ABSTRACT
The prevalence of osteoporosis among women aged 50 y and older is expected to reach 13.6 million by 2030. Alternative nonpharmaceutical agents for osteoporosis, including nutritional interventions, are becoming increasingly popular. Prunes (dried plums; Prunus domestica L.) have been studied as a potential whole-food dietary intervention to mitigate bone loss in preclinical models of osteoporosis and in osteopenic postmenopausal women. Sixteen preclinical studies using in vivo rodent models of osteopenia or osteoporosis have established that dietary supplementation with prunes confers osteoprotective effects both by preventing and reversing bone loss. Increasing evidence from 10 studies suggests that, in addition to antiresorptive effects, prunes exert anti-inflammatory and antioxidant effects. Ten preclinical studies have found that prunes and/or their polyphenol extracts decrease malondialdehyde and NO secretion, increase antioxidant enzyme expression, or suppress NF-κB activation and proinflammatory cytokine production. Two clinical trials have investigated the impact of dried plum consumption (50–100 g/d for 6–12 mo) on bone health in postmenopausal women and demonstrated promising effects on bone mineral density and bone biomarkers. However, less is known about the impact of prune consumption on oxidative stress and inflammatory mediators in humans and their possible role in modulating bone outcomes. In this review, the current state of knowledge on the relation between inflammation and bone health is outlined. Findings from preclinical and clinical studies that have assessed the effect of prunes on oxidative stress, inflammatory mediators, and bone outcomes are summarized, and evidence supporting a potential role of prunes in modulating inflammatory and immune pathways is highlighted. Key future directions to bridge the knowledge gap in the field are proposed.
Keywords: immunity, dried plums, prunes, bone density, inflammation, gut microbiota, nutritional intervention, osteoporosis, osteopenia
Statement of Significance: Osteoporosis represents a major public health issue and prunes (dried plums) have been extensively studied as a dietary intervention to mitigate bone loss in preclinical models of osteoporosis. In postmenopausal women, estrogen deficiency triggers an upregulation of oxidative stress and inflammatory pathways, which promotes bone loss, increasing risk of fracture. In this review, we summarize the evidence suggesting a potential role of prunes in modulating oxidative and inflammatory pathways, which may contribute to the protective effect on bone.
Introduction
Osteoporosis is a debilitating bone disease characterized by a significant reduction in bone mineral density (BMD) and deterioration of bone microstructure, predisposing individuals to increased fracture risk (1). Osteoporosis is estimated to affect over 200 million women worldwide, causing 8.9 million fractures annually (2), and recent projections indicate that the prevalence of osteoporosis among women aged 50 y and older will reach 13.6 million by 2030 (3, 4). Current pharmacological therapies include antiresorptive (bisphosphonates, denosumab, calcitonin, strontium ranelate), anabolic (teriparatide, abaloparatide, romosozumab), or selective estrogen receptor modulators (SERMs) to treat low BMD in women (5, 6). Although these interventions are effective, high cost, poor compliance, and negative side effects contribute to declining use and popularity (7, 8). Therefore, osteoporosis represents a major public health issue that necessitates effective prevention and treatment regimens that are safe, cost-effective, and are associated with fewer adverse effects than conventional pharmaceutical agents.
Hypoestrogenism manifests as a consequence of ovarian senescence during menopause and is responsible for the onset of a 3- to 5-y period of accelerated bone loss, followed by continued gradual bone loss, which involves loss of bone strength, density, and poor bone quality, thereby increasing the risk for osteoporosis and fracture (9). In addition, hypoestrogenism is a potent stimulus for increased production of inflammatory mediators from immune cells (10). In particular, estrogen deficiency can lead to activation of macrophages and T cells, which secrete inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, that stimulate osteoclast activity and inhibit osteoblast activity, thus collectively promoting bone resorption (11–13). Furthermore, aging is associated with increased concentrations of circulating proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, which partly contribute to the “inflamm-aging” phenomenon observed in older adults (14, 15). Last, changes in the gut microbiota can modulate inflammatory mediators in healthy individuals (16), and gut dysbiosis is linked to numerous chronic diseases (17–20), including osteoporosis (21, 22). Commensal bacteria play an important role in maintaining the integrity of tight junctions within the intestinal epithelium and in secreting metabolites, such as SCFAs, that exhibit anti-inflammatory effects within the intestinal mucosa (21, 23). While the link between the immune system and bone is well established (24, 25), emerging data suggest that changes in the gut microbiota may be modulating this relation (26, 27). Therefore, the likely factors that contribute to bone loss during postmenopausal osteoporosis appear to include increased oxidative stress and inflammatory cytokine production secondary to hypoestrogenism, and the effect of changes in the gut microbiota on inflammatory mediators, all which contribute to upregulated bone resorption.
There is increasing consumer interest in alternative, nonpharmacological therapies for osteoporosis, including nutritional interventions (28, 29), which might be used alone or in combination with pharmacological agents to reduce their dose or duration. Calcium and vitamin D supplementation are considered the minimal standard of care to maintain bone health in postmenopausal women and are associated with modest reduction in fracture risk among older adults (30, 31). Bone remodeling involves continuous turnover of the protein matrix and dietary protein is another nutritional factor required for maintaining bone health (32, 33). Fruits and vegetables rich in bioactive compounds, such as phenolic acid, flavonoids, and carotenoids, have potential osteoprotective effects in both animal studies and clinical trials (34–36), and many postmenopausal women use botanical supplements for osteoporosis management (37).
Of the functional foods and plant-derived compounds assessed for their effects on bone health, prunes (also known as dried plums; Prunus domestica L.) have gained increasing attention and these findings have been recently summarized (38, 39). Sixteen preclinical studies demonstrate that prune supplementation not only prevents but also reverses bone loss in several rodent models of gonadal hormone deficiency. To date, 4 randomized controlled trials (RCTs) (40–43) and 1 case study (44) assessed the effect of prune consumption on bone health outcomes in postmenopausal women. These studies demonstrated promising effects on bone turnover (40) and BMD (41, 42, 44), suggesting that supplementation with prunes may confer significant beneficial effects on bone outcomes in this at-risk population.
Prunes have been historically consumed for their purported gastrointestinal health benefits (45) and are a rich source of potassium, boron, copper, vitamin K, and phenolic compounds, such as chlorogenic acids, phenolic acids, and flavonoids (45, 46), which have antioxidant properties (47). Compared with prune juice, prunes have a higher content of dietary fiber and vitamins A and K, and total oxygen radical absorbance capacity. Furthermore, prunes have a higher content of total phenolics compared with fresh plums (45). Overall, prunes are considered a promising functional food for improving bone health and the bioactive components are thought to act synergistically within the whole food matrix to maintain bone health after menopause (39, 48). Additionally, prune consumption may potentially alter gut microbiota (49) and subsequently affect bone health (49–52). This review outlines the known associations between oxidative stress, inflammation, and bone health and summarizes findings from preclinical and clinical studies that demonstrate a potential role of prunes in modulating these pathways.
Current Status of Knowledge
Regulation of bone remodeling
Bone formation by osteoblasts and bone resorption by osteoclasts are tightly coupled processes that constitute bone remodeling, which maintains homeostasis of bone mass throughout adult life (53). However, with increasing age, there is uncoupling of bone turnover. After the age of 40, resorption begins to exceed formation, and this imbalance can be exaggerated by estrogen deficiency, radiation exposure, or long-term immobility (54). Inflammation and oxidative stress are other factors that can exacerbate bone loss, particularly in older adults (13, 55). Acute inflammation is an immune response that is normally mounted during infection and tissue repair. However, chronic inflammation can favor bone resorption, thus compromising bone structure and integrity (12), and contribute to bone loss independently of hypoestrogenism. Several inflammatory diseases, such as rheumatoid arthritis and inflammatory bowel disease (IBD), are associated with bone loss (13, 56) and increased fracture risk [rheumatoid arthritis—HR: 1.26; 95% CI: 1.15–1.38 (57); IBD—OR: 1.32; 95% CI: 1.20–1.45 (58)].
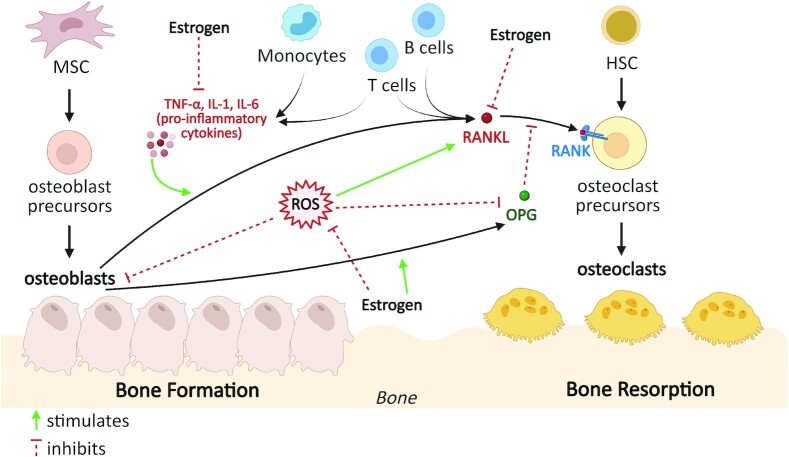
Bone remodeling is regulated by the coordinated activity and maturation of osteoblasts and osteoclasts, which are influenced by various factors, including estrogen, oxidative stress, immune mediators, and growth factors (Figure 1) (12, 59–61). Osteoclasts are derived from hematopoietic stem cells (HSCs) that differentiate into granulocyte–macrophage progenitor cells, and thus, like macrophages, osteoclasts express innate immune signaling receptors, which, upon activation, induce the release of proinflammatory cytokines IL-1β and IL-18 (62). Osteoclast differentiation is regulated by receptor activator of NF-κB ligand (RANKL), which is secreted by osteoblasts and various immune cell populations such as T and B cells (Figure 1), indicating that bone resorption and the immune response are tightly linked (63). Upon secretion, RANKL binds its cognate receptor RANK expressed by osteoclast precursors, triggering the RANKL/RANK cascade (63). This cascade stimulates downstream adaptor proteins called TNF receptor–associated factors (TRAFs), mainly TRAF6, that activate the transcription factor NF-κB, which induces expression of nuclear factor of activated T cells, cytoplasmic 1 (NFATc1) (54). NFATc1 is the major transcriptional factor that promotes expression of osteoclast-specific genes encoding tartrate-resistant acid phosphate (TRAP) 5b (TRAP-5b), cathepsin K, and calcitonin receptor (62, 64). Osteoblasts are derived from mesenchymal stem cells (MSCs) (Figure 1) and are regulated by transcriptional factors such as runt-related transcription factor (RUNX)-2, Osterix, and bone morphogenic proteins (BMPs), which orchestrate osteoblast differentiation (65). In addition to expressing RANKL, which promotes osteoclastogenesis, osteoblasts also express osteoprotegerin (OPG), a soluble decoy receptor for RANKL, thus preventing its binding to and activation of its cognate receptor RANK, inhibiting osteoclast differentiation, activation, and survival.
FIGURE 1.
Factors influencing bone remodeling. Bone remodeling is regulated by the coordinated activity and maturation of osteoblasts and osteoclasts, which are influenced by various factors including estrogen, oxidative stress, and inflammatory mediators. Estrogen 1) upregulates OPG production by osteoblasts and B cells (66); 2) inhibits proinflammatory cytokine (TNF-α, IL-1, IL-6) secretion from monocytes and T cells (12, 67, 68) and RANKL secretion from osteoblasts, T cells, and B cells (69, 70); and 3) blocks the production of ROS (71, 72), which upregulates RANKL expression and downregulates OPG expression, thus ultimately preventing bone resorption. HSC, hematopoietic stem cells; MSC, mesenchymal stem cells; OPG, osteoprotegerin; RANK, receptor activator of NF-κB; RANKL, receptor activator of NF-κB ligand; ROS, reactive oxygen species.
Estrogen regulates bone remodeling as an anabolic or antiresorptive agent that promotes OPG production by osteoblasts (66) and inhibits proinflammatory cytokine production by immune cells (12, 67, 68) and RANKL secretion from osteoblasts, T cells, and B cells (69, 70) (Figure 1), thereby inhibiting osteoclast formation and decreasing bone resorption. Furthermore, oxidative stress alters bone remodeling by impacting the activity of osteoclasts and osteoblasts (71, 72). Several studies in humans demonstrate that reactive oxygen species (ROS) and antioxidant systems are involved in bone loss in aged and/or osteoporotic subjects (73–75). High levels of ROS inhibit osteoblast activity and differentiation (76–78) and thus contribute to reduced bone mineralization. Increased ROS production also promotes bone loss by upregulating RANKL and downregulating OPG expression, thus activating osteoclast differentiation (78–80). Estrogen blocks the production of ROS (71), which prevents ROS-induced bone resorption.
Role of inflammation on bone turnover
The regulatory role of immune and inflammatory mediators in bone remodeling has been well studied (25, 81, 82). In addition to osteoclasts and osteoblasts, T cells, B cells, monocytes, macrophages, and dendritic cells can play a role in inflammation and bone loss (83). Macrophages play a major role in the activation and formation of osteoclasts (84), and the activation of macrophages may induce the production of IFN-γ, IL-1, TNF-α, and numerous inflammatory mediators (85). Osteoclast-mediated bone resorption is activated by oxidative and inflammatory stimuli such as NO, IL-1β, IL-6, IL-8, IL-18, IL-15, IL-17, IL-32, and TNF-α (62), many of which induce osteoclastogenesis by upregulating the release of RANKL (13). Conversely, osteoclast-mediated bone resorption is downregulated by anti-inflammatory cytokines, such as IL-4, IL-10, IL-13, and IL-3 (86). NO and anti-inflammatory cytokines influence osteoblasts by increasing OPG and decreasing RANKL production, thus generating a high OPG to RANKL ratio that favors inhibition of osteoclast differentiation (86). OPG is also secreted by B cells and dendritic cells, demonstrating that osteoclast formation is also regulated by additional components of the immune system (25, 87). T cells secrete pro-osteoclastic cytokines such as TNF-α and RANKL, as well as anti-osteoclastogenic cytokines such as IFN-γ. IFN-γ indirectly inhibits osteoclast activity by accelerating ubiquitin-proteasomal degradation of TRAF-6 (88), thus preventing downstream activation of NF-κB and c-Jun N-terminal kinase and attenuating osteoclast differentiation. B cells are also active regulators of the RANK/RANKL/OPG system and produce a number of regulatory cytokines and chemokines (25). Therefore, osteoclast maturation appears to be regulated by a balance of pro- and anti-inflammatory cytokines and chemokines (62, 67, 68, 86, 88–91).
Immune mediators play an important role in postmenopausal bone loss. Surgical menopause in women is associated with elevated IL-1 and TNF-α secretion from peripheral blood mononuclear cells (PBMCs) with a concomitant increase in urinary markers of bone resorption such as calcium:creatinine ratio (92). Furthermore, estrogen replacement therapy in these women resulted in decreased IL-1 and TNF-α secretion from PBMCs concomitantly with reduced urinary bone resorption markers (92). Hypoestrogenism after menopause is also associated with increased IL-1 activity from circulating human monocytes (93–95), IL-6 (96) and TNF-α (97) production by mononuclear cells, and increased secretion of TNF-α (98–100) and IL-17 by T cells (101). Estrogen deficiency also induces a shift in the T-cell lineage by increasing the ratio of T-helper (Th)-17 cells to T regulatory (Treg) cells, resulting in elevated concentrations of proinflammatory cytokines, primarily IL-17 and TNF-α, which stimulate the release of osteoclast-promoting RANKL (13, 87). Moreover, IL-1, IL-6, and TNF-α suppress osteoblast activity and formation (25), demonstrating that bone resorption is favored in the presence of cytokines that are upregulated during an inflammatory response. PBMCs isolated from women with low BMD produce higher concentrations of pro-resorptive cytokines, TNF-α, IL-6, IL-12, and IL-17, and lower concentrations of antiresorptive cytokines, IL-4, IL-10, and IL-23, compared with women with normal BMD (102), suggesting that the cytokine profile during postmenopausal osteoporosis may be associated with activation of bone resorption.
In addition to estrogen loss, IL-1, IL-6, and TNF-α increase with age (14, 91), which contributes to the state of “inflamm-aging” observed in older adults (10, 15). Postmenopausal women experience bone loss in 2 phases: an accelerated transient phase caused by estrogen loss and a gradual continuous phase attributed, in part, to inflamm-aging associated with increased production of proinflammatory cytokines IL-1, IL-6, and TNF-α (14, 103). Blocking TNF-α with an inhibitor in postmenopausal women reduces the concentrations of carboxyl-terminal telopeptide of type 1 collagen (CTX), a serum marker of bone resorption, suggesting that elevated TNF-α is key mediator of bone resorption (104). Estrogen deficiency accelerates the effect of aging on bone by not only elevating inflammatory mediators but also markers of oxidative stress (71). Two preclinical studies (105, 106) provide mechanistic evidence linking bone loss with estrogen deficiency, aging, oxidative stress, and inflammation. In a rat model of postmenopausal osteoporosis, hepatic expression of lipid peroxide, NO, and inducible NO synthase (iNOS) increased in aged intact rats compared with young intact rats, and these effects were more prominent in ovariectomized (OVX) animals (105). Furthermore, aging and OVX significantly increased liver TNF-α, IL-1β, and IL-6, and downregulated IL-10 (105). In femurs, OVX decreased BMD, impaired bone micro-architecture parameters, upregulated gene expression of proinflammatory cytokine monocyte chemoattractant protein (MCP)) and ROS-generating enzymes, and downregulated gene expression of anti-inflammatory IL-1 receptor 2 gene and antioxidant defense enzymes compared with sham-operated mice (106). Collectively, these preclinical data highlight that estrogen deficiency enhances markers of inflammation and oxidative stress in the bone microenvironment. Thus, targeting the age-related increase in inflammatory mediators and/or oxidative stress with novel treatments may be a strategy to reduce postmenopausal bone loss.
The gut microbiota are also emerging as an important factor in the pathogenesis of osteoporosis (22). The gut microbiota produce various immunomodulatory molecules that promote epithelial barrier integrity, maintain immune tolerance toward commensal bacteria, and confer anti-inflammatory effects on the intestinal mucosa (21). Dysregulation of the gut microbiota is associated with various chronic diseases (17–20), including rheumatoid arthritis (20) and osteoporosis (22), suggesting that microbial populations in the gut may communicate with distant organs such as the bone. Gut dysbiosis may lead to breakdown of epithelial barrier integrity, exposing commensal microbiota to the immune cells in the lamina propria of the intestine and entry of LPS, a component of the outer membrane in gram-negative bacteria, and bacterial peptides into the bloodstream (107). This triggers activation of immune cells, resulting in increased production of proinflammatory cytokines, including TNF-α, IL-1β, and IL-6 (108). These proinflammatory cytokines can promote differentiation and activation of osteoclasts, leading to increased bone resorption (22).
Obesity is a complex, multifactorial disease, involving genetic and environmental factors (including diet and physical inactivity), wherein increased energy intake and decreased energy expenditure contribute to pathological expansion of adipose tissue. Adipocyte hypertrophy can result in hypoxia, cellular and tissue stress, and ultimately increased production of proinflammatory cytokines, including TNF-α, IL-1β, and MCP-1 (109), and increased markers of oxidative stress (110). In both preclinical and clinical studies, increased adiposity resulted in the accumulation of macrophages in adipose tissue (111). Macrophages in adipose tissue localize around adipocytes undergoing hypertrophy-induced cellular stress and apoptosis (112). Macrophages infiltrating adipose tissue also contributed to the production and secretion of proinflammatory mediators and promoted both local and systemic proinflammatory status (113). In addition, obesity is associated with an altered composition of the microbiota, with a marked decrease in microbial diversity and an increase in intestinal permeability (114, 115), resulting in bacterial translocation which contributes to the proinflammatory milieu observed in obesity (116). Thus, obesity-induced inflammation and gut dysbiosis represent important factors that may potentially increase bone resorption through increased production of proinflammatory cytokines (TNF-α, IL-6), upregulation of the RANKL-OPG pathway, and subsequent osteoclast differentiation (86). Obesity increases mechanical loading of bone and elevates 17β-estradiol concentrations, which has been hypothesized to attenuate bone loss in postmenopausal women (117, 118). However, a recent meta-analysis demonstrated that risk for ankle fractures increased by 60% (risk ratio = 1.60; 95% CI: 1.52, 1.68) in postmenopausal women with obesity compared with lean postmenopausal women (119). These data suggest that obesity might not confer osteoprotective effects in postmenopausal women and may, in fact, have detrimental effects on bone.
Two preclinical studies provide mechanistic evidence linking obesity, gut dysbiosis, and inflammation to poor bone outcomes (120,121). High-fat/high-sucrose diet-induced obese rats developed more severe osteoarthritis compared with lean rats concurrently with elevated concentrations of proinflammatory cytokines in synovial fluid and serum, and changes in gut microbial populations (Methanobrevibacter and Lactobacillus spp.) (120). Moreover, high-fat-diet (HFD)-induced obesity in mice impaired osteoblastogenesis and augmented bone marrow adiposity, resulting in impaired bone architecture. The aforementioned outcomes were mediated by changes in the gut microbiota as antibiotic treatment partially reversed the HFD-induced effects on bone, and stool transplanted from HFD-fed mice induced the bone defects in lean, pellet-fed mice (121). Chronic inflammation, oxidative stress, and dysregulation of the gut microbiota are hallmarks of obesity (122). Thus, it is postulated that these factors might contribute to the adverse effects of obesity on bone outcomes; however, the exact mechanisms underlying the relation between obesity and bone loss are unknown.
In addition to obesity, diet is an important modulator of the intestinal microbiota and is a source of phytoestrogens and prebiotics that are metabolized by the gut microbiota (26). Numerous dietary factors alter intestinal microbial composition or metabolites (123, 124) and/or immune responses, which can contribute to systemic inflammation. Thus, accumulating evidence suggests that obesity, alterations in the gut microbiota, dietary factors, estrogen deficiency, and aging contribute to an increase in inflammatory mediators and markers of oxidative stress, which can enhance bone resorption and suppress bone formation, resulting in bone loss (Figure 2). Treatment strategies, including nutritional interventions that attenuate oxidative stress and inflammation, might potentially improve bone outcomes in postmenopausal women. While the osteoprotective effects of prune consumption have been extensively reviewed elsewhere (39, 48, 125), the potential role of prunes in improving bone outcomes via the modulations of antioxidant and anti-inflammatory mechanisms needs to be further explored. Therefore, the focus of this review is to summarize the findings from preclinical and clinical studies that evaluate antioxidant, anti-inflammatory, and other immune outcomes to outline key knowledge gaps in the field.
FIGURE 2.
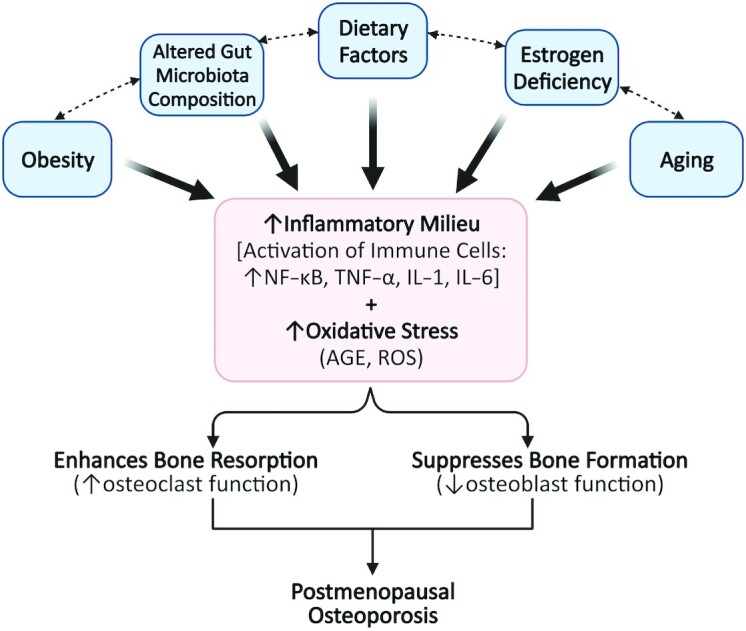
Factors contributing to an elevation in inflammation and oxidative stress, which may underlie postmenopausal osteoporosis. The pathogenesis of postmenopausal osteoporosis is attributed to several factors. Ovarian senescence during menopause results in estrogen deficiency, which is a potent stimulus for increased production of proinflammatory mediators and oxidative stress markers that promote bone loss. Aging is another factor that is associated with elevated inflammation and oxidative stress. The gut microbiome is emerging as an important modulator of the bone and immune system and the composition of the intestinal microbiota is affected by the diet, which is an important source of fiber and phytoestrogens. While these factors directly contribute to increased inflammation and oxidative stress (indicated by solid black lines), they also modulate one another (indicated by hashed lines). AGE, advanced glycation end products; ROS, reactive oxygen species.
Prunes (Dried Plums) and Bone Health
In vitro findings
Six in vitro studies (126–131) have evaluated the antiresorptive and/or anabolic effects of prunes using osteoclast (RAW 264.7) and osteoblast (MC3T3-E1) cell lines, and these results are summarized in Table 1. One study demonstrates that concurrent treatment of RAW 264.7 murine macrophages with prune polyphenol extracts (1000 μg/mL) prevented an increase in malondialdehyde secretion induced by ferrous sulfate and hydrogen peroxide (128). A significant reduction in LPS-induced NO secretion (126, 128) was reported in 2 studies, and 1 study demonstrated a reduction in iNOS protein expression following either pretreatment or concurrent treatment of RAW 264.7 cells with varying concentrations of prune polyphenol extracts (0.1–1000 μg/mL).
TABLE 1.
Effect of phenolic compounds of prunes (dried plum) on antioxidant, anti-inflammatory, and bone outcomes in osteoclast and osteoblast cell lines1
| In vitro model | Prune product | Dose | Method | Antioxidant, anti-inflammatory, or bone outcomes2 | Reference |
|---|---|---|---|---|---|
| RAW 264.7 cells | DPPE (Prunus domestica) | 0, 1000 μg/mL | Concurrent treatment with DPPE and FeSO4 (100 μg/mL) + H2O2 (1000 μg/mL) for 4 h | ↓MDA secretion | (128) |
| RAW 264.7 cells | DPPE (Prunus domestica) | 0, 10, 20, 30 μg/mL | Pretreatment with DPPE for 2 h followed by LPS (10 ng/mL) for 16 h | ↓NO secretion (dose-dependent)↓iNOS protein expression | (126) |
| RAW 264.7 cells | DPPE (Prunus domestica) | 0, 0.1, 1, 10, 100, 1000 μg/mL | Concurrent treatment with DPPE and LPS (1 μg/mL) for 12 h | ↓NO secretion (with 1000 μg/mL DPPE) | (128) |
| RAW 264.7 cells | DPPE (Prunus domestica) | 0, 10, 20, 30 μg/mL | Pretreatment with DPPE for 2 h followed by LPS (10 ng/mL) for 16 h | ↓COX-2 protein expression | (126) |
| RAW 264.7 cells | DPPE (Prunus domestica) | 0, 100, 1000 μg/mL | Concurrent treatment with DPPE and LPS (10 ng/mL) for 6 h | ↓COX-2 protein expression | (128) |
| Bone marrow-derived macrophages (C57BL/6 mice) | Neochlorogenic acid | 10 μM | Pretreatment with neochlorogenic acid for 1 h followed by TNF-α (10 ng/mL) for 0, 5, 15, 30, and 60 min | ↓NF-κB activation | (130) |
| RAW 264.7 cells | DPPE (Prunus domestica) | 0, 10, 20, 30 μg/mL | Cells stimulated with RANKL (30 ng/mL) for 4 d and then treated with DPPE for 2 h followed by LPS (10 ng/mL) for 24 h | ↓TNF-α secretion (dose-dependent) | (126) |
| Human synovial fibroblasts | Neochlorogenic acid | 10 μM | Pretreatment with neochlorogenic acid for 1 h followed by TNF-α (10 ng/mL) for 2 d | ↓TNF-α, IL-1β↓MCP-1, MIP-1α↓MMP-1, MMP-3 | (130) |
| RAW 264.7 cells | DPPE (Prunus domestica) | 0, 10, 20, 30 μg/mL | Pretreatment with DPPE for 2 h followed by LPS (10 ng/mL) for 4 h | ↓Tlr2 gene expression (with 30 μg/mL DPPE) | (126) |
| RAW 264.7 cells | DPPE (Prunus domestica) | 0, 10, 20, 30 μg/mL | Cells stimulated with RANKL (30 ng/mL) for 4 d, then treated with DPPE for 2 h followed by LPS (10 ng/mL) for 24 h | ↓Nfatc1 gene expression↓TRAP+ osteoclast number and size↓resorptive pit area | (126) |
| Primary bone marrow cells (C57BL/6 mice) | DPPF (Prunus domestica) | 0, 1, or 10 μg/mL | Cells stimulated with RANKL (50 ng/mL) for 4 d, then treated with DPPF and: TNF-α (1 ng/mL) for 1 h | ↓Nfatc1, Traf6, Sirpb1 gene expression | (129) |
| TNF-α (1 ng/mL) for 30 min–1 h | ↓p38, Erk1/2 protein expression | ||||
| TNF-α (1 ng/mL) for 1–7 d | ↓TRAP+ osteoclast number↓resorptive pit area | ||||
| Osteoclast-osteoblast co-cultures (C57BL/6 mice) | DPPF (Prunus domestica) | 0 or 10 μg/mL | Osteoclast-osteoblast co-cultures treated with DPPF and: TNF-α (1 ng/mL) for 6 d; TNF-α (1 ng/mL) for 10 d | ↓Rankl, Nfatc1, cFos gene expression↓TRAP+ osteoclast number | (129) |
| MC3T3-E1 cells | DPPE (Prunus domestica) | 0, 2.5, 5, 10, and 20 μg/mL | Pretreatment with DPPE for 24 h followed by: TNF-α (1 ng/mL) for 18 h; TNF-α (1 ng/mL) for 7–28 d | ↑Runx2, Osterix, Igf1, Lysyl oxidase gene expression↓Rankl gene expression↑ALP activity↑number and size of mineralized nodules | (127) |
| Primary osteoblast cells (C57BL/6 mice) | DPPF (Prunus domestica) | 10 μg/mL | Concurrent treatment with DPPF and TNF-α (1 ng/mL) for 14 d | ↑mineralized nodule formation | (131) |
| Primary osteoblast cells (C57BL/6 mice) | DPPF (Prunus domestica) | 10 μg/mL | Concurrent treatment with DPPF and TNF-α (1 ng/mL) for 1 h | ↔Bmp2, Runx2, Tak1, Smad1, Smad5 gene expression | (131) |
ALP, alkaline phosphatase; COX, cyclooxygenase; DPPE, dried plum polyphenol extracts; DPPF, dried plum polyphenol fractions; Erk, extracellular regulated kinase; Igf, insulin-like growth factor; iNOS, inducible NO synthase; MCP-1, monocyte chemoattractant protein 1; MDA, malondialdehyde; MIP, macrophage inflammatory protein; MMP, matrix metalloproteinases; Nfatc1, nuclear factor of activated T cells, cytoplasmic 1; RANKL, receptor activator of NF-κB ligand; Runx2, runt-related transcription factor; Sirpb1, signaling regulatory protein b1; Tak1, transforming growth factor-beta-activated kinase 1; Tlr, Toll-like receptor; Traf, TNF receptor–associated factor; TRAP, tartrate-resistant acid phosphatase; ↑, significant increase; ↓, significant decrease; ↔, no change.
Bone outcomes under an inflammatory stimulus (LPS or TNF-α).
A significant reduction in cyclooxygenase 2 (COX-2) protein expression was reported in 2 studies following pretreatment or concurrent treatment of RAW 264.7 cells with varying concentrations of prune polyphenol extracts (10-1000 μg/mL) (126, 128). A reduction in inflammatory cytokine production from either RANKL and TNF-α–stimulated RAW 264.7 cells or TNF-α–stimulated human synovial fibroblasts following treatment with prune polyphenol extracts (10–30 μg/mL) (126) or neochlorogenic acid (10 μM) (130), respectively, was also reported. One study found that pretreatment of TNF-α–stimulated bone marrow–derived macrophages with neochlorogenic acid (10 mM) reduced NF-κB activation (130). Another single study reported a reduction in Toll-like receptor 2 (Tlr2) gene expression in LPS-stimulated RAW 264.7 cells following pretreatment with prune phenolic extracts (30 μg/mL) (126).
Four studies explored the effect of dried plum phenolic fractions or extracts on bone-related outcomes using either bone marrow–derived macrophages, osteoclast-osteoblast co-cultures, RAW 264.7 cells, or the murine pre-osteoblast cell line MC3T3-E1, which were treated with inflammatory stimuli (RANKL and TNF-α or TNF-α alone; Table 1). A reduction in Nfatc1 gene expression, TRAP+ osteoclast number, and resorptive pit area following treatment with dried plum phenolic fractions or extracts (126, 129) was reported in 2 studies, suggesting that dried plum phenolic fractions or extracts can reduce markers associated with osteoclast differentiation and function. Two studies using MC3T3-E1 cells or primary osteoblast cells demonstrated that dried plum phenolic fractions or extracts increase the formation of mineralized nodules (127, 131), and one of these reported an increase in gene expression of modulators of osteoblastogenesis (Runx2, Osterix, Igf1) (127). Several polyphenolic fractions upregulate osteoblast activity by enhancing BMP signaling, and TNF-α–mediated inhibition of this cascade results in suppression of the osteogenic response (131). Furthermore, treatment of MC3T3-E1 cells with human serum collected from healthy women 1 to 2 h following prune consumption increases alkaline phosphatase (ALP) activity and gene expression of Runx2, connexin 43, and beta-catenin (132). Overall, these results suggest that the phenolic compounds in prunes may mediate the antioxidant and anti-inflammatory effects observed in osteoclasts and osteoblasts, and that the osteoprotective effects of prune polyphenols are mediated both via attenuation of osteoclastogenesis and augmentation of osteoblastogenesis.
Preclinical findings
The OVX rat model of osteoporosis is established as a suitable model for assessing bone loss due to ovarian hormone deficiency as it mimics the pattern of rapid bone loss followed by a period of more gradual bone loss observed in postmenopausal women (133). Both OVX rats and postmenopausal women respond similarly to antiresorptive and anabolic agents such as bisphosphonates, estrogen, SERMs, parathyroid hormone, calcium, vitamin D, and exercise (125). These similarities justify the use of the OVX rat model to evaluate the effects of prune consumption on bone health.
The effects of prunes on measures of bone metabolism have been widely studied in both rat and mouse models of bone loss (134–144) supplemented with varying doses of prunes, ranging from 5% to 25% wt:wt. To better understand the relation between prunes and attainment of peak bone mass, alterations in osteoclast and osteoblast precursor cells in both growing (1- to 2-mo-old) and skeletally mature (6-mo-old) C57BL/6 male mice were evaluated following prune consumption (142). The number of ALP+ osteoblast precursors increased in young growing mice after prune supplementation (5, 15, or 25% wt:wt). In adult mice, prune supplementation (25% wt:wt) increased bone volume, which was associated with decrease in osteoclast surface, serum CTX, and multinucleated TRAP+ osteoclast precursors. Although the number of ALP+ osteoblast precursors and serum procollagen type I N propeptide did not change with prune supplementation in adult mice, surface-based bone formation rate decreased, suggesting that increased bone volume in adult mice might be attributed to diminished bone resorption rather than bone formation. These findings demonstrate that prunes increase peak bone mass during growth and in adulthood (142). Short-term prune supplementation (5, 15, or 25% wt:wt) in an adult osteopenic OVX rat model not only restored the gene expression of osteoblast differentiation factors such as Bmp4 but also suppressed the gene expression of osteoclast differentiation factors such as Nfatc1 (136). Additionally, in comparison to other dried fruits, prunes (25% wt:wt) exerted a unique effect on bone by downregulating osteoclast differentiation in conjunction with upregulating osteoblast and glutathione activity in an osteopenic OVX mouse model (141). Combined, these data suggest that prunes may improve bone metabolism and biochemical properties in aging and reproductive hormone–deficient animal models.
In addition to improving measures of bone metabolism, prunes modulate bone structural properties. Overall, preclinical evidence suggests that higher doses of prune supplementation (15% and 25% wt:wt) prevent bone loss by improving bone strength, indicated by an increase in BMD of the femur and/or spine. Notably, Smith and colleagues demonstrated in both female (136) and male (137) rodent models of osteopenia that prune supplementation (25% wt:wt) improves BMD at the whole body, spine, and femur and confers anabolic effects on the trabecular bone by improving trabecular volume, number, and thickness at the lumbar and distal femur. Prunes also showed osteoprotective effects in a rat model of male osteoporosis, where 25% wt:wt supplementation increased vertebral and femoral BMD, vertebral trabecular bone volume, and cortical thickness (134). In a mouse model of age-related osteoporosis, prune supplementation (25% wt:wt) improved bone volume and restored bone loss due to aging in male mice (140). Overall, animal studies using male and female rodent models of osteopenia or osteoporosis demonstrate that dietary supplementation with prunes confers osteoprotective effects by not only preventing bone loss associated with age and estrogen deficiency but also by reversing pre-existing bone loss as a consequence of these conditions (134–147).
Prunes have also shown promising osteoprotective effects in other preclinical disease models of bone loss. Cancer patients undergoing radiotherapy, radiation workers, and astronauts are examples of populations who are at a higher risk for bone loss due to exposure to ionizing radiation. Prune supplementation (25% wt:wt) in mouse models of radiation-induced bone loss completely prevented cancellous bone loss, accompanied by reduced gene expression of pro-osteoclastogenic cytokines Rankl, Mcp1, and Tnfa (148). Prune supplementation ameliorated simulated spaceflight-induced damage in bone micro-architecture and mechanical properties, providing evidence of the use of prune supplementation as a potential strategy to mitigate bone loss induced by radiation exposure and microgravity. In addition, 25% prune supplementation attenuated spinal cord injury–induced bone loss in a mouse model, suggesting the beneficial effect of prunes on bone outcomes may be widely applicable.
Several studies have assessed the effect of bioactive components in prunes on bone outcomes in rat models of postmenopausal bone loss to determine the mechanisms underlying the protective effect of prune consumption. Prunes contain significant amounts of chlorogenic acids, which may confer beneficial effects on bone (45). However, a diet supplemented with prunes with a high chlorogenic acid content was not more effective at preventing bone loss than a diet supplemented with low chlorogenic acid containing prunes in an OVX rat model (149). These data suggest that a dose-dependent effect of chlorogenic acid on bone loss may not exist (149) and/or other bioactive components in the whole fruit might contribute to the osteoprotective effects of prunes. In an aged, osteopenic OVX rat model, prune polyphenols accounted for 60–80% of the anabolic effect of prunes on bone. However, when the polyphenolic extract was combined with vitamin K and potassium, the reversal of bone loss was equivalent to that observed with consumption of the whole fruit (150). These findings suggest that numerous bioactive compounds in prunes may be contributing their beneficial effect on bone.
A growing body of evidence suggests that prunes and their polyphenols also modulate inflammatory pathways and oxidative stress. Four preclinical studies (142, 148, 151, 152) have investigated the effect of prunes on modulating inflammatory and oxidative stress markers, findings of which have been summarized in Table 2. One study demonstrated that prune supplementation (5% or 10% wt:wt) for 8 wk significantly decreased markers of oxidative damage [serum malondialdehyde and advanced glycation end product (AGE)] and increased protein expression of nuclear factor, erythroid 2–like 2 (NRF2) and downstream antioxidant enzymes [catalase (CAT), superoxide dismutase (SOD)-2, and glutathione peroxidase (GPX)] in 12-wk-old male BALB/c mice (152). Prunes also exhibited immunomodulatory effects in an ovarian hormone deficiency–induced model of osteoporosis. Prune supplementation (15% or 25% wt:wt) for 4 wk increased peripheral blood leukocytes, CD31−Ly-6C+ granulocytes, and CD115+ committed monocytes but decreased bone marrow lymphoblasts in 12-wk-old OVX female C57BL/6J mice (151). Four preclinical studies in mouse models of aging and bone loss (148, 151–153) reported that prune supplementation (5–25% wt:wt for 1–8 wk) suppressed NF-κB expression and production of inflammatory cytokines (IL-1α, IL-1β, IL-10, IL-12-p70, IL-13, IL-17, TNF-α, and MCP-1). Prune supplementation (20% wt:wt) also restored bone loss and is associated with fewer TRAP + cells, indicating downregulation of osteoclastogenesis in a TNF-α-dependent rodent model of arthritis (130). Combined, these findings suggest prune supplementation may be modulating inflammatory and oxidative pathways.
TABLE 2.
Effect of prunes (dried plum) supplementation on antioxidant and anti-inflammatory outcomes in preclinical studies1
| Animal model | Intervention | Study groups | Antioxidant or anti-inflammatory effect | Reference |
|---|---|---|---|---|
| Aged male BALB/c mice (12-wk-old, n = 35) | 5% or 10% wt:wt DP for 8 wk | 5% or 10% wt:wt DP compared with ND5% wt:wt DP compared with ND5% or 10% wt:wt DP compared with ND5% or 10% wt:wt DP compared with ND5% wt:wt DP compared with ND | ↓Serum MDA↓Serum AGE↑NRF2 protein expression↓KEAP-1 protein expression↑CAT, SOD2 protein expression↑GPX protein expression | (152) |
| OVX female C57BL/6J mice (12-wk-old, n = 59) | 5%, 15%, or 25% wt:wt DP for 4 wk | 5%, 15%, or 25% wt:wt DP compared with ND15% or 25% wt:wt DP compared with ND15% wt:wt DP compared with ND | ↑Peripheral leukocyte count↑CD31− Ly-6C+granulocytes, CD115+ committed monocytes↓Bone marrow lymphoblasts | (151) |
| Aged male BALB/c mice (12-wk-old, n = 35) | 5% or 10% w/w DP for 8 weeks | 5% or 10% wt:wt DP compared with ND | ↓NF-κB protein expression in the liver | (152) |
| OVX female C57BL/6J mice (12-wk-old, n = 59) | 5%, 15%, or 25% wt:wt DP for 4 wk | Splenocytes stimulated with Con-A (2.5 μg/mL) for 48 h15% or 25% wt:wt DP compared with ND | ↓TNF-α secretion | (151) |
| Male C57BL/6 mice: skeletally mature (6-mo-old, n = 15/group) | 25% wt:wt DP for 4 wk | 25% wt:wt DP compared with ND | ↓Serum IL-1α, IL-1β, IL-10, IL-12 (p70), IL-13, IL-17, TNF-α, MCP-1 | (142) |
| Male C57BL/6J mice with radiation-induced bone loss (16-wk-old, n = 5–10/group) | 25% wt:wt DP for 7–21 d | 25% wt:wt DP compared with ND | ↓Tnfa, Mcp1 gene expression in bone marrow cells | (148) |
AGE, advanced glycation end products; CAT, catalase; Con-A, concanavalin A; DP, dried plum; GPX, glutathione peroxidase; KEAP-1, Kelch-like ECH associated protein 1; MCP-1, monocyte chemoattractant protein 1; MDA, malondialdehyde; ND, normal diet; NRF2, nuclear factor, erythroid 2–like 2; OVX, ovariectomized/ovariectomy; SOD, superoxide dismutase; ↑, significant increase; ↓, significant decrease.
A relation between the changes in gut microbiota and overt alterations in bone mass has been demonstrated in numerous preclinical studies (154–159). However, the mechanisms underlying this relation are unknown. In young, female germ-free (GF) mice, femoral bone volume fraction was 39% greater and was associated with a lower number of osteoclasts and reduced gene expression of Il6 and Tnfa in bone, likely contributing to reduced bone resorption compared with conventionally raised mice (154). Greater femoral cortical volume and cortical thickness were also observed in female GF mice compared with conventionally raised mice (156). Short-term colonization of 2-mo-old male and female GF mice with commensal bacteria reduced bone mass; however, long-term colonization of GF mice (i.e., 8 mo) resulted in increased femur length and bone mass compared with GF counterparts (155). Yan et al. (155) demonstrated that microbiota-regulated bone growth may be mediated by SCFA-induced modulation of IGF-1, a bone trophic hormone. Broad-spectrum antibiotic treatment depleted the microbiota and reduced SCFAs, as well as serum IGF-1, to approximately half of the concentrations observed in the vehicle-treated control. However, when SCFA-supplemented water was provided, serum IGF-1 increased by approximately 50% and bone mass was comparable to control mice (155). Furthermore, OVX in GF mice did not result in increased osteoclastogenic cytokine production, bone resorption, and trabecular bone loss, demonstrating that the gut microbiota plays a key role in OVX-induced bone loss (156). Together, these preclinical studies provide evidence that the gut microbiota influences bone outcomes.
In summary, there is sufficient preclinical evidence to suggest that the osteoprotective effects of prune consumption are due to a decrease in the rate of bone turnover, where resorption is downregulated more than formation. The dietary levels of prunes in some in vivo models are high—for example, 25% by weight, which is equivalent to approximately 20 prunes daily for a 2000-kcal diet in humans (142). Although the high doses of prunes used in animal models may not represent ideal quantities for human consumption, these preclinical findings suggest that the osteoprotective effects of prunes in animal models of osteopenia may be partly mediated by anti-inflammatory and antioxidative pathways.
Clinical Findings
Postmenopausal women represent a clinically relevant population in which bone health is a primary concern. As such, several investigators have administered prunes to women to test their osteoprotective effects by evaluating if prune consumption alters bone biomarkers and prevents bone loss over time. To date, the results from 4 RCTs (40–43) and 1 case study (44) investigating the effects of prune consumption on bone health outcomes in postmenopausal women indicate promising effects on bone turnover (40) and BMD (41, 42, 44). Prune consumption at a dose of 100 g/d for 3 mo significantly increased the serum concentrations of bone-specific alkaline phosphatase (BSAP) by 5.8% and IGF-1 by 17% in postmenopausal women who were not receiving any hormone replacement therapy (40). Additionally, prune consumption at the same dose of 100 g/d for 1 year improved BMD of the ulna and lumbar spine in comparison to the control (75 g/d dried apple) (41, 43), and decreased serum markers of bone turnover, BSAP, TRAP-5b, and osteocalcin (41). Prune consumption at 50 g/d and 100 g/d for 6 mo prevented loss of total BMD, but not ulnar BMD (reported as change from baseline), and decreased TRAP-5b compared with women consuming 0 g/d of prunes (n = 16/group) (42). In a recent case study describing changes in BMD over the course of 28 mo, a postmenopausal osteopenic woman participated in an ongoing RCT as a control participant (1200 mg calcium carbonate and 800 IU vitamin D3 daily for 12 mo) and then volitionally consumed 50 g prunes daily in addition to calcium and vitamin D3 for an additional 16 mo (44). During participation in the RCT, the participant experienced a 7.6% decrease in lumbar spine BMD; however, from month 12 to 28, which included voluntary prune consumption, there was a notable improvement in lumbar spine BMD (7.8%) while preventing further decline in total body and total hip BMD. Together, these results suggest that 50 g/d of prunes may be an effective dose to improve bone health in postmenopausal women, with longer duration of consumption (42, 44). However, data from the 6-mo RCT and case study should be interpreted with caution until replicated due to the limited sample size. Overall, the clinical findings to date suggest that prune consumption may be effective in improving bone outcomes in postmenopausal women, possibly by enhancing bone formation and reducing bone resorption.
In total, 3 clinical studies (41, 42, 160) have assessed the effect of prune consumption on markers of oxidative stress or inflammation in postmenopausal women, and findings are summarized in Table 3. One study (160) reported a significant increase in plasma total antioxidant capacity and plasma antioxidant enzyme activity of SOD in postmenopausal women after 6 mo of consuming 50 or 100 g/d prunes compared with baseline; however, no effects were observed on plasma CAT and GPX activities. Two studies (41, 42) investigated the effect of prune consumption in postmenopausal women with osteopenia on serum C-reactive protein (CRP), a widely used serum marker of systemic inflammation. One study reported a significant reduction in CRP in postmenopausal women after 3 mo of consuming 100 g/d of prunes compared with the control group consuming 75 g/d of dried apple; however, no difference in CRP between the prune- versus dried apple– consuming group was observed after 6 and 12 mo on the trial (41). The lack of difference in CRP concentrations at 6 and 12 mo in the prune group and dried apple control group was due to a reduction in CRP in the dried apple–consuming control group, which likely resulted because apples also contain phenolic compounds (161). The second study measuring CRP reported no effect of 50 g/d or 100 g/d of prune consumption on serum CRP at 3 and 6 mo compared with of 0 g/d of prune consumption (42). Although serum CRP is a widely used marker of inflammation, it may not be modulated by prune consumption. One study (160) investigated the effect of 50 g/d or 100 g/d prune consumption for 6 mo on additional markers of inflammation in the same study population as reported in Hooshmand et al. (42). Low-dose (50 g/d) prune consumption significantly decreased plasma proinflammatory cytokines IL-6 and TNF-α compared with baseline. Overall, these 3 clinical trials provide preliminary evidence suggesting potential antioxidant and anti-inflammatory effects of prune consumption in postmenopausal women with bone loss, but results vary depending on what oxidative or inflammatory marker is assessed.
TABLE 3.
Effect of prunes (dried plum) supplementation on antioxidant and anti-inflammatory outcomes in clinical studies1
| Study design | Study population | Intervention2 | Control2 | Duration of intervention | Antioxidant or anti-inflammatory effect | Reference |
|---|---|---|---|---|---|---|
| RCT, parallel arm | Postmenopausal women (n = 40, completed) | 50 g/d DP consumption (n = 14, age = 68.5 y)100 g/d DP consumption (n = 13, age = 70.4 y) | 0 g/d DP consumption (n = 13, age = 71 y) | 6 mo | ↓Plasma TBARS in 50-g/d and 100-g/d DP groups↑Plasma TAC in 50-g/d DP group↑Plasma SOD activity in 50-g/d and 100-g/d DP groups↑Plasma GST activity in 50-g/d DP group↔Plasma CAT and GPX activities | (160) |
| RCT, parallel arm | Postmenopausal women with osteopenia (n = 100, completed) | 100 g/d DP consumption (n = 55, age = 57.5 y) | 75 g/d dried apple consumption (n = 45, age = 55.6 y) | 3, 6, and 12 mo | ↓Serum CRP at 3 mo in DP group↔Serum CRP at 6 or 12 mo | (41) |
| RCT, parallel arm | Postmenopausal women with osteopenia (n = 42, completed) | 50 g/d DP consumption (n = 16, age = 68.5 y)100 g/d DP consumption (n = 13, age = 70.4 y) | 0 g/d DP consumption (n = 13, age = 71 y) | 6 mo | ↔Serum CRP | (42) |
| RCT, parallel arm | Postmenopausal women (n = 40, completed) | 50 g/d DP consumption (n = 14, age = 68.5 y)100 g/d DP consumption (n = 13, age = 70.4 y) | 0 g/d DP consumption (n = 13, age = 71 y) | 6 mo | ↓Plasma IL-6, TNF-α in 50-g/d DP group | (160) |
CAT, catalase; CRP, C-reactive protein; DP, dried plum; GPX, glutathione peroxidase; GST, glutathione S transferase; RCT, randomized controlled trial; SOD, superoxide dismutase; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances; ↑, significant increase; ↓, significant decrease; ↔, no change.
Includes 500 mg calcium + 400 IU (10 mg) vitamin D3. Ages are means.
There are limited data in humans on prune consumption and changes in the gut microbiota, as well as immune and bone health alterations. To date, 3 clinical studies (162–164) have investigated the effect of the gut microbiota on bone health in postmenopausal women. These studies demonstrate that postmenopausal women with low BMD have altered diversity and composition of the gut microbiota, with a higher abundance of Bacteroides (164). Plasma markers of gut barrier dysfunction, including LPS-binding protein and soluble CD14, increase during the menopausal transition and are associated with lower BMD. These data suggest that greater gut permeability may associated with elevated inflammatory mediators, which may contribute to postmenopausal bone loss (162). Additionally, reduced BMD in older adults with osteopenia and osteoporosis is associated with an altered microbiota (165–167). In the only study published to date examining prune consumption on gut microbiota, 120 healthy males and females were randomly assigned to 1 of 3 arms—control (no prunes), 80 g/d of prunes, or 120 g/d of prunes (n = 40/group)—for 9 wk (168). Changes in stool characteristics (i.e., stool weight and frequency) were reported, but no effect of prune consumption on gut bacterial composition, SCFA concentration, or stool pH were observed (168).
Overall, evidence from in vitro, preclinical studies, and limited clinical studies suggests the potential role of prunes in ameliorating bone loss. These findings may be attributed to altered bone turnover and by inhibiting inflammation-induced NF-κB and NFATc1 signaling and their downstream expression of cytokines in osteoclast precursors, attenuating TNF-α secretion from monocytes and T cells, and suppressing markers of oxidative stress.
Potential mechanisms and future directions
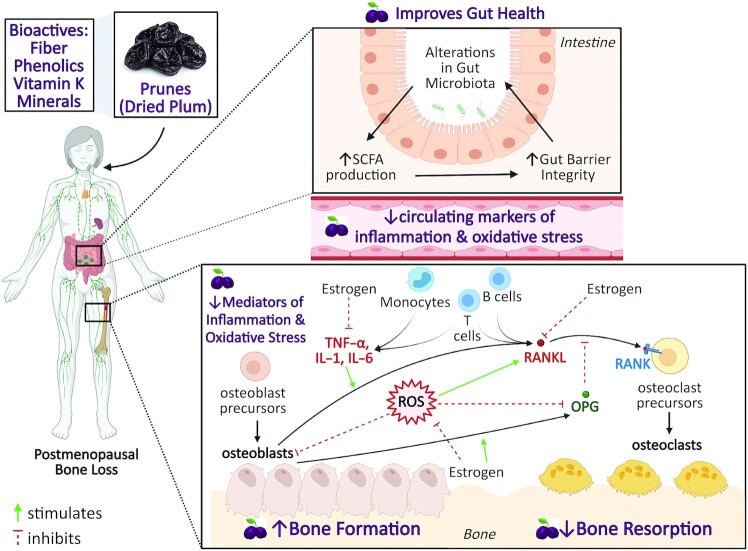
Potential mechanisms underlying the protective effects of prunes on bone health have been described by Arjmandi et al. (39), suggesting that the components of prunes, including minerals, vitamin K, phenolic compounds, and dietary fiber, might work additively or synergistically to mediate beneficial effects. Polyphenol-rich extracts have been shown to promote the growth of commensal bacteria (52). Dietary fiber and phenolic compounds may contribute to the potential anti-inflammatory properties of prunes by modulating the gut microbiota (169). These changes may include shifts in the microbiota composition and increased SCFA production upon microbial fermentation of dietary fiber. SCFAs have the potential to modulate host immune response and promote gut barrier integrity (52, 170). Reduction in chronic inflammation can potentially reduce the differentiation and activation of osteoclasts and prevent bone loss. Therefore, any potential anti-inflammatory effect of prune consumption may mediate, at least in part, the beneficial effect of prune on bone outcomes. Proposed mechanisms underlying the osteoprotective effects of prunes via changes in inflammatory and oxidative stress mediators are outlined in Figure 3. Prune consumption may lead to changes in the gut microbiota due to the fiber and phenolic content, promoting increased SCFA production, which decreases colonic inflammation and improves gut integrity (39, 170). This may lower secretion of proinflammatory cytokines (IL-1, IL-6, TNF-α) and oxidative damage markers from immune cells in the lamina propria and in circulation (169). Overall, reduction in mediators of inflammation (IL-1, IL-6, TNF-α) and oxidative stress (ROS) contributes to enhanced bone formation and reduced bone resorption, ameliorating bone loss. A substantial body of literature demonstrates the role of the gut–bone axis in the pathogenesis of osteoporosis, suggesting that dietary phytochemicals with osteoprotective properties improve the gut barrier integrity and favorably modulate the gut microbiota and mucosal immune cells (171). For example, prune consumption in healthy individuals significantly increases the growth of Bifidobacteria, a genus of bacteria generally thought to provide beneficial effects to the host (168). However, further studies are needed to determine whether prunes exert prebiotic potential to positively modulate the host gut microbiota and reduce inflammation, and whether these changes occur following prune interventions of longer duration. In addition to prunes, there is emerging evidence on the osteoprotective effects of other polyphenol-rich fruits such as tart cherry (172–175), blueberry (176–178), and watermelon (179), which may mitigate bone loss via similar biological mechanisms. The biological mechanisms underlying the potential effect of prunes on inflammatory and immune mediators require further investigation.
FIGURE 3.
Proposed mechanisms linking prune consumption to improved bone health via changes in inflammatory mediators, oxidative stress, and the gut microbiome. Prunes are rich in minerals, vitamin K, phenolic compounds, and dietary fiber, all of which might work additively or synergistically to mediate beneficial effects on bone (39). Dietary fiber and polyphenolic compounds found in prunes may alter the composition of the gut microbiota (169), increasing SCFA production and promoting increased gut barrier integrity. These changes in the gut may be associated with decreased secretion of proinflammatory cytokines (IL-1, IL-6, TNF-α) and oxidative damage markers from immune cells in the lamina propria and in the periphery (169). Preclinical evidence supports the role of prune polyphenols in enhancing osteoblast function and suppressing osteoclast function. It is hypothesized that reductions in markers of inflammation and oxidative stress are linked to improvements in bone outcomes. OPG, osteoprotegerin; RANK, receptor activator of NF-κB; RANKL, receptor activator of NF-κB ligand; ROS, reactive oxygen species.
Clinical trials assessing the effect of dietary interventions on inflammatory responses frequently measure plasma or serum cytokines; however, measurement of these circulating inflammatory markers alone might not adequately capture the immunomodulatory effects of nutritional interventions. We have demonstrated that in vitro stimulation of PBMCs with LPS, which mimics in vivo activation, may be an additional endpoint to include in human intervention studies to assess the inflammatory response following a dietary intervention (180, 181). Therefore, additional measurements of proinflammatory mediators, including serum cytokines and/or cytokines secreted from stimulated PBMCs collected from postmenopausal women following prune consumption, might provide a more comprehensive understanding of the effect of prunes on inflammatory outcomes in humans.
In addition to exploring the mechanistic role of prunes on bone health, it is important to determine the clinical feasibility of prunes as a nutritional intervention by addressing questions such as whether the osteoprotective effects of prunes are long-lasting and if they are associated with clinical endpoints such as reduction in fractures. Further studies are needed to determine the optimal dosage and duration associated with minimal adverse effects and to develop strategies for obtaining maximum compliance. Additional large-scale RCTs are needed to enhance the generalizability of these findings among postmenopausal women, as well as other target populations such as osteopenic men. Furthermore, investigating whether the osteoprotective effects of prunes are mediated through anti-inflammatory, antioxidant, and/or immunomodulatory mechanisms and measuring phenolic compounds in the plasma or urine after long-term prune consumption in conjunction with inflammatory mediators and fecal microbial populations are critical next steps. There is an emerging but poorly understood link between gut microbiota, inflammatory mediators, and bone health. Future mechanistic studies are warranted to advance our understanding of the complexities of these relations.
ACKNOWLEDGEMENTS
Images in the review were created with BioRender.com. The authors’ responsibilities were as follows—MJDS and CJR: designed the research; JJD, HLVE, and NCAS: conducted the research and collected data; JJD, HLVE, NCAS, MJDS, and CJR: participated in data analysis and interpretation and wrote the manuscript; CJR: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
Supported by the California Prune Board provided funding to MJDS and CJR. Publication funds came from the Hershey Company endowment, Department of Nutritional Sciences, Penn State University. California Dried Plum Board (grant no. 100804).
Author disclosures: CJR is member of the Nutrition Advisory Panel for the California Dried Plum Board. The other authors report no conflicts of interest.
Abbreviations used: AGE, advanced glycation end product; ALP, alkaline phosphatase; BMD, bone mineral density; BMP, bone morphogenic protein; BSAP, bone-specific alkaline phosphatase; CAT, catalase; CRP, C-reactive protein; CTX, carboxyl-terminal telopeptide of type 1 collagen; GF, germ-free; GPX, glutathione peroxidase; HFD, high-fat-diet; IBD, inflammatory bowel disease; iNOS, inducible NO synthase; MCP, monocyte chemoattractant protein; NFATc1, nuclear factor of activated T cells, cytoplasmic 1; OPG, osteoprotegerin; OVX, ovariectomized/ovariectomy; PBMC, peripheral blood mononuclear cell; RANK, receptor activator of NF-κB; RANKL, receptor activator of NF-κB ligand; RCT, randomized controlled trial; ROS, reactive oxygen species; RUNX, runt-related transcription factor; SERM, selective estrogen receptor modulator; SOD, superoxide dismutase; TRAF, TNF receptor–associated factor.
Contributor Information
Janhavi J Damani, Intercollege Graduate Degree Program in Integrative and Biomedical Physiology, Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA, USA.
Mary Jane De Souza, Department of Kinesiology, The Pennsylvania State University, University Park, PA, USA.
Hannah L VanEvery, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA.
Nicole C A Strock, Department of Kinesiology, The Pennsylvania State University, University Park, PA, USA.
Connie J Rogers, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA; Center for Molecular Immunology and Infectious Disease, Huck Institutes of the Life Sciences, The Pennsylvania State University, University Park, PA, USA.
References
- 1. NIH Consensus Development Panel on Osteoporosis Prevention Diagnosis, and Therapy . Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285(6):785–95. [DOI] [PubMed] [Google Scholar]
- 2. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–33. [DOI] [PubMed] [Google Scholar]
- 3. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, Dawson-Hughes B. The recent prevalence of osteoporosis and low bone mass in the united states based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29(11):2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–75. [DOI] [PubMed] [Google Scholar]
- 5. Pavone V, Testa G, Giardina SMC, Vescio A, Restivo DA, Sessa G. Pharmacological therapy of osteoporosis: a systematic current review of literature. Front Pharmacol. 2017;8:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barrionuevo P, Kapoor E, Asi N, Alahdab F, Mohammed K, Benkhadra K, Almasri J, Farah W, Sarigianni M, Muthusamy Ket al. Efficacy of pharmacological therapies for the prevention of fractures in postmenopausal women: a network meta-analysis. J Clin Endocrinol Metab. 2019;104(5):1623–30. [DOI] [PubMed] [Google Scholar]
- 7. Burger H. Hormone replacement therapy in the post-Women's Health Initiative era. Report of a meeting held in Funchal, Madeira, February 24-25, 2003. Climacteric. 2003;6(Suppl 1):11–36. [PubMed] [Google Scholar]
- 8. Tabatabaei-Malazy O, Salari P, Khashayar P, Larijani B. New horizons in treatment of osteoporosis. Daru. 2017;25(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khosla S. Pathogenesis of age-related bone loss in humans. J Gerontol A Biol Sci Med Sci. 2013;68(10):1226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ginaldi L, Di Benedetto MC, De Martinis M. Osteoporosis, inflammation and ageing. Immunity Ageing. 2005;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cenci S, Toraldo G, Weitzmann MN, Roggia C, Gao Y, Qian WP, Sierra O, Pacifici R. Estrogen deficiency induces bone loss by increasing t cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc Natl Acad Sci. 2003;100(18):10405–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11(8):1043–51. [DOI] [PubMed] [Google Scholar]
- 13. Sapir-Koren R, Livshits G. Postmenopausal osteoporosis in rheumatoid arthritis: the estrogen deficiency-immune mechanisms link. Bone. 2017;103:102–15. [DOI] [PubMed] [Google Scholar]
- 14. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 15. Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim S, Jazwinski SM. The gut microbiota and healthy aging: a mini-review. Gerontology. 2018;64(6):513–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol. 2018;11(1):1–10. [DOI] [PubMed] [Google Scholar]
- 19. Yang T, Richards EM, Pepine CJ, Raizada MK. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol. 2018;14(7):442–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maeda Y, Takeda K. Role of gut microbiota in rheumatoid arthritis. J Clin Med. 2017;6(6):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D'Amelio P, Sassi F. Gut microbiota, immune system, and bone. Calcif Tissue Int. 2018;102(4):415–25. [DOI] [PubMed] [Google Scholar]
- 22. Yatsonsky D, Pan K, Shendge VB, Liu JY, Ebraheim NA. Linkage of microbiota and osteoporosis: a mini literature review. World J Orthop. 2019;10(3):123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28(1):623–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rauner M, Sipos W, Pietschmann P. Osteoimmunology. Int Arch Allergy Immunol. 2007;143(1):31–48. [DOI] [PubMed] [Google Scholar]
- 25. Pietschmann P, Mechtcheriakova D, Meshcheryakova A, Foger-Samwald U, Ellinger I. Immunology of osteoporosis: a mini-review. Gerontology. 2016;62(2):128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weaver CM. Diet, gut microbiome, and bone health. Curr Osteoporos Rep. 2015;13(2):125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Locantore P DGV, Gelli S, Paragliola RM, Pontecorvi A. The interplay between immune system and microbiota in osteoporosis. Mediators Inflamm. 2020;2020;3686749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sansai K, Na Takuathung M, Khatsri R, Teekachunhatean S, Hanprasertpong N, Koonrungsesomboon N. Effects of isoflavone interventions on bone mineral density in postmenopausal women: a systematic review and meta-analysis of randomized controlled trials. Osteoporos Int. 2020;31(10):1853–64. [DOI] [PubMed] [Google Scholar]
- 29. Thaung Zaw JJ, Howe PRC, Wong RHX. Postmenopausal health interventions: time to move on from the Women's Health Initiative?. Ageing Res Rev. 2018;48:79–86. [DOI] [PubMed] [Google Scholar]
- 30. Medicine IO, Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Washington (DC): The National Academy Press; 2011. [Google Scholar]
- 31. Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Darling AL, Millward DJ, Lanham-New SA. Dietary protein and bone health: towards a synthesised view. Proc Nutr Soc. 2021;80(2):165–72. [DOI] [PubMed] [Google Scholar]
- 33. Wallace TC, Frankenfeld CL. Dietary protein intake above the current RDA and bone health: a systematic review and meta-analysis. J Am Coll Nutr. 2017;36(6):481–96. [DOI] [PubMed] [Google Scholar]
- 34. Shen CL, von Bergen V, Chyu MC, Jenkins MR, Mo H, Chen CH, Kwun IS. Fruits and dietary phytochemicals in bone protection. Nutr Res. 2012;32(12):897–910. [DOI] [PubMed] [Google Scholar]
- 35. Tucker KL, Chen H, Hannan MT, Cupples LA, Wilson PW, Felson D, Kiel DP. Bone mineral density and dietary patterns in older adults: the Framingham Osteoporosis Study. Am J Clin Nutr. 2002;76(1):245–52. [DOI] [PubMed] [Google Scholar]
- 36. Macdonald HM, New SA, Golden MH, Campbell MK, Reid DM. Nutritional associations with bone loss during the menopausal transition: evidence of a beneficial effect of calcium, alcohol, and fruit and vegetable nutrients and of a detrimental effect of fatty acids. Am J Clin Nutr. 2004;79(1):155–65. [DOI] [PubMed] [Google Scholar]
- 37. Kurzer MS. Phytoestrogen supplement use by women. J Nutr. 2003;133(6):1983S–6S. [DOI] [PubMed] [Google Scholar]
- 38. Wallace T. Dried plums, prunes and bone health: a comprehensive review. Nutrients. 2017;9(4):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arjmandi BH, Johnson SA, Pourafshar S, Navaei N, George KS, Hooshmand S, Chai SC, Akhavan NS. Bone-Protective effects of dried plum in postmenopausal women: efficacy and possible mechanisms. Nutrients. 2017;9(5):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Arjmandi BH, Khalil DA, Lucas EA, Georgis A, Stoecker BJ, Hardin C, Payton ME, Wild RA. Dried plums improve indices of bone formation in postmenopausal women. J Womens Health Gend Based Med. 2002;11(1):61–8. [DOI] [PubMed] [Google Scholar]
- 41. Hooshmand S, Chai SC, Saadat RL, Payton ME, Brummel-Smith K, Arjmandi BH. Comparative effects of dried plum and dried apple on bone in postmenopausal women. Br J Nutr. 2011;106(6):923–30. [DOI] [PubMed] [Google Scholar]
- 42. Hooshmand S, Kern M, Metti D, Shamloufard P, Chai SC, Johnson SA, Payton ME, Arjmandi BH. The effect of two doses of dried plum on bone density and bone biomarkers in osteopenic postmenopausal women: a randomized, controlled trial. Osteoporos Int. 2016;27(7):2271–9. [DOI] [PubMed] [Google Scholar]
- 43. Hooshmand S, Brisco JR, Arjmandi BH. The effect of dried plum on serum levels of receptor activator of NF-kappaB ligand, osteoprotegerin and sclerostin in osteopenic postmenopausal women: a randomised controlled trial. Br J Nutr. 2014;112(1):55–60. [DOI] [PubMed] [Google Scholar]
- 44. Strock NCA, Koltun KJ, Weaver CM, De Souza MJ. Dried plum consumption improves bone mineral density in osteopenic postmenopausal woman: a case report. Bone Rep. 2021;14:101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stacewicz-Sapuntzakis M. Dried plums and their products: composition and health effects—an updated review. Crit Rev Food Sci Nutr. 2013;53(12):1277–302. [DOI] [PubMed] [Google Scholar]
- 46. Treutter D, Wang D, Farag MA, Baires GD, Ruhmann S, Neumuller M. Diversity of phenolic profiles in the fruit skin of Prunus domestica plums and related species. J Agric Food Chem. 2012;60(48):12011–9. [DOI] [PubMed] [Google Scholar]
- 47. Del Caro A, Piga A, Pinna I, Fenu PM, Agabbio M. Effect of drying conditions and storage period on polyphenolic content, antioxidant capacity, and ascorbic acid of prunes. J Agric Food Chem. 2004;52(15):4780–4. [DOI] [PubMed] [Google Scholar]
- 48. Wallace TC. Dried plums, prunes and bone health: a comprehensive review. Nutrients. 2017;9(4):401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Noratto GD, Garcia-Mazcorro JF, Markel M, Martino HS, Minamoto Y, Steiner JM, Byrne D, Suchodolski JS, Mertens-Talcott SU. Carbohydrate-Free peach (Prunus persica) and plum (Prunus salicina) [corrected] juice affects fecal microbial ecology in an obese animal model. PLoS One. 2014;9(7):e101723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Voreades N, Kozil A, Weir TL. Diet and the development of the human intestinal microbiome.Front Microbiol. 2014;5:494, doi: 10.3389/fmicb.2014.00494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McCabe L, Britton RA, Parameswaran N. Prebiotic and probiotic regulation of bone health: role of the intestine and its microbiome. Curr Osteoporos Rep. 2015;13(6):363–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Anhe FF, Varin TV, Le Barz M, Desjardins Y, Levy E, Roy D, Marette A. Gut microbiota dysbiosis in obesity-linked metabolic diseases and prebiotic potential of polyphenol-rich extracts. Curr Obesity Rep. 2015;4(4):389–400. [DOI] [PubMed] [Google Scholar]
- 53. Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21(2):115–37. [DOI] [PubMed] [Google Scholar]
- 54. Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O'Karma M, Wallace TC, Zemel BS. The National Osteoporosis Foundation's position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65(12):S147–151. [DOI] [PubMed] [Google Scholar]
- 56. Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discovery. 2012;11(3):234–50. [DOI] [PubMed] [Google Scholar]
- 57. Kim SY, Schneeweiss S, Liu J, Daniel GW, Chang C-L, Garneau K, Solomon DH. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res Ther. 2010;12(4):R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hidalgo DF, Boonpheng B, Phemister J, Hidalgo J, Young M. Inflammatory bowel disease and risk. of osteoporotic fractures: a meta-analysis. Cureus. 2019;11(9):e5810, doi: 10.7759/cureus.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Suda T, Takahashi N, Martin TJ. Modulation of osteoclast differentiation. Endocr Rev. 1992;13(1):66–80. [DOI] [PubMed] [Google Scholar]
- 60. Mundy GR. Cytokines and local factors which affect osteoclast function. Int J Cell Cloning. 1992;10(4):215–22. [DOI] [PubMed] [Google Scholar]
- 61. Manolagas SC, Jilka RL. Bone marrow, cytokines, and bone remodeling. Emerging insights into the pathophysiology of osteoporosis. N Engl J Med. 1995;332(5):305–11. [DOI] [PubMed] [Google Scholar]
- 62. Cappariello A, Maurizi A, Veeriah V, Teti A. The great beauty of the osteoclast. Arch Biochem Biophys. 2014;558:70–8. [DOI] [PubMed] [Google Scholar]
- 63. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5. [DOI] [PubMed] [Google Scholar]
- 64. Guerrini MM, Takayanagi H. The immune system, bone and RANKL. Arch Biochem Biophys. 2014;561:118–23. [DOI] [PubMed] [Google Scholar]
- 65. Komori T. Regulation of osteoblast differentiation by transcription factors. J Cell Biochem. 2006;99(5):1233–9. [DOI] [PubMed] [Google Scholar]
- 66. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. [DOI] [PubMed] [Google Scholar]
- 67. Walsh MC, Choi Y. Biology of the RANKL-RANK-OPG system in immunity, bone, and beyond. Front Immunol. 2014;5:511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23(1):90–119. [DOI] [PubMed] [Google Scholar]
- 69. Khosla S, Oursler MJ, Monroe DG. Estrogen and the skeleton. Trends Endocrinol Metab. 2012;23(11):576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Streicher C, Heyny A, Andrukhova O, Haigl B, Slavic S, Schüler C, Kollmann K, Kantner I, Sexl V, Kleiter Met al. Estrogen regulates bone turnover by targeting RANKL expression in bone linin. g cells. Sci Rep. 2017;7(1):6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Manolagas SC. From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31(3):266–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Domazetovic V MG, Iantomasi T, Brandi ML, Vincenzini MT. Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab. 2017;14(2):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Maggio D, Barabani M, Pierandrei M, Polidori MC, Catani M, Mecocci P, Senin U, Pacifici R, Cherubini A. Marked decrease in plasma antioxidants in aged osteoporotic women: results of a cross-sectional study. J Clin Endocrinol Metab. 2003;88(4):1523–7. [DOI] [PubMed] [Google Scholar]
- 74. Yousefzadeh G, Larijani B, Mohammadirad A, Heshmat R, Dehghan G, Rahimi R, Abdollahi M. Determination of oxidative stress status and concentration of TGF-β1 in the blood and saliva of osteoporotic subjects. Ann NY Acad Sci. 2006;1091(1):142–50. [DOI] [PubMed] [Google Scholar]
- 75. Östman B, Michaëlsson K, Helmersson J, Byberg L, Gedeborg R, Melhus H, Basu S. Oxidative stress and bone mineral density in elderly men: antioxidant activity of alpha-tocopherol. Free Radical Biol Med. 2009;47(5):668–73. [DOI] [PubMed] [Google Scholar]
- 76. Bai X-C, Lu D, Bai J, Zheng H, Ke Z-Y, Li X-M, Luo S-Q. Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-κB. Biochem Biophys Res Commun. 2004;314(1):197–207. [DOI] [PubMed] [Google Scholar]
- 77. Lee DH, Lim B-S, Lee Y-K, Yang H-C. Effects of hydrogen peroxide (H2O2) on alkaline phosphatase activity and matrix mineralization of odontoblast and osteoblast cell lines. Cell Biol Toxicol. 2006;22(1):39–46. [DOI] [PubMed] [Google Scholar]
- 78. Romagnoli C, Marcucci G, Favilli F, Zonefrati R, Mavilia C, Galli G, Tanini A, Iantomasi T, Brandi ML, Vincenzini MT. Role of GSH/GSSG redox couple in osteogenic activity and osteoclastogenic markers of human osteoblast-like Saos-2 cells. FEBS J. 2013;280(3):867–79. [DOI] [PubMed] [Google Scholar]
- 79. Filaire E, Toumi H. Reactive oxygen species and exercise on bone metabolism: friend or enemy?. Joint Bone Spine. 2012;79(4):341–6. [DOI] [PubMed] [Google Scholar]
- 80. Fontani F, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT, Glutathione, N-acetylcysteine and lipoic acid down-regulate starvation-induced apoptosis, RANKL/OPG ratio and sclerostin in osteocytes: involvement of JNK and ERK1/2 signalling. Calcif Tissue Int. 2015;96(4):335–46. [DOI] [PubMed] [Google Scholar]
- 81. Zhang W DK, Huai Y, Qian A. Osteoimmunology: the regulatory roles of T lymphocytes in osteoporosis. Front Endocrinol. 2020;11:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Saxena Y RS, Mukhopadhaya A. Immunoporosis: role of innate immune cells in osteoporosis. Front Immunol. 2021;12:687037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Long CL, Humphrey MB. Osteoimmunology: the expanding role of immunoreceptors in osteoclasts and bone remodeling. Bonekey Rep. 2012;1(4):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Pereira M, Petretto E, Gordon S, Bassett JHD, Williams GR, Behmoaras J. Common signalling pathways in macrophage and osteoclast multinucleation. J Cell Sci. 2018;131(11):jcs216267. [DOI] [PubMed] [Google Scholar]
- 85. Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79(1):541–66. [DOI] [PubMed] [Google Scholar]
- 86. Lee SH, Kim TS, Choi Y, Lorenzo J. Osteoimmunology: cytokines and the skeletal system. BMB Rep. 2008;41(7):495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lorenzo J. Interactions between immune and bone cells: new insights with many remaining questions. J Clin Invest. 2000;106(6):749–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka Ket al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408(6812):600–5. [DOI] [PubMed] [Google Scholar]
- 89. Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408(6812):535–6. [DOI] [PubMed] [Google Scholar]
- 90. Pfeilschifter J. Role of cytokines in postmenopausal bone loss. Curr Osteoporos Rep. 2003;1(2):53–8. [DOI] [PubMed] [Google Scholar]
- 91. Goldring SR. Inflammatory mediators as essential elements in bone remodeling. Calcif Tissue Int. 2003;73(2):97–100. [DOI] [PubMed] [Google Scholar]
- 92. Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, McCracken R, Avioli LV. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci. 1991;88(12):5134–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pacifici R, Rifas L, McCracken R, Avioli LV. The role of interleukin-1 in postmenopausal bone loss. Exp Gerontol. 1990;25(3-4):309–16. [DOI] [PubMed] [Google Scholar]
- 94. Pacifici R, Rifas L, McCracken R, Vered I, McMurtry C, Avioli LV, Peck WA. Ovarian steroid treatment blocks a postmenopausal increase in blood monocyte interleukin 1 release. Proc Natl Acad Sci. 1989;86(7):2398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pacifici R, Vannice JL, Rifas L, Kimble RB. Monocytic secretion of interleukin-1 receptor antagonist in normal and osteoporotic women: effects of menopause and estrogen/progesterone therapy. J Clin Endocrinol Metab. 1993;77(5):1135–41. [DOI] [PubMed] [Google Scholar]
- 96. Scheidt-Nave C, Bismar H, Leidig-Bruckner G, Woitge H, Seibel MJ, Ziegler R, Pfeilschifter J. Serum interleukin 6 is a major predictor of bone loss in women specific to the first decade past menopause. J Clin Endocrinol Metab. 2001;86(5):2032–42. [DOI] [PubMed] [Google Scholar]
- 97. D'Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, Giribaldi G, Ulliers D, Pescarmona GP, Isaia G. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43(1):92–100. [DOI] [PubMed] [Google Scholar]
- 98. Pietschmann P, Grisar J, Thien R, Willheim M, Kerschan-Schindl K, Preisinger E, Peterlik M. Immune phenotype and intracellular cytokine production of peripheral blood mononuclear cells from postmenopausal patients with osteoporotic fractures. Exp Gerontol. 2001;36(10):1749–59. [DOI] [PubMed] [Google Scholar]
- 99. Cenci S, Weitzmann MN, Roggia C, Namba N, Novack D, Woodring J, Pacifici R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-alpha. J Clin Invest. 2000;106(10):1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R. Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci. 2001;98(24):13960–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Molnar I, Bohaty I, Somogyine-Vari E. High prevalence of increased interleukin-17A serum levels in postmenopausal estrogen deficiency. Menopause. 2014;21(7):749–52. [DOI] [PubMed] [Google Scholar]
- 102. Azizieh F, Raghupathy R, Shehab D, Al-Jarallah K, Gupta R. Cytokine profiles in osteoporosis suggest a proresorptive bias. Menopause. 2017;24(9):1057–64. [DOI] [PubMed] [Google Scholar]
- 103. Bruunsgaard H. Effects of tumor necrosis factor-alpha and interleukin-6 in elderly populations. Eur Cytokine Netw. 2002;13(4):389–91. [PubMed] [Google Scholar]
- 104. Charatcharoenwitthaya N, Khosla S, Atkinson EJ, McCready LK, Riggs BL. Effect of blockade of TNF-alpha and interleukin-1 action on bone resorption in early postmenopausal women. J Bone Miner Res. 2007;22(5):724–29. [DOI] [PubMed] [Google Scholar]
- 105. Kireev RA, Tresguerres ACF, Garcia C, Borras C, Ariznavarreta C, Vara E, Vina J, Tresguerres JAF. Hormonal regulation of pro-inflammatory and lipid peroxidation processes in liver of old ovariectomized female rats. Biogerontology. 2010;11(2):229–43. [DOI] [PubMed] [Google Scholar]
- 106. Spilmont M, Léotoing L, Davicco M-J, Lebecque P, Mercier S, Miot-Noirault E, Pilet P, Rios L, Wittrant Y, Coxam V. Pomegranate and its derivatives can improve bone health through decreased inflammation and oxidative stress in an animal model of postmenopausal osteoporosis. Eur J Nutr. 2014;53(5):1155–64. [DOI] [PubMed] [Google Scholar]
- 107. Belkaid Y, Hand T. Role of the microbiota in immunity and inflammation. Cell. 2014;157(1):121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yoo J, Groer M, Dutra S, Sarkar A, Mcskimming D. Gut microbiota and immune system interactions. Microorganisms. 2020;8(10):1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Blüher M. Adipose tissue inflammation: a cause or consequence of obesity-related insulin resistance?. Clin Sci (Colch). 2016;130(18):1603–14. [DOI] [PubMed] [Google Scholar]
- 110. Biobaku F, Ghanim H, Batra M, Dandona P. Macronutrient-mediated inflammation and oxidative stress: relevance to insulin resistance, obesity, and atherogenesis. J Clin Endocrinol Metab. 2019;104(12):6118–28. [DOI] [PubMed] [Google Scholar]
- 111. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46(11):2347–55. [DOI] [PubMed] [Google Scholar]
- 113. Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol. 2016;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Moran CP, Shanahan F. Gut microbiota and obesity: role in aetiology and potential therapeutic target. Best Pract Res Clin Gastroenterol. 2014;28(4):585–97. [DOI] [PubMed] [Google Scholar]
- 115. Cornejo-Pareja I, Muñoz-Garach A, Clemente-Postigo M, Tinahones FJ. Importance of gut microbiota in obesity. Eur J Clin Nutr. 2019;72(S1):26–37. [DOI] [PubMed] [Google Scholar]
- 116. Nagpal R, Yadav H. Bacterial translocation from the gut to the distant organs: an overview. Ann Nutr Metab. 2017;71(Suppl 1):11–6. [DOI] [PubMed] [Google Scholar]
- 117. Hou J HC, He W, Yang M, Luo X, Li C. Obesity and bone health: a complex link. Front Cell Dev Biol. 2020;8:600181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Turcotte A-F, O'Connor S, Morin SN, Gibbs JC, Willie BM, Jean S, Gagnon C. Association between obesity and risk of fracture, bone mineral density and bone quality in adults: a systematic review and meta-analysis. PLoS One. 2021;16(6):e0252487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Collins KH, Paul HA, Reimer RA, Seerattan RA, Hart DA, Herzog W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: studies in a rat model. Osteoarthritis Cartilage. 2015;23(11):1989–98. [DOI] [PubMed] [Google Scholar]
- 121. Luo Y, Chen G-L, Hannemann N, Ipseiz N, Krönke G, Bäuerle T, Munos L, Wirtz S, Schett G, Bozec A. Microbiota from obese mice regulate hematopoietic stem cell differentiation by altering the bone niche. Cell Metab. 2015;22(5):886–94. [DOI] [PubMed] [Google Scholar]
- 122. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29(1):415–45. [DOI] [PubMed] [Google Scholar]
- 123. Kolodziejczyk AA, Zheng D, Elinav E. Diet–microbiota interactions and personalized nutrition. Nat Rev Microbiol. 2019;17(12):742–53. [DOI] [PubMed] [Google Scholar]
- 124. Jamar G, Pisani LP. High-fat or high-sugar diets as trigger inflammation in the microbiota-gut-brain axis. Crit Rev Food Sci Nutr. 2021;61(5):836–54. [DOI] [PubMed] [Google Scholar]
- 125. Hooshmand S, Arjmandi BH. Viewpoint: dried plum, an emerging functional food that may effectively improve bone health. Ageing Res Rev. 2009;8(2):122–7. [DOI] [PubMed] [Google Scholar]
- 126. Bu SY, Lerner M, Stoecker BJ, Boldrin E, Brackett DJ, Lucas EA, Smith BJ. Dried plum polyphenols inhibit osteoclastogenesis by downregulating NFATc1 and inflammatory mediators. Calcif Tissue Int. 2008;82(6):475–88. [DOI] [PubMed] [Google Scholar]
- 127. Bu SY, Hunt TS, Smith BJ. Dried plum polyphenols attenuate the detrimental effects of TNF-α on osteoblast function coincident with up-regulation of Runx2, Osterix and IGF-I. J Nutr Biochem. 2009;20(1):35–44. [DOI] [PubMed] [Google Scholar]
- 128. Hooshmand S, Kumar A, Zhang JY, Johnson SA, Chai SC, Arjmandi BH. Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food Funct. 2015;6(5):1719–25. [DOI] [PubMed] [Google Scholar]
- 129. Graef JL, Rendina-Ruedy E, Crockett EK, Ouyang P, Wu L, King JB, Cichewicz RH, Lin D, Lucas EA, Smith BJ. Osteoclast differentiation is downregulated by select polyphenolic fractions from dried plum via suppression of MAPKs and nfatc1 in mouse C57BL/6 primary bone marrow cells. Curr Dev Nutr. 2017;1(10):e000406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Mirza F, Lorenzo J, Drissi H, Lee FY, Soung DY. Dried plum alleviates symptoms of inflammatory arthritis in TNF transgenic mice. J Nutr Biochem. 2018;52:54–61. [DOI] [PubMed] [Google Scholar]
- 131. Graef JL, Rendina-Ruedy E, Crockett EK, Ouyang P, King JB, Cichewicz RH, Lucas EA, Smith BJ. Select polyphenolic fractions from dried plum enhance osteoblast activity through BMP-2 signaling. J Nutr Biochem. 2018;55:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Delgado Cuenca P, Almaiman L, Schenk S, Kern M, Hooshmand S. Dried plum ingestion increases the osteoblastogenic capacity of human serum. J Med Food. 2017;20(7):653–8. [DOI] [PubMed] [Google Scholar]
- 133. Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15(3):175–91. [DOI] [PubMed] [Google Scholar]
- 134. Bu SY, Lucas EA, Franklin M, Marlow D, Brackett DJ, Boldrin EA, Devareddy L, Arjmandi BH, Smith BJ. Comparison of dried plum supplementation and intermittent PTH in restoring bone in osteopenic orchidectomized rats. Osteoporos Int. 2007;18(7):931–42. [DOI] [PubMed] [Google Scholar]
- 135. Rendina E, Lim YF, Marlow D, Wang Y, Clarke SL, Kuvibidila S, Lucas EA, Smith BJ. Dietary supplementation with dried plum prevents ovariectomy-induced bone loss while modulating the immune response in C57BL/6J mice. J Nutr Biochem. 2012;23(1):60–8. [DOI] [PubMed] [Google Scholar]
- 136. Smith BJ, Bu SY, Wang Y, Rendina E, Lim YF, Marlow D, Clarke SL, Cullen DM, Lucas EA. A comparative study of the bone metabolic response to dried plum supplementation and PTH treatment in adult, osteopenic ovariectomized rat. Bone. 2014;58:151–9. [DOI] [PubMed] [Google Scholar]
- 137. Smith BJ, Graef JL, Wronski TJ, Rendina E, Williams AA, Clark KA, Clarke SL, Lucas EA, Halloran BP. Effects of dried plum supplementation on bone metabolism in adult C57BL/6 male mice. Calcif Tissue Int. 2014;94(4):442–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Arjmandi BH, Johnson CD, Campbell SC, Hooshmand S, Chai SC, Akhter MP. Combining fructooligosaccharide and dried plum has the greatest effect on restoring bone mineral density among select functional foods and bioactive compounds. J Med Food. 2010;13(2):312–9. [DOI] [PubMed] [Google Scholar]
- 139. Johnson CD, Lucas EA, Hooshmand S, Campbell S, Akhter MP, Arjmandi BH. Addition of fructooligosaccharides and dried plum to soy-based diets reverses bone loss in the ovariectomized rat. Evid Based Complement Altern Med. 2011;2011:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Halloran BP, Wronski TJ, VonHerzen DC, Chu V, Xia X, Pingel JE, Williams AA, Smith BJ. Dietary dried plum increases bone mass in adult and aged male mice. J Nutr. 2010;140(10):1781–7. [DOI] [PubMed] [Google Scholar]
- 141. Rendina E, Hembree KD, Davis MR, Marlow D, Clarke SL, Halloran BP, Lucas EA, Smith BJ. Dried plum's unique capacity to reverse bone loss and alter bone metabolism in postmenopausal osteoporosis model. PLoS One. 2013;8(3):e60569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shahnazari M, Turner RT, Iwaniec UT, Wronski TJ, Li M, Ferruzzi MG, Nissenson RA, Halloran BP. Dietary dried plum increases bone mass, suppresses proinflammatory cytokines and promotes attainment of peak bone mass in male mice. J Nutr Biochem. 2016;34:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Pawlowski JW, Martin BR, McCabe GP, Ferruzzi MG, Weaver CM. Plum and soy aglycon extracts superior at increasing bone calcium retention in ovariectomized Sprague Dawley rats. J Agric Food Chem. 2014;62(26):6108–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. El-Wakf AM, El-Komy MA, Hassan DG. Preventetive effect of dried plum extract against dexamethasone-induced osteoporosis in male rats through inhibiting cathepsin-K activity, lipogenesis and trabecular bone loss. J Innov Pharm Biol Sci. 2019;6(2):52–61. [Google Scholar]
- 145. Arjmandi BH, Lucas EA, Juma S. A. S., Stoecker BJ, Khalil DA, Smith BJ, Wang C. Dried plums prevent ovariectomy-induced bone loss in rats. JANA. 2001;4(1):50–6. [Google Scholar]
- 146. Deyhim F, Stoecker BJ, Brusewitz GH, Devareddy L, Arjmandi BH. Dried plum reverses bone loss in an osteopenic rat model of osteoporosis. Menopause. 2005;12(6):755–62. [DOI] [PubMed] [Google Scholar]
- 147. Franklin M, Bu SY, Lerner MR, Lancaster EA, Bellmer D, Marlow D, Lightfoot SA, Arjmandi BH, Brackett DJ, Lucas EAet al. Dried plum prevents bone loss in a male osteoporosis model via IGF-I and the RANK pathway. Bone. 2006;39(6):1331–42. [DOI] [PubMed] [Google Scholar]
- 148. Schreurs AS, Shirazi-Fard Y, Shahnazari M, Alwood JS, Truong TA, Tahimic CGT, Limoli CL, Turner ND, Halloran B, Globus RK. Dried plum diet protects from bone loss caused by ionizing radiation. Sci Rep. 2016;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Leotoing L, Wauquier F, Davicco MJ, Lebecque P, Gaudout D, Rey S, Vitrac X, Massenat L, Rashidi S, Wittrant Yet al. The phenolic acids of Agen prunes (dried plums) or Agen prune juice concentrates do not account for the protective action on bone in a rat model of postmenopausal osteoporosis. Nutr Res. 2016;36(2):161–73. [DOI] [PubMed] [Google Scholar]
- 150. Graef JL, Ouyang P, Wang Y, Rendina-Ruedy E, Lerner MR, Marlow D, Lucas EA, Smith BJ. Dried plum polyphenolic extract combined with vitamin K and potassium restores trabecular and cortical bone in osteopenic model of postmenopausal bone loss. J Funct Foods. 2018;42:262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Rendina E, Lim YF, Marlow D, Wang Y, Clarke SL, Kuvibidila S, Lucas EA, Smith BJ. Dietary supplementation with dried plum prevents ovariectomy-induced bone loss while modulating the immune response in C57BL/6J mice. J Nutr Biochem. 2012;23(1):60–8. [DOI] [PubMed] [Google Scholar]
- 152. Jeong H, Liu Y, Kim H-S. Dried plum and chokeberry ameliorate d-galactose-induced aging in mice by regulation of Pl3k/Akt-mediated Nrf2 and Nf-kB pathways. Exp Gerontol. 2017;95:16–25. [DOI] [PubMed] [Google Scholar]
- 153. Shahnazari M, Turner RT, Iwaniec UT, Wronski TJ, Li M, Ferruzzi MG, Nissenson RA, Halloran BP. Dietary dried plum increases bone mass, suppresses proinflammatory cytokines and promotes attainment of peak bone mass in male mice. J Nutr Biochem. 2016;34:73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27(6):1357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci. 2016;113(47):E7554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Li JY, Chassaing B, Tyagi AM, Vaccaro C, Luo T, Adams J, Darby TM, Weitzmann MN, Mulle JG, Gewirtz ATet al. Sex steroid deficiency-associated bone loss is microbiota dependent and prevented by probiotics. J Clin Invest. 2016;126(6):2049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yu M, Pal S, Paterson CW, Li J-Y, Tyagi AM, Adams J, Coopersmith CM, Weitzmann MN, Pacifici R. Ovariectomy induces bone loss via microbial-dependent trafficking of intestinal TNF+ T cells and Th17 cells. J Clin Invest. 2021;131(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Ma S, Qin J, Hao Y, Shi Y, Fu L. Structural and functional changes of gut microbiota in ovariectomized rats and their correlations with altered bone mass. Aging. 2020;12(11):10736–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hsu E, Pacifici R. From osteoimmunology to osteomicrobiology: how the microbiota and the immune system regulate bone. Calcif Tissue Int. 2018;102(5):512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Hong MY, Nakamichi-Lee M, Abbaspour N, Ahouraei Far A, Hooshmand S. Dried plum consumption improves total cholesterol and antioxidant capacity and reduces inflammation in healthy postmenopausal women. J Med Food. 2021;24(11):1161–8. [DOI] [PubMed] [Google Scholar]
- 161. Yan H, Kerr WL. Total phenolics content, anthocyanins, and dietary fiber content of apple pomace powders produced by vacuum-belt drying. J Sci Food Agric. 2013;93(6):1499–504. [DOI] [PubMed] [Google Scholar]
- 162. Shieh A, Epeldegui M, Karlamangla AS, Greendale GA. Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight. 2020;5(2):e134092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Palacios-González B, Ramírez-Salazar EG, Rivera-Paredez B, Quiterio M, Flores YN, Macias-Kauffer L, Moran-Ramos S, Denova-Gutiérrez E, Ibarra-González I, Vela-Amieva Met al. A multi-omic analysis for low bone mineral density in postmenopausal women suggests a relationship between diet, metabolites, and microbiota. Microorganisms. 2020;8(11):1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Rettedal EA, Ilesanmi-Oyelere BL, Roy NC, Coad J, Kruger MC. The gut microbiome is altered in postmenopausal women with osteoporosis and osteopenia. JBMR Plus. 2021;5(3):e10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Das M, Cronin O, Keohane DM, Cormac EM, Nugent H, Nugent M, Molloy C, O'Toole PW, Shanahan F, Molloy MGet al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology (Oxf). 2019;58(12):2295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. He J, Xu S, Zhang B, Xiao C, Chen Z, Si F, Fu J, Lin X, Zheng G, Yu Get al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging. 2020;12(9):8583–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Ozaki D, Kubota R, Maeno T, Abdelhakim M, Hitosugi N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos Int. 2021;32(1):145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Lever E, Scott SM, Louis P, Emery PW, Whelan K. The effect of prunes on stool output, gut transit time and gastrointestinal microbiota: a randomised controlled trial. Clin Nutr. 2019;38(1):165–73. [DOI] [PubMed] [Google Scholar]
- 169. Duda-Chodak A, Tarko T, Satora P, Sroka P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: a review. Eur J Nutr. 2015;54(3):325–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Correa-Oliveira R, Fachi JL, Vieira A, Sato FT, Vinolo MA. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunol. 2016;5(4):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Keirns BH, Lucas EA, Smith BJ. Phytochemicals affect T helper 17 and t regulatory cells and gut integrity: implications on the gut-bone axis. Nutr Res. 2020;83:30–48. [DOI] [PubMed] [Google Scholar]
- 172. Moon N, Effiong L, Song L, Gardner TR, Soung DY. Tart cherry prevents bone loss through inhibition of RANKL in TNF-overexpressing mice. Nutrients. 2018;11(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Smith BJ, Crockett EK, Chongwatpol P, Graef JL, Clarke SL, Rendina-Ruedy E, Lucas EA. Montmorency tart cherry protects against age-related bone loss in female C57BL/6 mice and demonstrates some anabolic effects. Eur J Nutr. 2019;58(8):3035–46. [DOI] [PubMed] [Google Scholar]
- 174. Thomas A, South S, Vijayagopal P, Juma S. Effect of tart cherry polyphenols on osteoclast differentiation and activity. J Med Food. 2020;23(1):56–64. [DOI] [PubMed] [Google Scholar]
- 175. Dodier T, Anderson KL, Bothwell J, Hermann J, Lucas EA, Smith BJ. U.S. Montmorency tart cherry juice decreases bone resorption in women aged 65-80 years. Nutrients. 2021;13(2):544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Devareddy L, Hooshmand S, Collins JK, Lucas EA, Chai SC, Arjmandi BH. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J Nutr Biochem. 2008;19(10):694–9. [DOI] [PubMed] [Google Scholar]
- 177. Zhang J, Lazarenko OP, Blackburn ML, Shankar K, Badger TM, Ronis MJJ, Chen J-R. Feeding blueberry diets in early life prevent senescence of osteoblasts and bone loss in ovariectomized adult female rats. PLoS One. 2011;6(9):e24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Zhang J, Lazarenko OP, Kang J, Blackburn ML, Ronis MJJ, Badger TM, Chen J-R. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS One. 2013;8(8):e70438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lucas EA, Yuhas M, White K, Perkins-Veazie P, Beebe M, Peterson S, Payton ME, Smith BJ. Freeze-dried watermelon supplementation has modest effects on bone and lipid parameters of ovariectomized mice. Prev Nutr Food Sci. 2020;25(1):41–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Meng H, Ba Z, Lee Y, Peng J, Lin J, Fleming JA, Furumoto EJ, Roberts RF, Kris-Etherton PM, Rogers CJ. Consumption of Bifidobacterium animalis subsp. lactis BB-12 in yogurt reduced expression of TLR-2 on peripheral blood-derived monocytes and pro-inflammatory cytokine secretion in young adults. Eur J Nutr. 2017;56(2):649–61. [DOI] [PubMed] [Google Scholar]
- 181. Oh ES, Petersen KS, Kris-Etherton PM, Rogers CJ. Spices in a high-saturated-fat, high-carbohydrate meal reduce postprandial proinflammatory cytokine secretion in men with overweight or obesity: a 3-period, crossover, randomized controlled trial. J Nutr. 2020;150(6):1600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]