ABSTRACT
Gastroparesis (Gp) is a delay in gastric emptying in the absence of a mechanical obstruction and has the capacity to cause symptoms that significantly impact a patient's quality of life. Dietary interventions are the first-line treatment in Gp, but the efficacy of different diets is unclear. This systematic review seeks to determine the effectiveness of dietary interventions on clinical outcomes in Gp. A literature search of MEDLINE Ovid from 1 March 2008 to 1 October 2021 was conducted to identify randomized controlled trials, cohort studies, and cross-sectional studies that reported dietary interventions in Gp. From the initial search, 2789 studies resulted. These were assessed by 2 independent reviewers and selected based on the primary outcomes of interest: changes in symptom-specific patient-reported outcomes and changes in gastric emptying time. A third reviewer resolved any discrepancies. Six adult studies (185 subjects) met the inclusion criteria, whereas no pediatric study did. Five of the included studies were randomized controlled trials and one was an observational study. The systematic review suggested low-fat diets, small-particle diets, diets with isoflavones, and foods considered bland, starchy, sweet, and salty did not exacerbate Gp symptoms. Small-particle diets and diets with isoflavones were found to improve gastric emptying time in patients. Additionally, small-particle diets were shown to reduce anxiety in comparison to large-particle diets. Of the randomized controlled trials, 80% were low risk of bias and 20% were fair risk of bias. The observational study was considered fair quality. The data presented in this review suggest specific dietary interventions could potentially improve Gp symptoms and gastric emptying in adult patients, particularly low-fat and small-particle diets. For pediatric Gp, data are lacking. The limited data available highlights a critical gap in the literature.
Keywords: gastroparesis, diet, gastric emptying, gastrointestinal symptoms, diet modification, nutrition
Statement of Significance: This systematic review reports how certain dietary modifications affect symptoms and gastric emptying in patients with gastroparesis to help guide management. Our findings highlight gaps in the knowledge that should be addressed in future studies.
Introduction
Gastroparesis (Gp) is a delay in gastric emptying of fluids or solids in the absence of a mechanical obstruction (1). Both adult and pediatric patients with Gp typically develop symptoms, such as early satiety, anorexia, bloating, abdominal pain, nausea, and vomiting (1). Depending on the severity of symptoms, patients may develop significant weight loss and nutritional deficiencies (1). In addition, Gp can negatively affect other areas of one's life; symptoms can lead to significant financial and mental health burdens.
In adult patients with Gp, when patients reflect on their quality of life using a validated questionnaire, their scores are comparable to patients with chronic mental health disorders and depression (2). Gp patients have more difficulty managing daily activities and are more likely to reach out for medical care or become hospitalized (2). When evaluating personal financial burden, patients reported that Gp reduced their annual income by 28.5% and 11% of patients with Gp had to apply for disability (2). Adult Gp-related hospitalizations accounted for millions of dollars and over 900,000 hospital days between 1995 and 2004 (3). In children, several studies have indicated that the number of hospitalizations due to pediatric Gp increased by 130 per year between 2004 and 2013 (4). These findings reflect the significant burden Gp has on patients and the health care system and the need for more research to improve outcomes.
Although there are multiple potential treatment options for Gp (e.g., prokinetic agents, botulinum toxin injections into the pylorus, and gastric neurostimulation), none are uniformly effective and may have significant side effects (4). Currently, the recommended first-line treatment for Gp for pediatric and adult patients is dietary intervention (4). However, a systematic review of the efficacy of dietary interventions is lacking. Hence, our goal was to conduct such a review of the literature assessing the efficacy of dietary interventions carried out in patients with Gp.
Methods
Data sources and searches
We conducted this systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (5). We created a search engine using Medical Subject Heading (MeSH) terms including “diet,” “diet therapy,” “feeding behavior,” and “gastroparesis.” A medical librarian applied the initial search in Medline Ovid, Embase, Cochrane, and Web of Science databases from 1 March 2008 to 1 October 2021. Equivalent keywords and phrases also were searched for within-article titles, abstracts, and keywords. Articles reviewed met the consensus definition of Gp published by the American Neurogastroenterology and Motility Society (6).
Study selection
Gp was diagnosed by either gastric emptying scintigraphy, breath test, or based on clinical symptoms and an upper gastrointestinal endoscopy to exclude obstruction. Studies on dietary interventions in patients with Gp evaluating their effect on symptoms, quality of life, and rate of gastric emptying were included. The settings included hospitals, clinics, and nursing facilities. The types of studies included interventional studies (blinded or not) and observational studies.
Exclusion criteria included non–English-language studies, animal studies, abstracts, patients with a history of gastrointestinal comorbidities including inflammatory bowel disease, celiac disease, eosinophilic esophagitis, history of malignancy, history of ileal resection, and patients exclusively on total parenteral nutrition.
Using the Covidence™ web-based software, 2 independent reviewers (DE and TS) screened each reference collected by the librarian from the initial search (7). The initial screen reviewed the titles and abstracts only, and the article then was categorized as “eligible,” “maybe eligible,” or “not eligible.” Of those considered “eligible” by both independent reviewers, the full texts were examined further to determine final article selection. A third reviewer (RJS) resolved any discrepancies during any of the phases of the article selection.
Study outcomes, data extraction, data synthesis, quality assessment
Within Covidence™, the 2 independent reviewers extracted the outcomes of interest—namely, gastric emptying time, general health outcomes (including psychosocial health outcomes and quality of life), change in Gp symptoms, and number of hospitalizations/length of stay. Gastric emptying time was measured via gastric scintigraphy or breath test. For studies in adults, general health and quality-of-life outcomes were measured by the Hospital Anxiety and Depression Scale (HADS) and the 36-item Short-Form Health Survey (SF-36). The HADS questionnaire is an effective screening tool for assessing anxiety and depression in patients in nonpsychiatric clinical settings (8). The SF-36 has been tested in primary care settings as well as other patient populations and is a valid tool in examining general health and differentiating groups with health differences (9). While the HADS examines depression and anxiety, the SF-36 focuses on the perception of physical and mental health (10). Gp symptoms were measured by surveys such as the Patient Assessment of Upper Gastrointestinal Symptoms (PAGI-SYM) score or the more focused Gastroparesis Cardinal Symptom Index (GCSI) score. Both scoring systems have been validated in identifying and monitoring the dynamic changes in symptoms of adult patients with Gp as well as other functional gastrointestinal disorders (11, 12). Other surveys used included the Food Tolerance and Aversion Survey, which has not been validated in the literature but was developed by gastroenterologists, diabetic educators, and nutritionists to gain a better understanding of patient diet and diet-related symptoms (13).
Two independent reviewers (DE and TS) determined the risk of bias per the Risk-of-Bias 2 tool for randomized controlled trials and the Risk Of Bias In Non-Randomized Studies of Interventions tool for nonrandomized studies, which are both described in the Cochrane Handbook for Systematic Reviews of Interventions (14). The tools categorize the risk of bias as “low risk,” “fair risk,” and “high risk” regarding each specific outcome analyzed.
Results
Overview of trials
The initial electronic search yielded 2789 studies. Of these studies, only 6 adult studies (185 patients) met the eligibility criteria to be included in the systematic review (Table 1). No pediatric study met the eligibility criteria. Given the heterogeneity of the data (e.g., interventions and outcomes assessed) among the various studies, we opted not to perform a meta-analysis. The completed search is outlined in the PRISMA flow diagram (Figure 1) (15).
TABLE 1.
Summary of dietary intervention study designs1
| First author, year (ref); country; groups and age (y); number of participants | Sample size, n | Study design | Interventions/control | Duration |
|---|---|---|---|---|
| Olausson et al., 2008 (17); Sweden; diabetic Gp: 59 ± 9; 7 (3 M, 4 F); healthy: 59 ± 9; 7 (3 M, 4 F)All participants treated with cisapride (stopped 1 wk prior to study) | 14 | Crossover randomized trial | Diets provided:Large-particle diet: slices of roast beef, pasta boiled for 14 min, raw carrots, and canola oil impregnated with radiotracerSmall-particle diet: minced and baked beef, pasta and carrots boiled and mixed in a food processor with canola oil impregnated with radiotracerMeal composition: Both meals contained 100 g meat, 40 g pasta, 150 g carrots, 5 g oilMacronutrient content: Both meals were 375 kcal; contained 26 g protein, 13 g fat (25–30% of total energy intake), 38 g carbohydrate, 4.8 g fiberEnergy intake: Both meals contained 375 kcal | Single meal ingested 7–14 d apart |
| Setchell et al., 2013 (18); USA; diabetic Gp: 63 ± 2; 10 (5 M, 5 F) | 10 | Crossover randomized controlled trial | Diets provided:Intervention: 80 g soy germ pasta consumed once a day containing 31–33 mg of isoflavonesControl: 80 g conventional pasta consumed once a day | 8 wk per diet with a 4-wk washout in between |
| Olausson et al., 2014 (10); Sweden; intervention: 52 ± 12; 28; control: 55 ± 11; 28; total: 20 M, 36 F | 56 | Parallel randomized controlled trial | Dietary advice alone:Intervention diet: foods with small particle size or food items that could easily be processed into small particle size with a blender if needed (foods with a size that could be mashed with a fork)Control diet: allowed foods with large particle size and low glycemic index that adhered to recommendations for diabetic dietNutritional composition of the diets the same: fat content reduced to 25–30% of total energy and fiber content to 15 g/1000 kcal | 20 wk |
| Homko et al., 2015 (16); USA; idiopathic Gp: 44 ± 17; 9; diabetic Gp: 44 ± 17; 3; total: 2 M, 10 F | 12 | Crossover randomized trial | Diets provided:Interventions (260 kcal each):High-fat solid meal—2 medium eggs, 1 slice of toast, 4.2 g butter, 236.6 mL of water (13 g fat)Low-fat solid meal—118.3 mL Eggbeaters® (Bob Evans Farms©),1 slice toast, 6.3 g grape jelly, 236.6 mL skim milk (1.5 g fat)High-fat liquid meal—236.6 mL whole milk, 85.1 g ice cream (13g fat)Low-fat liquid meal—118.3 mL vanilla Boost®(Nestle Global©) and 236.6 mL juice nectar (2 g fat) | Each meal on separate days |
| Wytiaz et al., 2015 (13); USA; idiopathic Gp: 41 ± 17; 39; diabetic Gp: 41 ± 17; 6; total: 4 M, 41 F | 45 | Cross-sectional study | Habitual diet:Participants ate their usual diet | Not applicable; participants completed the questionnaires once |
| Mahjoub et al., 2018 (19); Iran; intervention: 53 ± 12; 24 (4 M, 20 F); control group: 49 ± 10; 24 (4 M, 20 F) | 48 | Parallel randomized controlled trial | Diets provided:Intervention: Pistacia atlantica kardum gum 2 g twice a dayControl: sugar-free chewing gum, twice a day | 1 mo |
Gp, gastroparesis; ref, reference.
FIGURE 1.
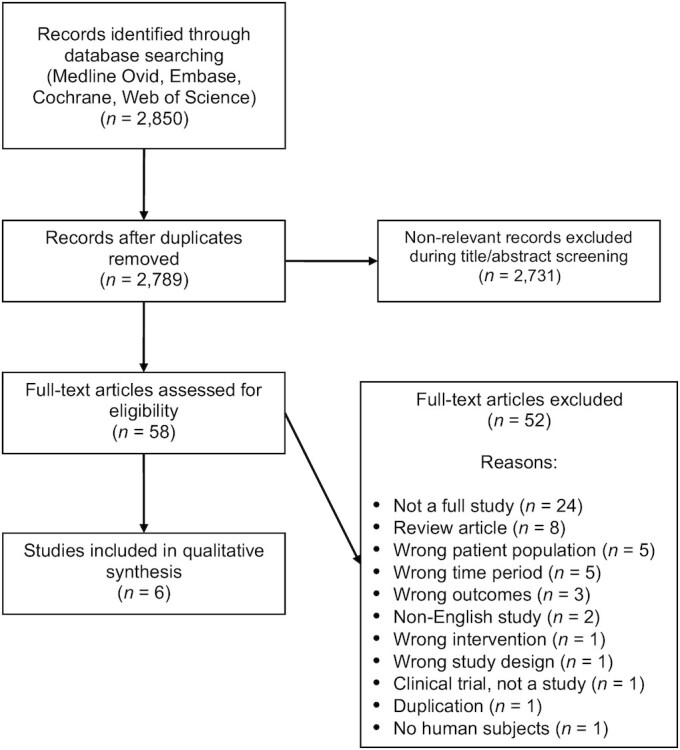
PRISMA flow diagram (15). PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Three of the included studies were conducted in the United States, 2 were conducted in Sweden, and 1 study was conducted in Iran. Most of the studies were funded by institutional research councils and country-specific diabetes associations. The patient demographic was largely women (140 patients) in comparison to men (45 patients) and the ages ranged from 18 to 70 y. The primary etiology of Gp was diabetes (133 patients; 72%), whereas the remaining patients had idiopathic Gp (45 patients; 24%). The remaining parti-cipants in the cohort were healthy controls (7 patients; 4%).
Fifty-seven study participants (13, 16) were diagnosed with Gp, defined as having gastric retention >60% at 2 h or >10% at 4 h during gastric scintigraphy (6). Seventy participants were diagnosed with Gp using gastric scintigraphy according to the published standards; however, in 2 studies, delayed gastric emptying was diagnosed if patients had retention >66% at 2 h (0–44% for men, 9–66% for women aged <40 y, 0–52% for women aged >40 y) (10, 17). Ten study participants were diagnosed with Gp using the 13C-octanoic breath tests, defining delayed gastric emptying as longer than 120 min (18). Forty-eight participants were diagnosed with Gp by symptoms and endoscopy ruling out mechanical obstruction (19).
Design
Five of the studies were randomized controlled trials (10, 16–19), of which 3 were crossover studies (16–18) and 2 were parallel-group designs (10, 19). One of the randomized controlled trials was designed as a double-blinded trial (18) and another study was designed as a triple-blinded trial (blinding the participants, investigators, and the statisticians) (19). The remaining 3 randomized controlled trials were open-label (unblinded) due to the type of intervention used (10, 16, 17). The 1 observational study consisted of a cross-sectional study (13).
Interventions
The median duration of the interventions within the studies was 22 d, with a range of 1 d to 140 d. Two of the studies focused on altering the food consistency as the study intervention, comparing large- with small-particle diets (17, 10). One study altered the food consistency as well as the fat content in each meal (16). Two studies introduced dietary products that contained natural ingredients found in legumes and nuts (18, 19). One used Pistacia atlantica, which is a species of pistachio tree that can be used for edible oils or as gum (19). The other study used soy germ–enriched pasta (18). The final included study did not alter the participants’ diets but requested that they recall the various foods they regularly ate over 2 wk (13).
Gp-specific patient-reported outcomes
Of the 6 studies (Table 2), 5 (171 patients) focused on assessing clinical symptoms associated with Gp based on the patient's current diet or the one being investigated. The studies either used the PAGI-SYM or the GCSI. In the Homko et al. study (16), 12 subjects (9 with diabetes-related Gp, 3 with idiopathic Gp) were given a high-fat solid meal, high-fat liquid meal, low-fat solid meal, and low-fat liquid meal in random order on 4 separate days. Patients were monitored for 4 h after meal ingestion while completing the PAGI-SYM every 15 min. The high-fat solid meal resulted in the most significant increase in total symptom score postprandially compared with the other meals tested. There were no significant differences in total symptom scores among the other meals, but the low-fat liquid meal resulted in the numerically smallest increase in total symptoms postprandially compared with the other meals (16).
TABLE 2.
Assessed outcomes in the selected studies
| First author, year (reference), country | Outcomes | Outcomes assessment |
|---|---|---|
| Olausson et al., 2008 (17), Sweden | • Gastric lag phase (the amount of time between the patient finishing the meal and the stomach emptying the food) and percent retention measured by scintigraphy• Changes in biochemical analysis (blood glucose and insulin concentrations) | • After meal ingestion, gastric lag phase and gastric retention measured every 2 min for up to 180 min from the beginning of the meal• Ingestion time corrected for individuals who took longer to complete the meal• Blood glucose and insulin collected at the time of ingestion and every 15 min up to 180 min |
| Setchell et al., 2013 (18), USA | • Gastric emptying time measured by 13C-octanoic acid breath test• GCSI scores | • Gastric emptying time measured at baseline, 8 wk, 12 wk, and 20 wk• GCSI measured at baseline, 8 wk, 12 wk, and 20 wk |
| Olausson et al., 2014 (10), Sweden | • Gastric emptying measured by scintigraphy• PAGI-SYM questionnaire to measure patient symptoms• HADS to evaluate anxiety and depression• Quality of life measured by the SF-36• Patients filled out a 4-d food diary at home after training by dietitians• Weight, height, and HbA1c | • Gastric retention at 2 h prior to study start and at final visit• PAGI-SYM, HADS, SF-36 completed at the initial visit and before the final visit (20 wk)• 4-d dietary record completed 3 d during the week and 1 d on the weekend• Body weight, height, and HbA1c measured at study start and at final visit |
| Homko et al., 2015 (16), USA | • PAGI-SYM questionnaire to measure patient symptoms• FFQ to evaluate nutritional intake• Exercise habits questionnaire (Paffenbarger survey) to measure activity level and number of calories expended per week• Body-composition measurement to determine BMI, percent lean mass and fat mass | • Full PAGI-SYM, FFQ, and Paffenbarger survey completed at baseline• Body composition at baseline• At subsequent visits weight measured prior to intervention• Before intervention, participants completed 2 abbreviated versions of the PAGI-SYM twice, 15 min apart• Abbreviated PAGI-SYM given immediately after completing test meal and completed every 15 min for 4 h |
| Wytiaz et al., 2015 (13), USA | • Food Tolerance and Aversion Survey• PAGI-SYM questionnaire to measure patient symptoms• Nutritional content and food quality reported on the Food Tolerance and Aversion Survey assessed using the USDA National Nutrient Database | • PAGI-SYM assessed at the time the Food Tolerance and Aversion Survey completed• Nutritional analysis done after the Food Tolerance and Aversion Survey completed |
| Mahjoub et al., (19), Iran | • GCSI• Biochemical analysis: complete blood count, fasting blood sugar, HbA1c• Systolic and diastolic blood pressure• BMI | • Measured at baseline and 4 wk• GCSI• Biochemical analysis• Blood pressure• BMI |
GCSI, Gastroparesis Cardinal Symptom Index; HADS, Hospital Anxiety Depression Scale; HbA1c, glycated hemoglobin; PAGI-SYM, Patient Assessment of Upper Gastrointestinal Symptoms; SF-36, 36-item Short-Form Survey.
Within the study by Homko et al. (16), when evaluating specific symptoms, the high-fat solid meal was associated with significantly greater symptoms of upper and lower abdominal pain, chest discomfort, stomach fullness, bloating, nausea, and the stomach becoming visibly larger at 4 h postprandially compared with the other 3 meals. The low-fat liquid meal was associated with a significantly lower nausea score than the high-fat liquid meal or the low-fat solid meal (16).
The study by Olausson et al. (10) recruited 56 patients with diabetic Gp and randomly assigned them to receive dietary advice to follow 1 of 3 diets: 2 that had a reduced particle size but differed in the magnitude of the reduction (food already processed into small particles vs. foods that can be easily broken down into small particles) and a control diabetic diet that contained normal-size particles. For all 3 diets, the recommended fat content was 25–30% of total energy and the recommended fiber content was 15 g/1000 kcal. The patients were monitored over 20 wk and asked to complete the PAGI-SYM at the baseline visit and again at the 20-wk mark. The subjects following the small-particle diet showed significant improvement in nausea/vomiting, fullness, bloating, lower abdominal pain, and heartburn/regurgitation scores. In contrast, subjects following the normal-size particle diet showed no improvement in symptoms (10).
The study by Mahjoub et al. (19) recruited 48 patients with diabetic Gp diagnosed clinically (using the GCSI questionnaire as clinical criteria) and by upper endoscopy to exclude mechanical obstruction; gastric emptying tests were not performed. Patients were randomly assigned to P. atlantica kurdica gum vs. placebo used twice daily for 1 mo. Patients given the P. atlantica kurdica gum had significantly greater improvement in total GCSI scores compared with those treated with the placebo (19). Although there is mention that some of the improvement was related to male sex, the data are not provided to clarify the statement further. Only 8 of the 48 patients were male (19). Given the shortcomings of the study (e.g., lack of scintigraphic Gp confirmation), interpretation of the results is hampered.
In a pilot study, Setchell et al. (18) and colleagues evaluated the effect of soy germ pasta enriched in isoflavones compared with conventional pasta in 10 patients with diabetic Gp in a crossover study spanning 20 wk. Based on total GCSI scores, there was a statistically significant improvement with the soy germ pasta compared with the conventional pasta (18).
In the study by Wytiaz et al. (13), the investigators sought to identify and characterize foods provoking or alleviating Gp symptoms assessed using the PAGI-SYM in 39 patients with idiopathic and 6 with diabetic Gp. They used a survey they developed for the study, which was not independently validated (13). Foods that provoked symptoms were those that were fatty, acidic, spicy, and roughage-based, whereas foods that alleviated symptoms were those that were bland, sweet, salty, and starchy (13).
Effect of dietary interventions on gastric emptying outcomes
Two of the selected studies (24 patients) assessed gastric emptying rates following dietary interventions (17, 18). The study published by Olausson et al. (17) assessed gastric emptying time on meals that differed only in their particle size in a crossover study of diabetic Gp patients (n = 7) and healthy patients (n = 7). In Gp patients, gastric emptying was significantly faster through 180 min after the small-particle meal compared with the large-particle meal (17). Emptying was faster for the healthy subjects at 120 min with the small-particle meal. Gastric retention tended to be greater for the Gp patients than the healthy subjects following the small-particle meal (P = 0.09) but was significantly worse than healthy controls following the large-particle meal (P = 0.018) (17).
The study by Setchell et al. (18) cited above also measured gastric emptying time following the meal of soy germ pasta enriched with isoflavones compared with conventional pasta. Half-emptying time was statistically faster with the isoflavone-enriched pasta than when consuming conventional standard pasta (18).
General health scoring, psychosocial health, and quality-of-life outcomes
Of the eligible Gp studies, the trial by Olausson and colleagues (10) examined the effect of the dietary interventions on anxiety and depression using the HADS and quality of life using the SF-36. They did not assess other aspects of general health. The patients following a small-particle diet had a reduction in anxiety symptoms compared with those following the large-particle diet but the change from baseline to treatment was not different between groups. Depression and quality of life remained unchanged in both groups and there was no difference between the small-particle-diet and the control-diet groups (10).
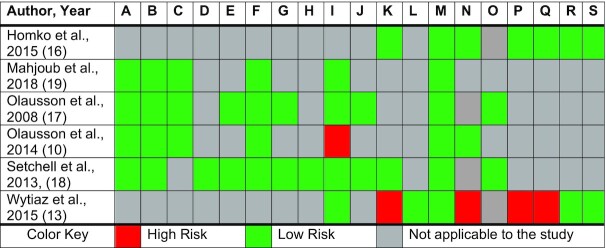
Risk of bias
Risk-of-bias assessments are summarized in Figure 2 (20). Of the 5 randomized controlled trials, 4 (80%) were of good quality with an overall low risk of bias and 1 study (20%) was designated as fair (10). This latter study was identified as fair because of the concern for attrition bias as many subjects from the control group dropped out of the study (11%) (10). Three of the randomized controlled trials did not blind the participants or study personnel because the interventions were changes in food consistency; however, the risk of bias was low because it did not affect the interventions given to the subjects as each subject received each of the food consistencies during the study, nor did it affect the guidance provided by the investigators conducting the study (10, 16, 17). The 1 cross-sectional study was of fair quality with an overall moderate risk of bias (13). Reasons that the Wytiaz et al. (13) cross-sectional study was graded as fair quality include concerns for incomplete outcome data, bias in measuring outcomes, confounding factors, and recall bias. Subjects who participated in this study were asked to complete a survey regarding their dietary habits over the last month; however, the survey was conducted either before or after an appointment with the gastroenterologist, with some previously being counseled on diets for Gp and others not being counseled.
FIGURE 2.
Graded risk of bias for the selected studies (20). A, Random sequence generation (selection bias). B, Allocation concealment (selection bias). C, Blinding of participants and personnel (performance bias). D, Blinding of participants and personnel in gastroparesis symptom scores. E, Blinding of participants and personnel in gastric emptying rate. F, Blinding of outcome assessment (detection bias). G, Blinding of outcome assessments in gastric emptying rate. H, Blinding of outcome assessments in gastroparesis symptom scores. I, Incomplete outcome data (attrition bias). J, Incomplete outcome data on gastric emptying rate. K, Incomplete outcome data on gastroparesis symptom scores. L, Incomplete outcome data on general health questionnaires. M, Selective reporting (reporting bias). N, Measurement outcome. O, Carryover bias. P, Bias in classification of intervention. Q, Confounding. R, Bias in selecting study participants. S, Bias due to deviation from intended intervention.
Discussion
We completed a systematic review to assess the literature investigating the efficacy of dietary interventions carried out in patients with Gp to help identify which interventions might be beneficial and potentially impact nutrition-management recommendations. Our systematic review identified 6 studies (185 patients) undergoing dietary interventions for Gp. All patients were adults as no pediatric studies met the inclusion criteria. Through this review, we found that dietary modifications may improve Gp-related symptoms, improve gastric emptying time, and possibly improve psychosocial health, as reflected by anxiety.
Based on our review, a small-particle diet appears to reduce Gp symptoms (10), likely, in part, because of a reduced need for gastric accommodation, which is impaired in some patients with Gp (21). In addition, a smaller-particle-size diet may facilitate more rapid gastric emptying (21). Although debate in the literature continues related to the correlation between gastric retention and Gp symptoms, a recent meta-analysis of studies that used optimal scintigraphic methods to assess gastric emptying found an inverse relation between gastric emptying rate and Gp symptoms; the faster the stomach is able to empty gastric content, the less severe the Gp symptoms (22).
Lowering the fat content of the diets also appeared to be associated with improvement in Gp symptoms. Dietary addition of lipids may slow the rate of gastric emptying through physiologic mechanisms such as the ileal brake mechanism, leading to early satiety (23). A negative feedback loop occurs when lipids reach the ileum, causing an increased production of the hormones glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) from the L cells of the ileum (23). These hormones decrease gastric acid, bile acid, and pancreatic enzyme secretion; decrease intestinal contractions; and increase pyloric sphincter pressure, resulting in slowing of gastric emptying (23). However, foods that are considered starchy, sweet, and bland also appeared beneficial (13). It is unclear why patients seemed to respond better to these foods, as, like lipids, carbohydrates also are associated with physiologic responses, including the ileal brake mechanism, increasing GLP-1 and PYY, and slowing of gastric emptying (24, 25). Although this association is not as strong as with lipids, one would anticipate complex carbohydrates to cause symptoms similar to lipids. More research in this area investigating other possibilities, such as different chemosensitivity responses to lipids compared with other macronutrients, is needed.
There was some preliminary evidence that food additives like P. atlantica and soy germ isoflavones may improve symptoms in patients with Gp (18, 19). Pistacia atlantica has been used in Iran and other Middle Eastern countries for several years as gum or oils ingested in meals to treat gastrointestinal symptoms such as stomach aches and dyspepsia (26). The mechanism by which it improves these symptoms is unknown; however, studies suggest that components within P. atlantica such as d-limonene may improve peristalsis, whereas components such as ɑ-pinene may have anti-inflammatory properties (27, 28). Whether improved peristalsis, addressing inflammation, or other mechanisms related to P. atlantica are playing a role remains to be determined.
Likewise, there are limited data evaluating the potential benefit of soy germ isoflavone in Gp. In mouse studies, soy isoflavones have been shown to have anti-inflammatory properties that affect monocytes, granulocytes, and lymphocytes (29). Additionally, flavonoids such as genistein, quercetin, myricetin, and epigallocatechin promote intestinal barrier function, enhancing the function of tight junctions (30). Gp pathophysiology has been associated with increased inflammation (both systemic and within gastric tissue). Although it remains to be proven, by reducing inflammation in gastric tissue, gastric emptying may improve, potentially contributing to symptom improvement (31, 32). More research with larger cohorts assessing the effects of soy isoflavones in humans with Gp is needed to assess the potential utility of this dietary additive and its mode of action.
There are no available published data that explains how soy isoflavones improve gastric emptying in humans, only speculation that soy isoflavones may alter genes affecting prostaglandin synthesis, which can affect motility (18). This speculation is in contrast to mouse models evaluating genistein, a soy isoflavone, which was found to block interstitial cells of Cajal pacemaker activity in the colon and other isoflavones having relaxant effects on gastrointestinal musculature (30). More research evaluating the role of soy isoflavones in gastric emptying is important to better understand its mechanism and whether it should be used as a treatment for Gp.
Additionally, the association between improvement in gastric emptying time and improvement in upper gastrointestinal symptoms remains controversial. Previous studies have been unable to find a significant correlation between improved gastrointestinal symptoms and gastric emptying rate (33, 34), whereas other studies have shown varying positive correlations between the severity of nausea and vomiting and loss of appetite and the degree of delay in gastric emptying (35, 36). As noted above, a recent meta-analysis suggests that discrepancy among studies lies in the use of less than optimal scintigraphic gastric emptying studies (22).
There are some limitations to this study. None of the studies assessed certain clinical outcomes on the treatments, such as readmissions, hospitalizations, and length of stay. A complicating factor to assessing such data is that hospitalizations may be complicated by comorbid conditions contributing to rates of admission, length of stay, and readmission. Bielefeldt (37) reported that length of stay was 1 d longer with Gp as a secondary diagnosis than with Gp as a primary diagnosis. Shahsavari et al. (38) found that readmission for Gp was associated with longer initial inpatient stay. Whether dietary interventions as identified in this systematic review may lead to decreases in hospitalizations or length of stay remains to be determined. Future studies should examine the impact of dietary interventions on these measures and the clinical course of Gp. Another issue is that there was a wide variety of interventions, making comparisons across studies difficult. As a result, combining data across studies for statistical analysis was not possible. That said, some common general principles, such as low-fat and/or small-particle diets improving Gp symptoms, were found across articles. Most studies were small, and this combined with incomplete data due to drop-out affected the rigor of the data and limited our ability to perform a meta-analysis. Some studies used unvalidated surveys, which was a limitation but still provided insight into assessing Gp symptom outcomes. Biases noted in certain studies reviewed were a limitation, as some studies were open-label, which may have led to bias in patient-reported outcomes, and other studies required subjects to recount their symptoms and diets within extended periods of time, which may have led to recall bias. Finally, not all studies documented Gp using the recommended 4-h scintigraphic study.
Unfortunately, despite our intent to include pediatric studies, none met the inclusion criteria. The lack of studies prospectively examining nutritional treatment in children with Gp has been noted in a recent review of Gp in children (39). The most common etiologies of Gp in children are idiopathic, medications, and postsurgical, while, for adults, the most common etiologies are diabetes and idiopathic (40). These differences in etiology between children and adults may affect patient response to treatment and highlight the importance of having data from pediatric studies. A study measuring symptoms in children during gastric emptying scintigraphy highlights the fact that children with Gp develop meal-related symptoms. Febo-Rodriguez et al. (41) showed that nausea after the scintigraphic meal correlated with the percentage retention at 4 h in children with Gp. Symptoms overall were worse in females (41). Preterm infants are known to have delayed gastric emptying and a propensity for feeding intolerance (42). Older studies have shown that the use of medium-chain triglycerides in place of long-chain triglycerides and glucose polymers substituted for glucose can accelerate gastric emptying in this population (43). In another study of preterm infants, a hydrolyzed protein formula (539 mOsm/L) emptied more slowly than an intact protein formula (211 mOsm/L); however, the difference in emptying rate related to the protein compared with osmolality could not be determined from this study (44). Whether these studies in preterm infants are relevant to older children with Gp needs to be determined.
Our review has several strengths. We followed the recommended strategy for carrying out systematic reviews (including the use of PRISMA and Covidence™) (7, 15). The review used the assistance of an expert librarian trained in the initial search methodology. The potentially included studies were reviewed by 2 investigators with a third used to resolve any conflicts. In addition, almost all of the studies had a low risk of bias.
In conclusion, this review highlights the effectiveness of dietary modifications on symptomatic and physiologic improvement in adult patients with Gp. The literature is limited but some data-based recommendations can be made, particularly related to small-particle-size and low-fat dietary interventions.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—DE, RJS, BPC, and RH: designed the research project; KL: developed the search strategy, conducted the literature search, and removed the duplicate studies; DE and TS: conducted the abstract and full text review of the selected articles and RJS acted as the third reviewer to assist with any disagreements; DE: completed the analysis of the selected studies; DE and TS: wrote the manuscript; RJS, BPC, and RH: assisted in editing and finalizing the manuscript; and all authors: read and approved the final manuscript.
Notes
Supported by U01 DK112194 (RJS) from the National Institutes of Health; the USDA/Agricultural Research Service (USDA/ARS) under cooperative agreement no. 58-3092-0-001; P30 DK56338, which funds the Texas Medical Center Digestive Disease Center; and the Daffy's Foundation.
Author disclosures: RJS is an associate editor of Advances in Nutrition but played no role in the Journal's evaluation of the manuscript. The authors report no conflicts of interest. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital. The contents do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
The protocol can be accessed on this site: https://www.crd.york.ac.uk/PROSPEROFILES/210536_PROTOCOL_20200921.pdf. An amendment was made to the protocol to extend time frame of the literature search.
DE and TS are joint first co-authors.
Abbreviations used: GCSI, Gastroparesis Cardinal Symptom Index; GLP-1, glucagon-like peptide 1; Gp, gastroparesis; HADS, Hospital Anxiety and Depression Scale; PAGI-SYM, Patient Assessment of Upper Gastrointestinal Symptoms; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PYY, peptide YY; SF-36, 36-item Short-Form Health Survey.
Contributor Information
Debra Eseonu, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA; USDA/ARS Children's Nutrition Research Center, Houston, TX, USA.
Tanya Su, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA.
Keya Lee, Texas Medical Center Library, Houston, TX, USA.
Bruno P Chumpitazi, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA; USDA/ARS Children's Nutrition Research Center, Houston, TX, USA.
Robert J Shulman, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA; USDA/ARS Children's Nutrition Research Center, Houston, TX, USA.
Ruben Hernaez, Section of Gastroenterology and Hepatology, Department of Medicine, Baylor College of Medicine and Michael E De Bakey Veterans Affairs Medical Center, Houston, TX, USA.
Data Availability
Data, analytic methods, and study materials will be made available to other researchers.
References
- 1. Islam S. Gastroparesis in children. Curr Opin Pediatr. 2015;27(3):377–82. [DOI] [PubMed] [Google Scholar]
- 2. Lacy BE, Crowell MD, Mathis C, Bauer D, Heinberg LJ. Gastroparesis: quality of life and health care utilization. J Clin Gastroenterol. 2018;52(1):20–4. [DOI] [PubMed] [Google Scholar]
- 3. Wang YR, Fisher RS, Parkman HP. Gastroparesis-related hospitalizations in the United States: trends, characteristics, and outcomes, 1995–2004. Am J Gastroenterol. 2008;103(2):313–22. [DOI] [PubMed] [Google Scholar]
- 4. Febo-Rodriguez L, Chumpitazi BP, Shulman RJ. Childhood gastroparesis is a unique entity in need of further investigation. Neurogastroenterol Motil. 2020;32(3):e13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abell TL, Camilleri M, Donohoe K, Hasler WL, Lin HC, Maurer AHet al. Consensus recommendations for gastric emptying scintigraphy: a joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. J Nucl Med Technol. 2008;36(1):44–54. [DOI] [PubMed] [Google Scholar]
- 7. Covidence systematic review software [database on the Internet]. Veritas Health Innovation. Available from: www.covidence.org. [Google Scholar]
- 8. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. [DOI] [PubMed] [Google Scholar]
- 9. Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood Tet al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olausson EA, Störsrud S, Grundin H, Isaksson M, Attvall S, Simrén M. A small particle size diet reduces upper gastrointestinal symptoms in patients with diabetic gastroparesis: a randomized controlled trial. Am J Gastroenterol. 2014;109(3):375–85. [DOI] [PubMed] [Google Scholar]
- 11. Revicki DA, Rentz AM, Dubois D, Kahrilas P, Stanghellini V, Talley NJet al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18(1):141–50. [DOI] [PubMed] [Google Scholar]
- 12. Rentz AM, Kahrilas P, Stanghellini V, Tack J, Talley NJ, de la Loge Cet al. Development and psychometric evaluation of the Patient Assessment of Upper Gastrointestinal Symptom Severity Index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13(10):1737–49. [DOI] [PubMed] [Google Scholar]
- 13. Wytiaz V, Homko C, Duffy F, Schey R, Parkman HP. Foods provoking and alleviating symptoms in gastroparesis: patient experiences. Dig Dis Sci. 2015;60(4):1052–8. [DOI] [PubMed] [Google Scholar]
- 14. Higgins JPT, Chander J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2. Cochrane; 2021. Available from [Internet]: www.www.training.cochrane.org/handbook. [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30. [PMC free article] [PubMed] [Google Scholar]
- 16. Homko CJ, Duffy F, Friedenberg FK, Boden G, Parkman HP. Effect of dietary fat and food consistency on gastroparesis symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2015;27(4):501–8. [DOI] [PubMed] [Google Scholar]
- 17. Olausson EA, Alpsten M, Larsson A, Mattsson H, Andersson H, Attvall S. Small particle size of a solid meal increases gastric emptying and late postprandial glycaemic response in diabetic subjects with gastroparesis. Diabetes Res Clin Pract. 2008;80(2):231–7. [DOI] [PubMed] [Google Scholar]
- 18. Setchell KD, Nardi E, Battezzati PM, Asciutti S, Castellani D, Perriello Get al. Novel soy germ pasta enriched in isoflavones ameliorates gastroparesis in type 2 diabetes: a pilot study. Diabetes Care. 2013;36(11):3495–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mahjoub F, Salari R, Yousefi M, Mohebbi M, Saki A, Rezayat KA. Effect of Pistacia atlantica kurdica gum on diabetic gastroparesis symptoms: a randomized, triple-blind placebo-controlled clinical trial. Electronic Physician. 2018;10(7):6997–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman ADet al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Camilleri M, Chedid V, Ford AC, Haruma K, Horowitz M, Jones KLet al. Gastroparesis. Nat Rev Dis Primers. 2018;4(1):41. [DOI] [PubMed] [Google Scholar]
- 22. Vijayvargiya P, Jameie-Oskooei S, Camilleri M, Chedid V, Erwin PJ, Murad MH. Association between delayed gastric emptying and upper gastrointestinal symptoms: a systematic review and meta-analysis. Gut. 2019;68(5):804–13. [DOI] [PubMed] [Google Scholar]
- 23. Shin HS, Ingram JR, McGill AT, Poppitt SD. Lipids, CHOs, proteins: can all macronutrients put a ‘brake’ on eating?. Physiol Behav. 2013;120:114–23. [DOI] [PubMed] [Google Scholar]
- 24. Jain NK, Boivin M, Zinsmeister AR, Brown ML, Malagelada JR, DiMagno EP. Effect of ileal perfusion of carbohydrates and amylase inhibitor on gastrointestinal hormones and emptying. Gastroenterology. 1989;96(2 Pt 1):377–87. [DOI] [PubMed] [Google Scholar]
- 25. Layer P, Holst JJ, Grandt D, Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig Dis Sci. 1995;40(5):1074–82. [DOI] [PubMed] [Google Scholar]
- 26. Mahjoub F, Rezayat Akhavan K, Yousefi M, Mohebbi M, Salari R. Pistacia atlantica desf. A review of its traditional uses, phytochemicals and pharmacology. J Med Life. 2018;11(3):180–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Minaiyan M, Karimi F, Ghannadi A. Anti-inflammatory effect of Pistacia atlantica subsp. kurdica volatie oil and gum on acetic acid-induced acute colitis in rats. Res J Pharmacognosy. 2015;2:1–12. [Google Scholar]
- 28. Sun J. D-Limonene: safety and clinical applications. Altern Med Rev. 2007;12(3):259–64. [PubMed] [Google Scholar]
- 29. Yu J, Bi X, Yu B, Chen D. Isoflavones: anti-inflammatory benefit and possible caveats. Nutrients. 2016;8(6):361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-Nakkash L, Kubinski A. Soy isoflavones and gastrointestinal health. Curr Nutr Rep. 2020;9(3):193–201. [DOI] [PubMed] [Google Scholar]
- 31. Grover MDS, Bernard C, Chikkamenahalli L, Yates K, Parischa P, Sarosiek Iet al. Proteomics in gastroparesis: unique and overlapping protein signatures in diabetic and idiopathic gastroparesis. Am J Physiol Gastrointest Liver Physiol. 2019;317(5):G716–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parischa PGM, Yates K, Abell T, Bernard C, Koch K, McCallum Ret al. Functional dyspepsia and gastroparesis in tertiary care are interchangeable syndromes with common clinical and pathologic features. Gastroenterology. 2021;160(6):2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janssen P, Harris MS, Jones M, Masaoka T, Farré R, Törnblom Het al. The relation between symptom improvement and gastric emptying in the treatment of diabetic and idiopathic gastroparesis. Am J Gastroenterol. 2013;108(9):1382–91. [DOI] [PubMed] [Google Scholar]
- 34. Kong MF, Horowitz M. Gastric emptying in diabetes mellitus: relationship to blood-glucose control. Clin Geriatr Med. 1999;15(2):321–38. [PubMed] [Google Scholar]
- 35. Parkman HP, Yates K, Hasler WL, Nguyen L, Pasricha PJ, Snape WJet al. Clinical features of idiopathic gastroparesis vary with sex, body mass, symptom onset, delay in gastric emptying, and gastroparesis severity. Gastroenterology. 2011;140(1):101–115.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cassilly DW, Wang YR, Friedenberg FK, Nelson DB, Maurer AH, Parkman HP. Symptoms of gastroparesis: use of the gastroparesis cardinal symptom index in symptomatic patients referred for gastric emptying scintigraphy. Digestion. 2008;78(2-3):144–51. [DOI] [PubMed] [Google Scholar]
- 37. Bielefeldt K. Factors influencing admission and outcomes in gastroparesis. Neurogastroenterol Motil. 2013;25(5):389–98, e294. [DOI] [PubMed] [Google Scholar]
- 38. Shahsavari D, Zhao H, Ehrlich AC, Zoll BE, Lu X, Malik Zet al. Factors associated with hospital admissions and readmissions in patients with gastroparesis using the nationwide readmission database. J Clin Gastroenterol. 2020;54(9):801–5. [DOI] [PubMed] [Google Scholar]
- 39. Kovacic K, Elfar W, Rosen JM, Yacob D, Raynor J, Mostamand Set al. Update on pediatric gastroparesis: a review of the published literature and recommendations for future research. Neurogastroenterol Motil. 2020;32(3):e13780. [DOI] [PubMed] [Google Scholar]
- 40. Lu P, Lorenzo C. Gastroparesis in the pediatric patient: children are not little adults. Gastrointest Disord. 2020;2(2):86–95. [Google Scholar]
- 41. Febo-Rodriguez L, Chumpitazi BP, Musaad S, Sher AC, Shulman RJ. Meal-induced symptoms in children with dyspepsia-relationships to sex and the presence of gastroparesis. J Pediatr. 2021;231:117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fanaro S. Feeding intolerance in the preterm infant. Early Hum Dev. 2013;89:S13–20. [DOI] [PubMed] [Google Scholar]
- 43. Siegel M, Krantz B, Lebenthal E. Effect of fat and carbohydrate composition on the gastric emptying of isocaloric feedings in premature infants. Gastroenterology. 1985;89(4):785–90. [DOI] [PubMed] [Google Scholar]
- 44. Pascale JA, Mims LC, Greenberg MG, Alexander JB. Gastric response in low birth weight infants fed various formulas. Neonatology. 1978;34(3–4):150–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data, analytic methods, and study materials will be made available to other researchers.