ABSTRACT
This systematic review and meta-analysis aimed to investigate the effect of dietary-based lifestyle modification interventions (“diet,” or “diet + exercise,” or “diet + exercise + behavioral” intervention) on the measures of anthropometric and dietary intake parameters in women with breas cancer (BC). Databases were searched until June 2021. Inclusion criteria were randomized controlled trials that enrolled only women with BC. Studies that used exercise or behavioral interventions alone were not included. Mean ± SD changes were extracted for each outcome, and pooled using a random-effects model; 7315 studies were identified. Fifty-one studies (n = 7743) were included. The median ± SD duration of treatment was 24 ± 16.65 wk. Dietary-based interventions significantly reduced body weight [45 studies (n = 7239), weighted mean difference (WMD) (95% CI): −2.6 (−3.2, −2.1) kg], BMI [31 studies (n = 5384); WMD (95% CI): −1.0 (−1.3, −0.7) kg/m2], lean body mass [15 studies (n = 1194); WMD (95% CI): −0.6(−0.7, −0.4) kg], fat mass [11 studies (n = 913); WMD (95% CI): –2.6 (−3.3, −1.8) kg], fat percentage [17 studies (n = 897); WMD (95% CI): −1.5 (−1.9, −1.3)%], hip circumference [9 studies (n = 489); WMD (95% CI): −2.43 (−3.34, −1.54) cm], and waist circumference [7 studies (n = 309); WMD (95% CI): 0.02 (−0.03, −0.005) cm]. Significant reductions in energy intakes [20 studies (n = 4608), WMD (95% CI): −162 (−220, 104) kcal/d] and fat intakes [7 studies (n = 4316), WMD (95% CI): −7.5 (−7.8, −7.2)% of energy/d], and an increase in fiber intakes [11 studies (n = 4241), WMD (95% CI): 2.4 (0.7, 4.1) g/d] were observed. No significant changes were seen in protein, carbohydrate, and fruit and vegetable intakes. Subgroup analyses showed that changes in anthropometric and dietary intake indices were significant in studies that enrolled patients with both obesity and normal weight, studies that used diet therapy in combination with exercise and behavioral therapy, and studies that started the intervention during the treatment period. Overall, a multimodal dietary-based lifestyle intervention had significant effects on anthropometric and dietary intake parameters in women with BC, specifically when started as early as the diagnosis. This meta-analysis was registered at PROSPERO as CRD42021291488.
Keywords: nutrition therapy, body weight, nutritional intake, breast neoplasms, cancer survivors, epidemiologic research design
Statement of Significance: The summary analysis of 51 eligible randomized controlled trials indicated that a multimodal, diet-based intervention with a median intervention duration of 24 wk resulted in a significant decrease in weight, BMI, waist circumference, hip circumference, body fat percentage, and lean body mass in women with breast cancer, regardless of their baseline BMI values, specifically when started as early as the diagnosis. In addition, daily caloric and fat intake were significantly reduced, while dietary fiber intake increased significantly.
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer among women. The 5-y relative survival rate of BC is 89.7% and improving (1). A series of recent surveys found that women with BC gain weight during and after cancer therapy (2). In fact, an increased body weight, an unhealthy dietary intake, and physical inactivity are reported in women with BC who are either normal weight or overweight and obese (3), which can increase both the expected duration of treatment and the risk of BC recurrence (3). The dietary intake status of patients with BC is a critical factor for maintaining an appropriate nutritional status and a healthy body weight both during and after cancer treatment (4, 5). For these reasons, lifestyle modification intervention in this population is important in order to achieve ideal body weight and a balanced dietary intake status, all of which will improve the overall survival rate (6). Changes in dietary intake characteristics, as well as physical activity and behavioral status, are all part of a comprehensive lifestyle modification approach, especially for noncommunicable diseases (7, 8). In fact, the improvements in dietary and physical activity parameters in women with BC may be more easily achieved with behavioral counseling support, which enhances the likelihood of long-term successful adoption of these habits (9).
Several studies have found beneficial effects of different dietary-based lifestyle modification approaches, including dietary interventions alone (10, 11), or in combination with either exercise (12, 13) or exercise and behavioral therapy during or after the treatment period in women with BC (14, 15). In fact, studies have investigated the impact of different lifestyle modification strategies, such as restricting dietary energy intake (16, 17), changing eating behaviors (9, 18), and increasing energy expenditure through physical activity among women with BC (19, 20), and have found both significant and nonsignificant findings.
A recent meta-analysis of 5 randomized controlled trials (RCTs), including 722 participants, reported that a dietary intervention alone or in combination with physical activity led to a significant reduction in weight, waist circumference (WC), and body fat mass in patients with colorectal cancer and BC (21). Another meta-analysis of 9 RCTs (with 462 participants) assessed the effects of different dietary management, including nutritional education and low-fat or low-calorie diets on anthropometric indices in women with BC and reported that dietary management strategies significantly reduced BMI and hip circumference (HC) but had no effect on weight and other anthropometric indices (22).
Given the lack of consensus on the role of dietary-based lifestyle modification strategies in maintaining optimal weight, body composition, and dietary intake status in women with BC, it is critical to identify the most appropriate and acceptable diet-based lifestyle modification strategy for this population. The goal of our systematic review and meta-analysis of RCTs is to synthesize the literature on the effects of various dietary-based lifestyle modification strategies, such as dietary intervention alone or in combination with physical activity, or physical activity and behavioral therapy as a comprehensive lifestyle modification intervention, on anthropometric and body composition indices, as well as dietary intake parameters, in women with BC either during or after the treatment period. The present study will suggest the appropriate strategy to recommend and for which group of women with BC regarding their treatment status, BMI, and other baseline characteristics in order to assist both women with BC and health care providers in achieving a comprehensive guide in survivorship care.
Methods
This systematic review and meta-analysis was previously registered at the International Prospective Register of Systematic Reviews (PROSPERO; registered on 15 November 2021 with the registration number CRD42021291488) (23). It was also conducted in accordance with the format of the Cochrane Handbook for Systematic Reviews of Intervention following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (24).
Data sources and search strategy
According to the PICO (Population, Intervention, Comparison, and Outcomes) tool for performing search strategies in systematic reviews and meta-analysis endorsed by Cochrane Collaborations (24), the related components consisted of “women” with BC, dietary-based lifestyle modification approaches as the “Intervention,” any type of control group as the “Comparator,” and anthropometric parameters and dietary intake indices as the “Outcomes” of measure. However, the related keywords for the present systematic review and meta-analysis consisted of only 2 separate components, including the associated keywords for women with BC (the population type) and the keywords for dietary-based lifestyle modification (the intervention type), which were concatenated separately, with the Boolean operator “OR,” and “AND” for the combination between them. The keywords were selected based on the Medical Subject Headings (MeSH) database and other related non-MeSH terms. In addition, we conducted gray literature searches and a manual search of conference abstracts, theses, and clinical trial registries.
MEDLINE via PubMed, Scopus, ISI Web of Science, Cochrane, the Central Register of Controlled Trials (CENTRAL), and Embase were searched until June 2021. Supplemental Table 1 contains all of the details on the precise keywords and the search strategy.
Eligibility criteria
Inclusion criteria consisted of RCTs involving women with BC who were 18 y or older and had been diagnosed with the condition (either during or after cancer therapy) and which met the following:
Assessed the effect of dietary-based lifestyle modification approaches, including “dietary intervention,” “dietary and physical activity intervention,” “dietary and behavioral therapy,” or “dietary intervention with physical activity and behavioral therapy.”
Reported changes in any of the anthropometric indices including weight, BMI, WC, HC, waist-to-hip ratio (WHR), lean body mass, fat mass, and fat percentage or dietary intake parameters, including daily levels of energy, carbohydrate, protein, fat, fiber, and fruit and vegetable intakes.
Exclusion criteria were as follows: animal or in vitro studies; retrospective, pretest–post-test, prospective cohort, and cross-sectional investigations; studies that did not assess either anthropometric or dietary intake parameters as primary or secondary outcomes; studies without full texts; articles that reported the same results of a previous publication; studies that did not report changes in any of the related outcomes; and studies without control groups. Furthermore, studies that included individuals with different forms of cancer or used interventions other than dietary-based lifestyle modification techniques were omitted (i.e., drug therapies, any kind of nutritional supplements, or specific diets, such as ketogenic or fasting diets).
No language restriction was considered. The titles and abstracts of the articles according to the eligibility criteria were screened by 3 main reviewers (MRL, SV, and MZ). Following the initial screening, the data were compared in a group discussion, and the final agreement was reached by consensus after the fourth reviewer (ER) resolved any disagreements about the eligibility of specific studies. The relevant articles were then retrieved for further screening. EndNote software was used to automatically eliminate duplicates (version 8.9, Thomson Reuters).
Outcomes and prioritization
Primary outcomes included mean changes in 1) body weight, 2) BMI, and 3) total energy intakes from the baseline measures, and secondary outcomes consisted of mean changes in 1) WC, 2) HC, 3) WHR, 4) lean mass, 5) fat mass, and 6) body fat percentage, as well as changes in 7) dietary carbohydrate, 8) protein, 9) total fat, 10) fiber, and 11) fruit and vegetable intakes compared with baseline levels.
Data extraction
Two review authors (MRL and SV) independently extracted the data from eligible studies. Except for the baseline information, the following data from every eligible article were collected: study design and setting; details of the intervention including the type of dietary-based lifestyle modification; as well as the study duration, outcomes of the measure, stage type of the disease, baseline BMI and the menopausal status of participants, and the status of treatment (i.e., participants were in the treatment period or had completed the treatment process).
The mean ± SD changes in the outcomes were used for the final meta-analysis. If SD was not provided, the SE was calculated from the available data and then converted to SDs, according to the formula reported in the Cochrane Handbook of Systematic Reviews (24) and the estimation methods obtained by Wan et al. (25). The investigators resolved any uncertainty and disagreement through group discussions with the fourth reviewer (ER). If essential information was missing from the published report, we contacted the study authors to obtain it. If we did not receive a response after 3 mo, we excluded that article.
Risk of bias in individual studies
The risk of bias for all included studies was independently evaluated by 1 reviewer (MRL) using the revised Cochrane risk-of-bias tool for randomized trials (RoB2) (26), which consists of 5 main domains, including bias arising from the randomization process, bias due to deviations from the intended interventions, bias due to missing outcome data, bias in the outcome measures, and bias related to the selection of reported results. Final judgments and overall risk of bias were defined as “low” or “high” risk of bias, or expressed as “some concerns.”
Quality of evidence
We also carried out the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to evaluate the overall quality of evidence according to the following domains: risk of bias, publication bias, imprecision of results, heterogeneity, and indirectness of evidence. Eventually, the quality of evidence was classified as “high,” “moderate,” “low,” or “very low” (27, 28).
Statistical analysis
When the optimal information size of every continuous outcome was calculated to be less than 400 participants in at least 1 of the 3 types of dietary-based interventions, the overall analyses for each outcome were performed using a combination of the 3 core elements of dietary-based lifestyle modification strategies (29).
The weighted mean difference (WMD) and its corresponding SE were extracted or calculated based on the mean differences between the intervention and control groups in each publication, and finally, they were pooled using the DerSimonian and Laird random-effects model, which takes the between-study variations into account. Between-studies heterogeneity was evaluated using Cochran's Q test and the I2 test. An I2 may represent moderate heterogeneity (if ranging from 30% to 50%), serious heterogeneity (if ranging from 50% to 75%), and very serious heterogeneity (if ranging from 75% to 100%) (30). Subgroup analyses were performed for those outcomes with more than 5 intervention comparisons. The following subgroup analyses were conducted to explain heterogeneity: the intervention duration (10–24 wk or >24 wk), the type of dietary-based lifestyle modification approaches (including dietary intervention alone, dietary and physical activity intervention, or dietary intervention with exercise and behavioral therapy), the composition of diet (low-calorie diet, nutrition education, or low-fat diet), the current status of the BC management (including the stage type of BC, and the status of surgery and chemotherapy or radiotherapy), the overall status of cancer treatment (i.e., participants were within the treatment period or had completed the treatment process), the type of control group, which was either a wait-list control or a control group without any specific intervention, and the baseline BMI categories of participants (participants with normal weight or overweight or obesity). Finally, the summary effect and the corresponding heterogeneity for each subgroup analysis were reported.
We assessed the presence of publication bias using visual inspection of funnel plots, and the degree of asymmetry was evaluated via Begg's adjusted rank correlation and Egger's tests (31). In the presence of publication bias, Duval and Tweedie's trim-and-fill method was used to correct funnel plot asymmetry (32). Sensitivity analyses were performed by excluding 1 study or a group of studies at a time to ensure the selected value did not influence the overall results. All statistical analyses were performed using Stata (version 14.2; StataCorp). Two-sided P values <0.05 were considered significant.
Results
Flow of studies
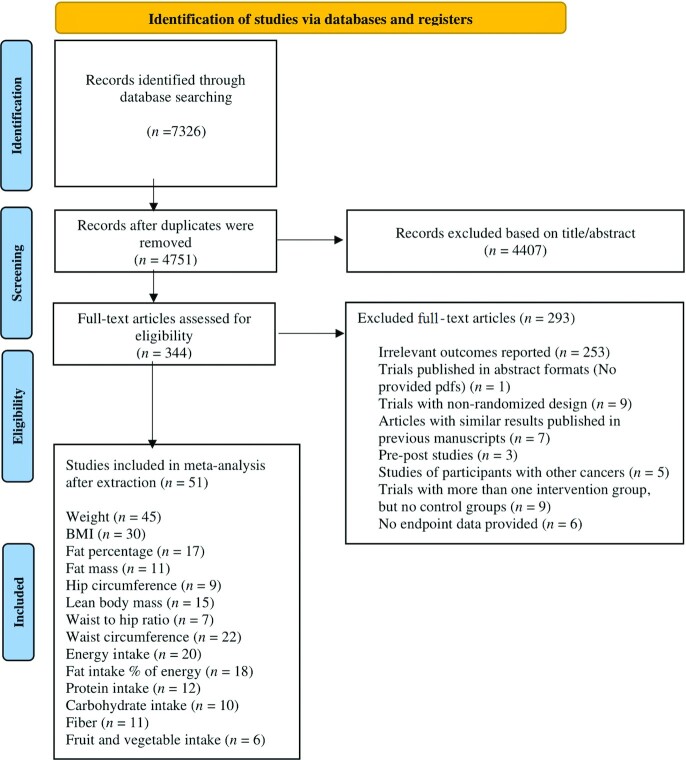
Figure 1 shows the selection of studies. Our literature search identified 7315 records. We removed 2575 duplicates, and after reviewing the titles and abstracts of the remaining articles, 344 records were subsequently selected for full-text screening. Of these articles, 253 studies were excluded due to irrelevant outcomes of measures or methodologies.
FIGURE 1.
Flow of the study.
Of the remaining 91 articles, 40 other studies were excluded for the following reasons: 9 studies did not use the randomization process (Supplemental References1–9), 5 articles included other types of cancer in addition to BC (Supplemental References 10–14), 9 studies did not have any control groups (Supplemental References 15–23), and 3 articles were pre-post studies (Supplemental References 24–26). In addition, 6 articles examined anthropometric and dietary intake parameters but did not publish final measurements, and none of the authors responded to our request for the final data (Supplemental References 27–32). The full text of 1 article was not available even after contacting the associated authors via e-mail (Supplemental Reference 33). Furthermore, some studies reported similar results, which were published previously and were excluded from the present study (Supplemental References 34–40).
Finally, 51 eligible articles were included in the present systematic review and meta-analysis (4, 5, 9–20, 33–69). The characteristics of the included studies are listed in Supplemental Table 2. Excluded articles are also reported in Supplemental Table 3.
Risk of bias and quality of evidence
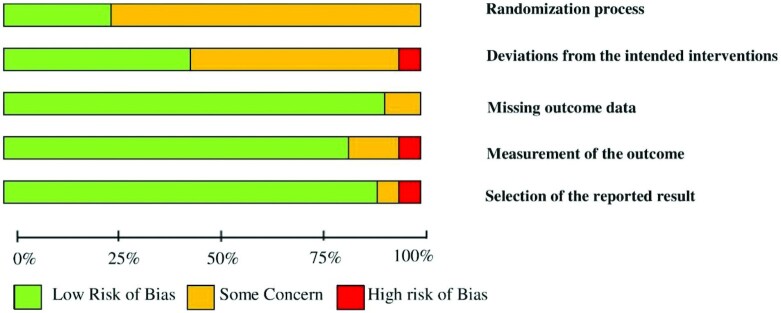
According to the revised RoB2 (Supplemental Table 4), only 10 studies were classified as “low” risk of bias (12, 13, 17, 18, 35, 57, 58, 60, 63, 66), 30 articles were assessed as “some concerns” (4, 5, 9, 11, 14, 16, 20, 33, 34, 36, 40–42, 44–49, 52–54, 56, 61, 62, 64, 65, 67–69), and 11 studies were judged to be at “high” risk of bias (10, 15, 19, 37–39, 43, 50, 51, 55, 59). Sufficient details on allocation concealment and randomization were not reported for 30 studies. Based on “the risk of bias due to deviations from the intended interventions,” 23 records represented a “low” risk of bias, and only 1 article reported a “high” risk of bias. One study was judged to be of “some concern” for “missing outcome data.” Moreover, 2 articles showed a “high” risk of bias and 6 studies tended to have “some concerns” after assessing the risk of bias based on the “measurement of the outcome.” In the assessment of the risk of bias based on “selection of the reported result,” 7 studies had a “some concerns” risk of bias, and 1 study had a “high” risk of bias (Supplemental Table 4). The summary of the risk of bias of included studies is shown in Figure 2. The GRADE quality of evidence was “low” for body weight and body fat percentage, as well as the intake of energy, dietary protein, fat, carbohydrates, and fruit and vegetables. “Moderate” quality of evidence was observed for BMI, WC, WHR, body fat mass, and dietary fiber intake, whereas only HC was found to have a “high” quality of evidence (Supplemental Table 5).
FIGURE 2.
Summary of risk of bias of the included studies.
Study characteristics
Supplemental Table 2 provides details on the characteristics of the included studies. Two studies had a crossover design (50, 53). The participants’ mean age ranged between 53 and 67 y old. Eleven studies were performed among patients with BC who were in their treatment process (10, 13, 19, 20, 38, 48, 54–57, 65). The remaining 40 studies included women with BC who completed the active treatment process, of which 3 studies were performed among women with lymphedema (17, 44, 45). The duration of the intervention varied from 5 to 100 wk. Twenty-eight studies assessed the effects of a low-calorie diet (4, 5, 9, 14–18, 20, 38, 44, 45, 47–54, 58, 62–64, 66–68). Eleven articles examined the effects of a low-fat diet (19, 33–36, 39, 42, 43, 46, 60, 65), and 12 studies investigated the effect of nutrition education (10, 11, 40, 41, 55–57, 59, 61, 69). Overall, 17 articles evaluated the effects of dietary intervention alone (10, 11, 13, 17, 33–46), 19 articles evaluated both dietary and physical activity interventions (12, 13, 16, 19, 20, 47–60), and 15 studies assessed the simultaneous effects of dietary intervention with physical activity and behavioral therapy (4, 5, 9, 14, 15, 61–69). Thirty-four studies included women with BC with overweight and obesity, while the remaining 17 articles further included participants with normal weight. None of the included studies only enrolled participants with normal weight.
Meta-analysis
Except for “body weight,” the optimal information size (OIS) was calculated to be less than 400 participants for at least 1 of the 3 types of dietary-based lifestyle modification approaches for every outcome (Supplemental Table 6). The data from the split analyses based on the 3 core elements of dietary-based interventions are included in Supplemental Table 7. The heterogeneities between the included studies for “body weight” were shown to be high in each of the 3 split analyses; as a result, a combined analysis was also performed for body weight to find out the overall sources of heterogeneities based on the main objectives of the present investigation.
Anthropometric factors
Body weight
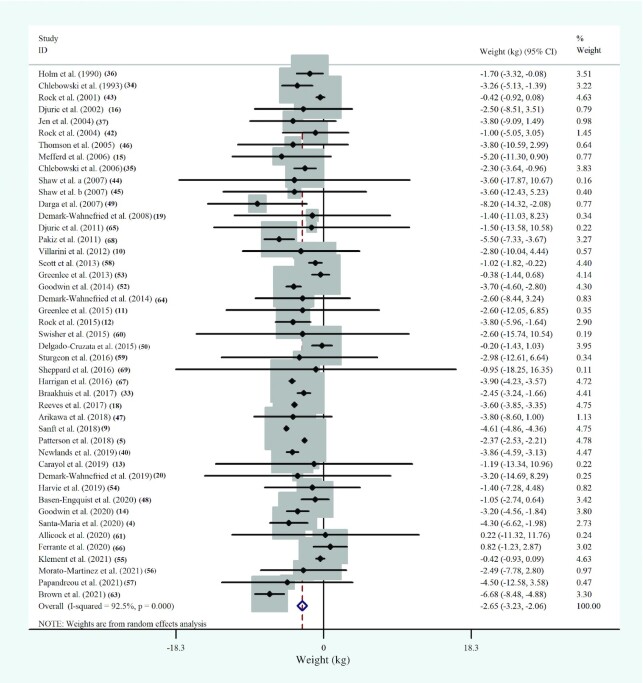
Among the included studies, 45 RCTs, including 7239 participants, evaluated the relation between dietary-based lifestyle modification interventions and body weight among women with BC. The WMDs in body-weight changes between the intervention and the control groups of studies are shown in Figure 3. Dietary-based lifestyle modification approaches significantly reduced weight in women with BC, while the between-study heterogeneity was reported to be high (WMD: −2.6; 95% CI: −3.2, −2.1 kg; P < 0.001; I2 = 92.5%; P-heterogeneity < 0.001) (Figure 3, Supplemental Table 7).
FIGURE 3.
Forest plot of randomized controlled trial studies showing weighted mean differences in “weight” change (in kg) between dietary-based lifestyle modification intervention and control groups for all eligible studies. Analysis was conducted using a random-effects model. Squares depict the weight assigned to the corresponding study; the diamond represents the summary effect. ES, effect size.
According to the subgroup analyses, body weight was reduced significantly in studies that included postmenopausal women with BC [10 studies (5, 14, 33–35, 47, 52, 57, 59, 64), n = 3228; WMD: −2.6; 95% CI: −3.0, −2.3 kg; P < 0.001; I2 = 16.0%; P-heterogeneity = 0.30]. Similar changes were found in studies that considered women who were before the surgery [7 studies (19, 20, 48, 54–56, 65), n = 499; WMD: −0.5; 95% CI: –1.0, –0.02 kg; P = 0.041; I2 = 0.0%; P-heterogeneity = 0.96] or chemotherapy and radiotherapy process [10 studies (13, 19, 20, 48, 54–57, 65), n = 772; WMD: −0.5; 95% CI: −1.0, –0.05 kg; P = 0.031; I2 = 0.0%; P-heterogeneity = 0.97]. The subgroup analyses revealed a significant reduction in weight following dietary-based lifestyle modification approaches over control in studies that enrolled patients with BC who were in the middle of the cancer treatment period [10 studies (10, 13, 19, 48, 54–57, 65), n = 772, WMD: −0.5; 95% CI: −1.0, –0.05 kg; P = 0.031; I2 = 0.0%; P-heterogeneity = 0.97], as well as studies that included patients either with normal weight or overweight and obesity [14 studies (10, 11, 20, 34–36, 46, 48, 55–57, 59, 61, 65), n = 2974; WMD: −1.6; 95% CI: −2.3, –0.8 kg; P < 0.001; I2 = 24.1%; P-heterogeneity = 0.19], although this significant finding was not seen in studies that exclusively included participants with overweight and obesity (Supplemental Figure 1). In addition, the stage of BC was reported as another source of heterogeneity (Supplemental Table 8).
BMI
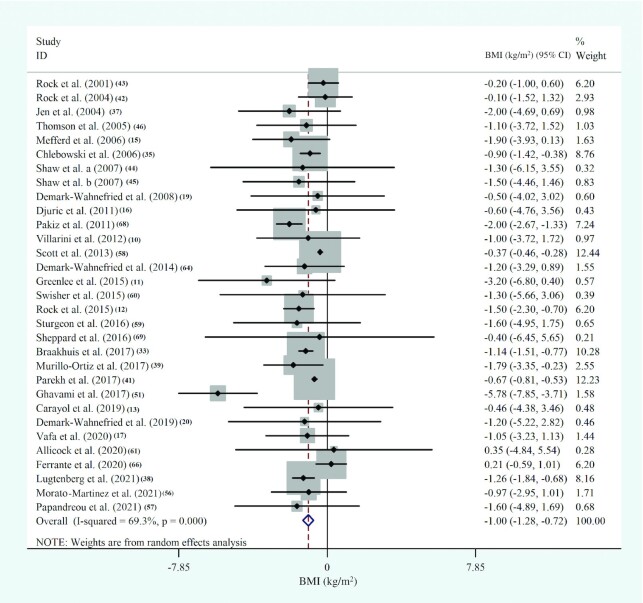
Thirty-one studies, with 5384 participants, evaluated the effects of diet-based lifestyle modification interventions on BMI in women with BC and showed a significant reduction in BMI (in kg/m2; WMD: −1.0; 95% CI: −1.3, –0.7; P < 0.001; I2 = 69.3%; P-heterogeneity < 0.001) (Figure 4, Supplemental Table 7). We repeated the overall analysis, excluding the article by Ghavami et al. (51), and the results were still significant (30 studies, n = 5304, WMD: −0.9; 95% CI: −1.1, –0.7; P < 0.001; I2 = 60.3%; P-heterogeneity < 0.001). Subgroup analyses showed similar changes in BMI in studies that enrolled postmenopausal women with BC [6 RCTs (33, 35, 39, 57, 59, 64), n = 2227; WMD: −1.1; 95% CI: −1.4, –0.8; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.9]. Similar findings were observed in investigations that included either patients who were within the treatment period (8 RCTs, n = 558; WMD: −1.2; 95% CI: −1.7, –0.7; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.9) or participants with normal weight or overweight and obesity (12 studies, n = 2588; WMD: −0.7; 95% CI: −0.8, –0.6; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.8). Moreover, BMI was considerably decreased when a low-fat diet was used as the dietary intervention (9 studies, n = 3568; WMD: −0.9; 95% CI: −1.2, –0.7; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.5), and when a combination of dietary intervention, physical activity, and behavioral therapy was used (6 studies, n = 260; WMD: −1.9; 95% CI: −2.4, –1.3; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.9) (Supplemental Figure 2). Other sources of heterogeneity were identified to be the status of surgery and chemotherapy and the stage of BC (Supplemental Table 9).
FIGURE 4.
Forest plot of randomized controlled trial studies showing weighted mean differences in BMI (in kg/m2) between dietary-based lifestyle modification intervention and control groups for all eligible studies. Analysis was conducted using a random-effects model. Squares depict the weight assigned to the corresponding study; the diamond represents the summary effect. ES, effect size.
Waist circumference
Meta-analysis of 22 studies (1654 participants) showed that the WC of women with BC was significantly reduced after the dietary-based lifestyle modification interventions compared with the control group, although a large between-study heterogeneity was noted (WMD: −3.8; 95% CI: −4.6, –3.0 cm; P < 0.001; I2 = 87.2%; P-heterogeneity < 0.001) (Supplemental Figure 3, Supplemental Table 7). Subgroup analyses showed that WC was considerably decreased when the intervention was administered for less than 24 wk (6 studies, n = 256; WMD: −2.9; 95% CI: −5.1, –0.7 cm; P = 0.009; I2 = 0.0%; P-heterogeneity = 0.7). Similar WC changes were observed in studies that included postmenopausal women (3 studies, n = 110; WMD: −3.3; 95% CI: −4.3, –2.4 cm; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.7) or considered a low-fat diet as the type of diet intervention (4 studies, n = 134; WMD: −3.2; 95% CI: −4.2, –2.3 cm; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.9) (Supplemental Figure 4). Other sources of heterogeneity were reported to be the status of chemotherapy and surgery, as well as the status of cancer treatment (patients who were within the treatment period compared with those who had completed the treatment process) and the baseline BMI of participants (studies that enrolled participants with both normal and high categories of BMI) (Supplemental Table 10).
Lean body mass
Fifteen studies with a total sample size of 1194 evaluated the effect of dietary-based lifestyle modification approaches on lean body mass among women with BC and reported a significant reduction in lean body mass after dietary-based lifestyle modifications (15 studies, n = 1194; WMD: −0.6; 95% CI: −0.7, –0.4 kg; P < 0.001; I2 = 36.6%; P-heterogeneity = 0.07) (Supplemental Figure 5, Supplemental Table 7). However, we conducted a subgroup analysis and found that there was a meaningful change in the lean body mass in studies that included patients who were within their treatment process (7 studies, n = 652; WMD: −0.4; 95% CI: −0.5, –0.3 kg; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.9), as well as studies that considered participants with all categories of BMI (6 studies, n = 305; WMD: −0.4; 95% CI: −0.5, –0.3 kg; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.9) (Supplemental Figure 6, Supplemental Table 11).
Fat mass
Overall, 11 studies including a total sample size of 913 participants showed that dietary-based lifestyle modification interventions significantly reduced fat mass among women with BC (WMD: −2.6; 95% CI: −3.3, –1.8 kg; P < 0.001; I2 = 38.3%; P-heterogeneity = 0.09) (Supplemental Figure 7, Supplemental Table 7). Despite a low between-study heterogeneity, we conducted subgroup analyses and observed that there was a significant decrease in body fat mass in studies that used a combination of dietary intervention and physical activity therapy (5 studies, n = 168; WMD: −1.6; 95% CI: −2.3, –0.8 kg; P < 0.001; I2 = 0.0%; P-heterogeneity = 0.6), as well as studies that did not limit the included participants to those with overweight and obesity (5 studies, n = 252; WMD: −2.7; 95% CI: −5.3, –0.2 kg; P = 0.03; I2 = 0.0%; P-heterogeneity = 0.9) (Supplemental Figure 8, Supplemental Table 12).
Fat percentage
The meta-analysis of 17 studies that included 897 participants with BC showed a significant reduction in fat percentage after the dietary-based lifestyle modification approaches with a very low between-study heterogeneity (WMD: −1.6%; 95% CI: −1.8%, –1.3%; P < 0.001; I2 = 24.2%; P-heterogeneity = 0.2) (Supplemental Figure 9, Supplemental Table 7).
Hip circumference
Combining 9 effect sizes from 9 studies including 489 participants demonstrated that dietary-based lifestyle modification reduced HC significantly (WMD: −2.43; 95% CI: −3.34, –1.54 cm; P < 0.001; I2 = 84.8%; P-heterogeneity < 0.001) (Supplemental Figure 10, Supplemental Table 7). According to the subgroup analysis, a significant reduction in HC was observed in studies that considered a combination of dietary and physical activity interventions (WMD: −1.04; 95% CI: −1.87, –0.21 cm; P = 0.014; I2 = 0.0%; P-heterogeneity = 0.96) (Supplemental Figure 11). Other sources of heterogeneity were found to be the composition of intervention based on the type of diet, the duration of the intervention, and the baseline BMI of participants (Supplemental Table 13).
Waist-to-hip ratio
Seven RCTs examined the effect of dietary-based lifestyle modification approaches on WHR (309 participants), and the overall analysis showed a significant reduction in WHR (WMD: −0.02; 95% CI: −0.03, –0.005; P = 0.003; I2 = 60.4%; P-heterogeneity = 0.02) (Supplemental Figure 12, Supplemental Table 7). The major sources of heterogeneity were found to be the duration of the intervention and the low-fat diet as the type of diet (Supplemental Table 14).
Dietary intake parameters
Energy intake
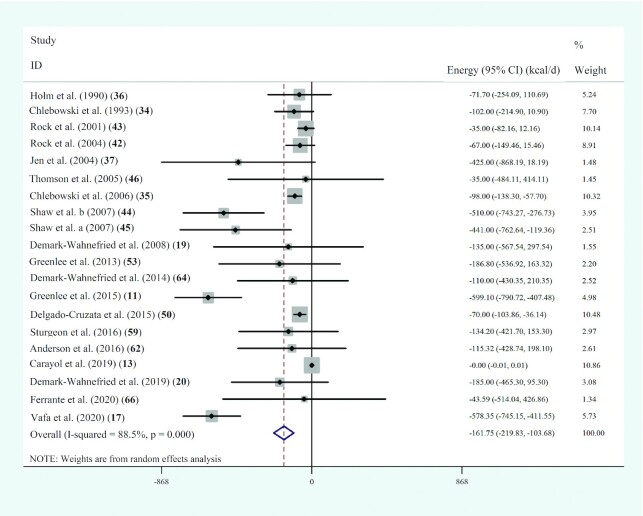
Twenty studies with 4608 participants assessed the effects of dietary-based lifestyle
modification interventions on energy intake levels in populations with BC, and the
pooled analysis showed a significant related reduction (WMD: –161.8; 95% CI: –219.8,
–103.7 kcal/d; P < 0.001; 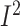 =88.5%;
P-heterogeneity < 0.001) (Figure 5, Supplemental Table 7).
=88.5%;
P-heterogeneity < 0.001) (Figure 5, Supplemental Table 7).
FIGURE 5.
Forest plot of randomized controlled trial studies showing weighted mean differences in daily energy intake (in kcal/d) between dietary-based lifestyle modification intervention and control groups for all eligible studies. Analysis was conducted using a random-effects model. Squares depict the weight assigned to the corresponding study; the diamond represents the summary effect. ES, effect size.
According to the subgroup analyses, a similar significant reduction was observed in
daily energy intake in studies that used a low-fat diet (7 studies,
n = 3938; WMD: –72.9; 95% CI: –100.3, –45.4 kcal/d;
P < 0.001; 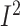 =0.0%;
P-heterogeneity = 0.6) (Supplemental Figure 13). Other important sources of the
heterogeneity were found to be the type of dietary-based lifestyle modification (the
combined intervention of diet, physical activity, and behavioral therapy), the status of
treatment (participants who were within the cancer treatment period), and the type of
menopausal status (Supplemental
Table 15).
=0.0%;
P-heterogeneity = 0.6) (Supplemental Figure 13). Other important sources of the
heterogeneity were found to be the type of dietary-based lifestyle modification (the
combined intervention of diet, physical activity, and behavioral therapy), the status of
treatment (participants who were within the cancer treatment period), and the type of
menopausal status (Supplemental
Table 15).
Dietary fat intake
Pooled analysis of 18 studies including 4458 participants showed that dietary-based
lifestyle modification interventions significantly reduced the percentage of the energy
consumed as fat per day in populations with BC, although the heterogeneity was shown to
be high (WMD: –6.0%; 95% CI: –7.2%, –4.9% of energy/d; P < 0.001;
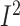 =85%;
P-heterogeneity < 0.001) (Supplemental Figure 14,
Supplemental Table 7). The
overall analysis was conducted again excluding the article by Holm et al. (36), and the final results still remained
significant (17 RCTs, n = 4289; WMD: –7.5%; 95% CI: –7.8%, −7.2% of
energy/d; P < 0.001;
=85%;
P-heterogeneity < 0.001) (Supplemental Figure 14,
Supplemental Table 7). The
overall analysis was conducted again excluding the article by Holm et al. (36), and the final results still remained
significant (17 RCTs, n = 4289; WMD: –7.5%; 95% CI: –7.8%, −7.2% of
energy/d; P < 0.001; 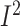 = 85.6%;
P-heterogeneity < 0.001). As shown in Supplemental Table 16,
dietary fat intake was considerably reduced in studies that included patients before the
surgical or chemotherapy and radiotherapy treatment process (3 studies,
n = 118; WMD: –5.9%; 95% CI: –8.4%, −3.6% of energy/d;
P < 0.001;
= 85.6%;
P-heterogeneity < 0.001). As shown in Supplemental Table 16,
dietary fat intake was considerably reduced in studies that included patients before the
surgical or chemotherapy and radiotherapy treatment process (3 studies,
n = 118; WMD: –5.9%; 95% CI: –8.4%, −3.6% of energy/d;
P < 0.001; 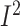 = 0.0%;
P-heterogeneity = 0.7). In addition, the dietary fat intake was
significantly decreased in investigations that used a combination of 3 approaches of
diet-based lifestyle modification (3 RCTs, n = 172; WMD: –3.6%; 95% CI:
–5.8%, –1.5% of energy/d; P < 0.001;
= 0.0%;
P-heterogeneity = 0.7). In addition, the dietary fat intake was
significantly decreased in investigations that used a combination of 3 approaches of
diet-based lifestyle modification (3 RCTs, n = 172; WMD: –3.6%; 95% CI:
–5.8%, –1.5% of energy/d; P < 0.001; 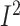 =0.0%;
P-heterogeneity = 0.9). Similar reductions in daily fat intake were
observed in studies that had an intervention duration of less than 24 wk (4 studies,
n = 196; WMD: –3.1%; 95% CI: –5.3%, −1.0% of energy/d;
P = 0.004;
=0.0%;
P-heterogeneity = 0.9). Similar reductions in daily fat intake were
observed in studies that had an intervention duration of less than 24 wk (4 studies,
n = 196; WMD: –3.1%; 95% CI: –5.3%, −1.0% of energy/d;
P = 0.004; 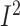 =0.9%;
P-heterogeneity = 0.4) (Supplemental Figure 15, Supplemental Table 16).
=0.9%;
P-heterogeneity = 0.4) (Supplemental Figure 15, Supplemental Table 16).
Considering the types of dietary fat, 7 studies (n = 2779
participants) reported changes in SFA intakes (11, 19, 34–36, 46,
62). The overall meta-analysis showed a
significant reduction in SFA intakes following the diet-based interventions, although a
high between-study heterogeneity was observed (WMD: –2.32%; 95% CI: –2.98%, –1.65% of
energy/d; P < 0.001; 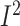 = 92.0%;
P-heterogeneity < 0.001) (Supplemental Figure 16A,
Supplemental Table 7). Based
on the subgroup analyses, the reduction in SFA intake was significant in studies that
used a combination of diet and exercise interventions (n = 137; WMD:
–1.39%; 95% CI: –2.44%, –0.35% of energy/d; P = 0.09;
= 92.0%;
P-heterogeneity < 0.001) (Supplemental Figure 16A,
Supplemental Table 7). Based
on the subgroup analyses, the reduction in SFA intake was significant in studies that
used a combination of diet and exercise interventions (n = 137; WMD:
–1.39%; 95% CI: –2.44%, –0.35% of energy/d; P = 0.09;
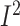 = 0.0%;
P-heterogeneity = 0.34) (Supplemental Table 17). Only 4 studies reported the changes
in dietary intakes of PUFAs and MUFAs (11,
34–36, 46) (Supplemental Table 7), and the overall meta-analyses revealed a significant
decrease in the intakes of both PUFAs (n = 2594; WMD: –1.47%; 95% CI:
–1.95%, –0.99% of energy/d; P < 0.001;
= 0.0%;
P-heterogeneity = 0.34) (Supplemental Table 17). Only 4 studies reported the changes
in dietary intakes of PUFAs and MUFAs (11,
34–36, 46) (Supplemental Table 7), and the overall meta-analyses revealed a significant
decrease in the intakes of both PUFAs (n = 2594; WMD: –1.47%; 95% CI:
–1.95%, –0.99% of energy/d; P < 0.001; 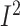 = 65.5%;
P-heterogeneity = 0.34) (Supplemental Figure 16B) and MUFAs (n = 2594; WMD:
–3.13%; 95% CI: –5.22%, –1.02% of energy/d; P = 0.004;
= 65.5%;
P-heterogeneity = 0.34) (Supplemental Figure 16B) and MUFAs (n = 2594; WMD:
–3.13%; 95% CI: –5.22%, –1.02% of energy/d; P = 0.004;
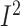 = 99.3%;
P-heterogeneity < 0.001) (Supplemental Figure 16C).
= 99.3%;
P-heterogeneity < 0.001) (Supplemental Figure 16C).
Dietary fiber intake
The meta-analysis of 11 studies, with 4241 participants, revealed that women with BC
who received dietary-based lifestyle modification interventions consumed more dietary
fiber per day (WMD: 2.4; 95% CI: 0.7, 4.1 g/d; P = 0.005;
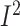 = 97.2%;
P-heterogeneity < 0.001) (Supplemental Figure 17A,
Supplemental Table 7).
Subgroup analyses showed that dietary fiber intake was significantly increased in
studies that included postmenopausal women with BC (3 studies,
n = 2408; WMD: 1.7; 95% CI: 1.2, 2.3 g/d;
P < 0.001;
= 97.2%;
P-heterogeneity < 0.001) (Supplemental Figure 17A,
Supplemental Table 7).
Subgroup analyses showed that dietary fiber intake was significantly increased in
studies that included postmenopausal women with BC (3 studies,
n = 2408; WMD: 1.7; 95% CI: 1.2, 2.3 g/d;
P < 0.001; 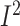 = 0.0%;
P-heterogeneity = 0.9) as well as studies that did not limit the
participants based on BMI (5 RCTs, n = 2625; WMD: 1.7; 95% CI: 1.2, 2.3
g/d; P < 0.001;
= 0.0%;
P-heterogeneity = 0.9) as well as studies that did not limit the
participants based on BMI (5 RCTs, n = 2625; WMD: 1.7; 95% CI: 1.2, 2.3
g/d; P < 0.001; 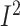 = 0.0%;
P-heterogeneity = 0.9) (Supplemental Figure 18, Supplemental Table
18).
= 0.0%;
P-heterogeneity = 0.9) (Supplemental Figure 18, Supplemental Table
18).
Dietary protein intake
As shown in Supplemental Figure
17B and Supplementary Table
7, the meta-analysis of 12 studies (with 2095 participants) reported that
diet-based lifestyle modification approaches had no significant effects on the measures
of dietary protein intakes, and the heterogeneity between studies was shown to be high
(WMD: 1.6; 95% CI: −0.6, 3.9 g/d; P = 0.1; 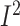 = 96.6%;
P-heterogeneity < 0.001) (Supplemental Figure 17B). The stage of BC was found to be the source
of heterogeneity (Supplemental
Table 19).
= 96.6%;
P-heterogeneity < 0.001) (Supplemental Figure 17B). The stage of BC was found to be the source
of heterogeneity (Supplemental
Table 19).
Dietary carbohydrate intake
Pooled analysis of 10 studies, including 2033 participants, showed no meaningful
changes in dietary carbohydrate intakes after the interventions. The heterogeneity was
observed to be high (WMD: 3.9; 95% CI: −12.7, 20.5 g/d; P = 0.6;
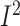 = 99.6%;
P-heterogeneity < 0.001) (Supplemental Figure 17C, Supplemental Table 7). As shown in Supplemental Table 20,
the sources of heterogeneity were reported to be the stage of BC, menopausal status,
duration of the intervention, and the baseline BMI categories of participants.
= 99.6%;
P-heterogeneity < 0.001) (Supplemental Figure 17C, Supplemental Table 7). As shown in Supplemental Table 20,
the sources of heterogeneity were reported to be the stage of BC, menopausal status,
duration of the intervention, and the baseline BMI categories of participants.
Dietary fruit and vegetable intake
Six studies (299 participants) assessed the effects of dietary-based lifestyle
modification interventions on the measures of fruit and vegetable intakes and no
significant related changes were observed (WMD: 1.0; 95% CI: −0.02, 2.1 servings/d;
P = 0.05; 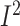 = 71.1%;
P-heterogeneity = 0.004) (Supplemental Figure 17D, Supplemental Table 7). Sources of
heterogeneity were found to be the type of dietary-based lifestyle modification and the
baseline BMI categories of participants (Supplemental Table 21).
= 71.1%;
P-heterogeneity = 0.004) (Supplemental Figure 17D, Supplemental Table 7). Sources of
heterogeneity were found to be the type of dietary-based lifestyle modification and the
baseline BMI categories of participants (Supplemental Table 21).
Outcomes not included in the meta-analysis
Two studies reported the effect of a low-calorie diet on the changes in skinfold thickness among survivors of BC with lymphedema (44, 45). One study assessed the effect of dietary-based lifestyle modification on the trunk and leg muscle mass, trunk and leg fat mass, as well as the muscle mass to fat mass ratio, and reported a significant reduction in trunk fat (22). Demark-Wahnefried et al. (19) showed a significant decrease in gynoid body fat percentage following a combination of diet and exercise intervention. Thomson et al. (46) reported no significant changes in dietary cholesterol intake after a low-fat diet intervention.
Sensitivity analysis and publication bias
An influence analysis was performed for every outcome, and the results showed that the omission of any individual study made no difference to the final result. Begg's and Egger's tests suggested publication bias for the effects of diet-based interventions on BMI (Begg's P value = 0.02, Egger's P value = 0.01) and energy intakes (Begg's P value = 0.048, Egger's P value = 0.02). However, the trim-and-fill method did not identify any missed “pseudostudies” for either analysis.
Discussion
In this review, we assessed the effects of dietary-based lifestyle modification strategies, including diet intervention, diet and physical activity intervention, or a combination of diet, physical activity, and behavioral intervention, on anthropometric indices and dietary intake parameters in women with BC. The summary analysis of 51 eligible RCTs, including 7743 participants, indicated that diet-based interventions with a median intervention duration of 24 wk resulted in a significant decrease in weight, BMI, WC, HC, body fat percentage, and lean body mass in the intervention group compared with the control group. In addition, daily caloric and fat intakes were significantly reduced, while dietary fiber intake increased significantly. However, all of the changes were so minor that they were almost imperceptible. No meaningful alterations were observed in dietary protein, carbohydrate, and fruit and vegetable intakes. The majority of findings from the subgroup analyses showed favorable changes in the anthropometric and dietary intake parameters in studies that used a combination of “diet and exercise” or “diet, exercise, and behavioral therapy.” Similar results were observed in studies involving patients with normal weight, overweight, and obesity, as well as studies involving participants in the middle of the cancer treatment period.
Although the therapeutic significance of optimal weight loss and reduced cancer recurrence risk is yet unknown, evidence suggests that even moderate weight loss is associated with improved overall quality of life (70). Shang et al. (71) found that a BMI decrease of 0.5 or more was associated with a more than 2-fold increase in overall and BC-specific survival, regardless of the patients’ baseline BMI levels. They found that this effect was even stronger than the increase in BMI and its link to a lower survival rate.
The considerable decrease in both WC and HC following dietary-based lifestyle modification interventions may be primarily due to total weight loss. According to research, losing weight results in a decrease in both WC and HC (72). However, the decrease in WC (–3.8 cm) was higher than the decrease in HC according to our data (–2.4 cm). WC represents harmful visceral fat and assesses subcutaneous and visceral adipose tissue, whereas HC represents healthy gluteal adipose tissue (73). A meta-analysis of 50 cohort studies with over 2 million individuals indicated that each 10-cm increase in WC was associated with an 11% increase in the risk of all-cause death, which was confirmed when the analysis was controlled for BMI (74).
The total weight loss is also accompanied by a decrease in both fat mass and lean body mass (72). That is why we observed a reduction in body fat mass and fat percentage as well as lean body mass following dietary-based lifestyle modification interventions. However, when compared with the decrease in body fat mass and fat percentage, the decrease in lean body mass was substantially smaller. According to research, patients with cancer who lost more than 5% of their weight, or 4 kg of lean body mass, were diagnosed with cancer cachexia, or sarcopenia (75). Because the current meta-analysis found only a 0.6-kg decrease in lean body mass after dietary-based lifestyle modification interventions, this decrease would not be associated with a poor prognosis among women with BC. Furthermore, despite the minor decrease in energy intake, a nonsignificant increase in dietary protein intake was observed in the final analysis, which could further explain the approximate preservation of lean body mass following dietary-based interventions.
In this study, there was a small but significant increase in dietary fiber intake. This finding would be of clinical significance since a previous meta-analysis of observational studies suggested that increasing fiber intake to more than 10 g/d would lower the incidence of BC (76). This important systematic review and meta-analysis showed that a diet-based intervention resulted in small but significant changes in anthropometric and dietary intake parameters, implying that patients are more inclined to adhere to moderate diets. This could convince more clinicians to recommend oncology nutrition if they realize that dietary and lifestyle modifications can be achieved with changes that are not so drastic.
Evidence suggests that, after a diagnosis of BC, women face a number of obstacles to maintaining a healthy body and diet, including anxiety, sadness, an elevated BMI, and so on (60). That is why following a healthy diet and regular physical activity as capacity dictates is critical for recently diagnosed women during the treatment period (77), and incorporating behavioral therapy into lifestyle modification is a novel approach that can assist women with BC to set goals and overcome barriers to having a healthier body (69). Our findings also suggest that a comprehensive diet-based lifestyle intervention would be better started as early as the cancer diagnosis and for all women with BC, regardless of their body weight. In fact, subgroup analyses showed more considerable changes in the majority of anthropometric and dietary intake parameters in studies that enrolled patients with both normal weight and overweight or obesity, as well as in studies that started the diet-based intervention during cancer treatment. In addition, the stated changes were also significant in investigations that included postmenopausal women. As a result, these guidelines should give special attention to premenopausal women with BC, who may be more emotionally affected by the cancer diagnosis and have poorer adherence to lifestyle changes (78).
Our findings also showed that the dietary-based interventions had considerable effects when they included a low-fat diet. A low-fat diet may lower intestinal dysbiosis and the related adverse impact on weight and metabolic variables (79). A meta-analysis of observational studies found that limiting fat consumption to less than 20% of total calories reduces the risk of breast cancer by 13.4% (80).
The present meta-analysis has several strengths. We used numerous databases and had no language restrictions to include all eligible RCTs. We did not limit the participants’ baseline BMI or treatment status to increase the generalizability of our findings to all women with a diagnosis of BC and to determine the optimal strategy to recommend. We conducted numerous subgroup analyses to identify the possible sources of heterogeneity. We also used both the updated RoB2 and the GRADE system to provide a clear assessment of the quality of the included studies. Finally, the systematic review part of the present study is thorough. Many studies that did not have a few eligibility criteria are listed in Supplemental Table 3.
The limitations of the present study should be considered. Twenty-four studies out of 51 included articles had “some concerns” risk of bias and an additional 17 were at “high risk of bias.” In many of the included studies, anthropometric indices were the secondary outcomes. Furthermore, the final analyses did not account for the effects of confounding factors, such as alcohol intake, smoking, or age, and the methods used to measure these parameters were not taken into account in the final analyses. In addition, despite contacting the authors, we were unable to obtain the full text of 1 eligible study. It is unclear, however, whether receiving the relevant data would have had a major impact on our findings.
It is strongly suggested that future studies include a follow-up duration of 2–3 y after the treatment period to see if the dietary-based lifestyle interventions were constant over the follow-up time. Evaluating the adherence to the intervention is essential since it greatly affects patients’ quality of life and survivorship. Furthermore, prospective RCTs should assess the baseline psychological status of women with BC before the study's initiation and throughout the follow-up period. Anxiety, depression, and fatigue are among the psychological barriers that affect adherence to the lifestyle interventions of women with BC, especially premenopausal women who are more affected by the emotional effects of cancer diagnosis. Future studies should use more reliable and validated methods, such as computed tomography scans, to evaluate if the changes in lean body mass are clinically meaningful. Direct evaluation of muscle and adiposity will help treatment plans and interventions optimize survival outcomes (81). Considering individual groups of diet-based interventions in future studies would also give a clear understanding of the conclusion that a comprehensive multimodal dietary-based lifestyle intervention had the most significant effect on anthropometric and dietary intake parameters in women with BC. According to the present meta-analysis, the majority of studies were conducted in American and European populations. As a result, additional research in Asian and African populations is required to account for country-specific differences and to update present findings.
In conclusion, our findings suggest that a comprehensive dietary-based intervention, which included a combination of diet and exercise or diet, exercise, and behavioral therapy, would have the most positive effects on anthropometric and dietary intake parameters in women with BC, especially when started as soon as the cancer was diagnosed. However, more research is needed to investigate the effect of multimodal dietary-based lifestyle modification interventions on BC recurrence.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ER and MZ: conceived of the study and designed the protocol; MRL and SV: performed the literature search; AS and MRL: selected the studies and extracted the relevant information; ER, SJ, and RJdS: synthesized the data; MRL: wrote the first draft of the manuscript; ER and MRL: are the study guarantors; ER: attests that all listed authors meet authorship criteria and that no others meeting the standards have been omitted; and all authors: critically revised successive drafts of the paper and read and approved the final version.
Notes
Supported by the Shahid Beheshti University of Medical Sciences (no. 28845). No external financial support was received.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–18, Supplemental Tables 1–21, and Supplemental References are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
MRL and ER contributed equally to this work.
Abbreviations used: BC, breast cancer; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; HC, hip circumference, MeSH, Medical Subject Headings; RCT, randomized controlled trial; RoB2, Cochrane risk-of-bias tool for randomized trials; WC, waist circumference; WHR, waist-to-hip ratio; WMD, weighted mean difference.
Contributor Information
Mahsa Raji Lahiji, Deparment of Integrative Oncology and Quality of Life, Breast Cancer Research Center, Motamed Cancer Institute, Academic Centre for Education, Culture, and Research, Tehran, Iran; Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
Saeideh Vafa, Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
Russell J de Souza, Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada; Population Health Research Institute, Hamilton, ON, Canada.
Mitra Zarrati, Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
Akram Sajadian, Deparment of Integrative Oncology and Quality of Life, Breast Cancer Research Center, Motamed Cancer Institute, Academic Centre for Education, Culture, and Research, Tehran, Iran.
Elham Razmpoosh, Nutrition and Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Shapour Jaberzadeh, Non-invasive Brain Stimulation and Neuroplasticity Laboratory, Department of Physiotherapy, School of Primary and Allied Health Care, Faculty of Medicine, Nursing and Health Science, Monash University, Melbourne, Australia.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request.
References
- 1. Maajani K, Jalali A, Alipour S, Khodadost M, Tohidinik HR, Yazdani K. The global and regional survival rate of women with breast cancer: a systematic review and meta-analysis. Clin Breast Cancer. 2019;19(3):165–77. [DOI] [PubMed] [Google Scholar]
- 2. Playdon MC, Bracken MB, Sanft TB, Ligibel JA, Harrigan M, Irwin ML. Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J Natl Cancer Inst. 2015;107(12):djv275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barone I, Giordano C, Bonofiglio D, Andò S, Catalano S. The weight of obesity in breast cancer progression and metastasis: clinical and molecular perspectives. Semin Cancer Biol. 2020;60:274–84. [DOI] [PubMed] [Google Scholar]
- 4. Santa-Maria CA, Coughlin JW, Sharma D, Armanios M, Blackford AL, Schreyer Cet al. . The effects of a remote-based weight loss program on adipocytokines, metabolic markers, and telomere length in breast cancer survivors: the POWER-Remote trial. Clin Cancer Res. 2020;26(12):3024–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patterson RE, Marinac CR, Sears DD, Kerr J, Hartman SJ, Cadmus-Bertram Let al. . The effects of metformin and weight loss on biomarkers associated with breast cancer outcomes. J Natl Cancer Inst. 2018;110(11):1239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hwang ES, Nho J-H. Lifestyle intervention for breast cancer women. J Lifestyle Med. 2019;9(1):12–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Male D, Fergus K, Yufe S. ‘Weighing’ losses and gains: evaluation of the Healthy Lifestyle Modification After Breast Cancer Pilot Program. Front Psychol. 2022;13:814671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Budreviciute A, Damiati S, Sabir DK, Onder K, Schuller-Goetzburg P, Plakys Get al. . Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health. 2020;8:574111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sanft T, Usiskin I, Harrigan M, Cartmel B, Lu L, Li FYet al. . Randomized controlled trial of weight loss versus usual care on telomere length in women with breast cancer: the Lifestyle, Exercise, And Nutrition (LEAN) study. Breast Cancer Res Treat. 2018;172(1):105–12. [DOI] [PubMed] [Google Scholar]
- 10. Villarini A, Pasanisi P, Raimondi M, Gargano G, Bruno E, Morelli Det al. . Preventing weight gain during adjuvant chemotherapy for breast cancer: a dietary intervention study. Breast Cancer Res Treat. 2012;135(2):581–9. [DOI] [PubMed] [Google Scholar]
- 11. Greenlee H, Gaffney AO, Aycinena AC, Koch P, Contento I, Karmally Wet al. . Cocinar para su salud! Randomized controlled trial of a culturally based dietary intervention among Hispanic breast cancer survivors. J Acad Nutr Diet. 2015;115(5):709–23, e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rock CL, Flatt SW, Byers TE, Colditz GA, Demark-Wahnefried W, Ganz PAet al. . Results of the Exercise and Nutrition to Enhance Recovery and Good health for You (ENERGY) trial: a behavioral weight loss intervention in overweight or obese breast cancer survivors. J Clin Oncol. 2015;33(28):3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carayol M, Ninot G, Senesse P, Bleuse J-P, Gourgou S, Sancho-Garnier Het al. . Short-and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: the “APAD1” randomized controlled trial. BMC Cancer. 2019;19(1):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux Aet al. . The LISA randomized trial of a weight loss intervention in postmenopausal breast cancer. NPJ Breast Cancer. 2020;6(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mefferd K, Nichols JF, Pakiz B, Rock CL. A cognitive behavioral therapy intervention to promote weight loss improves body composition and blood lipid profiles among overweight breast cancer survivors. Breast Cancer Res Treat. 2007;104(2):145–52. [DOI] [PubMed] [Google Scholar]
- 16. Djuric Z, DiLaura NM, Jenkins I, Darga L, Jen CKL, Mood Det al. . Combining weight-loss counseling with the weight watchers plan for obese breast cancer survivors. Obes Res. 2002;10(7):657–65. [DOI] [PubMed] [Google Scholar]
- 17. Vafa S, Zarrati M, Malakootinejad M, Totmaj AS, Zayeri F, Salehi Met al. . Calorie restriction and synbiotics effect on quality of life and edema reduction in breast cancer-related lymphedema, a clinical trial. The Breast. 2020;54:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reeves M, Winkler E, Mccarthy N, Lawler S, Terranova C, Hayes Set al. . The Living Well after Breast Cancer™ pilot trial: a weight loss intervention for women following treatment for breast cancer. Asia Pac J Clin Oncol. 2017;13(3):125–36. [DOI] [PubMed] [Google Scholar]
- 19. Demark-Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz Net al. . Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clin Breast Cancer. 2008;8(1):70–9. [DOI] [PubMed] [Google Scholar]
- 20. Demark-Wahnefried W, Rogers LQ, Gibson JT, Harada S, Frugé AD, Oster RAet al. . Randomized trial of weight loss in primary breast cancer: impact on body composition, circulating biomarkers and tumor characteristics. Int J Cancer. 2020;146(10):2784–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Orange ST, Hicks KM, Saxton JM. Effectiveness of diet and physical activity interventions amongst adults attending colorectal and breast cancer screening: a systematic review and meta-analysis. Cancer Causes Control. 2021;32(1):13–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Yang T, Qiang W, Shen A, Zhao Z, Liu X. Benefits of dietary management in breast cancer patients: a systematic review and meta-analysis. Nutr Cancer. 2022;74(5):1580–92. [DOI] [PubMed] [Google Scholar]
- 23. Raji M, Razmpoosh E, Zarrati M, Vafa S, Sajadian A. The effect of dietary-based lifestyle modification approaches on anthropometric indices and dietary intake parameters in women with breast cancer: a systematic review and meta-analysis of randomized controlled trials [Internet]. 2021; Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=291488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJet al. . Cochrane handbook for systematic reviews of interventions. United Kingdom: John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Method. 2014;14(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron Iet al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:14898. [DOI] [PubMed] [Google Scholar]
- 27. Waters E, Doyle J. Systematic reviews of public health in developing countries are in train. BMJ. 2004;328(7439):585.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek Jet al. . GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind Det al. . GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol. 2011;64(12):1283–93. [DOI] [PubMed] [Google Scholar]
- 30. Deeks J, Higgins J, Altman D. Cochrane handbook of systematic reviews of interventions. [Internet]. 2011. Available from: www. training.cochrane.org/handbook. [Google Scholar]
- 31. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 33. Braakhuis A, Campion P, Bishop K. The effects of dietary nutrition education on weight and health biomarkers in breast cancer survivors. Med Sci. 2017;5(2):12. doi: 10.3390/medsci5020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chlebowski RT, Blackburn GL, Buzzard IM, Rose DP, Martino S, Khandekar Jet al. . Adherence to a dietary fat intake reduction program in postmenopausal women receiving therapy for early breast cancer. The Women's Intervention Nutrition Study. J Clin Oncol. 1993;11(11):2072–80. [DOI] [PubMed] [Google Scholar]
- 35. Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MKet al. . Dietary fat reduction and breast cancer outcome: interim efficacy results from the Women's Intervention Nutrition Study. J Natl Cancer Inst. 2006;98(24):1767–76. [DOI] [PubMed] [Google Scholar]
- 36. Holm L-E, Nordevang E, Ikkala E, Hallström L, Callmer E. Dietary intervention as adjuvant therapy in breast cancer patients—a feasibility study. Breast Cancer Res Treat. 1990;16(2):103–9. [DOI] [PubMed] [Google Scholar]
- 37. Jen KLC, Djuric Z, DiLaura NM, Buison A, Redd JN, Maranci Vet al. . Improvement of metabolism among obese breast cancer survivors in differing weight loss regimens. Obes Res. 2004;12(2):306–12. [DOI] [PubMed] [Google Scholar]
- 38. Lugtenberg RT, de Groot S, Kaptein AA, Fischer MJ, Kranenbarg EM-K, Duijm-de Carpentier Met al. . Quality of life and illness perceptions in patients with breast cancer using a fasting mimicking diet as an adjunct to neoadjuvant chemotherapy in the phase 2 DIRECT (BOOG 2013–14) trial. Breast Cancer Res Treat. 2021;185(3):741–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murillo-Ortiz B, Martínez-Garza S, Landeros VC, Velázquez GC, Garcia DS. Effect of reduced dietary fat on estradiol, adiponectin, and IGF-1 levels in postmenopausal women with breast cancer. Breast Cancer. 2017;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Newlands RS, Ntessalen M, Clark J, Fielding S, Hoddinott P, Heys SDet al. . Pilot randomised controlled trial of Weight Watchers® referral with or without dietitian-led group support for weight loss in women treated for breast cancer: the BRIGHT (BReast cancer weIGHT loss) trial. Pilot Feasibility Stud. 2019;5(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Parekh N, Jiang J, Buchan M, Meyers M, Gibbs H, Krebs P. Nutrition literacy among cancer survivors: feasibility results from the Healthy Eating and Living Against Breast Cancer (HEAL-BCa) study: a pilot randomized controlled trial. J Cancer Educ. 2018;33(6):1239–49. [DOI] [PubMed] [Google Scholar]
- 42. Rock CL, Flatt SW, Thomson CA, Stefanick ML, Newman VA, Jones LAet al. . Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J Clin Oncol. 2004;22(12):2379–87. [DOI] [PubMed] [Google Scholar]
- 43. Rock CL, Thomson C, Caan BJ, Flatt SW, Newman V, Ritenbaugh Cet al. . Reduction in fat intake is not associated with weight loss in most women after breast cancer diagnosis: evidence from a randomized controlled trial. Cancer. 2001;91(1):25–34. [PubMed] [Google Scholar]
- 44. Shaw C, Mortimer P, Judd PA. A randomized controlled trial of weight reduction as a treatment for breast cancer-related lymphedema. Cancer. 2007;110(8):1868–74. [DOI] [PubMed] [Google Scholar]
- 45. Shaw C, Mortimer P, Judd PA. Randomized controlled trial comparing a low-fat diet with a weight-reduction diet in breast cancer-related lymphedema. Cancer. 2007;109(10):1949–56. [DOI] [PubMed] [Google Scholar]
- 46. Thomson CA, Rock CL, Giuliano AR, Newton TR, Cui H, Reid PMet al. . Longitudinal changes in body weight and body composition among women previously treated for breast cancer consuming a high-vegetable, fruit and fiber, low-fat diet. Eur J Nutr. 2005;44(1):18–25. [DOI] [PubMed] [Google Scholar]
- 47. Arikawa AY, Kaufman BC, Raatz SK, Kurzer MS. Effects of a parallel-arm randomized controlled weight loss pilot study on biological and psychosocial parameters of overweight and obese breast cancer survivors. Pilot Feasibility Stud. 2018;4(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Basen-Engquist KM, Raber M, Carmack CL, Arun B, Brewster AM, Fingeret Met al. . Feasibility and efficacy of a weight gain prevention intervention for breast cancer patients receiving neoadjuvant chemotherapy: a randomized controlled pilot study. Support Care Cancer. 2020;28(12):5821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Darga LL, Magnan M, Mood D, Hryniuk WM. Quality of life as a predictor of weight loss in obese, early-stage breast cancer survivors. Oncol Nurs Forum. 2007;34(1):86. [DOI] [PubMed] [Google Scholar]
- 50. Delgado-Cruzata L, Zhang W, McDonald JA, Tsai WY, Valdovinos C, Falci Let al. . Dietary modifications, weight loss, and changes in metabolic markers affect global DNA methylation in Hispanic, African American, and Afro-Caribbean breast cancer survivors. J Nutr. 2015;145(4):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ghavami H, Akyolcu N. The impact of lifestyle interventions in breast cancer women after completion of primary therapy: a randomized study. J Breast Health. 2017;13(2):94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goodwin PJ, Segal RJ, Vallis M, Ligibel JA, Pond GR, Robidoux Aet al. . Randomized trial of a telephone-based weight loss intervention in postmenopausal women with breast cancer receiving letrozole: the LISA trial. J Clin Oncol. 2014;32(21):2231–9. [DOI] [PubMed] [Google Scholar]
- 53. Greenlee HA, Crew KD, Mata JM, McKinley PS, Rundle AG, Zhang Wet al. . A pilot randomized controlled trial of a commercial diet and exercise weight loss program in minority breast cancer survivors. Obesity (Silver Spring). 2013;21(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harvie M, Pegington M, McMullan D, Bundred N, Livingstone K, Campbell Aet al. . The effectiveness of home versus community-based weight control programmes initiated soon after breast cancer diagnosis: a randomised controlled trial. Br J Cancer. 2019;121(6):443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Klement RJ, Koebrunner PS, Krage K, Weigel MM, Sweeney RA. Short-term effects of a Paleolithic lifestyle intervention in breast cancer patients undergoing radiotherapy: a pilot and feasibility study. Med Oncol. 2021;38(1):1–13. [DOI] [PubMed] [Google Scholar]
- 56. Morato-Martínez M, Santurino C, López-Plaza B, Arcos-Castellanos L, Clavero-Fraile M, Palma-Milla Set al. . A standardized, integral nutritional intervention and physical activity program reduces body weight in women newly diagnosed with breast cancer. Nutr Hosp. 2021;38(3):575–84. [DOI] [PubMed] [Google Scholar]
- 57. Papandreou P, Gioxari A, Nimee F, Skouroliakou M. Application of clinical decision support system to assist breast cancer patients with lifestyle modifications during the COVID-19 pandemic: a randomised controlled trial. Nutrients. 2021;13(6):2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Scott E, Daley A, Doll H, Woodroofe N, Coleman R, Mutrie Net al. . Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: a randomized controlled trial. Cancer Causes Control. 2013;24(1):181–91. [DOI] [PubMed] [Google Scholar]
- 59. Sturgeon KM, Dean LT, Heroux M, Kane J, Bauer T, Palmer Eet al. . Commercially available lifestyle modification program: randomized controlled trial addressing heart and bone health in BRCA1/2+ breast cancer survivors after risk-reducing salpingo-oophorectomy. J Cancer Survivorship. 2017;11(2):246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian Set al. . Exercise and dietary advice intervention for survivors of triple-negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Support Care Cancer. 2015;23(10):2995–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Allicock M, Kendzor D, Sedory A, Gabriel KP, Swartz MD, Thomas Pet al. . A pilot and feasibility mobile health intervention to support healthy behaviors in African American breast cancer survivors. J Racial Ethn Health Disparities. 2021;8(1):157–65. [DOI] [PubMed] [Google Scholar]
- 62. Anderson C, Harrigan M, George SM, Ferrucci LM, Sanft T, Irwin MLet al. . Changes in diet quality in a randomized weight loss trial in breast cancer survivors: the Lifestyle, Exercise, And Nutrition (LEAN) study. NPJ Breast Cancer. 2016;2(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Brown JC, Sarwer DB, Troxel AB, Sturgeon K, DeMichele AM, Denlinger CSet al. . A randomized trial of exercise and diet on body composition in survivors of breast cancer with overweight or obesity. Breast Cancer Res Treat. 2021;189(1):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Demark-Wahnefried W, Jones LW, Snyder DC, Sloane RJ, Kimmick GG, Hughes DCet al. . Daughters and Mothers Against Breast Cancer (DAMES): main outcomes of a randomized controlled trial of weight loss in overweight mothers with breast cancer and their overweight daughters. Cancer. 2014;120(16):2522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Djuric Z, Ellsworth JS, Weldon AL, Ren J, Richardson CR, Resnicow Ket al. . A diet and exercise intervention during chemotherapy for breast cancer. Open Obesity J. 2011;3(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ferrante JM, Devine KA, Bator A, Rodgers A, Ohman-Strickland PA, Bandera EVet al. . Feasibility and potential efficacy of commercial mHealth/eHealth tools for weight loss in African American breast cancer survivors: pilot randomized controlled trial. Transl Behav Med. 2020;10(4):938–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Harrigan M, Cartmel B, Loftfield E, Sanft T, Chagpar AB, Zhou Yet al. . Randomized trial comparing telephone versus in-person weight loss counseling on body composition and circulating biomarkers in women treated for breast cancer: the Lifestyle, Exercise, And Nutrition (LEAN) study. J Clin Oncol. 2016;34(7):669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pakiz B, Flatt SW, Bardwell WA, Rock CL, Mills PJ. Effects of a weight loss intervention on body mass, fitness, and inflammatory biomarkers in overweight or obese breast cancer survivors. Int J Behav Med. 2011;18(4):333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sheppard VB, Hicks J, Makambi K, Hurtado-de-Mendoza A, Demark-Wahnefried W, Adams-Campbell L. The feasibility and acceptability of a diet and exercise trial in overweight and obese Black breast cancer survivors: the Stepping STONE study. Contemp Clin Trials. 2016;46:106–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Warkentin LM, Das D, Majumdar SR, Johnson JA, Padwal RS. The effect of weight loss on health-related quality of life: systematic review and meta-analysis of randomized trials. Obes Rev. 2014;15(3):169–82. [DOI] [PubMed] [Google Scholar]
- 71. Shang L, Hattori M, Fleming G, Jaskowiak N, Hedeker D, Olopade OIet al. . Impact of post-diagnosis weight change on survival outcomes in Black and White breast cancer patients. Breast Cancer Res. 2021;23(1):18. doi: 10.1186/s13058-021-01397-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ashtary-Larky D, Bagheri R, Abbasnezhad A, Tinsley GM, Alipour M, Wong A. Effects of gradual weight loss v. rapid weight loss on body composition and RMR: a systematic review and meta-analysis. Br J Nutr. 2020;124(11):1121–32. [DOI] [PubMed] [Google Scholar]
- 73. Xing Z, Peng Z, Wang X, Zhu Z, Pei J, Hu Xet al. . Waist circumference is associated with major adverse cardiovascular events in male but not female patients with type-2 diabetes mellitus. Cardiovasc Diabetol. 2020;19(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jayedi A, Soltani S, Zargar MS, Khan TA, Shab-Bidar S. Central fatness and risk of all cause mortality: systematic review and dose-response meta-analysis of 72 prospective cohort studies. BMJ. 2020;370:m3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Horstman AMH, Olde Damink SW, Schols A, van Loon LJC. Is cancer cachexia attributed to impairments in basal or postprandial muscle protein metabolism?. Nutrients. 2016;8(8):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Aune D, Chan DSM, Greenwood DC, Vieira AR, Rosenblatt DAN, Vieira Ret al. . Dietary fiber and breast cancer risk: a systematic review and meta-analysis of prospective studies. Ann Oncol. 2012;23(6):1394–402. [DOI] [PubMed] [Google Scholar]
- 77. Xing M-Y, Xu S-Z, Shen P. Effect of low-fat diet on breast cancer survival: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(3):1141–4. [DOI] [PubMed] [Google Scholar]
- 78. Anderson DJ, Yates P, McCarthy A, Lang CP, Hargraves M, McCarthy Net al. . Younger and older women's concerns about menopause after breast cancer. Eur J Cancer Care (Engl). 2011;20(6):785–94. [DOI] [PubMed] [Google Scholar]
- 79. Koliaki C, Spinos T, Spinou Μ, Brinia Μ-E, Mitsopoulou D, Katsilambros N. Defining the optimal dietary approach for safe, effective and sustainable weight loss in overweight and obese adults. Healthcare. 2018;6(3):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wu AH, Pike MC, Stram DO. Meta-analysis: dietary fat intake, serum estrogen levels, and the risk of breast cancer. J Natl Cancer Inst. 1999;91(6):529–34. [DOI] [PubMed] [Google Scholar]
- 81. Caan BJ, Cespedes Feliciano EM, Prado CM, Alexeeff S, Kroenke CH, Bradshaw Pet al. . Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4(6):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request.