Abstract
Background
The importance of autoimmune encephalitis and its overlap with infectious encephalitides are not well investigated in South-East Asia.
Methods
We report autoantibody testing, using antigen-specific live cell-based assays, in a series of 134 patients (cerebrospinal fluid and sera) and 55 blood donor controls (sera), undergoing lumbar puncture for suspected meningoencephalitis admitted in Vientiane, Lao People's Democratic Republic (PDR).
Results
Eight of 134 (6%) patients showed detectable serum neuronal autoantibodies, against the N-methyl-D-aspartate and gamma-aminobutyric acid A receptors (NMDAR and GABAAR), and contactin-associated protein-like 2 (CASPR2). Three of eight patients had accompanying autoantibodies in cerebrospinal fluid (two with NMDAR and one with GABAAR antibodies), and in two of these the clinical syndromes were typical of autoimmune encephalitis. Three of the other five patients had proven central nervous system infections, highlighting a complex overlap between diverse infectious and autoimmune causes of encephalitis. No patients in this cohort were treated with immunotherapy, and the outcomes were poor, with improvement observed in a single patient.
Conclusions
In Lao PDR, autoimmune encephalitis is underdiagnosed and has a poor prognosis. Empiric immunotherapy should be considered after treatable infectious aetiologies are considered unlikely. Awareness and diagnostic testing resources for autoimmune encephalitis should be enhanced in South-East Asia.
Keywords: autoimmune, Laos, LGI1, meningoencephalitis, neuroimmunology, NMDAR
Introduction
Encephalitis, inflammation of the brain, is a medical emergency with high morbidity and mortality and it predominantly occurs secondary to infectious and immune-mediated causes.1–9 The incidence of all-cause encephalitis has been estimated at 3–7 cases per 100 000 person-years in the UK and USA. In these regions, several aetiological descriptions of encephalitis have focused on infections, with no known cause described in approximately 60% of patients. 1–9 However, over the last few years, a range of neuroglial surface-directed autoantibodies has been discovered in patients with forms of autoimmune encephalitis (AE). As these autoantibodies target the extracellular domains of key neuroglial proteins, they are likely to be pathogenic in humans. The most common autoantibody targets include the N-methyl-D-aspartate receptor (NMDAR), leucine-rich glioma-inactivated 1 (LGI1), the gamma-aminobutyric acid receptor A and B receptors (GABAAR, GABABR) and contactin-associated protein-like 2 (CASPR2).2–5 These autoantibodies explain a substantial proportion of previously idiopathic cases and, importantly, typically associate with immunotherapy-responsive conditions: meaning they are considered ‘not to miss’ diagnoses.2–6
This shift is reflected by a recent USA study, in which autoimmune aetiologies of encephalitis were described at least as frequently as infectious causes.3 In this study, the incidence of AE increased around threefold in a comparison of 1995–2005 with 2006–2015, likely reflecting increased awareness of AE and a growing emphasis on neuronal autoantibody testing. In contrast, the incidence of all infectious aetiologies remained stable at 1.0 per 100 000 person-years.
In addition, overlaps have been recognised between infectious and autoimmune causes of encephalitis. In particular, herpes virus simplex encephalitis (HSVE) and Japanese encephalitis (JE) have been described to precede AE associated with autoantibodies to NMDAR, GABAAR and others.7–9
Epidemiological data about meningoencephalitis in southeast Asian countries are sparse with cohorts examining its causes described in Taiwan, Thailand, China, Vietnam, Cambodia, Korea and the Lao People's Democratic Republic (Lao PDR, Laos).10–11 These studies show a predominance of unknown aetiologies, with infectious aetiologies only confirmed in 17–54% of patients and few reports of autoimmune encephalitis.12–17 Here, we present the first examples of neuronal autoantibodies and AE in patients with meningoencephalitis in Laos, describe their phenotypes and identify those with potentially novel infectious–autoimmune overlaps.
Materials and Methods
Patient and clinical data
A prospective study during January 2003–August 2011 recruited all consenting inpatients with suspected central nervous system (CNS) infection in Mahosot Hospital, Vientiane Capital, for whom diagnostic lumbar puncture was indicated in the opinion of the responsible physician. Patient history and examination findings were recorded on standardised forms and meningitis, meningoencephalitis and encephalitis were defined as previously described.11,18
Cerebrospinal fluid (CSF) and sera were aliquoted and immediately stored at −80°C. All patient samples underwent a panel of laboratory tests including full blood count, biochemistry panel, culture and serologic and molecular assays for a range of bacteria, viruses, parasites and fungi.11 HIV-1 and HIV-2 rapid diagnostic tests were performed when requested. CT brain scans were available starting in 2002 but were rarely used, especially for intensive care patients, because of difficulties in transferring patients. MRI and EEG were not available.
Clinical case descriptions were prepared (MM, SR, PNN) and reviewed (CEU, SRI), blind to autoantibody results, to determine whether they met 2016 consensus criteria for AE (Table 1).19
Table 1.
Clinical and paraclinical features of patients with encephalitis-associated autoantibodies in Laos
| IgG-specificity and endpoint titres | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient age (y) and gender | Clinical features and CNS syndrome | Serum | CSF | CSF WBC (cells/uL) | CSF: serum glucose ratio | CSF protein (mg/dL) | Identified infection | Outcome | Meeting criteria for possible autoimmune encephalitis?19 |
| A. 22M | Prodromal fever then acute confusion, neck stiffness, agitation, delirium, hypersomnolence/insomnia, catatonia; meningoencephalitis | NMDAR 1:200 |
NMDAR 1:200 |
15 | 0.50 | 55 | None | Discharged, not improved - diagnosis catatonic schizophrenia | YES |
| B. 45F | Prodromal fever, headache, neck stiffness then acute confusion, drowsiness, agitation, delirium; admitted initially to psychiatry; meningoencephalitis | NMDAR 1:500 | NMDAR 1:2 | 50 | 0.81 | 28 | None | Discharged moribund | YES |
| C. 35M | 7-d history of fever and headache followed by acute confusion, drowsiness; meningoencephalitis | NMDAR 1:500 | Neg. | 85 | 0.35 | 55 | Pus in ears, culture negative | Deceased | NO |
| D. 53M | 10-d history of fever, headache, neck stiffness, cough, vomiting, confusion, right leg weakness; meningoencephalitis | NMDAR 1:400 | Neg. | 610 | CSF 2.0 mmol/L* | 276 | TB culture CSF positive | Deceased, diagnosis TB meningitis | NO |
| E. 45M | Acute onset of fever, headache, neck stiffness, agitation, drowsiness, confusion, with single convulsion; meningoencephalitis | NMDAR 1:200 | Neg. | 10 | 0.43 | 38 | Serum murine typhus IgM+, Rickettsia typhi PCR negative | Discharged well | NO |
| F. 73M | Acute onset of fever, neck stiffness, delirium, agitation and altered consciousness; admitted initially to psychiatry; meningoencephalitis | NMDAR 1:200 | Neg. | 5 | CSF 5.2 mmol/L* | 30 | None | Discharged moribund | NO |
| G. 51M | 1-mo weight loss preceding acute onset fever, headache and neck stiffness; meningitis; suspected underlying malignancy | CASPR2 1:800 | Neg. | 0 | 1.09 | 72 | N. meningitidis PCR positive on CSF (culture negative) | Discharged AMA, no improvement | NO |
| H. 57M | 7-d fever, headache, cough and rash without seizures or confusion | GABAAR 1:160 | GABAAR 1:8 | 18 | 0.34 | 20 | None | Unknown | NO |
Abbreviations: AMA, against medical advice; CASPR2, contactin-associated protein-like 2; CSF, cerebrospinal fluid; GABAAR, gamma-amino butyric acid A receptor; NMDAR, N-methyl-D-aspartate receptor; WBC, white blood cells.
The following cut-offs were used for positive serum neuronal autoantibody titres: NMDAR antibodies >1:100, CASPR2 antibodies >1:500, GABAAR antibodies >1:50. In CSF, any antigen-specific reactivity is considered positive.
*no concurrent serum glucose available for these patients.
See Dubot-Pérès et al. (2019)11 for diagnostic techniques for the pathogens given in the Identified infection column.
The criteria for possible autoimmune encephalitis used are = diagnosis can be made when all three of the following criteria have been met: (a) subacute onset (rapid progression of less than 3 mo) of working memory deficits (short-term memory loss), altered mental status or psychiatric symptoms; (b) at least one of the following: new focal CNS findings, seizures not explained by a previously known seizure disorder, CSF pleocytosis (white blood cell count of more than five cells per mm3), MRI features suggestive of encephalitis; and (c) reasonable exclusion of alternative causes.19
Autoantibody testing
Data on the infectious disease aetiologies have been published.11 Autoantibodies were tested in available CSF and serum samples from 134 patients: 92 patients without an identified infectious aetiology and 42 patients with a confirmed infectious diagnosis. In addition, sera from 55 healthy blood donor controls from Vientiane were tested. Samples were tested on antigen-specific live cell-based assays, against LGI1, CASPR2 and the NMDA and GABAA receptors, the commonest neuroglial surface-directed autoantibodies encountered in clinical practice, as previously described.20–22 Positive results were titrated to endpoint dilutions.
Results
Clinical findings
From the 134 paired CSF/serum samples, 42 (31%) had a confirmed infectious aetiology that included Japanese encephalitis virus (JEV; n=7), Cryptococcus spp. (n=7), Mycobacterium tuberculosis (n=5), Herpes simplex virus (n=5), Dengue virus (n=4), Streptococcus pneumoniae (n=3), Rickettsia typhi (n=3), Orientia tsutsugamushi (n=3), Neisseria meningitidis (n=2), Streptococcus suis (n=2) and Leptospira spp. (n=1), and two had suspected infectious aetiologies (JEV n=1, mumps/measles n=1).11 The remaining 90 patients had meningoencephalitis of unknown aetiology. The 55 healthy controls had a median (range) age of 19 (17–19) y and 16.4% were female.
Patients with autoantibodies
Overall, none of the 55 healthy controls but 8/134 (6%) patients had detectable neuroglial surface autoantibodies (Table 1 and Figure 1); 0/8 fulfilled definite AE criteria, lacking MRI and EEG findings, which are part of the formal diagnostic requirements. These eight patients were aged 0.08–77 (median 32) y and one was female. These demographics were similar to the overall seronegative case series, in which 44/126 (35%) were female and the median age was 45 (22–73) y. Six of eight seropositive patients had serum NMDAR autoantibodies (endpoint dilutions from 1:200 to 1:500). In two (patients A and B), the CSF also harboured NMDAR antibodies with serum:CSF NMDAR antibody ratios of 1 and 250. Both these patients had syndromes compatible with definite NMDAR antibody encephalitis (and fulfilled the criteria for possible AE) and were discharged with catatonic schizophrenia and moribund, respectively.
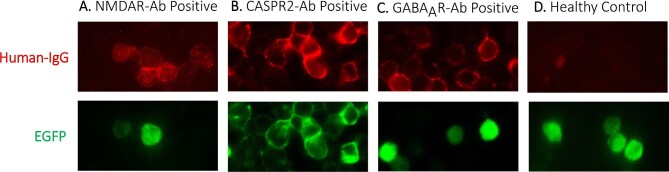
Figure 1.
Live cell-based assays in antigen-expressing HEK293T cells demonstrate surface binding of autoantibodies from the serum of patients with suspicion of CNS infection in Laos. Autoantibodies shown against the (A) N-methyl-D-aspartate receptor (NMDAR), (B) contactin-associated protein-like 2 (CASPR2) and (C) gamma-amino butyric acid A receptor (GABAAR). All at 100x magnification. (D) Absence of staining shown in the serum of a representative healthy control, using NMDAR-transfected HEK293T cells. For each assay, CNS autoantibody staining is shown in red and co-transfected or antigen-tagged enhanced green fluorescent protein (EGFP) in green.
Two other seropositive patients had either serum CASPR2 antibodies (without CSF CASPR2 antibodies, patient G) or serum GABAAR antibodies with accompanying CSF GABAAR antibodies (patient H). The latter patient had a serum:CSF autoantibody ratio of 20, indicative of marked intrathecal synthesis of the GABAAR antibodies, with a meningoencephalitis syndrome but no seizures.
Six of the eight patients had neck stiffness, consisting of 5/6 with NMDAR antibodies and one with CASPR2 antibodies (Table 1). Six patients with suspected CNS infection were classified as having meningoencephalitis, one as having meningitis (patient G) and one (patient H) did not fulfil WHO encephalitis or meningitis case definitions.18 Of the 134 patients tested for autoantibodies, 76 (57%) met WHO criteria for encephalitis and 34 (26%) met WHO criteria for meningitis.
Infectious overlaps
As described in Table 1, two of the eight seropositive patients had definite infectious diagnoses (M. tuberculosis in patient D and N. meningitidis in patient G) with serum, but not CSF, NMDAR and CASPR2 antibodies, respectively. These findings of an alternative aetiology exclude them from criteria-based diagnoses of AE. Another patient had serum NMDAR antibodies and anti-R. typhi IgM (patient E) detected but a negative peripheral blood EDTA buffy coat PCR for R. typhi, leading to uncertainty with the diagnosis of acute murine typhus.23 One patient (patient C) had a moderate (1:500) serum NMDAR antibody level and purulent discharge from the ears with a history of chronic otitis media; hence, a primary infectious aetiology from contiguous spread was considered more likely than a primary autoimmune condition.
Outcomes
None of the eight patients with autoantibodies received immunotherapy, including adjunctive corticosteroids for infectious disease indications. At last follow-up at a median (range) interval of 10 (2–40) d, two were deceased, two discharged moribund, two discharged without improvement and one was in an unknown clinical condition. Only one patient (13%) improved during their hospitalisation (Table 1).
Discussion
We provide the first description of neuronal autoantibodies in patients in Laos. Although limited clinical and investigation data were available in these patients, our findings suggest that cases of AE in this population likely remain undiagnosed and untreated. This observation may apply to many other countries where widespread autoantibody testing is not available, and infectious diagnoses are historically considered more frequent. Yet, autoimmune causes of encephalitis appeared as common as infectious aetiologies in adjacent Thailand.12
Of interest, we detected moderate-titre autoantibodies, absent from the healthy controls tested, which target the extracellular domains of key neuronal proteins, exclusively in the serum of the small subset of patients with proven infectious aetiologies, namely, M. tuberculosis and N. meningitidis. These findings extend the possible infections that may be associated with neuronal autoantibodies, beyond the recognised prodromal associations with HSVE and JE.7–9 Future studies should systematically and longitudinally evaluate the evolution of these reactivities in diverse infectious settings. We hypothesise that such CNS infections may expose CNS antigens, which drain through meningeal lymphatics to stimulate peripheral autoantibody-reactive B cells,24 with cervical lymph nodes representing the most likely site for initiation of this reaction. CSF autoantibodies are highly specific in the diagnosis of these conditions and their absence in the CSF of these patients supports that they are likely an epiphenomenon of infection rather than due to a primary autoimmune process.
The limitations of the study include the relatively small sample size, that patients were from a single centre, the lack of MRI and EEG data and investigations for systemic tumours associated with autoantibodies and the absence of testing by a ‘confirmatory’ detection method of rodent brain section immunohistochemistry due to insufficient sample volumes.25 Nevertheless, the exclusive binding of any serum/CSF sample to a single autoantigen confirms a significant degree of specificity.26
Neck stiffness is unusual in AE but 6/8 patients described here had neck stiffness, including one patient meeting WHO criteria for meningitis but not encephalitis.11,18 This suggests that future studies should search for autoantibodies in patients with meningitic presentations.27 Further, CNS autoantibodies were detected in one patient who did not fulfil either WHO encephalitis or meningitis case definitions.
These data, plus several emerging reports, collectively suggest that AE is not uncommon worldwide.3,19 AE results in significant morbidity and mortality but usually responds to immunotherapies. Hence, the trajectory and outcome of these patients can be significantly improved with appropriate recognition and care. Indeed, clinical recognition of AE is key: individual forms show distinctive clinical features such as their psychopathological features,28 profile of cognitive deficits,29 phenomenology of movement disorders30 and highly characteristic seizure semiologies.26,31 Accurate identification of these features, in parallel with autoantibody testing, permits rational administration of immunotherapies. Engagement with general, infectious disease, neurology and psychiatry health workers and policymakers will be essential. Some AE patients are given a variety of alternative diagnoses that include delirium, functional disorders and catatonia. Indeed, one patient in this series with catatonia and CSF NMDAR antibodies was likely to have been misdiagnosed with this primary psychiatric condition.28
In mainland South-East Asia, as far as we are aware, laboratory diagnosis of AE is only available in Thailand, Vietnam, Singapore and Malaysia, at very few specialised centres. Autoantibody detection utilises simple 2–3 colour fluorescent microscopy, already widely available within South-East Asia for diagnosis of infectious diseases such as rabies, with trained diagnostic staff and systems that could be repurposed for AE diagnosis. Creation of laboratory networks for the diagnosis of AE, using existing equipment and staff trained in indirect immunofluorescence assays, would raise awareness, increase our understanding of AE risk factors, epidemiology and the infectious disease–autoimmune interface and provide key information for the optimal management of individual patients.
Immunotherapies such as intravenous immunoglobulin, plasmapheresis, azathioprine, mycophenolate, cyclophosphamide and monoclonal antibody therapy (e.g. rituximab, tocilizumab) may be prohibitively expensive or unavailable in resource-poor settings.4 However, corticosteroids are widely available and highly effective in many patients with AE.5,6 In Vientiane, only corticosteroids and cyclophosphamide were available medications as of January 2021. Adverse consequences of lone corticosteroid therapy in some conditions that are relatively common in South-East Asia (e.g. M. tuberculosis CNS infection) mean careful consideration will be needed to exclude these infections. In addition, surgical resection of an underlying tumour can improve outcomes in patients with paraneoplastic AE, and such operations can be performed in many South-East Asian hospitals.
Taken together, our study suggests that a proportion of patients in Laos with meningoencephalitis and poor outcomes have a treatable form of AE. Others represent potentially novel infectious–autoimmune overlaps, an area for future study. Recognition of AE may remain relatively neglected in much of the world and should be promoted with appropriate clinical training and autoantibody testing facilities. The risk factors and epidemiology in these regions may provide important insights for the optimal management of individual patients worldwide.
Acknowledgements
We thank the patients, Bounthaphany Bounxouei (Associate Professor and former Director) and the staff of Mahosot Hospital, especially those of the Microbiology Laboratory and the wards, the late Rattanaphone Phetsouvanh, and Vayouly Vidhamaly, Konnie Bellingham, Kum Thong Wong, Rogier van Doorn, Guy Thwaites and Paul Turner, for their advice and technical help. We also thank Bounnack Saysanasongkham (Associate Professor and former Director of Department of Health Care, Ministry of Health) and H.E. Bounkong Syhavong (Associate Professor and former Minister of Health), Laos, for their kind help and support. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health, UBC, or Vancouver Coastal Health. This research was funded in whole, or in part, by the Wellcome Trust. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Contributor Information
Christopher E Uy, Oxford Autoimmune Neurology Group, Nuffield Department of Clinical Neurosciences, Nuffield Department of Medicine, Oxford University, John Radcliffe Hospital, Oxford OX3 9DU, UK; Division of Neurology, Department of Medicine, University of British Columbia Hospital, Vancouver, British Columbia V6T 2B5, Canada; Department of Neurology, Oxford University Hospitals, John Radcliffe Hospital, Oxford OX3 9DU, UK.
Mayfong Mayxay, Lao-Oxford-Mahosot Hospital-Wellcome Research Unit OX3 7JX (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR; Center for Tropical Medicine and Global Health, Nuffield Department of Medicine, New Richards Building, Oxford University, Oxford OX3 7LG, UK; Institute of Research and Education Development (IRED), University of Health Sciences, Ministry of Health, Vientiane, Lao PDR.
Ruby Harrison, Oxford Autoimmune Neurology Group, Nuffield Department of Clinical Neurosciences, Nuffield Department of Medicine, Oxford University, John Radcliffe Hospital, Oxford OX3 9DU, UK.
Adam Al-Diwani, Oxford Autoimmune Neurology Group, Nuffield Department of Clinical Neurosciences, Nuffield Department of Medicine, Oxford University, John Radcliffe Hospital, Oxford OX3 9DU, UK; Department of Psychiatry, Warneford Hospital, University of Oxford, Oxford, UK.
Leslie Jacobson, Oxford Autoimmune Neurology Group, Nuffield Department of Clinical Neurosciences, Nuffield Department of Medicine, Oxford University, John Radcliffe Hospital, Oxford OX3 9DU, UK.
Sayaphet Rattanavong, Lao-Oxford-Mahosot Hospital-Wellcome Research Unit OX3 7JX (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR.
Audrey Dubot-Pérès, Lao-Oxford-Mahosot Hospital-Wellcome Research Unit OX3 7JX (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR; Center for Tropical Medicine and Global Health, Nuffield Department of Medicine, New Richards Building, Oxford University, Oxford OX3 7LG, UK; Unité des Virus Émergents (UVE: Aix-Marseille Univ-IRD 190-INSERM 1207), IHU Méditerranée Infection, 19-21, Bd Jean Moulin, Marseille 13005, France.
Manivanh Vongsouvath, Lao-Oxford-Mahosot Hospital-Wellcome Research Unit OX3 7JX (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR.
Viengmon Davong, Lao-Oxford-Mahosot Hospital-Wellcome Research Unit OX3 7JX (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR.
Vilada Chansamouth, Lao-Oxford-Mahosot Hospital-Wellcome Research Unit OX3 7JX (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR; Center for Tropical Medicine and Global Health, Nuffield Department of Medicine, New Richards Building, Oxford University, Oxford OX3 7LG, UK.
Koukeo Phommasone, Lao-Oxford-Mahosot Hospital-Wellcome Research Unit OX3 7JX (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR.
Patrick Waters, Oxford Autoimmune Neurology Group, Nuffield Department of Clinical Neurosciences, Nuffield Department of Medicine, Oxford University, John Radcliffe Hospital, Oxford OX3 9DU, UK.
Sarosh R Irani, Oxford Autoimmune Neurology Group, Nuffield Department of Clinical Neurosciences, Nuffield Department of Medicine, Oxford University, John Radcliffe Hospital, Oxford OX3 9DU, UK; Department of Neurology, Oxford University Hospitals, John Radcliffe Hospital, Oxford OX3 9DU, UK.
Paul N Newton, Lao-Oxford-Mahosot Hospital-Wellcome Research Unit OX3 7JX (LOMWRU), Microbiology Laboratory, Mahosot Hospital, Vientiane, Lao PDR; Center for Tropical Medicine and Global Health, Nuffield Department of Medicine, New Richards Building, Oxford University, Oxford OX3 7LG, UK.
Authors’ contributions
The authors wish it to be known that, in their opinion, CEU, MM and RH should be regarded as joint First Authors. PNN, MM, SR, AD-P, MV, VD, VC, KP and SRI conceived and designed the study; SR, MV, VD, VC, KP, MM and PNN carried out the clinical assessment; RH, AA-D, LJ, PW, CEU and SRI carried out and interpreted the autoantibody assays; CEU, SRI and PNN drafted the manuscript; and CEU, MM, RH, AA-D, LJ, SR, AD-P, MV, VD, VC, KP, PW, SRI and PNN revised the manuscript for intellectual content. PNN and SRI are guarantors of the paper.
Funding
This work in Laos was supported by the Wellcome Trust [grant number 104079/Z/14/Z]. SRI is supported by the BMA Research Grants - Vera Down grant [2013] and Margaret Temple [2017], Epilepsy Research UK [P1201], the Fulbright UK-US commission [MS Society research award] and by the NIHR Oxford Biomedical Research Centre. CEU is supported by the Friedman Award for Scholars in Health (University of British Columbia, Canada) and received salary support from the UBC Division of Neurology (Vancouver, Canada). AA-D is supported by the Wellcome Trust [205126/Z/16/Z], National Institute for Health Research (NIHR) Oxford and Oxford Health Biomedical Research Centres (BRC) and by a British Medical Association (BMA) foundation for medical research Margaret Temple prize 2017.
Competing interests
SRI and PW are coapplicants and receive royalties on patent application WO/2010/046716 (UK patent no. PCT/GB2009/051441) entitled ‘Neurological Autoimmune Disorders’. The patent has been licensed commercially for the development of assays for LGI1 and other VGKC-complex antibodies and on a patent application entitled Diagnostic Strategy to improve specificity of CASPR2 antibody detection (PCT/GB2019/051257, publication number WO/2019/211633 and UK1807410.4). SRI has received honoraria from UCB, MedImmun, ADC therapeutics and Medlink Neurology, and research support from CSL Behring, UCB and ONO Pharma. PW has received honoraria from Biogen Idec, Mereo Biopharma, Retrogenix, University of British Columbia, F. Hoffmann-La Roche and Alexion. The other authors have no relevant conflicts of interest to declare.
Ethical approval
The study was conducted in accordance with the ethical standards of the Helsinki Declaration (1964, amended most recently in 2008) of the World Medical Association. Verbal (2003–2006) or written (2006–2011) informed consent was obtained from all patients or their close relatives and written consent for use of aliquots of sera from sequential blood bank donors in 2004. Ethical approvals were granted by the Ethical Review Committee of the former Faculty of Medical Sciences, National University of Laos – now University of Health Sciences, the National Ethics Committee for Health Research (Vientiane, Laos) and the Oxford University Tropical Ethics Research Committee (Oxford, UK). Research ethics committee approval (REC16/YH/0013) was obtained by the Oxford Autoimmune Neurology Group for testing samples from patients consented elsewhere.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Granerod J, Cousens S, Davies NWSet al. New estimates of incidence of encephalitis in England. Emerg Infect Dis. 2013;19(9):1455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Glaser CA, Honarmand S, Anderson LJet al. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin Infect Dis. 2006;43(12):1565–77. [DOI] [PubMed] [Google Scholar]
- 3. Dubey D, Pittock SJ, Kelly CRet al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83(1):166–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ramanathan S, Al-Diwani A, Waters Pet al. The autoantibody-mediated encephalitides: from clinical observations to molecular pathogenesis. J Neurol. 2019;1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson J, Bi M, Murchison AGet al. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain. 2018;141(2):348–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Titulaer MJ, McCracken L, Gabilondo Iet al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Armangue T, Leypoldt F, Málaga Iet al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol. 2014;75(2):317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hacohen Y, Deiva K, Pettingill Pet al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord. 2014;29(1):90–6. [DOI] [PubMed] [Google Scholar]
- 9. Ma J, Zhang T, Jiang L. Japanese encephalitis can trigger anti-N-methyl-d-aspartate receptor encephalitis. J Neurol. 2017;264(6):1127–31. [DOI] [PubMed] [Google Scholar]
- 10. Lee TC, Tsai CP, Yuan CLet al. Encephalitis in Taiwan: a prospective hospital-based study. Jpn J Infect Dis. 2003;56(56):193–9. [PubMed] [Google Scholar]
- 11. Dubot-Pérès A, Mayxay M, Phetsouvanh Ret al. Management of central nervous system infections, Vientiane, Laos, 2003-2011. Emerg Infect Dis. 2019;25(5):898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Saraya AW, Worachotsueptrakun K, Vutipongsatorn Ket al. Differences and diversity of autoimmune encephalitis in 77 cases from a single tertiary care center. BMC Neurol. 2019;19:273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thepwiwatjit S, Jitprapaikulsan J. Anti-NMDA-receptor encephalitis of Thai patients: description of a consecutive series of patients over 10 years and a literature review. J Med Assoc Thail. 2018;101(2):163–71. [Google Scholar]
- 14. Lim JA, Lee ST, Jung KHet al. Anti-N-methyl-D-aspartate receptor encephalitis in Korea: clinical features, treatment, and outcome. J Clin Neurol. 2014;10(2):157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Duan BC, Weng WC, Lin KLet al. Variations of movement disorders in anti-N-methyl-D-aspartate receptor encephalitis: a nationwide study in Taiwan. Medicine. 2016;95(37):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gu Y, Zhong M, He Let al. Epidemiology of antibody-positive autoimmune encephalitis in Southwest China: a multicenter study. Front Immunol. 2019;10:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoang MNT, Hoan PN, Le Van TE, et al. First reported cases of anti-NMDA receptor encephalitis in Vietnamese adolescents and adults. J Neurol Sci. 2017;373:250–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization . WHO-recommended standards for surveillance of vaccine-preventable diseases. Geneva, Switzerland: World Health Organization;2003. [Google Scholar]
- 19. Graus F, Titulaer MJ, Balu Ret al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Irani SR, Bera K, Waters Pet al. N-methyl-d-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain. 2010;133(6):1655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Irani SR, Alexander S, Waters Pet al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain. 2010;133(9):2734–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pettingill P, Kramer HB, Coebergh JAet al. Antibodies to GABAA receptor α1 and γ2 subunits: clinical and serologic characterization. Neurology. 2015;84(12):1233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dittrich S, Castonguay-Vanier J, Moore CEet al. Loop-mediated isothermal amplification for Rickettsia typhi (the causal agent of murine typhus): problems with diagnosis at the limit of detection. J Clin Microbiol. 2014;52(3):832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Makuch M, Wilson R, Al-Diwani Aet al. N-methyl-D-aspartate receptor antibody production from germinal center reactions: therapeutic implications. Ann Neurol. 2018;83(3):553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gresa-Arribas N, Titulaer MJ, Torrents Aet al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. 2014;13(2):167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGinty RN, Handel A, Moloney Tet al. Clinical features which predict neuronal surface autoantibodies in new-onset focal epilepsy: implications for immunotherapies. J Neurol Neurosurg Psych. 2021;92(3):291–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stavrou M, Yeo JM, Slater ADet al. Case report: meningitis as a presenting feature of anti-NMDA receptor encephalitis. BMC Infect Dis. 2020;20(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al-Diwani A, Handel A, Townsend Let al. The psychopathology of NMDAR-antibody encephalitis in adults: a systematic review and phenotypic analysis of individual patient data. Lancet Psych. 2019;6(3):235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finke C, Prüss H, Heine Jet al. Evaluation of cognitive deficits and structural hippocampal damage in encephalitis with leucine-rich, glioma-inactivated 1 antibodies. JAMA Neurol. 2017;74(1):50–9. [DOI] [PubMed] [Google Scholar]
- 30. Varley JA, Webb AJS, Balint Bet al. The movement disorder associated with NMDAR antibody-encephalitis is complex and characteristic: an expert video-rating study. J Neurol Neurosurg Psych. 2019;90(6):724–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Irani SR, Michell AW, Lang Bet al. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol. 2011;69(5):892–900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.