ABSTRACT
Fish consumption is associated with a reduced risk of cardiovascular diseases (CVDs) partly ascribed to the high content of long-chain (LC) n–3 PUFAs; however, not all fish types are equally rich in these components. To date, it is not clear whether the beneficial effects of fish consumption are shared by fatty and lean fish. Therefore, the aim of this meta-analysis was to synthesize knowledge regarding the relation between the intake of fatty fish or lean fish and the risk of cardiovascular events and all-cause mortality. We conducted a systematic search in PubMed, Web of Science, and Embase until May 2021 for full text with a prospective design involving humans providing data for the highest compared with the lowest fish consumption categories. Summary risk ratios (RRs) and 95% CIs were estimated using a random-effects model. Out of 1902 articles retrieved from the literature search, 19 reports met the criteria for inclusion in the meta-analysis. Altogether, studies on fatty fish comprised 1,320,596 person-years of follow-up, 20,531 incident coronary heart disease (CHD) cases, 9256 incident CVD cases, and 104,763 total deaths. Studies on lean fish comprised 937,362 person-years of follow-up, 21,636 incident CHD cases, 7315 incident CVD cases, and 16,831 total deaths. An inverse association was present for fatty fish with CHD incidence (RR: 0.92; 95% CI: 0.86, 0.97), CHD mortality (RR: 0.83; 95% CI: 0.70, 0.98), and total mortality (RR: 0.97; 95% CI: 0.94, 0.99). This was not the case for lean fish. The summary estimates for CVD incidence and mortality did not show significant association with both fatty fish and lean fish consumption. The study findings are innovative in highlighting that the health benefits so far linked to fish consumption are, in fact, driven by fatty fish.
Keywords: fatty fish, lean fish, CHD incidence, mortality, meta-analysis, cohort studies, all-cause mortality, cardiovascular disease, coronary heart disease
Statement of Significance: Current guidelines for cardiovascular disease prevention in the healthy adult population recommend the habitual consumption of fish with special emphasis on fatty fish. Over the years, a number of prospective cohort studies has provided data on the association between fatty fish or lean fish and cardiovascular events, but to date, a comprehensive evaluation of the evidence relative to distinct fish categories is lacking.
Introduction
Cardiovascular diseases (CVDs) are the major cause of death and disability worldwide. It has recently been estimated that coronary heart disease (CHD) alone accounts for 16% of all deaths globally (1), and that the prevalence of death from CVD will exceed 23.6 million people by 2030 (2).
Against this scenario, a balanced diet has been identified as a potential key lever to avoid premature deaths. Indeed, a large body of evidence from both epidemiological and intervention studies indicates that appropriate food consumption may markedly reduce CVD risk and, in particular, the development and progression of atherosclerosis (3). It has been estimated that by optimizing dietary patterns, 1 in every 5 premature cardiovascular deaths in Europe could have been prevented in the year 2016 (4).
Fish consumption has been consistently associated with a reduced risk of CHD and total CVD in a number of meta-analyses of prospective cohort studies and, therefore, its habitual consumption is usually recommended in the context of a balanced and healthy diet (5–11). Data from dose-response analyses indicate that the consumption of fish associated with the greatest CHD risk reduction (–12%), ranges from 3 to 4 servings per week (11). Smaller amounts (100–150 g/wk) are also associated with a significant CHD risk reduction, although of a lesser magnitude (–4% to –7%) (5, 8–10). Conversely, for higher intakes (>4 servings/wk) the available data do not indicate any further advantage and, if anything, there are suggestions of possible drawbacks for health; this has to be taken into consideration, also in light of the relevant ecological impact of fishing and aquafarming.
The cardiovascular benefits of moderate fish consumption are generally ascribed, at least in part, to its high content of long-chain (LC) n–3 PUFAs: EPA (20:5 n–3) and DHA (22:6 n–3) (12). However, not all fish species are equally rich in these components; fatty fish provides ≤10-fold higher amounts of n–3 PUFAs than lean fish (13). To date, there is no standard definition for fatty fish or lean fish, but a cut-off of 4 g/100 g has been widely used in prior studies to distinguish fatty (≥4 g/100 g; salmon, tuna, herring, kippers, mackerel, eel, and sardines) from lean fish (<4 g/100 g; cod, plaice, shellfish, hake, saithe, seabass, seabream, sole) (13–24). Notably, fat quality also differs between these 2 categories as well as the content of other nutrients such as cholesterol, calcium, sodium, potassium, iron, and vitamin D (Supplemental Table 1). These relevant nutritional differences may in turn have an impact on the health effects associated with fish consumption (25).
Current guidelines for CVD prevention in the healthy adult population (26, 27) recommend the habitual consumption of fish with special emphasis on fatty fish. However, this specification is mainly based on the extensive available evidence supporting the beneficial effects of n–3 PUFAs, rather than on a clear demonstration of an inverse relation of fatty fish consumption with clinical outcomes (26–28). On the other hand, there is no clear evidence of whether lean fish is also associated with a reduced cardiovascular risk. Over the years, a number of prospective cohort studies (13, 14, 15–24, 30–36) has provided data on the association between fatty fish or lean fish and cardiovascular events, but to date, a comprehensive evaluation of the evidence relative to distinct fish categories is lacking.
The aim of this work was to meta-analyze cohort studies exploring the relation of fish consumption with CVD-related morbidity and mortality, as well as all-cause mortality, in order to provide comprehensive evidence for the association of fatty fish or lean fish consumption with CVD-related endpoints and all-cause mortality.
Methods
Search strategy
We performed a literature search (from the earliest available online indexing year until 30 May, 2021) in PubMed, Embase, Scopus, and Cochrane Library databases for prospective cohort studies examining the association between either fatty fish or lean fish and risk of selected cardiovascular outcomes according to PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines (36). We used this specific search strategy (fish or lean fish or fatty fish or shellfish or canned fish or cod or mussels or prawns or mackerel or eel or herring or salmon or bluefish or tuna or halibut) and (consumption or intake or serving or eating) and (myocardial[title] or coronary[title] or mortality[title] or cardiovascular[title] or ischemic[title] or stroke[title] or cerebrovascular[title] or death[title] or fatal[title] or fatality[title] or vascular[title] or heart[title] or events[title] or event[title] or prognosis[title] or prognostic[title] or survival[title]). We also performed additional manual searches through the reference lists of original publications and review articles to identify further pertinent studies. The search was limited to human studies and was restricted to articles written in English. This meta-analysis has been registered on Prospero (https://www.crd.york.ac.uk/PROSPERO) as CRD42021265134.
Selection criteria
Studies were considered for inclusion in the present meta-analysis if they met the following criteria: 1) the authors reported data from an original, peer-reviewed study (not reviews, conferences, and letters); 2) the study had a prospective design; 3) the authors reported risk ratios (RRs), or HRs, with 95% CIs for fatty fish and/or lean fish consumption; 4) the investigators reported ≥1 of the outcomes of CVD risk, including incidence of total CVD and CHD, or CVD and CHD mortality, or all-cause mortality. We included only prospective cohorts to minimize recall and selection bias.
Two investigators (IC and AG) conducted a 2-stage selection process to identify eligible studies: an initial screening of titles and abstracts, followed by an evaluation of all potentially relevant full-length articles. Any discrepancy was resolved by discussions with another investigator (MV). Studies were excluded if they failed to meet the criteria detailed above.
Data extraction and quality assessment
Two investigators (IC and AG) independently reviewed each eligible study, and the following data were extracted: first author's name, publication year, cohort name, geographical location, age of participants at baseline, duration of follow-up, the number of CVD events, the number of participants/person-years of follow-up, method of assessment of fish consumption, categories of fish consumption, outcome ascertainment, and adjusted covariates. If the numbers of participants/person-years and cases were not provided, the corresponding author(s) were contacted for further information. All requested data was provided upon contacting the corresponding authors.
Study quality assessment was performed according to the Newcastle–Ottawa Quality Assessment Scale (37). Two investigators independently extracted and assessed the quality for each study and then compared the results. Scores ranged from 0 to 9 points, with higher scores indicating higher study quality.
Data synthesis and analysis
In this meta-analysis, fatty fish and lean fish were considered the main exposures of interest. Fatty fish was defined as any fish with a total fat content ≥4 g/100 g (e.g., salmon, tuna, herring, kippers, mackerel, eel, and sardines); lean fish included all kinds of fish with a total fat content <4 g/100 g (e.g., cod, plaice, shellfish, hake, saithe, seabass, seabream, sole). This cut-off was derived from prior studies, as there is no standard definition for fatty or lean fish. Notably, fat quality also differs between these 2 categories, as well as the content of other nutrients such as cholesterol, calcium, sodium, potassium, iron, and vitamin D. The nutritional composition per 100 g of fatty fish or lean fish according to this definition is provided in Table 1.
TABLE 1.
Quality of the evidence by the NutriGrade scoring system
| Fatty fish | Lean fish | |
|---|---|---|
| Total CHD incidence (fatal and nonfatal) | Moderate (score: 6.4) | Moderate (score: 6.8) |
| CHD mortality | Low (score: 5.9) | Low (score: 5.9) |
| Total CVD incidence (fatal and nonfatal) | Low (score: 5.0) | Low (score: 5.0) |
| All-cause mortality | Low (score: 5.0) | Low (score: 5.0) |
CHD, coronary heart disease; CVD, cardiovascular disease.
RRs were used as the common measure of association across studies. Studies conducted in 2 independent cohorts were treated as separate reports. We used the forest plots to evaluate RRs and 95% CIs of outcomes in the groups with high versus low fish consumption. Random-effects meta-analyses were used for all comparisons due to high heterogeneity in the fixed-effects models, based on the I2 cut-off. The inverse variance method was used to generate study weights. The heterogeneity among studies was estimated by Cochran's Q test (P <0.05 to be indicative of statistically significant heterogeneity) and I2 statistic (I2 values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively) (38). Additional sensitivity analyses were preplanned and performed by systematically omitting each study 1 at a time and recalculating the summary association to test the robustness of the results and the influence of individual studies on heterogeneity. Potential publication bias was assessed by the Egger regression symmetry test with significant bias for P <0.10 (39).
All statistical analyses were performed with RevMan 5.4 (Review Manager RevMan—Computer program; Version 5.4; The Cochrane Collaboration, 2020) and R 4.1.0 (The R Project for Statistical Computing; Version 4.1.0, 2021), and all tests were 2-sided with a significance level of 0.05 unless otherwise stated.
In order to evaluate the quality of our meta-analyses we utilized the NutriGrade scoring system, adapted from the Grading of Recommendations Assessment, Development, and Evaluation, and designed specifically to assess the quality of evidence of meta-analyses of randomized controlled trials and cohort studies in nutrition research. This scoring system evaluates the risk of bias, precision, heterogeneity, directness, publication bias, funding bias, effect size, and dose response (details are reported in Table 1); accordingly, the quality of a meta-analysis would be viewed as high-, moderate-, low-, or very-low when it received 8 to 10, 6 to <8, 4 to <6, or 0 to <4 points, respectively (40).
Results
Literature search
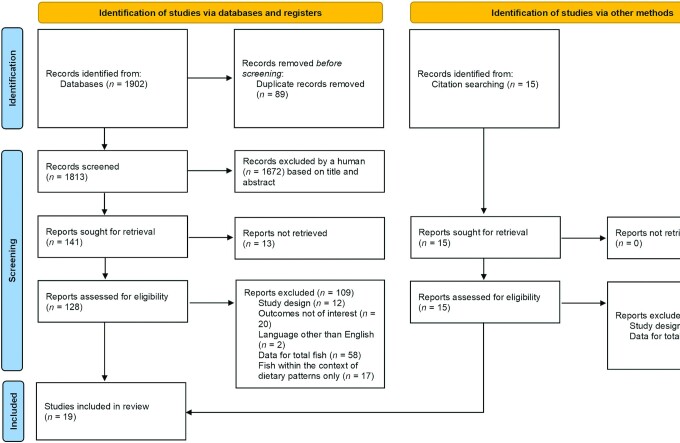
The results from the literature search and study selection process are shown in Figure 1. We identified 1902 articles from PubMed, Embase, Scopus, and Cochrane Library databases by 30 May, 2021. After 2 rounds of review and searching citations of retained articles, 128 potentially relevant studies were initially selected. After evaluating the full texts, we further excluded 109 studies: 12 articles were not based on a prospective study design, 20 articles lacked data for CHD and CVD incidence or CHD and CVD mortality, 2 articles were not written in English, 58 articles reported data for total fish only, and 17 articles examined the consumption of fish only within the context of complex dietary patterns.
FIGURE 1.
Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) flow chart of the systematic review and meta-analysis, indicating the results of the search strategy (65).
Study characteristics
The characteristics of identified studies are shown in Supplemental Table 2. The included studies for fatty fish comprised 1,320,596 person-years of follow-up, 20,531 incident CHD cases, 9256 incident CVD cases, and 104,763 total deaths (811 deaths from CHD). The included studies for lean fish comprised 937,362 person-years of follow-up, 21,636 incident CHD cases, 7315 incident CVD cases, and 16,831 total deaths (1474 deaths from CHD). The mean follow-up periods ranged from 4.0 to 40 y. Among the 19 articles included, 8 were conducted in the USA (13, 14, 16, 18–20, 24, 30), 9 in Europe (15, 17, 22, 23, 29, 31–33, 35), and 2 in Asia (21, 34). Fish consumption was assessed with validated FFQs in all studies. The validation method was the 24-h recall or 7-d food records. The amount of fish in the higher exposure category was ∼25 g/d for fatty fish and 29 g/d for lean fish. In the lower exposure group, the amounts ranged from 0 to 6 g/d both for fatty and lean fish.
All studies except 2 (19, 24) adjusted for smoking, most of the studies adjusted for age (13–16, 18–21, 23, 24, 29–34) and physical activity (13–20, 29–31, 33–35). Most studies controlled for other risk factors, including BMI (14–23, 29, 31, 33–35), education (14, 15, 17–19, 21, 23, 29–35), alcohol consumption (14–23, 30, 31, 33–35), total energy intake (14–23, 29–35), and dietary quality (14–18, 20, 22, 23, 30–35).
The majority of the included studies were rated as high quality as indicated by the Newcastle–Ottawa Quality Assessment Scale score (>8), and the mean study quality scores were 7.8 and 7.9 for fatty fish or lean fish, respectively (Supplemental Table 3).
Fatty fish, lean fish, and total CHD incidence
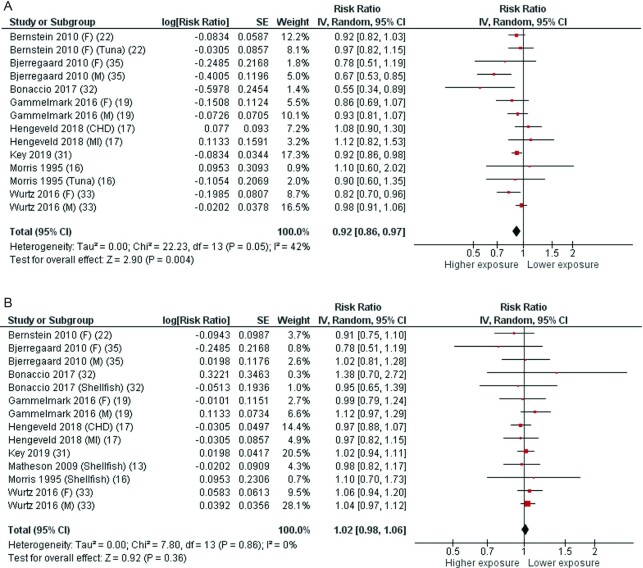
Eight articles (15, 17, 20, 24, 31–33, 35) with 14 cohorts were included in the analysis of fatty fish intake and total CHD incidence (fatal and nonfatal). Two studies reported separate data for tuna (20, 24), and 3 reported data by gender (17, 33, 35). The average follow-up period for the pooled studies was 12.5 y (ranging from 4 to 26 y). The pooled RR of CHD incidence for the highest compared with the lowest category of fatty fish consumption was 0.92 (95% CI: 0.86, 0.97), with moderate heterogeneity (I2 = 42%, P = 0.05) (Figure 2A). By systematically omitting each study 1 at a time, the heterogeneity was generated by 4 articles (15, 32, 33, 35), and when these reports were excluded, the association remained statistically significant with no significant heterogeneity. According to the NutriGrade scoring system, the quality of evidence on the associations of fatty fish intake with total CHD incidence (fatal and nonfatal) was moderate (Table 1).
FIGURE 2.
Forest plots summarizing the RR with 95% CI of coronary heart disease incidence between the highest and lowest categories of fatty fish intake (A) and lean fish intake (B). CHD, coronary heart diseases; F, females; M, males; MI, myocardial infarction; RR, risk ratio.
Nine articles (13, 15, 17, 20, 24, 31–33, 35) with 14 cohorts were included in the analysis of lean fish intake and total CHD incidence (fatal and nonfatal). Three studies reported separate data for shellfish (13, 24, 32). The average follow-up period for the pooled studies was 12.6 y (ranging from 4 to 26 y). The pooled RR for the highest compared with the lowest category of lean fish consumption was 1.02 (95% CI: 0.98, 1.06), with no significant heterogeneity (I2 = 0%, P = 0.86) (Figure 2B). The pooled RR remained stable when the 3 studies reporting data for shellfish were excluded (Supplemental Figure 1). Similarly, the meta-analysis of the 3 studies on shellfish did not show any significant association with CHD (Supplemental Figure 2). According to the NutriGrade scoring system, the quality of evidence on the associations of lean fish intake with total CHD incidence (fatal and nonfatal) was moderate (Table 1).
Studies on the association between either fatty or lean fish and total CHD were grouped according to the quality of the study performance and meta-analyses were performed separately for higher and lower quality studies (Supplemental Figure 3). This evaluation did not significantly influence the findings, confirming that in both high- and low-quality studies a higher consumption of fatty fish is associated with significantly lower CHD incidence [NOS ≥8: RR 0.93 (95% CI: 0.88, 0.99) (15, 17, 20, 31–33); NOS <8: RR 0.76 (95% CI: 0.63, 0.92) (24, 35)], whereas no such significant association was present for lean fish [NOS ≥8: RR 1.02 (95% CI: 0.98, 1.06) (13, 15, 17, 20, 31–33); NOS <8: RR 0.98 (95% CI: 0.82, 1.18) (24, 35)] (Supplemental Figure 3).
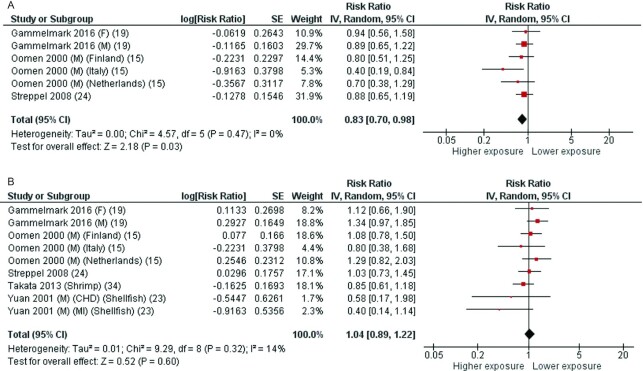
Some articles reported data for CHD mortality only. In more detail, 3 articles (17, 22, 23) with 6 cohorts were included in the analysis of fatty fish intake and CHD mortality. The average follow-up period for the pooled studies was 18.5 y (ranging from 17 to 20 y). The pooled RR of CHD mortality for the highest compared with the lowest category of fatty fish consumption was 0.83 (95% CI: 0.70, 0.98), with no significant heterogeneity (I2 = 0%, P = 0.47) (Figure 3A). According to the NutriGrade scoring system, the quality of evidence on the associations of fatty fish intake with CHD mortality was low (Table 1).
FIGURE 3.
Forest plots summarizing the RR with 95% CI of coronary heart disease mortality between the highest and lowest categories of fatty fish intake (A) and lean fish intake (B). CHD, coronary heart diseases; F, females; M, males; MI, myocardial infarction; RR, risk ratio.
Five articles (17, 21–23, 34) with 9 cohorts were included in the analysis of lean fish intake and CHD mortality. One study reported separate data for shellfish (21), 1 for shrimp (34), and 1 reported data by geographical area (23). The average follow-up period for the pooled studies was 14.3 y (ranging from 8.4 to 20 y). The pooled RR of CHD mortality for the highest compared with the lowest category of lean fish consumption was 1.04 (95% CI: 0.89, 1.22), with low heterogeneity (I2 = 14%, P = 0.32) (Figure 3B). According to the NutriGrade scoring system, the quality of evidence on the associations of lean fish intake with CHD mortality was low (Table 1).
Fatty fish, lean fish, and total CVD incidence
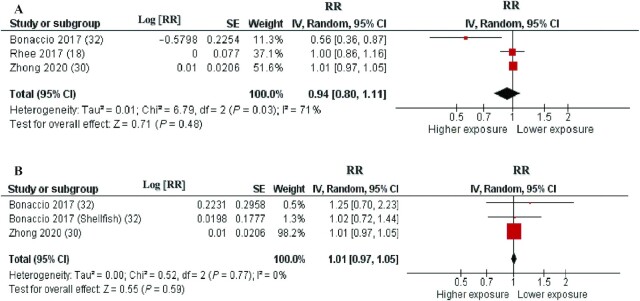
Three cohorts described in 3 articles (16, 30, 32) were included in the analysis of fatty fish intake and total CVD incidence (fatal and nonfatal). The average follow-up period for the pooled studies was 16.6 y (ranging from 4.3 to 19 y). The pooled RR of total CVD incidence for the highest compared with the lowest category of fatty fish consumption was 0.94 (95% CI: 0.80, 1.11), with moderate heterogeneity (I2 = 71%, P = 0.03) (Figure 4A). The heterogeneity was generated by 1 article (32), and when this report was excluded, the association remained stable, RR 1.01 (95% CI: 0.97, 1.05), with no significant heterogeneity (I2 = 0%, P = 0.90). According to the NutriGrade scoring system, the quality of evidence on the associations of fatty fish intake with CVD incidence was low (Table 1).
FIGURE 4.
Forest plots summarizing the RR with 95% CI of total cardiovascular disease incidence between the highest and lowest categories of fatty fish intake (A) and lean fish intake (B). RR, risk ratio.
Three cohorts described in 2 articles (30, 32) were included in the analysis of lean fish intake in relation to total CVD incidence (fatal and nonfatal). The average follow-up period for the pooled studies was 16.6 y (ranging from 4.3 to 19 y). The pooled RR of total CVD incidence for the highest compared with the lowest category of lean fish consumption was 1.01 (95% CI: 0.97, 1.05), with no significant heterogeneity (I2 = 0%, P = 0.77) (Figure 4B). According to the NutriGrade scoring system, the quality of evidence on the associations of lean fish intake with CVD incidence was low (Table 1).
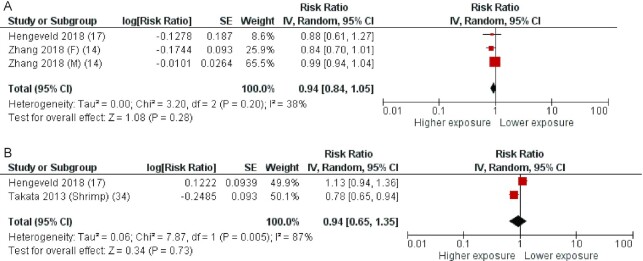
Some articles reported data for CVD mortality only. In more detail, 2 articles (14, 15) with 3 cohorts were included in the analysis of fatty fish intake and CVD mortality. The average follow-up period for the pooled studies was 15.5 y (ranging from 15.1 to 16 y). The pooled RR of CVD mortality for the highest compared with the lowest category of fatty fish consumption was 0.94 (95% CI: 0.84, 1.05), with no significant heterogeneity (I2 = 38%, P = 0.20) (Figure 5A).
FIGURE 5.
Forest plots summarizing the RR with 95% CI of total cardiovascular disease mortality between the highest and lowest categories of fatty fish intake (A) and lean fish intake (B). F, females; M, males; RR, risk ratio.
Two articles (15, 34) with 2 cohorts were included in the analysis of lean fish intake and CVD mortality. One study reported only data for shrimp (34). The average follow-up period for the pooled studies was 11.7 y (ranging from 8.4 to 15.1 y). The pooled RR of CVD mortality for the highest compared with the lowest category of lean fish consumption was 0.94 (95% CI: 0.82, 1.07), with significant high heterogeneity (I2 = 87%, P = 0.005) (Figure 5B).
Fatty fish, lean fish, and all-cause mortality
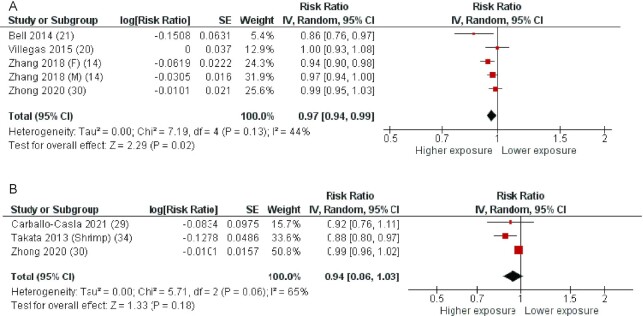
Five cohorts described in 4 articles (14, 18, 19, 30) were included in the analysis of fatty fish intake and all-cause mortality. The average follow-up period for the pooled studies was 11.3 y (ranging from 5 to 19 y). The pooled RR of all-cause mortality for the highest compared with the lowest category of fatty fish consumption was 0.97 (95% CI: 0.94, 0.99), with no statistically significant heterogeneity (I2 = 44%, P = 0.13) (Figure 6A). According to the NutriGrade scoring system, the quality of evidence on the associations of fatty fish intake with all-cause mortality was low (Table 1).
FIGURE 6.
Forest plots summarizing the RR with 95% CI of all-cause mortality between the highest and lowest categories of fatty fish intake (A) and lean fish intake (B). F, females; M, males; RR, risk ratio.
Three cohorts described in 3 articles (29, 30, 34) were included in the analysis of lean fish intake and all-cause mortality. One study reported separate data for shrimp (34). The average follow-up period for the pooled studies was 12.7 y (ranging from 8.4 to 19 y). The pooled RR of all-cause mortality for the highest compared with the lowest category of lean fish consumption was 0.94 (95% CI: 0.86, 1.03), with borderline statistically significant heterogeneity (I2 = 65%, P = 0.06) (Figure 6B). The pooled RR remained not statistically significant when the study reporting separate data for shrimp was excluded [0.99 (95% CI: 0.96, 1.02)] (Supplemental Figure 4). According to the NutriGrade scoring system, the quality of evidence on the associations of lean fish intake with all-cause mortality was low (Table 1).
Publication bias and quality of evidence
Publication bias was assessed using funnel plots (Supplemental Figure 5). Visual analysis of the funnel plots suggested that the associations between fatty fish, lean fish, and risk of selected cardiovascular outcomes (i.e., total CHD incidence, CHD mortality, total CVD incidence, and all-cause mortality) were symmetric and thus at low risk of publication bias. This was confirmed by the Egger's linear regression test.
According to the NutriGrade scoring system, the quality of evidence on the associations of fatty fish intake with total CHD incidence (fatal and nonfatal) was moderate and the quality of evidence on the associations of fatty fish intake with CHD mortality, total CVD incidence (fatal and nonfatal), and all-cause mortality was low (Table 1). Regarding lean fish intake, the quality of evidence on the associations with total CHD incidence (fatal and nonfatal) was moderate and the quality of evidence on the associations of lean fish intake with CHD mortality, total CVD incidence (fatal and nonfatal), and all-cause mortality was low (Table 1).
Discussion
Prior studies in the healthy adult population have shown the benefits of total fish consumption on coronary events and composite CVD outcomes (41–43); however, it remains unclear whether the beneficial health effects associated with total fish consumption are shared by both fatty and lean fish (42). This meta-analysis is the first to focus on the relation between fatty fish and lean fish consumption considered separately, and CHD, CVD, and total mortality. The results indicate that while lean fish is not associated with CHD, CVD, or total mortality, fatty fish consumption is significantly associated with a lower risk of coronary events and total mortality. As for CVD-related mortality, the few available studies did not show a significant association with fatty fish intake. However, due to the paucity of data, we cannot draw firm conclusions. The magnitude of the risk reduction ranges from 17% for fatal CHD to 8% for total CHD incidence (fatal and nonfatal), and to 3% for all-cause mortality; no significant relation with CVD risk was observed.
These findings are coherent with prior studies, which have suggested that the benefits of total fish consumption are strongest for coronary events [such as myocardial infarction (MI) and CHD death], rather than for composite CVD outcomes that include stroke (41). Indeed, in a meta-analysis of observational cohort studies that separately evaluated fatty and lean fish in relation to the risk of stroke, no significant beneficial association emerged with fatty fish (44) and lean fish. The content of LC n-3 PUFAs is the most relevant difference in the nutritional composition of fatty or lean fish (Supplemental Table 1), which could explain, at least in part, their different relation with health outcomes. LC n–3 PUFAs — EPA and DHA — have been linked to the promotion of cardiovascular health (45). In fact, LC n–3 PUFAs contribute to limit atherosclerotic plaque progression and to stabilize it, mainly through the inhibition of both smooth muscle cell proliferation and neovascularization (46), and controlling the release of matrix metalloproteinases by macrophages (12, 47). Fish-derived LC n–3 PUFAs also have beneficial effects on heart electrophysiology thus decreasing the risk of arrhythmias (48, 49) and sudden cardiac death (50, 51). These effects have been observed within the ranges of usual dietary intakes of LC n–3 PUFAs reported for the adult population (i.e., <750 mg/d), with smaller additional benefits for higher intakes (52).
Furthermore, a dietary intervention with 500 g per week of fatty fish (i.e., equivalent to ∼1 g/d of LC n–3 PUFAs) exerts a cardioprotective effect by inhibiting platelet-monocyte aggregation (53); a higher dietary LC n–3 PUFA intake also improves endothelial function (52) by increasing endothelium-derived vasodilators (50, 54) and reducing circulating markers of endothelial dysfunction (i.e., E-selectin, vascular cell adhesion molecule-1) (52). Finally, the well-known anti-inflammatory properties of LC n–3 PUFAs (12, 55–57) further contribute to cardiovascular health.
These data have supported current dietary guidelines which encourage the consumption of a variety of fish, preferably fatty types — mainly as a dietary source of LC n–3 PUFAs — for CVD prevention (27, 58). The use of supplements with doses of EPA plus DHA that are substantially higher than those obtained by dietary fish intake are not recommended for the general population; however, the American Heart Association indicates fish oil supplements as a reasonable (but not recommended) treatment for the secondary prevention of CVD in people with pre-existing CHD (28, 59).
Our findings not only give further support to dietary guidelines in promoting fatty fish consumption, but they can also lead to an improvement of the adherence to dietary recommendations, since cardiovascular benefits could be achieved with a smaller quantity of fish consumption — provided that fatty fish is chosen. Indeed, an amount of 1–2 servings of fatty fish per week could be more feasible for most people, as compared to the currently recommended 3–4 servings of total fish. Looking at the limited available evidence on the relation between intake of fatty fish and cardiovascular benefits, we could speculate that a weekly intake of fatty fish starting from 100 g to 150 g may be sufficient to contribute to CHD prevention. On the other hand, although lean fish does not provide cardioprotective benefit, it could be consumed, if desired, within a varied and balanced diet as a good source of animal protein as well as other foods of animal origin such as poultry, eggs, and dairy products.
The preferential consumption of fatty fish should also be viewed in the context of the so-called “planetary health” (60, 61), which aims to harmonize human health and environmental preservation. In fact, the chance to achieve the largest health advantages with a smaller quantity of fish contributes to limit further overexploitation of fisheries (62, 63). Moreover, current evidence linking fish for human consumption to environmental sustainability (64) supports the advice of shifting towards the smallest types of fish with the shortest lifecycle that mainly belong to the fatty type and, therefore, should also be preferred for their health benefits.
A major strength of our study is that it summarizes, for the first time in meta-analyses, up-to-date evidence of the associations between the consumption of different types of fish (fatty or lean) with CHD/CVD incidence and mortality and all-cause death. The utilization of meta-analyses allows a comprehensive and weighted balanced summary of the available evidence. Furthermore, all the included studies used a prospective cohort design, which allows a more efficient control for confounders than other observational studies, and often represent the best source of evidence in nutritional epidemiology, as randomized controlled trials are rarely available. The evidence here provided is moderate, largely due to the lack of dose-response assessment and the effect size; it is however, consistent and is supported by biologically plausible mechanisms of action.
However, several limitations should be acknowledged. First, residual confounding may persist in our meta-analysis, though the estimates with the maximum extent of adjustment for confounders from each study were used in our analyses to reduce the potential of confounding. Furthermore, it remains unclear the extent to which fish consumption reduces risk per se or by substituting “unhealthy foods.” Second, except for the analysis of the association between fatty fish and lean fish intakes and total CHD incidence, other analyses included a limited number of studies; for this reason, it was not possible to test a dose-response relation. In addition, the analyses were of low-to-moderate quality, as indicated by the NutriGrade score. The lack of dose-response assessment and the effect size were the main components contributing to the low-moderate range of the NutriGrade system for CHD and all-cause mortality, and CVD incidence. In addition, a small number of studies included in the relations with CVD incidence and all-cause mortality, also contributed to the low-moderate range. Third, the types of fatty fish and lean fish consumed varied among countries; moreover, the evaluation of their consumption was based on self-administered questionnaires that did not include specific questions on fatty fish and lean fish intake. Fourth, the mean follow-up period across the studies is quite wide (4–40 y); however, to partially account for time effect, for CHD incidence (the outcome with the large number of studies) we performed a subgroup analysis based on median of follow-up (i.e., above or below median) and the findings did not change (data not shown). Fifth, the studies included in this meta-analysis did not adjust for the use of supplements that contained fish oil, a potentially relevant confounder. This applies to both fatty fish and lean fish, therefore, the risk of bias is the same for all the included studies and the outcomes analyzed. In this regard it is also relevant to underline that the included studies were conducted involving people in primary prevention for CHD and CVD for whom the use of fish oil supplements is not specifically recommended and the reported percentage of use is rather low, ∼8% of the population. Finally, some results were affected by significant heterogeneity. Although it was not possible to definitely identify the origin of such heterogeneity, we excluded the presence of publication bias and, moreover, confirmed the outcomes of the analyses after excluding the studies with the highest contribution to the heterogeneity of the meta-analyses.
Conclusion
The study findings are innovative in highlighting that the health benefits so far linked to fish consumption are, in fact, limited to fatty fish. Hence, the preferential consumption of this type of fish, and particularly the species more sustainable for ecological reasons, should be recommended.
The evidence we provide could also substantiate future guidelines on diet and CHD prevention: 1 or 2 servings per week of fatty fish, which are consistent with features of the traditional Mediterranean diet, are feasible for the majority of the adult population and could give a significant contribution to the prevention of CHD at the population level. Moreover, if fatty fish were properly chosen among the different species, this recommendation would also contribute to the preservation of life below water. Future research is needed to address the unresolved questions around dose-response relations and in relation to the evaluation of all mechanisms linking LC n–3 fatty acids and other components of fatty fish to a reduced risk of CHD and total mortality.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—GR and OV: were responsible for study design; AG, IC, RL, GR, OV, and MV: were responsible for writing; GR, OV, and MV: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
The present analyses were supported by a research grant from the “Barilla Center for Food and Nutrition Foundation (BCFN)” within the framework of a project aimed at an evidence-based reformulation of the Food Pyramid for the prevention of cardiovascular disease. The funder had no role in study design, collection, analysis, and interpretation of data; in the writing of the manuscript and in the decision to submit the article for publication.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–5 and Supplemental Tables 1–3 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
AG and IC contributed equally to this work.
Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; LC, long-chain; RR, risk ratio.
Contributor Information
Annalisa Giosuè, Department of Clinical Medicine and Surgery, Federico II University of Naples, Naples, Italy.
Ilaria Calabrese, Department of Clinical Medicine and Surgery, Federico II University of Naples, Naples, Italy.
Roberta Lupoli, Department of Molecular Medicine and Medical Biotechnology, Federico II University of Naples, Naples, Italy.
Gabriele Riccardi, Department of Clinical Medicine and Surgery, Federico II University of Naples, Naples, Italy.
Olga Vaccaro, Department of Pharmacy, Federico II University of Naples, Naples, Italy.
Marilena Vitale, Department of Clinical Medicine and Surgery, Federico II University of Naples, Naples, Italy.
References
- 1. Meier T, Gräfe K, Senn F, Sur P, Stangl GI, Dawczynski C, März W, Kleber ME, Lorkowski S. Cardiovascular mortality attributable to dietary risk factors in 51 countries in the WHO European region from 1990 to 2016: a systematic analysis of the Global Burden of Disease study. Eur J Epidemiol. 2019;34(1):37–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SRet al. . Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–528. [DOI] [PubMed] [Google Scholar]
- 3. Riccardi G, Giosuè A, Calabrese I, Vaccaro O. Dietary recommendations for prevention of atherosclerosis. Cardiovasc Res. 2021;cvab173. [DOI] [PubMed] [Google Scholar]
- 4. Riccardi G, Vitale M, Vaccaro O. Are Europeans moving towards dietary habits more suitable for reducing cardiovascular disease risk?. Nutr Metab Cardiovasc Dis. 2020;30(11):1857–60. [DOI] [PubMed] [Google Scholar]
- 5. Whelton SP, He J, Whelton PK, Muntner P. Meta-analysis of observational studies on fish intake and coronary heart disease. Am J Cardiol. 2004;93(9):1119–23. [DOI] [PubMed] [Google Scholar]
- 6. He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109(22):2705–11. [DOI] [PubMed] [Google Scholar]
- 7. Zheng J, Huang T, Yu Y, Hu X, Yang B, Li D. Fish consumption and CHD mortality: an updated meta-analysis of seventeen cohort studies. Public Health Nutr. 2012;15(4):725–37. [DOI] [PubMed] [Google Scholar]
- 8. Leung Yinko SS, Stark KD, Thanassoulis G, Pilote L. Fish consumption and acute coronary syndrome: a meta-analysis. Am J Med. 2014;127(9):848–857.e2. [DOI] [PubMed] [Google Scholar]
- 9. Jayedi A, Zargar MS, Shab-Bidar S. Fish consumption and risk of myocardial infarction: a systematic review and dose-response meta-analysis suggests a regional difference. Nutr Res. 2019;62:1–12. [DOI] [PubMed] [Google Scholar]
- 10. Zhang B, Xiong K, Cai J, Ma A. Fish consumption and coronary heart disease: a meta-analysis. Nutrients. 2020;12(8)::2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, De Henauw S, Michels N, Devleesschauwer B, Schlesinger Set al. . Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019;59(7):1071–90. [DOI] [PubMed] [Google Scholar]
- 12. Goel A, Pothineni NV, Singhal M, Paydak H, Saldeen T, Mehta JL. Fish, fish oils and cardioprotection: promise or fish tale?. Int J Mol Sci. 2018;19(12):3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matheson EM, Mainous AG, Hill EG, Carnemolla MA. Shellfish consumption and risk of coronary heart disease. J Am Diet Assoc. 2009;109(8):1422–26. [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Zhuang P, He W, Chen JN, Wang WQ, Freedman ND, Abnet CC, Wang JB, Jiao JJ. Association of fish and long-chain omega-3 fatty acids intakes with total and cause-specific mortality: prospective analysis of 421 309 individuals. J Intern Med. 2018;284(4):399–417. [DOI] [PubMed] [Google Scholar]
- 15. Hengeveld LM, Praagman J, Beulens JWJ, Brouwer IA, van der Schouw YT, Sluijs I. Fish consumption and risk of stroke, coronary heart disease, and cardiovascular mortality in a Dutch population with low fish intake. Eur J Clin Nutr. 2018;72(7):942–50. [DOI] [PubMed] [Google Scholar]
- 16. Rhee JJ, Kim E, Buring JE, Kurth T. Fish consumption, omega-3 fatty acids, and risk of cardiovascular disease. Am J Prev Med. 2017;52(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gammelmark A, Nielsen MS, Bork CS, Lundbye-Christensen S, Tjønneland A, Overvad K, Schmidt EB. Association of fish consumption and dietary intake of marine n-3 PUFA with myocardial infarction in a prospective Danish cohort study. Br J Nutr. 2016;116(1):167–77. [DOI] [PubMed] [Google Scholar]
- 18. Villegas R, Takata Y, Murff H, Blot WJ. Fish, omega-3 long-chain fatty acids, and all-cause mortality in a low-income US population: results from the Southern Community cohort study. Nutr Metab Cardiovasc Dis. 2015;25(7):651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bell GA, Kantor ED, Lampe JW, Kristal AR, Heckbert SR, White E. Intake of long-chain ω-3 fatty acids from diet and supplements in relation to mortality. Am J Epidemiol. 2014;179(6):710–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122(9):876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154(9):809–16. [DOI] [PubMed] [Google Scholar]
- 22. Streppel MT, Ocké MC, Boshuizen HC, Kok FJ, Kromhout D. Long-term fish consumption and n-3 fatty acid intake in relation to (sudden) coronary heart disease death: the Zutphen study. Eur Heart J. 2008;29(16):2024–30. [DOI] [PubMed] [Google Scholar]
- 23. Oomen CM, Feskens EJ, Räsänen L, Fidanza F, Nissinen AM, Menotti A, Kok FJ, Kromhout D. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am J Epidemiol. 2000;151(10):999–1006. [DOI] [PubMed] [Google Scholar]
- 24. Morris MC, Manson JE, Rosner B, Buring JE, Willett WC, Hennekens CH. Fish consumption and cardiovascular disease in the physicians’ health study: a prospective study. Am J Epidemiol. 1995;142(2):166–75. [DOI] [PubMed] [Google Scholar]
- 25. Costabile G, Della Pepa G, Vetrani C, Vitaglione P, Griffo E, Giacco R, Vitale M, Salamone D, Rivellese AA, Annuzzi Get al. . An oily fish diet improves subclinical inflammation in people at high cardiovascular risk: a randomized controlled study. Molecules. 2021;26(11):3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Horn L, Carson JA, Appel LJ, Burke LE, Economos C, Karmally W, Lancaster K, Lichtenstein AH, Johnson RK, Thomas RJet al. . Recommended dietary pattern to achieve adherence to the American Heart Association/American College of Cardiology (AHA/ACC) guidelines: a scientific statement from the American Heart Association. Circulation. 2016;134(22):e505–29. [DOI] [PubMed] [Google Scholar]
- 27. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton Cet al. . 2016 European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siscovick DS, Barringer TA, Fretts AM, Wu JH, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HMet al. . Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135(15):e867–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carballo-Casla A, Ortolá R, García-Esquinas E, Oliveira A, Sotos-Prieto M, Lopes C, Lopez-Garcia E, Rodríguez-Artalejo F. The Southern European Atlantic diet and all-cause mortality in older adults. BMC Medicine. 2021;19(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhong VW, Van Horn L, Greenland P, Carnethon MR, Ning H, Wilkins JT, Lloyd-Jones DM, Allen NB. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern Med. 2020;180(4):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Key TJ, Appleby PN, Bradbury KE, Sweeting M, Wood A, Johansson I, Kühn T, Steur M, Weiderpass E, Wennberg Met al. . Consumption of meat, fish, dairy products, and eggs and risk of ischemic heart disease. Circulation. 2019;139(25):2835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bonaccio M, Ruggiero E, Di Castelnuovo A, Costanzo S, Persichillo M, De Curtis A, Cerletti C, Donati MB, de Gaetano G, Iacoviello Let al. . Fish intake is associated with lower cardiovascular risk in a Mediterranean population: prospective results from the Moli-Sani study. Nutr Metab Cardiovasc Dis. 2017;27(10):865–73. [DOI] [PubMed] [Google Scholar]
- 33. Würtz AML, Hansen MD, Tjønneland A, Rimm EB, Schmidt EB, Overvad K, Jakobsen MU. Substitution of meat and fish with vegetables or potatoes and risk of myocardial infarction. Br J Nutr. 2016;116(9):1602–10. [DOI] [PubMed] [Google Scholar]
- 34. Takata Y, Zhang X, Li H, Gao YT, Yang G, Gao J, Cai H, Xiang YB, Zheng W, Shu XO. Fish intake and risks of total and cause-specific mortality in 2 population-based cohort studies of 134,296 men and women. Am J Epidemiol. 2013;178(1):46–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bjerregaard LJ, Joensen AM, Dethlefsen C, Jensen MK, Johnsen SP, Tjønneland A, Rasmussen LH, Overvad K, Schmidt EB. Fish intake and acute coronary syndrome. Eur Heart J. 2010;31(1):29–34. [DOI] [PubMed] [Google Scholar]
- 36. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, Atkins D, Barbour V, Barrowman N, Berlin JAet al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264. [DOI] [PubMed] [Google Scholar]
- 37. Wells G, Shea B, O'Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON: Ottawa Hospital Research Institute; 2000. [Google Scholar]
- 38. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwingshackl L, Knüppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopantelis E, Iqbal Ket al. . Perspective: Nutrigrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7(6):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jayedi A, Soltani S, Abdolshahi A, Shab-Bidar S. Healthy and unhealthy dietary patterns and the risk of chronic disease: an umbrella review of meta-analyses of prospective cohort studies. Br J Nutr. 2020;124(11):1133–44. [DOI] [PubMed] [Google Scholar]
- 42. Jayedi A, Shab-Bidar S, Eimeri S, Djafarian K. Fish consumption and risk of all-cause and cardiovascular mortality: a dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2018;21(7):1297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wan Y, Zheng J, Wang F, Li D. Fish, long chain omega-3 polyunsaturated fatty acids consumption, and risk of all-cause mortality: a systematic review and dose-response meta-analysis from 23 independent prospective cohort studies. Asia Pac J Clin Nutr. 2017;26:939–56. [DOI] [PubMed] [Google Scholar]
- 44. Qin Z-Z, Xu J-Y, Chen G-C, Ma Y-X, Qin L-Q. Effects of fatty and lean fish intake on stroke risk: a meta-analysis of prospective cohort studies. Lipids Health Dis. 2018;17(1):264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schulze MB, Minihane AM, Saleh RNM, Risérus U. Intake and metabolism of omega-3 and omega-6 polyunsaturated fatty acids: nutritional implications for cardiometabolic diseases. Lancet Diabetes Endocrinol. 2020;8(11):915–30. [DOI] [PubMed] [Google Scholar]
- 46. Yagi S, Fukuda D, Aihara K-I, Akaike M, Shimabukuro M, Sata M. n-3 polyunsaturated fatty acids: promising nutrients for preventing cardiovascular disease. J Atheroscler Thromb. 2017;24(10):999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–26. [DOI] [PubMed] [Google Scholar]
- 48. London B, Albert C, Anderson ME, Giles WR, Van Wagoner DR, Balk E, Billman GE, Chung M, Lands W, Leaf Aet al. . Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office of Dietary Supplements omega-3 fatty acids and their role in Cardiac Arrhythmogenesis Workshop. Circulation. 2007;116(10):e320–35. [DOI] [PubMed] [Google Scholar]
- 49. Mozaffarian D, Prineas RJ, Stein PK, Siscovick DS. Dietary fish and n-3 fatty acid intake and cardiac electrocardiographic parameters in humans. J Am Coll Cardiol. 2006;48(3):478–84. [DOI] [PubMed] [Google Scholar]
- 50. Rimm EB, Appel LJ, Chiuve SE, Djoussé L, Engler MB, Kris-Etherton PM, Mozaffarian D, Siscovick DS, Lichtenstein AH. Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138(1):e35–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. McLennan PL, Abeywardena MY. Membrane basis for fish oil effects on the heart: linking natural hibernators to prevention of human sudden cardiac death. J Membr Biol. 2005;206(2):85–102. [DOI] [PubMed] [Google Scholar]
- 52. Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–67. [DOI] [PubMed] [Google Scholar]
- 53. Din JN, Harding SA, Valerio CJ, Sarma J, Lyall K, Riemersma RA, Newby DE, Flapan AD. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis. 2008;197(1):290–6. [DOI] [PubMed] [Google Scholar]
- 54. Cottin SC, Sanders TA, Hall WL. The differential effects of EPA and DHA on cardiovascular risk factors. Proc Nutr Soc. 2011;70(2):215–31. [DOI] [PubMed] [Google Scholar]
- 55. Endo J, Arita M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J Cardiol. 2016;67(1):22–7. [DOI] [PubMed] [Google Scholar]
- 56. Calder PC. The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res. 2012;56(7):1073–80. [DOI] [PubMed] [Google Scholar]
- 57. Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr. 2012;32(1):203–27. [DOI] [PubMed] [Google Scholar]
- 58. Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JWet al. . 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hu Y, Hu FB, Manson JE. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc. 2019;8(19):e013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Whitmee S, Haines A, Beyrer C, Boltz F, Capon AG, de Souza Dias BF, Ezeh A, Frumkin H, Gong P, Head Pet al. . Safeguarding human health in the Anthropocene epoch: report of the Rockefeller Foundation-Lancet commission on planetary health. Lancet North Am Ed. 2015;386(10007):1973–2028. [DOI] [PubMed] [Google Scholar]
- 61. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen S, Garnett T, Tilman D, DeClerck F, Wood Aet al. . Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet North Am Ed. 2019;393(10170):447–92. [DOI] [PubMed] [Google Scholar]
- 62. McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. Marine defaunation: animal loss in the global ocean. Science. 2015;347(6219):1255641. [DOI] [PubMed] [Google Scholar]
- 63. Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423(6937):280–3. [DOI] [PubMed] [Google Scholar]
- 64. Petersson T, Secondi L, Magnani A, Antonelli M, Dembska K, Valentini R, Varotto A, Castaldi S. A multilevel carbon and water footprint dataset of food commodities. Scientific Data. 2021;8(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SEet al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical research ed). 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.