ABSTRACT
It is widely believed that diet and the gut microbiota are strongly related to the occurrence and progression of inflammatory bowel disease (IBD), but the effects of the interaction between dietary patterns and the gut microbiota on IBD have not been well elucidated. In this article, we aim to explore the complex relation between dietary patterns, gut microbiota, and IBD. We first comprehensively summarized the dietary patterns associated with IBD and found that dietary patterns can modulate the occurrence and progression of IBD through various signaling pathways, including mammalian target of rapamycin (mTOR), mitogen-activated protein kinases (MAPKs), signal transducer and activator of transcription 3 (STAT3), and NF-κB. Besides, the gut microbiota performs a vital role in the progression of IBD, which can affect the expression of IBD susceptibility genes, such as dual oxidase 2 (DUOX2) and APOA-1 , the intestinal barrier (in particular, the expression of tight junction proteins), immune function (especially the homeostasis between effector and regulatory T cells) and the physiological metabolism, in particular, SCFAs, bile acids (BAs), and tryptophan metabolism. Finally, we reviewed the current knowledge on the interaction between dietary patterns and the gut microbiota in IBD and found that dietary patterns modulate the onset and progression of IBD, which is partly attributed to the regulation of the gut microbiota (especially SCFAs-producing bacteria and Escherichia coli). Faecalibacteria as “microbiomarkers” of IBD could be used as a target for dietary interventions to alleviate IBD. A comprehensive understanding of the interplay between dietary intake, gut microbiota, and IBD will facilitate the development of personalized dietary strategies based on the regulation of the gut microbiota in IBD and expedite the era of precision nutritional interventions for IBD.
Keywords: dietary patterns, gut microbiota, short chain fatty acids, bile acids, tryptophan, inflammatory bowel disease, dysbacteriosis
Statement of Significance: Diet and the gut microbiota are strongly related to the occurrence and progression of inflammatory bowel disease. A complete understanding of the crosstalk between dietary patterns, the gut microbiota, and inflammatory bowel disease will facilitate the development of personalized dietary strategies based on regulation of the gut microbiota in inflammatory bowel disease.
Introduction
Inflammatory bowel disease (IBD), which mainly comprises ulcerative colitis (UC) and Crohn's disease (CD), is a chronic, relapsing, and relieved inflammatory intestinal disorder, characterized by abdominal pain, diarrhea, and weight loss (1, 2). IBD has evolved into a global disease with increasing prevalence in every continent, especially North America (>2 million) and Europe (>3.2 million) (3). The global prevalence of IBD was >0.3% at the beginning of the 21st century, and 57% of the 6.8 million cases of IBD in 2017 occurred in female subjects (4, 5). The precise pathogenesis of IBD remains unclear, but the most accepted hypothesis is that environmental factors trigger an abnormal immune response against the gut microbiota in genetically susceptible hosts (6). Various environmental factors could affect intestinal microbial homeostasis, which is associated with human health (7). Diet, as one of the environmental factors, is widely considered to play a crucial role in the development of IBD (8).
Numerous studies have shown that dietary patterns, including the Western diet (WD) (9, 10), the Mediterranean diet (MED) (11), the semi-vegetarian diet (SVD) (12), the IBD anti-inflammatory diet (IBD-AID) (13), the specific carbohydrate diet (SCD) (14), the low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet (Low FODMAP diet) (15), the gluten-free diet (GFD) (16), etc., have significant effects on the development of IBD. Although the exact mechanisms by which dietary interventions affect IBD remain unclear, some plausible explanations have been proposed. First, the diet may modulate mucosal barrier function, which is a critical factor in the pathogenesis of IBD (17). Second, the diet may be involved in the inflammatory response and immune function (18, 19). Third, the diet may contribute to shaping the gut microbiota and its metabolites (20). A growing body of evidence suggests that the effects of dietary patterns on IBD may be attributable to the alteration of the gut microbiota (17, 21, 22) and gut microbiota-derived metabolites (23), because the gut microbiota acts as an effector between the diet and IBD, and bacterial flagellin serves as an antigen to modulate the host immune response (22, 24).
Most studies of diets, the gut microbiota, and IBD have mainly focused on their interrelations, for instance, diet and IBD (1, 25–28), or the gut microbiota and IBD (2, 29–32), but few studies have regarded all 3 factors as a whole. Considering that diet may affect immune homeostasis via the gut microbiota in individuals with a genetic susceptibility to IBD, a complete understanding of the crosstalk between diet, the gut microbiota, and IBD would have great significance for the prevention and/or treatment of IBD. Therefore, this review first summarizes the relations between various dietary patterns and IBD, and we further present the underlying mechanisms. Alterations of the gut microbiota and its function in IBD patients are then discussed, and the mechanisms by which the gut microbiota contributes to IBD are further proposed. Finally, the interactions between dietary patterns and the gut microbiota that underlie the occurrence and development of IBD are highlighted. The aim of this article is to summarize the complex relations among dietary patterns, the gut microbiota, and IBD to facilitate the formulation of personalized dietary strategies based on individual gut microbiota characteristics and to expedite the era of precision nutritional interventions for IBD.
Dietary Patterns and IBD
WD
The WD features a high intake of red and processed meat, high-fat dairy products, high-sugar drinks, refined grains, butter, etc., and a low intake of dietary fiber, which has been considered a possible cause of the increased incidence of IBD (4, 5, 10, 33, 34). A large (n = 366,351) European prospective cohort study found a positive correlation between the intake of sugar and soft drinks and the risk of UC (Ptrend = 0.02) (35). A cross-sectional study among adults with and without IBD in the USA found that adopting a healthy well-balanced diet (especially limiting added sugars) may be beneficial to the overall health of women with IBD (36). Similarly, a systematic review (including 2609 IBD patients and over 4000 controls) indicated that a high intake of total fat, PUFAs, and meat is positively associated with the risk of UC and CD. Conversely, high vegetables or fiber and fruit consumption could decrease the risk of UC and CD, respectively (10), which indicates that the pathogenesis of IBD may be related to a variety of dietary factors. However, another study demonstrated that controlling the consumption of red and processed meat in patients with CD in remission does not reduce the incidence of disease (time to any (P= 0.61) or moderate/severe (P= 0.50) relapse) (37). Interestingly, a recent study showed that a WD significantly aggravated dextran sodium sulfate (DSS)-induced experimental colitis in BALB/c mice, but switching to a healthy diet reduced colon inflammation (38). A recent Dutch cohort study with 2-y longitudinal clinical follow-up found that a dietary pattern considered to be a “Western” pattern was associated with the exacerbation of IBD (39). Subsequently, they conducted another large prospective population-based cohort, which further found that Western dietary patterns and carnivorous patterns were associated with the development of CD and UC, respectively (40).
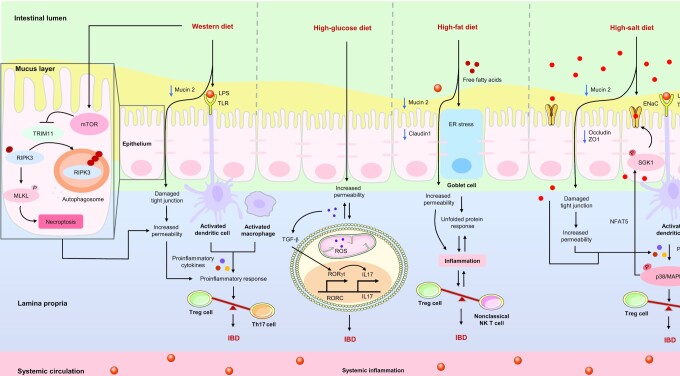
A WD could inhibit the expression of mucin 2 (Muc2), the first line of defense against microbial invasion in the intestine, thereby increasing the permeability of the epithelial barrier (41). In addition, a WD may upregulate the concentrations of Toll-like receptors (TLRs), such as TLR2 and TLR4, which are also significantly elevated in patients with IBD (42, 43). A recent study revealed that activation of the mammalian target of rapamycin (mTOR) is the basis of the intestinal dysfunction and inflammation caused by a WD (44), and that the excessive inflammatory responses further disrupt the balance of intestinal T helper 17 (Th17) and regulatory T (Treg) cells, ultimately increasing the risk of IBD (25, 45), as shown in Figure 1.
FIGURE 1.
Potential mechanisms of proinflammatory diets on IBD. The Western diet not only increases the permeability of the epithelial barrier by inhibiting the expression of mucin 2 and upregulating the concentrations of TLRs and LPS, but also activates the mTOR, which further causes intestinal dysfunction and the excessive release of proinflammatory cytokines, and the inflammatory response further disrupts the balance between Treg and Th17 cells in the intestine, ultimately leading to an increased risk of IBD. The high-glucose diet increases the permeability of the epithelial barrier and the sensitivity of proinflammatory cytokines, and activates TGF-β by upregulating the production of ROS in T cells, thereby promoting the differentiation of Th17 cells and ultimately triggering IBD. The high-fat diet could increase oxidative stress in the ER of goblet cells and decrease the expression of mucin 2 and claudin1, thereby disrupting the intestinal barrier and triggering an inflammatory response, and further disrupting the balance between NK T and Treg cells, ultimately exacerbating IBD. The high-salt diet could destroy the colonic barrier by reducing the expression of mucin 2, occluding, and ZO1, which induces excessive release of proinflammatory cytokines, thereby activating the p38/MAPK signaling pathway, disrupting the balance between Treg and Th17 cells and ultimately aggravating IBD. In addition, p38/MAPK can activate SGK1, which promotes the accumulation of salt in the colon via epithelial sodium channels (ENaC) and triggers a proinflammatory “vicious cycle.” →: Pro; ┤: Anti. ENaC, epithelial sodium channels; ER, endoplasmic reticulum; IBD, inflammatory bowel disease; MLKL, mixed lineage kinase domain like pseudokinase; mTOR, mammalian target of rapamycin; Na+, sodium; NFAT5, nuclear factor of activated T cells 5; NK T cells, natural killer T cells; P, phosphorylate; p38/MAPK, p38/mitogen-activated protein kinase; RIPK3, receptor-interacting serine-threonine kinase 3; RORC, retinoic-acid-related orphan nuclear receptor C; RORγt, retinoid-related orphan nuclear receptor γ t; ROS, reactive oxygen species; SGK1, serum glucocorticoid kinase 1; TGF-β, transforming growth factor-β; Th17, T helper 17 cells; TLR, Toll-like receptor; Treg, regulatory T cells; TRIM11, tripartite motif containing 11; ZO1, zonula occludens 1.
Protein, fat, and sugar have been widely studied as the main ingredients of a WD. A recent case-control study indicated that a high-protein dietary pattern is positively related to the risk of IBD, whereas a high-vegetable dietary pattern may reduce IBD risk (46). Previous studies have demonstrated that high glucose intake can increase the permeability of the gut barrier and the sensitivity of proinflammatory cytokines, and that it can activate transforming growth factor-β (TGF-β) by upregulating reactive oxygen species (ROS) in T cells, thereby promoting the differentiation of Th17 cells and triggering colitis in mice (18), as illustrated in Figure 1. Similarly, a high-protein or high-fat diet was shown to increase oxidative stress in the endoplasmic reticulum (ER) and decrease the expression of Muc2 and claudin 1 in the colon tissues of mice, thereby disrupting the intestinal barrier and triggering an inflammatory response, and further disrupting the balance between nonclassical natural killer T cells and Treg cells, ultimately exacerbating experimental colitis (47, 48), as summarized in Figure 1.
High-salt diet
The high-salt diet (HSD) is generally defined as a diet that includes a total salt intake of >6 g per day (49), the current average global daily intake of sodium is between 5 and 6 g (equivalent to ∼12–15 g of salt), which is nearly 3 times the amount recommended by the WHO (50). The only clinical study (n = 3,220,247) of salt consumption and IBD risk showed that dietary supplementation with potassium (Ptrend = 0.005) was negatively correlated with the risk of IBD (51). A number of studies have investigated the effects of salt on experimental colitis in various mouse models (19, 52, 53), suggesting that salt is a potential dietary risk factor for IBD. This is mainly because salt may accumulate in the colon of mice, which has a direct effect on colitis, and the excessive production of IL17 triggers an inflammatory response in the colon (54, 55). In addition, an HSD could destroy the colonic barrier by reducing the expression of occludin, zonula occludens 1 (ZO1), and Muc2, and aggravate experimental colitis in mice by activating the p38/mitogen-activated protein kinases (p38/MAPKs) signaling pathways, disturbing intestinal immune homeostasis, and stimulating the intestinal Th17 response, but inhibiting the function of Treg cells (19, 52, 53). The potential mechanisms of the HSD on IBD are illustrated in Figure 1.
MED
The MED is a mostly plant-based dietary strategy that is considered to be among the healthiest dietary patterns (56). The MED encourages a high consumption of vegetables, fruits, beans, nuts, and whole grains and moderate consumption of seafood, red wine, and olive oil (57). A meta-analysis showed that a high consumption of fruits could reduce the risk of CD (OR: 0.57, 95% CI: 0.44–0.74, n = 10 studies) and UC (OR: 0.69, 95% CI: 0.49–0.96, n = 8 studies) and that a high consumption of vegetables is negatively correlated with the risk of UC (OR: 0.71, 95% CI: 0.58–0.88, n = 9 studies) (58). A large prospective cohort study (n = 83,147) with 17 y of follow-up revealed that a higher modified MED score was related to a lower risk of late-onset CD (Ptrend = 0.03) but not UC (Ptrend = 0.61) (11). A recent systematic review conducted by Tian et al. implied that a high score on the MED was inversely associated with the risk and development of IBD (59). The MED can reduce intestinal permeability (60) and benefit patients with IBD after pouch surgery by decreasing calprotectin concentrations and modifying intestinal inflammation (61).
The MED is a balanced nutritional diet that features the high and frequent consumption of fibers (such as grains, vegetables, and fruits) and antioxidants [such as wine and extra virgin olive oil (EVOO)] (62). A prospective study (n = 170,776 women) with 26 y of follow-up indicated that a long-term intake of dietary fiber, especially from fruits, resulted in a 40% reduction in the risk of CD (63). Desai et al. (64) found that the regular consumption of dietary fiber protects the intestinal mucus barrier, inhibits pathogenic infection, and reduces the incidence of colitis in mice, whereas a fiber-free diet has the opposite effects. Valcheva et al. revealed that a 15 g/d dose of inulin-type fructans was beneficial in patients with mild/moderately active UC (65). Singh et al. (66) recently examined the effects of various dietary fibers on colitis in mice and found that inulin aggravated the severity of IL10 receptor-induced colitis, in part by the activation of NOD-, LRR- and pyrin domain-containing 3 (NLRP3), whereas pectin alleviated colitis. Another study suggests that different types of fibers have differing effects on IBD, which may depend on the existing baseline gut microbiota (67).
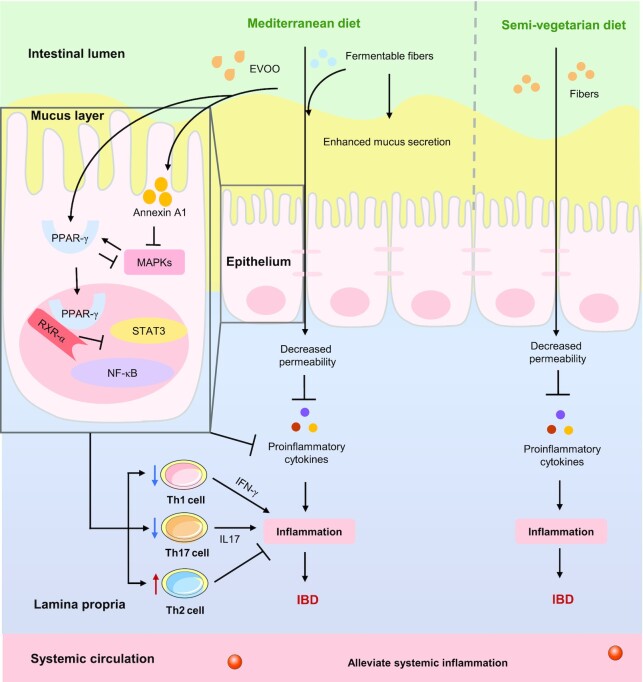
Wine and EVOO have been widely studied as the main antioxidant ingredients in the MED. Previous studies have shown that the regular consumption of moderate doses of wine (especially those rich in resveratrol and proanthocyanins) could protect against DSS-induced experimental colitis (68, 69). EVOO is considered to be one of the main contributors to the MED's health benefits (70), because of its potential for antioxidant, anti-inflammatory, and immunomodulatory activities. A diet rich in EVOO could reduce the inflammatory cascade via upregulation of peroxisome proliferator-activated receptor-γ (PPAR-γ) and annexin A1, and inhibition of the activators and signal transducer of STAT3, NF-κB, and MAPK signaling pathways, respectively, thereby decreasing the proportion of CD4+RORγt+cells (Th17 cells) expressing IFNγ+IL17+, inhibiting the concentrations of IFN-γ and IL17, and ultimately relieving colitis in mice (71–74). The potential mechanisms of the MED on IBD are summed up in Figure 2.
FIGURE 2.
Potential mechanisms of plant-based diets on IBD. Fermentable fibers (such as pectin) in the Mediterranean diet could enhance the secretion of mucus and decrease the permeability of the epithelial barrier, which in turn inhibits the inflammatory response. EVOO in the Mediterranean diet could reduce the inflammatory cascade by upregulating PPAR-γ and annexin A1, which in turn inhibits the activators and signal transducers of STAT3, NF-κB, and MAPK signaling pathways, respectively, thereby decreasing the proportion of CD4+RORγt+ cells (Th17 cells) expressing IFNγ+IL17+ and the concentrations of IFN-γ and IL17, and ultimately alleviating IBD; fibers in the semivegetarian diet could decrease the permeability of the epithelial barrier, which in turn inhibits the inflammatory response, and ultimately, reducing the risk of IBD. →: Pro; ┤: Anti. EVOO, extra virgin olive oil; IBD, inflammatory bowel disease; MAPK, mitogen-activated protein kinase; PPAR-γ, peroxisome proliferator-activated receptor-γ; RXR-α, retinoid X receptor α; STAT3, signal transducers and activators of transduction-3; T cell, T lymphocyte cell; Th, T helper.
Vegetarian diet or SVD
A vegetarian diet (VD) is characterized by a high consumption of cereals, pulses, nuts, fruits, and vegetables, and exclusion of meat, poultry, and fish (75), whereas an SVD allows eggs, milk, and halved fish (weekly) and all meat (fortnightly) (12), both are plant-based dietary patterns that are usually rich in dietary fiber but low in protein. A case-control study conducted in Canada indicated that the imbalance in the intake of fatty acids, vegetables, and fruits is related to an increased risk of CD in children. More specifically, the higher consumption of long-chain ω-3 fatty acids (OR: 0.44, 95% CI: 0.19–1.00, P < 0.001), vegetables (OR: 0.69, 95% CI: 0.33–1.44, P = 0.03), and fruits (OR: 0.49, 95% CI: 0.25–0.96, P = 0.02) was significantly correlated with lower risks of CD (33). In contrast, the considerable intake of vegetables and fruits seemed to reduce the risk of CD (RR: 0.36 and 0.66) and UC (RR: 0.30 and 0.38) (76). Recently, Khorshidi et al. performed a meta-analysis, and the results indicated that a healthy dietary pattern, characterized by a high intake of vegetables, fruits, dietary fiber, etc., could reduce the risk of CD (OR/RR: 0.39, 95% CI: 0.16–0.62, P = 0.014) but not UC (77). A few prospective studies highlighted that a VD or SVD can serve as a protective factor against IBD [OR: 0.29, 95% CI: 0.27–0.39 (UC); OR: 1.179, 95% CI: 0.88–1.57 (CD)] (78), and CD patients following an SVD had a remission rate of 92% at 2 y, and a significantly lower cumulative relapse rate than the omnivorous group (P = 0.0003) (12), which could reduce the incidence of CD complications relative to a diet containing meat (42.4% compared with 60.5%, P = 0.039) (79). An SVD also has the potential to assist IBD drugs in reducing the relapse rate of IBD (80). Although the exact mechanism of these observed benefits has yet to be determined, a plant-based diet exerts anti-inflammatory effects (81), as shown in Figure 2. A case-control study from Iran found that the dietary inflammatory index was positively associated with the risk of UC (P = 0.04) (82). Therefore, the consumption of plant-based foods rich in fiber and phytochemicals is a potential strategy to reduce the occurrence of UC. Although a vegetarian lifestyle can improve the health of adults, it may also bring certain risks, such as lower blood pressure (83) and nutritional deficiencies (84). Therefore, a VD or SVD must be carefully designed to provide adequate nutrition to avoid the risk of malnutrition, and additional randomized controlled trials are still needed to confirm their efficacy and safety in patients with IBD.
SCD
The SCD is not a low-carbohydrate diet, but one that consists mainly of monosaccharides (glucose, fructose, and galactose), solid proteins, fat, nuts, fruits, and vegetables (high ratio of amylose) but excludes grains, processed meats, additives (such as emulsifiers, preservatives, etc.), starches (such as wheat, barley, corn, rice, etc.), and most dairy products, except for fully fermented yogurts (85–87). The SCD permits the consumption of only monosaccharides in patients with IBD based on the theory that disaccharides and polysaccharides enter the colon without digestion, resulting in excessive growth of bacteria and fungi and secondary harmful effects (88). Studies have shown that following an SCD can significantly reduce IBD disease activity scores and the incidence of intestinal inflammation (14), and as the duration of SCD treatment increased (from 2 to 13 mo), the patients with IBD experienced mucosal healing (40% mucosal healing rate at 52 wk of SCD) (89) and varying degrees of symptom relief (87). The mechanism by which an SCD affects IBD remains unknown, but it is hypothesized that an SCD could reduce the occurrence of intestinal inflammation by restoring the gut microbiome from a proinflammatory state to a normal state (90). Because an SCD makes an obvious contribution to a positive clinical response in patients with IBD, Obih et al. (14) recommended that patients maintain such a diet for 1 y during active disease and for another year after symptom relief. However, many patients find it difficult to maintain an SCD for the long term, resulting in poor adherence and nutritional deficiencies.
IBD-AID
The IBD-AID, a modified version of the SCD, was developed by Olendzki et al. (13), is divided into 4 phases, and patients are advised to initially consume soft, well-cooked foods without seeds and to eat more whole foods as their symptoms improve. The IBD-AID encourages the intake of prebiotics and probiotics, but it restricts lactose and refined or processed complex carbohydrates, regulates fat intake (reducing the ratio of SFAs to ω-3 PUFAs), identifies nutritional deficiencies and food intolerances, and finally improves food texture to increase nutrient absorption and minimize intact fiber. Only 1 small retrospective case series study has been conducted for the IBD-AID, and the results were encouraging. All patients who followed the diet for ≥4 wk experienced symptom remission and were able to discontinue ≥1 of their medications (13). Peter et al. (91) recently designed a MELODY (Modulating Early Life Microbiome through Dietary Intervention in Pregnancy) trial to investigate whether an IBD-AID™ dietary intervention during the last trimester of pregnancy could reshape the microbiome of patients with CD and their infants, thereby reducing the risk of CD in the offspring. Interventional studies of the IBD-AID remain very rare and are limited by age, small samples, and (or) retrospective studies; therefore, a large number of randomized prospective studies are needed to further investigate its efficacy, mechanism of action, and applicability.
Low FODMAP diet
The low FODMAP diet specifically allows the use of sucrose, but restricts fructose, lactose, polyols, galactose, and fructans, which can exacerbate gastrointestinal symptoms in patients with IBD (92). The theory behind the low FODMAP diet is to eliminate short-chain carbohydrates that can be fermented by the gut microbiota, which results in gas, bloating, abdominal pain, and changes in bowel habits (93). A low FODMAP diet not only improved common symptoms of irritable bowel syndrome (IBS) in patients with IBD (15), but was also shown to relieve gastrointestinal symptoms in 52% of quiescent IBD patients (94) and 96% of IBD patients (95). Recent clinical practice for the treatment of functional gastrointestinal symptoms of IBD released by the American Gastroenterological Association noted that patients with IBD should consume adequate nutrition while following a low FODMAP diet to manage their functional symptoms (96). Patients are advised to follow a strict low FODMAP diet for the first 4–6 wk under the supervision of a dietitian and then to reintroduce moderate FODMAP foods while still controlling the patient's symptoms to avoid the risk of micronutrient deficiencies or malnutrition (97). Recently, de Castro et al. performed a cross-sectional study and found that “traditional + FODMAP” and “snacks and processed foods” were associated with duration of the CD, but not disease stage (98). Systematic studies are still needed to investigate the potential effects of the low FODMAP diet on intestinal inflammation, mucosal healing, and the gut microbiota in IBD patients.
GFD
The GFD eliminates gluten, which exists mainly in wheat, barley, rye, triticale, and related processed foods, because gluten sensitivity is common in patients with IBD (23.6% and 27.3% of patients with CD and UC, respectively) (99, 100). A cross-sectional study showed that 40 of 145 participants with IBD (27.6%) exhibited nonceliac gluten sensitivity and that 9 (6.2%) followed the GFD, and patients with CD who had self-reported nonceliac gluten sensitivity had higher disease activity index scores than those without self-reported nonceliac gluten sensitivity (101). A clinical study showed that gluten has the potential to directly activate the innate immune system (102). Given the potential immunogenicity and poor absorption of gluten, which can lead to osmotic diarrhea and even malnutrition in patients with IBD (103), it seems reasonable for patients with IBD to try the GFD. Herfarth et al. (16) conducted a cross-sectional study to assess the prevalence of, and adherence to, the GFD in 1647 patients with IBD found that 314 (19.1%) had followed and 135 (8.2%) were still following a GFD. In addition, 65.6% of patients on the GFD experienced relief of ≥1 specific clinical symptom related to gluten exposure (such as bloating, diarrhea, abdominal pain, etc.), 38.3% experienced a reduction in severe IBD flares, 23.6% had less need for medication, and 41.5% of patients who were still following a GFD reported good compliance. Taken together, these observations suggest that the exacerbation of symptoms in patients with IBD may be attributable in part to gluten or wheat sensitivity and that the GFD has potential benefits for patients with IBD (especially CD). This is mainly based on the following 2 hypotheses, one is that gluten causes inflammation, and the alternative hypothesis is that gastrointestinal symptoms are caused by noninflammatory mechanisms, and more specifically, that IBD-related malnutrition, inflammation, and anatomical structural changes lead to abnormal digestion of wheat-related compounds (104). However, long-term adherence to a GFD can lead to nutritional inadequacies (such as fiber, calcium, and vitamin A, etc.) and a high economic burden (105, 106). Therefore, it is recommended that a GFD be followed only after careful consideration of the potential adverse factors and only under the supervision and guidance of a dietitian.
Crohn's disease exclusion diet
The Crohn's disease exclusion diet (CDED) is a whole-food diet that includes fruits, vegetables, meats, and both simple and complex carbohydrates, but eliminates certain animal fats, certain types of meats, and reduces exposure to food additives (such as emulsifiers and maltodextrins), as these dietary components can disrupt the intestinal mucus layer or cause dysbiosis (28). Several prospective randomized controlled trials have shown that in children with mild to moderate CD, the proportion of sustained remission was significantly higher in the CDED plus partial enteral nutrition (PEN) than exclusive enteral nutrition (EEN) (107, 108). Recently, a clinical study revealed that both CDED and EEN induce a rapid clinical response (by week 3) and remission in pediatric patients with active CD (109). In addition, CDED with or without combined PEN was equally effective for remission in adults with mild to moderate biologic naïve CD (110).
Other dietary patterns
There are other dietary patterns that are directly or indirectly related to IBD, including intermittent fasting (IF), fasting-mimicking diet (FMD), paleolithic diet (PD), and ketogenic diet (KD). Although these dietary strategies may not be as well-studied as those listed above, the available data lead us to suspect that these dietary patterns also have the potential to alleviate IBD.
IF is usually a form of an eating pattern that includes a pure water or very low-calorie period of <24 h followed by a normal feeding period of 1 or 2 d (111). A recent study demonstrated that time-restricted fasting and intermittent energy restriction, but not alternate day fasting, could improve intestinal barrier integrity and colon length, relieve intestinal inflammatory responses, alter the composition of the gut microbiota, thereby preventing gut leak, and ultimately reverse the pathological progression of DSS-induced experimental colitis in mice (112), which suggests that appropriate IF may be an effective strategy for nutritional interventions to prevent and treat colitis.
The FMD is a dietary strategy that can be used to maintain a fasting-like physiological state by reducing caloric intake and changing dietary ingredients, but without necessarily fasting (113). Rangan et al. (21) revealed that an FMD can not only relieve intestinal inflammation, accelerate intestinal regeneration, and regulate the gut microbiota in DSS-induced experimental colitis, but also reduce systemic inflammation and the concentrations of immune cells in clinical trials.
The PD consists mainly of lean, nondomesticated meats and plant-based foods, but excludes grains, cereals, refined sugars, domestic meats, and processed fruits and vegetables (114). It is characterized by high intakes of fiber and protein, lower intakes of refined carbohydrates, and comparable intakes of fat (primarily unsaturated fat) and cholesterol, with an emphasis on the source and balance of caloric intake (115). This diet is based on the assumption that modern humans are poorly evolved to adapt to the dietary changes of modern food, and these undigested and agriculturally produced foods contribute to inflammation and ultimately result in modern diseases (116). So far, clinical remission has been reported in only 1 severe case of CD in a patient from Hungary within 2 wk of following a paleolithic ketogenic diet, and this patient remained asymptomatic and off medications for 15 mo (117). However, the PD is accompanied by a risk of deficiencies in folate, calcium, vitamin D, vitamin B-6, and thiamine (103); therefore, reliable scientific evidence should be obtained before this diet is widely advocated.
The KD is a high-fat, low-carbohydrate diet with adequate protein that induces a switch to fatty acid oxidation as an energy source (118). A recent study showed that the KD improved gastrointestinal symptoms and restored fecal calprotectin to the normal range (from 123 to 19 μg/g) in a patient with UC, but it increased the concentrations of total cholesterol and LDL cholesterol (119). The KD could alleviate gastrointestinal inflammation not only by inhibiting NLRP3 inflammasome activity (120), and promoting intestinal stem cell regeneration and gut healing (121), but also by promoting intestinal immune system homeostasis via excessive secretion of bile acids (BAs) (122). As the KD is a high-fat diet, it is necessary to track the serum lipids of those who follow it.
Gut microbiota and IBD
Alterations of the gut microbiota and function in patients with IBD
Growing evidence suggests that the gut microbiota is a crucial actor in IBD (123, 124). This is supported by the finding that colitis is not spontaneously induced in genetically susceptible mice in sterile facilities (125), whereas the fecal microbiota of mice with colitis can induce colitis in normal mice (126). Numerous studies have paid attention to alterations in the composition of the gut microbiota during the development of IBD. These studies have shown reduced fecal and mucosal microbial diversity in IBD, and disturbance of the species composition, which is characterized by a reduction in beneficial bacteria and an increase in pathogens (30, 124, 127–130). At the phylum level, Firmicutes were markedly reduced in IBD patients, whereas Proteobacteria presented the opposite trend, and inconsistencies in the abundance of Bacteroidetes have been seen in different studies (131–133). At the genus or species level, Clostridium and Escherichia were commonly increased, whereas Phascolarctobacterium, Faecalibacterium, and Roseburia intestinalis were markedly decreased in IBD (22, 124, 129, 130, 134). Intestinal inflammation can alter gut microbiota and induce dysbiosis, which is characterized by a marked decrease in the representation of obligate anaerobic bacteria and an increased relative abundance of facultative anaerobic bacteria. The generation of S-oxides, N-oxides, and nitrate as by-products of the host inflammatory response selectively enhances growth of facultative anaerobic bacteria (135). For example, the inflammatory host response selectively enhances growth of commensal Enterobacteriaceae, especially Escherichia coli (E. coli) by generating nitrate (136). Of note, epithelial-derived ROS enable AppBCX-mediated aerobic respiration of E. coli during intestinal inflammation (137). Previous studies have indicated that alterations in colonocyte metabolism allows the outgrowth of pathogenic bacteria due to increased oxygen in the gut lumen (138). The details of abnormal gut microbiota associated with IBD are summarized in Table 1. In summary, in CD patients, Roseburia intestinalis were consistently decreased, whereas E. coli were consistently increased, and the abundance of Ruminococcus gnavus was inconsistent in different studies. Faecalibacterium were commonly reduced in CD and ileum Crohn's disease (ICD) patients but increased in colonic Crohn's disease (CCD) patients. Faecalibacterium prausnitzii were commonly decreased in CD and UC patients. Roseburia were decreased in ICD patients, whereas inconsistent abundance of Roseburia and Shigella were observed in IBD (CD and UC) and ICD patients, respectively. Rehman et al. (139) speculated that Faecalibacteria and Papillibacter could be used as “microbiomarkers” for IBD, because they show a consistent pattern of reduction in disease status. However, the reduction in Papillibacter has only been demonstrated in that study to date and this finding has not yet been reproduced.
TABLE 1.
Overview of changes in the gut microbiota associated with IBD in humans
| Subjects | Samples | Microbiota changes | References |
|---|---|---|---|
| 15 twin pairs for UC23 twin pairs for CDvs.2 healthy pairs | Stool | Bacteroidetes Hallella ∆ Prevotella◊ Porphyromonadaceae●◊ Proteobacteria♦ Aeromonas♦ Citrobacter♦ Shigella♦ Enterobacteriaceae♦ ActinobacteriaBifidobacterium▲ Collinsella▲ Firmicutes▲ Ruminococcus▲ Anaerovorax♦ Faecalibacterium▲◊ Ruminococcaceae▲◊ Roseburia◊ Lachnospiraceae◊ Peptococcus◊ Tenericutes○▲◊ Asteroleplasma○▲◊ | (134) |
| 75 UC patients121 CD patients8 indeterminatevs.27 healthy controls | Stool | Bacteroidetes Odoribacter□Proteobacteria■Enterobacteriaceae■Escherichia◊ Shigella◊ Firmicutes Ruminococcaceae↓ Veillonellaceae■Faecalibacterium◊ Roseburia□ Leuconostocaceae○ Phascolarctobacterium□ | (129) |
| 447 CD patients (children)vs.221 healthy controls | Stool | Bacteroidetes↓ Porphyromonadaceae↓ Bacteroides↓ Bacteroides vulgatus↓Bacteroides caccae↓ Proteobacteria↑ Neisseriaceae↑Eikenella corrodens↑ Enterobacteriaceae↑ Pasteurellaceae↑ Haemophilus parainfluenzae↑Escherichia coli↑ Actinobacteria↓ Micrococcaceae↑ Bifidobacteriaceae↓ Bifidobacterium bifidum↓ Bifidobacterium longum↓ Bifidobacterium adolescentis↓ Bifidobacterium dentum↓ Coriobacteriaceae↓ Firmicutes↓ Gemellaceae↑ Gemella moribillum↑ Faecalibacterium↓ Faecalibacterium prausnitzii↓ Streptococcaceae↑ Veillonellaceae↑ Veillonella parvula↑ Christensenellaceae↓ Clostridiaceae↓ Clostridium bolteae↓ | (130) |
| Clostridium nexile↓ Lachnospiraceae↓ Blautia ↓ Blautia hansenii↓ Ruminococcaceae↓ Ruminococcus↓ Ruminococcus gnavus↓ Erysipelotrichaceae↓ Roseburia↓ Roseburia intestinalis↓ Coprococcus↓ Coprococcus comes↓ Eubacterium rectale↓ Tenericutes Verrucomicrobiaceae↓ Fusobacteria Fusobacteriaceae↑ Fusobacterium nucleatum↑ | |||
| 107 UC patients188 CD patients18 IBDI/IBDU patientsvs.582 healthy controls | Stool | Bacteroidetes■ Porphyromonadaceae↑ Proteobacteria■ Enterobacteriaceae↑ Escherichia coli■ Shigella■ Actinobacteria↓ Actinomycetaceae○ Bifidobacteriaceae↓ Coriobacteriaceae□ Firmicutes○ Faecalibacterium prausnitzii□ Mogibacteriaceae□ Christensenellaceae□ Clostridiaceae□ Dehalobacteriaceae□ Ruminococcaceae□ Erysipelotrichacea□ Lachnospiraceae○ Roseburia intestinalis□ Peptococcaceae↓ Peptostreptococcaceae↓ Enterococcaceae■ Lactobacillaceae● Aerococcaceae↑ Tenericutes↓ Mollicutes□ | (124) |
| 68 CD patients53 UC patientsvs.34 healthy controls | Stool | Proteobacteria Escherichia coli ↑Actinobacteria Bifidobacterium breve ●Firmicutes Coprococcus catus □ Faecalibacterium prausnitzii □ Ruminococcus lactaris□ Roseburia■ Clostridium symbiosum● Clostridium clostridioforme↑ Lactobacillus gasseri↑ Lachnospiraceae bacterium↑ Enterococcus faecium↑ Ruminococcus gnavus↑ Pediococcus acidilactici↑ Blautia producta↑ | (141) |
■□Represents a significant increase or decrease in both CD and UC patients, respectively; ●○represents a significant increase or decrease in UC patients, respectively; ↑↓ represents a significant increase or decrease in CD patients, respectively; ▲∆ represents a significant increase or decrease in CCD patients, respectively; ♦◊ represents a significant increase or decrease in ICD patients, respectively.
CCD, colonic Crohn's disease; CD, Crohn's disease; IBD, inflammatory bowel disease; IBDI, inflammatory bowel disease intermediate; IBDU, inflammatory bowel disease undetermined; ICD, ileum Crohn's disease; UC, ulcerative colitis.
Imbalance of the microbiota in patients with IBD can affect the basic metabolism, the abundance of virulence factors, and antibiotic resistance (129, 140, 141). For example, the basic metabolism (such as amino acid biosynthesis and carbohydrate metabolism), the production of SCFAs, and pyruvate synthase were substantially reduced in patients with IBD, whereas biosynthesis and transport of compounds that favor oxidative stress (such as sulfate transport and glutathione metabolism) were increased. Similar intestinal dysfunction was also found in 2,4,6-trinitrobenzenesulfonic acid-induced experimental colitis in rats (142). In addition, bacterial virulence factors (especially enterotoxins) were found to be significantly enriched in patients with IBD, and most of these virulence factors were derived from E. coli and showed a correlation with the abundance of E. coli (143). These features imply a “pathogen-like” invasive metagenome. Furthermore, patients with CD showed an increase in the antibiotic resistance protein TolC, which was positively correlated with the abundance of Escherichia, whereas one of the most variable antibiotic resistance proteins in patients with UC was cepA (140).
As mentioned above, these results suggest that dysbacteriosis exists in patients with IBD and is characterized by bacteria with less anti-inflammatory capacity and more inflammatory capacity. This can further lead to gut microbiome dysfunction, which mainly includes a reduction in basic metabolism and increased virulence factors and antibiotic resistance. Overall, these results suggest the destruction of the core microbiome and microbiome function in patients with IBD.
Mechanisms of the gut microbiota contribute to IBD
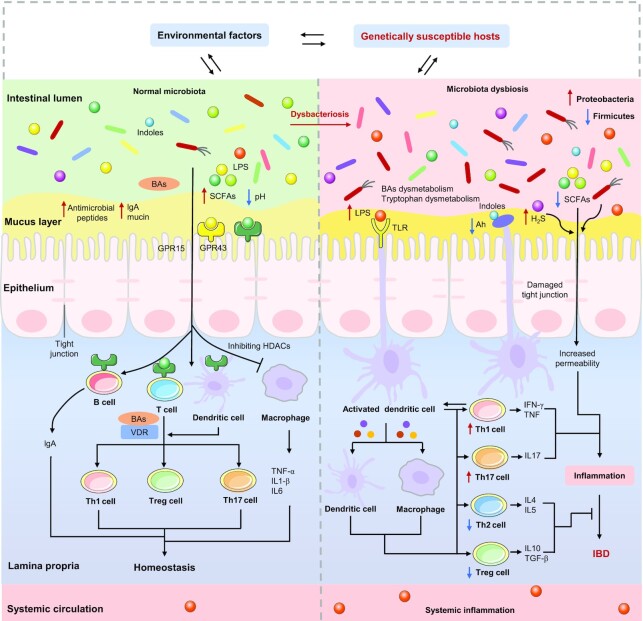
Dysbacteriosis, as a result of decreased diversity of the gut microbiota, has been reported in some patients with IBD, which can affect barrier integrity and the host immune system, leading to chronic inflammation and abnormal immune responses (144). We propose 4 mechanisms by which the gut microbiota may be involved in IBD, including the effects of an imbalance in the microbiota on the expressions of IBD susceptibility genes, the host intestinal barrier, immune function, and physiological metabolism. These mechanisms may contribute simultaneously to the pathogenesis of IBD. The mechanisms of the gut microbiota in the pathogenesis of IBD are summed up in Figure 3.
FIGURE 3.
Mechanisms of the gut microbiota in the pathogenesis of IBD. Under normal conditions, the gut microbiota and its metabolites are in a dynamic balance, which contributes to the maintenance of immune homeostasis. In genetically susceptible hosts, certain environmental factors (such as the Western diet) can trigger microbiota dysbacteriosis, which can affect the expressions of IBD susceptibility genes (such as DUOX2 and APOA-1) and further trigger metabolic dysfunction (such as SCFAs, BAs, and tryptophan) and intestinal dysfunction, as well as immune dysfunction, ultimately leading to the occurrence of IBD.→: Pro; ┤: Anti. Ah, aryl hydrocarbon receptor; B cell, B lymphocyte cell; BAs, bile acids; GPR15, G-protein coupled receptor 15; GPR43, G-protein coupled receptor 43; H2S, hydrogen sulfide; HDAC, histone deacetylase; IBD, inflammatory bowel disease; T cell, T lymphocyte cell; TGF-β, transforming growth factor-β; TLR, Toll-like receptor; VDR, vitamin D receptor.
Gut microbiota, host genes, and IBD
More than 200 susceptible loci associated with IBD have been identified to constitute the complex genetic architecture of IBD (145, 146). Researchers have recently begun to focus on the complex interaction between host genetics and the gut microbiota in patients with IBD. The shift in the gut microbiota in genetically susceptible hosts could result in increased susceptibility to IBD (147). It has been reported that the expressions of dual oxidase 2 (DUOX2) and APOA-1 are positively correlated with the abundance of Proteobacteria and Firmicutes, respectively (148). Even in healthy control subjects, the variabilities in IBD genetic risk are related to adverse variations in the gut microbiota. In particular, a reduced abundance of Roseburia is associated with an increased number of IBD risk alleles (124). In addition to the influence of the gut microbiota on the expression of IBD risk alleles, certain host genes are also involved in shaping the gut microbiota (32). For example, patients with IBD who carry NOD2 and ATG16L1 can exhibit a significant decrease in the abundance of Faecalibacterium and an increase in the abundance of Escherichia (149). As mentioned above, complex interactions occur between the gut microbiota and host genes, which are involved in IBD susceptibility. However, population-level studies have revealed that host genetics has a minor impact on microbiota composition (150) and IBD is probably not an exception.
Gut microbiota, intestinal barrier, and IBD
The intestinal barrier is critical for patients with IBD and is closely related to tight junction proteins and the gut microbiota. Turpin et al. performed a prospective study and found that increased intestinal permeability is associated with the later development of CD (151). Decreases in the expression and redistribution of tight junction proteins have been observed in patients with IBD (30, 129, 152). Destruction of the composition of the gut microbiota can affect the interaction between the host and the micro-organisms, as well as the intestinal physiology, thereby weakening the intestinal barrier (153). The abundance of E. coli is significantly elevated in patients with IBD (130, 141), which can exacerbate barrier dysfunction by destroying the tight junction proteins, thereby triggering inflammatory responses (154), and ultimately leading to IBD (155). In addition, the increased abundance of sulfate-reducing bacteria in patients with IBD could lead to the production of hydrogen sulfate, which damages intestinal epithelial cells (IECs), increases intestinal permeability, and induces mucosal inflammation (156, 157). Bacterial recognition is dependent on transmembrane pattern recognition receptors, including TLRs and Nod-like receptors (NLRs), the ligation of these bacterial receptors stimulates a series of central signaling cascades (158).
Gut microbiota, immune function, and IBD
The gut microbiota also plays crucial roles in immune maturation and homeostasis. An abnormal gut microbiota has been observed in patients with IBD and can result in alterations of immune system homeostasis (159). Britton et al. (160) recently demonstrated that fecal microbiotas from patients with IBD disturbed CD4+ T cell homeostasis in germ-free mice, which was characterized by an increase in Th2 and Th17 cells and a decrease in RORγt+ Treg cells, whereas microbiotas from healthy individuals induced more RORγt+ Treg cells. F. prausnitzii and Bifidobacterium are significantly decreased in patients with IBD (124, 141), which can modulate the immune response after activating dendritic cells by binding pattern recognition receptors (such as TLRs) via pathogen-associated molecular patterns (PAMPs) (161, 162), subsequently stimulating the corresponding T-cell responses, such as Th1, Th2, or Treg pathways (163). As mentioned above, these studies support the hypothesis that the altered composition of the gut microbiota disturbs the homeostasis between effector T cells and Treg cells (especially Th17 and RORγt+ Treg cells), and thus contributes to the risk of colitis.
Gut microbiota, metabolites, and IBD
As the vital molecular mediators between the microbiota and host, microbial metabolism has been widely reported to be disrupted in IBD, which is manifested mainly by reduced concentrations of SCFAs (164) and medium-chain fatty acids (165), dysregulation of the metabolism of BAs (2, 141), and alteration of the concentrations of tryptophan (166), amino acids (167), polyamines (168), acylcarnitines (2), triacylglycerols, sphingolipids, and tetrapyrroles (141). The following sections focus on the effects of disorders of SCFAs, BAs, and tryptophan metabolism on the progression of IBD.
The concentrations of SCFAs are significantly decreased in patients with IBD (2), in particular, the decrease in butyrate is consistent with the previously observed depletion of butyrate producers, such as R. hominis and F. prausnitzii (169). Butyrate is a major energy source for colonic epithelial cells, which can contribute to the homeostasis of epithelial cells with other SCFAs by activating the inflammasome to produce IL18 (170, 171), but it also acts as a natural ligand of PPAR-γ, which is involved in the interaction between PPAR-γ and the host-microbe relationship, thereby preventing inflammation in experimental colitis (172). The positive effects of butyrate were further highlighted by Geirnaert et al. (173), who indicated that supplementation with butyrate-producing bacteria could improve the integrity of the intestinal epithelial barrier in patients with CD. SCFAs act as the primary energy source for IECs (174), which affects the host health primarily via binding to metabolite-sensing G-protein coupled receptors (GPCRs) and (or) inhibiting histone deacetylases (HDACs) (175). Smith et al. (176) revealed that the effects of SCFAs on colonic Treg cells are mediated by GPCR15, GPCR43, and the inhibition of HDAC6 and HDAC9. One of the health effects of SCFAs is a consequent decrease in the luminal pH, which in turn, suppresses pathogenic micro-organisms and increases the absorption of certain nutrients (177). SCFAs are also involved in maintaining the intestinal barrier function, which can improve the integrity of tight junctions (178). Furthermore, SCFAs are also involved in maintaining intestinal homeostasis, which contributes to the growth of epithelial cells, the response of B cells, and the differentiation of T cells (179–181).
BAs, as anti-inflammatory molecules, contribute to lipid absorption and cholesterol homeostasis and serve as signaling molecules to regulate their own biosynthesis (182), energy metabolism (183), inflammatory responses (184), and immune homeostasis (185). The impairment of microbiota enzyme activity (such as Firmicutes-associated bile salt hydrolase) is observed in patients with IBD (186), and leads to alterations in the composition of the luminal BA pool, with an increase in primary BAs (including cholate and chenodeoxycholate) at the expense of secondary BAs, and consequent exacerbation of the intestinal epithelial inflammatory response, thus worsening IBD (187, 188). In contrast, secondary BAs can suppress experimental colitis in mice (189). BAs exert numerous metabolic and immune effects by binding to receptors, such as the farnesoid X receptor, the pregnane X receptor, and the vitamin D receptor (VDR) (190). Microbial BA metabolites modulate the homeostasis of intestinal RORγ+ Treg via the VDR, which in turn, mediates host susceptibility to inflammatory colitis in mice (185). These results support the hypothesis that IBD-associated dysbacteriosis could result in dysmetabolism of the BAs and consequently contribute to chronic inflammation, which suggests that BAs are key actors in the proinflammatory “vicious circle” between the gut microbiota and the host.
Studies have demonstrated that dietary tryptophan deficiency contributes to the deterioration of colitis both in a mouse model and patients with IBD (166, 191). Indoles produced by the gut microbiota that metabolize tryptophan can induce the expression of downstream cytokines by binding and activating the aryl hydrocarbon receptor (Ah), thereby regulating intestinal homeostasis (192). Interestingly, the concentrations of Ah were reduced in the inflamed mucosa of patients with CD (193). In contrast, Ah agonists and Ah-activating strains of Lactobacillus can alleviate experimental colitis (194, 195). Commensal Peptostreptococcus russellii can also decrease the sensitivity of colitis, which is attributable to the metabolism of tryptophan to indoleacrylic acid (an Ah agonist) (196). Overall, dysregulation of tryptophan metabolism in patients with IBD exacerbates the progression of IBD, whereas activation of Ah alleviates experimental colitis.
Diet patterns, gut microbiota, and IBD
IBD is a chronic and relapsing intestinal inflammatory disease triggered by environmental factors (such as the diet) and occurs in genetically susceptible individuals who are intolerant to dysbacteriosis (6). The composition of the human gut microbiota is shaped by multiple factors, especially the diet, which can strongly affect the composition and function of the trillions of microbes that reside in the human gut (197, 198), in turn, the nutritional value of food is affected by the composition and operation of the consumer's gut microbiota (199).
Proinflammatory diets, gut microbiota, and IBD
The WD (high-fat, high-carbohydrate, low-fiber), and HSD consistently reduce the abundance of beneficial species (such as Bifidobacterium or Lactobacillus) and promote the bloom of pathogenic bacteria, such as adherent-invasive Escherichia coli (AIEC), which in turn, leads to dysbacteriosis and metabolic dysfunction, resulting in intestinal dysfunction and inflammation, and ultimately exacerbating the development of IBD.
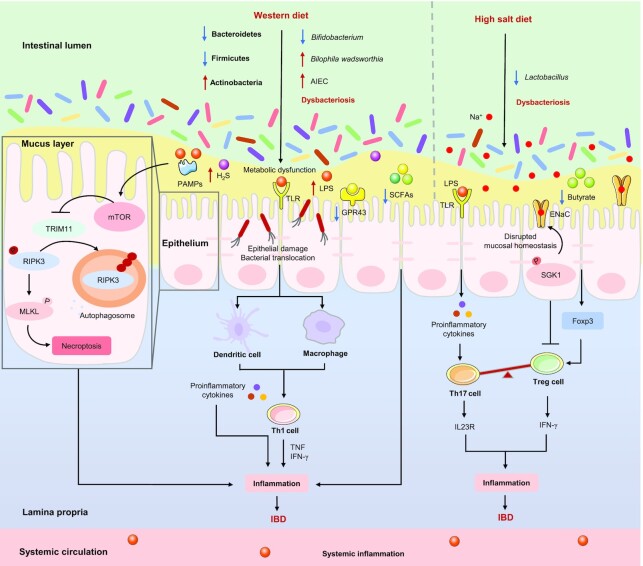
It is well-demonstrated that the WD can cause dysbacteriosis, as characterized by the expansion and colonization of AIEC, an increased abundance of Actinobacteria, and reduced abundance of Bacteroidetes and Firmicutes, resulting in metabolic dysfunction (such as SCFAs and BAs) that in turn, causes intestinal barrier dysfunction and excessive release of proinflammatory cytokines and ultimately exacerbates experimental colitis in mice (17, 200, 201). Abnormal expansion of the AIEC is primarily seen in patients with CD rather than UC, and the concentration of SCFAs is consistently reduced in IBD patients (141, 169, 202). In addition, the abundance of Bifidobacterium (a producer of acetate), which showed a positive correlation with mucus barrier function, is significantly decreased after a WD, whereas the mucus growth rate was restored in mice fed a WD supplemented with B. longum (203, 204). The WD promotes colonization with AIEC and leads to chronic inflammation in susceptible mice by enhancing the concentrations of microbial LPS and flagellin, and eventually contributing to the occurrence of IBD (17, 205). A recent study revealed that the activation of mTOR is the basis of intestinal dysfunction and inflammation caused by WD, and the activation of mTOR in the IECs depends on microbial-derived PAMPs (such as LPS and flagellin) (44). A WD rich in saturated (milk-derived) fat uniquely promotes Bilophila wadsworthia, which can increase the incidence of colitis in genetically susceptible IL10−/− mice by promoting Th1-mediated immune responses, and the conjugation of taurine and hepatic BAs (206). These studies suggest that WD-induced dysbacteriosis can activate mTOR via microbial-derived PAMPs, which leads to intestinal dysfunction, intestinal inflammation, and immune imbalance, ultimately exacerbating colitis, as illustrated in Figure 4.
FIGURE 4.
Proinflammatory diets affect IBD by altering certain gut microbiota. The Western diet (WD) promotes the expansion and colonization of AIEC and Actinobacteria, decreases the abundance of Bacteroidetes, Firmicutes, and Bifidobacterium, resulting in metabolic dysfunction (for example, H2S, acts as gut mucosal “barrier-breakers” is increased) and the activation of mTOR (depends on microbial-derived PAMPs), thus causing intestinal dysfunction and chronic inflammation, and ultimately contributing to the occurrence of IBD. In addition, a WD could lead to the bloom of Bilophila wadsworthia, which can ultimately exacerbate IBD by promoting Th1cell-mediated immune responses. The high-salt diet (HSD) could decrease the abundance of Lactobacillus and the concentrations of butyrate (which can promote the expression of Foxp3 and the differentiation of Treg cells). On the other hand, an HSD could affect the T cell phenotype and its effector functions in an SGK1-dependent manner, which in turn triggers an inflammatory response and ultimately exacerbates IBD. →: Pro; ┤: Anti. AIEC, adherent-invasive Escherichia coli; ENaC, epithelial sodium channels; Foxp3, forkhead box protein p3; GPR43, G-protein coupled receptor 43; H2S, hydrogen sulfide; IBD, inflammatory bowel disease; MLKL, mixed lineage kinase domain like pseudokinase; mTOR, mammalian target of rapamycin; Na+, sodium; P, phosphorylate; PAMPs, pathogen-associated molecular patterns; RIPK3, receptor-interacting serine-threonine kinase 3; SGK1, serum glucocorticoid kinase 1; T cell, T lymphocyte cell; Th, T helper; TLR, Toll-like receptor; TRIM11, tripartite motif containing 11.
Previous studies have revealed that the deterioration of colitis in mice trigged by an HSD is related to a reduction in Lactobacillus and butyrate, which can promote the expression of forkhead box protein p3 (Foxp3) and the differentiation of Treg (23), whereas the adverse effects of an HSD were not found in germ-free mice, which means that the gut microbiota perform a vital role in the exacerbation of HSD-induced experimental colitis (53). In addition, HSD not only triggers the secretion of IL23R of Th17 cells, thereby inducing pathogenicity (207), but also promotes the secretion of IFN-γ of Treg cells, thus inhibiting the activity and function of Treg cells in a serum glucocorticoid kinase1 (SGK1)-dependent manner both in vitro and in vivo (208). As stated above, HSD affects the T cell phenotype and its effector functions in microbiota-dependent and SGK1-dependent manners, which in turn, triggers an inflammatory response and eventually exacerbates colitis, as shown in Figure 4.
Plant-based diets, gut microbiota, and IBD
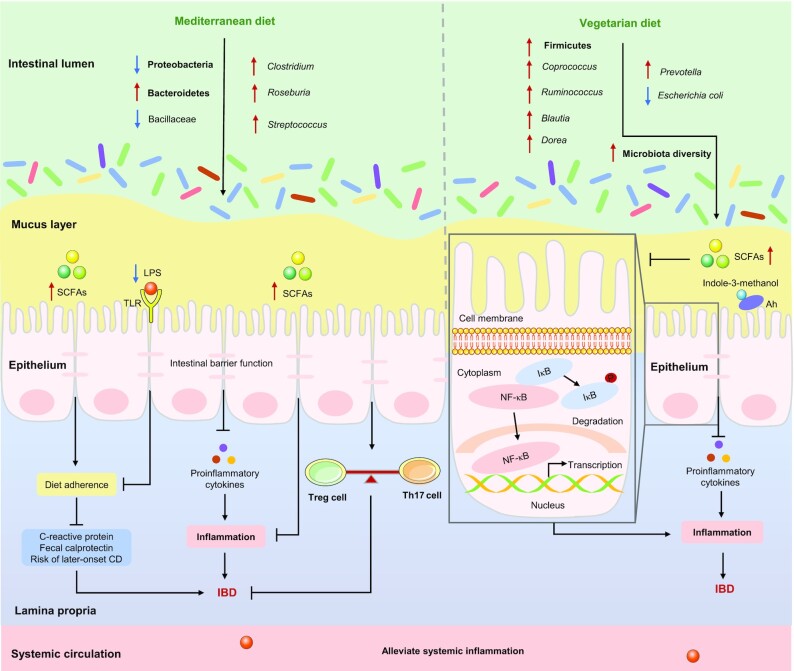
Both MED (high-unsaturated fats, high-fiber) and VD (high-fiber, low-protein) are plant-based dietary patterns that decrease the abundance of pathogenic bacteria (especially E. coli), and consistently increase the abundance of beneficial species (such as Roseburia or Ruminococcus), thereby increasing the concentrations of SCFAs, which modulate intestinal barrier and immune function, as well as intestinal inflammation, and ultimately alleviate IBD.
The MED has been claimed to exert beneficial effects on the gut microbiota and associated metabolomes, thereby contributing to human health (209). Studies have demonstrated that the MED contributes to decreased concentrations of C-reactive protein (CRP) and fecal calprotectin and normalization of the gut microbiota, which is characterized by a decrease in Proteobacteria and Bacillaceae and an increase in Bacteroidetes, Streptococcus, Clostridium, and Roseburia (a producer of SCFAs, especially butyrate) in patients with IBD (61, 210, 211). The abundance of Bacteroidetes and the concentration of SCFAs were positively associated with MED adherence (212), and good adherence to a MED can reduce the risk of later-onset CD (11). Overall, the mechanisms by which a MED contributes to the alleviation of IBD may be attributable to its ability to normalize the gut microbiota and increase the concentrations of SCFAs, which in turn, improves patient compliance with the MED and modulates the intestinal barrier and immune function, as illustrated in Figure 5.
FIGURE 5.
Plant-based diets affect IBD by altering certain gut microbiota. The Mediterranean diet decreases the abundance of Proteobacteria and Bacillaceae, increases the abundance of Bacteroidetes, Streptococcus, Clostridium, and Roseburia (a producer of SCFAs), and thus increases the concentrations of SCFAs. SCFAs can not only improve patient compliance to MED, thereby reducing the concentrations of CRP and fecal calprotectin, and decreasing the risk of later-onset CD, but it also can modulate the intestinal barrier and immune function, ultimately alleviating IBD. The vegetarian diet increases the abundance of Firmicutes (especially Coprococcus, Ruminococcus,Blautia, and Dorea), decreases the abundance of pathogenic bacteria (especially Escherichia coli), and thus increasing the concentrations of SCFAs, thereby inhibiting the transcription of NF-κB and the excessive release of proinflammatory cytokines, ultimately alleviating IBD. In addition, indole-3-methanol, which is present in fruits and cruciferous vegetables, activates the aryl hydrocarbon receptors, thereby reducing the risk of IBD. →: Pro; ┤: Anti. Ah, aryl hydrocarbon receptor; CD, Crohn’s disease; CRP, C-reactive protein; IBD, inflammatory bowel disease; IκB, inhibitor of NF-κB; MED, Mediterranean diet; Th, T helper; TLR, Toll-like receptor; Treg, regulatory T cells.
A decrease in microbial diversity, with an increase in Bacteroidetes and a reduction in the proportion of Firmicutes, have been reported in patients with IBD (124, 213). Conversely, the abundance of Firmicutes (especially Blautia, Coprococcus, Ruminococcus, and Dorea) in the VD group was higher than that in the meat diet group among patients with UC (79). Dietary fiber from a VD is beneficial for patients with IBD (27, 63), which could be explained by several potential mechanisms. First, dietary fiber contributes to the integrity of the epithelial barrier and the expansion of commensal bacteria, as well as to the limitation of access by pathogenic bacteria (especially E. coli) to the gut epithelium, which is called “competitive exclusion” (214, 215). In contrast, an increase in E. coli has been observed in patients with CD (124). In addition, fiber from fruits is more soluble or fermentable fiber, which can be metabolized by the gut microbiota into SCFAs, thereby inhibiting the transcription of proinflammatory mediators and NF-κB (216, 217). Finally, indole-3-methanol, which is present in fruits and cruciferous vegetables, activates the Ah and attenuates experimental colitis (218). Overall, the benefits of a VD for patients with IBD are a result of the remission of intestinal inflammation by the expansion of commensal bacteria and the limitation of pathogenic bacteria, as well as the increase in SCFAs and indole-3-methanol, as summarized in Figure 5.
Carbohydrate-restrictive diets, gut microbiota, and IBD
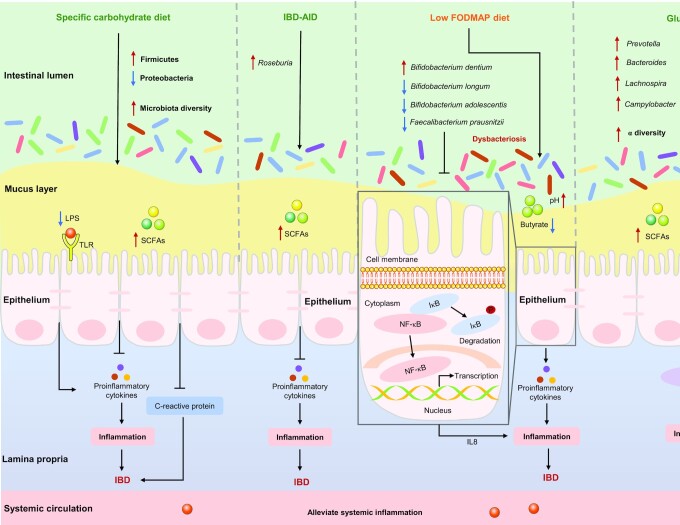
SCD, IBD-AID, low FODMAP diet, and GFD are carbohydrate-restrictive diets. Except for the low FODMAP diet, the other 3 dietary patterns increase the concentrations of SCFAs by modulating the gut microbiota, which in turn, inhibits the excessive release of proinflammatory cytokines, and ultimately alleviates IBD. The low FODMAP diet reduces the potentially beneficial bacterial species (especially F. prausnitzii), which in turn leads to reduced concentrations of SCFAs, ultimately increasing susceptibility to colitis.
Few studies have explored the effects of an SCD on the gut microbiota of patients with IBD. Kakodkar et al. (87, 219, 220) demonstrated that patients with IBD who followed the SCD had lower symptom scores, better quality of life, and greater fecal microbiome biodiversity than those who consumed a WD. In addition, a prospective multicenter study of the SCD in children with CD or UC demonstrated that SCD contributes to normalization of CRP (from 24.1 ± 22.3 mg/L to 7.1 ± 0.4 mg/L), an increase in Firmicutes and a decrease in Proteobacteria (221), which expand abnormally in patients with IBD and may cause intestinal inflammation by attacking impaired mucosal surfaces and ultimately exacerbating clinical symptoms (124), as illustrated in Figure 6.
FIGURE 6.
Carbohydrate-restrictive diets affect IBD by altering certain gut microbiota. The specific carbohydrate diet increases the abundance of Firmicutes, decreases the abundance of Proteobacteria, and thus increasing the concentration of SCFAs, thereby reducing the concentrations of CRP and the excessive release of proinflammatory cytokines, ultimately alleviating IBD. The IBD anti-inflammatory diet increases the abundance of Roseburia (a producer of SCFAs), thus increasing the concentrations of SCFAs, thereby inhibiting the excessive release of proinflammatory cytokines, ultimately alleviating IBD. The low FODMAP diet increases the abundance of Bifidobacterium dentium, decreases the abundance of Bifidobacterium longum, Bifidobacterium adolescentis, and Faecalibacterium prausnitzii, and thus decreasing the concentrations of butyrate. F. prausnitzii or its supernatant could inhibit the activation of NF-κB and the secretion of IL8, which in turn suppresses the inflammatory response; The gluten-free diet increases the abundance of Prevotella, Bacteroides, Lachnospira, and Campylobacter, thereby increasing the concentrations of SCFAs, which can suppress the inflammatory response by inhibiting the activation of NF-κB, and ultimately alleviates IBD. →: Pro; ┤: Anti. CRP, C-reactive protein; FODMAP, fermentable oligosaccharide, disaccharide, monosaccharide and polyol; IBD, inflammatory bowel disease; IBD-AID, IBD anti-inflammatory diet; IκB, inhibitor of NF-κB; TLR, Toll-like receptor.
Only 1 study has explored the effects of an IBD-AID on the gut microbiota of patients with IBD, showing that Roseburia (a producer of SCFAs), which was significantly reduced in IBD patients, increased significantly after the dietary intervention (22), as shown in Figure 6. Furthermore, a reduction of Roseburia contributed to the IBD genetic risk score in healthy subjects (124). These results imply that Roseburia may be the key bacteria in the prevention and treatment of IBD.
Numerous clinical studies have demonstrated that a low FODMAP diet alters the composition of the gut microbiota in patients with digestive disease (including IBD and IBS), resulting in a reduction in total bacterial abundance (especially B. longum, B. adolescentis, and F. prausnitzii) and an increase in B. dentium, and contributes to greater microbial diversity in IBS patients (94, 222, 223). However, the concern is that following a low FODMAP diet may lead to a reduction in SCFAs, which may involve an increased sensitivity of mice to colitis (224). This theoretical concern was confirmed in a randomized controlled crossover feeding trial, which showed that a low FODMAP diet reduced the potentially beneficial bacterial species (especially F. prausnitzii) and butyrate in patients with CD (223). A double-blind controlled trial demonstrated that F. prausnitzii or its supernatant exerts anti-inflammatory effects on Caco-2 cells and 2,4,6-trinitrobenzene sulfonic acid-induced colitis in mice, which was attributed to the inhibition of NF-κB activation and IL8 production (132). Overall, the low FODMAP diet may cause a reduction in Bifidobacterium and an increase in fecal pH, which may contribute to the colonization of intestinal pathogenic bacteria and a reduction in anti-inflammatory bacteria, thus aggravating an imbalance in the microbiota, as described in Figure 6. A restrictive low FODMAP diet is not recommended, except during the “induction” phase of prescribed dietary adjustments, because it contributes to the alleviation of functional gastrointestinal symptoms, and patients who do not respond to the diet should stop FODMAP restrictions (94, 225).
A large, prospective, and comprehensive cohort study indicated that the α-diversity and Campylobacter were significantly increased in patients with CD who followed a GFD, whereas the abundances of Bacteroides, Prevotella, and Lachnospira were significantly increased in patients with UC who followed a GFD (79); however, these bacteria, which are positively associated with the production of SCFAs, were reduced in patients with IBD (134). As a result of reduced SCFAs producers, decreased SCFAs concentrations were frequently observed in patients with IBD (169). SCFAs are currently considered a promising adjunctive therapy for the clinical management of patients with IBD, which can improve clinical symptoms by inhibiting the expression of LPS-induced cytokines and the activation of NF-κB (226, 227), as shown in Figure 6. However, the effects of SCFAs intervention are inconsistent in mouse models of experimental colitis, possibly due to differences in species specificity, colitis models, commensal bacteria depletion, butyrate dosing, and administration routes (228). These results suggest that following a GFD has potential benefits for patients with IBD by restoring gut microbiota-metabolism homeostasis, but it should not be recommended for healthy individuals, because a GFD adversely alters the gut microbiome composition, the activity of microbial pathways, and immune function in healthy adult humans (229–231).
Other dietary patterns, gut microbiota, and IBD
A small number of animal studies have shown that IF (high-carbohydrate, low-fat, low-protein with low calories) and FMD (high-fat, low-carbohydrate, low-protein with low calories) could relieve IBD by regulating the gut microbiota. Although the evidence for these dietary strategies may not be as strong as those mentioned above, based on the available data, we suspect that these dietary patterns may also have the potential to alleviate IBD, which is partly attributable to modulation of the gut microbiota.
A recent study demonstrated that intermittent energy restriction uniquely reduced the enrichment of Peptostreptococcaceae, whereas time-restricted fasting and intermittent energy restriction not only suppressed the abundance of E. coli and Shigella (both of which were increased in DSS-induced mice), but also promoted the abundance of SCFAs-producing bacteria (including Ruminococcus, Lactobacillus, and Coprococcus), and ultimately increased the production of SCFAs (112). Overall, the potential benefits of IF for patients with IBD may be attributable in part to the suppression of harmful microbiota and the expansion of beneficial microbiota and their related metabolites (especially SCFAs).
Only 1 study has investigated the effects of an FMD on the gut microbiota of DSS-induced colitis in mice (21), and the results showed a noteworthy decrease in S24-7 and conspicuous increases in Bifidobacteriaceae and Lactobacillaceae, which are involved in the modulation of T-cell activity, thereby alleviating the severity of IBD symptoms in an experimental model (232).
Conclusions
Dietary patterns can modulate the occurrence and progression of IBD through various signaling pathways, including mTOR, MAPKs, STAT3, and NF-κB. For example, proinflammatory diets (such as the WD and the HSD) could trigger inflammatory responses and immune imbalances through activation of mTOR and p38/MAPK signaling pathways, thereby exacerbating the progression of IBD. In contrast, the MED could modulate immune function by inhibiting activators and signal transduction of STAT3, NF-κB, and MAPK signaling pathways, thereby ultimately alleviating IBD. In general, IBD patients should avoid excessive intake of animal fats and promote a diet high in fruits and vegetables. It may be wise to maintain a well-balanced and varied diet.
The gut microbiota plays a vital role in the progression of IBD, which affects the expression of IBD susceptibility genes, such as DUOX2 and APOA-1, the intestinal barrier (in particular, the expression of tight junction proteins), immune function (especially the homeostasis between effector and Treg cells), and physiological metabolism, in particular, SCFAs, BAs, and tryptophan metabolism.
Both clinical trials and animal studies support the notion that dietary patterns modulate the onset and progression of IBD, which is partly attributable to modulation of the gut microbiota. Proinflammatory diets such as the WD and the HSD consistently reduce the abundance of beneficial species (for instance, Bifidobacterium or Lactobacillus) and promote the bloom of pathogenic bacteria (such as AIEC). WD-induced dysbacteriosis can activate mTOR via microbial-derived PAMPs, which leads to intestinal dysfunction, intestinal inflammation, and immune imbalance, ultimately exacerbating colitis. An HSD affects the T cell phenotype and its effector functions in microbiota-dependent and SGK1-dependent manners, which in turn triggers an inflammatory response and eventually exacerbates colitis. Plant-based dietary patterns, such as the MED and VD, can reduce the abundance of pathogenic bacteria (especially E. coli) and consistently increase the abundance of beneficial species (e.g. Roseburia or Ruminococcus), thereby increasing the concentrations of SCFAs, which modulate the intestinal barrier and immune function as well as intestinal inflammation and ultimately alleviate IBD. Carbohydrate-restrictive diets, including the SCD, IBD-AID, and GFD could increase the abundance of SCFAs-producing bacteria (such as Roseburia and Prevotella), and thus increase the concentrations of SCFAs, which improve clinical symptoms in IBD patients by inhibiting LPS-induced cytokine expression and NF-κB activation. The low FODMAP diet reduces the potentially beneficial bacterial species (especially F. prausnitzii), which in turn leads to reduced concentrations of SCFAs, ultimately increasing susceptibility to colitis. Furthermore, the IF and FMD may also have the potential to alleviate IBD. Overall, the potential benefits of the plant-based dietary patterns and carbohydrate-restrictive diets (except the low FODMAP diet) for patients with IBD may be partly attributed to the suppression of harmful microbiota (especially E. coli) and the expansion of beneficial microbiota (especially SCFAs-producing bacteria) and the accompanying increased concentration of SCFAs. Faecalibacteria as “microbiomarkers” of IBD could be used as a target for dietary interventions that combine top-down and bottom-up approaches to modulate microbial communities with the aim of alleviating IBD.
There is now a broad consensus that a more individualized approach to the treatment of IBD is needed. Attempts have been made to construct microbiota simulation models to predict potential dietary intervention strategies by integrating metagenomic data and metabolomics data from patients with IBD (233). Recently, Sasson et al. presented a viewpoint on the role of precision nutrition in the modulation of microbial composition and function in IBD patients, and provided constructive advice for the design of future research to develop precision nutrition in patients with IBD (234). The continuous development of big data, nutrigenomics, systems biology, bioinformatics, and artificial intelligence (such as machine learning) will contribute to the integration and analysis of clinical data, imaging data, and multi-omics data of patients with IBD, which may contribute to the development of more accurate predictive models to move toward the era of precision nutritional interventions for IBD.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—PH, LY, FT, HZ, WC, and QZ: designed the reviews; PH and QZ: were involved in searching the literature and writing the manuscript; PH and QZ: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This work was supported by the National Natural Science Foundation of China (No. 32122067; U1903205; No. 32001665), the Natural Science Foundation of Jiangsu Province (BK20200084), and Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: Ah, aryl-hydrocarbon receptor; AIEC, adherent-invasive Escherichia coli; BAs, bile acids; CD, Crohn's disease; CDED, Crohn's disease exclusion diet; CRP, C-reactive protein; DSS, dextran sodium sulfate; DUOX2, dual oxidase 2; E. coli, Escherichia coli; EEN, exclusive enteral nutrition; EVOO, extra virgin olive oil; FMD, fasting-mimicking diet; GFD, gluten-free diet; GPCR, G-protein coupled receptor; HDAC, histone deacetylase; HSD, high-salt diet; IBD, inflammatory bowel disease; IBD-AID, IBD anti-inflammatory diet; IBS, irritable bowel syndrome; ICD, ileum Crohn's disease; IEC, intestinal epithelial cell; IF, intermittent fasting; KD, ketogenic diet; low FODMAP diet, low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet; MAPK, mitogen-activated protein kinase; MED, Mediterranean diet; mTOR, mammalian target of rapamycin; Muc2, mucin 2; NLRP3, NOD-, LRR-, and pyrin domain-containing 3; NLR, Nod-like receptor; PAMPs, pathogen-associated molecular patterns; PD, paleolithic diet; PEN, partial enteral nutrition; PPAR-γ, peroxisome proliferator-activated receptor γ; RORγt, retinoid-related orphan nuclear receptor-γt; ROS, reactive oxygen species; SCD, specific carbohydrate diet; SGK1, serum glucocorticoid kinase 1; STAT3, signal transducer and activator of transcription 3; SVD, semivegetarian diet; T cell, T lymphocyte cell; TLR, Toll-like receptor; Treg cells, regulatory T cells; UC, ulcerative colitis; VD, vegetarian diet; VDR, vitamin D receptor; WD, Western diet.
Contributor Information
Pandi He, State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China; School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China.
Leilei Yu, State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China; School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China.
Fengwei Tian, State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China; School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China.
Hao Zhang, State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China; School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China; National Engineering Research Center for Functional Food, Jiangnan University, Wuxi, Jiangsu, China; Wuxi Translational Medicine Research Center, Jiangsu Translational Medicine Research Institute Wuxi Branch, Wuxi, Jiangsu, China.
Wei Chen, State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China; School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China; National Engineering Research Center for Functional Food, Jiangnan University, Wuxi, Jiangsu, China.
Qixiao Zhai, State Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China; School of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu, China.
References
- 1. Lee D, Albenberg L, Compher C, Baldassano R, Piccoli D, Lewis JD, et al. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology. 2015;148(6):1087–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lloyd-Price J, Arze C, Ananthakrishnan AN, Schirmer M, Avila-Pacheco J, Poon TWet al. . Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ananthakrishnan AN, Kaplan GG, Ng SC. Changing global epidemiology of inflammatory bowel diseases: sustaining health care delivery into the 21st century. Clin Gastroenterol Hepatol. 2020;18(6):1252–60. [DOI] [PubMed] [Google Scholar]
- 4. GBD 2017 Inflammatory Bowel Collaborators . The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EIet al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet North Am Ed. 2017;390(10114):2769–78. [DOI] [PubMed] [Google Scholar]
- 6. Reddavide R, Rotolo O, Caruso MG, Stasi E, Notarnicola M, Miraglia Cet al. . The role of diet in the prevention and treatment of inflammatory bowel diseases. Acta Biomed. 2018;89(9-S):60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang BH, Yao MF, Lv LX, Ling ZX, Li LJ. The human microbiota in health and disease. Engineering. 2017;3(1):71–82. [Google Scholar]
- 8. Richards JL, Yap YA, McLeod KH, Mackay CR, Marino E. Dietary metabolites and the gut microbiota: an alternative approach to control inflammatory and autoimmune diseases. Clinical & Translational Immunology. 2016;5(5):e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Fuchs CS, et al. Long-term intake of dietary fat and risk of ulcerative colitis and Crohn's disease. Gut. 2014;63(5):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–73. [DOI] [PubMed] [Google Scholar]
- 11. Khalili H, Hakansson N, Chan SS, Chen Y, Lochhead P, Ludvigsson JF, et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn's disease: results from two large prospective cohort studies. Gut. 2020;69(9):1637–44. [DOI] [PubMed] [Google Scholar]
- 12. Chiba M, Abe T, Tsuda H, Sugawara T, Tsuda S, Tozawa H, et al. Lifestyle-related disease in Crohn's disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010;16(20):2484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Olendzki BC, Silverstein TD, Persuitte GM, Ma Y, Baldwin KR, Cave D. An anti-inflammatory diet as treatment for inflammatory bowel disease: a case series report. Nutrition Journal. 2014;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Obih C, Wahbeh G, Lee D, Braly K, Giefer M, Shaffer ML, et al. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic IBD center. Nutrition. 2016;32(4):418–25. [DOI] [PubMed] [Google Scholar]
- 15. Kakodkar S, Mutlu EA. Diet as a therapeutic option for adult inflammatory bowel disease. Gastroenterol Clin North Am. 2017;46(4):745–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Herfarth HH, Martin CF, Sandler RS, Kappelman MD, Long MD. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20(7):1194–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinez-Medina M, Denizot J, Dreux N, Robin F, Billard E, Bonnet R, et al. Western diet induces dysbiosis with increased E. coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation. Gut. 2014;63(1):116–24. [DOI] [PubMed] [Google Scholar]
- 18. Zhang DF, Jin WW, Wu RQ, Li J, Park SA, Tu Eet al. . High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-beta cytokine activation. Immunity. 2019;51(4):671–81.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei YF, Lu C, Chen JN, Cui GY, Wang L, Yu TMet al. . High salt diet stimulates gut Th17 response and exacerbates TNBS-induced colitis in mice. Oncotarget. 2017;8(1):70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albenberg LG, Wu GD. Diet and the intestinal microbiome: associations, functions, and implications for health and disease. Gastroenterology. 2014;146(6):1564–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rangan P, Choi I, Wei M, Navarrete G, Guen E, Brandhorst Set al. . Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 2019;26(10):2704–19.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mutlu E, Mikolaitis S, Sedghi S, Chakradeo PS, Engen P, Chlipala G, et al. Mo1795 dietary treatment of Crohn's disease: a randomized, placebo-controlled, double-blinded clinical trial. Gastroenterology. 2016;150(4, Supplement 1):S778. [Google Scholar]
- 23. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi Det al. . Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–50. [DOI] [PubMed] [Google Scholar]
- 24. Lodes MJ, Cong YZ, Elson CO, Mohamath R, Landers CJ, Targan SR, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113(9):1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khalili H, Chan SSM, Lochhead P, Ananthakrishnan AN, Hart AR, Chan AT. The role of diet in the aetiopathogenesis of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2018;15(9):525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Levine A, Sigall Boneh R, Wine E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut. 2018;67(9):1726–38. [DOI] [PubMed] [Google Scholar]
- 27. Yao CK, Staudacher HM. The low-fibre diet: contender in IBD, or has it had its time?. Lancet Gastroenterol Hepatol. 2019;4(5):339. [DOI] [PubMed] [Google Scholar]
- 28. Sasson AN, Ananthakrishnan AN, Raman M. Diet in treatment of inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2021;19(3):425–35..e3. [DOI] [PubMed] [Google Scholar]
- 29. Caruso R, Lo BC, Núñez G. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Immunol. 2020;20(7):411–26. [DOI] [PubMed] [Google Scholar]
- 30. Sokol H, Leducq V, Aschard H, Pham HP, Jegou S, Landman Cet al. . Fungal microbiota dysbiosis in IBD. Gut. 2017;66(6):1039–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heinken A, Ravcheev DA, Baldini F, Heirendt L, Fleming RMT, Thiele I. Systematic assessment of secondary bile acid metabolism in gut microbes reveals distinct metabolic capabilities in inflammatory bowel disease. Microbiome. 2019;7(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu S, Vich Vila A, Gacesa R, Collij V, Stevens C, Fu JMet al. . Whole exome sequencing analyses reveal gene-microbiota interactions in the context of IBD. Gut. 2021;70(2):285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Amre DK, D'Souza S, Morgan K, Seidman G, Lambrette P, Grimard Get al. . Imbalances in dietary consumption of fatty acids, vegetables, and fruits are associated with risk for Crohn's disease in children. Am J Gastroenterol. 2007;102(9):2016–25. [DOI] [PubMed] [Google Scholar]
- 34. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152(2):313–21..e2. [DOI] [PubMed] [Google Scholar]
- 35. Racine A, Carbonnel F, Chan SS, Hart AR, Bueno-de-Mesquita HB, Oldenburg Bet al. . Dietary patterns and risk of inflammatory bowel disease in Europe: results from the EPIC study. Inflamm Bowel Dis. 2016;22(2):345–54. [DOI] [PubMed] [Google Scholar]
- 36. Xu F, Park S, Liu Y, Greenlund KJ. Dietary intake patterns among adults with inflammatory bowel disease in the United States, 2015. PLoS One. 2021;16(4):e0250441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Albenberg L, Brensinger CM, Wu QF, Gilroy E, Kappelman MD, Sandler RS, et al. A diet low in red and processed meat does not reduce rate of Crohn's disease flares. Gastroenterology. 2019;157(1):128–36.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Groschel C, Prinz-Wohlgenannt M, Mesteri I, Karuthedom George S, Trawnicek L, Heiden Det al. . Switching to a healthy diet prevents the detrimental effects of Western diet in a colitis-associated colorectal cancer model. Nutrients. 2019;12(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peters V, Spooren CEGM, Pierik MJ, Weersma RK, van Dullemen HM, Festen EAMet al. . Dietary intake pattern is associated with occurrence of flares in IBD patients. J Crohns Colitis. 2021;15(8):1305–15. [DOI] [PubMed] [Google Scholar]
- 40. Peters V, Bolte L, Schuttert E, Andreu-Sánchez S, Dijkstra G, Weersma R, et al. Western and carnivorous dietary patterns are associated with greater likelihood of IBD development in a large prospective population-based cohort. J Crohns Colitis. 2021; Epub ahead of print: PMID: 34864946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14(3):141–53. [DOI] [PubMed] [Google Scholar]
- 42. Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn's disease. Lancet North Am Ed. 2017;389(10080):1741–55. [DOI] [PubMed] [Google Scholar]
- 43. De Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27. [DOI] [PubMed] [Google Scholar]
- 44. Xie YD, Zhao YF, Shi L, Li W, Chen K, Li Met al. . Gut epithelial TSC1/mTOR controls RIPK3-dependent necroptosis in intestinal inflammation and cancer. J Clin Invest. 2020;130(4):2111–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schroeder BO, Birchenough GMH, Stahlman M, Arike L, Johansson MEV, Hansson GC, et al. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host & Microbe. 2018;23(1):27–40..e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tayyem RF, Qalqili TR, Ajeen R, Rayyan YM. Dietary patterns and the risk of inflammatory bowel disease: findings from a case-control study. Nutrients. 2021;13(6):1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ma X, Torbenson M, Hamad AR, Soloski MJ, Li Z. High-fat diet modulates non-CD1d-restricted natural killer T cells and regulatory T cells in mouse colon and exacerbates experimental colitis. Clin Exp Immunol. 2007;151(1):130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gulhane M, Murray L, Lourie R, Tong H, Sheng YH, Wang Ret al. . High fat diets induce colonic epithelial cell stress and inflammation that is reversed by IL-22. Sci Rep. 2016;6(1):28990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hou YJ, Zheng RM. High salt diet and immunity. Advances in Physiological Science. 2020;51(03):228. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=SLKZ202003018&DbName=CJFQ2020. [Google Scholar]
- 50. GBD 2017 Diet Collaborators . Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khalili H, Malik S, Ananthakrishnan AN, Garber JJ, Higuchi LM, Joshi Aet al. . Identification and characterization of a novel association between dietary potassium and risk of Crohn's disease and ulcerative colitis. Front Immunol. 2016;7:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Monteleone I, Marafini I, Dinallo V, Di Fusco D, Troncone E, Zorzi Fet al. . Sodium chloride-enriched diet enhanced inflammatory cytokine production and exacerbated experimental colitis in mice. J Crohns Colitis. 2017;11(2):237–45. [DOI] [PubMed] [Google Scholar]
- 53. Miranda PM, De Palma G, Serkis V, Lu J, Louis-Auguste MP, McCarville JLet al. . High salt diet exacerbates colitis in mice by decreasing lactobacillus levels and butyrate production. Microbiome. 2018;6(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tubbs AL, Liu B, Rogers TD, Sartor RB, Miao EA. Dietary salt exacerbates experimental colitis. J Immunol. 2017;199(3):1051–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aguiar SLF, Miranda MCG, Guimarães MAF, Santiago HC, Queiroz CP, Cunha PDSet al. . High-salt diet induces IL-17-dependent gut inflammation and exacerbates colitis in mice. Front Immunol. 2017;8:1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Capurso A, Crepaldi G, Capurso C. The Mediterranean diet: a pathway to successful aging. Aging Clin Exp Res. 2020;32(6):1187–8. [DOI] [PubMed] [Google Scholar]
- 57. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92(5):1189–96. [DOI] [PubMed] [Google Scholar]
- 58. Li F, Liu XQ, Wang WJ, Zhang DF. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2015;27(6):623–30. [DOI] [PubMed] [Google Scholar]
- 59. Tian ZY, Zhuang XJ, Zhao M, Zhuo SY, Li XZ, Ma RQet al. . Index-based dietary patterns and inflammatory bowel disease: a systematic review of observational studies. Adv Nutr. 2021;12(6):2288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Molendijk I, van der Marel S, Maljaars PWJ. Towards a food pharmacy: immunologic modulation through diet. Nutrients. 2019;11(6):1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Godny L, Reshef L, Pfeffer-Gik T, Goren I, Yanai H, Tulchinsky H, et al. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur J Nutr. 2020;59(7):3183–90. [DOI] [PubMed] [Google Scholar]
- 62. Bifulco M. Mediterranean diet: the missing link between gut microbiota and inflammatory diseases. Eur J Clin Nutr. 2015;69(9):1078. [DOI] [PubMed] [Google Scholar]
- 63. Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JRet al. . A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Desai MS, Seekatz AM, Koropatkin NM, Kamada N, Hickey CA, Wolter Met al. . A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell. 2016;167(5):1339–53..e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Valcheva R, Koleva P, Martínez I, Walter J, Gänzle MG, Dieleman LA. Inulin-type fructans improve active ulcerative colitis associated with microbiota changes and increased short-chain fatty acids levels. Gut Microbes. 2019;10(3):334–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Singh V, Yeoh BS, Walker RE, Xiao X, Saha P, Golonka RMet al. . Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut. 2019;68(10):1801–12. [DOI] [PubMed] [Google Scholar]
- 67. Armstrong H, Mander I, Zhang ZX, Armstrong D, Wine E. Not all fibers are born equal; variable response to dietary fiber subtypes in IBD. Frontiers in Pediatric. 2020;8:620189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Singh UP, Singh NP, Singh B, Hofseth LJ, Price RL, Nagarkatti M, et al. Resveratrol (trans-3,5,4'-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther. 2010;332(3):829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Larrosa M, Yanez-Gascon MJ, Selma MV, Gonzalez-Sarrias A, Toti S, Ceron JJet al. . Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem. 2009;57(6):2211–20. [DOI] [PubMed] [Google Scholar]
- 70. Covas MI, de la Torre R, Fito M. Virgin olive oil: a key food for cardiovascular risk protection. Br J Nutr. 2015;113(S2):S19–28. [DOI] [PubMed] [Google Scholar]
- 71. Sanchez-Fidalgo S, Cardeno A, Sanchez-Hidalgo M, Aparicio-Soto M, de la Lastra CA. Dietary extra virgin olive oil polyphenols supplementation modulates DSS-induced chronic colitis in mice. J Nutr Biochem. 2013;24(7):1401–13. [DOI] [PubMed] [Google Scholar]
- 72. Giner E, Recio MC, Rios JL, Giner RM. Oleuropein protects against dextran sodium sulfate-induced chronic colitis in mice. J Nat Prod. 2013;76(6):1113–20. [DOI] [PubMed] [Google Scholar]
- 73. Giner E, Recio MC, Rios JL, Cerda-Nicolas JM, Giner RM. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol Nutr Food Res. 2016;60(2):242–55. [DOI] [PubMed] [Google Scholar]
- 74. Perretti M, D'Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9(1):62–70. [DOI] [PubMed] [Google Scholar]
- 75. Key TJ, Appleby PN, Rosell MS. Health effects of vegetarian and vegan diets. Proc Nutr Soc. 2006;65(1):35–41. [DOI] [PubMed] [Google Scholar]
- 76. Bianchi Porro G, Panza E. Smoking, sugar, and inflammatory bowel disease. BMJ. 1985;291(6500):971–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Khorshidi M, Djafarian K, Aghayei E, Shab-Bidar S. A posteriori dietary patterns and risk of inflammatory bowel disease: a meta-analysis of observational studies. Int J Vitam Nutr Res. 2020;90(3–4):376–84. [DOI] [PubMed] [Google Scholar]
- 78. Amarapurkar AD, Amarapurkar DN, Rathi P, Sawant P, Patel N, Kamani Pet al. . Risk factors for inflammatory bowel disease: a prospective multi-center study. Indian J Gastroenterol. 2018;37(3):189–95. [DOI] [PubMed] [Google Scholar]
- 79. Schreiner P, Yilmaz B, Rossel JB, Franc Y, Misselwitz B, Scharl Met al. . Vegetarian or gluten-free diets in patients with inflammatory bowel disease are associated with lower psychological well-being and a different gut microbiota, but no beneficial effects on the course of the disease. United European Gastroenterol J. 2019;7(6):767–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chiba M, Tsuji T, Nakane K, Tsuda S, Ishii H, Ohno Het al. . Induction with infliximab and a plant-based diet as first-line (IPF) therapy for Crohn disease: a single-group trial. Perm J. 2017;21:17–009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BEet al. . Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505(7484):559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shivappa N, Hébert JR, Rashvand S, Rashidkhani B, Hekmatdoost A. Inflammatory potential of diet and risk of ulcerative colitis in a case-control study from Iran. Nutr Cancer. 2016;68(3):404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa Aet al. . Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014;174(4):577–87. [DOI] [PubMed] [Google Scholar]
- 84. Haider LM, Schwingshackl L, Hoffmann G, Ekmekcioglu C. The effect of vegetarian diets on iron status in adults: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2018;58(8):1359–74. [DOI] [PubMed] [Google Scholar]
- 85. Pigneur B, Ruemmele FM. Nutritional interventions for the treatment of IBD: current evidence and controversies. Therapeutic Advances in Gastroenterology. 2019;12:175628481989053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lewis JD, Albenberg L, Lee D, Kratz M, Gottlieb K, Reinisch W. The importance and challenges of dietary intervention trials for inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(2):181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kakodkar S, Farooqui AJ, Mikolaitis SL, Mutlu EA. The specific carbohydrate diet for inflammatory bowel disease: a case series. Journal of the Academy of Nutrition and Dietetics. 2015;115(8):1226–32. [DOI] [PubMed] [Google Scholar]
- 88. Knight-Sepulveda K, Kais S, Santaolalla R, Abreu MT. Diet and inflammatory bowel disease. Gastroenterol Hepatol (NY). 2015;11(8):511–20. [PMC free article] [PubMed] [Google Scholar]
- 89. Cohen SA, Gold BD, Oliva S, Lewis J, Stallworth A, Koch Bet al. . Clinical and mucosal improvement with specific carbohydrate diet in pediatric Crohn disease. Journal of Pediatric Gastroenterology & Nutrition. 2014;59(4):516–21. [DOI] [PubMed] [Google Scholar]
- 90. Vindigni SM, Zisman TL, Suskind DL, Damman CJ. The intestinal microbiome, barrier function, and immune system in inflammatory bowel disease: a tripartite pathophysiological circuit with implications for new therapeutic directions. Therapeutic Advances in Gastroenterology. 2016;9(4):606–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Peter I, Maldonado-Contreras A, Eisele C, Frisard C, Simpson S, Nair Net al. . A dietary intervention to improve the microbiome composition of pregnant women with Crohn's disease and their offspring: the MELODY (Modulating Early Life Microbiome through Dietary Intervention in Pregnancy) trial design. Contemporary Clinical Trials Communications. 2020;18:100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cox SR, Prince AC, Myers CE, Irving PM, Lindsay JO, Lomer MCet al. . Fermentable carbohydrates [FODMAPs] exacerbate functional gastrointestinal symptoms in patients with inflammatory bowel disease: a randomised, double-blind, placebo-controlled, cross-over, re-challenge trial. J Crohns Colitis. 2017;11(12):1420–9. [DOI] [PubMed] [Google Scholar]
- 93. Rao SS, Yu S, Fedewa A. Systematic review: dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment Pharmacol Ther. 2015;41(12):1256–70. [DOI] [PubMed] [Google Scholar]
- 94. Cox SR, Lindsay JO, Fromentin S, Stagg AJ, McCarthy NE, Galleron Net al. . Effects of low FODMAP diet on symptoms, fecal microbiome, and markers of inflammation in patients with quiescent inflammatory bowel disease in a randomized trial. Gastroenterology. 2020;158(1):176–88..e7. [DOI] [PubMed] [Google Scholar]
- 95. Zhan YL, Zhan YA, Dai SX. Is a low FODMAP diet beneficial for patients with inflammatory bowel disease? A meta-analysis and systematic review. Clin Nutr. 2018;37(1):123–9. [DOI] [PubMed] [Google Scholar]
- 96. Colombel JF, Shin A, Gibson PR. AGA clinical practice update on functional gastrointestinal symptoms in patients with inflammatory bowel disease: expert review. Clin Gastroenterol Hepatol. 2019;17(3):380–90.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Barrett JS. How to institute the low-FODMAP diet. J Gastroenterol Hepatol. 2017;32:8–10. [DOI] [PubMed] [Google Scholar]
- 98. de Castro MM, Corona LP, Pascoal LB, Miyamoto JE, Ignacio-Souza LM, de Lourdes Setsuko Ayrizono Met al. . Dietary patterns associated to clinical aspects in Crohn's disease patients. Sci Rep. 2020;10(1):7033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Britto S, Kellermayer R. Carbohydrate monotony as protection and treatment for inflammatory bowel disease. J Crohns Colitis. 2019;13(7):942–8. [DOI] [PubMed] [Google Scholar]
- 100. Limketkai BN, Sepulveda R, Hing T, Shah ND, Choe M, Limsui Det al. . Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand J Gastroenterol. 2018;53(2):147–51. [DOI] [PubMed] [Google Scholar]
- 101. Aziz I, Branchi F, Pearson K, Priest J, Sanders DS. A study evaluating the bidirectional relationship between inflammatory bowel disease and self-reported non-celiac gluten sensitivity. Inflamm Bowel Dis. 2015;21(4):847–53. [DOI] [PubMed] [Google Scholar]
- 102. Sapone A, Lammers KM, Casolaro V, Cammarota M, Giuliano MT, De Rosa Met al. . Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Medicine. 2011;9(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hwang C, Ross V, Mahadevan U. Popular exclusionary diets for inflammatory bowel disease: the search for a dietary culprit. Inflamm Bowel Dis. 2014;20(4):732–41. [DOI] [PubMed] [Google Scholar]
- 104. Potter MD, Walker MM, Jones MP, Koloski NA, Keely S, Talley NJ. Letter: gluten sensitivity in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48(10):1167–8. [DOI] [PubMed] [Google Scholar]
- 105. Shepherd SJ, Gibson PR. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J Hum Nutr Diet. 2013;26(4):349–58. [DOI] [PubMed] [Google Scholar]
- 106. Lee AR, Ng DL, Zivin J, Green PH. Economic burden of a gluten-free diet. J Hum Nutr Diet. 2007;20(5):423–30. [DOI] [PubMed] [Google Scholar]
- 107. Levine A, Wine E, Assa A, Sigall Boneh R, Shaoul R, Kori Met al. . Crohn's disease exclusion diet plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157(2):440–50.e8..e8. [DOI] [PubMed] [Google Scholar]
- 108. Dickson I. Exclusion diet plus partial enteral nutrition sustains remission in children with Crohn's disease. Nat Rev. Gastroenterol Hepatol. 2019;16:454. [DOI] [PubMed] [Google Scholar]
- 109. Sigall Boneh R, Van Limbergen J, Wine E, Assa A, Shaoul R, Milman Pet al. . Dietary therapies induce rapid response and remission in pediatric patients with active Crohn's disease. Clin Gastroenterol Hepatol. 2021;19(4):752–9. [DOI] [PubMed] [Google Scholar]
- 110. Yanai H, Levine A, Hirsch A, Boneh RS, Kopylov U, Eran HBet al. . The Crohn's disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn's disease (CDED-AD): an open-label, pilot, randomised trial. Lancet. Gastroenterol Hepatol. 2022;7(1):49–59. [DOI] [PubMed] [Google Scholar]
- 111. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhang X, Zou QH, Zhao BT, Zhang JW, Zhao WY, Li YTet al. . Effects of alternate-day fasting, time-restricted fasting and intermittent energy restriction DSS-induced on colitis and behavioral disorders. Redox Biol. 2020;32:101535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hsieh MS, Hsu WH, Wang JW, Wang YK, Hu HM, Chang WKet al. . Nutritional and dietary strategy in the clinical care of inflammatory bowel disease. J Formos Med Assoc. 2020;119(12):1742–9. [DOI] [PubMed] [Google Scholar]
- 115. Konner M, Eaton SB. Paleolithic nutrition: twenty-five years later. Nutr Clin Pract. 2010;25(6):594–602. [DOI] [PubMed] [Google Scholar]
- 116. Eaton SB, Konner M. Paleolithic nutrition. A consideration of its nature and current implications. N Engl J Med. 1985;312(5):283–9. [DOI] [PubMed] [Google Scholar]
- 117. Tóth C, Dabóczi A, Howard M, Miller N, Clemens Z. Crohn's disease successfully treated with the paleolithic ketogenic diet. International Journal of Case Reports and Images. 2016;7(9):570–8. [Google Scholar]
- 118. Tognini P, Murakami M, Liu Y, Eckel-Mahan KL, Newman JC, Verdin Eet al. . Distinct circadian signatures in liver and gut clocks revealed by ketogenic diet. Cell Metab. 2017;26(3):523–38.e5. [DOI] [PubMed] [Google Scholar]
- 119. Norwitz NG, Loh V. A standard lipid panel is insufficient for the care of a patient on a high-fat, low-carbohydrate ketogenic diet. Frontiers in Medicine. 2020;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Youm Y-H, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim Det al. . The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cheng CW, Biton M, Haber AL, Gunduz N, Eng G, Gaynor LTet al. . Ketone body signaling mediates intestinal stem cell homeostasis and adaptation to diet. Cell. 2019;178(5):1115–31.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hang SY, Paik D, Yao LN, Kim E, Jamma T, Lu JPet al. . Bile acid metabolites control T(H)17 and T-reg cell differentiation. Nature. 2019;576(7785):143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Yilmaz B, Juillerat P, Oyas O, Ramon C, Bravo FD, Franc Yet al. . Microbial network disturbances in relapsing refractory Crohn's disease. Nat Med. 2019;25(2):323–36. [DOI] [PubMed] [Google Scholar]
- 124. Imhann F, Vila AV, Bonder MJ, Fu JY, Gevers D, Visschedijk MCet al. . Interplay of host genetics and gut microbiota underlying the onset and clinical presentation of inflammatory bowel disease. Gut. 2018;67(1):108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish Eet al. . Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66(11):5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Schaubeck M, Clavel T, Calasan J, Lagkouvardos I, Haange SB, Jehmlich Net al. . Dysbiotic gut microbiota causes transmissible Crohn's disease-like ileitis independent of failure in antimicrobial defence. Gut. 2016;65(2):225–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul Let al. . Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55(2):205–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Martinez-Medina M, Aldeguer X, Gonzalez-Huix F, Acero D, Garcia-Gil LJ. Abnormal microbiota composition in the ileocolonic mucosa of Crohn's disease patients as revealed by polymerase chain reaction-denaturing gradient gel electrophoresis. Inflamm Bowel Dis. 2006;12(12):1136–45. [DOI] [PubMed] [Google Scholar]
- 129. Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DVet al. . Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Gevers D, Kugathasan S, Denson LA, Vazquez-Baeza Y, Van Treuren W, Ren Bet al. . The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15(3):382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci. 2007;104(34):13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJet al. . Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci. 2008;105(43):16731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gophna U, Sommerfeld K, Gophna S, Doolittle WF, Veldhuyzen van Zanten SJ. Differences between tissue-associated intestinal microfloras of patients with Crohn's disease and ulcerative colitis. J Clin Microbiol. 2006;44(11):4136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Willing BP, Dicksved J, Halfvarson J, Andersson AF, Lucio M, Zheng ZLet al. . A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology. 2010;139(6):1844–54..e1. [DOI] [PubMed] [Google Scholar]
- 135. Winter SE, Lopez CA, Bäumler AJ. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013;14(4):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AMet al. . Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science. 2013;339(6120):708–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Chanin RB, Winter MG, Spiga L, Hughes ER, Zhu WH, Taylor SJet al. . Epithelial-derived reactive oxygen species enable appbcx-mediated aerobic respiration of Escherichia coli during intestinal inflammation. Cell Host & Microbe. 2020;28(6):780–8..e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cani PD. Gut cell metabolism shapes the microbiome. Science. 2017;357(6351):548–9. [DOI] [PubMed] [Google Scholar]
- 139. Rehman A, Rausch P, Wang J, Skieceviciene J, Kiudelis G, Bhagalia Ket al. . Geographical patterns of the standing and active human gut microbiome in health and IBD. Gut. 2016;65(2):238–48. [DOI] [PubMed] [Google Scholar]
- 140. Vich Vila A, Imhann F, Collij V, Jankipersadsing SA, Gurry T, Mujagic Zet al. . Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci Transl Med. 2018;10(472):eaap8914. [DOI] [PubMed] [Google Scholar]
- 141. Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker Set al. . Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nature Microbiology. 2019;4(2):293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Zheng HH, Chen MY, Li Y, Wang YY, Wei L, Liao ZQet al. . Modulation of gut microbiome composition and function in experimental colitis treated with sulfasalazine. Frontiers in Microbiology. 2017;8:1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Moustafa A, Li WZ, Anderson EL, Wong EHM, Dulai PS, Sandborn WJet al. . Genetic risk, dysbiosis, and treatment stratification using host genome and gut microbiome in inflammatory bowel disease. Clinical and Translational Gastroenterology. 2018;9(1):e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clinical Journal of Gastroenterology. 2018;11(1):1–10. [DOI] [PubMed] [Google Scholar]
- 145. Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KYet al. . Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Liu JZ, van Sommeren S, Huang HL, Ng SC, Alberts R, Takahashi Aet al. . Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015;47(9):979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Leone V, Chang EB, Devkota S. Diet, microbes, and host genetics: the perfect storm in inflammatory bowel diseases. J Gastroenterol. 2013;48(3):315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Haberman Y, Tickle TL, Dexheimer PJ, Kim M-O, Tang D, Karns Ret al. . Pediatric Crohn disease patients exhibit specific ileal transcriptome and microbiome signature. J Clin Invest. 2014;124(8):3617–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Frank DN, Robertson CE, Hamm CM, Kpadeh Z, Zhang TY, Chen HYet al. . Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17(1):179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Rothschild D, Weissbrod O, Barkan E, Kurilshikov A, Korem T, Zeevi Det al. . Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555(7695):210–5. [DOI] [PubMed] [Google Scholar]
- 151. Turpin W, Lee SH, Raygoza Garay JA, Madsen KL, Meddings JB, Bedrani Let al. . Increased intestinal permeability is associated with later development of Crohn's disease. Gastroenterology. 2020;159(6):2092–100.e5. [DOI] [PubMed] [Google Scholar]
- 152. Zeissig S, Buergel N, Guenzel D, Richter J, Mankertz J, Wahnschaffe Uet al. . Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut. 2007;56(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Natividad JMM, Verdu EF. Modulation of intestinal barrier by intestinal microbiota: pathological and therapeutic implications. Pharmacol Res. 2013;69(1):42–51. [DOI] [PubMed] [Google Scholar]
- 154. Pagano E, Romano B, Iannotti FA, Parisi OA, D'Armiento M, Pignatiello Set al. . The non-euphoric phytocannabinoid cannabidivarin counteracts intestinal inflammation in mice and cytokine expression in biopsies from UC pediatric patients. Pharmacol Res. 2019;149:104464. [DOI] [PubMed] [Google Scholar]
- 155. Shawki A, McCole DF. Mechanisms of intestinal epithelial barrier dysfunction by adherent-invasive Escherichia coli. Cellular and Molecular Gastroenterology and Hepatology. 2017;3(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Loubinoux J, Bronowicki JP, Pereira IAC, Mougenel JL, Le Faou AE. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol Ecol. 2002;40(2):107–12. [DOI] [PubMed] [Google Scholar]
- 157. Rowan F, Docherty NG, Murphy M, Murphy B, Coffey JC, O'Connell PR. Desulfovibrio bacterial species are increased in ulcerative colitis. Diseases of the Colon & Rectum. 2010;53(11):1530–6. [DOI] [PubMed] [Google Scholar]
- 158. Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134(2):577–94. [DOI] [PubMed] [Google Scholar]
- 159. Lutter L, Hoytema van Konijnenburg DP, Brand EC, Oldenburg B, van Wijk F. The elusive case of human intraepithelial T cells in gut homeostasis and inflammation. Nat Rev Gastroenterol Hepatol. 2018;15(10):637–49. [DOI] [PubMed] [Google Scholar]
- 160. Britton GJ, Contijoch EJ, Mogno I, Vennaro OH, Llewellyn SR, Ng Ret al. . Microbiotas from humans with inflammatory bowel disease alter the balance of gut Th17 and RORγt(+) regulatory T cells and exacerbate colitis in mice. Immunity. 2019;50(1):212–24..e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Lindsay JO, Whelan K, Stagg AJ, Gobin P, HO Al-H, Rayment Net al. . Clinical, microbiological, and immunological effects of fructo-oligosaccharide in patients with Crohn's disease. Gut. 2006;55(3):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Stagg AJ, Hart AL, Knight SC, Kamm MA. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut. 2003;52(10):1522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Lecerf JM, Dépeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi Let al. . Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr. 2012;108(10):1847–58. [DOI] [PubMed] [Google Scholar]
- 164. Bjerrum JT, Wang YL, Hao FH, Coskun M, Ludwig C, Günther Uet al. . Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn's disease and healthy individuals. Metabolomics. 2015;11(1):122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. De Preter V, Machiels K, Joossens M, Arijs I, Matthys C, Vermeire Set al. . Faecal metabolite profiling identifies medium-chain fatty acids as discriminating compounds in IBD. Gut. 2015;64(3):447–58. [DOI] [PubMed] [Google Scholar]
- 166. Nikolaus S, Schulte B, Al-Massad N, Thieme F, Schulte DM, Bethge Jet al. . Increased tryptophan metabolism is associated with activity of inflammatory bowel diseases. Gastroenterology. 2017;153(6):1504–16..e2. [DOI] [PubMed] [Google Scholar]
- 167. Kolho K-L, Pessia A, Jaakkola T, de Vos WM, Velagapudi V. Faecal and serum metabolomics in paediatric inflammatory bowel disease. J Crohns Colitis. 2017;11(3):321–34. [DOI] [PubMed] [Google Scholar]
- 168. Le Gall G, Noor SO, Ridgway K, Scovell L, Jamieson C, Johnson ITet al. . Metabolomics of fecal extracts detects altered metabolic activity of gut microbiota in ulcerative colitis and irritable bowel syndrome. J Proteome Res. 2011;10(9):4208–18. [DOI] [PubMed] [Google Scholar]
- 169. Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut Vet al. . A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–83. [DOI] [PubMed] [Google Scholar]
- 170. Kaiko GE, Ryu SH, Koues OI, Collins PL, Solnica-Krezel L, Pearce EJet al. . The colonic crypt protects stem cells from microbiota-derived metabolites. Cell. 2016;165(7):1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong Set al. . Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6(1):6734. [DOI] [PubMed] [Google Scholar]
- 172. Annese V, Rogai F, Settesoldi A, Bagnoli S. PPARγ in inflammatory bowel disease. PPAR Research. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe Get al. . Butyrate-producing bacteria supplemented in vitro to Crohn's disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep. 2017;7(1):11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de los Reyes-Gavilan CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Frontiers in Microbiology. 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Tan J, McKenzie C, Potamitis M, Thorburn AN, Mackay CR, Macia L. The role of short-chain fatty acids in health and disease. Adv Immunol. 2014;121:91–119. [DOI] [PubMed] [Google Scholar]
- 176. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YMet al. . The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Macfarlane GT, Macfarlane S. Bacteria, colonic fermentation, and gastrointestinal health. JAOAC Int. 2012;95(1):50–60. [DOI] [PubMed] [Google Scholar]
- 178. Peng LY, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. 2009;139(9):1619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa Het al. . T-reg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–6. [DOI] [PubMed] [Google Scholar]
- 180. Kim M, Qie YQ, Park J, Kim CH. Gut microbial metabolites fuel host antibody responses. Cell Host & Microbe. 2016;20(2):202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sun M, Wu W, Liu ZJ, Cong YZ. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol. 2017;52(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Holt JA, Luo GZ, Billin AN, Bisi J, McNeill YY, Kozarsky KFet al. . Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes Dev. 2003;17(13):1581–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato Het al. . Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9. [DOI] [PubMed] [Google Scholar]
- 184. Pavlidis P, Powell N, Vincent RP, Ehrlich D, Bjarnason I, Hayee B. Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence?. Aliment Pharmacol Ther. 2015;42(7):802–17. [DOI] [PubMed] [Google Scholar]
- 185. Song XY, Sun XM, Oh SF, Wu M, Zhang YB, Zheng Wet al. . Microbial bile acid metabolites modulate gut RORγ(+) regulatory T cell homeostasis. Nature. 2020;577(7790):410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Labbé A, Ganopolsky JG, Martoni CJ, Prakash S, Jones ML. Bacterial bile metabolising gene abundance in Crohn's, ulcerative colitis and type 2 diabetes metagenomes. PLoS One. 2014;9(12):e115175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Duboc H, Rajca S, Rainteau D, Benarous D, Maubert MA, Quervain Eet al. . Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62(4):531–9. [DOI] [PubMed] [Google Scholar]
- 188. Das P, Marcišauskas S, Ji B, Nielsen J. Metagenomic analysis of bile salt biotransformation in the human gut microbiome. BMC Genomics. 2019;20(1):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Van den Bossche L, Hindryckx P, Devisscher L, Devriese S, Van Welden S, Holvoet Tet al. . Ursodeoxycholic acid and its taurine- or glycine-conjugated species reduce colitogenic dysbiosis and equally suppress experimental colitis in mice. Appl Environ Microbiol. 2017;83(7):e02766–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Schaap FG, Trauner M, Jansen PLM. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11(1):55–67. [DOI] [PubMed] [Google Scholar]
- 191. Lavelle A, Sokol H. Gut microbiota-derived metabolites as key actors in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2020;17(4):223–37. [DOI] [PubMed] [Google Scholar]
- 192. Sun MG, Ma N, He T, Johnston LJ, Ma X. Tryptophan (Trp) modulates gut homeostasis via aryl hydrocarbon receptor (AhR). Crit Rev Food Sci Nutr. 2020;60(10):1760–8. [DOI] [PubMed] [Google Scholar]
- 193. Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone Let al. . Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology. 2011;141(1):237–48..e1. [DOI] [PubMed] [Google Scholar]
- 194. Lamas B, Richard ML, Leducq V, Pham HP, Michel ML, Da Costa Get al. . CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat Med. 2016;22(6):598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Takamura T, Harama D, Fukumoto S, Nakamura Y, Shimokawa N, Ishimaru Ket al. . Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunology & Cell Biology. 2011;89(7):817–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Wlodarska M, Luo CW, Kolde R, d'Hennezel E, Annand JW, Heim CEet al. . Indoleacrylic acid produced by commensal peptostreptococcus species suppresses inflammation. Cell Host & Microbe. 2017;22(1):25–37..e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SAet al. . Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Muegge BD, Kuczynski J, Knights D, Clemente JC, Gonzalez A, Fontana Let al. . Diet drives convergence in gut microbiome functions across mammalian phylogeny and within humans. Science. 2011;332(6032):970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lea JC, Lee HY, Kim TK, Kim MS, Park YM, Kim Jet al. . Obesogenic diet-induced gut barrier dysfunction and pathobiont expansion aggravate experimental colitis. PLoS One. 2017;12(11):e0187515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Agus A, Denizot J, Thévenot J, Martinez-Medina M, Massier S, Sauvanet Pet al. . Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci Rep. 2016;6(1):19032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Kamali Dolatabadi R, Feizi A, Halaji M, Fazeli H, Adibi P. The prevalence of adherent-invasive Escherichia coli and its association with inflammatory bowel diseases: a systematic review and meta-analysis. Frontiers in Medicine. 2021;8:730243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Puertollano E, Kolida S, Yaqoob P. Biological significance of short-chain fatty acid metabolism by the intestinal microbiome. Curr Opin Clin Nutr Metab Care. 2014;17(2):139–44. [DOI] [PubMed] [Google Scholar]
- 204. Birchenough G, Schroeder BO, Bäckhed F, Hansson GC. Dietary destabilisation of the balance between the microbiota and the colonic mucus barrier. Gut Microbes. 2019;10(2):246–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Chassaing B, Koren O, Carvalho FA, Ley RE, Gewirtz AT. AIEC pathobiont instigates chronic colitis in susceptible hosts by altering microbiota composition. Gut. 2014;63(7):1069–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli Aet al. . Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10(−/−) mice. Nature. 2012;487(7405):104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Yet al. . Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hernandez AL, Kitz A, Wu C, Lowther DE, Rodriguez DM, Vudattu Net al. . Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125(11):4212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi Let al. . High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65(11):1812–21. [DOI] [PubMed] [Google Scholar]
- 210. Marlow G, Ellett S, Ferguson IR, Zhu ST, Karunasinghe N, Jesuthasan AC, et al. Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn's disease patients. Hum Genomics. 2013;7(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Duncan SH, Hold GL, Barcenilla A, Stewart CS, Flint HJ. Roseburia intestinalis sp nov., a novel saccharolytic, butyrate-producing bacterium from human faeces. Int J Syst Evol Microbiol. 2002;52:1615–20. [DOI] [PubMed] [Google Scholar]
- 212. Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on gut microbiota associated to Mediterranean diet adherence and specific dietary intakes on general adult population. Frontiers in Microbiology. 2018;9:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ott SJ, Schreiber S. Reduced microbial diversity in inflammatory bowel diseases. Gut. 2006;55(8):1207. [PMC free article] [PubMed] [Google Scholar]
- 214. Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “Western-lifestyle” inflammatory diseases. Immunity. 2014;40(6):833–42. [DOI] [PubMed] [Google Scholar]
- 215. Roberts CL, Keita AV, Duncan SH, O'Kennedy N, Söderholm JD, Rhodes JMet al. . Translocation of Crohn's disease Escherichia coli across M-cells: contrasting effects of soluble plant fibres and emulsifiers. Gut. 2010;59(10):1331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Maslowski KM, Mackay CR. Diet, gut microbiota and immune responses. Nat Immunol. 2011;12(1):5–9. [DOI] [PubMed] [Google Scholar]
- 217. Quivy V, Van Lint C. Regulation at multiple levels of NF-kappaB-mediated transactivation by protein acetylation. Biochem Pharmacol. 2004;68(6):1221–9. [DOI] [PubMed] [Google Scholar]
- 218. Monteleone I, MacDonald TT, Pallone F, Monteleone G. The aryl hydrocarbon receptor in inflammatory bowel disease: linking the environment to disease pathogenesis. Curr Opin Gastroenterol. 2012;28(4):310–3. [DOI] [PubMed] [Google Scholar]
- 219. Kakodkar S, Mikolaitis SL, Engen P, Forsyth CB, Mutlu E. The effect of the SCD diet on gut bacterial fingerprints in IBD. Gastroenterology. 2012;142(5):S–395. [Google Scholar]
- 220. Kakodkar S, Mikolaitis S, Engen P, Mutlu E. The bacterial microbiome of IBD patients on the specific carbohydrate diet (SCD). Am J Gastroenterol. 2013;108:S552. [Google Scholar]
- 221. Suskind DL, Cohen SA, Brittnacher MJ, Wahbeh G, Lee D, Shaffer MLet al. . Clinical and fecal microbial changes with diet therapy in active inflammatory bowel disease. J Clin Gastroenterol. 2018;52(2):155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. 2015;64(1):93–100. [DOI] [PubMed] [Google Scholar]
- 223. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Muir JG, Gibson PR. Consistent prebiotic effect on gut microbiota with altered FODMAP intake in patients with Crohn's disease: a randomised, controlled cross-over trial of well-defined diets. Clinical and Translational Gastroenterology. 2016;7(4):e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Gill A, Fedorak R, Park H, Hotte N, Ginter R, Keshteli AHet al. . Short-term exposure to a high sugar diet reduces short chain fatty acid production and increases susceptibility to colitis. Journal of the Canadian Association of Gastroenterology. 2018;1(suppl_1):16–7. [Google Scholar]
- 225. Gearry RB, Irving PM, Barrett JS, Nathan DM, Shepherd SJ, Gibson PR. Reduction of dietary poorly absorbed short-chain carbohydrates (FODMAPs) improves abdominal symptoms in patients with inflammatory bowel disease—a pilot study. J Crohns Colitis. 2009;3(1):8–14. [DOI] [PubMed] [Google Scholar]
- 226. Lührs H, Gerke T, Müller JG, Melcher R, Schauber J, Boxberge Fet al. . Butyrate inhibits NF-kappaB activation in lamina propria macrophages of patients with ulcerative colitis. Scand J Gastroenterol. 2002;37(4):458–66. [DOI] [PubMed] [Google Scholar]
- 227. Segain JP, Raingeard de la Blétière D, Bourreille A, Leray V, Gervois N, Rosales Cet al. . Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn's disease. Gut. 2000;47(3):397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra Get al. . Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Bonder MJ, Tigchelaar EF, Cai XH, Trynka G, Cenit MC, Hrdlickova Bet al. . The influence of a short-term gluten-free diet on the human gut microbiome. Genome Medicine. 2016;8(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. De Palma G, Nadal I, Collado MC, Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult human subjects. Br J Nutr. 2009;102(8):1154–60. [DOI] [PubMed] [Google Scholar]
- 231. Sanz Y. Effects of a gluten-free diet on gut microbiota and immune function in healthy adult humans. Gut Microbes. 2010;1(3):135–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Damaskos D, Kolios G. Probiotics and prebiotics in inflammatory bowel disease: microflora ‘on the scope’. Br J Clin Pharmacol. 2008;65(4):453–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Bauer E, Thiele I. From metagenomic data to personalized in silico microbiotas: predicting dietary supplements for Crohn's disease. NPJ Systems Biology and Applications. 2018;4(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Sasson AN, Ingram RJM, Zhang Z, Taylor LM, Ananthakrishnan AN, Kaplan GGet al. . The role of precision nutrition in the modulation of microbial composition and function in people with inflammatory bowel disease. Lancet Gastroenterol Hepatol. 2021;6(9):754–69. [DOI] [PubMed] [Google Scholar]