communications . 2014;9(1):45–50. doi: 10.1177/1934578X1400900115. [DOI] [PubMed] [Google Scholar]
- 42.Caballero-Salazar S., Riverón-Negrete R., Ordáz-Tellez M. G., Abdullaev F., Espinosa-Aguirre J. J. Evaluation of the antimutagenic activity of different vegetable extracts using an In Vitro screening test. Proceedings of the Western Pharmacology Society . 2022;45:101–103. [PubMed] [Google Scholar]
- 43.Erkan N. Antioxidant activity and phenolic compounds of fractions from _Portulaca oleracea_ L. Food Chemistry . 2012;133(3):775–781. doi: 10.1016/j.foodchem.2012.01.091. [DOI] [Google Scholar]
- 44.Tian J. L., Liang X., Gao P. Y., et al. Two new alkaloids fromPortulaca oleraceaand their cytotoxic activities. Journal of Asian Natural Products Research . 2014;16(3):259–264. doi: 10.1080/10286020.2013.866948. [DOI] [PubMed] [Google Scholar]
- 45.Meng Y., Ying Z., Xiang Z., et al. The anti-inflammation and pharmacokinetics of a novel alkaloid fromPortulaca oleraceaL. Journal of Pharmacy and Pharmacology . 2016;68(3):397–405. doi: 10.1111/jphp.12526. [DOI] [PubMed] [Google Scholar]
- 46.Yang Z., Liu C., Xiang L., Zheng Y. Phenolic alkaloids as a new class of antioxidants in Portulaca oleracea. Phytotherapy Research . 2009;23(7):1032–1035. doi: 10.1002/ptr.2742. [DOI] [PubMed] [Google Scholar]
- 47.Elkhayat E. S., Ibrahim S. R. M., Aziz M. A. Portulene, a new diterpene fromPortulaca oleraceaL. Journal of Asian Natural Products Research . 2008;10(11):1039–1043. doi: 10.1080/10286020802320590. [DOI] [PubMed] [Google Scholar]
- 48.Chen C.-J., Wang W.-Y., Wang X.-L., et al. Anti-hypoxic activity of the ethanol extract from Portulaca oleracea in mice. Journal of Ethnopharmacology . 2009;124:246–250. doi: 10.1016/j.jep.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 49.Uddin M. K., Juraimi A. S., Ali M. E., Ismail M. R. Evaluation of antioxidant properties and mineral composition of purslane (Portulaca oleracea L.) at different growth stages. International Journal of Molecular Sciences . 2012;13(8):10257–10267. doi: 10.3390/ijms130810257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu L., Howe P., Zhou Y. F., Xu Z. Q., Hocart C., Zhang R. Fatty acids and β-carotene in Australian purslane (_Portulaca oleracea_) varieties. Journal of Chromatography A . 2000;893(1):207–213. doi: 10.1016/S0021-9673(00)00747-0. [DOI] [PubMed] [Google Scholar]
- 51.Desta M., Molla A., Yusuf Z. Characterization of physico-chemical properties and antioxidant activity of oil from seed, leaf and stem of purslane (Portulaca oleracea L.) Biotechnology Reports . 2020;27:1–5. doi: 10.1016/j.btre.2020.e00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiveron A. P., Melo P. S., Bergamaschi K. B., Vieira T. M. F. S., Regitano-d’Arce M. A. B., Alencar S. M. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. International Journal of Molecular Sciences . 2012;13(7):8943–8957. doi: 10.3390/ijms13078943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Melilli M. G., Stefano V. D., Sciacca F., et al. Improvement of fatty acid profile in durum wheat breads supplemented with Portulaca oleracea L. quality traits of purslane-fortified bread. Food . 2020;9(6) doi: 10.3390/foods9060764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melilli M. G., Pagliaro A., Scandurra S., Gentile C., di Stefano V. Omega-3 rich foods: durum wheat spaghetti fortified with _Portulaca oleracea_. Food Bioscience . 2020;37, article 100730 doi: 10.1016/j.fbio.2020.100730. [DOI] [Google Scholar]
- 55.Lee J. Y., Sim T. B., Lee J. E., Na H. K. Chemopreventive and chemotherapeutic effects of fish oil derived omega-3 polyunsaturated fatty acids on colon carcinogenesis. Clinical Nutrition Research . 2017;6(3):147–160. doi: 10.7762/cnr.2017.6.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delvarianzadeh L. N., Nafchi M., Ebrahimi H. Physicochemical, rheological, and sensory evaluation of voluminous breads enriched by purslane (Portulaca oleracea L.) Italian Journal of Food Science . 2020;32:815–830. [Google Scholar]
- 57.Santiago-Saenz Y. O., López-Palestina C. U., Gutiérrez-Tlahque J., Monroy-Torres R., Pinedo-Espinoza J. M., Hernández-Fuentes A. D. Nutritional and functional evaluation of three powder mixtures based on mexican quelites: alternative ingredients to formulate food supplements. Food Science and Technology . 2020;40(4):1029–1037. doi: 10.1590/fst.28419. [DOI] [Google Scholar]
- 58.Di Cagno R., Filannino P., Vincentini O., Cantatore V., Cavoski I., Gobbetti M. Fermented portulaca oleracea L. juice: a novel functional beverage with potential ameliorating effects on the intestinal inflammation and epithelial injury. Nutrients . 2019;11(2):p. 248. doi: 10.3390/nu11020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moreau A. G., Savage G. P. Oxalate content of purslane leaves and the effect of combining them with yoghurt or coconut products. Journal of Food Composition and Analysis . 2009;22(4):303–306. doi: 10.1016/j.jfca.2009.01.013. [DOI] [Google Scholar]
- 60.Apostol L. C., Ropciuc S., Prisacaru A. E., Albu E. haracterization of tomato sauce enriched with purslane (Portulaca oleracea) leaves. Journal of Hygienic Engineering and Design . 2020;31:127–132. [Google Scholar]
- 61.Yan J., Sun L. R., Zhou Z. Y., et al. Homoisoflavonoids from the medicinal plant _Portulaca oleracea_. Phytochemistry . 2012;80:37–41. doi: 10.1016/j.phytochem.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Zhao R., Shao X., Jia G., et al. Anti-cervical carcinoma effect of Portulaca oleracea L. polysaccharides by oral administration on intestinal dendritic cells. BMC Complementary and Alternative Medicine . 2019;19(1):1–10. doi: 10.1186/s12906-019-2582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ye Q., Zhang N., Chen K., Zhu J., Jiang H. Effects of portulacerebroside a on apoptosis of human leukemia HL60 cells and p38/JNK signaling pathway. International Journal of Clinical and Experimental Pathology . 2015;8(11):13968–13977. [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao R., Zhang T., Ma B., Li X. Antitumor activity ofPortulaca OleraceaL. polysaccharide on HeLa cells through inducing TLR4/NF-κB signaling. Nutrition and Cancer . 2017;69(1):131–139. doi: 10.1080/01635581.2017.1248294. [DOI] [PubMed] [Google Scholar]
- 65.Guo G., Yue L., Fan S., Jing S., Yan L. H. Antioxidant and antiproliferative activities of purslane seed oil. Journal of Hypertension . 2016;5(2):01–19. doi: 10.4172/2167-1095.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin H., Chen L., Wang S., Chao D. Portulaca oleraceaextract can inhibit nodule formation of colon cancer stem cells by regulating gene expression of the notch signal transduction pathway. Tumor Biology . 2017;39(7):101042831770869–101042831770869. doi: 10.1177/1010428317708699. [DOI] [PubMed] [Google Scholar]
- 67.Mali P. Y. Assessment of cytotoxicity of Portulaca oleracea Linn. Against human colon adenocarcinoma and vero cell line. Ayu . 2015;36(4):432–436. doi: 10.4103/0974-8520.190691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


