Abstract
Astrocytes are the most abundant glial cell in the central nervous system and occupy a wide range of roles that are essential for brain function. Over the last few years, evidence has emerged that astrocytes exhibit cellular and molecular heterogeneity, raising the possibility that subsets of astrocyte are functionally distinct and that transcriptional mechanisms are involved in encoding this prospective diversity. In this review, we will focus on three emerging areas of astrocyte biology: i) region-specific circuit regulation, ii) molecular diversity and iii) transcriptional regulation. This review highlights our nascent understanding of how molecular diversity is converted to functional diversity of astrocytes through the lens of brain region specific circuits. We will articulate our understanding of how transcriptional mechanisms regulate this diversity and key areas that need further exploration to achieve the overarching goal of a functional taxonomy of astrocytes in the brain.
Introduction
The brain is comprised of a seemingly infinite ensemble of neuronal circuits responsible for integrating and processing sensory information into consequential behavioral outputs. In many ways, the human experience reflects the summation of brain circuit activity. While synaptic connections between neurons drives circuit function, they do not act in isolation. Coordinating neuronal function into circuits and higher-level neural networks requires a specialized cellular infrastructure to support these complex, yet delicate facets of brain function. Glial cells are estimated to comprise at least half of the cellular constituency of the adult brain and occupy these support roles, subserving a wide range of neuronal functions (Allen 2014; Khakh and Deneen 2019; Volterra and Meldolesi 2005). There are two types of central nervous system (CNS)-derived glia in the adult brain: oligodendrocytes and astrocytes. Astrocytes are the most abundant type of glial cell in the brain and play a variety of roles to support neuronal functions from neurotransmission and synapse formation to metabolic support and maintenance of the blood brain barrier (Allen 2014). Anatomically, astrocytes exhibit a highly complex morphology characterized by an elaborate network of branches, processes, and leaflets that are in close proximity to neuronal synapses (Figure 1). Indeed, it has been estimated that a single astrocyte can contact up to 100,00 synapses in CA1 of the hippocampus, though these numbers may vary across brain regions (BushongMartoneJones and Ellisman 2002). This proximity to neuronal synapses, coupled with their key roles in synaptic function, has led astrocyte processes to be included as the third component of the synapse (Araque and others 2014).
Figure 1. Astrocyte Exhibit Complex Morphology and Proximity to Neurons.
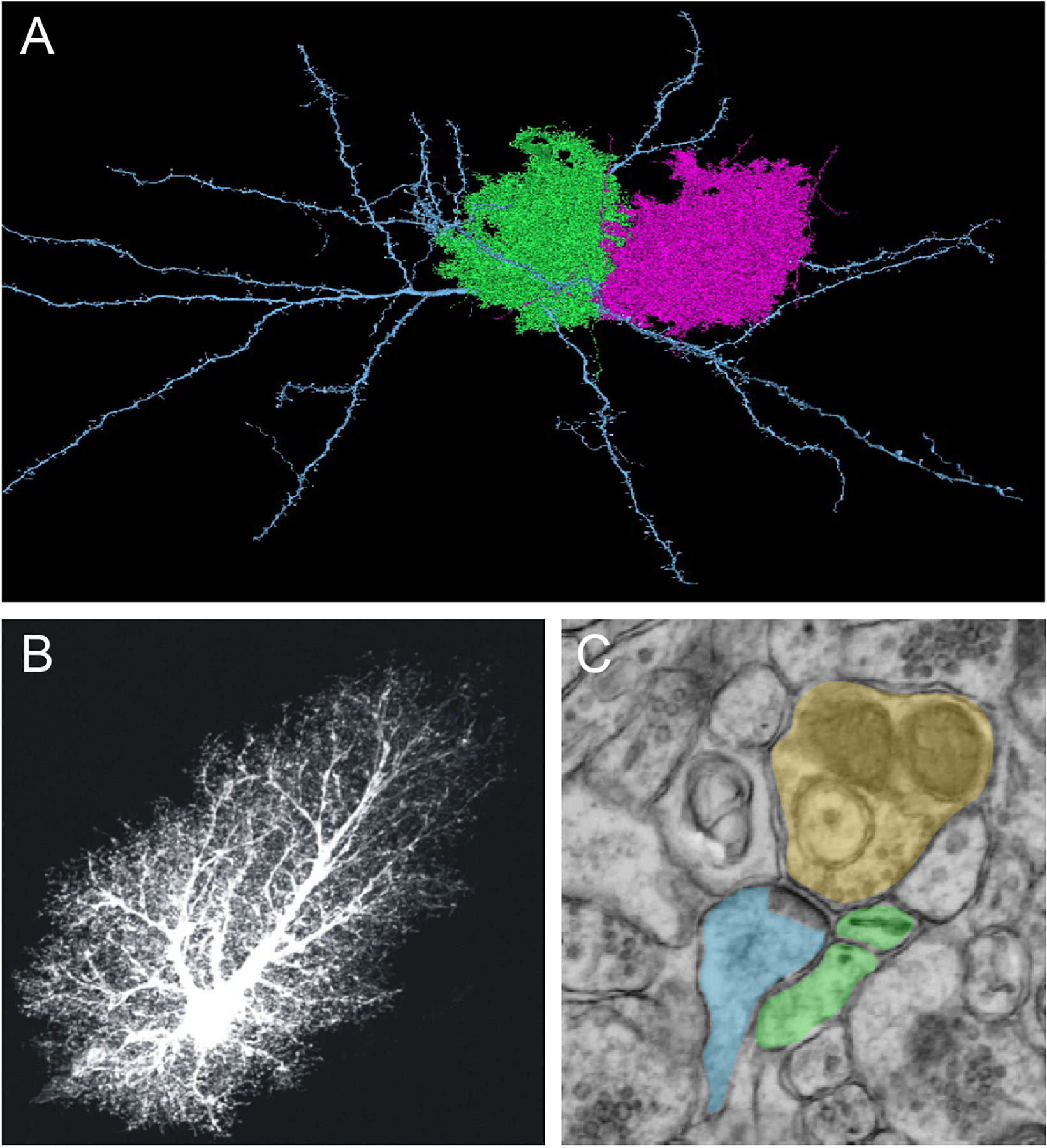
A) High resolution, serial EM reconstruction of astrocytes in layer 2/3 of mouse visual cortex. Image generated as part of the IARPA MICrONS project.(Consortium and others 2021)
B) A prototypical astrocyte from the mouse hippocampus, loaded with fluorescent dye (Lucifer yellow). The images are from the Cell Centered Database, , at the National Center for Microscopy and Imaging Research (http://ccdb.ucsd.edu/index.shtm) and have Accession #1066, 1063; also shown in ref (Khakh and Sofroniew 2015). Note the complex morphology, featuring thousands of processes and leaflets that are peripheral to the soma.
C) Electron microscopy image of an astrocyte process in close proximity to a neuron. Presynaptic (yellow), postsynaptic (blue), and astrocyte leaflet (green).
Astrocytes are not electrically excitable, which has made studying their physiological properties challenging and contributed to the historical view that they are a static, monolithic cell type (Haydon 2001). Traditional entry points for studying astrocyte function may be another limitation for understanding how their diversity is encoded, as most investigations to date have focused not on their unique molecular properties or affiliations with distinct subtypes of neurons but rather on core astrocytic properties including glutamate processing, buffering potassium, and Ca2+ signaling. However, new tools and approaches for examining cellular and molecular heterogeneity in the brain have highlighted a potentially rich reservoir of diverse astrocyte subtypes (YuNagai and Khakh 2020). The emerging molecular diversity of astrocytes, in conjunction with the spectrum of functions they perform, suggest the existence of functionally distinct subsets of astrocytes that serve unique roles in brain function (Khakh and Deneen 2019).
A series of recent studies have begun to tackle this question through the lens of regional diversity, where astrocytes from different brain regions exhibit unique molecular profiles (John Lin and others 2017). These molecular observations suggest that astrocytes from different brain regions may be endowed with unique functions (Chai and others 2017; Morel and others 2017). Complementary cellular studies identified regionally distinct astrocyte-neuron interactions, where astrocyte function is tuned to neighboring neurons and these interactions are often region-dependent (Batiuk and others 2020; Herrero-Navarro and others 2021). At the molecular level, these region-specific functions are regulated in part by transcription factors, where astrocytes exhibit region-specific dependencies on unique sets of transcription factors to encode diverse functions (Huang and others 2020; Huang and others 2021; Ung and others 2021). Together, these cellular and molecular observations highlight how the complex interplay between intrinsic transcriptional programs and the extrinsic neuronal milieu shapes astrocyte function across diverse brain regions.
In this review, we will focus on three emerging areas of astrocyte biology: i) region-specific circuit regulation, ii) molecular diversity and iii) transcriptional regulation. In the first section, we will discuss region-specific roles for astrocytes in circuit function and how interactions with neurons shape these functions. In the second section, we will examine the nature of astrocyte diversity from a cellular and molecular perspective, and we will describe how regional diversity of astrocytes is regulated by unique transcription factor codes in the third section. Our review highlights our nascent understanding of how molecular diversity is converted to functional diversity of astrocytes, articulating the current state of this field and key areas that need further exploration.
Astrocytes Regulate Region-Specific Circuit Function
Despite not possessing dynamic electrical properties, astrocytes fulfill a host of roles that complement neuronal function and are essential for neurotransmission, maintaining synaptic function, and preserving circuit integrity (Haydon 2001; Haydon and Carmignoto 2006; Volterra and Meldolesi 2005). Many of these core functions rely upon astrocyte proximity to neuronal synapses and their capacity to buffer neurotransmitters and ions (Figure 2). For example, astrocytes express a host of glutamate transporters and potassium channels that “clean up” excess neuroactive agents in the synaptic space after neurotransmission, ensuring proper synaptic activity. Critically, astrocyte uptake of neuroactive compounds (i.e., glutamate and GABA) via transporters and sensing via receptors elicits dynamic calcium (Ca2+) responses. These astrocyte responses are often coordinated with neuronal activity (Bazargani and Attwell 2016; Simard and Nedergaard 2004). While the precise role of increased Ca2+ activity in astrocytes remains an open question (Khakh and McCarthy 2015), as it is it likely tied to the ligand/receptor signaling mechanism, there is evidence that Ca2+ can regulate the release of neuroactive compounds from astrocytes, called “gliotransmitters”, that further modulate synaptic and neuronal activity (Araque and others 2014) (Figure 2).
Figure 2. Astrocyte-Neuron Communication Regulates Synaptic Activity.
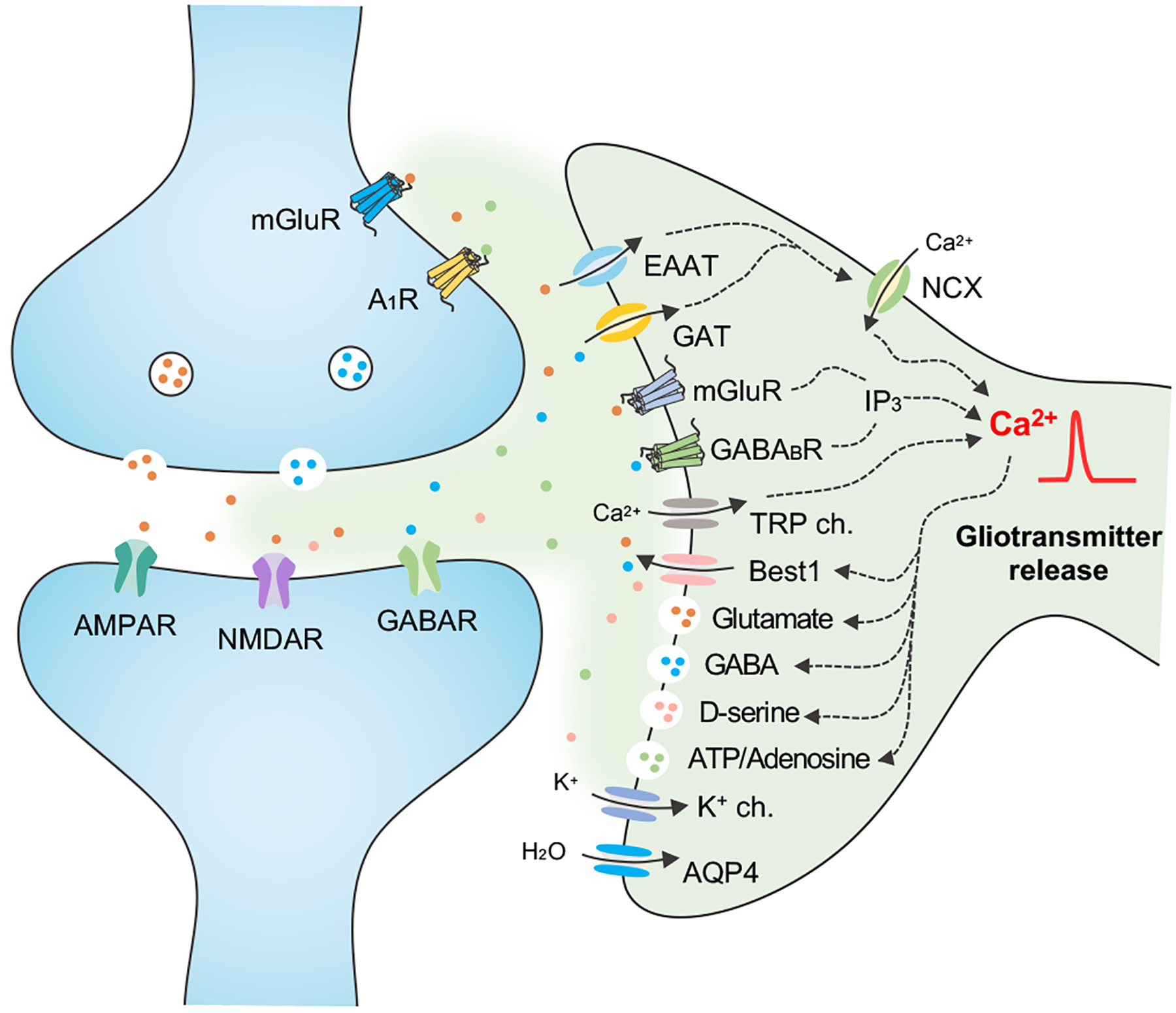
Astrocytes maintain synaptic function through the uptake of ions and neuroactive molecules via channels, transporters, and receptors. This can elicit increases in Ca2+ activity through direct or indirect pathways. In response to Ca2+ activity astrocytes release neuroactive compounds, called ‘gliotransmitters’ such as glutamate, GABA, D-serine and ATP/adenosine to pre- and post- synaptic neurons. EAAT, excitatory amino acid (glutamate) transporter; GAT, GABA transporter; NCX, Na+-Ca2+ exchanger; mGluR, metabotropic glutamate receptor; IP3, inositol trisphosphate; TRP ch, transient receptor potential channel; Best1, bestrophin1 channel permeable to glutamate/GABA/D-serine; AQP4, aquaporin-4.
This bi-directional communication between astrocytes and neurons is a hallmark of synaptic function (Eroglu and Barres 2010; HalassaFellin and Haydon 2007), which has critical implications in the function of a host of circuits across brain regions. Critically, there is accumulating evidence that astrocytes play direct roles in modulating various facets of neuronal physiology and associated behavioral outputs (Kofuji and Araque 2021). We will note that investigations into astrocyte regulation of neuronal circuits are often quite challenging because they require knowledge of astrocyte biology, as well as neuronal physiology and behavior. Moving forward, comprehensive analysis of the contributions of each cellular component of the synapse and how defined perturbations to one influence another, as well as their effects at the circuit and behavioral levels, should become more commonplace. In this section, we will discuss recent studies of astrocytic mechanisms regulating neuronal circuits and associated behaviors, with an emphasis on region-specific circuits (Figure 3).
Figure 3. Astrocyte Regulation of Circuits and Behaviors Across Brain Regions.

Sagittal brain section demonstrating various brain regions and their associated behavioral function, summarized molecular mechanism, and the molecular/genetic tools used in functional studies. OB, olfactory bulb; ST, striatum; HP, hippocampus; AG, amygdala; SP, spinal cord.
Hippocampus
The association of the hippocampus with learning and memory behaviors, coupled with long-term potentiation (LTP) as a physiological correlate, make it an attractive region in which to examine astrocyte contributions to neuronal function and circuit activities. Indeed, much of our knowledge about astrocytic regulation of neuronal circuits comes from the hippocampus and the well-defined CA3-CA1 circuit, featuring Schaffer Collaterals as the key neuronal substrate (Bliss and Collingridge 1993). Some key concepts that underlie astrocyte-neuron communication in the hippocampus include gliotransmission, where astrocytes release neuroactive compounds that act at the synapse to regulate neuronal circuit activities. These studies showed that astrocytes release glutamate, activate the pre/postsynaptic glutamate receptors, and regulate synaptic plasticity (LTP & LTD) and hippocampal-associated behaviors (Ota and others 2013; Volterra and Meldolesi 2005). Other studies demonstrated that astrocyte release of ATP, which is rapidly hydrolyzed to adenosine and activates the adenosine receptor on presynaptic terminals, regulates LTP and host of associated behaviors, including learning, memory, and sleep (Halassa and Haydon 2010; Haydon 2001; Haydon and Carmignoto 2006). Further studies on gliotransmitters identified d-serine, a co-agonist of the NMDA receptor, as a neuroactive compound released by astrocytes that stimulates LTP in the hippocampus (Henneberger and others 2010; Shigetomi and others 2013). Critically, these studies also demonstrated release of d-serine from astrocytes is Ca2+ dependent, linking astrocytic Ca2+ signaling to the modulation of neuronal circuits in the hippocampus (Figure 3).
The link between astrocytic calcium and neuronal activity ushered in a series of studies that identified key roles for astrocytic Ca2+ in regulating neuronal circuits in the hippocampus (Khakh and McCarthy 2015). More recently, the role of Ca2+ in hippocampal astrocytes has been examined using chemogenetic approaches that employ hM3Dq and hM4Di DREADDs to manipulate Ca2+ signaling associated with the Gq/Gi pathways, respectively (Adamsky and others 2018; Kol and others 2020). These studies revealed that activation of astrocytic Gq pathways via hM3Dq in the hippocampus results in enhanced learning and memory, while hM4Di conversely impairs these same behaviors and associated neuronal physiology. However, whether this Gq/Gi, Ca2+ associated phenomenon is dependent upon gliotransmitter release or other mechanisms warrants future investigation. Nevertheless, a model emerges from these studies, wherein Ca2+ dependent release of gliotransmitters from hippocampal astrocytes plays a critical role in neuronal circuit function in CA1-CA3 and directly influences behavioral outcomes associated with learning and memory.
Striatum
The striatum is the major input nucleus of the basal ganglia in the forebrain and contributes to action selection, motor function, and repetitive and habitual behaviors, among other outputs. At the cellular level, the striatum is predominately populated by GABAergic projection neurons called medium spiny neurons (MSNs) (Kreitzer and Malenka 2008). MSNs are defined by the expression of D1-dopamine receptor or D2-dopamaine receptor. D1 or D2 neurons comprise clearly defined circuits that receive excitatory inputs from the cortex, while projecting to a host of regions, including the substantia nigra and globus pallidus, which ultimately provide inputs into the thalamus. Thus, the striatum is an excellent venue in which to assess the contributions of astrocytes to complex macrocircuits that span multiple brain regions (Khakh 2019).
Recent papers manipulated astrocytic Ca2+ to demonstrate astrocyte contributions to striatal circuit function. Among these studies, one study used a newly devised plasma membrane pump that exports Ca2+ from astrocytes (termed CalEx) to reveal that reducing Ca2+ activity in striatal astrocytes results in excessive self-grooming behaviors (Yu and others 2018). Mechanistically, decreased Ca2+ led to an increase in the uptake of extracellular GABA into astrocytes via GAT-3, which resulted in decreased activity of MSNs. Another study reported that chemogenetic activation of striatal astrocytes with hM4Di DREADD increases Ca2+ activity, and that this increase is coupled with up-regulation of TSP-1, a synaptogenic cue, in astrocytes (Nagai and others 2019). The net result of these changes is increased firing of MSNs and the induction of hyperlocomotion and repetitive behavioral outputs in the mice. Together, these studies reinforce the role of astrocytic Ca2+ as a regulator of circuit level functions and highlight the utility of the striatum as model system for examining astrocyte-neuron interactions (Figure 3)
Olfactory bulb
The olfactory bulb (OB) is localized in the forebrain and controls the sensory processing of odors. During olfaction, volatile odor molecules bind to receptors on olfactory sensory neurons (OSN) that project into glomeruli in the OB. The OSNs make synaptic connections with mitral/tufted (M/Ts) cells that occupy deeper layers of the OB, with specific odors activating spatially defined M/T cells (Nagayama and others 2014). These connections can be visualized as a topographical sensory map in the OB (Meister and Bonhoeffer 2001; Vassar and others 1994). Moreover, odor detection, learning, and habituation are well defined (Mori and Sakano 2021). The robust input/output relationships between odors, neuronal populations, and behaviors of the olfactory system, along with its experimental tractability make the OB an ideal model for studying astrocyte regulation of dynamic sensory circuits.
Initial studies in the OB focused on Ca2+ activity in astrocytes in response to OSN stimulation, demonstrating that Ca2+ activity in glomeruli astrocytes is coordinated with OSN activity. These changes in Ca2+ activity precede hyperemia, coupling astrocytic Ca2+ with neurovascular regulation in the OB. One recent study used dual Ca2+ imaging in astrocytes (w/jRCaMP1) and neurons (with GCaMP) to reveal that neuronal sensory maps are topographically aligned with neighboring astrocyte domains that exhibit increased Ca2+. This work further supports the idea that signaling between active neurons and astrocytes is coordinated (Ung and others 2020). Furthermore, hM3Dq and hM4Di manipulations in OB astrocytes were shown to influence odor detection, supporting a role for astrocytic Ca2+ activity in olfaction. Studies also demonstrated that knockout of the Wnt-related gene Daam2 results in enhanced astrocyte morphology and increased Ca2+ activity, coupled with decreased excitatory activity and impaired olfactory behaviors (Jo and others 2021). Altogether, these studies show that coordination between neurons and astrocytes contributes to sensory processing in the OB (Figure 3).
Amygdala
The amygdala is associated with regulation of emotions including fear and anxiety and, along with the hippocampus and thalamus, is a core component of the limbic system. Amygdala nuclei are divided into two main sub-regions, the basolateral amygdala (BLA) and the central amygdala (CeA) (Ehrlich and others 2009; Janak and Tye 2015). These sub-regions are mostly comprised of neuronal populations, with the BLA containing spiny glutamatergic neurons, while the CeA is predominately composed of GABAergic projection neurons. The CeA can be further divided into lateral (CeL) and medial (CeM) compartments, with the CeM serving as the primary output center. The CeM also receives excitatory inputs from the BLA and inhibitory inputs from the CeL. These clearly defined input-output relationships between amygdala subregions and other brain regions make it an attractive system to examine roles for astrocytes.
A recent study demonstrated that increased intracellular Ca2+ in CeM, whether induced by endogenous endocannabinoid or exogenous activation using hM3Dq, leads to release of ATP/adenosine, decreased CeM neuronal firing, and reduced fear responses (Martin-Fernandez and others 2017). This study also found specific astrocyte-neuron interactions in the CeM, where astrocytes suppressed excitatory synapses from BLA via adenosine receptor A1 and activated inhibitory neurons from CeL via adenosine receptor A2. In addition to changes in Ca2+ activity in astrocytes, other studies have implicated astrocytic metabolic pathways and Rac1, a Rho GTPase involved in cytoskeletal remodeling, in amygdala fear responses (Liao and others 2017). Other mechanisms utilized by astrocytes to regulate amygdala circuits and associated fear memory include release of gliotransmitters via connexin 43 hemichannels in the BLA (Stehberg and others 2012). These studies highlight a diverse range of mechanisms used by astrocytes to influence amygdala circuits. (Figure 3).
Spinal Cord
The spinal cord plays an essential role in processing sensory inputs from the periphery, including pain, mechanosensation, and heat. In addition, key spinal cord outputs include movement via motor neurons. Put simply, the dorsal laminae of the spinal cord receive inputs from peripheral sensor neurons, while motor neurons reside in the ventral spinal cord and project to neuromuscular junctions. Clearly defined circuit systems associated with robust behavioral output measurements, coupled with its relatively simple anatomy, make the spinal cord an attractive model for examining astrocyte-neuron interactions associated with circuit function. In addition, developmental patterning mechanisms have been rigorously defined in the spinal cord (discussed below), resulting in a well annotated cellular diversity that oversees diverse circuits. Early studies demonstrated that astrocytes in the spinal ventral horn that exhibit enriched Sema3a and Kir4.1 expression are critical for motor circuit function(Kelley and others 2018; Molofsky and others 2014). Moreover, it has been reported that Hes5+ astrocytes and a population of LFNG-GFP astrocytes that reside in specific lamina layers in the dorsal spinal cord mediate mechanosensory processing (Akdemir and others 2021; Kohro and others 2020). These studies not only identify region-specific markers, but also suggest functional roles for astrocytes in the murine spinal cord (Figure 3).
To date, a range of mechanisms used by astrocytes to subserve neuronal function have been discovered across brain regions, underscoring the necessity of astrocytes to neurotransmission and circuit function. In addition to the regions discussed above, numerous studies have demonstrated critical roles for astrocytes in circuit function in other brain regions, including the cortex (prefrontal, somatosensory, motor, anterior cingulate cortex), hypothalamus, thalamus, brain stem, midbrain, and cerebellum (HwangLeeSeo and Lee 2021). One open question that arises from these collective studies is whether all astrocytes or subsets of distinct astrocyte subpopulations execute this diverse range of functions. For example, gliotransmission via Ca2+ dependent release of d-serine was established in the hippocampus, but whether it is restricted to hippocampal astrocytes or even subsets of hippocampal astrocytes remains unknown. Also, the issue of gliotransmission remains somewhat controversial, as important aspects of this phenomenon remain unresolved (Fiacco and McCarthy 2018, Bazargani and Attwell 2016). Nevertheless, the extent to which this prospective functional diversity is specific to the local neuronal milieu also remains a critical question. Finally, whether astrocytes are endowed with functional plasticity to respond to dynamic neuronal states warrants further investigation.
Molecular and Cellular Diversity of Astrocytes
Astrocytes were first described by Virchow in 1846 (Virchow, 1846) and subsequent drawings by Gustaf Retzius and Ramon Cajal described the existence of morphologically diverse astrocyte subpopulations in the human cortex. Despite these seminal observations, astrocytes have largely been grouped into two broad categories: fibrous and protoplasmic, with fibrous astrocytes residing in the white matter and protoplasmic astrocytes in the grey matter (Sofroniew and Vinters 2010; Verkhratsky and Nedergaard 2018). The distinct anatomical and morphological features of these broad classes of astrocytes suggests functional distinctions as well, however whether fibrous and protoplasmic have discrete functions in circuit activity remains poorly defined.
Over the past decade new tools to probe astrocyte biology and the constant refinement of technologies enabling high resolution molecular profiling of diverse cell populations have ushered in a new appreciation for astrocyte heterogeneity in the brain (Table I). At the core of these studies is an understanding of intra- and inter- regional molecular diversity of astrocytes as an entry point for dissecting whether and how they exhibit functional diversity. In this section we will discuss recent studies that examine the molecular and cellular diversity of astrocytes at the local and regional level under non-pathological conditions, highlighting attempts to link molecular diversity with functional diversity
Table 1.
Functional, molecular, and transcriptional heterogeneoty of astrocutes across brain regions.
| HP |
|
|
|
(Batiuk and others 2020; Chai and others 2017; Huang and others 2020; Tang, Taniguchi and Kofuji 2009) |
| ST | N/A |
|
(Chai and others 2017) | |
| OB |
|
|
|
(John Lin and others 2017; Ung and others 2021) |
| BS | N/A |
|
|
(John Lin and others 2017; Lozzi and others 2020) |
| CX |
|
|
|
(Batiuk and others 2020; Blanco-Suarez and others 2018; Farhy-Tselnicker and others 2021; John Lin and others 2017; Morel and others 2019) |
| SP |
|
|
|
(Akdemir and others 2021; Hochstim and others 2008; Kelley and others 2018; Kohro and others 2020; Molofsky and others 2014) |
HP: hippocampus; ST: striatum; OB: olfactory bulb; BS: brain stem; CX: cortex; SP: spinal cord
Developmental Patterning as a Paradigm for Astrocyte Diversity
Some of the first studies to examine the molecular diversity of astrocytes were performed in the developing spinal cord, leveraging developmental patterning mechanisms as an organizing principle for cellular diversity (Hochstim and others 2008; Tsai and others 2012). A defining feature of the developing spinal cord is the combinatorial expression of transcription factors along the dorsal-ventral axis that endow the progenitor domains with a unique neuronal identity. Applying these patterning principles to astrocytes revealed that transcription factors Pax6 and Nkx6.1, which regulate the generation of neuronal subtypes in the spinal cord, also endow spinal cord astrocytes with positional identities, compartmentalizing astrocytes with different molecular identities in the ventral spinal cord (Hochstim and others 2008). Ventral astrocyte subtypes 1, 2, and 3 (VA1–3) express different combinations of Pax6 and Nkx6.1, which further regulate the expression of Reelin and Slit. Pax6 promotes Reelin expression and inhibits Slit1, while Nkx6.1 promotes Slit1 expression. Thus, ventral astrocyte subtypes have the following combinatorial code: VA1 astrocytes express Reelin and Pax6; VA2 astrocytes express Nkx6.1, Pax6, Reelin, and Slit1; VA3 astrocytes express Nkx6.1 and Slit1. Additional lineage tracing studies corroborated these findings by showing that their allocation along the dorsal ventral axis of the adult spinal cord is dependent upon their embryonic domain of origin, where, for example, ventral astrocytes are derived from ventral progenitor domains (Tsai and others 2012). Taken a step further, the same developmental mechanisms apply to cortical astrocytes, where regional domains of origin oversee astrocyte distribution in the adult cortex. Together, these studies highlight the important role of developmental mechanisms in the specification of astrocyte identity and their diversity. However, whether molecularly distinct astrocyte subtypes are endowed with distinct functions in the adult spinal cord remains an open question.
Regional Diversity of Astrocytes in the Adult Brain
The adult brain is a highly compartmentalized organ system that consists of numerous, distinct regions that are further divided into subnuclei comprised of unique populations of neurons. Therefore, neuronal diversity across distinct brain regions contributes to the unique function of a given brain region. Given the close association between astrocytes and neurons, it stands to reason astrocytes may exhibit analogous molecular, cellular and functional heterogeneity across brain regions.
A series of recent papers have begun to examine the molecular and cellular differences of astrocytes from different brain regions. One of the first studies to systematically compare a host of astrocyte features from different brain region focused on striatal and hippocampal astrocytes (Chai and others 2017). At the cellular level, striatal astrocytes exhibit a larger volume than hippocampal astrocytes, however hippocampal astrocytes contact more excitatory synapses while striatal astrocytes predominately contact neuronal soma. DREADD-based approaches to activate the Gi pathway in astrocytes revealed that striatal astrocytes exhibit stronger calcium responses than their hippocampal counterparts. At the molecular level, RNA-sequencing revealed widespread transcriptomic differences, highlighted by an enrichment of GFAP expression in hippocampal astrocytes and μ-Crystallin in striatal astrocytes (Figure 4). In a related study, Morel et al. profiled astrocytes in cortical and subcortical regions, including cortex, hippocampus, caudate-putamen, nucleus accumbens, thalamus, and hypothalamus (Morel and others 2017). The authors found that synaptogenic modulator Sparc is differentially expressed in astrocytes from these brain regions, exhibiting an enrichment in cortex, hippocampus, and hypothalamus but not the others (Morel and others 2017). Interestingly, functional studies using in vitro neuron and astrocyte co-culture showed that cortical astrocytes selectively promote neurite growth of cortical neurons, while subcortical astrocytes selectively promote neurite growth of subcortical neurons (Figure 4). This finding suggests that astrocytes are tuned to neurons from the same region.
Figure 4. Regional Astrocyte Diversity.
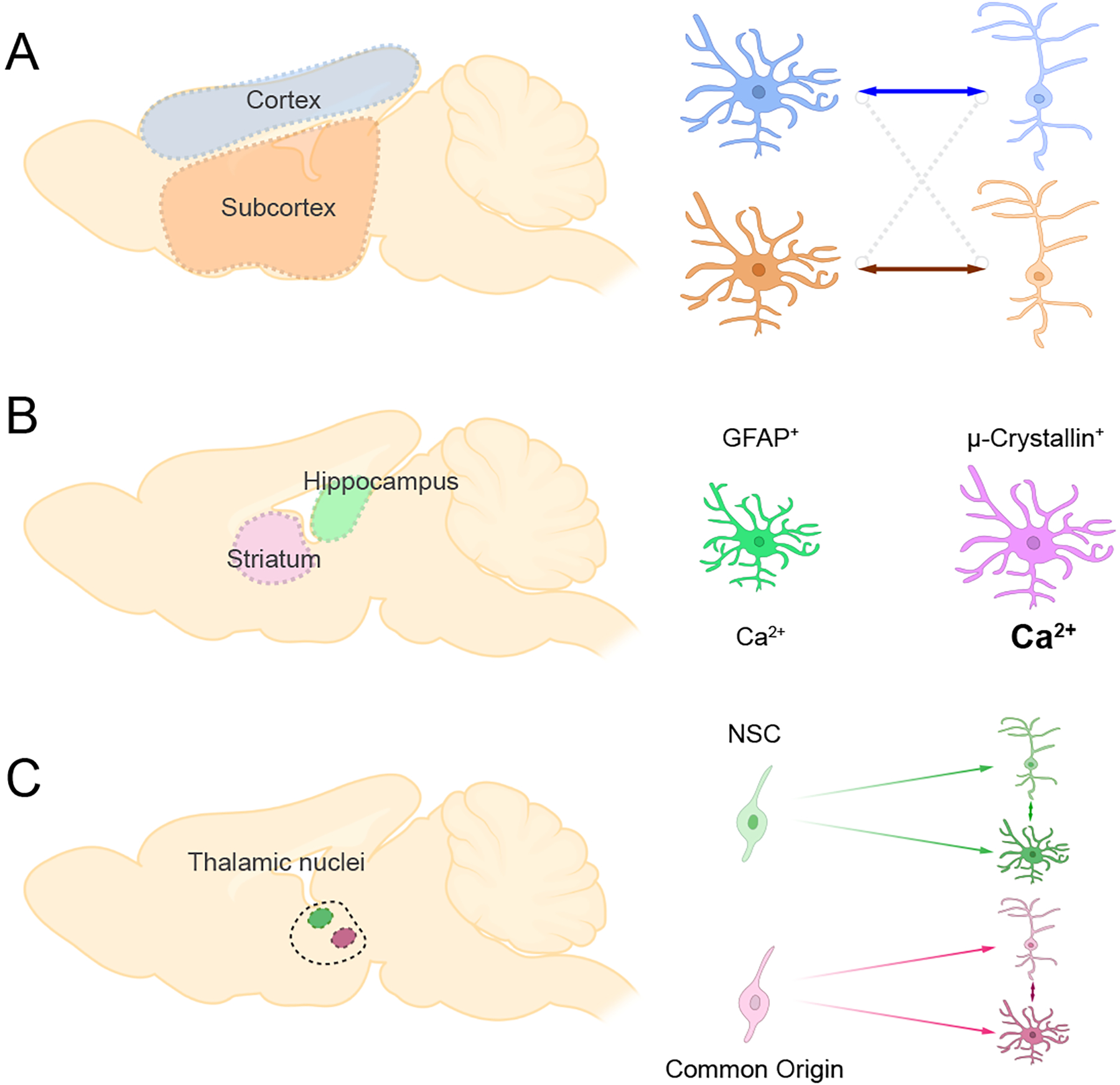
A) Astrocytes preferentially support neurons from matched brain regions. Cortical astrocytes support cortical neurons, while subcortical astrocytes support subcortical neurons
B) Striatal and hippocampal astrocytes exhibit distinct molecular and functional properties.
C) Astrocytes and neurons that share common origin are clustered in the same thalamic nucleus
The forgoing studies illustrate that astrocytes exhibit region-specific gene expression profiles and this diversity may be in part due to brain regions’ distinct neuronal environments. A recent study compared the transcriptional profiles of astrocytes and neurons from the murine thalamus and cortex, finding that astrocytes and neurons share region-specific gene expression profiles (Herrero-Navarro and others 2021). Further analysis of astrocyte and neuron transcriptomes from different thalamus nuclei revealed that astrocytes and neurons also share nucleus-specific gene signatures. By tracing the clonality during thalamic development, the authors found that neural and astrocyte progenitor cells from the same position ended up at the same thalamus nucleus (Figure 4). This finding suggests that neuronal and astrocyte diversity is tied to their common embryonic origins, like the patterning mechanisms that oversee local astrocyte diversity in the developing spinal cord.
While aspects of astrocyte heterogeneity may be hard wired and tuned to embryonic origins, it is important to note that astrocyte heterogeneity is not static. Recent studies have shown alterations in the molecular profiles of astrocytes across a host of regions during aging, particularly in synaptic-regulating genes (Boisvert and others 2018; Clarke and others 2018).
Collectively, these studies provide a framework for understanding molecular differences between astrocytes from diverse brain regions. Importantly, while molecular distinctions imply functional differences, they do not prove a priori that astrocytes from different regions have distinct functional properties. Converting this wealth of information into a functional taxonomy that accounts for these regional differences, as well as changes that occur during aging, remains a major challenge for the field.
Local Diversity of Astrocytes in the Adult Brain
In addition to diversity between brain regions, recent studies have revealed intra-regional astrocyte diversity. The archetype for this form of “local diversity” is the spinal cord, where molecularly distinct subpopulations exist in relative proximity within a given region (Hochstim and others 2008; Tsai and others 2012). Given that astrocytes execute a wide range of functions, coupled with an emerging appreciation of their molecular diversity, a division of labor may exist at the local level. In this model, subsets of astrocytes may be endowed with specialized roles that serve specific neuronal functions or maintain essential neurovascular interactions.
One approach to defining astrocyte subpopulations within a given region is to examine the expression of established markers and use combinatorial expression to segregate prospective astrocyte subtypes. For example, one study used a bacterial artificial chromosome (BAC) to express EGFP under the control of Kir4.1 promoter and found that hippocampal astrocytes have different expression levels of Kir4.1 and Kir4.1 expression determines the resting conductance of astrocytes (TangTaniguchi and Kofuji 2009). Similarly, a recent study examined astrocyte heterogeneity in the cortex using eaat2-Tdtomato transgenic animals, finding that Eaat2+ astrocytes are mostly located at cortical layer III-V and have higher Kir4.1 expression, which correlates with the resting membrane potential and passive conductance of these populations (Morel and others 2019). Furthermore, it has been shown that astrocytes from different cortical layers exhibit different morphological characteristics and proximity to synapses, with astrocytes from Layer II/III exhibiting enhanced contact with synapses (Figure 5). However, whether these morphologically and molecularly distinct astrocytes exhibit functional diversity remains unknown (Lanjakornsiripan and others 2018). To address this question, a recent paper combined the Aldh1l1-GFP reporter mouse line with cell surface markers and FACS to identify five subpopulations of astrocytes, each of which is present in the cortex, brainstem, and olfactory bulb (John Lin and others 2017). Using FACS to isolate these prospective populations enabled both transcriptional profiling and interrogation of their functional properties in vitro (Figure 5). These studies identified a unique subpopulation of astrocytes endowed with an enhanced synaptogenic signature that more efficiently promotes synapse formation between neurons compared to other populations. These studies suggest that astrocytes exhibit functional heterogeneity with respect to supporting synapse formation.
Figure 5. Local Astrocyte Diversity.

Features of local astrocyte diversity in the brain. Local astrocyte subpopulations within a given brain region are heterogeneous at the molecular and functional levels. Astrocytes in the same region also demonstrate differences that are related to adjacent neurons, such as excitatory (green) or inhibitory (pink) neurons. Cortical astrocytes exhibit diverse morphological properties and gene expression gradients based on cortical layering.
The finding that astrocytes can differentially support synapse formation is supported by a recent study demonstrating that that Chrdl1 (Chordin-like 1) is a secreted protein enriched in astrocytes of upper cortical layers and functions to promote synapse maturation (Blanco-Suarez and others 2018). In Chrdl1 knockout (KO) animals, the number of GluA2 AMPA receptor synapses is reduced in the visual cortex, and synaptic plasticity is increased in the visual cortex after monocular enucleation. Another study reported that synaptic-regulating genes have differential spatial-temporal expression patterns across cortical layers during development. Further, manipulating thalamic input into cortex or astrocyte calcium activity influenced the expression of synaptic-regulating genes (Farhy-Tselnicker and others 2021). For example, Glypican 4 expression in cortical layer I is decreased after P7, whereas Chrdl1 expression is increased in cortical layer II-III after P7. Whether these molecular profiles represent transient states or distinct populations remains unknown; however, they point to a degree of functional heterogeneity or plasticity that is tied to their roles in developmental synaptogenesis.
A host of studies using single cell RNA-sequencing (scRNA-seq) on brain tissues from various regions and across various developmental and aging timepoints have identified distinct astrocyte subpopulations (Tasic and others 2016; Zeisel and others 2015). While a powerful approach for prospective identification of astrocyte subpopulations, scRNA-seq analysis on its own does not allow for functional interrogation of diverse populations. However, some recent studies combined scRNA-seq with complementary validation experiments (Batiuk and others 2020; Bayraktar and others 2020; Zhu and others 2018). One study performed scRNA-seq on astrocytes from the cortex and hippocampus and identified five distinct subpopulations that demonstrated distinct morphology and calcium dynamics (Batiuk and others 2020). Among these five astrocyte subtypes, one was predominantly in the cortex, two were predominantly in the hippocampus, and two were shared in both regions, supporting the concept of regional and local diversity. Another study combined scRNA-seq and fluorescent in situ hybridization (FISH) to identify domain-associated subtypes of excitatory and inhibitory neurons and their relationship with astrocytes (Zhu and others 2018). After classifying neuronal domains, the authors found that astrocytes associated with each domain have a unique molecular profile. These results suggesting that neuronal inputs sculpt astrocyte heterogeneity. Similarly, one study used scRNA-seq to reveal unique molecular markers for astrocytes in different cortical layers and associated molecular mechanisms that shape cortical laminar architecture (Bayraktar and others 2020) (Figure 5). Applying large-area spatial transcriptomic (LaST) maps revealed that astrocytes adjacent to excitatory or inhibitory neurons exhibited distinct molecular profiles and that altering the lamina structure also altered the molecular profiles of astrocytes. Along these same lines, while the selective expression of glutamate or GABA transporters on subsets of astrocytes is an attractive mode of astrocyte diversification that has functional significance, there remains no clear evidence that these neurotransmitter transporters exhibit segregated expression amongst subsets of astrocytes.
Together, these studies illustrate how neurons influence the molecular diversity of astrocytes, suggesting that astrocyte diversity is tuned to the surrounding neuronal milieu. The identification of astrocyte populations endowed with enhanced synaptogenic functions provides further evidence for these links and indicates that cross communication with neurons can shape the functional properties of astrocytes. While far from decoding a functional taxonomy of molecularly diverse astrocyte subpopulations, interactions with neurons provide a framework for future studies in this area.
Transcriptional Regulation of Astrocyte Function
Historically, the study of astrocytes has focused on channels, transporters, or ligand/receptors because these classes of proteins directly influence cell physiology and cross communication with neurons (Khakh and Deneen 2019; Sofroniew and Vinters 2010; Verkhratsky and Nedergaard 2018). While transcription factors regulate nearly every facet of cell physiology through gene regulation, their roles in astrocyte function have been largely overlooked because they operate in the nucleus and their activities do not have an immediate, real time impact on physiology or neuron interactions. However, the identification of local and regional molecularly diverse astrocyte populations raises the question of how this diversity is encoded. Neuronal substrates can serve as key extrinsic cues, but astrocyte intrinsic mechanisms that oversee this diversity remain unknown and are likely to be regulated by transcription factors and associated epigenetic mechanisms. Evidence for this can be found in the spinal cord, where the transcription factors Pax6 and Nkx6.1 play critical roles in specifying VA1-VA3 identities in the ventral spinal cord (see above and Figure 6). Genetic knockout of Pax6 results in complete loss of VA2-VA3 but expansion of VA1 astrocytes, and overexpression of Nkx6.1 similarly expands VA1 astrocytes (Hochstim and others 2008). In this section, we will discuss how transcription factors regulate astrocyte function, highlighting region specific roles in the adult brain (Table I).
Figure 6. Transcriptional Regulation of Astrocyte Function.
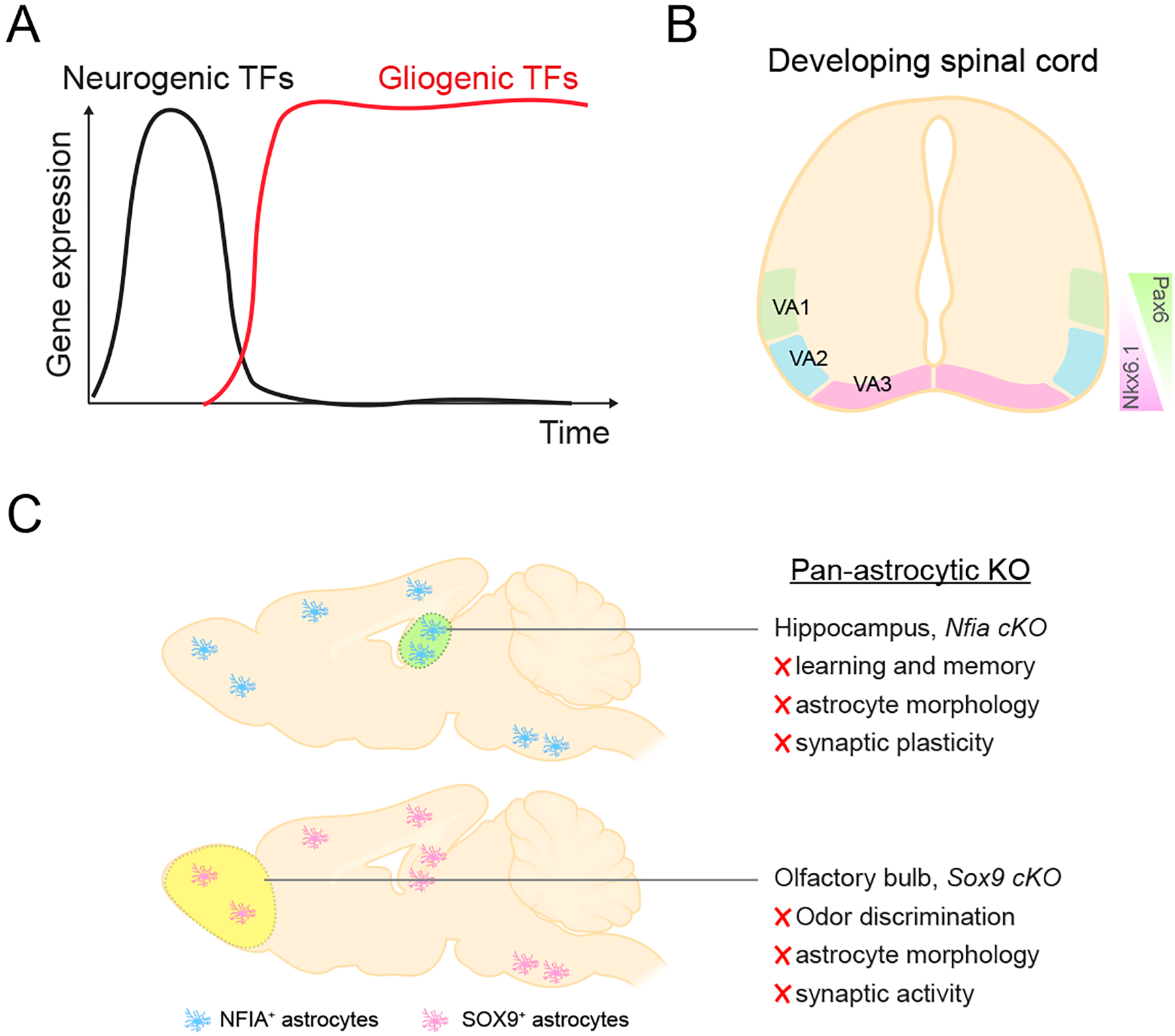
A) Expression dynamics of transcription factors that regulate the generation of neuronal and glial fates in the developing and adult CNS.
B) Patterned expression of development transcription factors Pax6 and Nkx6.1 in subsets of astrocytes in the developing spinal cord.
C) Summary of region specific transcriptional dependencies for NFIA and Sox9. NFIA and Sox9 are universally expressed in astrocytes throughout the brain, but when knocked out specifically impact astrocyte function and associated circuits in a region specific manner.
Developmental Transcription Factors are Expressed in Adult Astrocytes
Despite numerous and extensive transcriptomic profiling studies on astrocytes across brain regions and developmental timepoints, identifying markers that are specifically expressed in a defined astrocyte subpopulation or in a region-specific manner has remained elusive. One reason for this is that most differentially expressed genes exhibit a gradient of expression, rather than an “all or nothing” expression pattern. For example, astrocytes in the cortex exhibit gene expression gradients that extend across cortical layers, where core astrocyte genes exhibit differential expression patterns in superficial and deep cortical layers (Batiuk and others 2020) (Figure 5).This trend holds for the expression of transcription factors. A recent study compared transcription factor expression profiles in astrocytes from the cortex, brainstem, olfactory bulb and hippocampus, only finding selective expression of Nkx6.1 in brainstem astrocytes (Lozzi and others 2020). Surprisingly, this study identified universal transcription factor expression profiles across all astrocytes, where Nuclear Factor I (NFI) family transcription factors (NFIA, NFIB and NFIX) are each expressed in >80% of astrocytes across all brain regions. NFI-family members play pivotal roles in glial specification and astrocyte differentiation during development and the maintenance of their expression in the adult brain suggests they may play a separate role in mature astrocytes.
During embryonic development, NFIA and Sox9 promote the switch from neurogenesis to gliogenesis; like NFIA, Sox9 expression is also maintained in mature astrocytes in the adult brain (Deneen and others 2006; Kang and others 2012; Lozzi and others 2020; Stolt and others 2003; Sun and others 2017). The maintenance of developmental transcription factors in mature astrocytes extends to other members of the Sox-family, including Sox2 and Sox3, suggesting that transcription factors governing astrocyte development are reutilized in mature astrocytes (Cheah and Thomas 2015; Chen and others 2019) (Figure 6). Similarly, transcription factors Sox10 and Olig2 are essential for oligodendrocyte development with maintained expression in mature oligodendrocytes in the adult brain, where they contribute to myelin homeostasis (Arnett and others 2004; Stolt and others 2002). Importantly, this reutilization appears to be a general feature of glial development that is in contrast to neuronal development. Neurogenesis is by governed sets of transcription factors that are restricted to defined developmental windows, where, for example, neurogenin1/2 are exclusively expressed in proliferating neuroblasts but not in mature neurons (Bertrand and others 2002) (Figure 6). These observations illustrate key differences in transcriptional control of neuronal and glial development, while also indicating transcription factors that govern astrocyte development have prospective roles in mature astrocytes.
Region Specific Roles for NFIA in Hippocampal Astrocytes
Several independent studies revealed that NFI-family genes exhibit broad expression in mature astrocytes (Huang and others 2020; Lozzi and others 2020), raising the question of whether they contribute to astrocyte function in the adult brain. To investigate the role of NFI-family members in mature astrocytes, a recent study conditionally knocked out NFIA in astrocytes in the adult brain using the Aldh1l1-CreER mouse line (Huang and others 2020). Despite NFIA exhibiting expression in a vast majority of astrocytes across the cortex, brainstem, hippocampus, and olfactory bulb, its loss only impacted astrocytes from the hippocampus (Figure 6). Analysis of astrocyte morphology revealed that loss of NFIA resulted in reduced morphological complexity specifically in the hippocampus. This “stunted” morphology was also correlated with decreased Ca2+ activities and detection of extracellular glutamate in the hippocampus. At the circuit level, these alterations in hippocampal astrocytes resulted in a complete loss of long term potentiation (LTP) and impaired learning and memory behaviors. Importantly, behaviors associated with the olfactory bulb (odor detection, odor discrimination) and brainstem (breathing) were unaffected, further highlighting the specific effects of NFIA in hippocampal astrocytes.
The observation that hippocampal astrocytes are specifically dependent upon NFIA to maintain their structural and functional integrity raises the question of how this region-specific dependency is conferred. RNA-seq revealed that loss of NFIA results in widespread changes in gene expression in the hippocampus, while the cortex, brainstem, and olfactory bulb exhibited very modest changes in gene expression. ChIP-seq studies further revealed that NFIA exhibits selective DNA binding, where it can efficiently bind DNA in the hippocampus but not in the olfactory bulb. Finally, direct interactions with NFIB in the olfactory bulb inhibited the ability of NFIA to bind DNA. These studies indicate that region-specific protein interactions may be responsible for conferring transcriptional dependencies in astrocytes.
Regional Specific Roles for Sox9 in Olfactory Bulb Astrocytes
Sox9 is expressed in the majority of mature astrocytes, raising the question of how it contributes to astrocyte function in the adult brain. Similar to studies on NFIA, a recent paper employed temporal conditional approaches to knockout Sox9 in adult astrocytes and found that astrocytes from the olfactory bulb exhibited a loss of morphological complexity, while astrocytes from the cortex were unaffected (Ung and others 2021) (Figure 6). Subsequent analysis of olfactory circuits in Sox9-mutants revealed a decrease in synaptic activity in mitral/tufted (M/T) cells, coupled with aberrant activation of olfactory sensory maps in response to a host of odors. Interestingly, these changes in olfactory topographical sensory maps culminated in increased odor detection at lower thresholds and impaired odor discrimination. Mechanistically, Sox9-deficient astrocytes exhibited decreased expression of glutamate transporters and defects in glutamate transporter currents, suggesting that defects in olfactory circuits are due to impaired glutamate handling in astrocytes.
Together, studies on NFIA and Sox9 highlight the region-specific roles played by transcription factors in mature astrocytes in the adult brain. While NFIA and Sox9 exhibit near universal expression in astrocytes across all brain regions, their impact on astrocyte morphology and function is region-specific. These observations suggest that deciphering how transcription factors differentially interact across regions to drive the functional specificity of astrocytes may shed light on how astrocytes acquire and maintain their functional diversity. While scRNA-seq is a powerful front-line tool to identify determinants of cellular heterogeneity, sequencing alone could not have predicted the region-specific dependencies for NFIA and Sox9, which further reinforces the need for rigorous functional interrogation of candidate genes and pathways identified in transcriptomic studies, along with advanced proteomics.
Perspectives and Future Directions
A newfound appreciation for astrocyte diversity has emerged over the past decade, however numerous open questions remain. The first set of questions involves deciphering whether molecularly diverse astrocyte populations represent: i) functional sub-classes, ii) a form of plasticity, or iii) stochastic patterns of gene expression. The recent explosion of transcriptomic profiling studies has highlighted extensive molecular heterogeneity at the local and regional level. Still, the eventual goal of these studies will be to convert molecular diversity into functional diversity. The second set of questions is related to the functional interrogation of prospective subpopulations and requires rigorous genetic perturbations of genes selectively expressed in defined subsets of astrocytes in the native brain and the development of new mouse tools to selectively target defined astrocyte subsets. These tools and associated studies also will shed new light on core astrocyte functions, which remains an active area of discovery. The third set of questions involves identifying criteria to define a functionally distinct class of astrocytes. Recent studies pointed to interactions with neurons as a key driver of this diversity. Perhaps viewing the functional taxonomy of astrocytes through the lens of neuronal- and circuit-level interactions is an appropriate starting point. Critically, many functions of astrocytes that influence neurotransmission and synaptogenesis appear to be either region-specific or enhanced in defined subsets of astrocytes, suggesting that a bona fide division of labor may exist among subsets. While this is admittedly a neuron-centric view of astrocyte function, we must also consider the functional diversity at end-feet, as these interactions may confer a degree of diversity as well.
Finally, we must re-evaluate the utility of relying on transcriptomic studies as tool for defining astrocyte diversity. As noted by the studies on NFIA and Sox9, transcription factors that exhibit broad expression in astrocytes can have region-specific and selective function in subsets of astrocytes that would otherwise be overlooked by standard profiling strategies. Front-line tools that define molecular diversity must be matched with independent validation and expanded upon with proteomic and other approaches that reflect active cell physiology. Ultimately, in vivo functional interrogation serves as the gold standard for defining diversity. Despite being time consuming and challenging, in vivo functional interrogation remains the gold standard for defining diversity.
In summary, recent molecular and functional studies indicate that astrocytes are a diverse population of cells that occupy key roles in a host of essential brain functions. However, there is an urgent need to evaluate how astrocyte diversity is defined and how this diversity relates to their physiological activity. Given the current trajectory of the field, we are sure to learn more about the wide-ranging roles of astrocytes in the functioning brain and continue the process of defining their functional taxonomy.
Acknowledgments
We thank Catherine Gillespie for editorial assistance. We also thank Bal Khakh, Sonia Mayoral, Jacob Reimer, and Cameron Smith for assistance with Figure 1. This work was supported by US National Institutes of Health grants NS071153 and AG071687.
References
- Adamsky A, Kol A, Kreisel T, Doron A, Ozeri-Engelhard N, Melcer T and others. 2018. Astrocytic Activation Generates De Novo Neuronal Potentiation and Memory Enhancement. Cell 174(1):59–71 e14. [DOI] [PubMed] [Google Scholar]
- Akdemir ES, Woo J, Bosquez Huerta NA, Lozzi B, Groves AK, Harmanci AS and others. 2021. Lunatic Fringe-GFP Marks Lamina-Specific Astrocytes That Regulate Sensory Processing. J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NJ. 2014. Astrocyte regulation of synaptic behavior. Annu Rev Cell Dev Biol 30:439–63. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A. 2014. Gliotransmitters travel in time and space. Neuron 81(4):728–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S and others. 2004. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306(5704):2111–5. [DOI] [PubMed] [Google Scholar]
- Batiuk MY, Martirosyan A, Wahis J, de Vin F, Marneffe C, Kusserow C and others. 2020. Identification of region-specific astrocyte subtypes at single cell resolution. Nat Commun 11(1):1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann N, Pham-Dinh D. 2001. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev 81(2):871–927. [DOI] [PubMed] [Google Scholar]
- Bayraktar OA, Bartels T, Holmqvist S, Kleshchevnikov V, Martirosyan A, Polioudakis D and others. 2020. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat Neurosci 23(4):500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N, Attwell D. 2016. Astrocyte calcium signaling: the third wave. Nat Neurosci 19(2):182–9. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. 2002. Proneural genes and the specification of neural cell types. Nat Rev Neurosci 3(7):517–30. [DOI] [PubMed] [Google Scholar]
- Blanco-Suarez E, Liu TF, Kopelevich A, Allen NJ. 2018. Astrocyte-Secreted Chordin-like 1 Drives Synapse Maturation and Limits Plasticity by Increasing Synaptic GluA2 AMPA Receptors. Neuron 100(5):1116–1132 e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. 1993. A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361(6407):31–9. [DOI] [PubMed] [Google Scholar]
- Boisvert MM, Erikson GA, Shokhirev MN, Allen NJ. 2018. The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Rep 22(1):269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. 2002. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22(1):183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H, Diaz-Castro B, Shigetomi E, Monte E, Octeau JC, Yu X and others. 2017. Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95(3):531–549 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah PS, Thomas PQ. 2015. SOX3 expression in the glial system of the developing and adult mouse cerebellum. Springerplus 4:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhong X, Smith DK, Tai W, Yang J, Zou Y and others. 2019. Astrocyte-Specific Deletion of Sox2 Promotes Functional Recovery After Traumatic Brain Injury. Cereb Cortex 29(1):54–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Munch AE, Heiman M, Barres BA. 2018. Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A 115(8):E1896–E1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium M, Bae JA, Baptiste M, Bodor AL, Brittain D, Buchanan J and others. 2021. Functional connectomics spanning multiple areas of mouse visual cortex. bioRxiv:2021.07.28.454025. [Google Scholar]
- Deneen B, Ho R, Lukaszewicz A, Hochstim CJ, Gronostajski RM, Anderson DJ. 2006. The transcription factor NFIA controls the onset of gliogenesis in the developing spinal cord. Neuron 52(6):953–68. [DOI] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, Luthi A. 2009. Amygdala inhibitory circuits and the control of fear memory. Neuron 62(6):757–71. [DOI] [PubMed] [Google Scholar]
- Eroglu C, Barres BA. 2010. Regulation of synaptic connectivity by glia. Nature 468(7321):223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, Boisvert MM, Liu H, Dowling C, Erikson GA, Blanco-Suarez E and others. 2021. Activity-dependent modulation of synapse-regulating genes in astrocytes. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiacco TA and McCarthy KD 2018. Multiple Lines of Evidence Indicate That Gliotransmission Does Not Occur under Physiological Conditions. J Neuroscience 38(1):3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. 2007. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13(2):54–63. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Haydon PG. 2010. Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol 72:335–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon PG. 2001. GLIA: listening and talking to the synapse. Nat Rev Neurosci 2(3):185–93. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. 2006. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86(3):1009–31. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. 2010. Long-term potentiation depends on release of D-serine from astrocytes. Nature 463(7278):232–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero-Navarro A, Puche-Aroca L, Moreno-Juan V, Sempere-Ferrandez A, Espinosa A, Susin R and others. 2021. Astrocytes and neurons share region-specific transcriptional signatures that confer regional identity to neuronal reprogramming. Sci Adv 7(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstim C, Deneen B, Lukaszewicz A, Zhou Q, Anderson DJ. 2008. Identification of positionally distinct astrocyte subtypes whose identities are specified by a homeodomain code. Cell 133(3):510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang AY, Woo J, Sardar D, Lozzi B, Bosquez Huerta NA, Lin CJ and others. 2020. Region-Specific Transcriptional Control of Astrocyte Function Oversees Local Circuit Activities. Neuron 106(6):992–1008 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TW, Iyer AA, Manalo JM, Woo J, Bosquez Huerta NA, McGovern MM and others. 2021. Glial-Specific Deletion of Med12 Results in Rapid Hearing Loss via Degradation of the Stria Vascularis. J Neurosci 41(34):7171–7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SN, Lee JS, Seo K, Lee H. 2021. Astrocytic Regulation of Neural Circuits Underlying Behaviors. Cells 10(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janak PH, Tye KM. 2015. From circuits to behaviour in the amygdala. Nature 517(7534):284–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Woo J, Cristobal CD, Choi JM, Wang CY, Ye Q and others. 2021. Regional heterogeneity of astrocyte morphogenesis dictated by the formin protein, Daam2, modifies circuit function. EMBO Rep 22(12):e53200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J and others. 2017. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci 20(3):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P, Lee HK, Glasgow SM, Finley M, Donti T, Gaber ZB and others. 2012. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron 74(1):79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Ben Haim L, Schirmer L, Tyzack GE, Tolman M, Miller JG and others. 2018. Kir4.1-Dependent Astrocyte-Fast Motor Neuron Interactions Are Required for Peak Strength. Neuron 98(2):306–319 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS. 2019. Astrocyte-Neuron Interactions in the Striatum: Insights on Identity, Form, and Function. Trends Neurosci 42(9):617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Deneen B. 2019. The Emerging Nature of Astrocyte Diversity. Annu Rev Neurosci 42:187–207. [DOI] [PubMed] [Google Scholar]
- Khakh BS, McCarthy KD. 2015. Astrocyte calcium signaling: from observations to functions and the challenges therein. Cold Spring Harb Perspect Biol 7(4):a020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, Sofroniew MV. 2015. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18(7):942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Araque A. 2021. Astrocytes and Behavior. Annu Rev Neurosci 44:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohro Y, Matsuda T, Yoshihara K, Kohno K, Koga K, Katsuragi R and others. 2020. Spinal astrocytes in superficial laminae gate brainstem descending control of mechanosensory hypersensitivity. Nat Neurosci 23(11):1376–1387. [DOI] [PubMed] [Google Scholar]
- Kol A, Adamsky A, Groysman M, Kreisel T, London M, Goshen I. 2020. Astrocytes contribute to remote memory formation by modulating hippocampal-cortical communication during learning. Nat Neurosci 23(10):1229–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. 2008. Striatal plasticity and basal ganglia circuit function. Neuron 60(4):543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanjakornsiripan D, Pior BJ, Kawaguchi D, Furutachi S, Tahara T, Katsuyama Y and others. 2018. Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat Commun 9(1):1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Tao Y, Guo X, Cheng D, Wang F, Liu X and others. 2017. Fear Conditioning Downregulates Rac1 Activity in the Basolateral Amygdala Astrocytes to Facilitate the Formation of Fear Memory. Front Mol Neurosci 10:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzi B, Huang TW, Sardar D, Huang AY, Deneen B. 2020. Regionally Distinct Astrocytes Display Unique Transcription Factor Profiles in the Adult Brain. Front Neurosci 14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fernandez M, Jamison S, Robin LM, Zhao Z, Martin ED, Aguilar J and others. 2017. Synapse-specific astrocyte gating of amygdala-related behavior. Nat Neurosci 20(11):1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. 2001. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci 21(4):1351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Kelley KW, Tsai HH, Redmond SA, Chang SM, Madireddy L and others. 2014. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature 509(7499):189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Chiang MSR, Higashimori H, Shoneye T, Iyer LK, Yelick J and others. 2017. Molecular and Functional Properties of Regional Astrocytes in the Adult Brain. J Neurosci 37(36):8706–8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel L, Men Y, Chiang MSR, Tian Y, Jin S, Yelick J and others. 2019. Intracortical astrocyte subpopulations defined by astrocyte reporter Mice in the adult brain. Glia 67(1):171–181. [DOI] [PubMed] [Google Scholar]
- Mori K, Sakano H. 2021. Olfactory Circuitry and Behavioral Decisions. Annu Rev Physiol 83:231–256. [DOI] [PubMed] [Google Scholar]
- Nagai J, Rajbhandari AK, Gangwani MR, Hachisuka A, Coppola G, Masmanidis SC and others. 2019. Hyperactivity with Disrupted Attention by Activation of an Astrocyte Synaptogenic Cue. Cell 177(5):1280–1292 e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama S, Homma R, Imamura F. 2014. Neuronal organization of olfactory bulb circuits. Front Neural Circuits 8:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota Y, Zanetti AT, Hallock RM. 2013. The role of astrocytes in the regulation of synaptic plasticity and memory formation. Neural Plast 2013:185463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Jackson-Weaver O, Huckstepp RT, O’Dell TJ, Khakh BS. 2013. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci 33(24):10143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. 2004. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129(4):877–96. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV. 2010. Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehberg J, Moraga-Amaro R, Salazar C, Becerra A, Echeverria C, Orellana JA and others. 2012. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J 26(9):3649–57. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A, Wegner M. 2003. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev 17(13):1677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolt CC, Rehberg S, Ader M, Lommes P, Riethmacher D, Schachner M and others. 2002. Terminal differentiation of myelin-forming oligodendrocytes depends on the transcription factor Sox10. Genes Dev 16(2):165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Cornwell A, Li J, Peng S, Osorio MJ, Aalling N and others. 2017. SOX9 Is an Astrocyte-Specific Nuclear Marker in the Adult Brain Outside the Neurogenic Regions. J Neurosci 37(17):4493–4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Taniguchi K, Kofuji P. 2009. Heterogeneity of Kir4.1 channel expression in glia revealed by mouse transgenesis. Glia 57(16):1706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, Menon V, Nguyen TN, Kim TK, Jarsky T, Yao Z and others. 2016. Adult mouse cortical cell taxonomy revealed by single cell transcriptomics. Nat Neurosci 19(2):335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H and others. 2012. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science 337(6092):358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung K, Huang TW, Lozzi B, Woo J, Hanson E, Pekarek B and others. 2021. Olfactory bulb astrocytes mediate sensory circuit processing through Sox9 in the mouse brain. Nat Commun 12(1):5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung K, Tepe B, Pekarek B, Arenkiel BR, Deneen B. 2020. Parallel astrocyte calcium signaling modulates olfactory bulb responses. J Neurosci Res 98(8):1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. 1994. Topographic organization of sensory projections to the olfactory bulb. Cell 79(6):981–91. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Nedergaard M. 2018. Physiology of Astroglia. Physiol Rev 98(1):239–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow R Uber das granulierte ansehen der wandungen der gerhirnventrikel. Allg Z Psychiatr. 1846;3:242–250 [Google Scholar]
- Volterra A, Meldolesi J. 2005. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci 6(8):626–40. [DOI] [PubMed] [Google Scholar]
- Yu X, Nagai J, Khakh BS. 2020. Improved tools to study astrocytes. Nat Rev Neurosci 21(3):121–138. [DOI] [PubMed] [Google Scholar]
- Yu X, Taylor AMW, Nagai J, Golshani P, Evans CJ, Coppola G and others. 2018. Reducing Astrocyte Calcium Signaling In Vivo Alters Striatal Microcircuits and Causes Repetitive Behavior. Neuron 99(6):1170–1187 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel A, Munoz-Manchado AB, Codeluppi S, Lonnerberg P, La Manno G, Jureus A and others. 2015. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347(6226):1138–42. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Shah S, Dries R, Cai L, Yuan GC. 2018. Identification of spatially associated subpopulations by combining scRNAseq and sequential fluorescence in situ hybridization data. Nat Biotechnol. [DOI] [PMC free article] [PubMed] [Google Scholar]


