Abstract
Microorganisms are exposed to a wide variety of stress factors in their natural environments. Under that stressful conditions, they move into a viable but nonculturable (VBNC) state to survive and maintain the vitality. At VBNC state, microorganisms cannot be detected by traditional laboratory methods, but they can be revived under appropriate conditions. Therefore, VBNC organisms cause serious food safety and public health problems. To date, it has been determined that more than 100 microorganism species have entered the VBNC state through many chemical and physical factors. During the last four decades, dating from the initial detection of the VBNC condition, new approaches have been developed for the induction, detection, molecular mechanisms, and resuscitation of VBNC cells. This review evaluates the current data of recent years on the inducing conditions and detection methods of the VBNC state, including with microorganisms on the VBNC state, their virulence, pathogenicity, and molecular mechanisms.
Keywords: Viable but nonculturable, Microbial survival, Food safety, Public health
Introduction
Microorganisms in their natural environment develop various strategies to cope with changing environmental conditions changes (Robben et al. 2018). These strategies include spore formation and protection mechanisms such as biofilm. However, under stress conditions, bacteria that lack of such mechanism pass into a state called dormancy, in which they are viable but nonculturable (VBNC) (Yamamoto 2000; Sachidanandham and Yew-Hoong Gin 2009). VBNC status is a survival strategy adopted by many bacteria in response to adverse environmental conditions (Ayrapetyan et al. 2018; Dong et al. 2020; Yoon and Lee 2020). In the case of VBNC, bacteria cannot grow on a routine culture medium, while their live and metabolic activities continue. When the environmental conditions are suitable again, VBNC cells become re-culturable (Oliver 2000).
Microbial diversity and factors causing VBNC state
VBNC was first identified in 1982 by Xu et al. in Escherichia coli and Vibrio cholerae (Xu et al. 1982). In many studies conducted in the following years, VBNC was observed in different bacterial species (Millet and Lonvaud-Funel 2000; Lemke and Leff 2006; Suzuki et al. 2006). To date, researchers have identified a total of more than 100 bacterial and fungal species that enter VBNC state (Oliver 2016; Dong et al. 2020; Zhang et al. 2021). In the list of bacteria that enter the VBNC form, there are many bacterial species such as Escherichia coli, Campylobacter jejuni, Helicobacter pylori, Legionella pneumophila, Listeria monocytogenes, Salmonella typhimurium, Vibrio cholerae, Yersinia pestis, and Mycobacterium tuberculosis, Klebsiella pneumoniae, Methicillin resistant Staphylococcus aureus, Pseudomonas aeruginosa, which cause nosocomial infections (Pinto et al. 2015; Zhao et al. 2017; Ayrapetyan et al. 2018; Dong et al. 2020; Ou et al. 2021; Zhang et al. 2021).
Although the VBNC state is wide a distribution across species, there is also variation in the conditions that induce VBNC. For the first time, VBNC state was detected in the sea and estuarine waters. After that this state has been determined by many studies to occur in different environments, such as swimming water, tap water, food, and soil too (Xu et al. 1982; Pommepuy et al. 1996; Defives et al. 1999; Maalej et al. 2004; Liu et al. 2008; Abdallah et al. 2008; Pawlowski et al. 2011).
Apart from these detected environments, many factors induce cells into the VBNC state, according to the available information in the literature. Factors such as temperature, starvation, and pH are among the most studied conditions. A study, S. aureus cells that treated with both citric acid and low temperature were induced to VBNC after 18 days (Bai et al. 2019). Similarly, in another study with E. coli O157:H7, it was determined that cells were induced to VBNC at two different temperatures at + 4 °C and − 20 °C (Li et al. 2020). It has been determined that V. vulnificus cells are induced into VBNC state in artificial seawater at low temperatures (Oliver 2016). Another factor that induces cells to VBNC state is nutrient starvation. Many bacterial species, such as E. coli (Pinto et al. 2011; Li et al. 2020), Shigella dysenteriae (Rahman et al. 1994, 1996; Oliver 2005), V. parahaemolyticus (Baffone et al. 2003), Aeromonas hydrophila (Maalej et al. 2004), and Klebsiella pneumoniae (Byrd et al. 1991), are induced to VBNC state under starvation conditions. Another environmental factor inducing into the VBNC state in bacteria is pH. Both acidic and alkaline pH conditions induce bacteria to VBNC state (Darcan et al. 2009; Capozzi et al. 2016; Bai et al. 2019; Li et al. 2020). In addition, increased salinity (Li et al. 2020), high carbon dioxide pressure (Zhao et al. 2016), thermosonication (Liao et al. 2018), sunlight (Pommepuy et al. 1996; Besnard et al. 2002), artificial different light sources (Idil et al. 2010; Darcan 2012), low oxygen content (Bovill and Mackey 1997; Pinto et al. 2011), drying (Dinu and Bach 2011; Jameelah et al. 2018), osmotic stress (Li et al. 2020), reactive oxygen species (ROS) (Zhou et al. 2022), food preservatives and heavy metals (del Campo et al. 2009; Aurass et al. 2011; Li et al. 2014), organic pollutants (Su et al. 2016), inorganic salts (Robben et al. 2018; Li et al. 2020), chlorination (Oliver 2005; Lin et al. 2017; Chen et al. 2018; Noll et al. 2020), UV radiation (Zhang et al. 2015; Xu et al. 2018), and under less studied stress conditions, such as aerosolization (Heidelberg et al. 1997), acetosyringone (Postnikova et al. 2015), lyophilization and cryopreservation (Hoefman et al. 2012), bacteria are induced into the VBNC state.
Moreover, VBNC state is also found in foodstuffs such as seafood (Lindba¨ck et al.; Asakura et al. 2002; Pan et al. 2020), pork (Han et al. 2018), poultry (Purevdorj-Gage et al. 2018), fruit juices (Nicolò et al. 2011; Pan et al. 2020), milk (Cunningham et al. 2009), wine (Millet and Lonvaud-Funel 2000; Capozzi et al. 2016), and beer (Liu et al. 2018; Xu et al. 2022). According to the above-mentioned literatures, it has been determined that VBNC formation is not only dependent on the stress conditions, but also microorganisms can switch to VBNC state in rich nutrient environments too.
VBNC detection methods
VBNC cells pose a significant public health problem as they remain potentially pathogenic under favorable conditions and cannot be detected by conventional culture methods (Ravel et al. 1995). Thus, it is extremely important to determine the level of microbial contamination from food, hospital, water, etc., and to use and develop appropriate techniques for detecting VBNC bacteria (Ramamurthy et al. 2014).
Bacteria in the VBNC state cannot be cultured despite metabolic activity continues. Therefore, it is impossible to detect VBNC cells with conventional methods. Various viability measurement methods, such as metabolism, cell membrane integrity, and respiratory activity, have been developed for detecting VBNC cells.
Initial studies of VBNC detection in bacteria were based on cell staining procedures. The first method adopted to detect VBNC cells is direct viable count (DVC), a microscopic method (Kogure et al. 1979). Direct viable count is based on the acridine orange staining of cells in the presence of antibiotics, such as nalidixic acid, aztreonam, and ciprofloxacin that inhibit DNA synthesis in rich medium (Kogure et al. 1979; Xu et al. 1982; Heidelberg et al. 1997). In this method, live and dead cells are distinguished by observation of the microscope due to the differences in their sizes (Kogure et al. 1979; Yokomaku et al. 2000). Although elongated cells are accepted as a live, small cells are accepted as a dead cell. The total of both dead and live cell are determined as total cell count.
Another method used to detect VBNC cells is the evaluation of cytoplasmic membrane integrity. In this method, the combination of two different fluorescent dyes, nucleic acid-based SYTO-9, and propidium iodide, is used. This dye makes it possible to distinguish between dead and living cells in fluorescent microscopy and flow cytometry. SYTO-9 gives a green fluorescent color that labels all bacteria in a population, while propidium iodide penetrates only damaged membranes and gives a red fluorescent color. In this way, cells with intact membranes fluoresce green, and cells with damaged membranes fluoresce red (Zhao et al. 2013; Pinto et al. 2015; Park and Kim 2018). Fluorescent green cell is live, while fluorescent red cell is dead. However, this method cannot distinguish culturable cell and unculturable cell (VBNC state) between fluorescent green cells. Therfore, this method must use with plaque count. Another method used to detect cellular activity in VBNC cells is CTC–DAPI staining. This method can be performed in two different ways, 5‐Cyano‐2,3‐Ditolyl Tetrazolium Chloride (CTC)-DAPI and 2‐p (Iodophenyl)‐3-p (Nitrophenyl) 5‐Phenyl Tetrazolium Chloride (INT)-DAPI (Denisova et al. 2022; Progulske-Fox et al. 2022; Qi et al. 2022). These formazan salts act as electron acceptors and are reduced to a precipitate as INT formazan or CTC formazan by taking electrons at the end of the electron transport chain. Dark red precipitates of INT formazan are visualized with an optical microscope, while red fluorescent precipitates of CTC formazan are visualized by epifluorescence microscopy (Fakruddin et al. 2013; Wideman et al. 2021). This method is not possible to distinguish culturable and nonculturable cells. As with the live–dead method, this method must use with plaque count.
In the recent years, various nucleic acid-based methods have been developed as viability markers in bacteria (Guo et al. 2021; Wen et al. 2022). However, it is not possible to distinguish dead bacteria from live bacteria by normal PCR or qPCR because these methods only target DNA. Because of this reason, ethidium monoazide (EMA) and propidium monoazide (PMA) were used in real-time PCR to measure the DNA of living cells (Wideman et al. 2021). EMA and PMA enter through the damaged cell membrane, penetrate dead bacteria, and react with the hydrocarbon portion of dead cell DNA, causing structural changes in DNA. With these changes, dead cell DNA cannot replicate in the PCR reaction, and only living cell DNA can replicate (Zhang et al. 2015; Ding et al. 2017; Zhong and Zhao 2018; Wideman et al. 2021). However, according to the results obtained, it has been determined that PMA has a higher affinity than EMA, and therefore the use of PMA has become widespread (Zhong and Zhao 2018; Fu et al. 2020; Hussein and Ghit 2021; Gao et al. 2021). Another method based on the DNA-based molecular diagnostic approaches is mRNA-based reverse transcription-quantitative PCR (RT-qPCR). In this method, a stably expressed target gene, genes such as 16S, relA, tuf, rpoS, and virulence genes are used as viability markers. Since the RT-qPCR method works with mRNA, this method limits the studies due to both the stability of mRNA and the difficulties in isolation (Lothigius et al. 2010; Zhang et al. 2015; Wideman et al. 2021). In both methods mentioned, they do not give numerical data as in microscope counts, but can only be used as a viability indicator.
Another method used to detect VBNC cells is the use of biosensors. A phage-based biochip using magnetic nanoparticles has been developed for the detection of VBNC cells. In the study, a broad-spectrum virulent phage (PVP-SE1) was selected and a phage-based biochip using magnetic nanoparticles was developed by adding Salmonella-specific antibodies (anti-Salmonella) to the surface probe regions of the phages. With this developed biochip, work was carried out with live, dead, and VBNC cells, and it was determined that dead cells were clearly separated from live cells (Fernandes et al. 2013). However, this method cannot separate culturable and nonculturable cell to each other. Again in a sensor-based study, Polyclonal anti-S. aureus antibody was immobilized on the gold electrode and an immunosensor was developed. With this developed immunosensor, bacteria at different concentrations were allowed to react, and the electrochemical response of the modified electrode was measured by impedance spectroscopy. In the measurement, it was observed that S. aureus cells of different densities, both stressed and resuscitated, could be successfully detected thanks to the specific antibody–bacteria interaction (Bekir et al. 2015).
Physical and molecular changes in bacteria in VBNC state
In the VBNC state, microorganisms show some changes to withstand environmental conditions. Although morphology (Zhu et al. 2022; Yang et al. 2022) and cell wall structure (Signoretto et al. 2000) vary in VBNC cells, protein synthesis (Rahman et al. 1994), gene expression (Lleò et al. 2001), membrane integrity (Lloyd and Hayes 1995), and respiratory activity (Oliver 2005; Li et al. 2014) still continue. It has been determined by electron microscopy that some changes occur in cell volume and cell envelope in bacteria in VBNC (Signoretto et al. 2000; Li et al. 2016). In the VBNC state, it was observed that rod-shaped microorganisms transitioned to coke and similar form, while arc-shaped microorganisms transitioned to spherical form (Kumar and Ghosh 2019; Dong et al. 2020). These changes are associated with extensive modifications in cell wall components, such as the cytoplasmic membrane, fatty acid composition, peptidoglycan crosslinks, lipoprotein, and glycan chains (Signoretto et al. 2000; Dong et al. 2020). These modifications are properties increase the stabilization of the cell wall and membrane in VBNC cells, and thus provide the stability of the cell. İn addition, there are several physiological and molecular differences between VBNC cells and culturable cells, including the amount of RNA, gene expression, protein profiles, ATP synthesis, virulence potential, metabolism, physical, and chemical resistance (González-Escalona et al. 2006; Darcan and Aydin 2012; Hung et al. 2013; Robben et al. 2018, 2019; Fu et al. 2020).
Enzyme activity in VBNC cells
Another important feature of VBNC organisms is the continuation of their metabolic activities. In some studies using the API ZYM kit with VBNC cells, the metabolic activities of VBNC cells were analyzed. In the study performed with Vibrio fluvialis in marine sediment, a decrease was observed in alkaline phosphatase, C4-esterase, and C8-esterase lipase activities, while leucine arylamidase, valine arylamidase, β-galactosidase, and β-glucosidase enzyme activities could not be detected. However, in the same study, only C8-esterase lipase, acid phosphatase, and naphthol-AS-BI-phosphohydrolase were found to be at the same level as normal cells in cells incubated in seawater (Amel et al. 2008). It was determined that all enzyme activities in E. coli O157:H7 cells induced to VBNC by high carbon dioxide pressure were lower than in cells growing in the logarithmic phase (Zhao et al. 2016). In a study with Rhodococcus biphenylivorans, some enzymes of VBNC and normal cells, such as α-glucosidase, β-glucosidase, acid phosphatase, naphthol-ASBI-phosphohydrolase, valine arylamidase, leucine arylamidase, esterase lipase, and esterase were found to have similar activities (Su et al. 2015). It was determined by API ZYM kit that acid phosphatase, alkaline phosphatase, and naphthol-ASBI-phosphohydrolase enzymes showed similar activities in S. aureus logarithmic phase cells and VBNC cells (Yan et al. 2021). The study with S. aureus, VBNC cells preserved the enzyme activities related to alkaline phosphatase, acid phosphatase, esterase lipase C8, esterase C4, and naphthol-AS-BI-phosphohydrolase like normal cells (Liao et al. 2021). Alkaline phosphatase, esterase (C4), esterase lipase (C8), β-glucosidase, amylase, lecitinase, and caseinase enzymes were activated in VBNC Bacillus sp. cells under fasting conditions as compared to normal cells (Mahdhi 2012).
Molecular mechanism of VBNC state
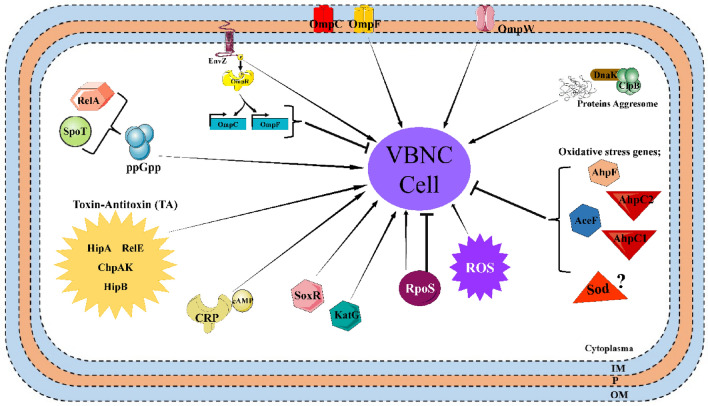
Although many studies have been conducted on the stress factors that trigger the entry of bacteria into VBNC and the bacterial species entering the VBNC state, the molecular mechanism of the VBNC state is still not fully elucidated (Li et al. 2014; Pinto et al. 2015; Dong et al. 2020; Yoon and Lee 2020). Consequently, in some studies carried out to determine the molecule that induces or controls the VBNC state, it has been stated that several proteins controlling the mechanism play an important role in VBNC cell formation. Some proteins involved in the formation of VBNC state in E. coli are summarized in Fig. 1.
Fig. 1.
Some proteins involved in VBNC state formation in E. coli
Proteins found to play a role in the VBNC state include the sensor histidine kinase EnvZ (Darcan et al. 2003, 2009), the alternative sigma factor RpoS (Boaretti et al. 2003; Kusumoto et al. 2012), and the polyphosphate kinase PPK1 (Gangaiah et al. 2009).
Darcan et al. (2009) found that E. coli envZ knockout strains did not enter the VBNC state in their study with seawater. Similarly, they found that this situation did not change under different stress conditions such as osmotic stress, alkaline pH, and the presence of osmotprotectan glycine betaine. That is, even if the stress factors change, the E. coli envZ mutant strain does not enter the VBNC state (Darcan et al. 2003, 2005, 2009). Furthermore, it has been determined in studies that outer membrane porin proteins and EnvZ sensor protein have a role in the formation of the VBNC state.
A significant increase in the synthesis of outer membrane proteins TolC, OmpW, and OmpF was detected in E. coli cells induced to VBNC by oxidative stress, high carbon dioxide pressure, chlorine, and low temperatures (Asakura et al. 2008; Muela et al. 2008; Li et al. 2016; Lin et al. 2017; Zhong and Zhao 2019). In addition, a decrease in the synthesis of the outer membrane protein OmpA was detected under high carbon dioxide pressure (Zhao et al. 2016). It was determined that the expression level of OmpA decreased in Cronobacter sakazakii cells induced to a VBNC state by drought stress (Jameelah et al. 2018).
In addition to outer membrane proteins, it has been determined that the alternative sigma factor RpoS, which is responsible for the regulation of many genes under different stress conditions, also plays an important role in the formation of VBNC. Boaretti et al. (2003) found that rpoS mutant E. coli cells did not enter the VBNC state in the artificial oligotrophic medium at + 4 °C, and died early under stress conditions (Boaretti et al. 2003). It was determined that rpoS expression increased in E. coli cells induced to VBNC state by chlorine (Lin et al. 2017; Chen et al. 2018). On the other hand, in studies performed with E. coli, S. typhimurium, S. enterica, C. sakazakii, and V. cholera under different stress conditions, it was determined that the expression level of RpoS decreased. Therefore, it is seen that there are variable results regarding the role of RpoS in different cells under different stress conditions (González-Escalona et al. 2006; Kusumoto et al. 2012; Jameelah et al. 2018; Xu et al. 2018).
Another enzyme associated with VBNC status is polyphosphate kinase 1 (PPK1), which has a key role in the synthesis of Poly-P. It has been determined in studies that this enzyme is the basic molecule for survival, the virulence and mediating stress responses of bacteria. Gangaiah et al (2009) and Kassem et al (2013) found that the ability of C. jejuni to form VBNC cells decreased with the decrease in the accumulation of poly-P in the Ppk1 mutant. This result indicates that poly-P can positively regulate VBNC formation (Gangaiah et al. 2009; Kassem et al. 2013).
Studies conducted that EnvZ, RpoS, and PPK1, as well as oxidative stress-related genes, LysR-type transcriptional regulator OxyR (Kong et al. 2004; Li et al. 2014), alkyl hydroperoxide reductase subunit AhpC (Asakura et al. 2007; Wang et al. 2013), glutathione S-transferase (GST) (Abe et al. 2007), and superoxide dismutase SodA (Noor et al. 2009) was determined as VBNC status-associated proteins. To determine the relationship of the LysR type transcriptional regulator OxyR with VBNC status, oxyR gene region mutant Vibrio vulnificus cells were exposed to cold, and loss of culturability was observed in the oxyR mutant strains. In other words, the oxyR gene prevents cells from entering the VBNC state (Kong et al. 2004). Asakura et al. (2007) found that the expression levels of ahpCF and aceF decreased in their study with MP37, a stress-sensitive variant of E. coli, under oxidative stress (Asakura et al. 2007). On the other hand, studies have also shown that GST affects the processes of VBNC status in response to oxidative stress (Abe et al. 2007). In the study performed with V. vulnificus in artificial seawater at low temperatures, it was determined that the cells were not induced to the VBNC state in the overexpression of GST (Abe et al. 2007; Li et al. 2014). The sod gene associated with oxidative stress was also found to be associated with VBNC status. In E. coli cells induced to VBNC under high carbon dioxide pressure, it was determined that the sod gene increased significantly in transcriptomic studies, but its expression changed insignificantly in proteomic analyzes (Zhao et al. 2016). Also, Masmoudi et al. reported that sodA inactivation can induce VBNC entry in S. aureus (Masmoudi et al. 2010). It was determined that the expression of katA, an oxidative stress gene, increased in V. cholerae cells induced to the VBNC state by low temperature (Zhao et al. 2017; Xu et al. 2018). When E. coli cells was induced to VBNC state in chlorine and chloramine, it was determined that soxR, and katG genes were expressed at high levels (Chen et al. 2018).
In the recent years, a good number of study have been carried out to unravel the molecular mechanism of VBNC with transcriptomic analyzes. This method provides the analysis of expression levels of all existing genes under the same conditions. Therefore, it can be determined which genes are active or inactive in the VBNC state. In the literature, there are transcriptomics-based studies carried out with different stress conditions and different microorganism species. In E. coli induced to VBNC state by low temperatures, a total of 2298 genes were detected, where the synthesis of 1735 genes increased and 563 genes decreased (Zhong and Zhao 2019). According to the RNA-seq and iTRAQ results in E. coli cells induced to VBNC under high CO2 pressure, it was revealed that 97 genes and 56 proteins were significantly altered in the VBNC state (Zhao et al. 2016).
In E. coli cells induced to VBNC using chlorine, it was found that the expression of 203 genes increased, 159 genes decreased, and the expression of 362 genes in total changed significantly (Ye et al. 2020). In another VBNC study with E. coli, cells were exposed to high-pressure carbon dioxide (HPDC), extracellular pH 3, and high pressure (HP), and their induction into the VBNC state was investigated. RNA-seq analyzes were performed with cells induced into the VBNC state. According to the results of the analysis, 85, 263, and 529 differently expressed genes were detected in HP, pH 3, and HPCD-treated cells compared to normal cells, respectively. 59 expressed genes were related in both pH 3 and HPCD treatment, and it was thought that these genes might be responsible for VBNC induction. It was emphasized that these detected genes are mainly involved in cellular transport and localization (Yang et al. 2022).
As a result of transcriptomic analyzes with S. cerevisiae cells induced to VBNC state by isomerized hop extract, while genes involved in carbohydrate, amino acid metabolism, DNA replication, and cell division were down-regulated in VBNC cells, but TCA cycle, ABC transporter, organic acid metabolism, and oxidoreductase activities were found to increase. Compared wıth normal cells, a total of 749 differently expressed genes were detected in VBNC cells, of which the synthesis of 499 genes increased, while the synthesis of 250 genes decreased. Cells that underwent resuscitation and VBNC cells were also compared, and a total of 1271 genes were found to be differentially expressed (Xiao et al. 2022).
In the microarray analysis with V. cholerae induced to VBNC state at low temperatures in artificial seawater, the proteins of transport, binding proteins, DNA metabolism, cell envelope proteins, energy metabolism, amino acid biosynthesis, fatty acid, and phospholipid metabolism, conserved proteins have been identified. These proteins increase and decrease the expression of many genes responsible for cellular processes were detected (Asakura et al. 2007). The study performed with V. cholerae in artificial seawater at low temperatures, it was determined that the synthesis of 17 genes, including lysine/cadaverine antiporter, 50S ribosomal protein L29, a serine protease, aminoimidazole riboside kinase, and DNA primase increased (Casasola-Rodríguez et al. 2018).
RNA-seq analysis of V. cholerae cells induced to VBNC state at low temperatures in artificial seawater, it was determined that the synthesis of 985 genes increased, the synthesis of 435 genes decreased, and the expression of 1420 genes in total changed. Biofilm formation (rbmA and bapl), chitin use (vc0769, vca0027, and vc1073), and synthesis of stress genes (groEL, groES, and dnaK) increased, while synthesis of cell division (ftsZ and DicC), morphology (mreBCD) and ribosomal activity genes decreased (Xu et al. 2018). The three studies mentioned above are based on the induction of V. cholerae into the VBNC state in artificial seawater at low temperatures. However, when these studies were compared, it was determined that the gene expression data did not give consistent results with each other. For example, Asakura et al. found that genes, such as mshM, vctc, rfbL, and malG are very highly expressed, Xu et al. found that these genes were decreased. When the studies are examined in this way, there is great diversity, and the studies cannot be confirmed by each other. Therefore, more studies are needed to unravel the molecular mechanism of VBNC.
This figure summarizes the genes associated with the VBNC condition in E. coli. Arrows indicate positive regulators of genes in VBNC formation, and T-shaped lines indicate negative regulators.
Virulence of bacteria in VBNC status
It has been determined by many studies that bacteria in the VBNC state maintain pathogenicity. It has been determined that many microorganism species such as E. coli, S. aureus, Vibrio sp, C. jejuni, Salmonella, Shigella, M. tuberculosis, and Cronobacter maintain their virulence in the case of VBNC.
In a study with S. typhimurium, it was determined that the cells were induced to VBNC state at − 20 °C and CuSO4 exposure. Then, both VBNC cells, resuscitated cells, and saline as a control was injected into mouse experimental groups. Mice inoculated with both VBNC cells and resuscitated cells after injection developed diarrhea, whereas none of the mice in the control group became ill. In addition, culturable S. typhimurium cells were isolated from mouse ascites of these two groups (Zeng et al. 2013). In another study, it was determined that V. parahaemolyticus cells induced in VBNC state in artificial seawater can colonize rat intestines, maintain their virulence in rat ileal loop model experiments, and can be isolated from the intestines of about half of mice (Baffone et al. 2003). The virulence of V. vulnificus cells induced into VBNC state at low temperatures was determined by mouse experiments and it was determined that V. vulnificus in VBNC state killed mice. In addition, resuscitated V. vulnificus cells could be isolated from dead mice (Oliver and Bockian 1995). In a study in the 4 °C microcosm with an attenuated strain of Vibrio cholerae, cells were induced into VBNC state. V. cholerae cells induced to VBNC state were then inoculated into a group of volunteers. It has been found that VBNC V. cholerae cells can be colonized and cultured in the human intestine (Colwell et al. 1996). Edwardsiella tarda, a fish pathogen, induced VBNC in seawater at low temperatures and its virulence properties were investigated. In virulence experiments with turbot fish, VBNC cells, normal cells, resuscitated cells, and sterile saline were injected into fish, and all fish died except those injected with saline. The cause of death was confirmed by re-culturing the bacterium from the acid fluid, kidneys and spleens of dead fish (Du et al. 2007). In another study, under copper stress in a nutrient-poor microcosm, E. coli cells were induced to VBNC and the cells were resuscitated as a result of relieving stress by chelation of copper ions. It has been determined that stx2, aggR, aggA genes, which are major virulence gene markers, are preserved in resuscitated cells (Aurass et al. 2011). Also, in another study with E. coli O157:H7, cells were induced into VBNC state at high temperature. It was found that Shiga toxin genes (stx1, stx2) and virulence genes (eae and hlyA) were highly expressed in cells induced to VBNC state. Among these genes, especially eae and stx2 genes were found to be expressed at a higher rate in VBNC conditions than in culturable condition, and as a result, higher virulence in VBNC cells was considered (Fu et al. 2020). Similarly, cytotoxicity tests were performed on Vero cells to quantitatively evaluate Stx production in E. coli O157:H7 cells induced to VBNC state by river water, PBS buffer, deionized water, and chloramine water. It has been found that VBNC cells retain their ability to produce Stx under all conditions and have different levels of Stx (Liu et al. 2010). In conclusion, virulence continues in E. coli O157:H7 cells both in VBNC state and in resuscitated cells.
Yoon and Lee (2018) found that three different strains of V. parahaemolyticus induced to VBNC state in artificial seawater maintained cellular toxicity against Vero and Caco-2 human cell lines and regained cytotoxicity even after resuscitation (Yoon and Lee 2018). In another cytotoxicity study with V. parahaemolyticus cells in VBNC condition, it was determined that VBNC V. parahaemolyticus cells showed cytotoxic effects on the HEp-2 cell line. In the same study, mouse experiments were also performed, and it was found that the infection time of cells in VBNC state was prolonged compared to cells in the exponential phase, but the mouse lethality and enteropathogenicity were preserved (Wong et al. 2004). The virulence and resuscitation of C. jejuni cells induced to VBNC state at low temperatures were investigated. Transcription levels of virulence-related genes (flaA, flaB, cadF, ciaB, cdtA, cdtB, and cdtC) were found to be low but maintained in VBNC C. jejuni cells, and VBNC C. jejuni cells were able to invade Caco-2 human intestinal epithelial cells (Chaisowwong et al. 2012). S. dysenteriae was induced into VBNC state under starvation stress and cytotoxicity study was performed using HeLa cells. It has been determined that pathogenicity is preserved in S. dysenteriae in the VBNC state and expression of the Shiga toxin (stx) gene is maintained (Rahman et al. 1994, 1996). However, in cytotoxicity studies, it is not known whether the pathogenicity originates from resuscitation or from VBNC cells. Pathogenicity may also be due to resuscitated bacteria. Therefore, in such pathogenicity studies, it is necessary to precisely determine whether the disease is caused by cells that have been resuscitated and become reproducible. As a result, in many studies, it has been determined that virulence persists in bacteria with VBNC state and differs according to bacterial species. Some bacteria in the VBNC state remain dormant under stress conditions until they regain their viability against various factors and can cause disease when resuscitated, while others continue to express their virulence/toxicity-related genes and remain pathogenic under the VBNC state (Zhang et al. 2015; Li et al. 2016; Zhao et al. 2017; Dong et al. 2020).
Resuscitation of bacteria in VBNC status
VBNC cells that cannot grow in routine culture environments but are metabolically active can return to an active and culturable state when the environmental conditions are favorable again or with some treatments that can be applied. This condition is known as resuscitation, and many factors can cause resuscitation. In cells induced to VBNC state by low or high temperature, keeping the temperature at the optimum level allows the cells to can be resuscitated (Mizunoe et al. 2000; Zeng et al. 2013; Fu et al. 2020). Also, factors play a role in reversing the VBNC condition, such as nutrient addition (Bekir et al. 2015; Song and Lee 2021), and increasing ambient humidity in drought (Se et al. 2021).
In addition, many chemical factors trigger the exit of cells from the VBNC state. For example, chemicals such as sodium pyruvate (Mizunoe et al. 2000), Tween 20 (Zeng et al. 2013; Jiang 2014), Tween 80 (Wei and Zhao 2018), NaCl (Casasola-Rodríguez et al. 2018), amino acids, such as (Pinto et al. 2011; Pienaar et al. 2016), glutamine, threonine, serine, some vitamins (Hommel et al. 2019), and active proteins (Mizunoe et al. 2000; Zeng et al. 2013; Jiang 2014; Pinto et al. 2015; Liu et al. 2018; Zhu et al. 2022), such as catalase, DnaK, Rpfs, YeaZ, autoinducers, norepinephrine (Pinto et al. 2011; Ayrapetyan et al. 2014) and antioxidizing compounds (Mizunoe et al. 2000; Liao et al. 2018) many substances such as can increase the culturability of VBNC cell population. However, culturability differs for each bacterial species, and sometimes multiple stimuli may be required for the culturability of a VBNC population. In bacteria such as V. parahaemolyticus, V. cholerae and V. vulnificus induced into VBNC state by cold stress, resuscitation occurs by elimination of cold stress (Yoon and Lee 2020). Enterohemorrhagic E. coli induced in VBNC by starvation and low temperature may resuscitate in environments modified with catalase or sodium pyruvate (Mizunoe et al. 2000). In cells that enter VBNC state with high temperatures, the exposure time to the temperature has different results on the resuscitation of the cells. Although the cells induced to VBNC by short-term exposure to high temperature can be resuscitation at optimum temperature values, cells exposed to high temperature for a long time cannot resuscitation (Fu et al. 2020). E. coli O157:H7 cells induced to VBNC state by electrolyzed oxidizing water were resuscitated in medium containing sodium pyruvate and Tween 20 (Afari and Hung 2018). Moreover, studies have shown that protein aggregation in E. coli may be associated with VBNC status, and this aggregation status may also be associated with resuscitation (Desnues et al. 2003; Pu et al. 2019; Fu et al. 2020). It has been determined that the DnaK–ClpB apparatus is required for resuscitation in VBNC cells (Pu et al. 2019).
Conclusion
VBNC state in bacteria is an important survival strategy developed against many physical and chemical factors such as temperature, pH, antibiotics, oxidation and heavy metals. Although VBNC state is predicted by some researchers as a result of genetic mechanism, some researchers suggest that it is random (Ravel et al. 1995; Desnues et al. 2003). There have been many studies on the VBNC state over the years. Many areas have been studied, including microorganisms entering VBNC state, conditions inducing VBNC state, physiology, virulence, resuscitation, and molecular mechanism of VBNC cells. However, as compiled above, conclusive and meaningful conclusions regarding the mechanism of VBNC state could not be drawn. Because, although the detected genes are logically related to VBNC, they do not give clear information about the mechanism. No clear information could be provided about how the genes determined in the studies regulate which genes in a molecular pathway, and different results were obtained regarding genes roles in different organisms and under different stress conditions. Despite the increase and decrease in the expression level of many genes in many transcriptome-based studies, a common metabolic pathway used in the molecular mechanism of VBNC has not been determined. Since different genes are prominent in cells entering VBNC state under different conditions, a common and valid mechanism could not be determined. Therefore, the mechanism of the VBNC state still contains serious loopholes. Moreover, VBNC poses a problem for public health and food safety, as cells retain their virulence and can resuscitation under appropriate conditions. Therefore, further studies are needed to unravel the mechanism involved in the induction and resuscitation of the VBNC state.
Declarations
Conflict of interest
The authors declared no financial conflict of interest.
Ethics approval
Not applicable.
Consent to participate
All authors consent to participate.
Consent for publication
All authors consent for publication.
Contributor Information
Özge Kaygusuz İzgördü, Email: ozge.kaygusuz@bilecik.edu.tr.
Cihan Darcan, Email: cihan.darcan@bilecik.edu.tr.
Ergin Kariptaş, Email: ergin.kariptas@samsun.edu.tr.
References
- Abdallah F, Lagha R, Bakhrouf A. Resuscitation and morphological alterations of Salmonella bovismorbificans cells under starvation in soil. World J Microbiol Biotechnol. 2008;24:1507–1512. doi: 10.1007/S11274-007-9633-Y. [DOI] [Google Scholar]
- Abe A, Ohashi E, Ren H, et al. Isolation and characterization of a cold-induced nonculturable suppression mutant of Vibrio vulnificus. Microbiol Res. 2007;162:130–138. doi: 10.1016/j.micres.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Afari GK, Hung YC. Detection and verification of the viable but nonculturable (VBNC) State of Escherichia coli O157:H7 and Listeria monocytogenes using flow cytometry and standard plating. J Food Sci. 2018;83:1913–1920. doi: 10.1111/1750-3841.14203. [DOI] [PubMed] [Google Scholar]
- Amel BKN, Amine B, Amina B. Survival of Vibrio fluvialis in seawater under starvation conditions. Microbiol Res. 2008;163:323–328. doi: 10.1016/J.MICRES.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Asakura H, Makino S, Takagi T, et al. Passage in mice causes a change in the ability of Salmonella enterica serovar Oranienburg to survive NaCl osmotic stress: resuscitation from the viable but non-culturable state. FEMS Microbiol Lett. 2002;212:87–93. doi: 10.1111/J.1574-6968.2002.TB11249.X. [DOI] [PubMed] [Google Scholar]
- Asakura H, Panutdaporn N, Kawamoto K, et al. Proteomic characterization of enterohemorrhagic Escherichia coli O157:H7 in the oxidation-induced viable but non-culturable state. Microbiol Immunol. 2007;51:875–881. doi: 10.1111/j.1348-0421.2007.tb03969.x. [DOI] [PubMed] [Google Scholar]
- Asakura H, Kawamoto K, Haishima Y, et al. Differential expression of the outer membrane protein W (OmpW) stress response in enterohemorrhagic Escherichia coli O157:H7 corresponds to the viable but non-culturable state. Res Microbiol. 2008;159:709–717. doi: 10.1016/j.resmic.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Aurass P, Prager R, Flieger A. EHEC/EAEC O104:H4 strain linked with the 2011 German outbreak of haemolytic uremic syndrome enters into the viable but non-culturable state in response to various stresses and resuscitates upon stress relief. Environ Microbiol. 2011;13:3139–3148. doi: 10.1111/j.1462-2920.2011.02604.x. [DOI] [PubMed] [Google Scholar]
- Ayrapetyan M, Williams TC, Oliver JD. Interspecific quorum sensing mediates the resuscitation of viable but nonculturable vibrios. Appl Environ Microbiol. 2014;80:2478. doi: 10.1128/AEM.00080-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayrapetyan M, Williams T, Oliver JD. Relationship between the viable but nonculturable state and antibiotic persister cells. J Bacteriol. 2018;200:2. doi: 10.1128/JB.00249-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baffone W, Citterio B, Vittoria E, et al. Retention of virulence in viable but non-culturable halophilic Vibrio spp. Int J Food Microbiol. 2003;89:31–39. doi: 10.1016/S0168-1605(03)00102-8. [DOI] [PubMed] [Google Scholar]
- Bai H, Zhao F, Li M, et al. Citric acid can force Staphylococcus aureus into viable but nonculturable state and its characteristics. Int J Food Microbiol. 2019 doi: 10.1016/j.ijfoodmicro.2019.108254. [DOI] [PubMed] [Google Scholar]
- Bekir K, Barhoumi H, Braiek M, et al. Electrochemical impedance immunosensor for rapid detection of stressed pathogenic Staphylococcus aureus bacteria. Environ Sci Pollut Res. 2015;22:15796–15803. doi: 10.1007/s11356-015-4761-7. [DOI] [PubMed] [Google Scholar]
- Besnard V, Federighi M, Declerq E, et al. Environmental and physico-chemical factors induce VBNC state in Listeria monocytogenes. Vet Res. 2002;33:359–370. doi: 10.1051/vetres:2002022. [DOI] [PubMed] [Google Scholar]
- Boaretti M, Lleò MDM, Bonato B, et al. Involvement of rpoS in the survival of Escherichia coli in the viable but non-culturable state. Environ Microbiol. 2003;5:986–996. doi: 10.1046/j.1462-2920.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- Bovill RA, Mackey BM. Resuscitation of “non-culturable” cells from aged cultures of Campylobacter jejuni. Microbiology. 1997;143:1575–1581. doi: 10.1099/00221287-143-5-1575. [DOI] [PubMed] [Google Scholar]
- Byrd JJ, Xu HS, Colwell RR. Viable but nonculturable bacteria in drinking water. Appl Environ Microbiol. 1991;57:875–878. doi: 10.1128/aem.57.3.875-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capozzi V, di Toro MR, Grieco F, et al. Viable but not culturable (VBNC) state of Brettanomyces bruxellensis in wine: new insights on molecular basis of VBNC behaviour using a transcriptomic approach. Food Microbiol. 2016;59:196–204. doi: 10.1016/j.fm.2016.06.007. [DOI] [PubMed] [Google Scholar]
- Casasola-Rodríguez B, Ruiz-Palacios GM, Pilar RC, et al. Detection of VBNC Vibrio cholerae by RT-real time PCR based on differential gene expression analysis. FEMS Microbiol Lett. 2018;365:1–8. doi: 10.1093/femsle/fny156. [DOI] [PubMed] [Google Scholar]
- Chaisowwong W, Kusumoto A, Hashimoto M, et al. Physiological characterization of Campylobacter jejuni under cold stresses conditions: its potential for public threat. J Vet Med Sci. 2012;74:43–50. doi: 10.1292/JVMS.11-0305. [DOI] [PubMed] [Google Scholar]
- Chen S, Li X, Wang Y, et al. Induction of Escherichia coli into a VBNC state through chlorination/chloramination and differences in characteristics of the bacterium between states. Water Res. 2018;142:279–288. doi: 10.1016/j.watres.2018.05.055. [DOI] [PubMed] [Google Scholar]
- Colwell RR, Brayton P, Herrington D, et al. Viable but non-culturable Vibrio cholerae O1 revert to a cultivable state in the human intestine. World J Microbiol Biotechnol. 1996;12:28–31. doi: 10.1007/BF00327795. [DOI] [PubMed] [Google Scholar]
- Cunningham E, O’Byrne C, Oliver JD. Effect of weak acids on Listeria monocytogenes survival: evidence for a viable but nonculturable state in response to low pH. Food Control. 2009;20:1141–1144. doi: 10.1016/J.FOODCONT.2009.03.005. [DOI] [Google Scholar]
- Darcan C (2005) An investigation on the effect of pH, starvation and osmotic stress on outer membrane porin protein synthesis level of Escherichia coli in the black sea water. Ph. D. Thesis, Deparment of Biology, Ondokuz Mayis University, 180 p.
- Darcan C. Expression of OmpC and OmpF porin proteins and survival of Escherichia coli under photooxidative stress in black sea water. Aquat Biol. 2012;17:97–105. doi: 10.3354/ab00458. [DOI] [Google Scholar]
- Darcan C, Aydin E. Fur- mutation increases the survival time of Escherichia coli under photooxidative stress in aquatic environments. Acta Biol Hung. 2012;63:399–409. doi: 10.1556/ABiol.63.2012.3.10. [DOI] [PubMed] [Google Scholar]
- Darcan C, Özkanca R, Flint KP. Survival of nonspecific porin-deficient mutants of Escherichia coli in black sea water. Lett Appl Microbiol. 2003;37:380–385. doi: 10.1046/J.1472-765X.2003.01418.X. [DOI] [PubMed] [Google Scholar]
- Darcan C, Özkanca R, I̧di̧l Ö, Flint KP Viable but non-culturable state (VBNC) of Escherichia coli related to EnvZ under the effect of pH, starvation and osmotic stress in sea water. Pol J Microbiol. 2009;58:307–317. [PubMed] [Google Scholar]
- Defives C, Guyard S, Oularé MM, et al (1999) Total counts, culturable and viable, and non-culturable microflora of a French mineral water: a case study [DOI] [PubMed]
- del Campo R, Russi P, Mara P, et al. Xanthomonas axonopodis pv. citri enters the VBNC state after copper treatment and retains its virulence. FEMS Microbiol Lett. 2009;298:143–148. doi: 10.1111/j.1574-6968.2009.01709.x. [DOI] [PubMed] [Google Scholar]
- Denisova V, Mezule L, Juhna T. The effect of chitosan nanoparticles on escherichia coli viability in drinking water disinfection. Water Pract Technol. 2022 doi: 10.2166/wpt.2022.012. [DOI] [Google Scholar]
- Desnues B, Cuny C, Grégori G, et al. Differential oxidative damage and expression of stress defence regulons in culturable and non-culturable Escherichia coli cells. EMBO Rep. 2003;4:400–404. doi: 10.1038/sj.embor.embor799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding T, Suo Y, Xiang Q, et al. Significance of viable but nonculturable Escherichia coli: Induction, detection, and control. J Microbiol Biotechnol. 2017;27:417–428. doi: 10.4014/jmb.1609.09063. [DOI] [PubMed] [Google Scholar]
- Dinu L-D, Bach S. Induction of viable but nonculturable Escherichia coli O157:H7 in the phyllosphere of lettuce: a food safety risk factor. Appl Envıron Mıcrobıol. 2011;77:8295–8302. doi: 10.1128/AEM.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K, Pan H, Yang D, et al. Induction, detection, formation, and resuscitation of viable but non-culturable state microorganisms. Comprehen Rev Food Sci Food Saf. 2020;19:149–183. doi: 10.1111/1541-4337.12513. [DOI] [PubMed] [Google Scholar]
- Du M, Chen J, Zhang X, et al. Characterization and resuscitation of viable but nonculturable Vibrio alginolyticus VIB283. Arch Microbiol. 2007;188:283–288. doi: 10.1007/S00203-007-0246-5/FIGURES/4. [DOI] [PubMed] [Google Scholar]
- Fakruddin Md, Mannan KS, Andrews S Viable but nonculturable bacteria: food safety and public health perspective. ISRN Microbiol. 2013;2013:1–6. doi: 10.1155/2013/703813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes E, Martins VC, Nóbrega C, et al. A bacteriophage detection tool for viability assessment of Salmonella cells. Biosens Bioelectron. 2013 doi: 10.1016/j.bios.2013.08.053. [DOI] [PubMed] [Google Scholar]
- Fu Y, Jia Y, Fan J, et al. Induction of Escherichia coli O157:H7 into a viable but non-culturable state by high temperature and its resuscitation. Environ Microbiol Rep. 2020;12:568–577. doi: 10.1111/1758-2229.12877. [DOI] [PubMed] [Google Scholar]
- Gangaiah D, Kassem II, Liu Z, Rajashekara G. Importance of polyphosphate kinase 1 for Campylobacter jejuni viable-but-nonculturable cell formation, natural transformation, and antimicrobial resistance. Appl Envıron Mıcrobıol. 2009;75:7838–7849. doi: 10.1128/AEM.01603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Liao X, Zhao X, et al. The diagnostic tools for viable but nonculturable pathogens in the food industry: current status and future prospects. Comprehens Rev Food Sci Food Saf. 2021;20:2146–2175. doi: 10.1111/1541-4337.12695. [DOI] [PubMed] [Google Scholar]
- González-Escalona N, Fey A, Höfle MG, et al. Quantitative reverse transcription polymerase chain reaction analysis of Vibrio cholerae cells entering the viable but non-culturable state and starvation in response to cold shock. Environ Microbiol. 2006;8:658–666. doi: 10.1111/j.1462-2920.2005.00943.x. [DOI] [PubMed] [Google Scholar]
- Guo L, Wan K, Zhu J, et al. Detection and distribution of vbnc/viable pathogenic bacteria in full-scale drinking water treatment plants. J Hazard Mater. 2021;406:124335. doi: 10.1016/J.JHAZMAT.2020.124335. [DOI] [PubMed] [Google Scholar]
- Han D, Hung YC, Wang L. Evaluation of the antimicrobial efficacy of neutral electrolyzed water on pork products and the formation of viable but nonculturable (VBNC) pathogens. Food Microbiol. 2018;73:227–236. doi: 10.1016/J.FM.2018.01.023. [DOI] [PubMed] [Google Scholar]
- Heidelberg JF, Shahamat M, Levin M, et al. Effect of aerosolization on culturability and viability of gram-negative bacteria. Appl Environ Microbiol. 1997;63:3585–3588. doi: 10.1128/aem.63.9.3585-3588.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoefman S, van Hoorde K, Boon N, et al. Survival or revival: long-term preservation induces a reversible viable but non-culturable state in methane-oxidizing bacteria. PLoS ONE. 2012;7:34196. doi: 10.1371/journal.pone.0034196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel B, Sturny-Leclère A, Volant S, et al. Cryptococcus neoformans resists to drastic conditions by switching to viable but non-culturable cell phenotype. PLoS Pathog. 2019;15:e1007945. doi: 10.1371/JOURNAL.PPAT.1007945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung WC, Jane WN, Wong HC. Association of a D-alanyl-D-alanine carboxypeptidase gene with the formation of aberrantly shaped cells during the induction of viable but nonculturable Vibrio parahaemolyticus. Appl Environ Microbiol. 2013;79:7305–7312. doi: 10.1128/AEM.01723-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein and Ghit Detection of viable but non-culturable (vbnc) escherichia coli from beef liver using Pma-PCR assay. Alexandr J Vet Sci. 2021;69:1. doi: 10.5455/ajvs.75980. [DOI] [Google Scholar]
- Idil Ö, Özkanca R, Darcan C, Flint KP. Escherichia coli: dominance of red light over other visible light sources in establishing viable but nonculturable state. Photochem Photobiol. 2010;86:104–109. doi: 10.1111/J.1751-1097.2009.00636.X. [DOI] [PubMed] [Google Scholar]
- Jameelah M, Dewanti-Hariyadi R, Nurjanah S. Expression of rpoS, ompA and hfq genes of Cronobacter sakazakii strain Yrt2a during stress and viable but nonculturable state. Food Sci Biotechnol. 2018 doi: 10.1007/s10068-018-0313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L. Low temperature and copper induce viable but nonculturable state of Salmonella typhi in the bottled drinking water. Adv Mater Res. 2014;893:492–495. doi: 10.4028/www.scıentıfıc.net/amr.893.492. [DOI] [Google Scholar]
- Kassem II, Chandrashekhar K, Rajashekara G, et al. Of energy and survival incognito: a relationship betwee viable but non-culturable cells formation and inorganic polyphosphate and formate metabolism in Campylobacter jejuni n. Front Microbiol. 2013 doi: 10.3389/fmicb.2013.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Kong IS, Bates TC, Hülsmann A, et al. Role of catalase and oxyR in the viable but nonculturable state of Vibrio vulnificus. FEMS Microbiol Ecol. 2004;50:133–142. doi: 10.1016/J.FEMSEC.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Ghosh AR. Assessment of bacterial viability: a comprehensive review on recent advances and challenges. Microbiology (united Kingdom) 2019;165:593–610. doi: 10.1099/mic.0.000786. [DOI] [PubMed] [Google Scholar]
- Kusumoto A, Asakura H, Kawamoto K. General stress sigma factor RpoS influences time required to enter the viable but non-culturable state in Salmonella enterica. Microbiol Immunol. 2012;56:228–237. doi: 10.1111/j.1348-0421.2012.00428.x. [DOI] [PubMed] [Google Scholar]
- Lemke MJ, Leff LG. Culturability of stream bacteria assessed at the assemblage and population levels. Microb Ecol. 2006;51:365–374. doi: 10.1007/s00248-006-9026-z. [DOI] [PubMed] [Google Scholar]
- Li L, Mendis N, Trigui H, et al. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. 2014;5:1–1. doi: 10.3389/fmicb.2014.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Xu Z, Zhao F, et al. Synergetic effects of high-pressure carbon dioxide and nisin on the inactivation of Escherichia coli and Staphylococcus aureus. Innov Food Sci Emerg Technol. 2016;33:180–186. doi: 10.1016/j.ifset.2015.11.013. [DOI] [Google Scholar]
- Li Y, Huang TY, Ye C, et al. Formation and control of the viable but non-culturable state of foodborne pathogen Escherichia coli O157:H7. Front Microbiol. 2020 doi: 10.3389/fmicb.2020.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Jiang L, Zhang R. Induction of a viable but non-culturable state in Salmonella Typhimurium by thermosonication and factors affecting resuscitation. FEMS Microbiol Lett. 2018;365:1–11. doi: 10.1093/femsle/fnx249. [DOI] [PubMed] [Google Scholar]
- Liao X, Hu W, Liu D, Ding T. Stress resistance and pathogenicity of nonthermal-plasma- induced viable-but-nonculturable staphylococcus aureus through energy suppression, oxidative stress defense, and immune-escape mechanisms. Appl Environ Microbiol. 2021;87:1–17. doi: 10.1128/AEM.02380-20/ASSET/483DC0EA-C10D-4EBF-B33A-72D60693EB74/ASSETS/GRAPHIC/AEM.02380-20-F0011.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Ye C, Chen S, et al. Viable but non-culturable E. coli induced by low level chlorination have higher persistence to antibiotics than their culturable counterparts. Environ Pollut. 2017;230:242–249. doi: 10.1016/j.envpol.2017.06.047. [DOI] [PubMed] [Google Scholar]
- Lindback T, Lindback L, Rottenberg ME, et al. The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among Listeria monocytogenes isolates from salmon, patients and environment. Vet Res. 2022 doi: 10.1051/vetres/2009056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gilchrist A, Zhang J, Li X-F. Detection of viable but nonculturable Escherichia coli O157:H7 bacteria in drinking water and river water. Appl Envıron Mıcrobıol. 2008;74:1502–1507. doi: 10.1128/AEM.02125-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang C, Tyrrell G, Li XF. Production of Shiga-like toxins in viable but nonculturable Escherichia coli O157:H7. Water Res. 2010;44:711–718. doi: 10.1016/J.WATRES.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Liu J, Deng Y, Soteyome T, et al. Induction and recovery of the viable but nonculturable state of hop-resistance lactobacillus brevis. Front Microbiol. 2018 doi: 10.3389/fmicb.2018.02076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleò MM, Bonato B, Tafi MC, et al. Resuscitation rate in different enterococcal species in the viable but non-culturable state. J Appl Microbiol. 2001;91:1095–1102. doi: 10.1046/j.1365-2672.2001.01476.x. [DOI] [PubMed] [Google Scholar]
- Lloyd D, Hayes AJ. Vigour, vitality and viability of microorganisms. FEMS Microbiol Lett. 1995;133:1–7. doi: 10.1016/0378-1097(95)00322-V. [DOI] [Google Scholar]
- Lothigius Å, Sjöling Å, Svennerholm AM, Bölin I. Survival and gene expression of enterotoxigenic Escherichia coli during long-term incubation in sea water and freshwater. J Appl Microbiol. 2010;108:1441–1449. doi: 10.1111/j.1365-2672.2009.04548.x. [DOI] [PubMed] [Google Scholar]
- Maalej S, Gdoura R, Dukan S, et al. Maintenance of pathogenicity during entry into and resuscitation from viable but nonculturable state in Aeromonas hydrophila exposed to natural seawater at low temperature. J Appl Microbiol. 2004;97:557–565. doi: 10.1111/J.1365-2672.2004.02336.X. [DOI] [PubMed] [Google Scholar]
- Mahdhi A. Change in physiological cellular state of halophilic Bacillus sp. under long marine stress starvation conditions. Afr J Microbiol Res. 2012 doi: 10.5897/AJMR12.1139. [DOI] [Google Scholar]
- Masmoudi S, Denis M, Maalej S. Inactivation of the gene katA or sodA affects the transient entry into the viable but non-culturable response of Staphylococcus aureus in natural seawater at low temperature. Mar Pollut Bull. 2010;60(60):2209–2214. doi: 10.1016/j.marpolbul.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Millet V, Lonvaud-Funel A (2000) The viable but non-culturable state of wine micro-organisms during storage [DOI] [PubMed]
- Mizunoe Y, Wai SN, Ishikawa T, et al. Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol Lett. 2000;186:115–120. doi: 10.1111/J.1574-6968.2000.TB09091.X. [DOI] [PubMed] [Google Scholar]
- Muela A, Seco C, Camafeita E, et al. Changes in Escherichia coli outer membrane subproteome under environmental conditions inducing the viable but nonculturable state. FEMS Microbiol Ecol. 2008;64:28–36. doi: 10.1111/j.1574-6941.2008.00453.x. [DOI] [PubMed] [Google Scholar]
- Nicolò MS, Gioffrè A, Carnazza S, et al. Viable but nonculturable state of foodborne pathogens in grapefruit juice: a study of laboratory. Foodborne Pathog Dis. 2011;8:11–17. doi: 10.1089/fpd.2009.0491. [DOI] [PubMed] [Google Scholar]
- Noll M, Trunzer K, Vondran A, et al. Benzalkonium chloride induces a VBNC state in Listeria monocytogenes. Microorganisms. 2020;8:4–6. doi: 10.3390/microorganisms8020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor R, Murata M, Yamada M. Oxidative stress as a trigger for growth phase-specific σ-dependent cell lysis in Escherichia coli. J Mol Microbiol Biotechnol. 2009;17:177–187. doi: 10.1159/000236029. [DOI] [PubMed] [Google Scholar]
- Oliver JD. The public health significance of viable but nonculturable bacteria. Noncult Microorgan Environ. 2000 doi: 10.1007/978-1-4757-0271-2_16. [DOI] [Google Scholar]
- Oliver JD. The viable but nonculturable state in bacteria. J Microbiol. 2005;43:93–100. [PubMed] [Google Scholar]
- Oliver JD. The viable but nonculturable state for bacteria: status update. Microbe. 2016;11:93–100. [Google Scholar]
- Oliver JD, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61:2620–2623. doi: 10.1128/aem.61.7.2620-2623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou A, Wang K, Mao Y, et al. First report on the rapid detection and identification of methicillin-resistant Staphylococcus aureus (MRSA) in viable but non-culturable (VBNC) under food storage conditions. Front Microbiol. 2021;11:1–7. doi: 10.3389/fmicb.2020.615875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, Dong K, Rao L, et al. Quantitative detection of viable but nonculturable state Escherichia coli O157:H7 by ddPCR combined with propidium monoazide. Food Control. 2020;112:107140. doi: 10.1016/J.FOODCONT.2020.107140. [DOI] [Google Scholar]
- Park SY, Kim CG. A comparative study of three different viability tests for chemically or thermally inactivated Escherichia coli. Environ Eng Res. 2018;23:282–287. doi: 10.4491/eer.2017.223. [DOI] [Google Scholar]
- Pawlowski DR, Metzger DJ, Raslawsky A, et al. Entry of Yersinia pestis into the viable but nonculturable state in a low-temperature tap water microcosm. PLoS ONE. 2011 doi: 10.1371/journal.pone.0017585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar JA, Singh A, Barnard TG. The viable but non-culturable state in pathogenic Escherichia coli: A general review. Afr J Lab Med. 2016 doi: 10.4102/AJLM.V5I1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto D, Almeida V, Almeida Santos M, Chambel LMM. Resuscitation of Escherichia coli VBNC cells depends on a variety of environmental or chemical stimuli. J Appl Microbiol. 2011;110:1601–1611. doi: 10.1111/j.1365-2672.2011.05016.x. [DOI] [PubMed] [Google Scholar]
- Pinto D, Santos MA, Chambel L. Thirty years of viable but nonculturable state research: unsolved molecular mechanisms. Crit Rev Microbiol. 2015;41:61–67. doi: 10.3109/1040841X.2013.794127. [DOI] [PubMed] [Google Scholar]
- Pommepuy M, Butin M, Derrien A, et al. Retention of enteropathogenicity by viable but nonculturable Escherichia coli exposed to seawater and sunlight. Appl Environ Microbiol. 1996;62:4621–4626. doi: 10.1128/AEM.62.12.4621-4626.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postnikova OA, Shao J, Mock NM, et al. Gene expression profiling in viable but nonculturable (VBNC) cells of Pseudomonas syringae pv. syringae. Front Microbiol. 2015;6:1419. doi: 10.3389/FMICB.2015.01419/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Progulske-Fox A, Chukkapalli S, Getachew H, et al. VBNC, previously unrecognized in the life cycle of Porphyromonas gingivalis? J Oral Microbiol. 2022 doi: 10.1080/20002297.2021.1952838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu Y, Li Y, Jin X, et al. ATP-dependent dynamic protein aggregation regulates bacterial dormancy depth critical for antibiotic tolerance. Mol Cell. 2019;73:143–156.e4. doi: 10.1016/J.MOLCEL.2018.10.022. [DOI] [PubMed] [Google Scholar]
- Purevdorj-Gage L, Nixon B, Bodine K, et al. Differential effect of food sanitizers on formation of viable but nonculturable Salmonella enterica in poultry. J Food Prot. 2018;81:386–393. doi: 10.4315/0362-028X.JFP-17-335. [DOI] [PubMed] [Google Scholar]
- Qi Z, Huang Z, Liu C. Metabolism differences of biofilm and planktonic Pseudomonas aeruginosa in viable but nonculturable state induced by chlorine stress. Sci Total Environ. 2022 doi: 10.1016/J.SCITOTENV.2022.153374. [DOI] [PubMed] [Google Scholar]
- Rahman I, Shahamat M, Kirchman PA, Colwell RR. Methionine uptake and cytopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol. 1994;60:3573–3578. doi: 10.1128/aem.60.10.3573-3578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Shahamat M, Chowdhury MAR. Potential virulence of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol. 1996;62(1):115–120. doi: 10.1128/aem.62.1.115-120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamurthy T, Ghosh A, Pazhani GP, Shinoda S. Current perspectives on viable but non-culturable (VBNC) pathogenic bacteria. Front Public Health. 2014;2:1–9. doi: 10.3389/fpubh.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J, Knight IT, Monahan CE, et al. Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: Growth or resuscitation? Microbiology (n y) 1995;141:377–383. doi: 10.1099/13500872-141-2-377. [DOI] [PubMed] [Google Scholar]
- Robben C, Fister S, Witte AK, et al. Induction of the viable but non-culturable state in bacterial pathogens by household cleaners and inorganic salts. Sci Rep. 2018;8:1–9. doi: 10.1038/s41598-018-33595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robben C, Witte AK, Schoder D, et al. A fast and easy ATP-based approach enables MIC testing for non-resuscitating VBNC pathogens. Front Microbiol. 2019;10:1–10. doi: 10.3389/fmicb.2019.01365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachidanandham R, Yew-Hoong Gin K. A dormancy state in nonspore-forming bacteria. Appl Microbiol Biotechnol. 2009;81:927–941. doi: 10.1007/s00253-008-1712-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Se J, Fu Y, Xie Y, et al. Proteomic changes of viable but nonculturable (VBNC) Escherichia coli O157:H7 induced by low moisture in an artificial soil. Biol Fertil Soils. 2021;57:219–234. doi: 10.1007/S00374-020-01520-6/FIGURES/8. [DOI] [Google Scholar]
- Signoretto C, Del M, Lleo L, et al (2000) Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state [DOI] [PMC free article] [PubMed]
- Song H, Lee SY. High concentration of sodium chloride could induce the viable and culturable states of Escherichia coli O157:H7 and Salmonella enterica serovar Enteritidis. Lett Appl Microbiol. 2021;72:741–749. doi: 10.1111/LAM.13468. [DOI] [PubMed] [Google Scholar]
- Su X, Sun F, Wang Y, et al. Identification, characterization and molecular analysis of the viable but nonculturable Rhodococcus biphenylivorans. Sci Rep. 2015 doi: 10.1038/SREP18590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Guo L, Ding L, et al. Induction of viable but nonculturable state in rhodococcus and transcriptome analysis using RNA-seq. PLoS ONE. 2016 doi: 10.1371/journal.pone.0147593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Iijima K, Asano S, et al. Induction of viable but nonculturable state in beer spoilage lactic acid bacteria. J Inst Brew. 2006;112:4. doi: 10.1002/j.2050-0416.2006.tb00734. [DOI] [Google Scholar]
- Wang HW, Chung CH, Ma TY, Wong HC. Roles of alkyl hydroperoxide reductase subunit C (AhpC) in viable but nonculturable Vibrio parahaemolyticus. Appl Environ Microbiol. 2013;79:3734–3743. doi: 10.1128/AEM.00560-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C, Zhao X. Induction of viable but nonculturable Escherichia coli O157:H7 by low temperature and its resuscitation. Front Microbiol. 2018;9:2728. doi: 10.3389/FMICB.2018.02728/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Tan Y, Zhao L, et al. Rapid on-site detection of viable Escherichia coli O157: H7 in lettuce using immunomagnetic separation combined with PMAxx-LAMP and nucleic acid lateral flow strip. Microchem J. 2022;178:107348. doi: 10.1016/J.MICROC.2022.107348. [DOI] [Google Scholar]
- Wideman NE, Oliver JD, Crandall PG, Jarvis NA. Detection and potential virulence of viable but non-culturable (VBNC) Listeria monocytogenes: a review. Microorganisms. 2021;9:1–11. doi: 10.3390/microorganisms9010194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HC, Shen CT, Chang CN, et al. Biochemical and virulence characterization of viable but nonculturable cells of Vibrio parahaemolyticus. J Food Prot. 2004;67:2430–2435. doi: 10.4315/0362-028X-67.11.2430. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Wang Z, Sun W, et al. Characterization and formation mechanisms of viable, but putatively non-culturable brewer’s yeast induced by isomerized hop extract. LWT. 2022;155:112974. doi: 10.1016/J.LWT.2021.112974. [DOI] [Google Scholar]
- Xu H-S, Roberts N, Singleton FL, et al. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhang C, Xu P, Wang XC. Mechanisms of ultraviolet disinfection and chlorination of Escherichia coli: culturability, membrane permeability, metabolism, and genetic damage. J Environ Sci (china) 2018;65:356–366. doi: 10.1016/J.JES.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wang K, Liu Z, et al. A novel procedure in combination of genomic sequencing, flow cytometry and routine culturing for confirmation of beer spoilage caused by Pediococcus damnosus in viable but nonculturable state. LWT. 2022;154:112623. doi: 10.1016/j.lwt.2021.112623. [DOI] [Google Scholar]
- Yamamoto H. Viable but nonculturable state as a general phenomenon of non- sporeforming bacteria, and its modeling. J Infect Chemother. 2000;6:112–114. doi: 10.1007/PL00012149. [DOI] [PubMed] [Google Scholar]
- Yan H, Li M, Meng L, Zhao F. Formation of viable but nonculturable state of Staphylococcus aureus under frozen condition and its characteristics. Int J Food Microbiol. 2021 doi: 10.1016/J.IJFOODMICRO.2021.109381. [DOI] [PubMed] [Google Scholar]
- Yang D, Wang Y, Zhao L, et al. Extracellular pH decline introduced by high pressure carbon dioxide is a main factor inducing bacteria to enter viable but non-culturable state. Food Res Int. 2022;151:110895. doi: 10.1016/J.FOODRES.2021.110895. [DOI] [PubMed] [Google Scholar]
- Ye C, Lin H, Zhang M, et al. Characterization and potential mechanisms of highly antibiotic tolerant VBNC Escherichia coli induced by low level chlorination. Sci Rep. 2020;10:1957. doi: 10.1038/s41598-020-58106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomaku D, Yamaguchi N, Nasu M. Improved direct viable count procedure for quantitative estimation of bacterial viability in freshwater environments. Appl Environ Microbiol. 2000;66:5544–5548. doi: 10.1128/AEM.66.12.5544-5548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J-H, Lee S-Y. Physiological characteristics of viable-but-nonculturable vibrio parahaemolyticus upon prolonged exposure to the refrigerator temperature. BioRxiv. 2018 doi: 10.1101/294744. [DOI] [Google Scholar]
- Yoon JH, Lee SY. Characteristics of viable-but-nonculturable Vibrio parahaemolyticus induced by nutrient-deficiency at cold temperature. Crit Rev Food Sci Nutr. 2020;60:1302–1320. doi: 10.1080/10408398.2019.1570076. [DOI] [PubMed] [Google Scholar]
- Zeng B, Zhao G, Cao X, et al. Formation and resuscitation of viable but nonculturable Salmonella typhi. Biomed Res Int. 2013 doi: 10.1155/2013/907170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Ye C, Lin H, et al. UV disinfection induces a vbnc state in Escherichia coli and Pseudomonas aeruginosa. Environ Sci Technol. 2015;49:1721–1728. doi: 10.1021/es505211e. [DOI] [PubMed] [Google Scholar]
- Zhang X-H, Ahmad W, Zhu X-Y, et al. Viable but nonculturable bacteria and their resuscitation: implications for cultivating uncultured marine microorganisms. Marine Life Sci Technol. 2021;3:189–203. doi: 10.1007/s42995-020-00041-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Bi X, Hao Y, Liao X. Induction of viable but nonculturable Escherichia coli O157:H7 by high pressure CO2 and its characteristics. PLoS ONE. 2013;8:e62388. doi: 10.1371/journal.pone.0062388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Wang Y, An H, et al. New insights into the formation of viable but nonculturable Escherichia coli O157:H7 induced by high-pressure CO2. Mbio. 2016;7:1–11. doi: 10.1128/mBio.00961-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhong J, Wei C, et al. Current perspectives on viable but non-culturable state in foodborne pathogens. Front Microbiol. 2017;8:580. doi: 10.3389/fmicb.2017.00580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Zhao X. Detection of viable but non-culturable Escherichia coli O157:H7 by PCR in combination with propidium monoazide. 3 Biotech. 2018;8:28. doi: 10.1007/s13205-017-1052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Zhao X. Transcriptomic analysis of viable but non-culturable Escherichia coli o157:H7 formation induced by low temperature. Microorganisms. 2019 doi: 10.3390/microorganisms7120634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Huang Y, Zhang Y, et al. Roles of virulence regulator ToxR in viable but non-culturable formation by controlling reactive oxygen species resistance in pathogen Vibrio alginolyticus. Microbiol Res. 2022;254:126900. doi: 10.1016/J.MICRES.2021.126900. [DOI] [PubMed] [Google Scholar]
- Zhu L, Shuai X, Xu L, et al. Mechanisms underlying the effect of chlorination and UV disinfection on VBNC state Escherichia coli isolated from hospital wastewater. J Hazard Mater. 2022;423:127228. doi: 10.1016/j.jhazmat.2021.127228. [DOI] [PubMed] [Google Scholar]