ABSTRACT
The global population is living longer; however, not everyone ages at the same rate with regard to their physical and cognitive abilities and their vulnerability to certain diseases and death. This review aimed to synthesize the contribution of biological age–predictive biomarkers to nutrition research and highlight the implications for future research and clinical practice. MEDLINE, CINAHL, and Cochrane CENTRAL were systematically searched on 30 September 2021 for randomized controlled trials and cross-sectional studies examining the association between nutrition and biological age in older adults reporting on genetic, clinical, or molecular biomarkers of biological aging. Cochrane's ROB 2 and ROBINS-I were used to assess the quality of included studies. Synthesis was undertaken narratively. Of 1245 records identified from the search, 13 studies from 8 countries and territories, involving 5043 participants, were included. Seven studies assessed associations between nutrient food intake and telomere attrition, reporting protective effects for branched-chain amino acids, calcium and vitamin D, and a diet of a lower inflammatory index; whereas they found shorter telomeres in people consuming more processed foods and arachidonic acid and other proinflammatory compounds. Five studies examined the associations between plasma nutrition biomarkers and cognitive function, and found a protective effect for HDL cholesterol, lycopene, carotenoids, ω-3 and ω-6 fatty acids, and vitamins B, C, D, and E; whereas trans fatty acids and fibrinogen correlated with a decline in cognitive function. One study used Horvath's clock and reported the epigenetic rejuvenation effect of a Mediterranean diet. In conclusion, biological aging was negatively associated with an anti-inflammatory diet. However, a few studies did not control for the confounding effect of other lifestyle factors. Future research should address this and also assess the synergistic effect of different nutrients, their combinations, and evaluate their dose–response relations. Nutrition practice can incorporate updated screening procedures for older people that include relevant biological aging nutrition markers, leading to anti-aging precision nutrition therapy. The methodology of this systematic review was registered in PROSPERO (CRD42021288122).
Keywords: aging, cognitive function, diet, dietetics, epigenetics, geriatrics, gerontology, nutritional epidemiology, telomere length
Statement of Significance: This review is the first to collate original findings on the associations between nutrients and dietary practices with biological aging, including their effects on telomere shortening, cognition, and epigenetic rejuvenation (measured using Horvath's clock) in order to provide a current synthesis of evidence and to discuss its implications for future research and clinical practice.
Introduction
Epidemiological insight combined with advances in medical technology and pharmacology have resulted in dramatic increases in life expectancy over recent years. Life expectancy increased, on average, by 5 y between 2000 and 2015, the greatest increase since the 1960s (1). Today, most people can expect to live into their 60s and beyond. The WHO predicts that, by 2030, 1-in-6 people in the world will be aged 60 y and over (2), and by 2050, the population of people aged 60 y and older will double, and the population of people aged 80 y and older will triple (2).
Although people are increasingly living longer, not everyone ages at the same rate with regard to their functional and intellectual ability. Aging is a complex process governed by molecular and cellular changes that mediate gradual decreases in physical and mental capacity, increasing the risk of disease and death (2). The rate at which these changes occur depends on a variety of genetic and environmental factors. Hence why, apart from “chronological age,” which denotes the number of years an individual has lived, the term “biological age” was coined, describing the rate of interindividual variation in loss of function, cognitive ability, and vulnerability to certain diseases and death.
Aging increases the risk for a plethora of conditions, such as diabetes (3), cancer (4), cardiovascular disease (5), neurodegenerative disorders (6, 7), osteoarthritis, joint pain and frailty (8), and chronic obstructive pulmonary disease (9), which pose a burden on health care systems. Therefore, understanding how to delay biological aging is paramount both at individual and population levels (10, 11). Importantly, the WHO states that evidence to date suggests that the proportion of life in good health has remained broadly constant, meaning that the additional years gained via the recent medical and technological advancements are in poor health (2). It is crucial to elucidate the factors that improve health and quality of life in the older population.
Hallmarks of biological aging include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, nutrient-sensing deregulation, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intracellular communication (12). Studies have demonstrated that biological age models can predict morbidity and mortality more accurately than chronological age (6, 13–15).
To delay aging, the WHO recommends “maintaining healthy behaviours throughout life, particularly eating a balanced diet, engaging in regular physical activity and refraining from tobacco use” (2). Although these recommendations have been consistent for years, the role of nutrition in delaying biological aging has not yet been clearly described. Research has attempted to describe associations between different nutrients and foods and the several markers of aging, such as frailty (16), cognition (17–19), physical strength (19), and “healthy aging” in general (20–21).
Recent findings in animals demonstrate that diet is a more potent weapon against biological aging than commonly used medications, such as metformin, resveratrol, and rapamycin, emphasizing the need for more nutritional evidence and tailored dietary interventions to delay aging (22). The objective of this review is to describe the contribution of biological age–predictive biomarkers to nutrition research by synthesizing the evidence in the literature in order to provide a complete summary of the current evidence on the associations between nutrition and biological aging, and highlight the implications for clinical practice and future research.
Methods
Search strategy
The protocol and reporting of this systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (23). EBSCOhost CINAHL complete, EMB Cochrane CENTRAL Register of Controlled Trials, and Ovid MEDLINE were searched on 30 September 2021 using broad search terms and Medical Subject Heading (MeSH) terms. The complete search strategy used for the databases is presented in Supplemental Table 1. The systematic review methodology was registered in PROSPERO (CRD42021288122) and is available at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=288122.
Study selection
Citations and abstracts of all retrieved studies were imported into Covidence systematic review software (Veritas Health Innovation). Duplicates were removed and the remaining studies were assessed for eligibility by both authors. The full list of inclusion and exclusion criteria is shown in Supplemental Table 2. Briefly, randomized controlled trials (RCTs), including cluster, pilot, crossover, and prospective RCTs, and observational, cross-sectional studies examining the association between nutrition and biological age in older adult populations (mean age ≥65 y) and reporting on genetic, clinical, or molecular biomarkers of biological aging were considered for inclusion. No restriction was imposed on the language or year of publication. The database searches were conducted on the 30th of September 2021 and therefore manuscripts published to this date have been considered in this review.
Data extraction
A comprehensive data-extraction form was developed, refined, and piloted based on the guidelines in the sixth version of the Cochrane Handbook for Systematic Reviews of Interventions (24). The following data were extracted: publication details (title, journal, year), authors’ details (names, affiliations, funding, conflict of interest), study details [start and end date, country, design, purpose, setting, methods for blinding, randomization and allocation concealment (if an RCT), retention rate, statistical analyses methods], participants’ characteristics [condition, severity of condition, comorbidities, inclusion and exclusion criteria, sample size, recruitment process, and demographics (i.e., age, sex, race, ethnicity, income, education, and remoteness of residence)], intervention (for RCTs, including type, duration, frequency, other details, primary and secondary outcome factors), comparison (details of care, other details), assessments (for non-RCTs, including dietary and biochemical assessments and measurements, methods of assessments and measurements, primary and secondary outcomes and methods of their measurements), results (time point for follow-up, primary and secondary outcomes and statistical significance, validated tool for measurement), conclusions, and limitations (Supplemental Table 3).
Risk of bias
The Cochrane Risk of Bias 2 (ROB 2) tool was used to assess the quality of the included RCT on aspects of selection (random-sequence generation and allocation concealment), performance (blinding of participants, personnel, and assessors) and deviations from intended interventions, appropriateness of analysis (missing outcome data, appropriate method for the measurement of the outcome), and selective reporting. The RCT was ranked by both authors as “low risk,” “high risk,” or “some concerns,” in accordance with the recommendations of the Cochrane Collaboration (25). Authors resolved discrepancies by discussion. The Risk Of Bias In Non-randomised Studies—of Interventions (ROBINS-I) tool was used to assess the quality of the included nonrandomized studies of interventions on aspects of confounding of the effect of the intervention, selection of participants, classification of interventions, deviations from intended interventions, appropriateness of analysis (missing outcome data, appropriate method for the measurement of the outcome), and selective reporting. The included studies were ranked by both authors as “low risk,” “moderate risk,” “serious risk,” or “severe risk,” in accordance with the recommendations of the Cochrane Collaboration (26). Authors resolved discrepancies by discussion.
Evidence synthesis and other analyses
The heterogeneity of study designs and outcomes did not permit a meta-analysis. Therefore, the evidence was presented in a table and summarized via a narrative synthesis. In the table, effect measures were presented as in the original manuscripts (e.g., ORs, r coefficient) and discussed accordingly. Descriptive statistics were calculated using IBM SPSS version 26.0 (IBM Corporation).
Results
Study selection
The search yielded 1245 records. Fifty-two duplicates were removed and the remaining 1193 titles and abstracts were assessed for eligibility (Figure 1). A total of 1146 abstracts were excluded during the first exclusion round, and a further 34 records were excluded during the second exclusion round, after assessing their full-text articles. The reasons for exclusion during the second round are shown in the Supplemental Results(page 9). Thirteen studies were included in the narrative synthesis.
FIGURE 1.
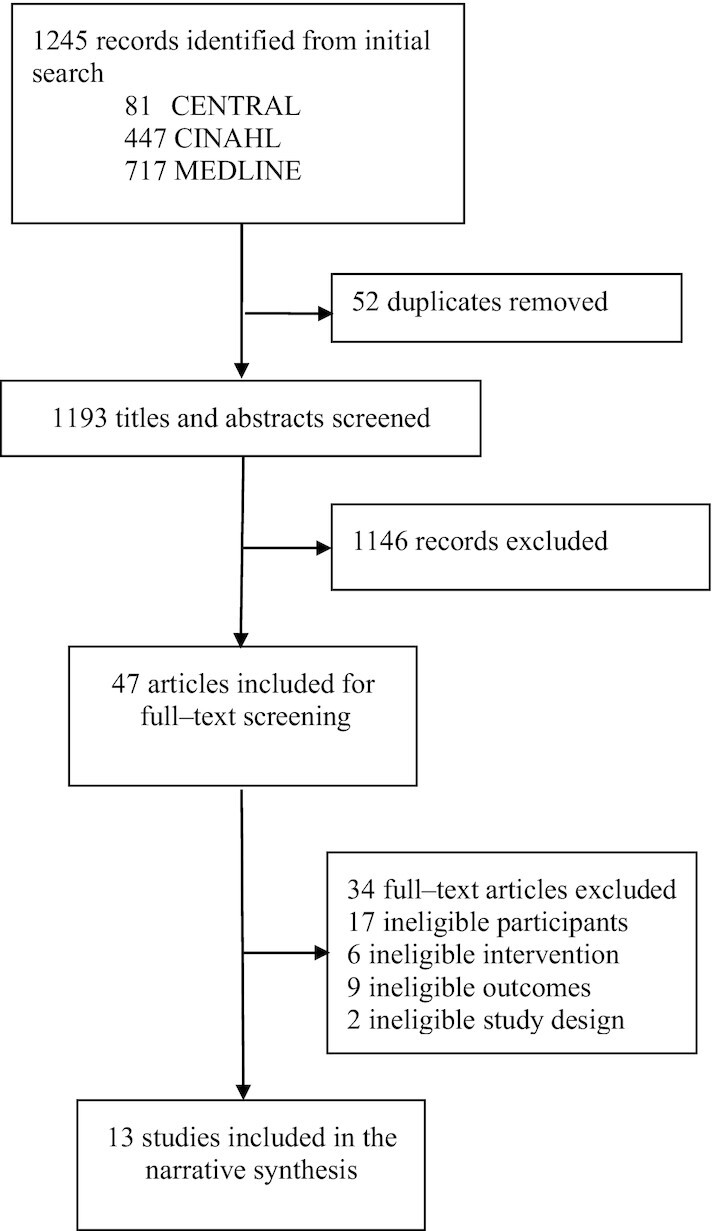
Flow diagram of the study selection process.
Characteristics of included studies
Table 1 summarizes the characteristics of the studies, including the country of conduct, study design, number of participants and their clinical and demographic characteristics at baseline, interventions (where these occurred) and assessment methods, primary and secondary findings, conclusions, and an assessment of the validity of these conclusions considering the study limitations.
TABLE 1.
Characteristics of included studies assessing the effect of nutritional biomarkers on biological aging in older adults with findings, conclusions, and assessment of the validity of conclusions1
| First author (ref), year, country | Study design and primary outcome | Participants and baseline characteristics | Assessments (for NRSI), intervention and control (for RCT) | Findings | Conclusions | Validity of conclusions |
|---|---|---|---|---|---|---|
| Alonso-Pedrero (28), 2020, Spain | Retrospective cross-sectional analysis; telomere attrition | n = 886 Spanish; mean age: 67.7 ± 6.1 y; mean BMI (in kg/m2): 25.85; 72.8% men; Spanish; 5.3 y at university (4 in 5 undergraduate degree); 3 in 4 married; 1 in 2 former smokers | UPF consumption assessed via a self-administered 136-item semi-quantitative FFQ, validated in Spain | Highest UPF consumption group [4.1 (±1.1) servings/d] had shorter telomeres vs. lowest consumption group [1.1 (±0.5) servings/d] (adjusted OR: 1.82; 95% CI: 1.05, 3.22; P-trend = 0.03). Participants in the highest UPF consumption group were more likely to have family history of CVD, diabetes, and dyslipidemia prevalence, and to snack more in between meals, and to consume more fats, SFAs, PUFAs, sodium, dietary cholesterol, fast food, and processed meat, and less carbohydrates, proteins, fiber, potassium, magnesium, calcium, phosphorus, olive oil, fruits, and vegetables, and overall adhere less to the Mediterranean diet, compared with participants in the lowest UPF consumption group. | Higher UPF consumption (≥3 servings/d) is associated telomere attrition in elderly Spanish | Conclusions are robust since a validated FFQ (that mitigates bias due to self-reporting) and a standard method to measure telomere length was used. But underreporting cannot be excluded due to the FFQ not including certain foods, e.g., energy bars |
| Atzmon (29), 2002, USA | Retrospective correlational analysis; cognitive function (MMSE) | n = 139 Ashkenazi Jews; mean age: F, 97.7 ± 0.2 y; M, 97.6 ± 0.4 y; 26.4% men | Plasma HDL cholesterol, TG, apo A-I | Plasma HDL-cholesterol concentrations correlated significantly with MMSE (r = 0.32; P < 0.0001).Each decrease in plasma HDL-cholesterol tertile (74.9 ± 2.1, 50.6 ± 0.5, and 36.8 ± 1.0 mg/dL) was associated with a significant decrease in MMSE (23.4 ± 1.5, 17.7 ± 1.8, and 12.4 ± 1.8; P < 0.04 for each plasma HDL cholesterol tertile). Increased plasma apo A-I and decreased plasma TG concentrations were also correlated with a significantly superior cognitive function. Biological markers of hydration and nutritional status did not differ between the groups with the higher or lower plasma HDL cholesterol or MMSE. | Cognitive dysfunction in centenarians is associated with a progressive decline in plasma HDL cholesterol | The study is robust, but the population studied is very homogeneous, limiting the applicability of findings to diverse populations |
| Bowman (30), 2012, USA | Retrospective cross-sectional analysis; cognitive function (MMSE, MRI, clinical dementia rating) | n = 104; mean age: 87±10 y; mean BMI: 25 ± 4; 38% men; 15±3 y of education; 2% smokers; 39% drinkers; 44% hypertension; 5% diabetes; 21% depression | 30 plasma dietary biomarkers that were grouped into 8 distinct NBPs via multivariate analysis (PCA) | Two NBPs associated with more favorable cognitive and MRI measures: one high in plasma B vitamins (thiamin, riboflavin, vitamin B-6, folate, and vitamin B-12) and vitamins C, D, and E, and another high in plasma marine ω-3 fatty acids. A third pattern characterized by high trans fat was associated with less favorable cognitive function and less total cerebral brain volume. Depression attenuated the relation between the marine ω-3 pattern and white matter hyperintensity volume. | Distinct plasma NBPs are associated with cognitive function and brain volume | Limitations are 1) the PCA that may require decisions with the data from the investigator; 2) selecting a priori nutrient biomarkers based on knowledge of their association with neurodegeneration; 3) the restriction of the sample population to a relatively healthy and well-educated cohort of white, non-Hispanic elders with minimal genetic risk for AD. Therefore, the results cannot be generalized |
| Chang (31), 2020, Taiwan | Case control post hoc analysis; leukocyte telomere attrition and TERRA expression | n = 72; sarcopenia (S), non-sarcopenia (NS); mean age: S, 75.7 ± 5.5 y; NS, 74.9 ± 6.7 y; mean BMI: S, 25.6 ± 2.9; NS, 21.5 ± 2.3; 22% men | Lower body strength exercise 2x per week + BCAAs (0.36 g valine, 0.54 g leucine, 0.43 g isoleucine), 0.65 g glutamine, 0.61 g arginine; 1.01 g of other amino acids + 600 mg calcium and 800 IU vitamin D-3 | No significant difference in telomere length between S and NS. TERRA expression was lower in S compared with NS (5.18 ± 2.98 vs. 2.51 ± 1.89; P < 0.001). Intervention significantly increased TERRA expression in the S group, but not telomere length. The GEE analysis demonstrated that TERRA expression was negatively associated with sarcopenia (β coefficient= –2.705, P < 0.001) but positively associated with intervention (β coefficient = 1.599, P = 0.023). | Sarcopenia is associated with decrease in TERRA expression in leukocytes. Rebound TERRA expression (to the level similar to the NS controls) was observed in the S group after exercise and nutrition intervention | Physical activity habits were not considered at baseline and their heterogeneity could be a confounder. Another limitation is the use of leukocyte telomere length as a surrogate marker for sarcopenia. Although muscle and leukocyte telomere lengths correlate, these are affected in different ways by aging. These limitations restrict the generalizability of the findings and the validity of the conclusion |
| Freitas-Simoes (32), 2019, Spain | Retrospective cross-sectional analysis; leukocyte telomere attrition | n = 344 Spanish; mean age: 68.8 ± 3.3 y; mean BMI: 27.1 ± 3.8; 31.4% men; 55% hypertension; 54% dyslipidemia, 35% on statins; 11% diabetes, 6% on metformin; 72% never smoked; PA (METs/wk): 2510 (1606–3888); 10 (8–15) y of education | RBC proportions of PUFAs | In multivariate models adjusted for age and gender, the RBC proportions of dietary PUFAs were unrelated to telomere length. In contrast, the RBC proportion of arachidonic acid was inversely related to telomere length (r = 0.10, 95% CI: 0.19, 0.01, P = 0.023). | Increased levels of C20:4 ω-6 in RBCs are associated with shorter telomeres | Strengths of the study include the methods of assessment of biomarkers and of the outcome. The main limitation is the relatively small number of participants and the homogeneity of the population. Further studies need to test the findings in more ethnically diverse and larger populations, as well as in more people without metabolic syndrome |
| García-Calzón (33), 2015, Spain | Retrospective cross-sectional analysis; leukocyte telomere attrition | n = 520; mean age: 67 ± 6 y; mean BMI: 29.1; 45% men; 83% hypertension; 66% dyslipidemia; 37% diabetes; 10% current smokers; PA (METs-min/d): T1 = 307.5 ± 205.9, T2 = 293.5 ± 206.8, T3 = 230.1 ± 165.0 | DII calculated from self-reported data using a 137-item FFQ that has been previously validated in Spain | Cross-sectional associations: Participants following a more anti-inflammatory diet (lowest DII score) had longer telomeres at baseline (P -trend = 0.012). Longitudinal associations: Greater anti-inflammatory potential of the diet (i.e., a decrease in the DII) significantly slowed down the rate of telomere shortening.Greater DII (greatest proinflammatory values) after 5 y of follow-up was associated with an almost 2-fold higher risk of accelerated telomere attrition compared with the highest decrease in DII (greatest anti-inflammatory values) during this period (P-trend = 0.025). | The cross-sectional and longitudinal associations between DII and telomere length in people with high CVD risk suggest that diet may modulate telomere length via pro- or anti-inflammatory mechanisms | The conclusions are strengthened by the standardized and validated assessment methods in this population but need to be tested in more diverse populations and also assessed for confounding, e.g., subjects with highest proinflammatory index had higher BMI and lower levels of PA (P < 0.001), and these may have confounded the effect of diet |
| Gensous (34), 2020, Italy and Poland | Retrospective cross-sectional analysis; epigenetic age (Horvath's clock) | n = 120; mean age: Italy: 72.2 ± 3.8 y, Poland: 71.1 ± 4 y; mean BMI: Italy, 26.99 ± 3.60; Poland, 28.07 ± 3.37; 73.9% men | Mediterranean diet assessed using 7-d food records | Epigenetic rejuvenation was seen after nutritional intervention. The effect was statistically significant in the group of Polish females (P = 0.0013) and in subjects who were epigenetically older at baseline. A genomewide association study of epigenetic age changes after the intervention did not return significant loci, but small-effect alleles were identified, mapping in genes enriched in pathways related to energy metabolism, regulation of cell cycle, and of immune functions. After 1 y of nutritional intervention, adherence to a Mediterranean diet significantly increased in both Italian and Polish participants, and a significant decrease in BMI was observed in Italian males (paired Student's t test, P = 0.008). | Mediterranean diet can promote epigenetic rejuvenation but with country-, sex-, and individual-specific effects, thus highlighting the need for a personalized approach to nutritional interventions | The small sample size limits the generalizability of the conclusions. The lack of analyses by demographic characteristics of the sample, other than sex and country (e.g., BMI, physical activity status, conditions), does not allow for exclusion of confounders |
| Handing (35), 2019, USA | Retrospective cross-sectional analysis; cognition (immediate and delayed story recall and word memory, digit subtraction, orientation questions; adapted from MMSE) | n = 1308; mean age: 70.8 y; 47% men; 68% overweight; 47% hypertension; 34% dyslipidemia; 13% diabetes; 70% self-rated their health good or excellent; 64% White; 74% less than college education; 33% drinkers; 14% smokers; 41% did PA | % of calories from fat, protein, and carbohydrate (via 24-h dietary recall), serum concentrations of vitamins B-12, C, D, and E, folate, iron, homocysteine, β-carotene, and inflammatory biomarkers (serum CRP, plasma fibrinogen, and serum ferritin) | Serum folate was positively significantly associated with cognitive score. Specifically, the interaction between age-cognition and folate indicated the associations of higher age and lower global cognition and lower immediate story recall were weaker in those with higher folate values (P values <0.05). A significant interaction between age and plasma fibrinogen indicated that the association between age and worse digit subtraction was stronger with values >3.1 g/L. | Folate and fibrinogen were significant moderators between age and cognition | The large sample size and the diversity of cognitive performance instruments strengthen the conclusion. Limitations are the cross-sectional design that does not allow for assessing causation, the inaccurate recall bias of the 24-h dietary recall, the non-inclusion of other inflammatory biomarkers linked to cognition, e.g., IL-1, IL-6, and TNF-α and ω-6 PUFAs and trans fats |
| Nettleton (36), 2008, USA | Retrospective cross-sectional analysis; leukocyte telomere attrition | n = 840; mean age: 65.3 ± 0.7 y; mean BMI: 29.0 ± 0.4; 48% men; 54% Hispanic adults; 27% African American; 19% White; 76.3 ≥high school; 37.6 to <$25K income; 11% smoking | Dietary intake patterns (whole grains, fruit and vegetables, low-fat dairy, nuts or seeds, non-fried fish, coffee, refined grains, fried foods, red and processed meat, sugar-sweetened soda) via 120-item FFQ | After adjustment for age, other demographics, lifestyle factors, and intakes of other foods or beverages, only processed meat intake was associated with telomere length. Categorical analysis showed that participants consuming 1 serving of processed meat each week had 0.017 smaller T/S ratios vs. nonconsumers.Age was strongly associated with telomere length. For every 1-y increment in age, the T:S ratio was 0.005 lower (β ± SE: –0.007 ± 0.001, P < 0.001).After adjustment for age, longer telomere length was associated with female sex, shorter duration of smoking, and less time spent in inactive leisure activities (P < 0.001–0.04). | Processed meat intake was inversely associated with telomere length, but other diet features did not display any associations | Limitations of the study include the lack of validation of the FFQ in the study population and the lack of analysis by SES, since people in lower SES may be likely to consume processed meat due to cost. Lower SES may be associated with greater life stress, and shorter telomeres. These limitations restrict the generalizability of the findings |
| O'Callaghan (37), 2014, Australia | Double-blinded, parallel-group RCT; 6 mo duration and 75% retention; telomere attrition | n = 44; mean age: C, 73 ± 4 y; DHA, 74.2 ± 7 y; EPA, 74.9 ± 5.1 y; mean BMI: C, 28.1 ± 5.3; DHA, 26.8 ± 2.6; EPA, 28.1 ± 4.1; C, 47% men; DHA, 72% men; EPA, 82% men; 14% diabetes; prehypertensive; mild cognitive impairment; C group slightly higher education (≥year 12) than I (years 11–12) | I group received 4 capsules/d of an ω-3 PUFA supplement; EPA group received 1.67 g EPA + 0.16 g DHA and DHA group received 1.55 g DHA + 0.40 g EPA; C group received ω-6 PUFA linoleic acid (LA) at 2.2 g/d for 6 mo | The intervention did not show an increase in telomere length with treatment and there was a trend toward telomere shortening during the intervention period. Telomere shortening was greater in the LA group (d = 0.21) than in the DHA (d = 0.12) and EPA groups (d = 0.06). Increased erythrocyte DHA concentrations were associated with reduced telomere shortening (r = –0.67; P = 0.02) in the DHA group. | Telomeric shortening may be attenuated by ω-3 PUFA supplement-ation, requiring further investigation in larger samples | Strength of the study is the blood sample collection method (in the morning, after an overnight fast, and before breakfast). Limitations include the use of whole blood to measure telomere length, as certain immune cell subpopulations have different proliferative rates and telomere lengths. The study was statistically underpowered to allow for meaningful conclusions, and this may be a reason for the lack of statistically significant differences |
| Praveen (38), 2020, India | Retrospective cross-sectional analysis; telomere attrition and mtCN | n = 428 Indian; median age (IQR): 65.0 (62.0–70.0) y; median BMI (IQR): 24.7 (22.1–27.2); 51.2% men; 83% abdominal obesity; 45% overweight; 22% obesity; 26% hypertension; 18% anemia; urban residents | Plasma folate and vitamin B-12 | Significant positive correlation was found between the telomere length and mtCN (r = 0.162, P < 0.001), and both of them were positively associated with plasma folate (r = 0.221, P < 0.001) and vitamin B-12 concentrations (r = 0.187, P < 0.001). | Folate and vitamin B-12 may delay aging by preventing the reduction in telomere length and mtCN | The homogeneous small sample restricts the generalizability of the conclusions. The lack of accounting for confounders, such as physical activity, smoking, and others, limits the validity of the conclusions |
| Seesen (39), 2020, Thailand | Nonrandomized intervention/control study; aging biomarkers (CRP, IL-6, IGF-I, and CD4+:CD8+ T-cell ratio) and cognitive status (MMSE) | n = 122; mean age: bran supplement (Br), 68.80 (±2.82) y; Exercise (Ex), 68.17 (±2.65) y; Br+Ex, 68.20 (±2.41) y; C, 69.06 (±2.74) y; 12% men; 49% with frailty; 10% smoking; 12.5% alcohol; 65% married | I group received 10 g black rice germ, Br providing 300 mg anthocyanins/d and Ex | During the 24-wk intervention, the combined Br+Ex group significantly (P < 0.05) decreased the inflammatory biomarkers (CRP and IL-6) and significantly increased IGF-I (P < 0.001). Additionally, the Br+Ex group significantly improved physical performance and muscle strength (P < 0.05). | A synergistic effect of the combined intervention produced sustainable improvement in physical performance, lower-body muscle strength, and positive inflammatory and endocrine changes | The study produced strong associations that warrant further investigation due to the small sample size and the lack of randomization. There was no reporting on habitual physical activity of participants and BMI and these are additional limitations |
| Zwilling (40), 2019, USA | Retrospective cross-sectional analysis; cognitive function (memory, executive function) | n = 116; mean age: 69.0 ± 3.2 y; mean BMI: 26.0 (±3.6); 31% men; Caucasian; 72% college degree; 54% to >$54K income | NBPs: plasma carotenoids, tocopherols, lipids, riboflavin, folate, vitamin B-12, and vitamin D | Five NBPs were associated with enhanced cognitive performance: 1) ω-3 and ω-6 PUFAs, 2) lycopene, 3) ω-3 PUFAs, 4) carotenoids, and 5) B vitamins (riboflavin, folate, vitamin B-12) and vitamin D. Three NBPs were associated with enhanced functional brain network efficiency: 1) ω-6 PUFAs, 2) ω-3 PUFAs, and 3) carotene. | NBPs account for a significant proportion of variance in measures of cognitive performance and functional brain network efficiency | Limitations are 1) the PCA that may require decisions with the data from the investigator; 2) selecting a priori NBP from the Mediterranean Diet based on knowledge of its association withneuroprotection; |
| ω-3 PUFAs moderated the frontoparietal network and general intelligence, while ω-6 PUFAs and lycopene moderated the dorsal attention network and executive function. | 3) the limitation of an observational study for causal relations; 4) the restriction of the sample to well-educated, Caucasian, neurologically healthy older adults. Therefore, the results cannot be generalized |
AD, Alzheimer disease; BCAA, branched-chain amino acid; C, control; CD4+/8+, cluster of differentiation 4+/8+; CRP, C-reactive protein; CVD, cardiovascular disease; d, Cohen's d; DII, Dietary Inflammatory Index; GEE, generalized estimating equation; I, intervention; IGF-I, insulin-like growth factor-I; MET, metabolic equivalent of task; MMSE, Mini-Mental State Examination; mtCN, mitochondrial DNA copy number; NBP, nutrient biomarker pattern; NRSI, nonrandomized studies of interventions; PA, physical activity; PCA, principal component analysis; r, Pearson's correlation coefficient; RCT, randomized controlled trial; ref, reference; SES, socioeconomic status; TERRA, telomeric repeat-containing RNA; TG, triglyceride; T/S: ratio of telomeric DNA (T) to the amount of a single-copy control DNA (S); UPF, ultra-processed food.
Included studies were undertaken in 7 countries (Australia, India, Italy, Poland, Spain, Thailand, and United States) and 1 territory (Taiwan) amassing a combined 5043 participants (Supplemental Figure 1). Eleven studies were conducted in high-income economies, according to the World Bank classification (27), with 1 study being conducted in an upper-middle and 1 in a lower-middle economy (Supplemental Figure 1). Despite not applying any publication year restrictions in the search for this review, most of the studies were recent, with 8 of them being published in the last 3 years (after 2019), and only 2 being older than 10 y, with the oldest having been published in 2002 (Table 1). Only 1 study was an RCT, 9 were cross-sectional studies, 1 was a case-control study, 1 was a nonrandomized intervention-control study, and 1 was a correlational study (Table 1).
The combined participant population was 5043 people with a baseline mean age of 71.7 y. The majority were women (56%), European White (49%), and had completed 1 y of tertiary education (Supplemental Table 4). The participants were, on average, overweight [average mean BMI (kg/m2): 26.6], the majority had hypertension (64%) and dyslipidemia (54%), more than one-quarter were current alcohol drinkers, nearly one-fifth were smokers, and nearly 1-in-6 had diabetes (Supplemental Table 5).
Summary of studies’ assessments, findings, and conclusions
The primary outcome reported by most of the included studies was telomere attrition (28, 31–33, 36–38), followed by cognitive function (29, 30, 35, 39, 40) assessed using the Mini-Mental State Examination test (29, 30, 35, 39) and/or MRI (29, 40) and/or clinical dementia rating (30); 1 study reported on telomeric repeat-containing RNA expression (31), 1 study on mitochondrial DNA copy number (38), 1 study on epigenetic age using Horvath's clock (34), and 1 study on select aging biomarkers (39) (Table 1).
Alonso-Pedrero et al. (28) tested the effect of ultra-processed food (UPF) consumption on telomere shortening, and found shorter telomeres in people who were consuming more UPFs. Atzmon et al. (29) assessed the effect of plasma lipids on cognitive function, reporting an association between cognitive dysfunction and decline in plasma HDL cholesterol. Bowman et al. (30) and Zwilling et al. (40) evaluated the associations between nutrient biomarker patterns and cognitive function. Bowman et al. (30) reported positive associations between plasma concentrations of B vitamins, and plasma vitamins C, D, and E, and cognitive function, and between plasma ω-3 fatty acids and cognitive function, whereas they found plasma trans fatty acids to be negatively associated with cognitive function. In agreement with Bowman et al. (30), Zwilling et al. (40) also reported positive associations between plasma ω-3 PUFAs and cognitive function, and between vitamins B and D and cognitive function. In addition, Zwilling et al. (40) reported positive associations between lycopene, carotenoids, and ω-6 PUFAs and cognitive function. Zwilling et al. (40) reported that ω-3 PUFAs moderated the fronto-parietal network and general intelligence, whereas ω-6 PUFAs and lycopene moderated the dorsal attention network and executive function. Chang et al. (31) examined the effect of regular-strength exercise and a combination of supplements that included branched-chain amino acids (BCAAs), calcium, and vitamin D on telomere length and on the expression of telomeric repeat-containing RNA (TERRA). They reported that the intervention significantly increased TERRA but did not alter telomere shortening. Freitas-Simoes et al. (32) looked at the associations between the RBC proportions of PUFAs and leukocyte telomere length and reported no associations, but found an association between the proportion of arachidonic acid (20:4n−6) and shorter telomeres. García-Calzón et al. (33) assessed the association between the Dietary Inflammatory Index (DII) and leukocyte telomere length and reported a protective effect of DII on telomere length. Gensous et al. (34) evaluated the effect of the Mediterranean diet on epigenetic age using Horvath's clock, and reported an epigenetic rejuvenation that was country (Poland) and sex (females) specific, emphasizing the need for personalized approaches to nutrition interventions. Handing et al. (35) examined the effect of macronutrient distributions, micronutrient intake, and serum inflammatory biomarkers on cognitive function. They found cognition to be positively associated with serum folate and negatively associated with fibrinogen. Nettleton et al. (36) assessed the associations between dietary intake patterns and leukocyte telomere length, reporting an association between processed meat and shorter telomeres. O'Callaghan et al. (37) tested the association between ω-3 fatty acid supplementation and telomere length, and found that telomere attrition may be attenuated by ω-3 PUFA supplementation. Praveen et al. (38) evaluated the associations between plasma folate and vitamin B-12 on telomere length and mitochondrial DNA copy number and reported protective effects. Seesen et al. (39) examined the effect of rice germ bran supplementation and exercise on cognition and select aging biomarkers and reported that the synergistic effect of the combined intervention improved physical performance, muscle strength, and the profile of inflammatory biomarkers and insulin growth factor-I (IGF-I). Figure 2 summarizes these findings.
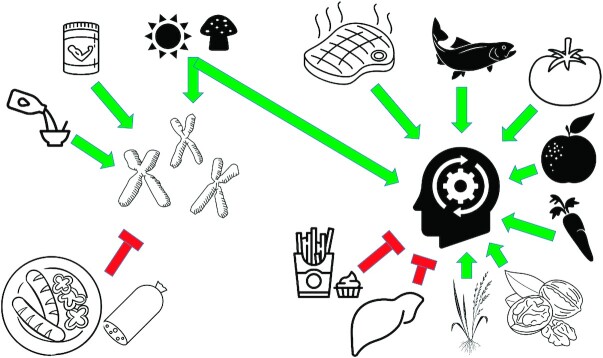
FIGURE 2.
Summary of findings of studies assessing the effect of nutritional biomarkers on biological aging in older adults Branched-chain amino acids (e.g., supplementation), calcium (e.g., milk), and vitamin D (e.g., mushrooms, sun exposure) protect the telomeric ends of chromosomes, whereas ultra-processed foods (e.g., salami, sausages), arachidonic acid, and other proinflammatory compounds are associated with shorter telomeres. Vitamin B-12 (e.g., red meat), vitamin C (e.g., oranges), vitamin D (e.g., mushrooms, sun), vitamin E (e.g., nuts), lycopene (e.g., tomatoes), carotenoids (e.g., carrots), ω-3 and ω-6 fatty acids (e.g., fish), and rice germ bran preserve cognitive function in older adults, whereas trans fatty acids (e.g., French fries) and fibrinogen (endogenously produced in the liver) correlate with cognitive function decline.
Quality appraisal
Risk of bias was assessed using the relevant tools developed by Cochrane for randomized and nonrandomized studies of interventions. The detailed results per domain assessed are presented in Table 2. Briefly, reasons for downgrading the quality included not using an intention-to-treat analysis method [e.g., Alonso-Pedrero et al. (28)], not reporting on and not analyzing by the sociodemographics of the population [e.g., Atzmon et al. (29)], not considering confounders such as physical activity [e.g., Bowman et al. (30), Chang et al. (31), Gensous et al. (34), Praveen et al. (38)], smoking and alcohol [Gensous et al. (34)], not reporting on subgroup results [Handing et al. (35)], not controlling habitual dietary intake in dietary interventions [Seesen et al. (39)], not checking the fidelity of the intervention [Seesen et al. (39)], not reporting on blinding [Seesen et al. (39)] or on the baseline differences between the intervention and control group and missingness of outcomes [O'Callaghan et al. (37)].
TABLE 2.
The risk-of-bias assessment of the included studies assessing the effect of nutritional biomarkers on biological aging in older adults, using the ROBINS-I tool for the nonrandomized studies and the ROB 2 tool for the randomized controlled study1
| First author (reference) | Year | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
|---|---|---|---|---|---|---|---|---|---|
| ROBINS-I | |||||||||
| Alonso-Pedrero (28) | 2020 | Serious | Low | Moderate | Low | Moderate | Low | Low | Serious |
| Atzmon (29) | 2002 | Serious | Low | Serious | Low | Moderate | Moderate | Low | Severe |
| Bowman (30) | 2012 | Moderate | Low | Low | Low | Serious | Low | Low | Serious |
| Chang (31) | 2020 | Serious | Moderate | Low | Moderate | Low | Low | Low | Serious |
| Freitas-Simoes (32) | 2019 | Low | Low | Low | Low | Low | Low | Low | Low |
| García-Calzón (33) | 2015 | Low | Low | Low | Moderate | Low | Low | Low | Moderate |
| Gensous (34) | 2020 | Serious | Low | Low | Low | Low | Low | Low | Moderate |
| Handing (35) | 2019 | Moderate | Low | Low | Low | Low | Low | Moderate | Moderate |
| Nettleton (36) | 2008 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Praveen (38) | 2020 | Serious | Low | Low | Low | Low | Low | Low | Moderate |
| Seesen (39) | 2020 | Serious | Low | Low | Moderate | Low | Moderate | Low | Serious |
| Zwilling (40) | 2019 | Moderate | Low | Low | Low | Low | Low | Low | Low |
| Year | Bias due to randomization process | Bias due to deviations from intended interventions | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias | |
|---|---|---|---|---|---|---|---|
| ROB 2 | |||||||
| O'Callaghan (37) | 2014 | Some concerns | High | High | Low | High | High |
ROB 2, Risk of bias 2; ROBINS-I, Risk Of Bias In Non-randomised Studies—of Interventions.
Conflicts of interest were analyzed and are presented in Supplemental Tables 6 and 7. Three studies did not include a conflict-of-interest statement and therefore no assessment could be made (29, 37, 38). Of the 10 studies that included a conflict-of-interest statement, 2 received funding from industry (32, 40), 6 from nonindustry sources (30, 31, 33, 34, 36, 39), 1 from both industry and nonindustry sources (28), and 1 study did not receive funding (35). All authors had academic affiliations, but in 3 studies the authors also reported industry affiliations (30, 32, 33). The authors of 1 study were deemed to have a conflict of interest (32), and a possible conflict of interest was determined for 3 other studies (28, 30, 33).
Discussion
This review aimed to describe the contribution of biological age–predictive biomarkers to nutrition research by synthesizing the evidence in the literature in order to provide a complete summary of the current evidence on the associations between nutrition and biological aging,
We identified 13 studies from 8 countries and territories, involving a total of 5043 participants. The majority of the studies were recent cross-sectional studies conducted in high-income countries involving older adults, many of whom had metabolic syndrome. Studies indicated protective effects for BCAAs, calcium and vitamin D, and a diet of a lower inflammatory index against telomere attrition, and for HDL cholesterol, B vitamins, vitamin C, vitamin D, vitamin E, lycopene, carotenoids, and ω-3 and ω-6 fatty acids against cognitive decline. Shorter telomeres were found in people consuming more UPFs, arachidonic acid, and other proinflammatory compounds and a cognitive decline correlated with plasma concentrations of trans fatty acids and fibrinogen. Epigenetic rejuvenation was observed in people following the Mediterranean diet.
The contradicting effect of arachidonic acid, an ω-6 fatty acid, on biological aging in terms of a positive effect on cognition [Zwilling et al. (40)] and a negative effect on telomere length [Freitas-Simoes et al. (32)] may be explained by virtue of its both proinflammatory and anti-inflammatory properties (41, 42). The relevant presence and proportions of proinflammatory- versus anti-inflammatory–mediator converting enzymes, such as cyclooxygenases and lipoxygenases and cytochrome P450, may be responsible for its opposite results in different tissues. It also needs to be noted that the presence of arachidonic acid in dietary fats is marginal and this compound is mainly endogenously produced. Therefore, instead of a reduction in consumption of its parent foods, such as meat, eggs, and poultry, intake of marine ω-3 fatty acids may be a better strategy to displace arachidonic acid from the plasma membranes of leukocytes and thus prevent telomere shortening.
Another contradicting finding was the report by Chang et al. (31) that BCAA and vitamin D supplementation combined with regular-strength exercise significantly increased TERRA but did not alter telomere shortening. TERRA is central to the modulation of telomere length. In cells with longer telomeres, TERRA competes with the telomerase's DNA substrate, or it enhances the catalytic reverse transcriptase subunit of the enzyme, to inhibit the elongation of telomeric repeats (43). On the contrary, in cells with shorter telomeres, TERRA promotes telomere lengthening by facilitating the recruitment of telomerase. It is possible that the findings reflect a lag in the increase in telomere size. The cross-sectional design of this study limits any causal associations between TERRA and telomere length, which need to be explored using a prospective study design.
Review findings are consistent with previously reported dietary habits in populations known for their longevity, such as people from Japan who eat fish high in ω-3 fatty acids (44) and people from the Mediterranean Basin who traditionally consume a diet high in antioxidants (olive oil, legumes, fruits and vegetables) and low in processed foods (45). It needs to be acknowledged, however, that extrapolating conclusions for food and dietary pattern with biological aging is risky, when most of the associations reported in the literature are focused on the effects of nutrients, either alone or in combination. Eleven out of the 13 included studies in this review reported on nutrient associations with biological aging, with only 1 study reporting on the effect of food processing on aging (28) and another one on the effect of diet as a whole on aging (34). Whole diets and dietary patterns display a complexity that cannot be reduced to the summed effect of their nutrient constituents, and therefore conclusions on the effects of foods and diet on biological aging should be tempered and treated with caution. Moreover, the mechanisms that underpin these associations are not yet understood in depth. A reason is that animal studies are usually used to assess these biological mechanisms, but the metabolism of certain nutrients can be different in humans compared with, for example, rodents. With regard to 1 of the compounds reported in this review, rats are able to derive arachidonic acid from dietary linoleic acid (18:2n–6) (46); however, this is not the case in humans (47), thus not allowing to extrapolate RBC arachidonic acid concentrations to dietary intake. Other factors, in addition to nutrition, have been proposed within the broader aging literature to contribute to longevity, and include warmer climates (48), ocean proximity (49), access to modern health care (50), and socioeconomic status (SES). In fact, the longest living people reside in Monaco, a very affluent sovereign city-state with a poverty rate of 0% (51) and a relaxed lifestyle (52).
Although longevity has been increasing worldwide, it is questioned whether these extra years gained are lived in good health (53). In 1948, the WHO defined health as “a state of complete physical, mental, and social well-being, and not merely the absence of disease or infirmity” (54). It is thus crucial to understand how different people age in relation to their physical, functional, and mental capacity, and their risk of disease, in addition to that of death. Several direct biomarkers of aging have been proposed to date, including genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, nutrient-sensing deregulation, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intracellular communication (12). Surrogate markers include markers of cognitive function, strength, and frailty (55). This review synthesized evidence from 8 studies that used direct markers—that is, telomere attrition and epigenetic changes (assessed using Horvath's clock)—and 5 that used surrogate markers—that is, cognitive function. The included studies pointed to a trend towards longer telomeres and functional improvements, both physical (e.g., strength), as well as intellectual (e.g., cognition), in people who were consuming diets with higher anti-inflammatory content and unprocessed foods. Surrogate markers including those diagnostic of sarcopenia and frailty were identified during the searches but were excluded as the research did not describe these markers in the context of biological aging.
This review synthesized novel research exploring the role of nutrition in biological aging. Studies are examining how diet can influence biological aging via assessing its association with direct aging markers, such as telomere shortening (56), or indirect ones, such as cognition or bone mineral density (57, 58). Additional studies are needed to elucidate the effects of the plethora of nutrients, foods, and importantly, their combinations on biological aging to obtain a more representative understanding of the real-life complex dietary patterns on aging. Although 2 studies included in this review reported on the effect of nutrient intake patterns on aging, this field warrants further study. Mechanistic studies are also warranted to assess the molecular pathways that mediate the effects of nutrition on aging—for example, the effects of ω-3 and ω-6 PUFA ratios on inflammation and telomere length (59). The potential dose–response relationship also warrants investigation to determine whether, in the advanced stages of life, certain nutrients or foods are needed in higher amounts to elicit their effects—for example, due to compromised absorption and bioavailability of these in the aging gastrointestinal tract (60, 61). Future research should examine the association between nutrients (e.g., vitamin D) and the immune system's function in aging populations. Also, given the accumulating number of supercentenarians who survived coronavirus disease 2019 (COVID-19) (62, 63), it is important to know whether dietary factors mediate or moderate these outcomes (64, 65), and whether the guidelines of increased risk for ill health for older people should be informed with biological, rather than chronological, age. Although the search strategies of this review were optimized to capture several biomarkers of aging, the current literature is focused on studies that assessed the effect of diet and nutrients on telomere length and on cognition. Future studies should aim to evaluate the effects of diet and nutrients on other markers of biological aging, such as mitochondrial dysfunction, nutrient-sensing deregulation, loss of proteostasis, and genomic instability, for which their potential associations with diet have not yet been explored. Further studies are also warranted to assess the effects of diet and nutrients on the epigenetic clock, as this review only managed to identify 1 such study [i.e., Gensous et al. (34)]. In addition to future nutrition research, the findings can inform nutrition practice. Future updates of nutrition screening procedures for older people should incorporate the relevant nutrients identified by the studies to associate with biological aging markers. Ultimately, this will pave the way for healthy aging precision nutrition therapy that could also benefit from the development of a pertinent algorithm with the use of artificial intelligence to assist in integrating genomic, epigenomic, physiologic, pathophysiological, and lifestyle data.
The strengths of this review include the use of standard methodology as documented in the PRISMA and Cochrane guidelines for conducting systematic reviews and the decision to not restrict the searches by language or year of publication. However, despite the robust methodology, the analyses presented here are limited by the methodological limitations of the included studies. First, the majority of the studies identified used a cross-sectional design that is not suited for inferring any causal association since the temporal relation is unattainable. Second, most studies assessed the effect of nutrition (environmental factor) on telomere attrition. However, telomere length is also under genetic control and shows interindividual variation at birth (66, 67). Therefore, studies should be taking into account these inherent differences, but this is not possible with a cross-sectional design as it does not permit the investigation of telomere changes over time. Third, most included studies in this review reported on nutrient associations with biological aging, limiting the ability to draw conclusions on the effect of foods, dietary patterns, and diets on biological aging. Fourth, although a number of studies assessed the effect of other modifiable factors on telomere length, such as obesity status, smoking, alcohol consumption, and physical activity, these potential confounders were not always accounted for and thus some of the results may have been over- or underestimated. Nine out of the 11 studies that reported on BMI included participants who were, on average, overweight (BMI = 25–29.9). This is another inherent limitation of this review as BMI has been reported to correlate with telomere attrition (68). In fact, obesity has been shown to promote telomere shortening from as early as the age of 8 y (69). For example, Alonso-Pedrero et al. (28) reported an association between UPFs and shorter telomeres and their participants were, on average, overweight. A recent study reported an association of UPFs with obesity (70). Therefore, this potential confounder needs to be accounted for when assessing the effects of UPF consumption on telomere shortening. García-Calzón et al. (33) and Gensous et al. (34) reported on the confounding effect of BMI. Seven studies reported on the smoking status of participants and 3 reported alcohol consumption status. These are also confounders that need to be carefully considered. A 2017 meta-analysis reported shorter telomere length in ever-smokers compared with never-smokers, as well as in current smokers compared with former smokers (71). An inverse trend between pack-years of smoking and telomere length was also found (71). Smoking has also been associated with cognitive decline, potentially due to the effects of nicotine on its brain receptors (72). A recent meta-analysis indicated that alcohol consumption per se is not associated with telomere attrition, in the absence of alcohol abuse or dependence (73). Similarly, frequent heavy consumption of alcohol has been associated with decreased cognitive performance, whereas regular light and moderate consumption may have protective effects (74). Interestingly, total abstainers show an inferior cognitive performance compared to people with moderate or light consumption (74). Since the consumption of alcohol was not quantitatively reported by the included studies, relevant evaluations and conclusions are not possible, presenting another limitation. Finally, physical activity is positively associated with telomere length (75), but only 3 studies reported on the physical activity status of their participants (32, 33, 35), and out of these, only 1 study assessed the confounding effect of physical activity (33). Fifth, most studies did not conduct subpopulation analyses, such as reporting stratified results by BMI, levels of alcohol intake, or smoking. Alonso-Pedrero et al. (28) reported that consumption of processed foods was associated with shorter telomeres; however, there was no analysis by SES, although it is known that people with lower SES may be more likely to consume these foods due to their perceived lower cost, and that people with lower SES may experience greater life stress, which has been associated with telomere attrition (76, 77). Sixth, the lack of consistency between the methods, analyses, and settings of the different studies meant that their results could not be pooled via a meta-analysis and therefore a quantitative synthesis was not possible. Seventh, there is no consensus on the definition of “older adult,” with some sources citing 60 y or more while others cite 65 y or more (2, 78–80). In this review, we adopted the 65 y or older cutoff of the United Nations old-age dependency ratio (81), but we do acknowledge this as a limitation since aging, in terms of biological aging mechanisms, may, in fact, commence at an earlier age, and thus some relevant studies may have been excluded. Finally, the lack of consistency between results in humans and animal models (46, 47) and the void in the literature between nutrient associations and foods, dietary patterns, and whole diets with biological aging limit the ability to propose biological mechanisms for the described epidemiological associations.
In conclusion, anti-inflammatory nutrients and unprocessed foods were associated with longer telomeres, epigenetic rejuvenation, and improved cognition. Future research should assess the synergistic effects of different nutrients and their combinations, and evaluate their dose–response relations, while controlling for the confounding effects of other modifiable factors, such as obesity status, physical activity, alcohol intake, and smoking. Nutrition practice can incorporate updated screening procedures for older people that include the relevant biological aging nutrition markers, paving the way for an anti-aging precision nutrition therapy.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—GS and JP: conceived the idea for the manuscript; GS: drafted the review protocol; GS and JP: refined the protocol for submission to PROSPERO; GS: devised and performed the database searches; GS and JP: screened the records for inclusion; GS: extracted the data from the included records; GS and JP: analyzed the data of the included records; GS: drafted the manuscript; GS and JP: refined the manuscript for submission; and both authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this work.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1, Supplemental Tables 1–7, and Supplemental Results are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations: BCAA, branched-chain amino acid; DII, Dietary Inflammatory Index; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; SES, socioeconomic status; TERRA, telomeric repeat-containing RNA; UPF, ultra-processed food.
Contributor Information
George Siopis, School of Life and Environmental Sciences, Faculty of Science, The University of Sydney, Sydney, New South Wales, Australia; Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Sciences, Deakin University, Geelong, Victoria, Australia.
Judi Porter, Institute for Physical Activity and Nutrition, School of Exercise and Nutrition Sciences, Deakin University, Geelong, Victoria, Australia.
Data Availability
Template data collection forms, data extracted from included studies, and data used for analyses can all be made available upon request to the corresponding author.
References
- 1. World Health Organization . Life expectancy increased by 5 years since 2000, but health inequalities persist [Internet]. Updated 16 May 2016. Available from: https://www.who.int/news/item/19-05-2016-life-expectancy-increased-by-5-years-since-2000-but-health-inequalities-persist (accessed 12 January2022).
- 2. World Health Organization . Ageing and health [Internet]. Updated 4 October 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed 12 January2022).
- 3. Tamura Y, Takubo K, Aida J, Araki A, Ito H. Telomere attrition and diabetes mellitus. Geriatrics Gerontol Int. 2016;16(Suppl 1):66–74. [DOI] [PubMed] [Google Scholar]
- 4. The ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium . Pan-cancer analysis of whole genomes. Nature. 2020;578(7793):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukherjee M, Brouilette S, Stevens S, Shetty KR, Samani NJ. Association of shorter telomeres with coronary artery disease in Indian subjects. Heart. 2009;95:(8):669–73. [DOI] [PubMed] [Google Scholar]
- 6. Wu JW, Yaqub A, Ma Y, Koudstaal W, Hofman A, Ikram MAet al. Biological age in healthy elderly predicts aging-related diseases including dementia. Sci Rep. 2021;11(1):15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown PJ, Wall MM, Chen C, Levine ME, Yaffe K, Roose SPet al. Biological age, not chronological age, is associated with late-life depression. J Gerontol A Biol Sci Med Sci. 2018;73(10):1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCulloch K, Litherland GJ, Rai TS. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16(2):210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Córdoba-Lanús E, Cazorla-Rivero S, Espinoza-Jiménez A, de-Torres JP, Pajares MJ, Aguirre-Jaime Aet al. Telomere shortening and accelerated aging in COPD: findings from the BODE cohort. Respir Res. 2017;18(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chang AY, Skirbekk VF, Tyrovolas S, Kassebaum NJ, Dieleman JL. Measuring population ageing: an analysis of the Global Burden of Disease Study 2017. Lancet Public Health. 2019;4(3):e159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Needham BL, Rehkopf D, Adler N, Gregorich S, Lin J, Blackburn EHet al. Leukocyte telomere length and mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology. 2015;26(4):528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine ME. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68(6):667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ashapkin VV, Kutueva LI, Vanyushin BF. Epigenetic clock: just a convenient marker or an active driver of aging?. Adv Exp Med Biol. 2019;1178:175–206. [DOI] [PubMed] [Google Scholar]
- 15. Cole JH, Ritchie SJ, Bastin ME, Valdés Hernández MC, Muñoz Maniega S, Royle Net al. Brain age predicts mortality. Mol Psychiatry. 2018;23(5):1385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lu WH, de Souto Barreto P, Rolland Y, Bouyahia A, Fischer C, Mangin JFet al. ; MAPT/DSA Group. Biological and neuroimaging markers as predictors of 5-year incident frailty in older adults: a secondary analysis of the MAPT study. J Gerontol A Biol Sci Med Sci. 2021;76:(11):e361–69. [DOI] [PubMed] [Google Scholar]
- 17. Zhang DM, Ye JX, Mu JS, Cui XP. Efficacy of vitamin B supplementation on cognition in elderly patients with cognitive-related diseases. J Geriatr Psychiatry Neurol. 2017;30(1):50–9. [DOI] [PubMed] [Google Scholar]
- 18. Tangvik RJ, Bruvik FK, Drageset J, Kyte K, Hunskår I. Effects of oral nutrition supplements in persons with dementia: a systematic review. Geriatr Nurs (Minneap). 2021;42(1):117–23. [DOI] [PubMed] [Google Scholar]
- 19. Coutts L, Ibrahim K, Tan QY, Lim SER, Cox NJ, Roberts HC. Can probiotics, prebiotics and synbiotics improve functional outcomes for older people: a systematic review. Eur Geriatr Med. 2020;11(6):975–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruxton CH, Derbyshire E, Toribio-Mateas M. Role of fatty acids and micronutrients in healthy ageing: a systematic review of randomised controlled trials set in the context of European dietary surveys of older adults. J Hum Nutr Diet. 2016;29(3):308–24. [DOI] [PubMed] [Google Scholar]
- 21. Derbyshire E. Brain health across the lifespan: a systematic review on the role of omega-3 fatty acid supplements. Nutrients. 2018;10:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Couteur DG, Solon-Biet SM, Parker BL, Pulpitel T, Brandon AE, Hunt NJet al. Nutritional reprogramming of mouse liver proteome is dampened by metformin, resveratrol, and rapamycin. Cell Metab. 2021;33(12):2367–79, e4. [DOI] [PubMed] [Google Scholar]
- 23. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPet al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(1):b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJet al. (editors). Cochrane handbook for systematic reviews of interventions. [Internet]. Version 6.0 (updated July 2019). Cochrane; 2019. Available from: www.training.cochrane.org/handbook (accessed 5 December 2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron Iet al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 26. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan Met al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The World Bank . World Bank country and lending groups—country classification. [Internet]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed 18 January2021).
- 28. Alonso-Pedrero L, Ojeda-Rodríguez A, Martínez-González MA, Zalba G, Bes-Rastrollo M, Marti A. Ultra-processed food consumption and the risk of short telomeres in an elderly population of the Seguimiento Universidad de Navarra (SUN) project. Am J Clin Nutr. 2020;111(6):1259–66. [DOI] [PubMed] [Google Scholar]
- 29. Atzmon G, Gabriely I, Greiner W, Davidson D, Schechter C, Barzilai N. Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol A Biol Sci Med Sci. 2002;57(11):M712–15. [DOI] [PubMed] [Google Scholar]
- 30. Bowman GL, Silbert LC, Howieson D, Dodge HH, Traber MG, Frei Bet al. Nutrient biomarker patterns, cognitive function, and MRI measures of brain aging. Neurology. 2012;78(4):241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang KV, Chen YC, Wu WT, Shen HJ, Huang KC, Chu HPet al. Expression of telomeric repeat-containing RNA decreases in sarcopenia and increases after exercise and nutrition intervention. Nutrients. 2020;12(12):3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freitas-Simoes TM, Cofán M, Blasco MA, Soberón N, Foronda M, Corella Det al. The red blood cell proportion of arachidonic acid relates to shorter leukocyte telomeres in Mediterranean elders: a secondary analysis of a randomized controlled trial. Clin Nutr. 2019;38(2):958–61. [DOI] [PubMed] [Google Scholar]
- 33. García-Calzón S, Zalba G, Ruiz-Canela M, Shivappa N, Hébert JR, Martínez JAet al. Dietary inflammatory index and telomere length in subjects with a high cardiovascular disease risk from the PREDIMED-NAVARRA study: cross-sectional and longitudinal analyses over 5 y. Am J Clin Nutr. 2015;102(4):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gensous N, Garagnani P, Santoro A, Giuliani C, Ostan R, Fabbri Cet al. One-year Mediterranean diet promotes epigenetic rejuvenation with country- and sex-specific effects: a pilot study from the NU-AGE project. Geroscience. 2020;42(2):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Handing EP, Small BJ, Andel R, McEvoy CL, Kumar N. Can nutrition or inflammation moderate the age-cognition association among older adults?. J Gerontol Ser B. 2019;74(2):193–201. [DOI] [PubMed] [Google Scholar]
- 36. Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR Jr.. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr. 2008;88(1):185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Callaghan N, Parletta N, Milte CM, Benassi-Evans B, Fenech M, Howe PR. Telomere shortening in elderly individuals with mild cognitive impairment may be attenuated with ω-3 fatty acid supplementation: a randomized controlled pilot study. Nutrition. 2014;30(4):489–91. [DOI] [PubMed] [Google Scholar]
- 38. Praveen G, Shalini T, Sivaprasad M, Reddy GB. Relative telomere length and mitochondrial DNA copy number variation with age: association with plasma folate and vitamin B12. Mitochondrion. 2020;51:79–87. [DOI] [PubMed] [Google Scholar]
- 39. Seesen M, Semmarath W, Yodkeeree S, Sapbamrer R, Ayood P, Malasao Ret al. Combined black rice germ, bran supplement and exercise intervention modulate aging biomarkers and improve physical performance and lower-body muscle strength parameters in aging population. Int J Environ Res Public Health. 2020;17:2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zwilling CE, Talukdar T, Zamroziewicz MK, Barbey AK. Nutrient biomarker patterns, cognitive function, and fMRI measures of network efficiency in the aging brain. Neuroimage. 2019;188:239–51. [DOI] [PubMed] [Google Scholar]
- 41. Wang B, Wu L, Chen J, Dong L, Chen C, Wen Zet al. Metabolism pathways of arachidonic acids: mechanisms and potential therapeutic targets. Signal Transduct Target Ther. 2021;6(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris WS, Mozaffarian D, Rimm E, Kris-Etherton P, Rudel LL, Appel LJet al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119(6):902–7. [DOI] [PubMed] [Google Scholar]
- 43. Maicher A, Kastner L, Luke B. Telomeres and disease: enter TERRA. RNA Biol. 2012;9(6):843–9. [DOI] [PubMed] [Google Scholar]
- 44. Willcox DC, Scapagnini G, Willcox BJ. Healthy aging diets other than the Mediterranean: a focus on the Okinawan diet. Mech Ageing Dev. 2014;136-137:148–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348(26):2599–608. [DOI] [PubMed] [Google Scholar]
- 46. Christophersen BO, Hagve TA, Norseth J. Studies on the regulation of arachidonic acid synthesis in isolated rat liver cells. Biochim Biophys Acta Lipids Lipid Metab. 1982;712(2):305–14. [DOI] [PubMed] [Google Scholar]
- 47. Rett BS, Whelan J. Increasing dietary linoleic acid does not increase tissue arachidonic acid content in adults consuming Western-type diets: a systematic review. Nutr Metab. 2011;8(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moore TG. Life, death, and climate. [Internet]. Available from: https://web.stanford.edu/∼moore/HealthBenefitsofWarmer.html (accessed 18 January2021).
- 49. Wheeler BW, White M, Stahl-Timmins W, Depledge MH. Does living by the coast improve health and wellbeing? Health Place. 2012;18:(5):1198–201. [DOI] [PubMed] [Google Scholar]
- 50. Siopis G, Jones A, Allman-Farinelli M. The dietetic workforce distribution geographic atlas provides insight into the inequitable access for dietetic services for people with type 2 diabetes in Australia. Nutr Diet. 2020;77:(1):121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Global Citizen . 5 Surprising countries with the longest-living populations. [Internet]. Available from: https://www.globalcitizen.org/en/content/the-secrets-to-a-long-life/ (accessed 18 January2021).
- 52. Bertozzi B, Tosti V, Fontana L. Beyond calories: an integrated approach to promote health, longevity, and well-being. Gerontology. 2017;63(1):13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. United Nations, Department of Economic and Social Affairs . Ageing populations: we are living longer lives, but are we healthier? [Internet]. Available from: https://desapublications.un.org/working-papers/ageing-populations-we-are-living-longer-lives-are-we-healthier(accessed 18 January2021).
- 54. World Health Organization . Constitution. [Internet]. Available from: https://www.who.int/about/governance/constitution (accessed 18 January2022).
- 55. MacDonald SW, DeCarlo CA, Dixon RA. Linking biological and cognitive aging: toward improving characterizations of developmental time. J Gerontol B Psychol Sci Soc Sci. 2011;66B(Suppl 1):i59–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Freitas-Simoes TM, Ros E, Sala-Vila A. Telomere length as a biomarker of accelerated aging: is it influenced by dietary intake? Curr Opin Clin Nutr Metab Care. 2018;21(6):430–6. [DOI] [PubMed] [Google Scholar]
- 57. Crowe-White KM, Phillips TA, Ellis AC. Lycopene and cognitive function. J Nutr Sci. 2019;8:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent Met al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxford). 2019;58(12):2295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kordinas V, Ioannidis A, Chatzipanagiotou S. The telomere/telomerase system in chronic inflammatory diseases. Cause or effect?. Genes. 2016;7(9):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lynch DB, Jeffery IB, Cusack S, O'Connor EM, O'Toole PW. Diet-microbiota-health interactions in older subjects: implications for healthy aging. Interdiscip Top Gerontol. 2015;40:141–54. [DOI] [PubMed] [Google Scholar]
- 61. Salazar N, Valdés-Varela L, González S, Gueimonde M, de Los Reyes-Gavilán CG. Nutrition and the gut microbiome in the elderly. Gut Microbes. 2017;8(2):82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Siopis G. Supercentenarians that survived COVID-19. Aging Dis. 2021;12(7):1539–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siopis G. Secrets to longevity: the Methuselahs that survived COVID-19. J Am Geriatr Soc. 2021;69(10):2788–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wardwell L, Chapman-Novakofski K, Herrel S, Woods J. Nutrient intake and immune function of elderly subjects. J Am Diet Assoc. 2008;108(12):2005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. High KP. Nutritional strategies to boost immunity and prevent infection in elderly individuals. Clin Infect Dis. 2001;33(11):1892–900. [DOI] [PubMed] [Google Scholar]
- 66. Thilagavathi J, Venkatesh S, Dada R. Telomere length in reproduction. Andrologia. 2013;45(5):289–304. [DOI] [PubMed] [Google Scholar]
- 67. Graakjaer J, Pascoe L, Der-Sarkissian H, Thomas G, Kolvraa S, Christensen Ket al. The relative lengths of individual telomeres are defined in the zygote and strictly maintained during life. Aging Cell. 2004;3(3):97–102. [DOI] [PubMed] [Google Scholar]
- 68. Gielen M, Hageman GJ, Antoniou EE, Nordfjall K, Mangino M, Balasubramanyam Met al. Body mass index is negatively associated with telomere length: a collaborative cross-sectional meta-analysis of 87 observational studies. Am J Clin Nutr. 2018;108(3):453–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Clemente DBP, Maitre L, Bustamante M, Chatzi L, Roumeliotaki T, Fossati Set al. Obesity is associated with shorter telomeres in 8 year-old children. Sci Rep. 2019;9(1):18739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rauber F, Chang K, Vamos EP, da Costa Louzada ML, Monteiro CA, Millett Cet al. Ultra-processed food consumption and risk of obesity: a prospective cohort study of UK Biobank. Eur J Nutr. 2021;60(4):2169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Astuti Y, Wardhana A, Watkins J, Wulaningsih W; PILAR Research Network . Cigarette smoking and telomere length: a systematic review of 84 studies and meta-analysis. Environ Res. 2017;158:480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Campos MW, Serebrisky D, Castaldelli-Maia JM. Smoking and cognition. Curr Drug Abuse Rev. 2016;9(2):76–9. [DOI] [PubMed] [Google Scholar]
- 73. Maugeri A, Barchitta M, Magnano San Lio R, La Rosa MC, La Mastra C, Favara Get al. The effect of alcohol on telomere length: a systematic review of epidemiological evidence and a pilot study during pregnancy. Int J Environ Res Public Health. 2021;18:5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gutwinski S, Schreiter S, Priller J, Henssler J, Wiers CE, Heinz A. Drink and think: impact of alcohol on cognitive functions and dementia—evidence of dose-related effects. Pharmacopsychiatry. 2018;51:136–43. [DOI] [PubMed] [Google Scholar]
- 75. Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L. Physical activity and telomere length: impact of aging and potential mechanisms of action. Oncotarget. 2017;8(27):45008–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JDet al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci. 2004;101(49):17312–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GLet al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5(5):361–5. [DOI] [PubMed] [Google Scholar]
- 78. World Health Organization . Ageing. [Internet]. Available from: https://www.who.int/westernpacific/health-topics/ageing (accessed 4 April2022). [Google Scholar]
- 79. Australian Government . Australian Institute of Health and Welfare. Older people. [Internet]. Available from: https://www.aihw.gov.au/reports-data/population-groups/older-people/overview (accessed 4 April2022). [Google Scholar]
- 80. Centers for Disease Control and Prevention . Indicator definitions—older adults. [Internet]. Available from: https://www.cdc.gov/cdi/definitions/older-adults.html (accessed 4 April2022).
- 81. United Nations, Department of Economic and Social Affairs . World population ageing 2019. [Internet]. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf (accessed 11 April2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Template data collection forms, data extracted from included studies, and data used for analyses can all be made available upon request to the corresponding author.