Abstract
Introduction: Oropharyngeal colonization with pathogenic organisms contributes to the development of ventilator-associated pneumonia (VAP) in intensive care units (ICUs). Oral hygiene care (OHC) is a very effective method for reducing the risk of VAP in these patients. This study aimed to evaluate recent OHC strategies to decrease VAP.
Methods: Randomized clinical trials (RCTs) published in the PubMed, Scopus, Embase, Cochrane Library, and Web of Science databases from inception to September 10, 2020 were reviewed to compare the effects of selective oropharyngeal decontamination (SOD) on the incidence of VAP in adult patients requiring mechanical ventilation.
Results: Out of a total of 1098 articles reviewed, 17 eligible studies were included for final analysis. The results showed that the use of chlorhexidine for oropharyngeal decontamination reduces the incidence of VAP. However, it had a small effect on gram-negative resistant bacteria. Also, it was observed that the combined use of colistin and chlorhexidine was more effective than chlorhexidine alone in preventing VAP. The results of studies on the use of toothbrushes to reduce the incidence of pneumonia are unclear since they used chlorhexidine at the same time. However, tooth brushing is one of the best ways to maintain oral hygiene. Using povidoneiodine, Nanosil, and non-absorbable topical antibiotics reduced the incidence of VAP, while Iseganan did not show a significant effect in this regard.
Conclusion: The prophylactic use of topical bactericidal agents in critically-ill patients is effective in reducing the incidence of VAP. However, the use of non-absorbable topical antibiotics is more effective than other methods in oropharyngeal decontamination.
Keywords: Decontamination, Oral hygiene, Oropharyngeal, Pneumonia ventilator-associated, Intensive care unit
Introduction
Among different complications, ventilator-associated pneumonia (VAP) is the most common nosocomial infection (NI) that occurs in the intensive care unit (ICU) and affects nearly 5 to 40% of patients with mechanical ventilation.1 Previous studies described the incidence of lung infection within 48 hours after the admission and artificial airway placement as VAP.2-4The aspiration of oropharyngeal organisms into the distal bronchial lumen is one of the most important mechanisms in the development of VAP.5,6 Intubation and critical illness reduce oral immunity, may be associated with mechanical injury of the mouth or respiratory tract, and increase the likelihood of dry mouth. Thus, mouth rinsing and dental plaque removal are effective nursing care for reducing the bacterial load in the mouth. However, the presence of the endotracheal tube makes it difficult to have access to the oral cavity for appropriate oral care.7-9 Therefore, it is essential to use antiseptic agents or topical antibiotics to reduce the bacterial load of the oral cavity.10However, the relationship between oral hygiene and the reduction of oropharyngeal colonization with pathogenic organisms is rarely recognized.11Previous systematic reviews recommend oral cavity disinfection with chlorhexidine for patients at risk of VAP.11,12 These reviews overlooked the type of microorganisms and their drug resistance. Aerobic gram-negative bacteria are the most common cause of VAP microorganisms in the ICU.13 Some studies showed that chlorhexidine has a relatively unknown effect on gram-negative bacteria.13,14 For selective oropharyngeal decontamination (SOD), antiseptic agents or topical antibiotics should be used with the least destructive effect on the normal flora and a highly destructive effect on the abnormal bacteria, such as gram-negative aerobic basil.2Some studies recommend using non-absorbable topical antibiotics such as polymyxin, neomycin, and colistin mixed with antifungal agents either in a solution or paste for the oropharyngeal cavity to prevent VAP.2,14 Topical antibiotics should not be widely used as there would be a risk of antibiotic-resistant organism development.15 In contrast to antibiotics, antiseptics act rapidly at multiple target sites and may be less prone to the induction of drug resistance.16 However, the best substance for oropharyngeal decontamination to prevent VAP with a good effect on pathogenic organisms is controversial, and numerous studies have shown that different organisms cause VAP in a critical care environment and have different patterns of resistance and sensitivity to similar organisms in other environments.17-19 Therefore, it is necessary to conduct a systematic review that not only determines the effects of antiseptic agents or topical antibiotics on the rate of VAP but also show the type of growing organisms so that health care providers could make decisions based on the type of common organisms in their environment and their pattern of antibiotic resistance or sensitivity. The antiseptic agent types should be identified for use in oral hygiene. Therefore, this systematic review seeks to find the best method of oropharyngeal decontamination to prevent VAP.
Materials and Methods
The current systematic review was conducted based on the preferred reporting items for systematic reviews and meta-analyses (PRISMA).
The primary objective was to investigate the effects of oropharyngeal decontamination in the prevention of VAP, while the second one was the evaluation of the effects of disinfectant agents on pathogenic organisms.
We searched articles indexed in the databases of PubMed, Scopus, Embase, Cochrane library, and Web of Science without publication date restriction from the inception of each database until September 10, 2020 (Table 1).
Table 1. Search parameters .
| Database | Parameters | Filters | Articles retrieved |
| Sep 10, 2020 | |||
| PubMed | ("decontamination"[MeSH Terms] or "decontamination" [all fields]) or "oral hygiene"[all fields]) or "oral rinse" [all fields]) or "oral decontamination"[all fields]) or "selective oral decontamination"[all fields]) and ("oropharynx"[mesh terms] or "oropharynx"[all fields])) or ("oropharynx"[mesh terms] or "oropharynx"[all fields] or "oropharyngeal"[all fields])) and (vap[all fields] or (("VAP"[all fields] or vap[all fields]) | None | 580 |
| Embase | ('Selective oral decontamination' or (selective and oral and ('decontamination'/exp or decontamination)) or 'oral Decontamination':ti,ab,kw or 'oropharyngeal Decontamination':ti,ab,kw or 'oropharynx Decontamination':ti,ab,kw or 'mouth hygiene':ti,ab,kw) and 'VAP':ti,ab,kw or 'VAP':ti,ab,kw) | None | 104 |
| Scopus | (TITLE-ABS-KEY (decontamination) or TITLE-ABS-KEY ("oral hygiene") or TITLE-ABS-KEY ("oral rinse") or TITLE-ABS-KEY ("oral decontamination") or TITLE-ABS-KEY ("selective oral decontamination") and TITLE-ABS-KEY (oropharynx) or TITLE-ABS-KEY (oropharyngeal) and TITLE-ABS-KEY ("VAP") or TITLE-ABS-KEY (VAP)) | None | 125 |
| Web of science | ("Selective oral decontamination") or TOPIC: ("oral hygiene") or TOPIC: ("oral rinse") or TOPIC: ("oral decontamination") and TOPIC: (oropharynx) or TOPIC: (oropharyngeal) and TOPIC: ("VAP") or TOPIC: (VAP) | None | 255 |
| Cochrane library | Decontamination 789 #2 "Oral hygiene" 3765 #3 "Oral rinse" 250 #4 "Oral decontamination" 45 #5 "Selective oral decontamination" 8 #6 #1 or #2 or #3 or #4 or #5 4729 #7 Oropharynx 2135 #8 Oropharyngeal 2536 #9 #7 or #8 3941 #10 "VAP" 1341 #11 #6 and #9 and #10 34 |
None | 34 |
The inclusion criteria were: 1-Original articles with randomized clinical trials (RCT) design in the English language, 2- Studies with at least two groups to compare the effects of any types of antibiotics or antiseptics (with placebo, routine care) for oropharyngeal decontamination, 3-Reporting the incidence of VAP or determining the type of the microorganism in oral and tracheal secretions, and 4-Studies conducted on adults over 16 years under mechanical ventilation.
The exclusion criteria were: 1- Clinical trials on the selective decontamination of the digestive tract,2- Observational studies, 3- Non-English studies, 4-Articles on patients below 16 years of age, 5- Articles with no full-text availability, 6-Abstracts of studies presented in congresses, seminars, and conferences, and 7- Letters to the editor-in-chief and short reports and case reports. It should be noted that some retrieved articles were reviewed and removed in several steps.
The prophylactic application of any type or combination of antibiotics or antiseptics in the oropharynx to the duration of undergoing mechanical ventilation and hospitalization time was systematically investigated in four steps within the PRISMA model to search the articles. Drawing on the above-mentioned keywords, a total of 1098 articles were retrieved; then, 957 studies were obtained after removing the duplicate ones. The titles and abstracts of the given articles were then reviewed; those related to oral decontamination patients admitted to ICUs were selected. Finally, 17 articles remained for the analysis with a focus on the effect of oropharyngeal decontamination on the incidence of VAP with respect to the research objectives as well as the consideration of the inclusion and exclusion criteria.
Two independent reviewers screened all the titles and abstracts for inclusion. Then, we independently assessed each selected reference for detailed evaluation. The two reviewers also independently abstracted relevant clinical trial characteristics, and disagreements were resolved by discussion and consensus with the third author. The two reviewers independently appraised the quality of theclinical trials, including randomization, allocation concealment, blinding techniques, clarity of inclusion and exclusion criteria and outcome definitions, withdrawals, and dropouts assess adverse effects and completeness of follow-up based on the criteria proposed in the scale of Jadad et al 20 for clinical trial quality assessment (Table 2).
Table 2. The Jadad scale for quality assessment of included trials .
| Author/s, year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Total |
| Pugin et al21 1991 | Y | ND | Y | Y | Y | Y | Y | Y | 7 |
| DeRiso et al22 1996 | Y | Y | Y | Y | N | Y | N | Y | 6 |
| Bergmans et al 23 2001 | Y | ND | Y | Y | Y | Y | N | Y | 6 |
| Fourrier et al 24 2005 | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| Kollef et al25 2006 | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| Seguin et al26 2006 | Y | Y | N | N | Y | Y | N | Y | 5 |
| Koeman et al27 2006 | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| Segers et al 28 2006 | Y | Y | Y | Y | Y | Y | Y | Y | 8 |
| Bellissimo-Rodrigues et al29 2009 | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| Munro et al 30 2009 | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| Özçaka et al31 2012 | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| Haghighi et al32 2016 | Y | ND | N | ND | ND | Y | N | Y | 3 |
| Nasiriani et al33 2016 | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| Fernanda de Lacerda Vidal et al34 2017 | Y | Y | Y | Y | Y | Y | N | Y | 7 |
| Zand et al35 2017 | Y | Y | N | N | Y | Y | Y | Y | 6 |
| Chacko et al36 2017 | Y | Y | Y | ND | Y | Y | N | Y | 6 |
| Khaky et al37 2018 | Y | ND | Y | ND | Y | Y | N | Y | 5 |
Q1 = Was the research described as randomized?
Q2 = Was the approach of randomization appropriate?
Q3 = Was the research described as blinding?
Q4 = Was the approach of blinding appropriate?
Q5 = Was there a presentation of withdrawals and dropouts?
Q6 = Was there a presentation of the inclusion/exclusion criteria?
Q7 = Was the approach used to assess adverse effects described?
Q8 = Was the approach of statistical analysis described?
Y: Yes, N: No, ND: Not described.
Results
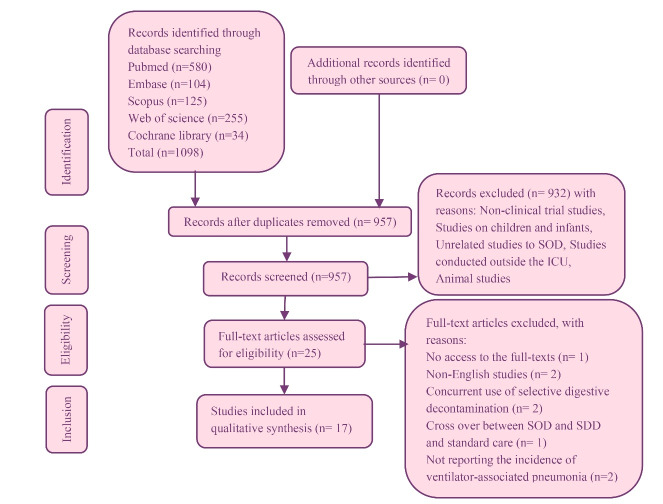
A total of 1098 articles were initially retrieved through searching based on the above-mentioned keywords. Then, 957 articles remained after the exclusion of duplicate ones. The titles and abstracts of the articles were also reviewed, selecting those relating to oropharyngeal decontamination patients admitted to ICUs. Finally, by focusing on the effect of oropharyngeal decontamination on the incidence of VAP based on the research objectives and considering the inclusion and exclusion criteria for further analysis, seventeen articles remained (Figure 1).
Figure 1.
PRISMA flow chart
The main outcome of the current study was the incidence of VAP in patients receiving oropharyngeal decontamination, and seventeen articles reviewed and reported the rate of VAP (Table 3).
Table 3. Comparison of incidence of VAP .
| Study design | Study group | Intervention | Comparison | Comparison | Result with respect to total participants (VAP) | Conclusion | |
| Pugin et al21 1991 |
Randomized, controlled, double-blind clinical trial | Mix | NATAa(150 mg polymyxin B sulfate, 1g neomycin sulfate, and 1g vancomycin hydrochloride) continued until extubation or death. | Placebo | Intervention | Control | Use of NATAdecreasing the prevalence of VAP |
| 25(4) | 27(21) | ||||||
| DeRiso et al221996 | Multicenter, prospective, randomized controlled trial | Cardiothoracic (open heart surgery) | CHXb 0.12% 15 ml preoperatively and twice daily postoperatively until discharge from intensive care or death | Placebo | 173(5) | 180(17) | SOD with CHX reduces the total HAPc rate. |
| Bergmans et al23 2001 | Prospective, randomized, double-blind, controlled trial | Mix | NATAconsisting 2% gentamicin, 2% colistin, and 2% vancomycin every 6 h, until extubation or death | Two Placebo group(A,B) | 87(9) | 78(24) and 61(14) |
Modulation of oropharyngeal colonization, reduces the incidence of late-onset VAP. |
| Fourrier et al24 2005 | Randomized, double-blind, controlled trial | Mix | The Specific gel containing CHX0.2%. The gel was left in place and the oral cavity was not rinsed until the next application. It was applied at least three times a day during the entire ICU stay until discharge or death. | Placebo | 114 (13) | 114 (12) | Significantly decreased the oropharyngeal colonization. However, its efficacy was insufficient to reduce the incidence of respiratory infections due to multi-resistant bacteria. |
| Kollef et al25 2006 |
Multinational, double-blind, randomized, controlled trial | 83% non-trauma, 27% trauma patients | Receive 3 ml Iseganand oral solution (9mg) or six times per day for up to 14 d. | Placebo | 282/(45) VAP in alive patients 80/(7) VAP in dead patients |
284/(57) in alive patients 63/(5) VAP in dead patients |
There were no significant differences in the rate of VAP. |
| Seguin et al26 2006 |
Prospective randomized controlled, trial | Severe closed head trauma | Nasopharynx and oropharynx rinsing with 20 mL of a 10% povidone-Iodine aqueous solution reconstituted in a 60-mL solution with sterile water, followed by aspiration of oropharyngeal secretions every 4 h until discharge from intensive care or death. | Nasopharynx and oropharynx rinsing with 60 mL of saline solution, followed by aspiration of oropharyngeal secretions and standard regimen without any instillation but with the aspiration of oropharyngeal secretions. | 36 (3) | 31 (12) and 31 (13) |
The regular administration of povidone-Iodine maybe an effective strategy for decreasing the prevalence of VAP. |
| Koeman et al27 2006 | Randomized, double-blind, controlled trial | Mixed and surgical ICUs | CHX 2% with COLe 2% in Vaseline was administered four times daily, after removing remnants of the previous dose with a gauze moistened with saline Until the diagnosis of VAP, death, extubation, or withdrawal of consent. | CHX 2% in petroleum jelly [Vaseline] And the placebo administered as same as the intervention group. | 128(16)/ | 127(13) and 130(23) |
SOD with either CHX or CHX/COL reduced and delayed the development of VAP. |
| Segers et al28 2006 | Prospective, randomized, double-blind, controlled clinical trial | Cardiothoracic surgery | 0.12% CHX was used as an oral rinse and as a gel for nasal application, 4 times daily continued until the nasogastric tube was removed. | Placebo | 485(45) | 469(74) | SOD with CHX be an effective method to reduce VAP. |
| Bellissimo-Rodrigues et al29 2009 | Double-blind, randomized, controlled trial | Mix | 0.12% CHX applied orally 3 times a day were continued as long as the patient remained in the ICU. | Placebo | 98(21) | 96(25) | 0.12% CHX does not prevent VAP. |
| Munro et al30 2009 | Randomized controlled factorial trial | Medical, surgical trauma, and neuroscience ICUs | 0.12% CHX 5 mL by oral swab twice daily. | Tooth brushing 3 times a day combination care (tooth brushing 3 times a day and CHX every 12 hours), or control (usual care). | 92(38) | 100(50) | CHX, but not tooth brushing, reduced early VAP Because dislodgement of dental plaque organisms during tooth brushing could provide a larger pool of organisms for translocation from the mouth to subglottic secretions or the lung |
| Özçaka et al31 2012 | Randomized, double-blind, controlled clinical trial | Respiratory ICU | Oral care was provided by swabbing the oral mucosa with either 30 mL of 0.2% CHX or saline on sponge pellets, four times a day. | Routine oral care provided by saline application. | 29(12) | 32(22) | Oral care with CHX swabbing reduces the risk of VAP. |
| Haghighi et al32 2016 | Randomized clinical trial | Mix | All surfaces of teeth and gums were brushed and rinsed with normal saline 0.9% solution. Then a syringe was used to spray 5cc CHX 0.2% on teeth, tongue, gum and mucosa following a mouth and throat deep suctioning after 30 s. Finally, Moisten lips and mouth with vitamin A+D. 2 to 6 times a day. CHX was sprayed every 12 hours. | Routine oral care including brushing the teeth with a toothpaste once a day and mouth washing with CHX 0.2% solution twice a day. | 50(5) | 50(7) | There was no significant difference in the incidence of VAP. |
| Nasiriani et al33 2016 | Randomized clinical trial | Trauma patients | In addition to routine oral care, the oral surface was brushed twice a day with a children’s soft toothbrush and distilled water, Subsequently, CHX was rubbed on the surface of the mouth twice daily for the next five days. | Routine oral care (control of endotracheal tube cuff, rinsing the mouth with normal saline, applying CHX to a swab and rubbing it on the surface of the tongue and oropharyngeal suction) three times a day. | 84(25) | 84(40) | Tooth brushing twice daily with distilled water reduced the incidence of VAP. |
| Fernanda de Lacerda Vidal et al34 2017 | Prospective, randomized clinical trial | Mix | Tooth brushing plus 0.12% CHX gel every 12 h, up to28 days. | Oral hygiene with 0.12%CHX every 12h | 105(17) | 108(28) | Patients undergoing tooth brushing there was a tendency to reduce the incidence of VAP. |
| Zand et al35 2017 | Randomized, clinical trial | Trauma, surgery neurosurgery, and general ICUs |
%2 CHX twice daily continued until discharge or death. | %0.2 CHX | 57(3) | 57(13) | SOD is more effective with 2% CHX than with 0.2% CHX in incidence of VAP. |
| Chacko et al36 2017 | Prospective, randomized, double-blind clinical trial | Medical ICU | Toothbrush and CHX 0.2% was instilled into the oral cavity Three times a day until discharge from ICU. | Routine oral care: the oral cavity was swabbed with sponges soaked in CHX 0.2%. | 104(5) | 102(7) | There was no significant difference in the incidence of VAP. |
| Khaky et al37 2018 | Prospective, a randomized clinical trial | Mix | Nanosilf mouthwash three times a day or until obtaining the exit criteria (death, extubation, transfer to other wards and performing any diagnostic and therapeutic procedures in the oral and throat areas). | CHX 0.12% mouthwash three times a day for five days that involved brushing the teeth, suctioning oral secretions, and rubbing the oropharyngeal mucosa. | 40(1) | 40(9) | Nanosil is better than CHX for the prevention of VAP. |
aNATA: non-absorbable topical antibiotic, bCHX: chlorohexidine, cHAP: Hospital-acquired pneumonia, dIseganan HCl is a synthetic protegrin analog that possesses a broad spectrum of activity in vitro against aerobic and anaerobic gram-positive and gram-negative bacteria and yeasts, is rapidly microbicidal in saliva, and has a low propensity for inducing resistance, eCOL: colistin, fNanosil mouthwashes are hydrogen peroxide and few silver ions. Hydrogen peroxide destroyed bacterial and viral protective membranes and therefore prevents anaerobic bacterial proliferation. Silver ions bind to bacterial proteins with extremely firm covalent bonds and causing bacterial deactivation.
The secondary outcome of the current study was oral and tracheal colonization; nine papers measured the bacterial colonization with bronchoalveolar lavage and mini- bronchoalveolar lavage.
A study examining the effect of Iseganan on oropharyngeal decontamination demonstrated that the distribution of bacterial pathogens causing VAP was similar in the two groups; Candida species. Were more frequently identified in the placebo group as compared to the Iseganan group. Oral cultures at the beginning and end of the study showed a greater reduction in total aerobes for Iseganan patients as compared to placebo patients, but no difference was found in the reduction of total gram-negative organisms and Staphylococcus Aureus between the groups.25 Also, another clinical trial analysis of the gram’s stains of organisms involved in total respiratory tract infections disclosed a clinically-significant reduction in gram-negative respiratory tract infections in the chlorhexidine-treated patients.22 Seguin et al used povidone-Iodine for oropharyngeal decontamination; most organisms responsible for early and late VAP were gram-positive, such as Staphylococcus aureus and Streptococcus pneumonia.26However, another study employed chlorhexidine for oropharyngeal decontamination and found that gram-negative bacilli with multidrug resistance were the most frequent cause of VAP.24 A clinical trial investigating the effect of chlorhexidine, chlorhexidine/colistin, and colistin on endotracheal colonization demonstrated that chlorhexidine and colistin had similar effects on the control of gram-positive bacteria, while the combination of chlorhexidine/colistin was more effective in gram-negative bacterial colonization.27 A clinical trial utilized chlorhexidine in oropharyngeal decontamination and observed that most of the organisms causing VAP were gram-negative organisms, such as Enterobacter, Acinetobacter, and Klebsiella.33 A trial conducted a long time ago employed Polymyxin, Neomycin, and Vancomycin and significantly reduced the rate of pneumonia caused by aerobic gram-negative bacilli and gram-positive organisms.21 Another clinical trial reported that most of the organisms observed in cultured tracheal secretions in chlorohexidine and placebo group were gram-positive, such as Haemophilus species, and Staphylococcus species, whereas gram-negative bacteria, such as Moraxella species, Pseudomonas species, Klebsiella species, Enterobacter species, and Escherichia coli accounted for a very small portion of the infections. However, the prevalence of all organisms, except for Klebsiella, was lower in the chlorhexidine group. Fungal pneumonia was also lower in the group of oropharyngeal decontamination with chlorhexidine than in the placebo group.28 Another clinical trial demonstrated that the frequency of colonization significantly decreased in the 2.0% chlorhexidine group as compared to the 0.2% chlorhexidine group. The most common microorganisms isolated from the tracheal samples of the patients with VAP included Acinetobacter, Staphylococcus aureus, Klebsiella, Candida albicans, and Escherichia coli. The oropharyngeal microorganism colonization was similar to tracheal colonies.35
Discussion
Twelve of the seventeen articles utilized different concentrations of chlorhexidine for oropharyngeal decontamination. Three studies compared chlorhexidine 0.12% to placebo. In two studies, oropharyngeal decontamination with chlorhexidine reduced VAP.22,28 Three studies compared chlorhexidine 0.12% with the simultaneous use of chlorhexidine 0.12% and toothbrushing. In one study, VAP was reduced in a group receiving only chlorhexidine. Because tooth brushing translocation of organisms from the mouth to subglottic secretions or the lung.30 But in two study, the rate of VAP was lower in the tooth brushing and chlorhexidine groups.33,34
In four studies, chlorhexidine 0.2 % was compared to placebo, tooth brushing and routine care; only in one study, the rate of VAP was reduced. 31 But in three studies, it was not able to reduce the incidence of VAP.24,32,36 The remaining study reported that chlorhexidine 2.0% had a greater effect than chlorhexidine 0.2% on the prevention of VAP.35 Koeman et al reported that the combination of chlorhexidine and colistin was more effective, even though chlorhexidine reduced VAP.27 These studies adopted suitable methodologies, and evidence suggests that oropharyngeal decontamination with chlorhexidine may be effective in the prevention of VAP. Other systematic reviews suggested that using chlorhexidine oral rinses is an effective way to prevent VAP.38-41 Most studies did not examine the side effects, and only a few studies reported side effects such as tooth discoloration and mucosal irritation.28,35 The analysis of the results of bacterial growth in the mouth and trachea showed that although chlorhexidine is effective on gram-positive and negative organisms, it has small effects on gram-negative organisms.24,27,33To improve the effectiveness of chlorhexidine, another antibacterial agent should be used simultaneously.27 A meta-analysis study indicated that 0.12% chlorhexidine had the best effect on the prevention of ventilator‐associated pneumonia; however, they did not assess the types of organisms grown in the oral and tracheal secretions.42Therefore, due to its low effect on the resistant gram-negative organism, we recommend that more high-quality clinical trials should be performed to determine the suitable concentration of chlorhexidine with the minimum side effects and maximum efficacy. Also, we recommend that more studies should be carried out to find the best drug combination with chlorhexidine in order to increase the antibacterial effect. Three trials used a simultaneous combination of tooth-brushing and various concentrations of chlorhexidine in comparison to routine care and chlorhexidine for oropharyngeal decontamination. Two articles reported a decreased incidence of VAP.33,34Since the use of a toothbrush could reduce dental plaque and bacterial accumulation in the mouth, evidence suggests that this method works best when routinely used in the ICU for oral care. However, different variables may contribute to these positive results. Consistent with the current study, another review recommended tooth-brushing to provide a higher standard of oral care to mechanically-ventilated patients and reduce VAP when used with chlorhexidine.43 A clinical trial used povidone-Iodine 10% in oropharyngeal decontamination, reducing the incidence of VAP.26 Chua et al also reported that the use of povidone-iodine 1% for oral rinse is effective in the reduction of VAP.44 Although both articles are of good quality, due to the small number of articles and the difference in the concentrations, further studies are needed to confirm the effects and to find the appropriate concentration for use. An article utilized Iseganan HCl for oropharyngeal decontamination; it did not affect the reduction of VAP.25 Other review articles have not been recommended for clinical use.40 A study employed Nanosil (containing hydrogen peroxide and silver ions) for mouthwash; it was found to be able to reduce the incidence of VAP better than chlorhexidine. Previous studies showed that hydrogen peroxide is more effective than distilled water, saline, and placebo in the prevention of oral plaque formation.45,46 However, hydrogen peroxide was significantly less effective than chlorhexidine.45-47 A number of studies reported complications such as abnormality in oral mucous.48Also, patient intolerance following hydrogen peroxide administration was reported.49However, some studies reported that the use of hydrogen peroxide had no side effects.47,50 The side effects of Nanosil were not evaluated. Therefore, further evidence is required for the utilization of Nanosil. Two articles used non-absorbable topical antibiotics for oropharyngeal decontamination. Both articles observed the reduction of VAP.21,23 Other studies employed this method; the rates of intra-oral bacterial colonization and VAP were found to reduce in all patients.2,51 In the long-term use of SOD, most of the gram-negative aerobic bacteria and fungi were reported to have been eliminated in the oral cavity and pharynx.51 A systematic review indicated that the use of non-absorbable topical antibiotics is effective in the prevention of respiratory infections.52 Also, it would not lead to increased antibiotic resistance.53Oropharyngeal decontamination helps nurses reduce VAP rates; however, it is not the main method of controlling VAP. There are three effective methods for preventing the colonization of organisms in the oropharynx and their translocation to the upper respiratory tract. Placing the patients in a semi-recumbent position to control the return of gastric secretions into the oropharynx has been widely advocated, particularly when patients receive enteral nutrition. A 30-45 degree position of the head prevents the returning contents of the stomach and translocation to the upper respiratory tract; the microaspiration prevention of secretions originating from the upper respiratory tract accumulating above the cuff of the endotracheal tube is the second effective method for the control of VAP. This is performed with a specific endotracheal tube (ETT) referred to as taper guard ETT. These tubes have a lumen behind the end of the endotracheal cuff connecting to the low-pressure suction. Finally, silver-coated tubes have been used to prevent bacteria originating from the upper respiratory tract from reaching the distal lung tissue. Silver has broad-spectrum antimicrobial activity and reduces bacterial adhesion and biofilm formation.54,55 Also, preserving the integrity of the gastrointestinal tract and using probiotics are good ways to prevent the translocation of microorganisms from the gastrointestinal tract to the lungs. This can reduce the rate of VAP and mortality.54,56 Another risk factor for VAP is normal saline instillation before endotracheal suctioning. This method leads to the transfer of pathogenic organisms from the upper respiratory tract to the lower respiratory tract. Therefore, using a humidifier and closed suction systems are a better way to dilute and suctioning of respiratory secretions and reduce the risk of VAP.57-60
Conclusion
The prophylactic use of the topical bactericidal agent in critically-ill patients is effective to decrease the incidence of VAP. Further studies are required to find the effective and safe amount of chlorhexidine for oropharyngeal decontamination. Chlorhexidine may be more effective when used with a solution that targets gram-negative bacteria. Although the povidone-Iodine and Nanosil contribute to the reduction of the incidence of VAP, few clinical trials have been performed, and further studies are required to investigate the effects and side effects of povidone-Iodine, Nanosil, and Iseganan. The use of non-absorbable topical antibiotics is the best method of oropharyngeal decontamination to reduce VAP in the ICU.
This systematic review had some limitations. Due to the considerable heterogeneity in studies, we could not perform a meta-analysis to statistically evaluate the contribution of each method of oropharyngeal decontamination to the rate of VAP. We did not search Google Scholar to avoid bias. Therefore, this review does not include all published articles in this field.
Acknowledgements
The authors are thankful to all researchers whose articles were used in Systematic Review study. Also, the cooperation and help of PhD students in nursing, library, and computer unit authorities of Mashhad University of Medical Sciences in the search for papers is appreciated.
Authors’ Contributions
AK: Determining the databases patterns to search and study design, reviewed titles, abstracts, full text and, quality assessment of articles, analysis, and interpretation of data for the work and writing-review and editing this article; HKM, MR, SHA: Consulting and supervision, reviewed titles, abstracts, and quality assessment of articles analysis and interpretation of data for the work. All authors have read and agreed to the published version of the manuscript.
Conflict of Interests
The authors declare no conflict of interest in this study.
Data Accessibility
The datasets are available from the first or corresponding author on reasonable request.
Ethical Issues
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences, Iran (Code: 1400/27208).
Funding
We have not received any funding for this project.
Research Highlights
What is the current knowledge?
Teeth brushing and rinsing the mouth with normal saline and using chlorhexidine for oral disinfection is sufficient to oral hygiene care (OHC) and prevent VAP.
What is new here?
The results of the current study showed that many common organisms in the oral cavity that cause VAP are resistant to chlorhexidine. OHC by rinsing with saline and toothbrush is not enough to prevent VAP. Health care providers should use topical antibiotics or antifungal agents to disinfect the mouth based on the common organisms that cause ventilator-induced pneumonia.
References
- 1.Papazian L, Klompas M, Luyt CE. Ventilator-associated pneumonia in adults: a narrative review. Intensive Care Med. 2020;46(5):888–906. doi: 10.1007/s00134-020-05980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barani M, Tabatabaei SM, Sarani H, Dahmardeh AR, Keykhah A. Investigating the effect of selective oropharyngeal decontamination using topical antibiotics on oropharyngeal and tracheal colonization in trauma patients admitted to the intensive care units of Zahedan, Iran: a clinical trial study. Med Surg Nurs J. 2018;7(3):e86895. doi: 10.5812/msnj.86895. [DOI] [Google Scholar]
- 3. Kohbodi GA, Rajasurya V, Noor A. Ventilator-associated Pneumonia. In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2021. [PubMed]
- 4.Klompas M, Branson R, Eichenwald EC, Greene LR, Howell MD, Lee G, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35 Suppl 2:S133–54. doi: 10.1017/s0899823x00193894. [DOI] [PubMed] [Google Scholar]
- 5.Modi AR, Kovacs CS. Hospital-acquired and ventilator-associated pneumonia: diagnosis, management, and prevention. Cleve Clin J Med. 2020;87(10):633–9. doi: 10.3949/ccjm.87a.19117. [DOI] [PubMed] [Google Scholar]
- 6.Alecrim RX, Taminato M, Belasco A, Longo MC, Kusahara DM, Fram D. Strategies for preventing ventilator-associated pneumonia: an integrative review. Rev Bras Enferm. 2019;72(2):521–30. doi: 10.1590/0034-7167-2018-0473. [DOI] [PubMed] [Google Scholar]
- 7.Zhao T, Wu X, Zhang Q, Li C, Worthington HV, Hua F. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2020;12(12):CD008367. doi: 10.1002/14651858.CD008367.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alhazzani W, Smith O, Muscedere J, Medd J, Cook D. Toothbrushing for critically ill mechanically ventilated patients: a systematic review and meta-analysis of randomized trials evaluating ventilator-associated pneumonia. Crit Care Med. 2013;41(2):646–55. doi: 10.1097/CCM.0b013e3182742d45. [DOI] [PubMed] [Google Scholar]
- 9.Hua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2016;10(10):CD008367. doi: 10.1002/14651858.CD008367.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Bassi G, Senussi T, Aguilera Xiol E. Prevention of ventilator-associated pneumonia. Curr Opin Infect Dis. 2017;30(2):214–20. doi: 10.1097/qco.0000000000000358. [DOI] [PubMed] [Google Scholar]
- 11.Johnny JD, Drury Z, Ly T, Scholine J. Oral care in critically ill patients requiring noninvasive ventilation: an evidence-based review. Crit Care Nurse. 2021;41(4):66–70. doi: 10.4037/ccn2021330. [DOI] [PubMed] [Google Scholar]
- 12.Silva PUJ, Paranhos LR, Meneses-Santos D, Blumenberg C, Macedo DR, Cardoso SV. Combination of toothbrushing and chlorhexidine compared with exclusive use of chlorhexidine to reduce the risk of ventilator-associated pneumonia: a systematic review with meta-analysis. Clinics (Sao Paulo) 2021;76:e2659. doi: 10.6061/clinics/2021/e2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao D, Song J, Gao X, Gao F, Wu Y, Lu Y, et al. Selective oropharyngeal decontamination versus selective digestive decontamination in critically ill patients: a meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2015;9:3617–24. doi: 10.2147/dddt.s84587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz MJ, Haas LE. Antibiotics or probiotics as preventive measures against ventilator-associated pneumonia: a literature review. Crit Care. 2011;15(1):R18. doi: 10.1186/cc9963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panchabhai TS, Dangayach NS, Krishnan A, Kothari VM, Karnad DR. Panchabhai TS, Dangayach NS, Krishnan A, Kothari VM, Karnad DROropharyngeal cleansing with 02% chlorhexidine for prevention of nosocomial pneumonia in critically ill patients: an open-label randomized trial with 001% potassium permanganate as control. Chest. 2009;135(5):1150–6. doi: 10.1378/chest.08-1321. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Yue J. Oral topical decontamination for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials--authors’ response. J Hosp Infect. 2014;86(4):278–9. doi: 10.1016/j.jhin.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Khoshfetrat M, Keykha A, Sedaghatkia M, Farahmandrad R. Determination of antibiotic resistance pattern of organisms isolated from endotracheal tube cultures of patients admitted to intensive care unit. Arch Anesth Crit Care. 2020;6(3):125–32. doi: 10.18502/aacc.v6i3.3996. [DOI] [Google Scholar]
- 18.Amini M, Ansari I, Vaseie M, Vahidian M. Pattern of antibiotic resistance in nosocomial infections with Gram-negative bacilli in ICU patients (Tehran, Iran) during the years 2012-2014. Journal of Basic and Clinical Pathophysiology. 2018;6(1):23–30. doi: 10.22070/jbcp.2018.3109.1092. [DOI] [Google Scholar]
- 19.Pandey M, Niranjan D, Pande R. Bacteriological profile and antimicrobial resistance of blood culture isolates from a 350 bedded hospital Lucknow, India. Int J Curr Microbiol Appl Sci. 2017;6(1):184–93. doi: 10.20546/ijcmas.2017.601.023. [DOI] [Google Scholar]
- 20.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17(1): 1-12. is blinding necessary? Control Clin Trials. 1996;17(1):is blinding necessary? Control Clin Trials 1996; 17(1). doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 21.Pugin J, Auckenthaler R, Lew DP, Suter PM. Oropharyngeal decontamination decreases incidence of ventilator-associated pneumonia: a randomized, placebo-controlled, double-blind clinical trial. JAMA. 1991;265(20):2704–10. doi: 10.1001/jama.1991.03460200084041. [DOI] [PubMed] [Google Scholar]
- 22.DeRiso AJ 2nd, Ladowski JS, Dillon TA, Justice JW, Peterson AC. DeRiso AJ 2nd, Ladowski JS, Dillon TA, Justice JW, Peterson ACChlorhexidine gluconate 012% oral rinse reduces the incidence of total nosocomial respiratory infection and nonprophylactic systemic antibiotic use in patients undergoing heart surgery. Chest. 1996;109(6):1556–61. doi: 10.1378/chest.109.6.1556. [DOI] [PubMed] [Google Scholar]
- 23.Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, van der Geest S, van Tiel FH, et al. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2001;164(3):382–8. doi: 10.1164/ajrccm.164.3.2005003. [DOI] [PubMed] [Google Scholar]
- 24.Fourrier F, Dubois D, Pronnier P, Herbecq P, Leroy O, Desmettre T, et al. Effect of gingival and dental plaque antiseptic decontamination on nosocomial infections acquired in the intensive care unit: a double-blind placebo-controlled multicenter study. Crit Care Med. 2005;33(8):1728–35. doi: 10.1097/01.ccm.0000171537.03493.b0. [DOI] [PubMed] [Google Scholar]
- 25.Kollef M, Pittet D, Sánchez García M, Chastre J, Fagon JY, Bonten M, et al. A randomized double-blind trial of iseganan in prevention of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173(1):91–7. doi: 10.1164/rccm.200504-656OC. [DOI] [PubMed] [Google Scholar]
- 26.Seguin P, Tanguy M, Laviolle B, Tirel O, Mallédant Y. Effect of oropharyngeal decontamination by povidone-iodine on ventilator-associated pneumonia in patients with head trauma. Crit Care Med. 2006;34(5):1514–9. doi: 10.1097/01.ccm.0000214516.73076.82. [DOI] [PubMed] [Google Scholar]
- 27.Koeman M, van der Ven AJ, Hak E, Joore HC, Kaasjager K, de Smet AG, et al. Oral decontamination with chlorhexidine reduces the incidence of ventilator-associated pneumonia. Am J Respir Crit Care Med. 2006;173(12):1348–55. doi: 10.1164/rccm.200505-820OC. [DOI] [PubMed] [Google Scholar]
- 28.Segers P, Speekenbrink RG, Ubbink DT, van Ogtrop ML, de Mol BA. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate: a randomized controlled trial. JAMA. 2006;296(20):2460–6. doi: 10.1001/jama.296.20.2460. [DOI] [PubMed] [Google Scholar]
- 29.Bellissimo-Rodrigues F, Bellissimo-Rodrigues WT, Viana JM, Teixeira GC, Nicolini E, Auxiliadora-Martins M, et al. Effectiveness of oral rinse with chlorhexidine in preventing nosocomial respiratory tract infections among intensive care unit patients. Infect Control Hosp Epidemiol. 2009;30(10):952–8. doi: 10.1086/605722. [DOI] [PubMed] [Google Scholar]
- 30.Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator-associated pneumonia in critically ill adults. Am J Crit Care. 2009;18(5):428–37. doi: 10.4037/ajcc2009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Özçaka Ö, Başoğlu OK, Buduneli N, Taşbakan MS, Bacakoğlu F, Kinane DF. Chlorhexidine decreases the risk of ventilator-associated pneumonia in intensive care unit patients: a randomized clinical trial. J Periodontal Res. 2012;47(5):584–92. doi: 10.1111/j.1600-0765.2012.01470.x. [DOI] [PubMed] [Google Scholar]
- 32.Haghighi A, Shafipour V, Bagheri-Nesami M, Gholipour Baradari A, Yazdani Charati J. The impact of oral care on oral health status and prevention of ventilator-associated pneumonia in critically ill patients. Aust Crit Care. 2017;30(2):69–73. doi: 10.1016/j.aucc.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Nasiriani K, Torki F, Jarahzadeh MH, Rashidi Maybodi F. The effect of brushing with a soft toothbrush and distilled water on the incidence of ventilator-associated pneumonia in the intensive care unit. Tanaffos. 2016;15(2):101–7. [PMC free article] [PubMed] [Google Scholar]
- 34.de Lacerda Vidal CF, Vidal AK, Monteiro JG Jr, Cavalcanti A, Henriques APC, Oliveira M, et al. Impact of oral hygiene involving toothbrushing versus chlorhexidine in the prevention of ventilator-associated pneumonia: a randomized study. BMC Infect Dis. 2017;17(1):112. doi: 10.1186/s12879-017-2188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zand F, Zahed L, Mansouri P, Dehghanrad F, Bahrani M, Ghorbani M. Zand F, Zahed L, Mansouri P, Dehghanrad F, Bahrani M, Ghorbani MThe effects of oral rinse with 02% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated pneumonia in adults’ intensive care units. J Crit Care. 2017;40:318–22. doi: 10.1016/j.jcrc.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 36.Chacko R, Rajan A, Lionel P, Thilagavathi M, Yadav B, Premkumar J. Oral decontamination techniques and ventilator-associated pneumonia. Br J Nurs. 2017;26(11):594–9. doi: 10.12968/bjon.2017.26.11.594. [DOI] [PubMed] [Google Scholar]
- 37.Khaky B, Yazdannik A, Mahjobipoor H. Evaluating the efficacy of nanosil mouthwash on the preventing pulmonary infection in intensive care unit: a randomized clinical trial. Med Arch. 2018;72(3):206–9. doi: 10.5455/medarh.2018.72.206-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Rabbany M, Zaghlol N, Bhandari M, Azarpazhooh A. Prophylactic oral health procedures to prevent hospital-acquired and ventilator-associated pneumonia: a systematic review. Int J Nurs Stud. 2015;52(1):452–64. doi: 10.1016/j.ijnurstu.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Xie D, Li A, Yue J. Oral topical decontamination for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. J Hosp Infect. 2013;84(4):283–93. doi: 10.1016/j.jhin.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Jelic S, Cunningham JA, Factor P. Clinical review: airway hygiene in the intensive care unit. Crit Care. 2008;12(2):209. doi: 10.1186/cc6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enwere EN, Elofson KA, Forbes RC, Gerlach AT. Impact of chlorhexidine mouthwash prophylaxis on probable ventilator-associated pneumonia in a surgical intensive care unit. Int J Crit Illn Inj Sci. 2016;6(1):3–8. doi: 10.4103/2229-5151.177368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang TT, Tang SS, Fu LJ. The effectiveness of different concentrations of chlorhexidine for prevention of ventilator-associated pneumonia: a meta-analysis. J Clin Nurs. 2014;23(11-12):1461–75. doi: 10.1111/jocn.12312. [DOI] [PubMed] [Google Scholar]
- 43.Roberts N, Moule P. Chlorhexidine and tooth-brushing as prevention strategies in reducing ventilator-associated pneumonia rates. Nurs Crit Care. 2011;16(6):295–302. doi: 10.1111/j.1478-5153.2011.00465.x. [DOI] [PubMed] [Google Scholar]
- 44.Chua JV, Dominguez EA, Sison CM, Berba RP. The efficacy of povidone-iodine oral rinse in preventing ventilator-associated pneumonia: a randomized, double-blind, placebo-controlled (VAPOR) trial: preliminary report. Philipp J Microbiol Infect Dis. 2004;33:153–61. [Google Scholar]
- 45.Wennström J, Lindhe J. Effect of hydrogen peroxide on developing plaque and gingivitis in man. J Clin Periodontol. 1979;6(2):115–30. doi: 10.1111/j.1600-051x.1979.tb02190.x. [DOI] [PubMed] [Google Scholar]
- 46.Binney A, Addy M, Newcombe RG. The effect of a number of commercial mouthrinses compared with toothpaste on plaque regrowth. J Periodontol. 1992;63(10):839–42. doi: 10.1902/jop.1992.63.10.839. [DOI] [PubMed] [Google Scholar]
- 47.Gusberti FA, Sampathkumar P, Siegrist BE, Lang NP. Microbiological and clinical effects of chlorhexidine digluconate and hydrogen peroxide mouthrinses on developing plaque and gingivitis. J Clin Periodontol. 1988;15(1):60–7. doi: 10.1111/j.1600-051x.1988.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 48.Tombes MB, Gallucci B. The effects of hydrogen peroxide rinses on the normal oral mucosa. Nurs Res. 1993;42(6):332–7. [PubMed] [Google Scholar]
- 49.Holberton P, Liggett G, Lundberg D. Researching mouth care in the ICU. Can Nurse. 1996;92(5):51–2. [PubMed] [Google Scholar]
- 50.Hasturk H, Nunn M, Warbington M, Van Dyke TE. Efficacy of a fluoridated hydrogen peroxide-based mouthrinse for the treatment of gingivitis: a randomized clinical trial. J Periodontol. 2004;75(1):57–65. doi: 10.1902/jop.2004.75.1.57. [DOI] [PubMed] [Google Scholar]
- 51. Rasoulinezhad F, Mohammadzadeh S, Piranfar V, Mirnejad R. Effect of selective oropharyngeal decontamination (SOD) on colonization of the oropharynx in hospitalized patients in intensive care units. Iran J Med Microbiol 2014; 8(3): 38-44. [Persian].
- 52.Pileggi C, Bianco A, Flotta D, Nobile CG, Pavia M. Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Crit Care. 2011;15(3):R155. doi: 10.1186/cc10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Plantinga NL, Bonten MJ. Selective decontamination and antibiotic resistance in ICUs. Crit Care. 2015;19(1):259. doi: 10.1186/s13054-015-0967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bonten MJ. Healthcare epidemiology: ventilator-associated pneumonia: preventing the inevitable. Clin Infect Dis. 2011;52(1):115–21. doi: 10.1093/cid/ciq075. [DOI] [PubMed] [Google Scholar]
- 55.Martini RP, Yanez ND, Treggiari MM, Tekkali P, Soelberg C, Aziz MF. Implementation of the TaperGuardTM endotracheal tube in an unselected surgical population to reduce postoperative pneumonia. BMC Anesthesiol. 2020;20(1):211. doi: 10.1186/s12871-020-01117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu D, Wu C, Zhang S, Zhong Y. Risk factors of ventilator-associated pneumonia in critically III patients. Front Pharmacol. 2019;10:482. doi: 10.3389/fphar.2019.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schults JA, Cooke M, Long DA, Mitchell ML. Normal saline and lung recruitment with paediatric endotracheal suction: a review and critical appraisal of practice recommendations. Dimens Crit Care Nurs. 2020;39(6):321–8. doi: 10.1097/dcc.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 58.Keykha A, Arbabshastan ME, Askari H, Abbaszadeh A, Khodadadi Hosseini BM. Arterial blood oxygen saturation and sedation level of the patients hospitalized in ICUs. Der Pharma Chem. 2016;8(1):483–90. [Google Scholar]
- 59.Afenigus AD, Mulugeta H, Bewuket B, Ayenew T, Getnet A, Akalu TY, et al. Skill of suctioning adult patients with an artificial airway and associated factors among nurses working in intensive care units of Amhara region, public hospitals, Ethiopia. Int J Afr Nurs Sci. 2021;14:100299. doi: 10.1016/j.ijans.2021.100299. [DOI] [Google Scholar]
- 60.Keykha A, Askari H, Abbaszadeh A, Enayatie H, Khodadadi Hosseini BM, Borhani F. Comparing the effects of standard suction and routine methods on vital signs, arterial blood oxygen saturation and pain level of patients hospitalized at the intensive care unit. Iran J Crit Care Nurs. 2016;9(2):e6619. doi: 10.17795/ccn-6619. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets are available from the first or corresponding author on reasonable request.