Abstract
In this paper we have analyzed the processing in vitro of the 16S rRNA of the thermophilic archaeon Sulfolobus solfataricus, using pre-rRNA substrates transcribed in vitro and different protein preparations as the source of processing enzymes. We show that the 5′ external transcribed spacer of the S. solfataricus pre-rRNA transcript contains a target site for a specific endonuclease, which recognizes a conserved sequence also existing in the early A0 and 0 processing sites of Saccharomyces cerevisiae and vertebrates. This site is present in other members of the kingdom Crenarchaeota but apparently not in the Euryarchaeota. Furthermore, S. solfataricus pre-16S RNA is processed within the double-helical stem formed by the inverted repeats flanking the 16S RNA sequence, in correspondence with a bulge-helix-bulge motif. The endonuclease responsible for this cleavage is present in both the Crenarchaeota and the Euryarchaeota. The processing pattern remained the same when the substrate was a 30S ribonucleoprotein particle instead of the naked RNA. Maturation of either the 5′ or the 3′ end of the 16S RNA molecule was not observed, suggesting either that maturation requires conditions not easily reproducible in vitro or that the responsible endonucleases are scarcely represented in cell extracts.
In both bacteria and eukaryotes, rRNAs are synthesized as large precursors which are processed to mature rRNA species. However, the maturation pathways, and the enzymatic machinery involved, differ in the two cell domains (for reviews, see references 7 and 9). The principal maturation enzyme in bacteria is RNase III, which cleaves within the long double-stranded stems formed by the inverted repeats flanking both large rRNA genes, releasing precursor 16S and 23S rRNAs, which are subsequently trimmed at both ends to yield the mature molecules. However, RNase III is not strictly essential, and mutants lacking this enzymatic activity are viable (although slow growing) because there exist alternative processing pathways for producing mature rRNAs, especially the 16S rRNA (9). In eukaryotes, the rRNA genes are not flanked by inverted repeats and there are no processing stems: rRNA maturation is performed by ribonucleoprotein enzymes that cut at specific sites within the transcribed spacers. The compositions and mechanisms of action of these enzymes are still largely unknown, although it is well established that they require the presence of several small nucleolar RNAs such as U3, U8, U14, and others (7, 25). However, eukaryotes also possess an RNase III homolog that in Saccharomyces cerevisiae has been shown to be involved in rRNA processing both in vitro and in vivo (1).
In contrast with the wealth of data available for the other two primary domains, our knowledge of rRNA processing in archaea is still very fragmentary. As in bacteria, the archaeal large rRNA genes are flanked by imperfect inverted repeats that pair, forming long double-helical stems. These stems are truncated by an enzyme which probably recognizes a specific structure, a bulge-helix-bulge (BHB) motif (4, 8, 13, 15). A peculiar situation seems to exist in the crenarchaeon Sulfolobus acidocaldarius, where 16S rRNA maturation was reported to be independent of the formation of the processing stem (6), thus resembling the early steps of eukaryotic 18S RNA maturation.
In this paper we discuss the in vitro processing of 16S rRNA in the extremely thermophilic archaeon Sulfolobus solfataricus, using pre-rRNA substrates transcribed in vitro and various protein preparations. We show that the 5′ external transcribed spacer (5′ETS) of the S. solfataricus pre-rRNA transcript contains a target site for a specific endonuclease, which recognizes a conserved sequence also present in the early A0 and 0 processing sites of yeast and vertebrates. This site is probably specific to the Crenarchaeota, as it is recognized and cleaved by heterologous cell extracts from this archaeal branch only. Furthermore, S. solfataricus pre-16S RNA is processed within the double-helical stem formed by the inverted repeats flanking the 16S sequence, in correspondence with the BHB motif. The endonuclease responsible for this cleavage is present in cell extracts from both Crenarchaeota and Euryarchaeota. The processing sites were the same regardless of whether the substrate was the naked RNA or a ribonucleoprotein particle. Under no experimental conditions was maturation of either the 5′ or the 3′ end of the 16S RNA observed, suggesting either that maturation requires conditions not easily reproducible in vitro or that the responsible endonucleases are scarcely represented in cell extracts.
MATERIALS AND METHODS
In vitro transcription.
The RNAs for in vitro processing were obtained by in vitro transcription with T7 RNA polymerase of the ribosomal DNA operon of S. solfataricus, cloned in such a way that the artificial transcription start site was very close to the natural one, except for the presence of some 15 nucleotides (nt) of a plasmid polylinker ahead of the archaeal sequence (21). To obtain the short transcript, which includes the 5′ETS and about 100 nt of the 16S sequence (see Fig. 1), the construct was linearized with AflIII. To obtain the entire 16S rRNA, which includes the 5′ETS and the internal transcribed spacer (ITS) (see Fig. 2), the construct was linearized with HpaI, whose first site from the 5′ end of the rDNA operon lies in the ITS 8 nt upstream from the 5′ extremity of the 23S RNA sequence.
FIG. 1.
In vitro processing of the short pre-rRNA by different protein preparations and processing of a minimal transcript. (A) Primer extension analysis of the processing cuts introduced in the short transcript by the following protein preparations: chaperonin (Ch), the postribosomal S-100 fraction (S100), and the RW fraction. The short transcript, which included the entire 5′ETS and about 100 nt of the 16S rRNA sequence, is illustrated on the right. The sequence and secondary structure of the 5′ETS are shown in full, while the remainder of the molecule is schematized as a box (16S, 100 nt). The primary processing site (site 0) and the location of the mature 5′ end of the 16S RNA are indicated. (B) In vitro processing patterns of uniformly labeled RNA molecules. Lanes 1 and 2, short transcript incubated with and without chaperonin, respectively; lanes 3 and 4, minimal transcript incubated in the same way. The structure of the minimal transcript (about 100 nt) is illustrated on the right.
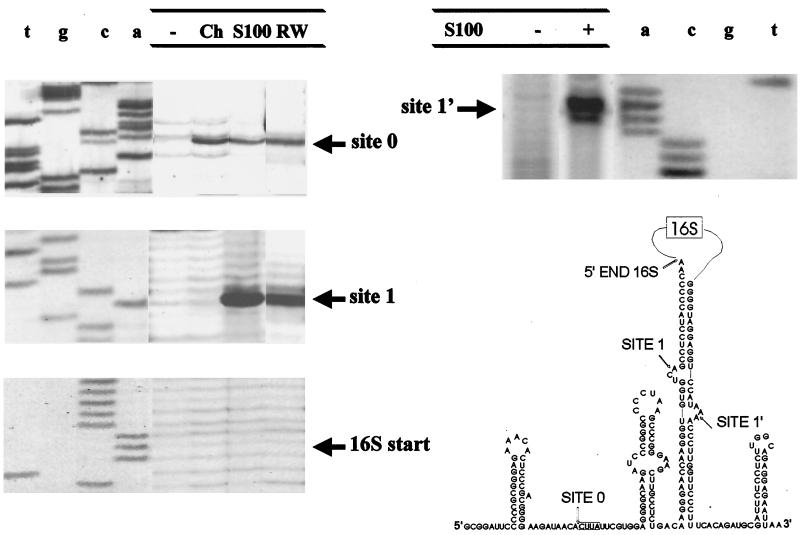
FIG. 2.
In vitro processing of the complete 16S pre-rRNA. The structure of the long transcript is schematized on the right. The sequences and secondary structures of the 5′ETS and of the ITS are shown in full, while the 16S sequence is represented schematically as a loop on top of the processing stem. The locations of the processing sites (site 0, site 1, and site 1′) and that of the 5′ terminus of the 16S rRNA are indicated. The left and top panels show the positions of the processing cuts as revealed by primer extension experiments. Ch, chaperonin.
Preparation of the S-100 and RW fractions.
S. solfataricus (strain MT4) cells were grown at 85°C as described by De Rosa et al. (5). Desulfurococcus mobilis and Thermococcus celer frozen cells were the kind gift of W. Zillig (Martinsried, Germany). To prepare the supernatant fraction, the cells were disrupted by alumina (Alcoa) grinding and crude cell lysates were obtained, as described by Londei et al. (17). The crude lysates were fractionated by centrifugation at 100,000 × g for 2 h; the resulting supernatants (S-100 fractions) were concentrated by precipitation with 70% ammonium sulfate. The precipitates were resuspended in 10 mM Tris-HCl (pH 7.2)–10% glycerol (one-fifth of the initial volume of the S-100 fraction), dialyzed extensively against the same buffer and stored at −80°C in small aliquots. To prepare the high-salt ribosome wash (RW) the crude ribosomes in the 100,000 × g pellets were resuspended in a buffer containing 500 mM NH4Cl, 20 mM Tris-HCl (pH 7.4), 10 mM Mg acetate, and 5 mM β-mercaptoethanol and again centrifuged at 100,000 × g for 12 h through a cushion of 0.5 M sucrose in the same buffer. The resulting pellets (purified ribosomes) were resuspended in a buffer containing 2 M NH4Cl, 20 mM Tris-HCl (pH 7.4), 10 mM Mg acetate, and 5 mM β-mercaptoethanol (high-salt buffer) and incubated on ice for 4 to 5 h with stirring. The samples were then centrifuged at 100,000 × g for 4 to 5 h; the resulting supernatant (high-salt RW fraction) was concentrated by precipitation with 70% ammonium sulfate and finally resuspended in 10 mM Tris-HCl (pH 7.2)–10% glycerol.
In vitro processing.
One to 2 μg of RNA, or of 30S ribonucleoprotein particles, was incubated for 10 min (or various amounts of time from 0 to 20 min when processing kinetics were determined) at 75°C with about 1 μg of chaperonin or 5 μg of either the S-100 fraction or of the high-salt RW fraction. When the experiments were performed with the radiolabeled RNA, the incubation mixtures contained 100 ng of each transcript (200,000 to 300,000 cpm). The reaction buffer contained 10 mM KCl (or 100 mM when the reconstituted 30S rRNAs were employed), 50 mM Tris (pH 8), and 10 mM MgCl2 (final volume, 30 μl). The reaction was stopped with 30 μl of 50 mM EDTA (pH 8) and 0.5% sodium dodecyl sulfate. The products were resolved by electrophoresis on 6% acrylamide gels containing 8 M urea in Tris-borate-EDTA or subjected to primer extension analysis.
Primer extension.
Primer extension determination of the processing cuts on the RNA transcripts was performed according to techniques described previously (24). To detect processing within the 5′ETS, a 17-mer oligonucleotide (5′ACTCCCATGGCTTAACC3′) complementary to the region containing nt 42 to 58 of the 16S RNA coding sequence was used as the primer. For the ITS, the primer was a 24-mer oligonucleotide (5′TAAGCGGCCTTTCGGCCCTAAGCC3′) complementary to the ITS tract from nt −20 to −43 relative to the 5′ end of the 23S rRNA.
Site-directed mutagenesis.
Site-directed mutagenesis was performed according to the method of Deng and Nickoloff (3), using a Transformer site-directed mutagenesis kit (Clontech Laboratories) and appropriate oligonucleotides containing the mutations to be inserted.
Chemical probing of RNA structure.
To obtain experimental information about the structure of the S. solfataricus 5′ETS, the short in vitro transcript was treated with 1-cyclohexyl-3-(2-morpholinoethyl)carbodiimide metho-p-toluene sulfonate (CMCT), which preferentially modifies unpaired uracil residues, essentially according to the protocol described by Stem et al. (24) except that chemical modification was performed at 70°C, close to the physiological temperature for Sulfolobus growth. The modified RNA was subjected to primer extension analysis with the same primer employed to analyze processing. The stop signals corresponding to the modified nucleotides were visualized by autoradiography.
In vitro assembly of 30S subunits.
About 2 μg (4 pmol) of the long transcript 16S RNA was incubated with an optimal amount of TP30 as determined experimentally. Incubation was carried out at 80°C for 15 min in the presence of a solution containing 100 mM KCl, 20 mM Tris-HCl (pH 7), and 20 mM Mg acetate, in a final volume of 15 μl. At the end of the incubation, 10 μl of the mixture was analyzed by centrifugation on sucrose density gradients to determine whether 30S particles had formed, while the rest was used for processing experiments.
RESULTS
In vitro processing of the S. solfataricus 5′ETS.
Previous work on the earlier steps of pre-rRNA processing in the thermophilic archaeon S. acidocaldarius (6) revealed the presence in vivo of three processing sites in the 5′ETS, one corresponding to the mature 5′ end of the 16S RNA and the other two located at nt −98 and −31 relative to it. These cleavage sites were also observed in vitro using unfractionated cell extracts and a short synthetic RNA substrate including the whole 5′ETS and only about 100 nt of the 16S rRNA sequence. This finding showed that processing within the 5′ETS did not require the presence of most of the 16S coding sequence or of the processing stem.
Recently, we found that a similar short pre-rRNA substrate from S. solfataricus (including the entire 5′ETS and about 100 nt of the 16S rRNA sequence) was cleaved in vitro at a single site (termed site 0) by a specific endonuclease tightly associated with the 60-kDa chaperonin (21) (Fig. 1). The processing site was located 94 nt upstream of the mature 5′ end of the 16S rRNA, corresponding to the most distal of the sites mapped in S. acidocaldarius. Importantly, site 0 is likely to be the earliest processing site in the S. solfataricus pre-rRNA, as precursor molecules ending at this site were observed in vivo (20).
Since no other cleavage sites were observed, the inference was that the chaperonin-associated endonuclease was responsible for the cut at site 0 only and that the rest of 16S rRNA 5′-end processing was carried out by other enzymatic activities located elsewhere in the cell. To detect these activities, and to elucidate the subsequent processing steps, we began with analyzing the processing of the short substrate in the presence of different protein fractions as the source of processing enzymes. These were a postribosomal supernatant (S-100) and a high-salt RW fraction obtained by treating purified S. solfataricus ribosomes with 2 M NH4Cl. RW fractions are known to be good sources of processing activities in Escherichia coli (23). As a control, we also employed a chaperonin preparation made as described by Ruggero et al. (21).
In the presence of the chaperonin, as observed previously (21), the short RNA was cleaved only at position −94 (site 0). The same occurred with both the S-100 fraction and the RW fraction (which contained a substantial amount of chaperonin) (Fig. 1). At incubation times higher than 10 min, the S-100 fraction also introduced a few cuts in the 16S rRNA coding sequence, probably because of the presence of unspecific nucleases (not shown). However, cleavage at either position −31 or at the mature 5′ end of the 16S rRNA was never observed. We concluded that the only bona fide in vitro processing site in the short substrate was site 0.
Processing of a complete pre-16S RNA.
We next analyzed the processing of a more physiological substrate, a longer transcript spanning the 5′ETS, the entire 16S gene, and the ITS. This RNA molecule contains both inverted repeats flanking the 16S rRNA and is therefore able to form the processing stem, including the canonical BHB motif (Fig. 2). The cleavage sites were mapped by primer extension analysis and confirmed by analyzing on a denaturing gel the fragments obtained upon processing of a uniformly labeled RNA substrate (not shown).
In the presence of the chaperonin, only processing at site 0 was observed, confirming that this site was uniquely recognized by a specific endonuclease. With the S-100 fraction, two distinct cleavage sites within the 5′ETS and one within the ITS were apparent (Fig. 2). The 5′ETS was cut at −94 (site 0) and also at −16 (site 1), within the upper bulge of the BHB motif in the processing stem. The ITS was cut at −146 relative to the 5′ end of the 23S RNA (site 1′), i.e., in the lower bulge of the BHB motif. Site 1 and site 1′ cleavages conformed to the canonical pattern of processing stem truncation in archaea, and both were probably introduced by the same enzyme, the BHB endonuclease. Essentially the same results were obtained with the RW fraction (Fig. 2). However, neither the S-100 fraction nor the RW fraction yielded any maturation of the 5′ or the 3′ end of the 16S RNA. Instead, as already noted with the short substrate, the S-100 proteins introduced several unspecific cuts within the 16S coding sequence, especially if incubation was prolonged above 10 min (not shown).
In summary, the results of the in vitro processing experiments showed that a pre-16S rRNA substrate complete with the 5′ETS and the ITS was cut at three distinct sites, all corresponding to expected processing sites on the basis of previous in vivo and/or in vitro observations with Sulfolobus itself (site 0) or other archaea (sites 1 and 1′). Thus, the situation in S. solfataricus differed from that observed in S. acidocaldarius (6), first because we did detect canonical processing at the BHB motif in the processing stem and second because no maturation at the 5′ (or 3′) end of the 16S rRNA was obtained.
RNA features determining cleavage site specificity.
The in vitro cleavage pattern illustrated in the previous paragraphs suggested that a minimum of two different enzymatic activities were at play. The first of these was a site-specific endonuclease, cleaving uniquely at site 0. The two symmetrical cuts at sites 1 and 1′ were probably introduced by the same structure-specific enzyme, predicted to be able to recognize the BHB motif in the processing stem. To obtain better insight into the nature of the processing nucleases, the RNA features required for the specific recognition of the cleavage sites were investigated by engineering a series of mutant RNA substrates.
(i) Site 0 cleavage is sequence specific.
In vitro cleavage at site 0, as demonstrated here and in previous works (6, 21), did not require the formation of the processing stem or most of the 16S coding sequence. To determine whether other sequences or structures in the 5′ETS were involved in site 0 recognition, we analyzed the processing behavior of a minimal pre-RNA derived from runoff transcription of a HindIII-linearized DNA substrate. This transcript was only about 100 nt long and lacked all of the 16S rRNA coding sequences plus a large tract of the 5′ETS comprising most of the secondary-structure elements downstream of site 0 (Fig. 1).
When incubated under the appropriate conditions with chaperonin, the minimal substrate was efficiently cleaved at site 0, yielding one 60-nt and one 40-nt fragment (Fig. 1). This result strongly suggested that the site itself contained its own recognition determinants. Indeed, as shown in Fig. 3, site 0 spans a conserved sequence, including a consensus CUU motif, that is found in the 5′ETSs of other archaeal pre-rRNAs as well as around the A0 and 0 sites of yeast and higher eukaryotes. Computer-aided secondary-structure modelling of the secondary structure of the 5′ETS of Sulfolobus (and other archaea) indicated that the tract containing the conserved sequence is single stranded (Fig. 1). To obtain direct experimental information about this point, however, we probed the structure of the pre-rRNA substrates prepared by in vitro transcription by means of chemical-modification and primer extension assays (24). Since the region containing site 0 is very uracil rich, the short pre-rRNA was treated with CMCT, which selectively modifies single-stranded uracils. As shown in Fig. 3, all uracil residues contained in, and surrounding, site 0 were modified, demostrating that they were fully accessible to the reagent and therefore not engaged in higher-order structures.
FIG. 3.
Site 0 is located in a single-stranded region, and cleavage is sequence specific. (Left panel) Primer extension analysis performed on an unmodified short transcript (lane −) and on the same transcript modified with CMCT (lane +). The main stop signals corresponding to modified uracil residues are indicated with arrows; the sequence at site 0 is evidenced. (Top middle panel) Alignment of the sequences around site 0 in several archaea and around sites A0 and 0 in yeast and mouse. The positions of the processing cuts, when known, are marked with arrows. The conserved CUU motif is underlined. (Bottom middle panel) Analysis by site-directed mutagenesis of the sequence determinants essential for processing at site 0. The nucleotides modified in each experiment are underlined; the efficiency of processing was assayed by incubating a uniformly labeled minimal transcript with the purified chaperonin. +, complete cleavage; +/−, partial cleavage; −, no cleavage. (Right panel) Structure of the short transcript. The uracil residues modified by CMCT are indicated with arrows.
The sequence determinants essential for processing were further analyzed in detail by site-directed mutagenesis, whereby the conserved 5′CUU3′ motif was systematically modified. As shown in Fig. 3, the modification of the first two nucleotides (C and U), both separately and together, completely abolished cleavage at site 0 while mutation of the third nucleotide (U) strongly impaired it. By contrast, the modification of a fourth nucleotide (A), which is also conserved in archaea, had essentially no effect. These data demonstrate that site 0 endonuclease is indeed sequence specific and that the CU motif immediately to the right of the cleavage site is required for processing. Also, it is worth noting that cleavage at site 1 of long substrates with an inactive site 0 remained undistrurbed (not shown), thus showing that the two sites behave in an independent fashion, at least in vitro.
It is known that the nucleases cutting at the eukaryotic sites A0 and 0 are ribonucleoproteins. To learn whether this was also true of S. solfataricus site 0 endonuclease, we treated with micrococcal RNase the chaperonin preparation containing the enzyme in order to destroy any catalytic RNA molecule that might be associated with it. This procedure, however, did not inhibit cleavage activity significantly (not shown), indicating that the site 0 endonuclease is probably independent of any trans-acting RNA molecules.
(ii) Cleavage at sites 1 and 1′ is structure specific.
Next, the determinants required for cleavage at sites 1 and 1′ were investigated. If both of these cuts were introduced by an endonuclease that recognized the BHB motif, we expected that any mutation destroying the integrity of the BHB structure would simutaneously abolish processing at both sites 1 and 1′.
To demonstrate this point, we created a mutant construct (mut 1) in which the sequence GG within the stem of the BHB motif was changed to UC. This mutation was expected to prevent the formation of the 4-bp stem, transforming the BHB structure into a large internal loop (Fig. 4). In fact, as shown in Fig. 4, a mut 1 long transcript could not be cleaved at either site 1 or site 1′, demonstrating that the enzyme indeed recognized the BHB structure and was therefore very likely to be the same endonuclease involved in the splicing of tRNA transcripts (18, 19). Cleavage at site 0 in a mut 1 transcript remained unaffected (Fig. 4).
FIG. 4.
Cleavage in the processing stem is structure specific. (Left panel) Sequence and predicted structure of the wild-type and mutated processing stems. The modified nucleotides (GG to CU) are boxed. (Right panels) Primer extension analysis of processing at sites 0 and 1 (top) and 1′ (bottom) by the S-100 fraction in the wild-type (wt) and mutated (mut 1) long transcripts.
Cleavage at site 0 and within the BHB motif are independent events.
Besides revealing the RNA features required for enzymatic recognition, the results with the mutant constructs showed that site 0 cleavage and processing stem truncation were independent of each other. In fact, an RNA with a mutated site 0 was normally cleaved at sites 1 and 1′ (not shown), while an RNA that had lost the BHB motif was still efficiently cut at site 0 (Fig. 4).
To determine whether in vitro processing on a wild-type substrate followed a definite order, a long transcript was incubated with the S-100 fraction and samples were withdrawn at increasing incubation times. The extents of cleavage at sites 0 and 1 were monitored at each time point by primer extension analysis (not shown). However, no correlation between the cleavage sites was apparent. Stop signals at both site 0 and site 1 increased linearly up to 15 min of incubation, indicating that the first cut in a given RNA molecule could be introduced at either location with equal likelihood.
Processing on ribonucleoprotein particles.
The observations described in the previous paragraphs were made with naked RNA molecules. However, it is known that in vivo pre-rRNA processing and ribosome assembly are contemporaneous, so that processing takes place on RNA substrates that are already coated to some extent with ribosomal proteins. Even in vitro, some aspects of rRNA processing in bacteria can be observed only on ribonucleoprotein substrates and are not reproducible with naked rRNA. Notably, this is true for terminal maturation of the 5′ and 3′ ends of the 16S rRNA in E. coli (23).
To determine whether the presence of the ribosomal proteins allowed the maturation of 16S RNA termini, in vitro processing was coupled with 30S rRNA subunit assembly. Functional in vitro reconstitution of S. solfataricus ribosomal subunits was achieved about a decade ago for the 50S particles (16). Recently, it was found that the 30S subunits can also be reconstructed under somewhat different conditions (D. Ruggero, A. Ciammaruconi, and P. Londei, unpublished data). Notably, in vitro assembly of structurally complete (albeit poorly active) 30S subunits may be achieved with a 16S RNA precursor still containing the 5′ETS and ITS, namely, the long transcript employed in this study (Fig. 4). Accordingly, the processing experiments were performed under reconstitution conditions in the presence of the small-ribosomal-subunit proteins. Preliminarily, we checked that the different ionic conditions did not impair the correct cleavage of a naked RNA substrate.
As shown in Fig. 5, the processing pattern of a ribonucleoprotein substrate in the presence of either chaperonin or the S-100 fraction was the same as that of a naked pre-16S RNA. Again, cleavages were detected at sites 0 and 1 while no maturation at the 5′ end of the 16S RNA was apparent. To determine whether the presence of the ribosomal proteins introduced a definite processing order, we also analyzed the cleavage kinetics (not shown). However, these were similar to those obtained with the naked RNA: the ribonucloprotein substrate was still randomly cut at either site 0 or site 1. The only difference was that processing was somewhat slower, presumably because the nucleases took longer to recognize their target sites on a ribonucleoprotein substrate.
FIG. 5.
Processing on ribonucleoprotein particles. (Top panel) Primer extension analysis of the processing cuts introduced by either the chaperonin (Ch) or the S-100 proteins on a long pre-rRNA, in the absence and in the presence of the total small-subunit proteins (TP30). (Bottom panel) Density gradient analysis showing that the TP30 and the long pre-rRNA assemble to form a 30S particle when incubated at 80°C under the conditions described in Materials and Methods. The gradients, 10 to 30% sucrose in reconstitution buffer, were run for 4 h at 38,000 rpm in a Beckman SW41 rotor.
Site 0 is a distinctive feature of the Crenarchaeota.
Cleavage within the BHB motif is probably a universal processing mode in archaea, since such motifs are found in pre-rRNAs from both major branches of archaeal descent, the Euryarchaeota and the Crenarchaeota. By contrast, the presence of a sequence-specific early processing site (site 0) has so far been demonstrated for only two species of the Sulfolobus genus. Therefore, the question is whether site 0 cleavage is a general feature of archaeal rRNA processing or whether it exists only in a subclass of archaea. Given the similarity of site 0 with the eukaryal site A0 or 0, this question has implications as regards archaeal evolution and the relationship of archaea with the eukaryotic lineage.
Sequence analysis of available archaeal pre-rRNA 5′ETS tracts revealed the presence of homologies with the site 0 consensus in several Crenarchaeota (Fig. 3) but not in Euryarchaeota. To assess experimentally the significance of this finding, we tested the ability of S-100 extracts from the euryarchaeon T. celer and the crenarchaeon D. mobilis to process S. solfataricus pre-rRNA. These organisms were chosen because they are extreme thermophiles, as is S. solfataricus.
As illustrated in Fig. 6, primer extension analysis following incubation of S. solfataricus pre-16S RNA with the heterologous extracts showed that both D. mobilis and T. celer proteins recognized the BHB motif (i.e., cut at site 1). However, only the D. mobilis S-100 fraction was able to cleave at site 0, in agreement with the observation that a site 0 consensus exists in the 5′ETS of D. mobilis but not in that of T. celer. The recognition of the site by the heterologous endonuclease was sequence specific, as demonstrated by the fact that a mutant transcript with an inactive site 0 was not cut (not shown). These results indicate that the occurrence of an early, sequence-specific, pre-rRNA processing site in the 5′ETS may be restricted to the Crenarchaeota.
FIG. 6.
Processing of the S. solfataricus pre-16S RNA by heterologous enzymes. The results of primer extension analysis of in vitro cleavage at sites 0 and 1 of a long transcript in the presence of the S-100 fractions from S. solfataricus (Sso), D. mobilis (Dmo), and T. celer (Tce) are shown.
DISCUSSION
In the present work we have analyzed the in vitro processing of the 16S RNA of the crenarchaeon S. solfataricus, using different in vitro transcripts and various protein preparations as the source of the processing enzymes. Our findings, along with earlier in vivo observations (20), suggest that the maturation of S. solfataricus pre-rRNA is initiated by a single-strand-specific endonuclease that recognizes a U-rich sequence located 94 nt upstream of the mature 5′ end of the 16S RNA. The sequence at this processing site (which we have termed site 0) is conserved in several members of the Crenarchaeota (but not in the Euryarchaeota); accordingly, we show that the relevant endonucleolytic activity is present in the crenarchaeon D. mobilis but not in the euryarchaeon T. celer. A homologous endonucleolytic cleavage site was also shown to exist in S. acidocaldarius (6, 22). We propose, therefore, that cleavage at site 0 is a common distinctive feature of early pre-rRNA processing in the Crenarchaeota.
Furthermore, we show that S. solfataricus pre-16S rRNA is also processed at the canonical archaeal site formed by the BHB motif within the processing stem and that the relevant nucleolytic acitivity is, as expected, universally distributed within archaea. Finally, we find that site 0 endonuclease and the BHB endonuclease are not sufficient to complete the maturation of the 5′ and 3′ ends of the 16S RNA. In fact, under conditions allowing cleavage by both activities, the 16S RNA termini remained unprocessed, regardless of whether the substrate was the naked RNA or a 30S ribonucleoprotein particle. This result may indicate that 16S rRNA terminal maturation in S. solfataricus requires specific enzymatic activities that are, however, too scarce in the protein preparations employed in this work. In this respect, it may be observed that in vitro terminal maturation of eukaryotic pre-rRNA transcripts can be reproduced only with nucleolar extracts (10). Alternatively, completing the processing of pre-16S RNA may require finely tuned conditions not easily reproducible in vitro, such as a precise coordination between transcription, processing, and ribosome assembly.
The present results differ in several aspects from those obtained from processing studies of the pre-rRNA of S. acidocaldarius, a species closely related to S. solfataricus (6, 22). In S. acidocaldarius pre-rRNA, the removal of the 5′ETS was found to entail three consecutive endonucleolytic cuts, the last of which generated the mature 5′ terminus of the 16S RNA. It was suggested that all three cleavages, including the one determining 5′ end maturation, were performed by the same site-specific endonuclease which recognized a common consensus sequence at the cleavage sites (6). It was subsequently shown that processing at one of these sites indeed required the presence of a specific sequence (22). However, in the S. solfataricus 5′ETS (and in those of other Crenarchaeota) the target sequence is found only at site 0, corresponding to the most distal of the three S. acidocaldarius sites. Therefore, even if the site 0 endonuclease does mature the 5′ end of S. acidocaldarius 16S RNA, this cannot be generalized to other archaea. Also, it was observed that the pre-16S RNA of S. acidocaldarius is not cleaved within the processing stem because it contains a defective BHB motif (6). Again, this is a specific feature of S. acidocaldarius, since, as we show here, the S. solfataricus pre-16S RNA does contain a canonical BHB motif which is regularly cleaved by the specific endonuclease. Therefore, the present results demonstrate that cleavage at site 0 is not an alternative to processing stem truncation.
Upon the whole, the present work supports the notion that certain similarities exist between the pre-rRNA maturation pathways in eukaryotes and in archaea, more specifically in the Crenarchaeota. In both cases, early pre-rRNA processing entails the introduction of a site-specific cleavage in the 5′ETS: indeed, we called 0 the archaeal site to stress its similarity with the A0 and 0 sites of yeast and vertebrates. Strikingly, the archaeal and eukaryotic A0 and 0 sites also share a consensus sequence (Fig. 3), which in archaea is essential for cleavage. However, a meaningful evaluation of the real degree of homology between the archaeal and the eukaryal early processing steps is hampered by our poor understanding of their functions and enzymology. In spite of its general occurrence in eukaryotes, site A0 or 0 does not seem to be essential for correct pre-rRNA processing; in Sulfolobus, we have shown that cleavage at site 0 is not a prerequisite for further processing, at least in vitro. As to the enzymes involved, much evidence indicates that processing at site A0 or 0 in eukaryotes is performed by ribonucleoproteins containing the essential small nucleolar RNA U3 (2, 11, 12). Recently, however, it has been found that, in yeast, cleavage at site A0 may also occur, both in vivo and in vitro, independently of U3 by an RNase homologous to bacterial RNase III (1). The relationship between the two modes of site A0 processing is not yet understood. In Sulfolobus, as we show here, site 0 cleavage very probably does not require a trans-acting catalytic RNA activity. However, the enzyme involved cannot be RNase III, which has no obvious homologues in archaea. Thus, Sulfolobus site 0 endonuclease may be a novel archaeon-specific protein or it may have homology with some component of the eukaryotic U3-containing RNase, although it dispenses with a trans-acting small RNA, just as RNase III does in eukaryotes.
As to cleavage within the processing stems, the resemblance of this process in yeast and eukaryotes to the corresponding process in bacteria is more apparent than real. In fact, in bacteria these stems are truncated by RNase III while in archaea the same task seems to be performed by an altogether different protein, specifically recognizing the BHB motif and very likely to be the same enzyme that removes tRNA and rRNA introns (although its identity, to the best of our knowledge, has never been demonstrated formally). The archaeal splicing endonuclease, which may be a dimer or a tetramer depending on the species, has no resemblance to RNase III but is homologous to one of the subunits of the eukaryotic tRNA-splicing endonuclease (14, 18). Thus, archaea may employ eukaryotic-like enzymes for a number of key pre-rRNA processing events.
Further work involving the isolation and characterization of archaeal processing enzymes is needed to trace the evolutionary history of pre-rRNA processing and help in the understanding of its mechanisms and pathways in the three primary domains of life.
ACKNOWLEDGMENTS
This work has been supported in part by funds from the Italian Ministry of University and Research (MURST). A.C. was the recipient of a fellowship from the Institute Pasteur-Cenci Bolognetti Foundation at the University of Rome “La Sapienza.”
We thank Laura Nicolini of the Istituto Superiore di Sanità (Rome, Italy) for her kind help in growing Sulfolobus cells.
REFERENCES
- 1.Abou Elela S, Igel H, Ares M., Jr RNase III cleaves eukaryotic pre-ribosomal RNA at a U3 snoRNP-dependent site. Cell. 1996;85:115–124. doi: 10.1016/s0092-8674(00)81087-9. [DOI] [PubMed] [Google Scholar]
- 2.Beltrame M, Tollervey D. Base-pairing between U3 and the pre-ribosomal RNA is required for 18S RNA synthesis. EMBO J. 1995;14:4350–4356. doi: 10.1002/j.1460-2075.1995.tb00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deng W P, Nickoloff J A. Site-directed mutagenesis of virtually any plasmid by eliminating a unique site. Anal Biochem. 1992;200:81–88. doi: 10.1016/0003-2697(92)90280-k. [DOI] [PubMed] [Google Scholar]
- 4.Dennis P P. Multiple promoters for the transcription of the ribosomal RNA gene cluster in Halobacterium cutirubrum. J Mol Biol. 1985;186:457–461. doi: 10.1016/0022-2836(85)90117-2. [DOI] [PubMed] [Google Scholar]
- 5.De Rosa M, Gambacorta A, Bu'lock J D. Extremely thermoacidophilic bacteria convergent with Sulfolobus acidocaldarius. J Gen Microbiol. 1975;86:156–164. doi: 10.1099/00221287-86-1-156. [DOI] [PubMed] [Google Scholar]
- 6.Durovic P, Dennis P P. Separate pathways for excision and processing of 16S and 23S rRNA from the primary rRNA operon transcript from the hyperthermophilic archaebacterium Sulfolobus acidocaldarius: similarities to eukaryotic rRNA processing. Mol Microbiol. 1994;13:229–242. doi: 10.1111/j.1365-2958.1994.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 7.Eichler D C, Craig N. Processing of eukaryotic ribosomal RNA. Prog Nucleic Acid Res Mol Biol. 1994;49:197–239. doi: 10.1016/s0079-6603(08)60051-3. [DOI] [PubMed] [Google Scholar]
- 8.Garrett R A, Dalgaard J, Larsen N, Kjems J, Mankin A S. Archaeal rRNA operons. Trends Biochem Sci. 1991;16:22–26. doi: 10.1016/0968-0004(91)90011-j. [DOI] [PubMed] [Google Scholar]
- 9.Gegenheimer P, Apirion D. Processing of procaryotic ribonucleic acid. Microbiol Rev. 1981;45:502–541. doi: 10.1128/mr.45.4.502-541.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hannon G J, Maroney P A, Branch A, Benenfield B J, Robertson H D, Nilsen T W. Accurate processing of human pre-rRNA in vitro. Mol Cell Biol. 1989;9:4422–4431. doi: 10.1128/mcb.9.10.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes J M, Ares M., Jr Depletion of U3 small nucleolar RNA inhibits cleavage in the 5′ external transcribed spacer of yeast pre-ribosomal RNA and impairs formation of 18S ribosomal RNA. EMBO J. 1991;10:4231–4239. doi: 10.1002/j.1460-2075.1991.tb05001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kass S, Tyc K, Steitz J A, Sollner-Webb B. The U3 small nucleolar ribonucleoprotein functions in the first step of preribosomal RNA processing. Cell. 1990;60:897–908. doi: 10.1016/0092-8674(90)90338-f. [DOI] [PubMed] [Google Scholar]
- 13.Kjems J, Leffers H, Garrett R A, Wich G, Leinfelder W, Bock A. Gene organization, transcription signals and processing of the single ribosomal RNA operon of the archaebacterium Thermoproteus tenax. Nucleic Acids Res. 1987;15:4821–4835. doi: 10.1093/nar/15.12.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleman-Leyer K, Armbruster D, Daniels C J. Properies of H. volcanii tRNA intron endonuclease reveal a relationship between the archaeal and eucaryal tRNA intron processing system. Cell. 1997;89:839–847. doi: 10.1016/s0092-8674(00)80269-x. [DOI] [PubMed] [Google Scholar]
- 15.Leffers H, Kjems J, Ostergaard L, Larsen N, Garrett R A. Evolutionary relationships amongst archaebacteria. A comparative study of 23 S ribosomal RNAs of a sulphur-dependent extreme thermophile, an extreme halophile and a thermophilic methanogen. J Mol Biol. 1987;195:43–61. doi: 10.1016/0022-2836(87)90326-3. [DOI] [PubMed] [Google Scholar]
- 16.Londei P, Teixido J, Acca M, Cammarano P, Amils R. Total reconstitution of functionally active ribosomal subunits of the extremely thermoacidophilic archaebacterium Sulfolobus solfataricus. Nucleic Acids Res. 1986;14:2269–2285. doi: 10.1093/nar/14.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Londei P, Altamura S, Cammarano P, Petrucci L. Differential properties of ribosomes and of poly(U) directed cell-free systems from sulphur-dependent archaebacterial species. Eur J Biochem. 1986;157:455–462. doi: 10.1111/j.1432-1033.1986.tb09689.x. [DOI] [PubMed] [Google Scholar]
- 18.Lykke-Andersen J, Garrett R A. RNA-protein interactions of an archaeal homotetrameric splicing endoribonuclease with an exceptional evolutionary history. EMBO J. 1997;16:6290–6300. doi: 10.1093/emboj/16.20.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lykke-Andersen J, Aagaard C, Semionenkov M, Garrett R A. Archaeal introns: splicing, intercellular mobility and evolution. Trends Biochem Sci. 1997;22:326–331. doi: 10.1016/s0968-0004(97)01113-4. [DOI] [PubMed] [Google Scholar]
- 20.Reiter W D, Palm P, Voos W, Kaniecki J, Grampp B, Schulz W, Zillig W. Putative promoter elements for the ribosomal RNA genes of the thermoacidophilic archaebacterium Sulfolobus sp. strain B12. Nucleic Acids Res. 1987;15:5581–5595. doi: 10.1093/nar/15.14.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruggero D, Ciammaruconi A, Londei P. The 60 kD chaperonin of the thermophilic archaeon Sulfoibus solfataricus is an RNA binding protein that participates in ribosomal RNA processing. EMBO J. 1998;17:3471–3477. doi: 10.1093/emboj/17.12.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell A G, Ebhard H, Dennis P P. Substrate requirements for a novel archaeal endonuclease that cleaves within the 5′ external transcribed spacer of Sulfolobus acidocaldarius precursor rRNA. Genetics. 1999;152:1373–1385. doi: 10.1093/genetics/152.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava A K, Schlessinger D. Processing pathway of Escherichia coli 16S precursor rRNA. Nucleic Acids Res. 1989;17:1649–1663. doi: 10.1093/nar/17.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stem S, Moazed D, Noller H F. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 1988;164:481–489. doi: 10.1016/s0076-6879(88)64064-x. [DOI] [PubMed] [Google Scholar]
- 25.Venema J, Tollervey D. Processing of pre-ribosomal RNA in Saccharomyces cerevisiae. Yeast. 1995;11:1629–1650. doi: 10.1002/yea.320111607. [DOI] [PubMed] [Google Scholar]