ABSTRACT
Recent scientific evidence has shown the importance of diet and lifestyle habits for the proper functioning of the human body. A balanced and healthy diet, physical activity, and psychological well-being have a direct beneficial effect on health and can have a crucial role in the development and prognosis of certain diseases. The Southern European Atlantic diet, also named the Atlantic diet, is a unique dietary pattern that occurs in regions that present higher life expectancy, suggesting that this specific dietary pattern is associated with positive health effects. In fact, it is enriched with nutrients of high biological value, which, together with its cooking methods, physical activity promotion, reduction in carbon footprint, and promoting of family meals, promote these positive effects on health. The latest scientific advances in the field of nutri-epigenetics have revealed that epigenetic markers associated with food or nutrients and environmental factors modulate gene expression and, therefore, are involved with both health and disease. Thus, in this review, we evaluated the main aspects that define the Southern European Atlantic diet and the potential epigenetic changes associated with them based on recent studies regarding the main components of these dietary patterns. In conclusion, based on the information existing in the literature, we postulate that the Southern European Atlantic diet could promote healthy aging by means of epigenetic mechanisms. This review highlights the necessity of performing longitudinal studies to demonstrate this proposal.
Keywords: Atlantic diet, biomarkers, bioactive compounds, epigenetics, healthy foods, lifestyle, nutri-epigenetics, prevention, precision nutrition
Statement of Significance: The Southern European Atlantic diet (SEAD) has features that are able to modulate epigenetic mechanisms, which could, in turn, promote healthy aging through the consumption of nutritional bioactive compounds, physical activity, promotion of psychological well-being, and reduction in the environmental production of endocrine disruptors. Therefore, this review provides evidence that the beneficial effect of the SEAD could be promoted by epigenetic mechanisms. This evidence strongly warrants further scientific studies to demonstrate the epigenetic value of the SEAD in promoting healthy aging by slowing down the molecular or physiological aging process, which is of the foremost relevance in the nutritional field of disease prevention.
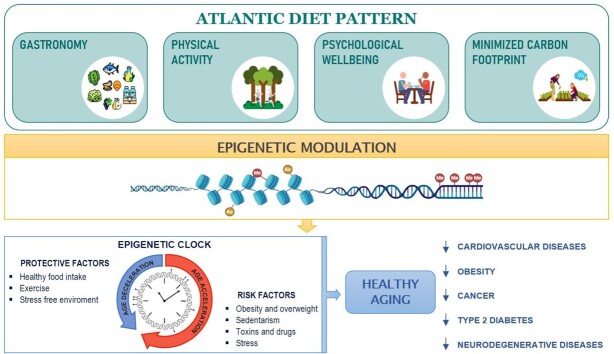
Graphical Abstract
Graphical Abstract.
Introduction
For centuries, it has been known that food can interfere with the health status of an individual and, hence, it has been used to treat different conditions and diseases throughout history (1). Currently, the scientific community shows a growing interest in how our genes respond to the different foods human beings consume. However, at present, the molecular basis by which dietary nutrients or dietary patterns modify the expression of genes has not yet been fully described. The most widely accepted hypothesis suggests that epigenetic regulation is responsible for these processes (2). Epigenetics involves the study of how environmental factors, such as lifestyle, physical activity, exposure to toxins, and diet, are capable of modulating the expression of genes without altering our DNA sequence (3–5). Epigenetic modifications are common in many diseases, such as obesity (6–8), type 2 diabetes (9, 10), metabolic syndrome (4, 11), insulin resistance (12, 13), and cancer (14–17). Importantly, these epigenetic markers can be reversed by different therapeutic strategies such as occur after following a low-calorie diet (18, 19), bariatric surgery (20, 21), or physical activity (22, 23) during obesity management. Nutrients can also act as a source of epigenetic modifications and reverse specific epigenetic markers associated with disease (24). Thus, nutritional epigenetics has emerged as a novel mechanism underlying gene–diet interactions, providing evidence of the modulating role of nutrition in aging and the development of age-related diseases (25–27).
Knowing what dietary components or dietary patterns influence the molecular regulatory mechanisms involved in gene expression would help advance research related to the prevention, prognosis, and treatment of pathologies such as obesity, diabetes, and cardiovascular diseases (CVDs) or cancer (28), and finally, to guarantee survival into old age.
Healthy dietary patterns are generally based on including health-promoting foods, such as plant-based foods, fresh fruits and vegetables, half-grain or whole-grain cereals, nuts, which are sources of omega-3 fatty acids and low in saturated fat and trans fat and refined added sugars, as well as different bioactive compounds. Examples of healthy dietary patterns are the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH). A Mediterranean diet occurs naturally in certain regions and is ingrained in local tradition, and is not only perceived as a healthy diet but also as a lifestyle (29). The DASH diet was created as a result of studies to improve certain pathologies, such as blood pressure and other CVDs (30). Both dietary patterns have been extensively assessed in relation to chronic diseases and were associated with beneficial effects on preventing several diseases. In the last few years other potentially healthy diets have been highlighted among traditional dietary patterns, such as the Nordic diet and Southern European Atlantic diet (SEAD).
The Nordic diet, based on typical Finnish foods (31), shares nutritional recommendations with the Mediterranean diet: both emphasize local seasonal foods and promote sustainability and preservation of the environment; their main difference is the type of oil used for cooking, where the Nordic diet preferably uses canola oil. In relation to the Nordic diet, epidemiological studies are still lacking to demonstrate its effects on health, as shown with the Mediterranean diet (32).
Particularly, in the northwestern Iberian Peninsula, mainly Galicia and northern Portugal, there is another dietary pattern, the SEAD (33). Although there are few scientific studies to date, the SEAD is also associated with health benefits similar to the Mediterranean diet.
The SEAD regions by themselves present higher life expectancy linked to a group of interconnected factors (socioeconomic, health, environmental, and genetic). Particularly in the Galicia region, higher survival rates were observed, with 1823 centenarians registered in 2020 according to the National Institute of Statistics (34–36). These data demonstrate that the SEAD regions have a high prevalence of survival into old age, which is strongly determined by mortality after 85 y of age, mainly caused by CVD. In fact, Europe accounts for 49% of the deaths which implies a great public health importance (37). Whereas, in the northwest of Spain low rates of CVD mortality were found, which is linked to significantly high rates of survival in recent years (34, 38, 39). This old-age survival observed in the SEAD region may be related to a healthy dietary pattern.
The aim of this review was to collect scientific evidence demonstrating the beneficial properties of the main components included in the SEAD pattern, such as foods, physical activity, healthy habits and behaviors and environmental sustainability, and the potential epigenetic changes associated with them based on recent studies regarding the main components of this dietary pattern.
Comparison between the SEAD and Other Studied Healthy Dietary Patterns
Although the Mediterranean diet, DASH, Nordic diet, and SEAD have common bases, there are also certain aspects that differentiate them and make each one a unique and independent dietary pattern. Table 1 shows the consumption of food servings and food groups in 4 dietary patterns.
TABLE 1.
Differences between servings in the SEAD, Mediterranean diet, DASH diet, and Nordic diet1
| Components | SEAD (40) | Mediterranean diet (41) | DASH diet (30) | Nordic diet (42, 43) |
|---|---|---|---|---|
| Whole-grain bread | ≥1 servings/d | ≥1 servings/d | Not specified | 3–4 servings/d |
| Rice and cereals | ≥1 servings/d, whole grain | 1–2 servings/main meal | Grains: 6–8 servings/d | Lunch cereals: 1.5 servings/d (muesli, oat bran, barley flakes)Whole grain: 3 servings/wk |
| Potatoes | ≥1 servings/d | ≤3 servings/wk | Not specified | >2.5 servings/d |
| Vegetable oil | Frequent use of olive oil | Frequent use of olive oil | Fat and oils: 2–3 servings/d | Frequent use of canola oil |
| Fresh fruit | ≥2 servings/d | 1–2 servings/main meal | 4–5 servings/d | >3 servings/d |
| Vegetables | ≥2 servings/dBrassicas, ≥3 servings/wk | ≥2 servings/main meal | 4–5 servings/d | >3 servings/d |
| Legumes | ≥2 servings/wk | ≥2 servings/wk | 4–5 servings/wk | High consumption but not a concrete recommendation |
| Fish | ≥3 servings/wk | ≥2 servings/wk | ≤ 2 servings/d | 3–5 servings/wk |
| Sea food | ≥1 servings/wk | Not specified | Not specified | High consumption but not a concrete recommendation |
| Meat | Pork, ≥1 servings/wkPoultry/game, ≥1 servings/wkVeal, ≥1 servings/wk | White meat, 2 servings/wkRed meat, <2 servings/wk | Lean meat, poultry: ≤ 2 servings/d | Meat, ≤5 servings/wkPoultry, ≤3 servings/wk |
| Dairy products | ≥2 servings/d | 2 servings/d | 2–3 servings/d (fat-free or low-fat) | 1–2 servings/wk |
| Eggs | ≥3 servings/wk | 2–4 servings/wk | ≤4 servings/wk | Without exceeding total cholesterol intake recommendation |
| Nuts | Preferably chestnuts, walnuts, and almonds, ≥2 servings/wk | 1–2 servings/d | 4–5 servings/wk | 0.5 servings/d (preferably almonds) |
| Sweets | Never or hardly ever | ≤2 servings/wk | ≤5 servings/wk | For weekends |
| Alcohol2 | Wine, ≥1 servings/d | Fermented beverages, 1–2 glass/d | ≤2 drinks/d for men and ≤1 drink/d for women | Beer in moderation |
DASH, Dietary Approaches to Stop Hypertension; SEAD, Southern European Atlantic diet.
Glass/drink: wine, 100 mL; beer, 200 mL.
In SEAD, as in the Nordic diet, the consumption of fish and shellfish is higher than in the Mediterranean diet (44–46); moreover, in the SEAD, the consumption of seaweed is gaining interest in the last few years (28). On the other hand, in DASH, the consumption of fish and shellfish is lower due to the low consumption of protein of animal origin (2). With regard to meat consumption, it is higher with the SEAD, mainly pork and veal (28, 29). On the contrary, in the Mediterranean diet, Nordic diet, and DASH diet, less meat is consumed, and the consumption of lean meat is encouraged (29, 30, 45, 46).
The consumption of fruits, vegetables, and legumes is high in these above-mentioned dietary patterns (45–47). Remarkably, the variety of vegetables consumed differs, however, with vegetables of the Brassicas genus being the predominant variety of vegetables consumed in SEAD (44). The same occurs with fruits, where, in SEAD, more apples, pears, or citrus fruits are consumed than in the Mediterranean diet (47) and the Nordic diet (45, 46). With regard to nuts, in the SEAD more chestnuts are consumed, which is a low-calorie nut; in the Mediterranean diet, more hazelnuts, almonds, or pistachios are consumed (44); and in the Nordic diet, more almonds are consumed (45, 46). However, high amounts of fruits and vegetables are consumed, as well as legumes and nuts to provide plant-based protein in the DASH diet (30).
Although bread is present as the basis of both SEAD and the Mediterranean diet, there are differences in the consumption of the sources of complex carbohydrates. The bread consumed in the SEAD is mostly unrefined flour. In SEAD and Nordic diets, the consumption of potatoes predominates, compared with rice and pasta that predominate in the Mediterranean diet (44–46). Also, the consumption of whole grains is highly consumed in the SEAD, Nordic diet, and DASH diets (30, 44–46).
Dairy products are highly consumed in the SEAD, particularly cheeses, compared with the Mediterranean diet and Nordic diet, where their consumption is low and moderate, respectively (29, 44–46), or in the DASH diet, where cheeses consumed are low in fat or without fat and consumed moderately (30).
The main source of fat is olive oil for cooking and dressing in the SEAD and the Mediterranean diet (29, 44). However, canola oil is the main source of fat for cooking and dressing in the Nordic diet (45, 46). Alcohol consumption is moderate in the 4 dietary patterns, based mainly on wine in the SEAD (44) and the Mediterranean diet (29) and in beer in Nordic diet (45, 46). In addition, in the SEAD, the Mediterranean diet, and the Nordic diet there is a moderate consumption of eggs (29, 44–46), and in the DASH diet, sodium consumption is limited to 2300 mg/d (30).
In addition to the differences in the food-group consumption, the SEAD, the Mediterranean diet, and the Nordic diet emphasize sustainable and seasonal foods (29, 45, 46, 48). Moreover the SEAD regions are found in rural areas where pollution is low. (34). The SEAD and Mediterranean diet promote a healthy lifestyle with daily physical activity and enjoying meals as a social act (29, 48).
Specific Properties of the SEAD
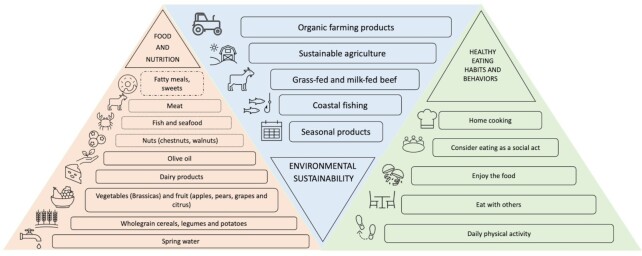
The SEAD, also called the “Atlantic diet,” represents a dietary pattern associated with the countries in Western Europe that border the Atlantic Ocean: Ireland, Scotland, Wales, southern England, Isle of Man, French Brittany, northern Portugal, and northwestern Spain (Galicia, Leon, and Asturias). Specifically, the SEAD is the characteristic eating pattern of the northwestern parts of the Iberian Peninsula, including Galicia (Spain) and northern Portugal (33, 49). In 2006, the “Baione Declaration of the Atlantic Diet” was signed by different institutions, which marked the first step towards the generation of the SEAD criteria. Currently, this declaration is known as the “Atlantic Diet Decalogue” and includes a high consumption of fish and seafood; high intake of cereals, potatoes, and legumes; high consumption of fruits and vegetables; olive oil as the primary source of fat; daily consumption of dairy products; moderate consumption of meat; water as the main beverage; simple cooking processes; maintenance of traditional Atlantic food habits; and daily exercise (Figure 1) (44, 50).
FIGURE 1.
Representation of the 3 main features of the SEAD pyramid corresponding to food and nutrition, environmental sustainability, and healthy eating habits and behavior. (Left) This triangle illustrates the typical SEAD based on a balanced, varied, healthy diet, including foods that are consumed daily (continuous line), 2–3 times/wk (dashed box)), and occasionally (dotted-dashed box). The recommended frequency for the intakes of the most important groups of foods is illustrated in ascending order, from the most to the least frequent. (Center) This triangle describes the typical habits and behaviors in the northwestern parts of the Iberian Peninsula that favor environmental sustainability. (Right) This triangle emphasizes the typical eating habits and behaviors of the SEAD that favor healthy living: cooking at home and family meals promote a stress-free environment. Daily physical activity is a part of daily routine in the northwestern parts of the Iberian Peninsula. SEAD, Southern European Atlantic diet.
Food and nutrition
Seafood and fish
The high intake of fish and seafood (mollusks and crustaceans) in this dietary pattern provides high biological value as proteins and omega-3 fatty acids have a protective effect on cardiovascular health (51), and the high-quality protein could have an important role in delaying sarcopenia (52), which is a frequent cause of poor quality of life and disability in older adults (53). In addition, fish, seafood and shellfish contain important amounts of minerals such as iodine, calcium, or selenium (54).
Meat
The moderate consumption of meat in the SEAD comes mainly from autochthonous bovine and porcine breeds from extensive livestock farms, where the animals are fed based on grass and milk, in the case of veal (Galician blonde calves and Cachena breed calves), and with chestnuts in the case of pork (Galician Celtic pig) (55, 56). This traditional and sustainable farming system gives the meat certain properties such as a rich content of proteins of high biological value and easy digestion (57), the high content of MUFAs and PUFAs (58, 59), and are an important source of minerals (iron, phosphorus, potassium, calcium, and zinc) and vitamins (mainly from group B) (44).
Vegetables and fruits
In the SEAD region, there is a high production of Brassica vegetables, a genus of plants that includes broccoli, cabbage, collard greens, and grelos (Brassica rapa L. var. rapa, broccoli raab, “nabizas,” and turnip). These vegetables are usually consumed cooked or prepared in a broth with pork. It is known that members of the Brassica are high in antioxidants and phytochemicals that contribute to the prevention of CVDs (49) and cancer (60).
The apple is the fruit with the highest production in the region and is part of a large number of staple dishes of the SEAD. Pears, grapes, and citrus fruits are also important in this dietary pattern. All of these fruits contain high amounts of fiber, and high fiber intake has known cardiovascular, metabolic (44), and constipation-preventing benefits (61).
Nuts
Although the consumption of walnuts is important, chestnuts are the most widely nut consumed under the SEAD. They have been used as a food source in Europe since the Middle Ages and in northwestern Spain; in communities that had low access to cereal flour, chestnuts were used as the main source of carbohydrates (62). Currently, chestnuts are consumed alone as snacks or used to prepare creams and soups. These nuts are rich in PUFAs (63) and tocopherols, and also have the highest concentrations of phytosterols compared with other nuts (62). In a recent study, mice that were supplemented with chestnuts had reduced abdominal adipose tissue and lower serum cholesterol concentration than those that were fed a standard diet (64). These results suggest that chestnuts may have an important role in regulating adipose tissue deposition and maintaining good health.
Cereals, complex carbohydrates
Carbohydrates contribute 50% of the caloric intake, with whole grains, legumes, and potatoes being the main sources of carbohydrates (49). In fact, the consumption of baked and cooked potatoes [nonfried potatoes, even with skin (cachelos)] in the northwest is the highest in the Iberian Peninsula and bread (mainly with unrefined flour) intake is higher than in other regions, which have a higher consumption of rice and pasta. It is known that baked potatoes have a lower glycemic index than rice and fried potatoes, and in a recent meta-analysis of prospective cohort studies diets with high glycemic indices and glycemic loads were predictive of the development of type 2 diabetes (65).
Dairy products
Dairy products, especially cheese, which occupy a relevant place in the SEAD in addition to milk, are consumed daily in the SEAD and are sources of high-value protein, calcium, and vitamins. Fermented milk products are probiotic foods that contain beneficial live microbiota (66). Also, dairy products increase bone mineral content during childhood (67).
Olive oil
The main source of fat in the SEAD is olive oil, which is used for seasoning and grilling. It provides MUFAs, antioxidants, and other bioactive components (phytosterols, tocopherols, and pigments) with known health benefits (44).
Healthy habits and behaviors
Exercise is an important component of a healthy lifestyle, and complements diet in this dietary pattern. In the SEAD geographical area, there are many factors that promote physical activity, such as warm weather, trails, rivers, and multiple spaces for hiking. Thus, the climate and geography make it a region where physical activity can be undertaken outdoors for most of the year (44).
As mentioned above, the SEAD is a dietary pattern; thus, in addition to the types of food that make up the SEAD, how the food is eaten is also important. Traditional Atlantic food habits include home cooking, eating meals together, enjoying the food, and considering eating food as a social act. In fact, gastronomic festivals are typical in this region and they are a main topic in the tourism policy of the Galician government (68, 69). All of these features contribute to improving well-being, which is becoming increasingly important as mental illness increases in a society subjected to many types of economic, social, and environmental stress.
General health and mental well-being are closely related to healthy behaviors, where SEAD plays an important role by including healthy and sustainable nutritional habits together with other lifestyles and social factors that have a direct and beneficial impact on health and society. A stress-free environment may be relevant for mental health disorders (70) and this fact highlights the need for further exploration of the link between SEAD and well-being in order to unravel the potential collateral benefits of this relationship, so that they can be implemented to improve the well-being of society.
Environmental sustainability
Food sustainability is a relevant topic in future policies on food production and consumption. It is quite important to promote sustainable consumption patterns in society. In this regard, it has been demonstrated that the SEAD can be considered as an example of a sustainable diet despite being a diet that includes animal-based foods at its core, including meats (71, 72). A recent analysis of the SEAD has shown that this dietary pattern has a significant effect on climate change. An evaluation was carried out by quantifying the carbon footprint following the Life Cycle Analysis methodology and identifying its nutritional quality, according to the value of the Nutrient-Rich Dietary index (NRD9.3). The carbon footprint of the SEAD was 3.01 kg CO2 eq · person−1 · d−1, and the SEAD had a high nutritional score (72). Esteve-Llorens et al. (72) reported that the SEAD had a better environmental and nutritional impact than the current diet in the region, concluding that the SEAD has a low environmental impact and promotes food safety and quality. This fact could be counterintuitive considering that higher consumption of meat has a higher impact on climate change through higher production of greenhouse gas (GHG) emissions (71). However, although the SEAD is an omnivorous diet with a significant presence of foods of animal origin, it has environmental indices such as the water footprint and carbon footprint that are in line with those corresponding to other diets with a lower intake of this type of food. One of the main reasons for these results is the characteristic of this diet that promotes the consumption of seasonal, local foods and a balanced intake of different food categories. This fact is especially favored by the size of the cities in the SEAD region, since the number of inhabitants living in 50% of them is less than 100,000 inhabitants and the population density in this region is 125.8 inhabitants/km2 (73, 74). Therefore, in times of global environmental change, there is an urgent need for a sustainable agricultural and livestock policy, such as the one based on traditional breeding schemes (75, 76). Extensive livestock farms based on autochthonous breeds promote this specialization of traditional, rural, and ecological production systems typical of the SEAD regions.
Scientific Evidence for the Beneficial Health Effects of the SEAD
To show the scientific evidence for the beneficial health effects of the SEAD, a systematic review based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol was conducted. For this purpose, an electronic search of PubMed was carried out using appropriate keywords, as follows: “Atlantic Diet” OR “Southern European Atlantic Diet.” The database was searched up to September 2021, without a time limitation. Thus, 24 articles were identified through this database search, and a total of 19 studies investigating beneficial health effects of the SEAD were finally assessed (Figure 2). The different scientific studies that have reported the beneficial health effects of SEAD are summarized in Table 2.
FIGURE 2.
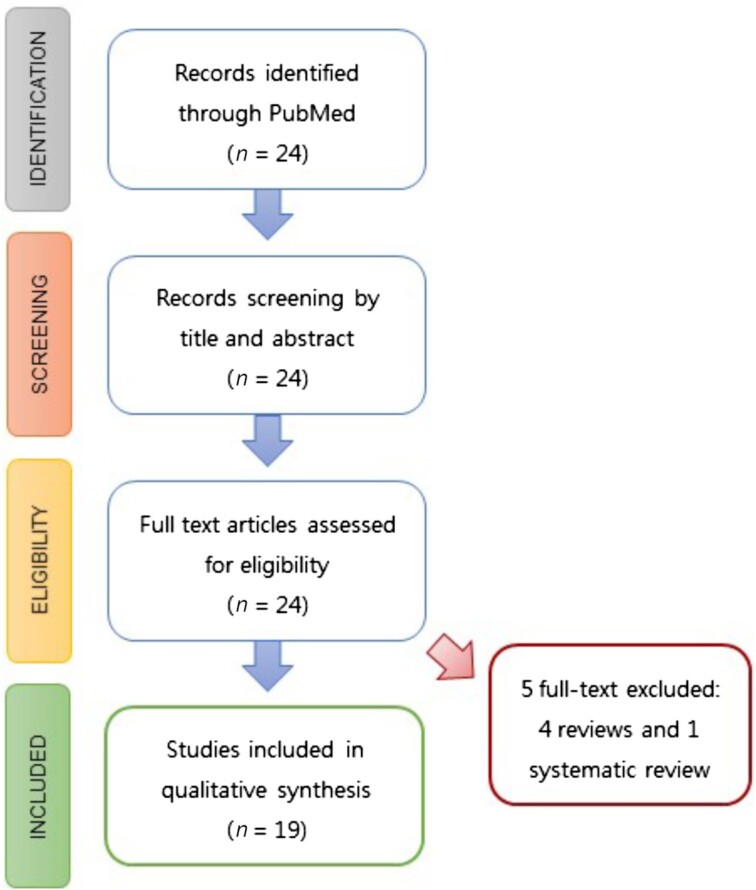
Systematic review flow chart.
TABLE 2.
Overview of observational and interventional studies that assessed the associations between adherence to the SEAD and health promotion in subjects aged 3–85 y1
| Reference | Objective | Study design | Measurements | FFQ | Time | Participants (age) | Outcomes |
|---|---|---|---|---|---|---|---|
| (77) | Adherence to the SEAD and occurrence of nonfatal AMI | Observational | Anthropometric measures, lifestyle information | Yes | 4 y (1999–2003) | 820 with AMI; 2196 without AMI (≥18 y) | SEAD was associated with lower odds of nonfatal AMI |
| (51) | Effects of a SEAD on coronary risk | Observational, cross-sectional | Blood and urine biomarkers, anthropometric measures | Yes | 2 y (2008–2010) | 10,231 (≥18 y) | Decreased in markers of inflammation, TGs, insulin, insulin resistance, and systolic blood pressure |
| (78) | Effects of CRF and SEAD on metabolic risk | Observational, cross-sectional | Blood biomarkers, blood pressure, anthropometric measures, 20-m shuttle-run test | Yes | — | 468 (15–18 y) | SEAD + CRF = decreased metabolic risk score |
| (50, 79) | Effects of the SEAD on anthropometric indices and lipid profile | Interventional, randomized. GALIAT study | Blood biomarkers, anthropometric measures | Yes | 6 mo | 720 (3–85 y) | Decreased in cholesterol, weight, BMI and % body fat mass |
| (66) | SEAD and gut microbiota | Observational | Fecal, anthropometric measures | Yes | — | 31 (18–65 y) | Increased of healthy microbiota |
| (80) | Effects of MF and SEAD on cardiometabolic risk | Cross-sectional “Azorean Physical Activity and Health Study II” | Blood biomarkers, blood pressure, anthropometric measures, 20-m shuttle-run test, curl-up and push-up test | Yes | 1 y (2009) | 467 (15–18 y) | SEAD + MF = decreased systolic blood pressure, waist circumference, metabolic risk factors |
| (81) | Effects of MF and SEAD on inflammation | Cross-sectional Azorean Physical Activity and Health Study II | Blood biomarkers, anthropometric measures, curl-up and push-up test | Yes | 1 y (2009) | 463 (15–18 y) | High adherence to SEAD decrease C-reactive protein |
| (82) | Effects of MF, CRF, and SEAD on AIP | Cross-sectional Azorean Physical Activity and Health Study II | Blood biomarkers, blood pressure, anthropometric measures, 20-m shuttle-run test, curl-up and push-up test | Yes | 1 y (2009) | 493 (15–18 y) | High adherence to SEAD decrease AIP |
| (83) | Analyze the relations of SEAD with cardiovascular risk factors, obesity indexes, and arterial stiffness markers | Interventional randomized subanalysis of EVIDENT-II study | Blood biomarkers, blood pressure, physical activity, anthropometric measures | Yes | 2 y (2014–2016) | 791 (20–70 y) | Decreased in cardiovascular risk, total cholesterol, TGs, and lower rates of obesity |
| (84) | Fatty acid profile of breast milk from lactating women in Galicia | Observational, cross-sectional | Human-milk mineral composition, anthropometric measures | Yes | 3 y (2016–2019) | 102 (≥18 y) | Increased iron in breast milk of women with SEAD |
| (85) | Association between adherence to the SEAD and all-cause mortality in older adults | Observational, prospective Seniors-ENRICA-1 study | Physical activity, BMI | Yes | 3 y (2008–2010, 2012) | 3165 (≥60 y) | Adherence to the SEAD is associated with lower risk of all-cause of mortality |
AIP, Atherogenic Index of Plasma; AMI, acute myocardial infarction; BMI, body mass index; CRF, cardiorespiratory fitness; FFQ, food-frequency questionnaire; GALIAT, Galicia Alimentación Atlántica (Galicia Atlantic Diet); MF, muscular fitness; SEAD, Southern European Atlantic diet; TG, triglyceride.
In a population-based, case-control study by Oliveira et al. (77) conducted in adults with (n = 820) and without (n = 2,196) a history of incident acute myocardial infarction, it was shown that a greater adherence to the SEAD was associated with a lower probability of nonfatal acute myocardial infarction. In addition, some components of the SEAD may contribute to the very low mortality from CVDs in northern Portugal and Galicia. Guallar-Castillón et al. (51) analyzed the association between the SEAD and coronary risk biomarkers in a cross-sectional study conducted in 10,231 individuals; the study showed that the SEAD was associated with lower concentrations of inflammation markers and a reduction in triglyceride concentrations, insulin concentrations, resistance to insulin, and blood pressure. In line with the aforementioned findings, in a recent subanalysis with 833 participants from the EVIDENT 2 study, Rodríguez-Martín et al. (83) showed that greater adherence to the SEAD was associated with a lower CVD disease risk, lower total cholesterol and triglyceride concentrations, and lower rates of obesity.
Adherence to the SEAD has also been associated with decreased myocardial infarction risk due to its links with reduced triglyceride concentrations, inflammatory markers, insulin concentrations, and blood pressure (51, 83). Some studies have investigated the combined effects of cardiorespiratory fitness (CRF) or muscular fitness (MF) and the SEAD (78). The first cross-sectional study assessing the combined associations of CRF and the SEAD on cardiometabolic risk was carried out in 468 adolescents aged 15–18 y. Different variables were studied, such as CRF, cholesterol concentrations, insulin concentrations, waist circumference, and systolic blood pressure, among others, from which the metabolic risk score was constructed (78, 86). In this study, it was shown that adherence to the SEAD combined with CRF decreased the metabolic risk score, whereas low adherence to the SEAD was significantly associated with a high metabolic risk score (78).
Although the clinical manifestations of CVD occur in adulthood, the development of atherosclerosis begins during early stages of life, often during childhood (87). A study carried out in an adolescent population evaluated the association of CRF, MF, and adherence to the SEAD with the atherogenic index of plasma (82). Agostinis-Sobrinho et al. (82) observed that the atherogenic index of plasma was inversely associated with CRF, MF, and adherence to the SEAD in adolescents. In addition, adolescents with high adherence to the SEAD, high MF, and high CRF had the lowest atherogenic index of plasma. As part of the same longitudinal school-based study, the same authors investigated the combined impact of MF, adherence to the SEAD, and C-reactive protein concentrations (81). This protein is one the most important inflammatory markers that is released as a proinflammatory acute-phase protein; thus, an anti-inflammatory dietary pattern, such as the SEAD, could exert an effect on the concentrations of the proinflammatory C-reactive protein (88). Several studies have demonstrated C-reactive protein to be a powerful predictor for the development of CVDs and type 2 diabetes (87, 88). The authors concluded that MF was inversely associated with C-reactive protein concentrations, and adolescents with low MF and low adherence to the Atlantic diet had the highest C-reactive protein values. For this reason, the combined effect of high MF and high adherence to the SEAD played a key role in low-grade inflammation in adolescents (81). Additionally, in the same Azorean population that was studied, it was observed that the SEAD was inversely correlated with systolic blood pressure, waist circumference, and the cardiometabolic risk score. In fact, low MF with low adherence to the SEAD was associated with the highest cardiometabolic risk score (80).
Although there is no scientific evidence on the role of the SEAD in cancer until now, certain components of this dietary pattern are associated with a decreased risk of the incidence of some types of cancer. The SEAD comprises foods rich in bioactive compounds with potential cancer-fighting effects, such as resveratrol, which is present in grapes and red wine, which has shown potential in vitro anticancer effects on various tissues and cell lines, including in breast cancer (14). Preclinical studies have shown that certain components, such as glucosinolates from Brassica vegetables, have anticancer properties (33, 60). In addition, the SEAD also has beneficial environmental impacts. As certain environmental pollutants are associated with complications that can manifest into serious illnesses over time, such as cancer, high adherence to the SEAD could reduce the risk of cancer (89, 90). More studies are warranted to evaluate the beneficial effects of this dietary pattern on cancer.
Interestingly, dietary quality also influences other important aspects, such as the nutritional composition of breast milk (91). It has been observed that the concentrations of some nutrients, such as PUFAs, in the mother's diet and in human milk are correlated (92). However, no correlation was observed for iron and other minerals (zinc and chromium) (93). Nevertheless, a recent analysis from 75 human-milk samples from Galicia in northwestern Spain showed a positive association between adherence to the SEAD and iron content in breast milk (84). As previously mentioned, this dietary pattern is characterized by a high consumption of meat; thus, maternal adherence to the SEAD could influence the iron content in breast milk, which has a fundamental role in infant development (94). Moreover, it has been shown that Galician breast milk has high concentrations of the PUFAs ɑ-linolenic acid (18:3n−3) and linoleic acid (18:2n−6), due to the high intake of especially fish, and also nuts. It is known that the quality of fats rather than the total amount plays an important role in an infants’ neurological and retina development (95, 96).
Another important aspect to consider is the role of dietary patterns in microbiota profiles. Gut bacteria play an important role in various metabolic processes and human diseases, such as obesity and its accompanying comorbidities such as diabetes and adverse cardiovascular events (97, 98). Both diet and physical activity influence different microbiota profiles. In fact, it is known that dietary patterns, probiotics, and other factors such as stress, age, exercise, and climatic conditions can dramatically affect the balance and diversity of the human gut microbiota (99). The proportion of different microbial populations in the human intestine in relation to adherence to the SEAD was studied, and it was observed that the only bacterial group that demonstrated statistically significant differences was the genus Bifidobacterium spp., whose concentrations were greater in participants who adhered to the SEAD (66). Bifidobacterium spp. plays an important role in the human body as it participates in important physiological functions, such as the development of the immune response (100), and several species are considered probiotics (101). Hence, it is believed that Bifidobacterium spp. has a protective effect on health. The genus has been linked to the concept of a healthy microbiota (102–104) and is closely associated with the consumption of dairy products. Fermented milks, which is 1 of the 10 points in the Atlantic Diet Decalogue (66, 105), have probiotics able to reduce lactose malabsorption (106), a fairly common problem in the Galician population (107).
Scientific evidence published to date shows that the SEAD is associated with improvements in general health. In line with this, in a representative cohort of noninstitutionalized persons aged ≥60 y in Spain, higher adherence to the SEAD was associated with lower all-cause mortality. Nevertheless, the associations between the food components of the SEAD and all-cause mortality were weak, except for moderate wine consumption (85). This result reinforces the idea that the benefits of this dietary pattern are due to the cumulative effect of individual foods and the interactions between the food components (108).
In the current coronavirus disease 2019 (COVID-19) era, studies linking dietary recommendations and the risk of infections are scarce. Viral infections are characterized by the production of reactive oxygen species (ROS), which can lead to decreased immunity in an organism (109). Thus, obtaining appropriate amounts of natural antioxidants from dietary sources could be of particular relevance during the current COVID-19 pandemic. In this context, healthy dietary patterns, such as the SEAD, provide the recommended amounts of essential vitamins, minerals, and phenolic compounds needed to activate antioxidant responses to maintain the immune system, with the exception of vitamins D and E, which should be supplemented, especially in vulnerable populations (110). Further studies are needed to evaluate the role of a dietary pattern based on the SEAD in the prevention and management of COVID-19.
Most of the studies performed to date that have assessed the beneficial effects of the SEAD have been observational, based on the evaluation of adherence to the SEAD. To the best of our knowledge, there is only 1 clinical trial that examined the effects of the SEAD on metabolic health, cardiovascular health, and adiposity: the GALIAT [Galicia Alimentación Atlántica (Galicia Atlantic Diet)] study (111). This was a randomized, controlled, dietary intervention clinical trial that evaluated the potential effects of this dietary pattern on glucose metabolism, inflammation markers, lipid profiles, and adiposity in 250 families (715 adults and children aged >3 y). The sample unit was the family, and each family was randomly assigned to either the control diet group or SEAD group (AD group) for a period of 6 mo. The families in the AD group received educational sessions on food, diet, and gastronomy. Moreover, they attended cooking lessons and were provided with a range of foods characteristic of the traditional SEAD. Some results of this nutritional intervention were decreases in cholesterol concentrations, body weight, BMI, and body fat mass percentages in the AD group (50, 111, 79). Therefore, more intervention studies with large sample sizes and longer follow-up are needed to evaluate the effects of the SEAD on human health.
Evidence of the Epigenetic Effects Induced by the SEAD Components
The term “epigenetics” refers to the study of the different molecular mechanisms that regulate the expression of genes without changes in the underlying DNA nucleotide sequence, ultimately determining the phenotype from the genotype and playing an important role in healthy development (112, 113). These changes in gene expression respond to the state of transcriptional activation or inactivation of genes and are regulated by the action of epigenetic mechanisms that are relevant throughout the life course and at different stages of embryonic development (114, 115). Epigenetic mechanisms are important molecular regulators that include DNA methylation, one of the most present in the organism and one of the most studied, histone post-translational modification and regulatory noncoding RNAs (ncRNAs), currently considered as health actors (116, 117) (Figure 3). They are characterized by being transmissible to the following generations (118), and by changing in response to specific stimuli of the organism (117). For these reasons, epigenetic mechanisms are characterized by being hereditary, dynamic, and reversible.
FIGURE 3.
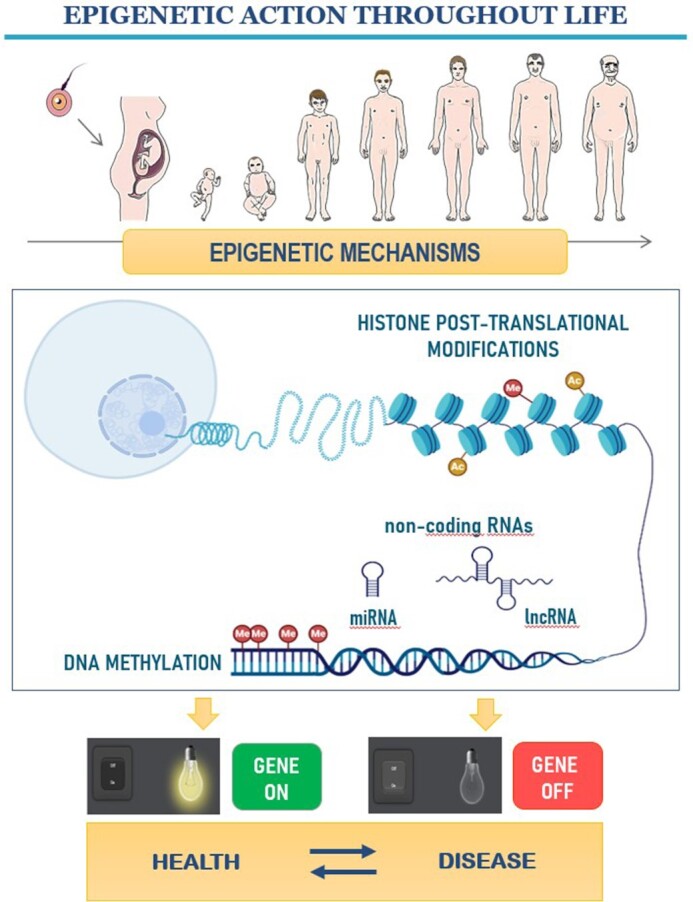
Role of epigenetic mechanisms during development and aging. Histone post-translational modifications, DNA methylation, and non-coding RNAs (miRNA and lncRNA) are involved in the regulation of gene expression, which induce the activation or inactivation of genes. This regulation is involved in the proper functioning of an organism and, if it becomes aberrant, it can promote disease onset. Part of this figure was produced using modified elements from BioRender.io (Toronto, ON, Canada). lncRNA, long-noncoding-RNA; miRNA, micro-RNA.
Epigenetic mechanisms are subject to the action of various environmental factors and external agents, such as lifestyle, nutrition, physical activity, stress, or exposure to pathogens and toxins, among others (5), with nutrition being a relevant environmental factor in health. In fact, nutritional epigenetics is currently postulated as a potential study tool for understanding the interconnection between genes and diet (24–26).
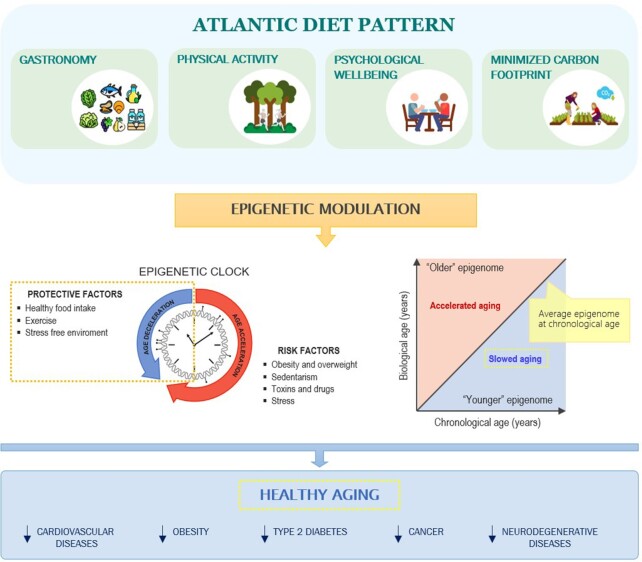
Along with the modulating role of the environment determined by our lifestyles, aging itself increases the susceptibility to a wide range of diseases (119, 120). The application of epigenetic clock models to data generated by epigenome-wide association studies that are focused on dietary intake and nutritional interventions is helping uncover the dietary determinants of healthy aging (121–124). Interestingly, a comparison of different molecular predictors of age indicated that the epigenetic clock had the highest correlation with biological age (125).
In this context, recent studies have shown that a low BMI, exercise, and consumption of fish, poultry, fruits, and vegetables (126) reduce the acceleration of the epigenetic clock. Dietary factors such as folate and related B vitamins are emerging as modifiers of epigenetic age because they can modulate DNA methylation (127). Therefore, we hypothesized that the components provided by the SEAD may have an important contribution in improving health outcomes with respect to diseases related to age and life expectancy.
Epigenetic effects of food and nutrients
The foods included in the SEAD contain bioactive and functional compounds, such as omega-3 fatty acids, sterols, flavonoids, carotenoids, and glucosinolates, that have beneficial health effects (49). In addition, the cooking techniques used in this dietary pattern preserve these bioactive compounds better than other culinary techniques such as frying (50). In this section, we focus on the actions that the main foods of the SEAD exert on health through epigenetic mechanisms (Table 3, Figure 4).
TABLE 3.
Summary of the bioactive compounds present in the SEAD and their effects on health in the general population1
| Bioactive compound | Dietary source | Effects on health |
|---|---|---|
| Sulfur compounds | ||
| Glucosinolates | Turnip greens, cabbage, collard greens | Anticancer Cholesterol-lowering (128) |
| Aliaceae | Onion, garlic, leek | Anticancer Cholesterol-lowering antihypertensive (128) |
| Omega-3 fatty acids | Fish, shellfish, walnuts | Anti-inflammatory (129, 130) |
| Bioactive peptides | Dairy products | Antihypertensive (131) |
| Polyphenols | ||
| Flavonoids | Onion, leek, tomato, apple, pear, grape, lettuce, or citrus | Antioxidant capacity Antithrombotic Anti-atherosclerotic Anti-inflammatory (132) |
| Resveratrol | Grape skin and wine | Antioxidant capacity Antithrombotic Anti-atherosclerotic Anti-inflammatory (132) |
| Carotenoids | ||
| Carotenes | Dark-green leafy vegetables, orange vegetables and fruits | Antioxidant capacity (132) Anticancer Cholesterol-lowering (133) |
| Xanthophylls | Green leafy vegetables, potatoes, tomato, red pepper, egg yolk | Antioxidant capacity (132) Anticancer Cholesterol-lowering (133) |
| Plant sterols | Olive oil, nuts | Cholesterol-lowering (134) |
| Vitamins | ||
| Vitamin C | Citrus, cabbage, peppers, peas | Antioxidant capacity (49) |
| Vitamin E | Tomato, peppers, lettuce, peas | Antioxidant capacity (49) |
| Folic acid | Green leafy vegetables | Anticancer Prevents degenerative diseases Important in embryonic development (49) |
SEAD, Southern European Atlantic diet.
FIGURE 4.
Representation of the epigenetic effects of bioactive compounds in foods of the Southern European Atlantic diet to promote health benefits.
Experimental studies in animal and cell models have shown that compounds contained in the Brassicaceae family and their derivatives exert beneficial health effects, such as preventing the development of cancer (135). This protective effect is mediated through epigenetic markers, which act as regulators and inhibitors of the expression factors present in most cancers (136). Glucosinolates induce changes in DNA methylation that reduce the risk of developing certain chronic diseases (137), and the chemopreventive and health-promoting properties of cruciferous vegetables could be mediated by mechanisms related to ncRNAs (138). More studies are needed, especially in humans, to determine which epigenetic markers occur as a result of the incorporation of these cruciferous vegetables into the diet, not only in cancer prevention but also in many other biological processes in chronic diseases that involve inflammation and elevated cell proliferation.
Carotenoids have anticancer properties that have been shown to be driven by changes in the DNA methylation of genes responsible for proangiogenic processes (139). Polyphenols, which include flavonoids and stilbenes such as resveratrol, can alter epigenetic cellular mechanisms associated with potential health-promoting effects (140, 141). Polyphenols reverse adverse epigenetic regulation by altering epigenetic markers, resulting in the reactivation of favorable genes (silenced tumor suppressors, antioxidant genes, and DNA repair genes) or inactivation of harmful genes (oncogenes involved in inflammation, cell cycle progression, proliferation, invasion, angiogenesis, and metastasis) (142).
The positive action of polyphenols on the body, mediated by epigenetic mechanisms, inhibits the development of pathologies such as CVDs (143–146), metabolic syndrome (147, 148), obesity (149), and cancer (150, 151), among others.
Omega-3 fatty acids and selenium contained in fish and shellfish possess anti-inflammatory and hypotriglyceridemic properties that are attributed to their alteration of genes carried out by epigenetic mechanisms such as DNA methylation (152) and microRNAs (153). Recently, anti-obesity effects and efficacy against metabolic syndrome have been attributed to omega-3 fatty acids, and research has shown that these effects are due to the epigenetic action carried out by these components when counteracting the associated metabolic changes (154, 155). Other beneficial actions of omega-3 fatty acids associated with epigenetic markers have been reported in research related to other disease areas such as cancer (156), Alzheimer disease (157), and CVD (158, 159). There are some studies on the favorable effects of selenium on health, which are also regulated by epigenetic modifications (160).
In addition, olive oil, dairy products, and foods rich in vitamins are present in the SEAD, which also have nutrients and bioactive components that promote proper functioning of the body and reduce the risk of developing obesity and chronic diseases (2). Studies have shown that epigenetics may be regulating these processes (161–165).
Epigenetic effects of exercise
Recent research shows that exposure of an organism to certain external agents, such as diet and physical exercise, can trigger alterations in the epigenome (126, 166, 167). This implies that nutrients and physical activity influence the expression of genes and, therefore, have a relevant role in health status and disease prevention in individuals. In this context, physical activity is able to induce a modulation in gene expression, and this modulation can be mediated by epigenetic mechanisms (168). Furthermore, the effect of physical exercise on gene expression depends on the type, intensity, duration of exercise, as well as on sex and age (169). Thus, the beneficial effects of moderate exercise on health, mainly its anti-inflammatory potential (169, 170), improvements in clinical outcomes (171), and reduction in the risk of noncommunicable diseases (170), may be mediated by epigenetic mechanisms (169, 170). Recent studies have reported that exercise causes a decrease in the methylation of many genes; in contrast, other studies have reported that the levels of methylation increase in some genes with physical activity (Table 4) (168–177).
TABLE 4.
Overview of observational and interventional studies that assessed the associations between adherence to physical exercise and epigenetic effects in adults aged >18 y1
| Reference | Type of physical exercise | Epigenetic modifications | Participants (age) | Sample |
|---|---|---|---|---|
| (173) | At least 30 min/d of physical activity≤10 min/d of physical activity | Increased level of global DNA methylation with physical activity | 131 (45–75 y) | Peripheral blood |
| (172) | Physical activity across 3 time periods (≥9.8 h/wk in childhood, 5.9 h/wk in adolescence, and 12.5 h/wk in past 12 mo) | Increased level of percent global DNA methylation | 647 non-Hispanic White women with a family history of breast cancer (35–74 y) | Peripheral blood |
| (178) | Physical exercise intervention for 6 wkResistance exercise plus walking | Changes in methylation of 756 CpG sites | 8 colorectal cancer survivors (>50 y) | Peripheral blood |
| (174) | 3 mo of aerobic physical exercise intervention | Increased level of global DNA methylationIncreased levels of DNA methylation in genes involved in blood pressure | 68 (22–70 y) | Peripheral blood |
| (22) | 6 mo of endurance exercise | Changes in global DNA methylation of genes important in muscle physiology | 13 men without family history of T2D; 15 men with family history of T2D (32–44 y) | Skeletal muscle |
| (176) | 1 wk of acute exercise | Changes in global DNA methylation of genes important in muscle physiology | 14 healthy and sedentary (25 ± 1 y) | Skeletal muscle |
| (23) | 6 mo of endurance exercise intervention | Changes in DNA methylation of adipocyte-specific genes | 16 healthy and sedentary men without family history of T2D; 15 healthy and sedentary men with family history of T2D (32–42 y) | Adipose tissue |
| (177) | Acute exercise plus 6 wk of intensive exercise program (45-min cycling sessions 5 d/wk) | Changes in DNA methylation of adipocyte-specific genes | 15 healthy and sedentary men (19–27 y) | Adipose tissue |
T2D, type 2 diabetes.
Several epigenetic markers were found to be altered in response to exercise, with a potential influence on skeletal muscle metabolism. However, whether these epigenetic markers play a role in the physiological impact of exercise is unclear (22). On the other hand, in men with a history of low physical activity levels, a 6-mo exercise intervention altered the level of DNA methylation of numerous CpG sites in genes that affect adipocyte lipogenesis (23). Currently, there is still a lack of clear data on the role of the type of exercise on epigenetic modulation. Therefore, the main objective of future studies should focus on developing panels of specific epigenetic markers that allow predicting the reaction of an individual to stimulation by a specific exercise-training regimen (168).
Epigenetic effects of psychological well-being
The tradition of family meals is an important aspect of the SEAD. Several studies have suggested that healthier family meals benefit the nutritional health of family members, with positive benefits on weight-related health, particularly in young individuals and children (179–181). It has currently been reported that frequent family meals help overcome several challenges, such as costs of healthy foods, scheduling, differences in food preferences, etc. The home environment is very important for mealtime, and lack of organization and a frantic pace of life are associated with an inadequate diet. On the contrary, low levels of household chaos, fixed dinnertime routines, and family cohesion have been linked to better nutrition and eating behaviors, with higher childhood intake of healthier foods such as fruits or vegetables, lower consumption of fast foods, and lower standardized BMI scores among children and parents being observed (179, 182, 183). Furthermore, adolescent involvement in food preparation has been associated with improved dietary quality and healthier eating patterns for the family.
Family meals also provide opportunities for communication and connections among members, which exerts a protective effect against adolescent participation in risky health behaviors and promotes social and psychosocial well-being (184–187). Thus, meal preparation and family mealtimes could be parts of an intervention program to improve family health and prevent obesity.
The protective role of family meals against disordered eating behaviors among young individuals has been demonstrated in numerous studies (188–191). In line with this, nutritional psychiatry is based on the relation between dietary patterns and psychiatric pathologies (depression, anxiety, and bipolar disorder). Dietary intervention studies, for example, the SMILES and HELFIMED trials (192–194), reported a reduction in depressive symptoms; however, the molecular mechanisms involved in this relation are still poorly understood. Oxidative stress, mitochondrial dysfunction, and inflammation are present in neuropsychiatric pathologies. Epigenetics also plays an important role in the pathogenesis of psychiatric disorders. Several studies have shown that environmental factors, such as stress, act through epigenetic mechanisms that lead to changes in gene expression and altered brain function (195, 196). A growing number of studies demonstrate DNA methylation of hypothalamic-pituitary-adrenal (HPA) axis genes as an important mechanism involved in stressful physical and social environments (197). Furthermore, a differential methylation profile was found in association with posttraumatic stress disorder, suggesting a relevant role of epigenetic mechanisms in the development of this pathology (198, 199).
A stress-free environment associated with dietary patterns such as the SEAD could prevent the activation of epigenetic mechanisms associated with mental health disorders.
Epigenetic effects of environmental contaminants
Dietary strategies promote health and well-being and reduce the incidence of diet-related diseases. However, in recent years, dietary strategies have increasingly been investigated as approaches to reduce the environmental impacts of the food system (200). Food production chains and consumption patterns account for one-third of the human impact on climate change; hence, it is important to fight climate change through sustainable diets that are more environmentally friendly (72). The sustainable diets concept, according to the FAO, encompasses those diets with low environmental impacts that contribute to food and nutrition security and to healthy lives for present and future generations. Sustainable diets are protective and respectful of biodiversity and ecosystems, culturally acceptable, accessible, economically fair and affordable, nutritionally adequate, and safe and healthy, while optimizing natural and human resources (201).
Approximately 50% of all GHG emissions from the food system are related to farming activities, which are attributed to emissions of nitrous oxide, methane, and carbon dioxide (202, 203). GHG emissions vary according to food products (especially those of animal origin, e.g., red meat) and the efficiency of the production chain.
The environmental footprints of some diets have been quantified according to the Life Cycle Assessment methodology (204–208) through the determination of the carbon footprint, which is considered an indicator of environmental impact. The SEAD has an approximately 8% lower carbon footprint than that of the Mediterranean diet, due to the SEAD prioritizing the consumption of plant-based, seasonal, fresh, and local products with limited cooking, which translates into a reduction in GHG emissions (72, 209, 210).
In the SEAD, the manufacturing stage is responsible for approximately 78% of the total GHG emissions, while the remaining amount is attributed to the household stage and transport activities. Due to the shorter distribution distances of Galician products, they are associated with low GHG emissions. With regard to food, meat and dairy production is responsible for 26% and 30% of GHG emissions, respectively, whereas the contribution of seafood is 15%. Grain products are basic components of the SEAD, and their contribution is 9% (72, 209).
Thus, the SEAD is not only beneficial for health but also for the environment due to the high intake of vegetables and fish compared with other dietary patterns, as well as the focus on consuming local products with simple cooking methods. These are important characteristics of the SEAD to consider because the presence of manmade chemical pollutants in the environment has increased rapidly over the past 70 y. The harmful effects of these pollutants are predominantly induced by endocrine-disrupting chemicals (211). These chemicals are environmental compounds that interfere with normal endocrine signaling and they are one of the largest classes of toxins that we are exposed to on a daily basis. Endocrine disruptors (EDCs) interfere with hormone signaling pathways in the body. They are synthetic or natural compounds present in the environment that can interfere with endocrine functions (212) as they mimic the actions of endogenous hormones and have impacts at various levels of organization in organisms, from physiological changes (phenotypes) to molecular alterations, including epigenetic modifications (211). EDCs in the environment can be classified as pesticides, plasticizers, industrial by-products, pharmaceuticals, flame retardants, phytoestrogens, or heavy metals such as cadmium (213–215). Environmental pollutants, such as pesticides, can cause both acute and delayed health effects in exposed participants (216). The molecular mechanisms underlying such effects are still under investigation; however, as epigenetic mechanisms such as DNA methylation have already been shown to be activated by external factors, they may be also triggered by environmental factors (217). Early exposure to EDCs has been associated with complications that manifest over time, such as obesity, diabetes, and cancer. The underlying factors for such associations are still unknown; however, they may be likely mediated by EDC-induced epigenetic changes (89, 90). Current evidence indicates that epigenetic markers may mediate the effects of pesticides on human health, including EDCs, persistent organic pollutants, arsenic, various herbicides, and insecticides (218).
Understanding the mechanistic links between EDC- and pesticide-induced epigenetic changes and phenotypic endpoints will be critical in providing improved strategies to better protect the environment and humans from exposure to these contaminants. In recent years, environmental epigenetics aims to analyze compounds, such as EDCs, responsible for inducing epigenetic effects (219, 220). Evidence suggests that environmental exposures markedly affect endocrine-related gene expression and, therefore, affect clinical endocrine outcomes (89). Several investigations have examined the effects of environmental exposures and epigenetic markers and have identified toxic substances that modify epigenetic states (221, 222). Thus, current evidence underscores that epigenetics has substantial potential to broaden our understanding of the molecular mechanisms of the effects of pesticides and EDCs on health, as well as to predict health-related risks due to environmental exposure and individual susceptibility. This risk of disease-promoting epigenetic mechanisms mediated by environmental factors could be prevented by adhering to the SEAD, which is associated with a low consumptive water footprint and carbon footprint (223).
Another major problem for the environment is the drastic increase in the production of plastics, used for food packaging and water bottling, which are easily incorporated into marine ecosystems due to their great stability and durability. These break down into small pieces called microplastics or nanoplastics depending on their size. These fragments are ingested by living beings, joining the food system. The ingestion of micro- and nanoplastics is associated with metabolic and intestinal problems, and some scientific evidence indicates that the ingestion of nanoplastics can induce important epigenetic changes (224). In this regard, due to the consumption of seasonal and local foods, the SEAD has a beneficial impact on the environment also by reducing the use of plastics in food packaging.
Conclusions
The SEAD is a specific and traditional dietary pattern from the Southern Atlantic area that shows beneficial effects from both a health and environmental perspective. These beneficial features could induce their effect on increased human survival by means of modulating epigenetic mechanisms. This warrants the necessity to explore the current adherence to a pattern of the SEAD in the population. To this end, there is a score for the study of the adherence to the SEAD, which was designed in 2010 by Oliveira et al. (77) based on population data collected in Porto (northern Portugal). The score has been widely used in different studies. However, the index has some limitations. It does not include the consumption of shellfish or olive oil, 2 basic foods in the Galician diet, as shown by dietary surveys carried out in this population (225, 226). Thus, it is necessary to design a new index of adherence to the Atlantic diet that represents the overall characteristics of the SEAD.
According to the scientific evidence so far, diet plays a critical role in both health and disease. Embracing and promoting healthy diets enriched in nutrients of high biological value, such as the SEAD, are crucial to guarantee the well-being of and disease prevention in the population. New scientific advances in epigenetics and nutrition are necessary to establish the mechanisms through which food interferes with the expression of genes. As epigenetic changes are reversible, epigenetic markers associated with nutrients have been postulated as attractive and promising tools for the prescription of personalized diets to treat diseases. The SEAD has features that are able to modulate epigenetic mechanisms, which could, in turn, promote healthy aging through the consumption of nutritional bioactive compounds, physical activity, promotion of psychological well-being, and reduction in EDCs (Figure 5). Therefore, the SEAD could be proposed as an epigenetic diet. The term “epigenetic diet” was coined for the first time in 2011 by Hardy and Tollefsbol (227), based on numerous studies delineating the impact of bioactive dietary compounds on changes in the epigenome. Considering that definition, we propose in this narrative review that the SEAD could be a potential “epigenetic diet” considering that there is scientific evidence regarding the epigenetic effect of its main components (nutrients, healthy habits and behaviors, and environmental sustainability). This proposal strongly warrants further scientific studies to demonstrate the epigenetic value of the SEAD as a whole in promoting healthy aging by slowing down the molecular or physiological aging process.
FIGURE 5.
Representation of the proposal of the SEAD as an “epigenetic diet.” The SEAD involves healthy food, physical activity, promotion of psychological well-being through a stress-free environment, and a reduction in endocrine disruptors by minimizing the carbon footprint. These factors may help modulate mechanisms of the epigenetic clock and, as a result, may improve life expectancy, promote healthy aging, and reduce the risk of several diseases. SEAD, Southern European Atlantic diet.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ABC: designed the study; PML, AGI, GR-C, AFP, and ABC: designed the study and prepared the figures and tables and wrote the first draft; PML, AGI, GR-C, AFP, AI, MCC, CT, and ABC: performed the literature search through scientific databases; DB, MAM-O, RL, FFC, and ABC: verified eligibility of the papers and critically revised the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by Centro de Investigación Biomedica en Red Fisiopatología de la Obesidad y Nutricion (CIBERobn), the Miguel Servet Project (CP17/0008), and research projects (PI20/00650; PI20/00628), under the initiative of Instituto de Salud Carlos III (ISCIII) and co-financed by the European Regional Development Fund (FEDER). PML is funded by a predoctoral grant from Xunta de Galicia (IN606-2020/013). ABC is a Miguel Servet researcher (ISCIII; CP17/0008).
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AD group, Southern European Atlantic diet group; COVID-19, coronavirus disease 2019; CRF, cardiorespiratory fitness; CVD, cardiovascular disease; DASH, Dietary Approaches to Stop Hypertension; EDC, endocrine disruptor; GHG, greenhouse gas; MF, muscular fitness; ncRNA, noncoding RNA; SEAD, Southern European Atlantic diet.
Contributor Information
Paula M Lorenzo, Epigenomics in Endocrinology and Nutrition Group, Epigenomics Unit, Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain; CIBER Fisiopatologia de la Obesidad y Nutricion (CIBERobn), Madrid, Spain.
Andrea G Izquierdo, Epigenomics in Endocrinology and Nutrition Group, Epigenomics Unit, Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain; CIBER Fisiopatologia de la Obesidad y Nutricion (CIBERobn), Madrid, Spain.
Gemma Rodriguez-Carnero, Epigenomics in Endocrinology and Nutrition Group, Epigenomics Unit, Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain; Endocrinology and Nutrition Division, Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain.
Antía Fernández-Pombo, Endocrinology and Nutrition Division, Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain.
Alba Iglesias, Epigenomics in Endocrinology and Nutrition Group, Epigenomics Unit, Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain.
Marcos C Carreira, CIBER Fisiopatologia de la Obesidad y Nutricion (CIBERobn), Madrid, Spain; Molecular and Cellular Endocrinology Group. Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS) and Santiago de Compostela University (USC), Santiago de Compostela, Spain.
Cristina Tejera, Epigenomics in Endocrinology and Nutrition Group, Epigenomics Unit, Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain; Endocrinology and Nutrition Unit, Complejo Hospitalario Universitario de Ferrol (CHUF/SERGAS), Ferrol, Spain.
Diego Bellido, Epigenomics in Endocrinology and Nutrition Group, Epigenomics Unit, Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain; Endocrinology and Nutrition Unit, Complejo Hospitalario Universitario de Ferrol (CHUF/SERGAS), Ferrol, Spain.
Miguel A Martinez-Olmos, Epigenomics in Endocrinology and Nutrition Group, Epigenomics Unit, Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain; CIBER Fisiopatologia de la Obesidad y Nutricion (CIBERobn), Madrid, Spain; Endocrinology and Nutrition Division, Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain.
Rosaura Leis, CIBER Fisiopatologia de la Obesidad y Nutricion (CIBERobn), Madrid, Spain; Department of Pediatrics, Complejo Hospitalario Universitario de Santiago de Compostela (CHUS); Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS) and Santiago de Compostela University (USC), Santiago de Compostela, Spain; Fundacion Dieta Atlántica, Santiago de Compostela, Spain.
Felipe F Casanueva, CIBER Fisiopatologia de la Obesidad y Nutricion (CIBERobn), Madrid, Spain; Molecular and Cellular Endocrinology Group. Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS) and Santiago de Compostela University (USC), Santiago de Compostela, Spain; Fundacion Dieta Atlántica, Santiago de Compostela, Spain.
Ana B Crujeiras, Epigenomics in Endocrinology and Nutrition Group, Epigenomics Unit, Instituto de Investigacion Sanitaria de Santiago de Compostela (IDIS), Complejo Hospitalario Universitario de Santiago de Compostela (CHUS/SERGAS), Santiago de Compostela, Spain; CIBER Fisiopatologia de la Obesidad y Nutricion (CIBERobn), Madrid, Spain.
References
- 1. Sales NMR, Pelegrini PB, Goersch MC. Nutrigenomics: definitions and advances of this new science. J Nutr Metab. 2014;2014:202759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ramos-Lopez O, Milagro FI, Allayee H, Chmurzynska A, Choi MS, Curi Ret al. . Guide for current nutrigenetic, nutrigenomic, and nutriepigenetic approaches for precision nutrition involving the prevention and management of chronic diseases associated with obesity. J Nutr. 2017;10:43–62. [DOI] [PubMed] [Google Scholar]
- 3. Crujeiras AB, Diaz-Lagares A. DNA methylation in obesity and associated diseases. In: Epigenetic biomarkers and diagnostics. García-Giménez JLeditor. London, UK: Academic Press, Elsevier; 2016. p.313–29. [Google Scholar]
- 4. Izquierdo AG, Crujeiras AB. Epigenetic biomarkers in metabolic syndrome and obesity. In: Prognostic epigenetics. Sharma Seditor. London, UK: Academic Press, Elsevier; 2019. p.269–87. [Google Scholar]
- 5. Liu L, Li Y, Tollefsbol TO. Gene-environment interactions and epigenetic basis of human diseases. Curr Issues Mol Biol. 2008;10:25–36. [PMC free article] [PubMed] [Google Scholar]
- 6. Sayols-Baixeras S, Subirana I, Fernández-Sanlés A, Sentí M, Lluís-Ganella C, Marrugat Jet al. . DNA methylation and obesity traits: an epigenome-wide association study. The REGICOR study. Epigenetics. 2017;12(10):909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crujeiras AB, Diaz-Lagares A, Sandoval J, Milagro FI, Navas-Carretero S, Carreira MCet al. . DNA methylation map in circulating leukocytes mirrors subcutaneous adipose tissue methylation pattern: a genome-wide analysis from non-obese and obese patients. Sci Rep. 2017;7(1):41903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samblas M, Milagro FI, Martínez A. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics. 2019;14(5):421–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen J, Zhu B. Integrated analysis of the gene expression profile and DNA methylation profile of obese patients with type 2 diabetes. Mol Med Rep. 2018;17:7636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martín-Núñez GM, Rubio-Martín E, Cabrera-Mulero R, Rojo-Martínez G, Olveira G, Valdés Set al. . Type 2 diabetes mellitus in relation to global LINE-1 DNA methylation in peripheral blood: a cohort study. Epigenetics. 2014;9(10):1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castellano-Castillo D, Moreno-Indias I, Sanchez-Alcoholado L, Ramos-Molina B, Alcaide-Torres J, Morcillo Set al. . Altered adipose tissue DNA methylation status in metabolic syndrome: relationships between global DNA methylation and specific methylation at adipogenic, lipid metabolism and inflammatory candidate genes and metabolic variables. J Clin Med. 2019;8(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Izquierdo AG, Crujeiras AB. Role of epigenomic mechanisms in the onset and management of insulin resistance. Rev Endocr Metab Disord. 2019;20(1):89–102. [DOI] [PubMed] [Google Scholar]
- 13. Crujeiras AB, Diaz-Lagares A, Moreno-Navarrete JM, Sandoval J, Hervas D, Gomez Aet al. . Genome-wide DNA methylation pattern in visceral adipose tissue differentiates insulin-resistant from insulin-sensitive obese subjects. Translat Res. 2016;178:13–24, e5. [DOI] [PubMed] [Google Scholar]
- 14. Lorenzo PM, Izquierdo AG, Diaz-Lagares A, Carreira MC, Macias-Gonzalez M, Sandoval Jet al. . ZNF577 methylation levels in leukocytes from women with breast cancer is modulated by adiposity, menopausal state, and the Mediterranean diet. Front Endocrinol. 2020;11:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crujeiras AB, Diaz-Lagares A, Stefansson OA, Macias-Gonzalez M, Sandoval J, Cueva Jet al. . Obesity and menopause modify the epigenomic profile of breast cancer. Endocr Relat Cancer. 2017;24:351–63. [DOI] [PubMed] [Google Scholar]
- 16. Crujeiras AB, Morcillo S, Diaz-Lagares A, Sandoval J, Castellano-Castillo D, Torres Eet al. . Identification of an episignature of human colorectal cancer associated with obesity by genome-wide DNA methylation analysis. Int J Obes. 2019;43(1):176–88. [DOI] [PubMed] [Google Scholar]
- 17. Cabrera-Mulero A, Crujeiras AB, Izquierdo AG, Torres E, Ayers D, Casanueva FFet al. . Novel SFRP2 DNA methylation profile following neoadjuvant therapy in colorectal cancer patients with different grades of BMI. J Clin Med. 2019;8(7): 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milagro FI, Campión J, Cordero P, Goyenechea E, Gómez-Uriz AM, Abete Iet al. . A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J. 2011;25(4):1378–89. [DOI] [PubMed] [Google Scholar]
- 19. Moleres A, Campión J, Milagro FI, Marcos A, Campoy C, Garagorri JMet al. . Differential DNA methylation patterns between high and low responders to a weight loss intervention in overweight or obese adolescents: the EVASYON study. FASEB J. 2013;27(6):2504–12. [DOI] [PubMed] [Google Scholar]
- 20. Izquierdo AG, Crujeiras AB. Obesity-related epigenetic changes after bariatric surgery. Front Endocrinol. 2019;10:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicoletti CF, Pinhel MAS, Diaz-Lagares A, Casanueva FF, Jácome A, Pinhanelli VCet al. . DNA methylation screening after Roux-en Y gastric bypass reveals the epigenetic signature stems from genes related to the surgery per se. BMC Med Genet. 2019;12(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nitert MD, Dayeh T, Volkov P, Elgzyri T, Hall E, Nilsson Eet al. . Impact of an exercise intervention on DNA methylation in skeletal muscle from first-degree relatives of patients with type 2 diabetes. Diabetes. 2012;61(12):3322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rönn T, Volkov P, Davegårdh C, Dayeh T, Hall E, Olsson AHet al. . A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLos Genet. 2013;9(6):e1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Arora I, Sharma M, Sun LY, Tollefsbol TO. The epigenetic link between polyphenols, aging and age-related diseases. Genes (Basel). 2020;11:1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Milenkovic D, Krga I, Aung HH, Leroux C. Molecular nutrition and epigenetics. Reference Module in Food Science. London, UK: Elsevier; 2018. [Google Scholar]
- 26. Park LK, Friso S, Choi S-W. Nutritional influences on epigenetics and age-related disease. Proc Nutr Soc. 2012;71(1):75–83. [DOI] [PubMed] [Google Scholar]
- 27. Divella R, Daniele A, Savino E, Paradiso A. Anticancer effects of nutraceuticals in the Mediterranean diet: an epigenetic diet model. Cancer Genomics Proteomics. 2020;17(4):335–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peña-Romero AC, Navas-Carrillo D, Marín F, Orenes-Piñero E. The future of nutrition: nutrigenomics and nutrigenetics in obesity and cardiovascular diseases. Crit Rev Food Sci Nutr. 2018;58(17):3030–41. [DOI] [PubMed] [Google Scholar]
- 29. Cena H, Calder PC. Defining a healthy diet: evidence for the role of contemporary dietary patterns in health and disease. Nutrients. 2020;12:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell AP. DASH eating plan: an eating pattern for diabetes management. Diabetes Spectrum. 2017;30(2):76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tertsunen H-M, Hantunen S, Tuomainen T-P, Virtanen JK. Adherence to a healthy Nordic diet and risk of type 2 diabetes among men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Eur J Nutr. 2021;60(7):3927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krznarić Ž, Karas I, Kelečić DL, Bender DV. The Mediterranean and Nordic diet: a review of differences and similarities of two sustainable, health-promoting dietary patterns. Front Nutr. 2021;8:683678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Velho MV, Pinheiro R, Rodrigues AS. The Atlantic diet—origin and features. Int J Food Stud. 2016;5:106–19. [Google Scholar]
- 34. Ribeiro AI, Krainski ET, Carvalho MS, Pina M de F. Where do people live longer and shorter lives? An ecological study of old-age survival across 4404 small areas from 18 European countries. J Epidemiol Community Health. 2016;70(6):561–8. [DOI] [PubMed] [Google Scholar]
- 35. Instituto Nacional de Estadística . Home page [Internet]. [cited 2021 Nov 22]. Available from: https://www.ine.es.
- 36. Mathers CD, Stevens GA, Boerma T, White RA, Tobias MI. Causes of international increases in older age life expectancy. Lancet North Am Ed. 2015;385(9967):540–8. [DOI] [PubMed] [Google Scholar]
- 37. Francula-Zaninovic S, Nola IA. Management of measurable variable cardiovascular disease’ risk factors. Curr Cardiol Rev. 2018;14(3):153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Müller-Nordhorn J, Binting S, Roll S, Willich SN. An update on regional variation in cardiovascular mortality within Europe. Eur Heart J. 2008;29(10):1316–26. [DOI] [PubMed] [Google Scholar]
- 39. Nichols M, Townsend N, Scarborough P, Rayner M. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J. 2014;35(42):2950–9. [DOI] [PubMed] [Google Scholar]
- 40. Casanueva FF, Bellido D, Crujeiras AB, Vaz-Velho M LR. Hacia un score de dieta Atlántica. In: Casanueva FF, editor. Bases científicas de la dieta Atlántica. Universidad de Santiago de Compostela, Santiago de Compostela (Spain); 2020:265–71. [Google Scholar]
- 41. Monteagudo C, Mariscal-Arcas M, Rivas A, Lorenzo-Tovar ML, Tur JA, Olea-Serrano F. Proposal of a Mediterranean diet serving score. PLoS One. 2015;10(6):e0128594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Olsen A, Egeberg R, Halkjær J, Christensen J, Overvad K, Tjønneland A. Healthy aspects of the Nordic diet are related to lower total mortality. J Nutr. 2011;141(4):639–44. [DOI] [PubMed] [Google Scholar]
- 43. Adamsson V, Reumark A, Cederholm T, Vessby B, Risérus U, Johansson G. What is a healthy Nordic diet? Foods and nutrients in the NORDIET study. Food Nutr Res. 2012;56(1): 18189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tejera-Pérez C, Sánchez-Bao A, Bellido-Guerrero D, Casanueva FF. The Southern European Atlantic diet, a review. Minerva Endocrinol. 2020;46:145–60. [DOI] [PubMed] [Google Scholar]
- 45. Mithril C, Dragsted LO, Meyer C, Blauert E, Holt MK, Astrup A. Guidelines for the new Nordic diet. Public Health Nutr. 2012;15(10):1941–7. [DOI] [PubMed] [Google Scholar]
- 46. Mithril C, Dragsted LO, Meyer C, Tetens I, Biltoft-Jensen A, Astrup A. Dietary composition and nutrient content of the new Nordic diet. Public Health Nutr. 2013;16(5):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ministerio de Agricultura, Pesca y Alimentación . Consumo en hogares. [cited 2021 Nov 22]. Available from:https://www.mapa.gob.es/app/consumo-en-hogares/resultado1.asp.
- 48. Fundación Dieta Atlántica . Caracteristicas. Fundación Dieta Atlántica. [cited 2021 Nov 22]. Available from: https://www.fundaciondietatlantica.com/caracteristicas.php.
- 49. Trabazo RL, Pérez C L, Castro Pérez X, Solla P. Atlantic diet. Nutrition and gastronomy in Galicia. Nutr Hosp. 2019;36:7–13. [DOI] [PubMed] [Google Scholar]
- 50. Calvo-Malvar MDM, Leis R, Benítez-Estévez AJ, Sánchez-Castro J, Gude F. A randomised, family-focused dietary intervention to evaluate the Atlantic diet: the GALIAT study protocol. BMC Public Health. 2016;16(1):820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Guallar-Castillón P, Oliveira A, Lopes C, López-García E, Rodríguez-Artalejo F. The Southern European Atlantic diet is associated with lower concentrations of markers of coronary risk. Atherosclerosis. 2013;226(2):502–9. [DOI] [PubMed] [Google Scholar]
- 52. Hanach NI, McCullough F, Avery A. The impact of dairy protein intake on muscle mass, muscle strength, and physical performance in middle-aged to older adults with or without existing sarcopenia: a systematic review and meta-analysis. Adv Nutr. 2019;10(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ethgen O, Beaudart C, Buckinx F, Bruyère O, Reginster JY. The future prevalence of sarcopenia in Europe: a claim for public health action. Calcif Tissue Int. 2017;100(3):229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kiczorowska B, Samolińska W, Grela ER,Bik-Małodzińska M. Nutrient and mineral profile of chosen fresh and smoked fish. Nutrients. 2019;11:1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ministerio de Agricultura, Pesca y Alimentación . Catálogo oficial de razas. [cited 2022 Feb 10]. Available from: https://www.mapa.gob.es/es/ganaderia/temas/zootecnia/razas-ganaderas/razas/catalogo-razas/.
- 56. García-Atance MA, Carleos C, Dunner S, Eusebi PG, Rivero CJ, Justo JRet al. . Genomic characterization of a set of Iberian Peninsula bovine local breeds at risk of extinction: Morenas gallegas. Animal. 2020;10(11):1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. De Smet S, Vossen E. Meat: the balance between nutrition and health. A review. Meat Sci. 2016;120:145–56. [DOI] [PubMed] [Google Scholar]
- 58. Moreno T, Varela A, Oliete B, Carballo JA, Sánchez L, Montserrat L. Nutritional characteristics of veal from weaned and unweaned calves: discriminatory ability of the fat profile. Meat Sci. 2006;73(2):209–17. [DOI] [PubMed] [Google Scholar]
- 59. Bispo E, Moreno T, Thomas A, Durand D, Monserrat L, Gonzalez Let al. . Effects of weaning and finishing feeding treatment on fatty acids, especially cis and trans C18:1 isomers, in the longissimus thoracis muscle of Galician blond calves. Animal. 2011;5(5):802–12. [DOI] [PubMed] [Google Scholar]
- 60. Charron CS, Vinyard BT, Jeffery EH, Ross SA, Seifried HE, Novotny JA. BMI is associated with increased plasma and urine appearance of glucosinolate metabolites after consumption of cooked broccoli. Front Nutr. 2020;7:575092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Boctor D. The role of dietary fibre and prebiotics in the paediatric diet. Paediatr Child Health. 2020;25(4):263–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. De Vasconcelos M, Bennett RN, Rosa EAS, Ferreira-Cardoso JV. Composition of European chestnut (Castanea sativa mill.) and association with health effects: fresh and processed products. J Sci Food Agric. 2010;90(10):1578–89. [DOI] [PubMed] [Google Scholar]
- 63. Zlatanov MD, Antova GA, Angelova-Romova MJ, Teneva OT. Lipid composition of Castanea sativa mill. and Aesculus hippocastanum fruit oils. J Sci Food Agric. 2013;93(3):661–6. [DOI] [PubMed] [Google Scholar]
- 64. Rodrigues P, Ferreira T, Nascimento-Gonçalves E, Seixas F, Gil da Costa RM, Martins Tet al. . Dietary supplementation with chestnut (Castanea sativa) reduces abdominal adiposity in FVB/n mice: a preliminary study. Biomedicines. 2020;8(4):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Livesey G, Taylor R, Livesey HF, Buyken AE, Jenkins DJA, Augustin LSAet al. . Dietary glycemic index and load and the risk of type 2 diabetes: a systematic review and updated meta-analyses of prospective cohort studies. Nutrients. 2019;11:1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Castro-Penalonga M, Roca-Saavedra P, Miranda JM, Porto-Arias JJ, Nebot C, Cardelle-Cobas Aet al. . Influence of food consumption patterns and Galician lifestyle on human gut microbiota. J Physiol Biochem. 2017;74:1–8. [DOI] [PubMed] [Google Scholar]
- 67. de Lamas C, de Castro MJ, Gil-Campos M, Gil Á, Couce ML, Leis R. Effects of dairy product consumption on height and bone mineral content in children: a systematic review of controlled trials. Adv Nutr. 2019;10(Suppl):S88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Turismo de G. Las ferias gastronómicas ocupan un lugar muy relevante en la política turística de la Xunta de Galicia [Internet]. 2015. [cited 2021 Nov 22]. Available from: https://www.turismo.gal/espazo-institucional/actualidade/detalle-nova?content=nova_0625.html.
- 69. Castro X. Yantares gallegos: historia de la dieta Atlantica. Santiago de Compostela, Spain: Universidade de Santiago de Compostela; 2013. [Google Scholar]
- 70. Marx W, Lane M, Hockey M, Aslam H, Berk M, Walder Ket al. . Diet and depression: exploring the biological mechanisms of action. Mol Psychiatry. 2020;26:134–50. [DOI] [PubMed] [Google Scholar]
- 71. González-García S, Green RF, Scheelbeek PF, Harris F, Dangour AD. Dietary recommendations in Spain—affordability and environmental sustainability?. J Cleaner Prod. 2020;254:120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Esteve-Llorens X, Darriba C, Moreira MT, Feijoo G, González-García S. Towards an environmentally sustainable and healthy Atlantic dietary pattern: life cycle carbon footprint and nutritional quality. Sci Total Environ. 2019;646:704–15. [DOI] [PubMed] [Google Scholar]
- 73. Instituto Galego de Estadística . Concellos e a súa poboación clasificados polo número dos seus habitantes. Padrón municipal de habitantes. Ano 2021 [Internet]. 2021. Available from:https://www.ige.eu/igebdt/esqv.jsp?paxina=001&c=0201001002&ruta=verPpalesResultados. [Google Scholar]
- 74. Instituto Galego de Estadística . [Internet]. Available from: https://www.ige.eu/web/mostrar_seccion.jsp?idioma=gl&codigo=0609.
- 75. Gicquel E, Boettcher P, Besbes B, Furre S, Fernández J, Danchin-Burge Cet al. . Impact of conservation measures on demography and genetic variability of livestock breeds. Animal. 2020;14(4):670–80. [DOI] [PubMed] [Google Scholar]
- 76. Medugorac I, Medugorac A, Russ I, Veit-Kensch CE, Taberlet P, Luntz Bet al. . Genetic diversity of European cattle breeds highlights the conservation value of traditional unselected breeds with high effective population size. Mol Ecol. 2009;18(16):3394–410. [DOI] [PubMed] [Google Scholar]
- 77. Oliveira A, Lopes C, Rodríguez-Artalejo F. Adherence to the Southern European Atlantic diet and occurrence of nonfatal acute myocardial infarction. Am J Clin Nutr. 2010;92(1):211–7. [DOI] [PubMed] [Google Scholar]
- 78. Moreira C, Santos R, Moreira P, Lobelo F, Ruiz JR, Vale Set al. . Cardiorespiratory fitness is negatively associated with metabolic risk factors independently of the adherence to a healthy dietary pattern. Nutr Metab Cardiovasc Dis. 2013;23(7):670–6. [DOI] [PubMed] [Google Scholar]
- 79. Calvo-Malvar M, Benítez-Estévez AJ, Sánchez-Castro J, Leis R, Gude F. Effects of a community-based behavioral intervention with a traditional Atlantic diet on cardiometabolic risk markers: a cluster randomized controlled trial (the GALIAT study). Nutrients. 2021;13:1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Agostinis-Sobrinho C, Abreu S, Moreira C, Lopes L, García-Hermoso A, Ramírez-Vélez Ret al. . Muscular fitness, adherence to the Southern European Atlantic diet and cardiometabolic risk factors in adolescents. Nutr Metab Cardiovasc Dis. 2017;27(8):695–702. [DOI] [PubMed] [Google Scholar]
- 81. Agostinis-Sobrinho C, Brand C, Moreira C, Lopes L, Oliveira-Santos J, Silva Pet al. . Muscular fitness, Southern European Atlantic diet and inflammation in adolescents. Azorean Physical Activity and Health Study II. Eur J Sport Sci. 2018;18(1):104–11. [DOI] [PubMed] [Google Scholar]
- 82. Agostinis-Sobrinho C, Dias AF, Brand C, Norkiene S, Abreu S, Gaya ACAet al. . Adherence to Southern European Atlantic diet and physical fitness on the atherogenic index of plasma in adolescents. Cad Saude Publica. 2019;35:e00200418. [DOI] [PubMed] [Google Scholar]
- 83. Rodríguez-Martín C, Garcia-Ortiz L, Rodriguez-Sanchez E, Maderuelo-Fernandez C, Lugones-Sanchez A, Martin-Cantera MSet al. . The relationship of the Atlantic diet with cardiovascular risk factors and markers of arterial stiffness in adults without cardiovascular disease. Nutrients. 2019;11:742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sánchez C, Fente C, Barreiro R, López-Racamonde O, Cepeda A, Regal P. Association between breast milk mineral content and maternal adherence to healthy dietary patterns in Spain: a transversal study. Foods (Basel). 2020;9:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Carballo-Casla A, Ortolá R, García-Esquinas E, Oliveira A, Sotos-Prieto M, Lopes Cet al. . The Southern European Atlantic diet and all-cause mortality in older adults. BMC Med. 2021;19(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Moreira C, Santos R, Vale S, Soares-Miranda L, Marques AI, Santos PCet al. . Metabolic syndrome and physical fitness in a sample of Azorean adolescents. Metab Syndr Relat Disorder. 2010;8(5):443–9. [DOI] [PubMed] [Google Scholar]
- 87. Balagopal P,de Ferranti SD, Cook S, Daniels SR, Gidding SS, Hayman LL, McCrindle BWet al. . Nontraditional risk factors and biomarkers for cardiovascular disease: mechanistic, research, and clinical considerations for youth. Circulation. 2011;123(23):2749–69. [DOI] [PubMed] [Google Scholar]
- 88. Blake GJ, Ridker PM. Novel clinical markers of vascular wall inflammation. Circ Res. 2001;89(9):763–71. [DOI] [PubMed] [Google Scholar]
- 89. Casati L, Sendra R, Sibilia V, Celotti F. Endocrine disrupters: the new players able to affect the epigenome. Front Cell Dev Biol. 2015;3:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Alavian-Ghavanini A, Rüegg J. Understanding epigenetic effects of endocrine disrupting chemicals: from mechanisms to novel test methods. Basic Clin Pharmacol Toxicol. 2018;122(1):38–45. [DOI] [PubMed] [Google Scholar]
- 91. Keikha M, Bahreynian M, Saleki M, Kelishadi R. Macro- and micronutrients of human milk composition: are they related to maternal diet? A comprehensive systematic review. Breastfeed Med. 2017;12(9):517–27. [DOI] [PubMed] [Google Scholar]
- 92. Morrow AL, Dawodu A. Fatty acids and fat-soluble vitamins in breast milk: physiological significance and factors affecting their concentrations. Nestle Nutr Inst Workshop Ser. 2019;90:57–67. [DOI] [PubMed] [Google Scholar]
- 93. Choi YK, Kim J-M, Lee J-E, Cho MS, Kang BS, Choi Het al. . Association of maternal diet with zinc, copper, and iron concentrations in transitional human milk produced by Korean mothers. Clin Nutr Res. 2016;5(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Cohen Kadosh K, Muhardi L, Parikh P, Basso M, Jan Mohamed HJ, Prawitasari Tet al. . Nutritional support of neurodevelopment and cognitive function in infants and young children—an update and novel insights. Nutrients. 2021;13(1):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Koletzko B. Human milk lipids. Ann Nutr Metab. 2016;69(Suppl 2):28–40. [DOI] [PubMed] [Google Scholar]
- 96. Campoy C, Chisaguano Tonato AM, de la Garza Puentes A, Sáenz de Pipaón M, Verduci E, Koletzko Bet al. [Controversy about the critical role of long-chain polyunsaturated fatty acids, arachidonic acid (ARA) and docosahexaenoic acid (DHA), during infancy]. Nutr Hosp. 2021;38:1101–12. [DOI] [PubMed] [Google Scholar]
- 97. Hills RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR. Gut microbiome: profound implications for diet and disease. Nutrients. 2019;11: 1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kim YA, Keogh JB, Clifton PM. Probiotics, prebiotics, synbiotics and insulin sensitivity. Nutr Res Rev. 2018;31(1):35–51. [DOI] [PubMed] [Google Scholar]
- 99. Roca-Saavedra P, Mendez-Vilabrille V, Miranda JM, Nebot C, Cardelle-Cobas A, Franco CMet al. . Food additives, contaminants and other minor components: effects on human gut microbiota—a review. J Physiol Biochem. 2018;74(1):69–83. [DOI] [PubMed] [Google Scholar]
- 100. Cresci GA, Bawden E. Gut microbiome: what we do and don't know. Nutr Clin Pract. 2015;30(6):734–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Conlon M, Bird A. The impact of diet and lifestyle on gut microbiota and human health. Nutrients. 2014;7(1):17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Trebichavsky I, Rada V, Splichalova A, Splichal I. Cross-talk of human gut with bifidobacteria. Nutr Rev. 2009;67(2):77–82. [DOI] [PubMed] [Google Scholar]
- 103. Taft DH, Liu J, Maldonado-Gomez MX, Akre S, Huda MN, Ahmad SMet al. . Bifidobacterial dominance of the gut in early life and acquisition of antimicrobial resistance. Msphere. 2018;3(5):e00441–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Underwood MA, German JB, Lebrilla CB, Mills DA. Bifidobacterium longum subspecies infantis: champion colonizer of the infant gut. Pediatr Res. 2015;77(1-2):229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Kok CR, Hutkins R. Yogurt and other fermented foods as sources of health-promoting bacteria. Nutr Rev. 2018;76(Suppl 1):4–15. [DOI] [PubMed] [Google Scholar]
- 106. Savaiano DA, Hutkins RW. Yogurt, cultured fermented milk, and health: a systematic review. Nutr Rev. 2021;79(5):599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Saborido R, Leis R. [Yogurt and dietary recommendations for lactose intolerance]. Nutrición Hospitalaria. 2018;35(6):45–8. [DOI] [PubMed] [Google Scholar]
- 108. Cespedes EM, Hu FB. Dietary patterns: from nutritional epidemiologic analysis to national guidelines. Am J Clin Nutr. 2015;101(5):899–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Beck MA, Levander OA. Dietary oxidative stress and the potentiation of viral infection. Annu Rev Nutr. 1998;18(1):93–116. [DOI] [PubMed] [Google Scholar]
- 110. Trujillo-Mayol I, Guerra-Valle M, Casas-Forero N, Sobral MMC, Viegas O, Alarcón-Enos Jet al. . Western dietary pattern antioxidant intakes and oxidative stress: importance during the SARS-CoV-2/COVID-19 pandemic.Adv Nutr. 2021;12:670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Calvo-Malvar M. Effect of an Atlantic diet on anthropometric indices and serum lipid profile [Internet]. 2015; [cited 2021 Feb 26]. Available from: https://clinicaltrials.gov/ct2/show/NCT02391701.
- 112. Wu CT, Morris JR. Genes, genetics, and epigenetics: a correspondence. Science. 2001;; 293(5532):1103–5. [DOI] [PubMed] [Google Scholar]
- 113. Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–63. [DOI] [PubMed] [Google Scholar]
- 114. Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447(7143):425–32. [DOI] [PubMed] [Google Scholar]
- 115. Aguilera O, Fernández AF, Muñoz A, Fraga MF. Epigenetics and environment: a complex relationship. J Appl Physiol. 2010;109(1):243–51. [DOI] [PubMed] [Google Scholar]
- 116. Xu W, Wang F, Yu Z, Xin F. Epigenetics and cellular metabolism. Genet Epigenet. 2016;8:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17(8):487–500. [DOI] [PubMed] [Google Scholar]
- 118. Skvortsova K, Iovino N, Bogdanović O. Functions and mechanisms of epigenetic inheritance in animals. Nat Rev Mol Cell Biol. 2018;19(12):774–90. [DOI] [PubMed] [Google Scholar]
- 119. Voisin S, Harvey NR, Haupt LM, Griffiths LR, Ashton KJ, Coffey VGet al. . An epigenetic clock for human skeletal muscle. J Cachexia Sarcopenia Muscle. 2020;11(4):887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Benayoun BA, Pollina EA, Brunet A. Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol. 2015;; 16(10):593–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Maegawa S, Lu Y, Tahara T, Lee JT, Madzo J, Liang Set al. . Caloric restriction delays age-related methylation drift. Nat Commun. 2017;8(1): 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Voisin S, Eynon N, Yan X, Bishop DJ. Exercise training and DNA methylation in humans. Acta Physiologica. 2015;; 213(1):39–59. [DOI] [PubMed] [Google Scholar]
- 123. Jacques M, Hiam D, Craig J, Barrès R, Eynon N, Voisin S. Epigenetic changes in healthy human skeletal muscle following exercise—a systematic review. Epigenetics. 2019;14(7):633–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Amenyah SD, Ward M, Strain JJ, McNulty H, Hughes CF, Dollin Cet al. . Nutritional epigenomics and age-related disease. Curr Dev Nutr. 2020;4(7):nzaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMedicine. 2017;; 21:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci Let al. . Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9(2):419–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF, Mills JLet al. . Biomarkers of Nutrition for Development—folate review. J Nutr. 2015;145(7):1636S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Martínez Roldán C, Carbajal Azcona Á. Componentes bioactivos de los alimentos conceptos clave. Available from: https://www.kelloggsnutrition.com/content/dam/globalnutrition/es_ES/assets/Manual_Nutricion_Kelloggs_Capitulo_02.2.pdf [Accessed 8 December 2020].
- 129. Guillamón E. Efecto de compuestos fitoquímicos del género allium sobre el sistema inmuno y la respuesta inflamatoria [Internet]. Ars Pharmaceutica. 2018;59(3):185–96. [Google Scholar]
- 130. Ácidos grasos omega-3—datos en español. Available from: https://ods.od.nih.gov/factsheets/Omega3FattyAcids-DatosEnEspanol/ (accessed 8 December 2020).
- 131. Castellanos T L, Rodriguez D M. El efecto de omega 3 en la salud humana y consideraciones en la ingesta. Revista Chilena De Nutriciã³N. 2015;42(1):90–5. [Google Scholar]
- 132. Ali MA, Kamal MM, Rahman MH, Siddiqui MN, Haque MA, Saha KKet al. . Functional dairy products as a source of bioactive peptides and probiotics: current trends and future prospectives. J Food Sci Technol. 2022;59(4):1263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lin T, Zirpoli GR, McCann SE, Moysich KB, Ambrosone CB, Tang L. Trends in cruciferous vegetable consumption and associations with breast cancer risk: a case-control study. Curr Dev Nutr. 2017;1(8):e000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Luteína y zeaxantina: los carotenoides del huevo. Granjas Redondo. Available from: http://www.avicolaredondo.com/luteina-zeaxantina-carotenoides-huevo/ (accessed 8 December 2020). [Google Scholar]
- 135. Kaufman-Szymczyk A, Majewski G, Lubecka-Pietruszewska K, Fabianowska-Majewska K. The role of sulforaphane in epigenetic mechanisms, including interdependence between histone modification and DNA methylation. Int J Mol Sci. 2015;16(12):29732–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Royston KJ, Tollefsbol TO. The epigenetic impact of cruciferous vegetables on cancer prevention. Curr Pharmacol Rep. 2015;1(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Baenas N, Wagner AE. Pharmacoepigenetics of Brassica-derived compounds. Cacabelos R. Translational epigenetics, pharmacoepigenetics. London, UK: Academic Press, Elsevier; 10;2019;Chapter 34,847–57. [Google Scholar]
- 138. Chapado LA, Martín-Hernández R, Hernández De La Red S, Tomé-Carneiro J, Gil-Zamorano J, Ruiz-Roso MBet al. . Connection between miRNA mediation and the bioactive effects of broccoli (Brassica oleracea var. italica): exogenous miRNA resistance to food processing and GI digestion. J Agric Food Chem. 2021;69(32):9326–37. [DOI] [PubMed] [Google Scholar]
- 139. Kiec-Wilk B, Sliwa A, Mikolajczyk M, Malecki MT, Mathers JC. The CpG island methylation regulated expression of endothelial proangiogenic genes in response to β-carotene and arachidonic acid. Nutr Cancer. 2011;63(7):1053–63. [DOI] [PubMed] [Google Scholar]
- 140. Busch C, Burkard M, Leischner C, Lauer UM, Frank J, Venturelli S. Epigenetic activities of flavonoids in the prevention and treatment of cancer. Clin Epigenet. 2015;7(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Declerck K, Szarc vel Szic K, Palagani A, Heyninck K, Haegeman G, Morand Cet al. . Epigenetic control of cardiovascular health by nutritional polyphenols involves multiple chromatin-modifying writer-reader-eraser proteins. Curr Top Med Chem. 2016;16(7):788–806. [DOI] [PubMed] [Google Scholar]
- 142. Pan M-H, Lai C-S, Wu J-C, Ho C-T. Epigenetic and disease targets by polyphenols. Curr Pharm Des. 2013;19(34):6156–85. [DOI] [PubMed] [Google Scholar]
- 143. Milenkovic D, Vanden, BW, Morand C, Claude S, van de Sandt A, Gorressen Set al. . A systems biology network analysis of nutri(epi)genomic changes in endothelial cells exposed to epicatechin metabolites. Sci Rep. 2018;8(1):15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Milenkovic D, Declerck K, Guttman Y, Kerem Z, Claude S, Weseler ARet al. . (-)-Epicatechin metabolites promote vascular health through epigenetic reprogramming of endothelial-immune cell signaling and reversing systemic low-grade inflammation. Biochem Pharmacol. 2020;173:113699. [DOI] [PubMed] [Google Scholar]
- 145. Ruskovska T, Maksimova V, Milenkovic D. Polyphenols in human nutrition: from the in vitro antioxidant capacity to the beneficial effects on cardiometabolic health and related inter-individual variability—an overview and perspective. Br J Nutr. 2020;123(3):241–54. [DOI] [PubMed] [Google Scholar]
- 146. Monfoulet L-E, Ruskovska T, Ajdžanović V, Havlik J, Vauzour D, Bayram Bet al. . Molecular determinants of the cardiometabolic improvements of dietary flavanols identified by an integrative analysis of nutrigenomic data from a systematic review of animal studies. Mol Nutr Food Res. 2021;65(16):2100227. [DOI] [PubMed] [Google Scholar]
- 147. Tauriainen E, Luostarinen M, Martonen E, Finckenberg P, Kovalainen M, Huotari Aet al. . Distinct effects of calorie restriction and resveratrol on diet-induced obesity and fatty liver formation. J Nutr Metab. 2011;2011:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Milagro FI, Mansego ML, De Miguel C, Martínez JA. Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol Aspects Med. 2013;34(4):782–812. [DOI] [PubMed] [Google Scholar]
- 149. Bruckbauer A, Zemel MB, Thorpe T, Akula MR, Stuckey AC, Osborne Det al. . Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr Metab. 2012;9(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Pudenz M, Roth K, Gerhauser C. Impact of soy isoflavones on the epigenome in cancer prevention. Nutrients. 2014;6(10):4218–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Lee P-S, Chiou Y-S, Ho C-T, Pan M-H. Chemoprevention by resveratrol and pterostilbene: targeting on epigenetic regulation. Biofactors. 2018;44(1):26–35. [DOI] [PubMed] [Google Scholar]
- 152. Hussey B, Lindley MR, Mastana SS. Omega 3 fatty acids, inflammation and DNA methylation: an overview. Clin Lipidol. 2017;12:24–32. [Google Scholar]
- 153. Ortega FJ, Cardona-Alvarado MI, Mercader JM, Moreno-Navarrete JM, Moreno M, Sabater Met al. . Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J Nutr Biochem. 2015;26(10):1095–101. [DOI] [PubMed] [Google Scholar]
- 154. Albracht-Schulte K, Kalupahana NS, Ramalingam L, Wang S, Rahman SM, Robert-McComb Jet al. . Omega-3 fatty acids in obesity and metabolic syndrome: a mechanistic update. J Nutr Biochem. 2018;58:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. D'Amore S, Vacca M, Cariello M, Graziano G, D'Orazio A, Salvia Ret al. . Genes and miRNA expression signatures in peripheral blood mononuclear cells in healthy subjects and patients with metabolic syndrome after acute intake of extra virgin olive oil. Biochim Biophys Acta Mol Cell Biol Lipid. 2016;1861(11):1671–80. [DOI] [PubMed] [Google Scholar]
- 156. Huang Q, Wen J, Chen G, Ge M, Gao Y, Ye Xet al. . Omega-3 polyunsaturated fatty acids inhibited tumor growth via preventing the decrease of genomic DNA methylation in colorectal cancer rats. Nutr Cancer. 2016;68(1):113–9. [DOI] [PubMed] [Google Scholar]
- 157. Karimi M, Vedin I, Freund Levi Y, Basun H, Faxén Irving G, Eriksdotter Met al. . DHA-rich n-3 fatty acid supplementation decreases DNA methylation in blood leukocytes: the OmegAD study. Am J Clin Nutr. 2017;106(4):1157–65. [DOI] [PubMed] [Google Scholar]
- 158. Tremblay BL, Guénard F, Rudkowska I, Lemieux S, Couture P, Vohl M-C. Epigenetic changes in blood leukocytes following an omega-3 fatty acid supplementation. Clin Epigenet. 2017;9(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Daimiel L, Micó V, Valls RM, Pedret A, Motilva MJ, Rubió Let al. . Impact of phenol-enriched virgin olive oils on the postprandial levels of circulating microRNAs related to cardiovascular disease. Mol Nutr Food Res. 2020;64(15):2000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Speckmann B, Grune T. Epigenetic effects of selenium and their implications for health. Epigenetics. 2015;10(3):179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Corominas-Faja B, Cuyàs E, Lozano-Sánchez J, Cufí S, Verdura S, Fernández-Arroyo Set al. . Extra-virgin olive oil contains a metabolo-epigenetic inhibitor of cancer stem cells. Carcinogenesis. 2018;39(4):601–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Ledesma-Martínez E, Aguíñiga-Sánchez I, Weiss-Steider B, Rivera-Martínez AR, Santiago-Osorio E. Casein and peptides derived from casein as antileukaemic agents. J Oncol. 2019;2019;1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Carlberg C. Nutrigenomics of vitamin D. Nutrients. 2019;11:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Bar-El Dadon S, Reifen R. Vitamin A and the epigenome. Crit Rev Food Sci Nutr. 2017;57(11):2404–11. [DOI] [PubMed] [Google Scholar]
- 165. Guz J, Oliński R. The role of vitamin C in epigenetic regulation. Postepy Hig Med Dosw (Online). 2017;71:747–60. [DOI] [PubMed] [Google Scholar]
- 166. Remely M, Stefanska B, Lovrecic L, Magnet U, Haslberger AG. Nutriepigenomics: the role of nutrition in epigenetic control of human diseases. Curr Opin Clin Nutr Metab Care. 2015;18(4):328–33. [DOI] [PubMed] [Google Scholar]
- 167. Ntanasis-Stathopoulos J, Tzanninis JG, Philippou A, Koutsilieris M. Epigenetic regulation on gene expression induced by physical exercise. J Musculoskelet Neuronal Interact. 2013;13:133–46. [PubMed] [Google Scholar]
- 168. Izquierdo AG, Portela M, Lorenzo PM, Mallo F, Crujeiras AB. Food components affecting the epigenome: “ergogenetic” aids for performance. PharmaNutrition. 2020;14:100231. [Google Scholar]
- 169. Ramos-Lopez O, Milagro FI, Riezu-Boj JI, Martinez JA. Epigenetic signatures underlying inflammation: an interplay of nutrition, physical activity, metabolic diseases, and environmental factors for personalized nutrition. Inflamm Res. 2020;1:29–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Horsburgh S, Robson-Ansley P, Adams R, Smith C. Exercise and inflammation-related epigenetic modifications: focus on DNA methylation. Exerc Immunol Rev. 2015;21:26–41. [PubMed] [Google Scholar]
- 171. Elsner V, Trevizol L, de Leon I, da Silva M, Weiss T, Braga Met al. . Therapeutic effectiveness of a single exercise session combined with Walkaide functional electrical stimulation in post-stroke patients: a crossover design study. Neural Regen Res. 2021;16(5):805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. White AJ, Sandler DP, Bolick SCE, Xu Z, Taylor JA, Deroo LA. Recreational and household physical activity at different time points and DNA global methylation. Eur J Cancer. 2013;49(9):2199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Zhang FF, Cardarelli R, Carroll J, Zhang S, Fulda KG, Gonzalez Ket al. . Physical activity and global genomic DNA methylation in a cancer-free population. Epigenetics. 2011;6(3):293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Ferrari L, Vicenzi M, Tarantini L, Barretta F, Sironi S, Baccarelli AAet al. . Effects of physical exercise on endothelial function and DNA methylation. Int J Environ Res Public Health. 2019;16(14):2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Ling C, Rönn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab. 2019;29(5):1028–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Barrès R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz Tet al. . Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–11. [DOI] [PubMed] [Google Scholar]
- 177. Fabre O, Ingerslev LR, Garde C, Donkin I, Simar D, Barrès R. Exercise training alters the genomic response to acute exercise in human adipose tissue. Epigenomics. 2018;10(8):1033–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hwang SH, Kang DW, Lee MK, Byeon JY, Park H, Park DHet al. . Changes in DNA methylation after 6-week exercise training in colorectal cancer survivors: a preliminary study. Asia Pac J Clin Oncol. 2022;18(1):52–60. [DOI] [PubMed] [Google Scholar]
- 179. Fulkerson JA, Telke S, Larson N, Berge J, Sherwood NE, Neumark-Sztainer D. A healthful home food environment: is it possible amidst household chaos and parental stress?. Appetite. 2019;142:104391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Horning ML, Fulkerson JA, Friend SE, Neumark-Sztainer D. Associations among nine family dinner frequency measures and child weight, dietary, and psychosocial outcomes. J Acad Nutr Diet. 2016;116(6):991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Utter J, Larson N, Berge JM, Eisenberg ME, Fulkerson JA, Neumark-Sztainer D. Family meals among parents: associations with nutritional, social and emotional wellbeing. Prev Med. 2018;113:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Sobal J, Hanson K. Family meals and body weight in US adults. Public Health Nutr. 2011;14(9):1555–62. [DOI] [PubMed] [Google Scholar]
- 183. Horning ML, Schow R, Friend SE, Loth K, Neumark-Sztainer D, Fulkerson JA. Family dinner frequency interacts with dinnertime context in associations with child and parent BMI outcomes. J Fam Psychol. 2017;31(7):945–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Elgar FJ, Craig W, Trites SJ. Family dinners, communication, and mental health in Canadian adolescents. J Adolesc Health. 2013;52(4):433–8. [DOI] [PubMed] [Google Scholar]
- 185. Berge JM, MacLehose RF, Larson N, Laska M, Neumark-Sztainer D. Family food preparation and its effects on adolescent dietary quality and eating patterns. J Adolesc Health. 2016;59(5):530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Utter J, Denny S, Lucassen M, Dyson B. Adolescent cooking abilities and behaviors: associations with nutrition and emotional well-being. J Nutr Educ Behav. 2016;48(1):35–41, e1. [DOI] [PubMed] [Google Scholar]
- 187. Utter J, Denny S, Peiris-John R, Moselen E, Dyson B, Clark T. Family meals and adolescent emotional well-being: findings from a national study. J Nutr Educ Behav. 2017;49(1):67–72, e1. [DOI] [PubMed] [Google Scholar]
- 188. Haines J, Gillman MW, Rifas-Shiman S, Field AE, Austin SB. Family dinner and disordered eating behaviors in a large cohort of adolescents. Eating Disord. 2009;18(1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Worobey J. Early family mealtime experiences and eating attitudes in normal weight, underweight and overweight females. Eat Weight Disord. 2002;7(1):39–44. [DOI] [PubMed] [Google Scholar]
- 190. Fulkerson JA, Story M, Mellin A, Leffert N, Neumark-Sztainer D, French SA. Family dinner meal frequency and adolescent development: relationships with developmental assets and high-risk behaviors. J Adolesc Health. 2006;39(3):337–45. [DOI] [PubMed] [Google Scholar]
- 191. Loth K, Wall M, Choi C-W, Bucchianeri M, Quick V, Larson Net al. . Family meals and disordered eating in adolescents: are the benefits the same for everyone?. Int J Eat Disord. 2015;48(1):100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi Met al. . Correction to: A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2018;16(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Jacka FN, O'Neil A, Opie R, Itsiopoulos C, Cotton S, Mohebbi Met al. . A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Parletta N, Zarnowiecki D, Cho J, Wilson A, Bogomolova S, Villani Aet al. . A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: a randomized controlled trial (HELFIMED). Nutr Neurosci. 2019;22(7):474–87. [DOI] [PubMed] [Google Scholar]
- 195. McGowan PO, Roth TL. Epigenetic pathways through which experiences become linked with biology. Dev Psychopathol. 2015;27(2):637–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos INet al. . Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Argentieri MA, Nagarajan S, Seddighzadeh B, Baccarelli AA, Shields AE. Epigenetic pathways in human disease: the impact of DNA methylation on stress-related pathogenesis and current challenges in biomarker development. EBioMedicine. 2017;18:327–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Snijders C, Maihofer AX, Ratanatharathorn A, Baker DG, Boks MP, Geuze Eet al. . Longitudinal epigenome-wide association studies of three male military cohorts reveal multiple CpG sites associated with post-traumatic stress disorder. Clin Epigenet. 2020;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Smith AK, Ratanatharathorn A, Maihofer AX, Naviaux RK, Aiello AE, Amstadter ABet al. . Epigenome-wide meta-analysis of PTSD across 10 military and civilian cohorts identifies methylation changes in AHRR. Nat Commun. 2020;11:5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Willett W, Rockström J, Loken B, Springmann M, Lang T, Vermeulen Set al. . Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet North Am Ed. 2019;393(10170):447–92. [DOI] [PubMed] [Google Scholar]
- 201. FAO . Opening speech at the International Scientific Symposium on Biodiversity and Sustainable Diets: United Against Hunger. Rome (Italy); FAO; 2010. [Google Scholar]
- 202. Friel S, Dangour AD, Garnett T, Lock K, Chalabi Z, Roberts Iet al. . Public health benefits of strategies to reduce greenhouse-gas emissions: food and agriculture. Lancet North Am Ed. 2009;374(9706):2016–25. [DOI] [PubMed] [Google Scholar]
- 203. Gustafson D, Gutman A, Leet W, Drewnowski A, Fanzo J, Ingram J. Seven food system metrics of sustainable nutrition security. Sustainability. 2016;8(3):196. [Google Scholar]
- 204. Heller MC, Keoleian GA, Willett WC. Toward a life cycle-based, diet-level framework for food environmental impact and nutritional quality assessment: a critical review. Environ Sci Technol. 2013;47(22):12632–47. [DOI] [PubMed] [Google Scholar]
- 205. Coelho CRV, Pernollet F, Van Der Werf HMG. Environmental life cycle assessment of diets with improved omega-3 fatty acid profiles. PLoS One. 2016;11:e0160397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Sáez-Almendros S, Obrador B, Bach-Faig A, Serra-Majem L. Environmental footprints of Mediterranean versus Western dietary patterns: beyond the health benefits of the Mediterranean diet. Environ Health. 2013;12(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Castañé S, Antón A. Assessment of the nutritional quality and environmental impact of two food diets: a Mediterranean and a vegan diet. J Cleaner Prod. 2017;167:929–37. [Google Scholar]
- 208. CTN 150–Gestión Ambiental . UNE-EN ISO 14040:2006. Gestión ambiental análisis del ciclo de vida principios y marco de referencia. 2006:29. [Internet]. Available from: https://www.iso.org/obp/ui#iso:std:iso:14040:ed-2:v1:es (accessed 18 December 2020).
- 209. Esteve-Llorens X, Moreira MT, Feijoo G, González-García S. Linking environmental sustainability and nutritional quality of the Atlantic diet recommendations and real consumption habits in Galicia (NW Spain). Sci Total Environ. 2019;683:71–9. [DOI] [PubMed] [Google Scholar]
- 210. González-García S, Esteve-Llorens X, Moreira MT, Feijoo G. Carbon footprint and nutritional quality of different human dietary choices. Sci Total Environ. 2018;644:77–94. [DOI] [PubMed] [Google Scholar]
- 211. Jacobs MN, Marczylo EL, Guerrero-Bosagna C, Rüegg J. Marked for life: epigenetic effects of endocrine disrupting chemicals. Annu Rev Environ Resour. 2017;42(1):105–60. [Google Scholar]
- 212. Uzumcu M, Zama AM, Oruc E. Epigenetic mechanisms in the actions of endocrine-disrupting chemicals: gonadal effects and role in female reproduction. Reprod Domest Anim. 2012;47:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AMet al. . Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30(4):293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B. 2009;12(3):206–23. [DOI] [PubMed] [Google Scholar]
- 215. Kortenkamp A, Martin O, Faust M, Evans R, Mckinlay R, Orton Fet al. . State of the art assessment of endocrine disrupters. Final Rep. 2011;1–135. [DOI] [PubMed]
- 216. Menouni A, Duca R-C, Gosh M, Zouine N, Lhilali I, Akroute Det al. . O1A.5 emergent role of epigenetic biomarkers of pesticides exposure: a case study among women of childbearing age living in Meknes (Morocco). Occup Environ Med. 2019;76(Suppl 1):A4.1–A4. [Google Scholar]
- 217. Tiffon C. The impact of nutrition and environmental epigenetics on human health and disease [Internet]. Int J Mol Sci. 2018;; 19(11)3425. [cited 2020 Dec 19]. Available from: https://pubmed.ncbi.nlm.nih.gov/30388784/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Collotta M, Bertazzi PA, Bollati V. Epigenetics and pesticides. Toxicology. 2013;307:35–41. [DOI] [PubMed] [Google Scholar]
- 219. Ho SM, Johnson A, Tarapore P, Janakiram V, Zhang X, Leung YK. Environmental epigenetics and its implication on disease risk and health outcomes. ILAR J. 2012;53:289–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Bollati V, Baccarelli A. Environmental epigenetics. Heredity. 2010;105(1):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr. 2009;21(2):243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kim M, Bae M, Na H, Yang M. Environmental toxicants induced epigenetic alterations and their reversers. J Environ Sci Health C. 2012;30(4):323–67. [DOI] [PubMed] [Google Scholar]
- 223. González-García S, Esteve-Llorens X, González-García R, González L, Feijoo G, Moreira MTet al. . Environmental assessment of menus for toddlers serviced at nursery canteen following the Atlantic diet recommendations. Sci Total Environ. 2021;770:145342. [DOI] [PubMed] [Google Scholar]
- 224. López de Las Hazas M-C, Boughanem H, Dávalos A. Untoward effects of micro- and nanoplastics: an expert review of their biological impact and epigenetic effects. Adv Nutr. 2021; doi: 10.1093/advances/nmab154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Porto-Arias JJ, Lorenzo T, Lamas A, Regal P, Cardelle-Cobas A, Cepeda A. Food patterns and nutritional assessment in Galician university students. J Physiol Biochem. 2018;74(1):119–26. [DOI] [PubMed] [Google Scholar]
- 226. Xunta de Galicia, Consellería de Sanidad . Encuesta sobre los hábitos alimentarios de la población adulta gallega, 2007. Santiago de Compostela: Xunta de Galicia; 2008. [Google Scholar]
- 227. Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3(4):503–18. [DOI] [PMC free article] [PubMed] [Google Scholar]