ABSTRACT
Recently developed ketone (monoester or salt) supplements acutely elevate blood β-hydroxybutyrate (BHB) exogenously without prolonged periods of fasting or carbohydrate restriction. Previous (small-scale) studies have found a blood glucose-lowering effect of exogenous ketones. This study aimed to systematically review available evidence and conduct meta-analyses of studies reporting on exogenous ketones and blood glucose. We searched 6 electronic databases on 13 December 2021 for randomized and nonrandomized trials of any length that reported on the use of exogenous ketones. We calculated raw mean differences (MDs) in blood BHB and glucose in 2 main analyses: 1) after compared with before acute ingestion of exogenous ketones and 2) following acute ingestion of exogenous ketones compared with a comparator supplement. We pooled effect sizes using random-effects models and performed prespecified subgroup analyses to examine the effect of potential explanatory factors, including study population, exercise, blood BHB, and supplement type, dosing, and timing. Risk of bias was examined using Cochrane's risk-of-bias tools. Studies that could not be meta-analyzed were summarized narratively. Forty-three trials including 586 participants are summarized in this review. Following ingestion, exogenous ketones increased blood BHB (MD = 1.73 mM; 95% CI: 1.26, 2.21 mM; P < 0.001) and decreased mean blood glucose (MD = –0.54 mM; 95% CI: –0.68, –0.40 mM; P < 0.001). Similarly, when compared with placebo, blood BHB increased (MD = 1.98 mM; 95% CI: 1.52, 2.45 mM; P < 0.001) and blood glucose decreased (MD = –0.47 mM; 95% CI: –0.57, –0.36 mM; P < 0.001). Across both analyses, significantly greater effects were seen with ketone monoesters compared with salts (P < 0.001). The available evidence indicates that acute ingestion of exogenous ketones leads to increased blood BHB and decreased blood glucose. Limited evidence on prolonged ketone supplementation was found.
Keywords: β-hydroxybutyrate, blood glucose, exercise, glycemia, heart failure, ketosis, meta-analysis, prediabetes, systematic review, type 2 diabetes
Statement of Significance: This is the first systematic evaluation of the effects of exogenous ketones on blood glucose, showing 1) elevated blood β-hydroxybutyrate and 2) decreased blood glucose following ingestion of oral ketone (monoester and salt) supplements.
Introduction
Low-carbohydrate and very-low-carbohydrate, high-fat ketogenic diets have regained popularity and are increasingly used for weight loss and metabolic health (1). Recently, ketogenic diets have been purported to improve glycemic control, particularly in individuals with type 2 diabetes (T2D) (2). Periods of carbohydrate restriction during such dietary interventions result in ketone bodies [acetoacetate, acetone, β-hydroxybutyrate (BHB)] being produced by the liver. These ketone bodies can also serve as signaling molecules (3). BHB, the most abundant ketone body found in circulation, is known to modulate various aspects of metabolism, including glucose metabolism (3).
Recently, exogenous BHB supplements in the form of orally administered ketone salts or ketone monoesters have been developed that allow for an acute increase in blood BHB without prolonged periods of fasting or adoption of a ketogenic diet (4). Emerging research suggesting an acute glucose-lowering effect following ingestion of exogenous BHB-containing supplements has resulted in interest around the therapeutic potential in individuals with impaired glucose metabolism, including individuals with prediabetes or type 2 diabetes (T2D) (5). However, despite numerous reports of lowered blood glucose following exogenous ketone ingestion in the literature, substantial heterogeneity in various aspects of study design (e.g., acute ingestion vs. prolonged supplementation, metabolic state of participants, type of supplement) makes interpretation of the findings challenging. A systematic appraisal of the literature on the effects of exogenous BHB supplementation in the form of ketone salts and ketone monoesters on measures of glycemia is therefore of considerable interest. Our purpose was to amalgamate the available evidence by performing a systematic review and meta-analysis on the impact of exogenous BHB supplementation on blood glucose in order to offer insight into potential application in hyperglycemia-related diseases.
Methods
Search strategy and selection criteria
We performed a systematic review and meta-analysis in accordance with recognized reporting guidelines (6). The review protocol was preregistered on PROSPERO (7). We included single-arm, parallel, and crossover randomized and nonrandomized clinical trials of any duration that reported on the use of an exogenous ketone (monoester or salt) supplement and assessed blood glucose. Eligible trials included those providing the supplement acutely (i.e., a single experimental session in a controlled setting) or for a prolonged period of time (i.e., for >1 d under free-living conditions). For consistent wording throughout the manuscript, the term “intervention” is used for the study arm providing the exogenous ketone supplement and, conversely, the term “comparator” is used for the study arm providing a control or placebo comparator supplement.
We prespecified a priori that only trials using BHB-containing supplements (in the form of ketone salts or ketone monoesters) would be eligible for inclusion in this review. This inclusion criterion was agreed upon in order to distinguish between exogenous ketones and other supplements that may act to raise blood BHB (e.g., medium-chain triglyceride [MCT] oil, ketogenic nutrition drinks). In particular, we use the term “ketone monoester” throughout the manuscript to refer to the (R)-3-hydroxybutyl (R)-3-hydroxybutyrate (i.e., butanediol/R-BHB monoester) ketone monoester.
If applicable, the comparator arm had to be a supplement not containing carbohydrates of any form (e.g., water with bitter agents and flavoring, long-chain fatty acids) to be included in the analyses. If no comparator arm was present or the comparator contained carbohydrates (which would independently raise blood glucose), the study was treated as a single-arm study in our analyses. The ketone supplement had to be delivered in the form of a ketone monoester or a ketone salt without additional active ingredients (e.g., caffeine, medium-chain triglycerides) or carbohydrates (e.g., mixed in a milkshake) unless the amount of carbohydrates was matched (i.e., a difference of ≤5 g carbohydrates) between intervention and comparator arms (e.g., as part of a sports drink during exercise, during an oral-glucose-tolerance test). Participants of eligible trials were adults (≥18 y without an upper age limit) of any sex and any intervention population (i.e., both healthy and individuals with medical conditions).
A search was performed to identify eligible trials examining the effect of exogenous ketone supplementation on blood glucose. Embase OVID, Web of Science, Cochrane Central Register of Controlled Trials (CENTRAL), International Clinical Trials Registry (ISRCTN), ClinicalTrials.gov, and Cochrane Database of Systematic Reviews (CDSR) were searched. Identified reviews and included studies were manually hand-searched for relevant references. The searches were limited to studies published in English. Searches were re-run by the first author prior to the final analysis on 13 December 2021. The search strategy is shown in Supplemental Table 1.
Reviewers identified eligible studies by screening titles and abstracts and, when required, full texts of articles. Two reviewers screened titles and abstracts and independently applied eligibility criteria, and selected studies for inclusion. Articles were rejected on initial screen when the authors could clearly determine that the article did not include ingestion of exogenous ketones, the study was not conducted in humans, the trial involved individuals less than 18 y old, or the exogenous ketone supplement contained carbohydrates that were not matched between the intervention and comparator arms. Reviewers used the Covidence platform (8) for screening and were blinded to each other's decisions throughout the process. In case of disagreement, the 2 reviewers jointly resolved the case by discussion.
Data collection
Two reviewers independently assessed risk of bias. We used the Cochrane Risk-of-Bias (RoB 2) tool to assess the randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results separately for parallel and crossover randomized trials (9). We used the Cochrane ROBINS-I tool to assess risk of bias in nonrandomized trials (10).
Data were extracted by 1 reviewer and confirmed by a second reviewer. Study design [country of investigation; number of participants; duration and details of intervention, including details of nutritional and/or exercise intervention(s), if applicable] and type and dosing strategy of ketone supplement and comparator, if applicable, were extracted alongside participant demographics (age, sex, body weight, maximal oxygen consumption (VO2max), BMI, glycated hemoglobin (HbA1c), fasting glucose, participant population). Data on blood glucose and potential effect modifiers, including blood BHB and insulin concentrations, were extracted for all available time points. Time points were defined relative to supplement ingestion (i.e., time of supplement consumption was considered “minute 0”). Data on adherence, dropout rates, and adverse events were collected as available.
Based on availability, we collected end data with a measure of variance (i.e., SD, SEM, or CI). In case an SD of 0 was reported (applicable only for blood BHB values of the comparator arm), we imputed a value of 0.01 because meta-analytic models do not assign any weight to effect sizes with nonpositive SDs. If values were reported as below the detection limit (similarly applicable only for 1 study reporting blood BHB values of the comparator arm), we imputed the value as the limit of detection divided by the square root of 2 (11).
If data were missing and it was not possible to calculate them from the reported data, study investigators were contacted for unreported data. If multiple attempts to contact study investigators failed (or for studies found while updating the search before final data analysis) and data were presented in graphical form in the manuscript, required values were extracted using online graph reading software (12). If data were still missing, studies were excluded.
Data analyses
All data analyses were performed in RStudio (version 3.6.1; R Foundation for Statistical Computing) (13, 14) with the packages meta (15), metafor (16), dmetar (17), esc (18), and netmeta (19). Analyses were performed according to our preregistered protocol (7) with 1 deviation: for better (clinical) interpretability of effect estimates, we used raw mean differences [MDs; as opposed to the originally specified standardized mean differences (SMDs)] as our effect measure of choice. This decision was made after collecting the full data set and prior to performing the presented analyses, as it was possible to obtain the underlying data in the same unit across all studies, except for insulin, for which analyses are presented using SMDs estimated using Hedges’ g. To calculate SEs of within-group (i.e., after compared with before consumption of the exogenous ketone supplement) MDs, we estimated the correlation between repeated measures at 0.8 based on observations from our own previous research and available individual participant data provided upon request by study authors. Additionally, we performed sensitivity analyses using correlation values of 0.5 and 0.9 to explore the robustness of the pooled estimate. Using a generic inverse-variance pooling method, we pooled effect sizes with a random-effects model using the Sidik-Jonkman τ2 estimator for between-study variance and the Hartung-Knapp adjustment (20). We calculated 95% CIs and prediction intervals, and assessed between-study heterogeneity with the I2 statistic (21) and Cochran's Q test (22). Statistical significance was set at P < 0.05. For exploratory purposes, we identified studies as statistical outliers when their CI did not overlap with the CI of the pooled effect, and assessed the effect of individual studies with an influence analysis using the leave-one-out method. Additionally, we assessed the effect-size heterogeneity pattern via Graphic Display of Study Heterogeneity (GOSH) plots (23). We explored potential publication bias visually via contour-enhanced funnel plots (24) as well as quantitatively via Egger's test of the intercept (25).
Because some studies contributed multiple effect sizes (e.g., by measuring multiple outcomes or having multiple eligible comparisons), we explored whether fitting a 3-level meta-analytic model would better represent the variability in our data to account for this dependency between effect sizes. Nesting individual effect sizes yielded very similar pooled effect estimates and did not provide a better fit compared with the 2-level model with level 3 heterogeneity constrained to zero. We therefore opted to perform all main and additional exploratory analyses using a conventional 2-level meta-analytic model.
If a study included multiple outcomes of interest, we included these as separate comparisons in the model and divided the number of participants in the groups evenly between the 2 study comparisons to avoid a unit-of-analysis error [(26) Chapter 23: “Including Variants on Randomized Trials”]. Similarly, if a study included a shared comparator arm (e.g., comparing a high dose of exogenous ketones with a low dose of exogenous ketones with placebo), we included all relevant comparisons (e.g., comparing a high dose of exogenous ketones with placebo and comparing a low dose of exogenous ketones with placebo) and divided the number of participants in the comparator group evenly between the 2 study comparisons. For crossover (paired) data, end-of-treatment values for each crossover period were analyzed as independent samples. If a study included multiple subsequent doses of exogenous ketone ingestion (e.g., at baseline and 30 min into an exercise protocol), we derived our outcome values based on all time points following the first dose, unless otherwise indicated.
We conducted 2 overall analyses on the effects of acute exogenous ketone consumption: a within-group analysis presented in the first part of the manuscript comparing (fasted) blood glucose after exogenous ketone ingestion with blood glucose before consumption of the ketone supplement but prior to the onset of exercise or ingestion of nutrients (e.g., initiation of an oral-glucose-tolerance test) and a between-condition analysis presented in the second part of the manuscript comparing blood glucose after ingestion of exogenous ketones with blood glucose after consumption of an appropriate (i.e., matched for carbohydrate content, if applicable) comparator (including during exercise and ingestion of nutrients via, e.g., an oral-glucose-tolerance test, if matched appropriately between arms). The combination of both within-group and between-condition analyses was prespecified and decided on in order to increase the robustness of presented findings by allowing the maximal number of studies (and therefore amount of data) across a variety of study designs to be included in this review, thereby presenting a comprehensive overview of the available literature.
Because of the multiple different study designs that assessed blood glucose at various time points and for various durations following supplement consumption, we averaged blood glucose values across the entire duration that blood glucose was assessed after consumption of the supplement for these 2 main analyses by pooling means and SDs according to Cochrane guidelines for combining groups [(26) Chapter 6: “Choosing Effect Measures and Computing Estimates of Effect”], as prespecified in our protocol. Outcome values for blood BHB and insulin were derived analogously. Because only aggregate (i.e., mean and corresponding SD) data were available, this pooling of means and SDs across time assumes independence of repeated measures and thereby potentially overestimates the pooled variance. As a second approach to amalgamating the data, we furthermore conducted meta-analyses on the time point with the greatest increase in BHB and the greatest decrease in blood glucose (i.e., the largest deviation compared with the baseline time point for within-group analyses or compared with the comparator for between-group analyses as opposed to pooled means across the postsupplementation period). This analysis was not preregistered but performed following suggestion in the peer review process. Both within-group and between-condition effect estimates for blood BHB and blood glucose with both approaches (i.e., pooled means across postsupplementation period and time point with largest deviation) are presented in Table 2 as an overview of the conducted analyses. Based on limited data available, studies of prolonged ketone supplementation are included as a separate narrative section in the review.
For the main analyses, we performed prespecified subgroup analyses investigating differences in effect estimates with regard to the type of supplement (ketone monoester or ketone salts), the population characteristics (i.e., participants at a healthy body weight, with overweight or obesity, with prediabetes, with T2D, with heart failure), the outcome (i.e., fasted or nonfasted blood glucose following acute supplement consumption, glucose during a mixed meal, or oral-glucose-tolerance test following acute supplement consumption), and whether exercise was part of the study design. We performed prespecified meta-regression analysis with a random-effects model to examine the effect of potential continuous explanatory factors (e.g., ketone dosage). The robustness of our evaluated associations was further determined by a permutation test in which the observed test statistic was compared with the sampling distribution obtained through rearranging the dataset.
We separately explored the effects of ketone ingestion on BHB and insulin as potential moderating variables of interest. Furthermore, we performed 1 exploratory analysis that was not preregistered but deemed of interest upon examining the type of data available: a time-based analysis of the found effect of exogenous ketone ingestion on blood glucose (i.e., a subgroup analysis based on the duration that blood glucose was assessed for and sensitivity analyses evaluating the pooled effect estimate at specific time points after supplement consumption).
Results
Search results
Figure 1 shows the flow diagram for the search and inclusion of studies: We assessed 16,094 titles and abstracts, which de-duplicated to 13,158 abstracts. Of these, 73 abstracts were considered potentially relevant for this review, and 71 were retrieved as full text. Of these, 34 full texts (not including trial registrations or conference abstracts) were excluded (Supplemental Table 2). Thirty-seven articles from the search, along with 6 articles identified via other methods, met the inclusion criteria and were ultimately considered relevant for this review.
FIGURE 1.
PRISMA flow chart detailing the literature search and study selection process. Six electronic databases were searched for eligible studies. A total of 13,158 records were screened, of which 71 were collected in full text. Of these, 34 were excluded and 37 studies were considered eligible along with 6 additional studies identified through citation searching and correspondence with authors. A total of 43 studies were included in this review. CDSR, Cochrane Database of Systematic Reviews; Central, Cochrane Central Register of Controlled Trials; ISRCTN, International Standard Randomized Controlled Trial Numbers; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; WoS, Web of Science.
Included studies
We included 43 trials comprising 36 randomized and 22 nonrandomized comparisons (because a single trial can contribute multiple study comparisons if, e.g., different dosages or types of ketone supplements are used) in this review, which resulted in a total of 58 study comparisons (Table 1). Of the randomized comparisons, 33 were conducted acutely (i.e., acute ingestion of exogenous ketones during a single experimental session in a controlled setting) and 3 chronically (i.e., supplementation for >1 d) compared to 20 and 2 of the nonrandomized comparisons, respectively. The publication date of included trials ranged from 2016 to 2021, with a total of 586 participants. Fifty-one comparisons were conducted with participants at a healthy weight, 3 with participants with overweight or obesity, 2 with participants with prediabetes, 1 with participants with T2D, and 1 with participants with heart failure. Forty-one comparisons used a ketone monoester and 17 used a ketone salt. Twenty-eight comparisons included an exercise intervention, 3 used an oral-glucose-tolerance test, and 1 a mixed-meal tolerance test. Supplemental Tables 3 and 4 provide further details on study designs and outcomes; Supplemental Table 5 provides an overview of potential conflicts of interest in conducted studies. Of the 58 comparisons, 39 were eligible for a within-group analysis (i.e., reported blood glucose values after compared with before consumption of exogenous ketones without concomitant exercise or nutrient ingestion), 33 comparisons were eligible for a between-condition analysis (i.e., reported blood glucose values after exogenous ketone supplement consumption compared with an eligible comparator), and 18 comparisons were eligible for both analyses.
TABLE 1.
Overview of the comparisons included in this review1
| Study | Within/between2 | Participants, n (% male) | Sample population | Ketone supplement | Dosing of supplement | Duration of intervention | Study type3 | Outcome of interest4 | Time points included5 | Exercise? |
|---|---|---|---|---|---|---|---|---|---|---|
| Bharmal 20216 (27) | W, B | 18 (67) | Prediabetes | Monoester | 395 mg⋅(kg LBW)–1 | Acute | X | 0, 30, 60, 90, 120, 150 | No | |
| Clark 20216,7 (28) | W, B | 9 (100) | Healthy | Salt | 300 mg⋅(kg BW)–1 | Acute | X | BL, PRE, steady state, time trial | Yes | |
| Cox 20168,9 (29) | W, B | 10 (100) | Healthy | Monoester | 573 mg⋅(kg BW)–1 | Acute | X | –10, 0, 2, 5, 10, 25, 35, 45, 50, 60 | Yes | |
| Crabtree 202110 (30) | — | 25 (48) | Overweight/obesity | Salt | 2 × 11.8 g daily | 6 wk | II | Fasting glucose | — | No |
| Dearlove 20198 (31) | W, B | 12 (75) | Healthy | Monoester | 330 mg⋅(kg BW)–1 | Acute | X | –60, 0, 3, 15, 27, 39 | Yes | |
| Dearlove 2020 (I)8,11 (32) | W, B | 6 (83) | Healthy | Monoester | 252 mg⋅(kg BW)–1 | Acute | X | Pre-drink, Post-drink, 25%, 50%, 75% | Yes | |
| Dearlove 2020 (II)8,11 (32) | W, B | 6 (83) | Healthy | Monoester | 752 mg⋅(kg BW)–1 | Acute | X | Pre-drink, Post-drink, 25%, 50%, 75% | Yes | |
| Dearlove 2021 (33)8,12 | W | 6 (100) | Healthy | Monoester | 573 mg⋅(kg BW) –1 | Acute | S | Fasting glucose | –60, –30, 0 | Yes |
| Evans 2018a6,11 (34) | W, B | 19 (63) | Healthy | Salt | 468 mg⋅(kg BW)–1 | Acute | X | –60, –30, 0, 8, 16, 24, 32, 40, 48 | Yes | |
| Evans 2018b6 (35) | B | 11 (100) | Healthy | Monoester | 750 mg⋅(kg BW)–1 | Acute | X | Mean nonfasted glucose | Start, block 1, block 2, block 3, block 4, block 5, end RTE | Yes |
| Evans 20196 (36) | B | 8 (88) | Healthy | Monoester | 573 mg⋅(kg BW)–1 | Acute | X | Mean nonfasted glucose | 0, 20, 40, 60, end of time trial | Yes |
| Fischer 20188 (37) | W | 5 (60) | Healthy | Salt | 308 mg⋅(kg BW)–1 | Acute | S | 0, 30, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 | No | |
| Greaves 20206 (38) | W, B | 19 (53) | Healthy | Monoester | 482 mg⋅(kg BW) -1 | Acute | X | Glucose during MMTT | –15, 0, 15, 30, 60, 90, 120 | No |
| Holdsworth 20178,13 (39) | W, B | 12 (100) | Healthy | Monoester | 615 mg⋅(kg BW) -1 | Acute | X | –30, 0 | Yes | |
| James 2018 (40) | B | 10 (30) | Healthy | Salt | 11.7 g | Acute | X | Baseline, before exercise, after exercise | Yes | |
| McCarthy 2020 (41) | B | 12 (53) | Healthy | Monoester | 600 mg⋅(kg BW) -1 | Acute | X | 0, 35 | Yes | |
| Monzo 2020 (I)6 (42) | W | 6 (100) | Healthy | Monoester | 9.6 g | Acute | S | Fasting, after KE | No | |
| Monzo 2020 (II)6 (42) | W | 11 (83) | Heart failure | Monoester | 9.6 g | Acute | S | Fasting, after KE | No | |
| Moore 2021 (I)14 (43) | W | 13 (46) | Healthy | Salt | 7 g | Acute | S | 0, 60 | No | |
| Moore 2021 (II)14 (43) | W | 13 (46) | Healthy | Salt | 7 g | Acute | S | 0, 60 | No | |
| Mose 2021 (44)8,11,15 | B | 8 (100) | Healthy | Monoester | 50 g | Acute | X | Fasting glucose | 240, 270, 300, 330, 360, 420 | No |
| Mujica-Parodi 2020 (I)16 (45) | W | 8 (63) | Healthy | Monoester | 395 mg⋅(kg BW)–1 | Acute | S | Pre, post 10 min, post 80 min | No | |
| Mujica-Parodi 2020 (II)16 (45) | W | 30 (40) | Healthy | Monoester | 395 mg⋅(kg BW)–1 | Acute | S | Pre, post 10 min, post 80 min | No | |
| Myette-Côté 20186 (46) | W, B | 20 (50) | Healthy | Monoester | 482 mg⋅(kg BW)–1 | Acute | X | Glucose during OGTT | –30, 0, 15, 30, 60, 90, 120 | No |
| Myette-Côté 20196 (47) | W, B | 15 (33) | Overweight/obesity | Monoester | 482 mg⋅(kg BW)–1 | Acute | X | Glucose during OGTT | –30, 0, 15, 30, 60, 90, 120 | No |
| Nakagata 20216 (48) | B | 9 (56) | Prediabetes17 | Monoester | 482 mg⋅(kg BW)–1 | Acute | X | Glucose during OGTT | 15, 30, 60, 90, 120, 180 | No |
| O'Connor 2018 (I)8 (49) | W, B | 10 (20) | Healthy | Salt | 11.7 g | Acute | X | 0, 15, 30, 60, 120, 180, 240 | No | |
| O'Connor 2018 (II)8 (49) | W, B | 10 (20) | Healthy | Salt | 5.85 g | Acute | X | 0, 15, 30, 60, 120, 180, 240 | No | |
| O'Malley 20176,18 (50) | W, B | 10 (100) | Healthy | Salt | 300 mg⋅(kg BW)–1 | Acute | X | Fasting, post-supplement, post-exercise, post-time trial | Yes | |
| Poffé 20196 (51) | — | 18 (100) | Healthy | Monoester | 1–3 ⋅ 25 g daily | 3 wk | II | Nonfasted glucose | — | Yes |
| Poffé 20206,11 (52) | (W), B | 12 (100) | Healthy | Monoester | 65 g | Acute | X | Mean nonfasted glucose | –60, –30, 0, 30, 60, 90, 120, 150, 180, 200 | Yes |
| Poffé 2021a (I)6,11,19 (53) | (W), B | 9 (100) | Healthy | Monoester | 65 g | Acute | X | Mean nonfasted glucose | –60, –30, 0, 30, 60, 90, 120, 150, 180, 200 | Yes |
| Poffé 2021a (II)6,11,19 (53) | B | 9 (100) | Healthy | Monoester | 65 g | Acute | X | Mean nonfasted glucose | –60, –30, 0, 30, 60, 90, 120, 150, 180, 200 | Yes |
| Poffé 2021b (I)6,11,19 (54) | B | 12 (100) | Healthy | Monoester | 50 g | Acute | X | Mean nonfasted glucose | 0, 15, 30 | Yes |
| Poffé 2021b (II)6,11,19 (54) | B | 12 (100) | Healthy | Monoester | 50 g | Acute | X | Mean nonfasted glucose | 0, 15, 30 | Yes |
| Poffé 2021c (I)6,11,19 (55) | B | 14 (100) | Healthy | Monoester | 75 g | Acute | X | Mean nonfasted glucose | 60, 90, 120, 150, 180, 200 | Yes |
| Poffé 2021c (II)6,11,19 (55) | B | 14 (100) | Healthy | Monoester | 75 g | Acute | X | Mean nonfasted glucose | 60, 90, 120, 150, 180, 200 | Yes |
| Rittig 20206,20 (56) | W | 8 (100) | Healthy | Salt | 36.5 g | Acute | S | 0, 15, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165, 180 | No | |
| Rodger8 2017 (57) | B | 12 (100) | Healthy | Salt | 23.4 g | Acute | X | 90, post 4PT | Yes | |
| Soto-Mota 201921 (58) | (W), — | 24 (50) | Healthy | Monoester | 3 ⋅ 26.8 g daily | 28 d | S | Mean nonfasted glucose | 30 | No |
| Soto-Mota 2021a (59) | — | 21 (67) | Type 2 Diabetes | Monoester | 3 ⋅ 25 g daily | 4 wk | S | Mean daily glucose | Without ketone monoester, with ketone monoester | No |
| Soto-Mota 2021b (60)22 | W | 10 (50) | Healthy | Monoester | 25 g | Acute | S | Fasting glucose | 0, 15, 30, 45, 60, 75, 90, 120 | No |
| Stubbs 2017a6 (61) | W | 15 (67) | Healthy | Monoester | 380 mg⋅(kg BW)–1 | Acute | S | 0, 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h | No | |
| Stubbs 2017b (I)6,23 (62) | W | 15 (60) | Healthy | Monoester | 141 mg⋅(kg BW)–1 | Acute | S | 0, 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h | No | |
| Stubbs 2017b (II)6,23 (62) | W | 15 (60) | Healthy | Monoester | 282 mg⋅(kg BW)–1 | Acute | S | 0, 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h | No | |
| Stubbs 2017b (III)6,23 (62) | W | 15 (60) | Healthy | Salt | 141 mg⋅(kg BW)–1 | Acute | S | 0, 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h | No | |
| Stubbs 2017b (IV)6,23 (62) | W | 15 (60) | Healthy | Salt | 282 mg⋅(kg BW)–1 | Acute | S | 0, 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h | No | |
| Stubbs 2017b (V)6,23 (62) | W | 16 (63) | Healthy | Monoester | 395 mg⋅(kg BW)–1 | Acute | S | 0, 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h | No | |
| Stubbs 2017b (VI)6,23 (62) | (W) | 16 (63) | Healthy | Monoester | 395 mg⋅(kg BW)–1 | Acute | S | Mean nonfasted glucose | 0, 0.5 h, 1 h, 1.5 h, 2 h, 3 h, 4 h | No |
| Svart 20206 (63) | B | 6 (100) | Healthy | Salt | 36.5 g | Acute | X | Glucose during clamp | 150, 180, 210, 240, 250, 260, 270 | No |
| Vandoorne 20176,11 (64) | B | 8 (100) | Healthy | Monoester | 1500 mg⋅(kg BW)–1 | Acute | X | Mean nonfasted glucose | 30, 60, 90, 120, 150, 180, 210, 240, 270, 300 | Yes |
| Vestergaard 20216,24 (65) | W, B | 10 (100) | Healthy | Monoester | 714 mg⋅(kg BW)–1 | Acute | X | 0, 30, 60, 120, 180, 240, 300 | No | |
| Waldman 2018 (66) | B | 15 (100) | Healthy | Salt | 11.38 g | Acute | X | Pre, post | Yes | |
| Waldman 20206,11 (67) | W, B | 16 (100) | Healthy | Salt | 468 mg⋅(kg BW)–1 | Acute | X | –60, –15, 10, 30 | Yes | |
| Walsh 2020 (I)6 (68) | — | 14 (29) | Overweight/obesity | Monoester | 3 ⋅ 12 g daily | 14 d | X | Fasting glucose, mean daily glucose | — | No |
| Whitfield 2021 (I)6,25 (69) | (W) | 3 (83) | Healthy | Monoester | 573 mg⋅(kg BW)–1 | Acute | S | Mean nonfasted glucose | Rest, post-KE | Yes |
| Whitfield 2021 (II)6,25 (69) | (W) | 3 (83) | Healthy | Monoester | 573 mg⋅(kg BW)–1 | Acute | S | Mean nonfasted glucose | Rest, post-KE | Yes |
| Whitfield 2021 (II)6,25 (69) | (W) | 3 (83) | Healthy | Monoester | 573 mg⋅(kg BW)–1 | Acute | S | Mean nonfasted glucose | –30, –5 | Yes |
BHB, β-hydroxybutyrate; BIC, bicarbonate; BL, baseline; BLG, beta-lactoglobulin; BW, body weight; CAT, catabolic condition; CHO, carbohydrate; CON, control; II, parallel study design;KD, ketogenic diet; KET, ketone supplementation; KS, ketone salt; LBW, lean body weight; MMTT, mixed-meal tolerance test; Monoester, (R)-3-hydroxybutyl (R)-3-hydroxybutyrate; OGTT, oral-glucose-tolerance test; PRE, end of the pre-exercise condition 30 minutes after supplement ingestion; RTE, shuttle run to exhaustion; S, single-arm study design; X, crossover study design; 4PT, 4-minutes maximal cycling performance test.
Refers to which main analysis the respective study is included in: B, between-condition analysis; W, within-group analysis; (W), within-group measurements in nonfasted state; therefore, included only in exploratory sensitivity analyses; —, narrative summary.
Study type refers to how the study is treated for this review (i.e., if the original study followed a crossover design but had an ineligible comparator arm, it was treated as a single-arm study for the purpose of this review and its analyses).
If nothing is indicated, outcome of interest is “mean fasted glucose” (i.e., average glucose following supplement consumption in a fasted state); “mean nonfasted glucose” refers to average glucose following supplement consumption in a nonfasted state or alongside nutrient consumption.
All time points following supplement consumption included in between-condition analyses, all time points before and following supplement consumption before onset of exercise or ingestion of nutrients included in within-group analyses (minutes, unless otherwise stated).
Data provided by authors upon request.
CON vs KET.
Data extracted via graph reader online (12).
Study 2/5.
KD + KS vs. KD + PL.
Ketone supplement was provided in multiple boluses.
KE + CHO.
Ketone vs. control drink before onset of hyperglycemic clamp.
Moore 2021 (I), racemic salts; (II), natural salts; average (mean) of both measurement devices used.
CAT-BLG vs. CAT-BLG + BHB.
Mujica-Parodi (I), MRS time course study; (II), fMRI bolus study.
Individuals had impaired glucose tolerance; treated as prediabetes for the purpose of this review.
Insulin data was provided by Neudorf et al. (70).
Poffé 2021a and 2021b and 2021c (I), KE vs. CON; (II), BIC vs. BIC + KE.
Oral condition.
Random glucose measurements before and 30 min following a 25-mL drink of ketone monoester averaged across weeks 1–4 included in (W) analysis.
KE; data extracted from preprint version of the paper (https://doi.org/10.21203/rs.3.rs-355173/v2; accessed 13 December 2021); only mean values were reported, therefore SD imputed as the average SD across studies using a similar design (no exercise, single-dose, monoester, healthy population).
Stubbs 2017b (I–IV), Study 1/3; (V), Study 2/3 in fasted state; (VI), Study 2/3 in fed state.
KE vs PBO.
Total sample size of n = 9 (in LCHF intervention arm) divided by 3 outcomes: Whitfield 2021 (I), Baseline + KE; (II), Adaptation + KE; (III), LCHF Adaptation + KE in 10,000-m race; 83% of total participants were male.
Risk of bias in included studies
The risk of bias of included studies is presented in Supplemental Tables 6 and 7. Six studies were identified as having an overall low risk of bias, while there was some concern for risk of bias for the remaining 37 studies. No study was judged to be at high risk of bias.
Effect of acute exogenous ketone ingestion on blood glucose
Within-group effects of acute exogenous ketone ingestion
Effects of acute exogenous ketone ingestion on BHB
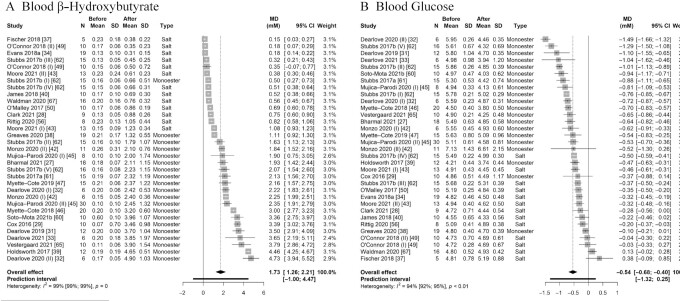
We first explored the effect of exogenous ketone ingestion on blood BHB. Pooling effect sizes from all 33 comparisons reporting fasted BHB after compared with before consumption of exogenous ketones (i.e., a within-group comparison) yielded a pooled effect estimate of MD = 1.73 mM (95% CI: 1.26, 2.21 mM; P < 0.001) with very high heterogeneity (Cochran's Q = 3349.44, I2 = 99.0%, P < 0.001) (Figure 2A). Separately exploring the pooled effect estimate for ketone monoesters (MD = 2.57 mM; 95% CI: 2.06, 3.08 mM; P < 0.001) and ketone salts (MD = 0.50 mM; 95% CI: 0.33, 0.67 mM; P < 0.001) showed a significantly greater effect estimate for ketone monoesters (χ12 = 64.82, P < 0.001), indicating greater BHB after consumption of a ketone monoester compared with a ketone salt (Table 2). Pooled effect sizes across time points with the greatest deviation from baseline (i.e., greatest increase in BHB) are shown in Table 2 and Supplemental Figure 1A. Pooled effect estimates for individual time points (i.e., 15, 30, 60, 90,120, 180, and 240 min following supplement consumption) are presented in Supplemental Table 8 (separately for ketone monoesters and ketone salts); in case of studies that included repeated ingestion of exogenous ketones (i.e., multiple doses within the same study protocol), we included only data up until the second dose to ensure the validity of the time-based analyses. Statistical heterogeneity remained high at all time points and in both subgroups. Pooled effect estimates over time are visually summarized in Figure 3A.
FIGURE 2.
Forest plot of comparisons quantifying the effect of exogenous ketone ingestion on average fasted (A) blood β-hydroxybutyrate and (B) blood glucose in a within-group analysis (i.e., after compared with before consumption of exogenous ketones). Effect sizes (raw MDs, mM) were pooled using a generic inverse-variance pooling method with a random-effects model using the Sidik-Jonkmann τ2 estimator for between-study variance and the Hartung-Knapp adjustment. Significant effects of (A) MD = 1.73 mM (95% CI: 1.26, 2.21 mM; P < 0.001) and (B) MD = –0.54 mM (95% CI: –0.68, –0.40 mM; P < 0.001) were found, indicating that ingestion of exogenous ketones acutely (A) raises blood β-hydroxybutyrate and (B) lowers blood glucose when compared with baseline values. Each square visually represents the weight of the study centered around the study effect size with the corresponding horizontal line showing the study CI. MD, mean difference; monoester, (R)-3-hydroxybutyl (R)-3-hydroxybutyrate ketone monoester.
TABLE 2.
Overview of the main analyses included in this review1
| Within-group | Between-condition | |||||
|---|---|---|---|---|---|---|
| Analysis of… | Mean difference (95% CI) | P | I 2 (95% CI) | Mean difference (95% CI) | P | I 2 (95% CI) |
| Mean across time | ||||||
| Blood BHB | ||||||
| • Combined | 1.73 mM (1.26, 2.21 mM) | < 0.001 | 99% (99%, 99%) | 1.98 mM (1.52, 2.45 mM) | < 0.001 | 98% (98%, 98%) |
| • Ketone salt | 0.50 mM (0.33, 0.67 mM) | < 0.001 | 96% (95%, 97%) | 0.48 mM (0.15, 0.81 mM) | < 0.01 | 88% (81%, 93%) |
| • Ketone monoester | 2.57 mM (2.06, 3.08 mM) | < 0.001 | 98% (98%, 98%) | 2.65 mM (2.23, 3.06 mM) | < 0.001 | 95% (93%, 96%) |
| Blood glucose | ||||||
| • Combined | –0.54 mM (–0.68, –0.40 mM) | < 0.001 | 94% (92%, 95%) | –0.47 mM (–0.57, –0.36 mM) | < 0.001 | 0% (0%, 35%) |
| • Ketone salt | –0.23 mM (–0.37, –0.09 mM) | < 0.01 | 83% (73%, 90%) | –0.24 mM (–0.37, –0.10 mM) | < 0.01 | 0% (0%, 20%) |
| • Ketone monoester | –0.76 mM (–0.92, –0.61 mM) | < 0.001 | 93% (91%, 95%) | –0.61 mM (–0.72, –0.51 mM) | < 0.001 | 0% (0%, 9%) |
| Largest difference | ||||||
| Blood BHB | ||||||
| • Combined | 2.05 mM (1.52, 2.58 mM) | < 0.001 | 99% (99%, 99%) | 2.54 mM (2.01, 3.07 mM) | < 0.001 | 99% (99%, 99%) |
| • Ketone salt | 0.63 mM (0.46, 0.81 mM) | < 0.001 | 97% (96%, 98%) | 0.73 mM (0.23, 1.23 mM) | < 0.01 | 99% (99%, 99%) |
| • Ketone monoester | 2.97 mM (2.42, 3.52 mM) | < 0.001 | 98% (98%, 99%) | 3.33 mM (2.92, 3.73 mM) | < 0.001 | 97% (97%, 98%) |
| Blood glucose | ||||||
| • Combined | –0.69 mM (–0.86, –0.52 mM) | < 0.001 | 97% (96%, 97%) | –0.70 mM (–0.83, –0.58 mM) | < 0.001 | 22% (0%, 50%) |
| • Ketone salt | –0.35 mM (–0.51, –0.20 mM) | < 0.001 | 93% (89%, 95%) | –0.41 mM (–0.55, –0.27 mM) | < 0.001 | 0% (0%, 38%) |
| • Ketone monoester | –0.93 mM (–1.14, –0.73 mM) | < 0.001 | 97% (96%, 97%) | –0.85 mM (–0.97, –0.73 mM) | < 0.001 | 0% (0%, 37%) |
BHB, β-hydroxybutyrate.
FIGURE 3.
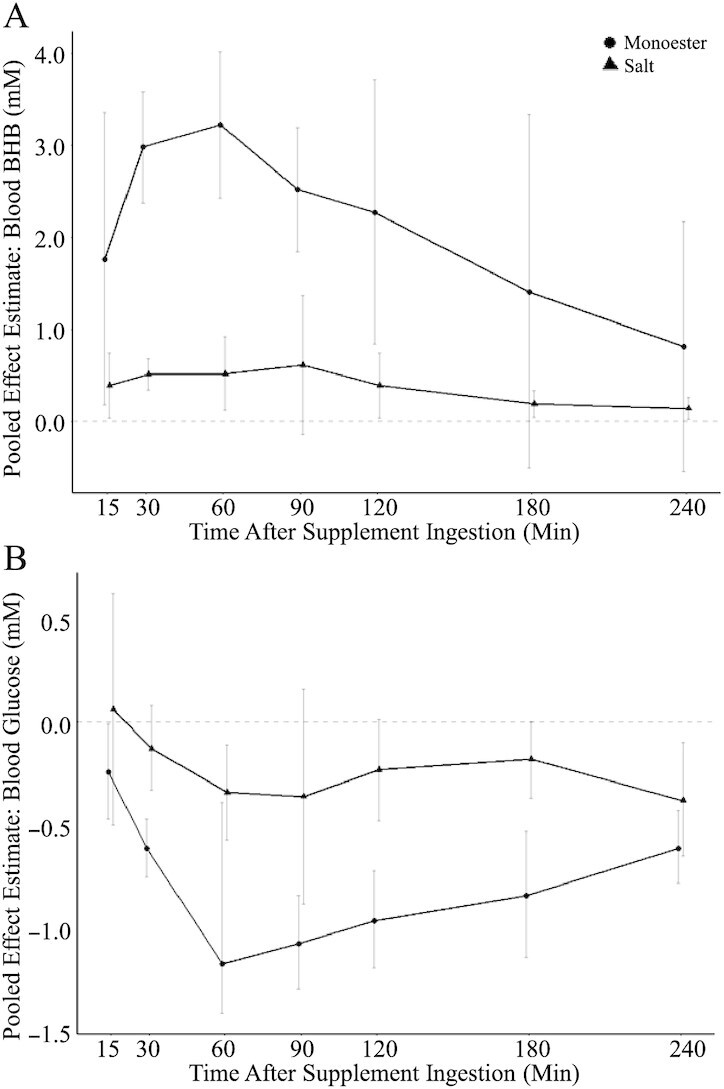
Pooled effect estimates and 95% CIs of comparisons reporting on (A) fasted blood BHB and (B) fasted blood glucose after compared with before consumption of an exogenous ketone (monoester, circle; salt, triangle) supplement. Effect sizes (raw mean differences, mM) were pooled separately at each time point using a generic inverse-variance pooling method with a random-effects model using the Sidik-Jonkmann τ2 estimator for between-study variance and the Hartung-Knapp adjustment. See Supplemental Table 8 for more details. BHB, β-hydroxybutyrate; monoester, (R)-3-hydroxybutyl (R)-3-hydroxybutyrate ketone monoester.
Effects of acute exogenous ketone ingestion on blood glucose
We pooled effect sizes from all 33 comparisons reporting on fasted blood glucose after compared with before exogenous ketone ingestion, which resulted in a pooled effect estimate of MD = –0.54 mM (95% CI: –0.68, –0.40 mM; P < 0.001), suggesting an acute decrease in blood glucose following ingestion of the ketone supplement (Figure 2B). Statistical heterogeneity was high (Cochran's Q = 528.98, I2 = 94.0%) and statistically significant (P < 0.001). For exploratory purposes, we therefore removed the identified 10 statistical outliers and overly influential studies; the pooled effect estimate of the remaining comparisons changed minimally to MD = –0.55 mM (95% CI: –0.65, –0.46 mM; P < 0.001) with reduced (Cochran's Q = 101.88, I2 = 78.4%) but still statistically significant heterogeneity (P < 0.001). Sensitivity analyses using different values for the estimated repeated-measures correlation did not affect the pooled effect estimate (Supplemental Figure 2).
An additional 7 comparisons were available that were conducted in a nonfasted (i.e., postprandial) state. Pooling effect sizes from all (i.e., fasted and nonfasted) comparisons resulted in a slightly greater pooled effect estimate of MD = –0.60 mM (95% CI: –0.75, –0.46 mM; P < 0.001). However, because the greater expected decrease in blood glucose in a postprandial (as compared with a fasted) state confounds the effect of exogenous ketones on blood glucose, all following (subgroup and exploratory) within-group analyses were conducted with all comparisons that assessed glucose in a fasted state only.
Similar to the analysis on BHB above, pooled effect estimates at the individual time points after supplement consumption are presented in Supplemental Table 8 (separately for ketone monoesters and ketone salts) and visually summarized in Figure 3B. Pooled effect estimates of the largest deviation from baseline (i.e., greatest decrease in glucose) are presented in Table 2 and Supplemental Figure 1B.
To assess potential publication bias, we visually inspected a contour-enhanced funnel plot (Supplemental Figure 3), which reflected the high degree of heterogeneity but did not show evidence of any asymmetry that would be suggestive of an existing small-study bias. This was corroborated by Egger's regression test (β = 0.15; 95% CI: –3.26, 3.56; t = 0.085, P = 0.93).
Moderating factors of the acute effect of exogenous ketones on blood glucose
To further investigate sources of the observed heterogeneity, we performed prespecified subgroup analyses evaluating the effect estimate of studies using ketone monoesters (MD = –0.76 mM; 95% CI: –0.92, –0.61 mM; P < 0.001) compared with those using ketone salts (MD = –0.23 mM; 95% CI: –0.37, –0.09 mM; P < 0.01), which showed a significantly stronger glucose-lowering effect of ketone monoesters (χ12 = 30.11, P < 0.001) (Table 2). However, heterogeneity was high across both subgroups, suggesting the existence of other moderating factors. Neither population characteristics (Supplemental Figure 4A) nor the duration of time that blood glucose was assessed for (and therefore averaged across) following consumption of the ketone supplement (Supplemental Figure 4B) resulted in statistically significant differences between subgroups or explained the high degree of heterogeneity.
Interactive effects of supplement dosage, blood glucose, blood BHB, and blood insulin
To investigate whether supplement dosage (calculated as the amount of “active ingredient” in each supplement per kilogram of body weight) would moderate the found effect of exogenous ketones on blood glucose after supplement ingestion (compared with before consumption of the supplement), we performed a meta-regression (separately for studies using ketone monoesters and ketone salts) that included all comparisons which provided the ketone supplement at a relative dose (i.e., relative to participants’ body weight). BHB dosage did not significantly moderate the effect on average glucose pooled across the postsupplementation period, irrespective of whether a ketone monoester (P = 0.96) or a ketone salt (P = 0.40) was used. These findings remained unchanged when only comparisons with healthy individuals were included, when regressing at 1 specific time point (i.e., 30 or 60 min after supplement consumption), and when comparisons using ketone monoesters and ketone salts were evaluated together. Similarly, no difference was found between a low [i.e., below the mean supplement dose used across comparisons of 431 mg · (kg body weight)–1)] and a high dose of BHB in a categorical subgroup analysis, regardless of whether ketone monoesters and ketone salts were evaluated jointly or separately; and no difference was found when evaluating the effect of supplement dosage on the largest decrease in glucose (as opposed to mean glucose over time).
To supplement these findings, we further explored whether supplement dosage moderated the effect of exogenous ketone ingestion on average blood BHB across time and peak BHB. The regression indicated that monoester supplement dosage was a statistically significant moderator of the effect on average blood BHB (P < 0.001) as well as peak BHB (P < 0.01), suggesting higher average (0.57 mM; 95% CI: 0.31, 0.82 mM) and peak (0.53 mM; 95% CI: 0.21, 0.84 mM) BHB values with higher supplement dosages. In contrast, ketone salt dosage showed no statistically significant relation to BHB values. Because only aggregate study-level data were available for most of the included studies, we were not able to further evaluate whether achieved blood BHB concentrations may have mediated the effect of exogenous ketone ingestion on blood glucose; however, the difference in average blood BHB (pooled across the postsupplementation period compared with before consumption of the exogenous ketone supplement) was a significant moderator of the effect on average glucose (P < 0.001) (Supplemental Figure 5A), as was peak BHB (P < 0.001) (Supplemental Figure 5B), suggesting higher blood BHB concentrations to be associated with lower blood glucose. Similar, yet slightly weaker, relations were found when evaluating the moderating effect of average (Supplemental Figure 5C) and peak (Supplemental Figure 5D) BHB on the largest decrease in glucose (as opposed to average glucose across time).
Fasting glucose significantly moderated the effect of exogenous ketones on average blood glucose (P = 0.02) (Supplemental Figure 6A) and greatest decrease in blood glucose (P = 0.03) (Supplemental Figure 6B), but not on average (P = 0.99; Supplemental Figure 6C) or peak (P = 0.69; Supplemental Figure 6D) blood BHB on a study level.
Similarly, it was not possible, using the aggregate data available, to evaluate whether exogenous ketones may have exerted the observed glucose-lowering effect through increasing insulin; however, pooling the effect sizes of 14 comparisons (conducted in a fasted state) reporting on insulin after compared with before ingestion of exogenous ketones yielded an overall effect estimate of SMD = 0.40 (95% CI: 0.18, 0.63; P < 0.01), suggesting an increase in insulin following exogenous ketone consumption (Supplemental Figure 7). Heterogeneity was high and statistically significant (Cochran's Q = 47.73, I2 = 72.8%, P < 0.001), and no statistically significant difference was found when studies using ketone monoesters (SMD = 0.32; 95% CI: 0.09, 0.54; P = 0.01) and ketone salts (SMD = 0.65; 95% CI: –0.20, 1.50; P = 0.09) were evaluated separately (χ12 = 1.36, P = 0.24).
Between-condition effects of acute exogenous ketone ingestion on blood glucose
Effects of acute exogenous ketone ingestion on BHB
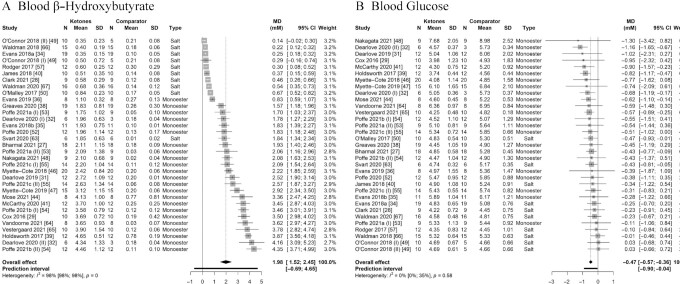
We first pooled effect sizes of all 33 comparisons evaluating exogenous ketone ingestion on average BHB compared with a comparator arm (i.e., a between-condition analysis), which yielded an overall effect estimate of MD = 1.98 mM (95% CI: 1.52, 2.45 mM; P < 0.001) with very high heterogeneity (Cochran's Q = 1760.87, I2 = 98.2%, P < 0.001) (Figure 4A). Subgroup analysis evaluating the effects of comparisons using a ketone monoester (MD = 2.65 mM; 95% CI: 2.23, 3.06 mM; P < 0.001) or a ketone salt (MD = 0.48 mM; 95% CI: 0.15, 0.81 mM; P < 0.01) separately revealed a significantly greater effect estimate for comparisons using a ketone monoester (χ12 = 76.18, P < 0.001) (Table 2). Pooled effect estimates of the effect of exogenous ketones on peak BHB (as opposed to averaged BHB across the postsupplementation period) are presented in Table 2 and Supplemental Figure 8A. Because of the variety of different study designs (including different exercise regimens, concomitant ingestion of nutrients, and/or repeated consumption of multiple doses of exogenous ketones), no further time-based analysis was conducted.
FIGURE 4.
Forest plot of comparisons quantifying the effect of exogenous ketone ingestion on (A) blood β-hydroxybutyrate and (B) blood glucose in a between-condition analysis (i.e., compared with consumption of a comparator supplement). Effect sizes (raw MDs, mM) were pooled using a generic inverse-variance pooling method with a random-effects model using the Sidik-Jonkmann τ2 estimator for between-study variance and the Hartung-Knapp adjustment. Significant effects of (A) MD = 1.98 mM (95% CI: 1.52, 2.45 mM; P < 0.001) and (B) MD = –0.47 mM (95% CI: –0.57, –0.36 mM; P < 0.001) were found, indicating that ingestion of exogenous ketones acutely (A) raises blood β-hydroxybutyrate and (B) lowers blood glucose when compared with ingestion of a comparator supplement. Each square visually represents the weight of the study centered around the study effect size with the corresponding horizontal line showing the study CI. MD, mean difference; monoester, (R)-3-hydroxybutyl (R)-3-hydroxybutyrate ketone monoester.
Effects of acute exogenous ketone ingestion on blood glucose
The pooled effect estimate of all 33 comparisons reporting on the effect of exogenous ketone ingestion on average blood glucose across the postsupplementation period compared with a comparator arm was MD = –0.47 mM (95% CI: –0.57, –0.36 mM; P < 0.001) with low heterogeneity (Cochran's Q = 29.74, I2 = 0.0%) that was not statistically significant (P = 0.58), and a prediction interval from MD = −0.90 mM to –0.04 mM (Figure 4B). These findings suggest a strong and consistent glucose-lowering effect of exogenous ketones. Given the low amount of overall heterogeneity, we did not search for statistical outliers or overly influential studies, and all following (subgroup and exploratory) analyses were conducted with all studies included. To assess publication bias, we visually inspected a contour-enhanced funnel plot (Supplemental Figure 9), which revealed no strong evidence for a small-study effect. Similarly, Egger's regression test (β = 0.04; 95% CI: –0.8, 0.88) was not statistically significant (t = 0.094, P = 0.93). Pooled effect estimates of the effect of ketone ingestion on the largest difference in blood glucose between the ketone and comparator group (as opposed to glucose averaged across time) are presented in Table 2 and Supplemental Figure 8B.
Moderating factors of the found acute effect of exogenous ketones on blood glucose
Despite the overall low heterogeneity in the main between-condition analysis of acute exogenous ketone ingestion on blood glucose, we performed a number of subgroup analyses to further evaluate the found effect in the context of different outcomes (i.e., mean fasted glucose following supplement consumption, mean nonfasted glucose following supplement consumption, mean glucose during an oral-glucose-tolerance test, mean glucose during a mixed-meal tolerance test, mean glucose during infusion of glucose tracers) (Supplemental Figure 10A), different study populations (i.e., individuals at healthy body weight, with overweight or obesity, or with prediabetes) (Supplemental Figure 10B), differing durations that blood glucose was assessed following consumption of the supplement (Supplemental Figure 10C), and whether or not exercise was performed as part of the study protocol (Supplemental Figure 10D). No statistically significant differences were found between any of the subgroups. We also explored whether the type of supplement (i.e., ketone monoester or salt) moderated the found effect, which showed a significantly stronger glucose-lowering effect in studies using ketone monoesters (MD = –0.61 mM; 95% CI: –0.72, –0.51 mM; P < 0.001) compared with ketone salts (MD = –0.24 mM; 95% CI: –0.37, –0.10 mM; P < 0.01) (χ12 = 23.39, P < 0.001) (Table 2).
Interactive effects of supplement dosage, blood glucose, blood BHB
To evaluate the effects of supplement dosage (calculated as the “active ingredient” in each supplement per kilogram of body weight) on the found effect of exogenous ketone ingestion on blood glucose, we performed a meta-regression with supplement dosage included as a continuous moderator of the effect on blood glucose averaged across the postsupplementation period (similar to the regression performed above for the within-group effect). Supplement dosage did not moderate the effect, irrespective of whether only comparisons using a ketone monoester (P = 0.86) or a ketone salt (P = 0.27) were included in the model, whether all comparisons were evaluated jointly, or whether the largest decrease in glucose (as opposed to average glucose) was considered. There was a statistically significant difference between subgroups comparing low [i.e., less than the average dose of 562 mg⋅(kg body weight)–1] (MD = –0.33 mM; 95% CI: –0.48, –0.19 mM; P < 0.001) to high doses (MD = –0.63 mM; 95% CI: –0.81, –0.45 mM; P < 0.001) of supplement when evaluated across all comparisons (χ12 = 7.70, P < 0.01), suggesting a greater decrease in blood glucose in studies using higher doses. This was supported by a significant difference between low and high doses when evaluating the effect of supplement dosage on largest difference in glucose (as opposed to glucose averaged across time) (P < 0.01). In contrast, no difference was found between subgroups when ketone monoesters and ketone salts were examined separately for either average glucose or largest difference in glucose.
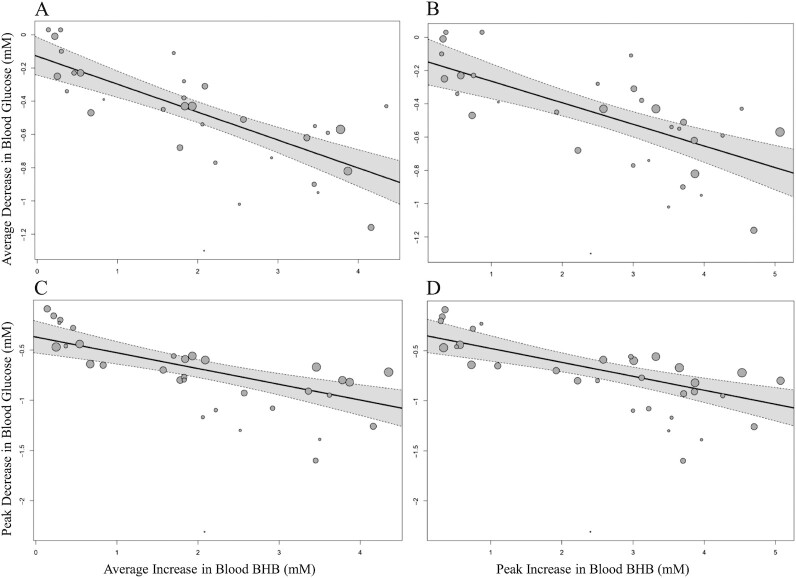
We further evaluated whether supplement dosage would moderate the effect of exogenous ketone ingestion on blood BHB concentrations. Regression analysis suggested that relative BHB dosage was not a statistically significant predictor for average or peak BHB, irrespective of whether studies evaluating ketone salts and ketone monoesters were evaluated jointly or separately. However, the differences in study-level average or peak blood BHB between treatment arms were found to be statistically significant moderators of the effect on average glucose and largest difference in glucose (all P < 0.001) (Figure 5).
FIGURE 5.
Meta-regression using random-effects models including (A) the average increase across time in blood BHB (–0.17 mM; 95% CI: –0.21, –0.12 mM; P < 0.001, R2 = 59.24%) or (B) the peak increase in blood BHB (–0.13 mM; 95% CI: –0.18, –0.08 mM; P < 0.001, R2 = 48.18%) as potential predictors of the average decrease in blood glucose across time and (C) the average increase across time in blood BHB (–0.16 mM; 95% CI: –0.22, –0.09 mM; P < 0.001, R2 = 42.45%) or (D) the peak increase in blood BHB (–0.14 mM; 95% CI: –0.20, –0.08 mM; P < 0.001, R2 = 41.55%) as potential predictors of the maximal decrease in blood glucose. All analyses are conducted in a between-condition manner (i.e., after ingestion of exogenous ketones compared with a comparator supplement). Each circle represents 1 comparison with its size corresponding to the weight attributed to it. The line represents the regression line, the shaded areas indicate 95% CIs. BHB, β-hydroxybutyrate.
Because of the significant differences in blood glucose between the intervention and comparator arms that would act as a confounding variable, no further analysis on the effect of exogenous ketones on insulin concentrations between groups was conducted.
Effects of prolonged exogenous ketone supplementation on blood glucose
Because only 5 trials have evaluated the prolonged (i.e., for >1 d) effect of exogenous ketone supplementation on blood glucose, the findings of these trials are summarized in this narrative section and presented in Supplemental Table 9. Of those trials, 3 trials compared the ketone supplement with a placebo comparator supplement, while 2 trials used a single-arm study design. Four trials used a ketone monoester, and 1 trial used a ketone salt. Across the 5 trials, no differences in fasting glucose following a period of exogenous ketone supplementation were found, but trials assessing glucose via continuous or intermittent scanning glucose monitoring found statistically significant decreases in mean daily glucose during thrice-daily supplementation with ketone monoesters, which was also reflected in statistically significant decreases in HbA1c in 2 studies and a decrease in fructosamine in 1 study.
Adverse events with consumption of exogenous ketones
An overview of reported adverse events (alongside additional information on adherence and dropout) in each comparison is presented in Supplemental Table 10. A total of 33 comparisons assessed and reported on adverse events, of which 16 (48.5%) reported no adverse events or no differences between the intervention and the comparator groups. Of the remaining comparisons, 15 reported gastrointestinal distress, including bloating, abdominal pain, diarrhea, vomiting, cramps, belching, flatulence, heartburn, urge to defecate, reflux, stitch, upset stomach, nausea, loose stool, and upper abdominal discomfort in the intervention group (with symptom incidence ranging from following <1% of drinks to 70% of participants experiencing symptoms). Additionally, light-headedness, dizziness, migraine, and headache were reported in 7 comparisons. A total of 3 participants across all studies withdrew due to side effects related to ingesting the exogenous ketone supplement. Of all comparisons using a ketone salt and assessing adverse events, approximately 85% of comparisons reported adverse side effects, in contrast to approximately 40% comparisons using a ketone monoester.
Discussion
Overview of main findings
In this systematic review we provide a comprehensive evaluation of the available evidence on the effects of exogenous ketone (monoester or salt) ingestion on blood glucose. Our findings suggest that acute ingestion of an exogenous ketone supplement increases blood BHB and decreases blood glucose both when evaluating average or maximal changes in blood glucose after compared with before consumption of exogenous ketones, and when comparing average or maximal changes in blood glucose after ingestion of exogenous ketones compared with a comparator supplement. The glucose-lowering effect in studies conducted in a fasted state and the attenuation of postprandial increases in glucose in studies conducted in a fed state were observed to last up to at least 4 h. Overall, ketone monoesters increased blood BHB approximately 5 times higher and lowered glucose approximately 3 times more than ketone salts. A high degree of heterogeneity was seen across all analyses, indicating the need for further investigation of potential moderating factors.
Moderating variables
The glucose-lowering effect of exogenous ketones appeared across multiple settings (including in a fasted state, during a mixed meal or oral-glucose-tolerance challenge, and during exercise) and in different study populations. However, our ability to draw conclusions on the moderating effect of these study-specific factors was limited given the high overall degree of heterogeneity and the potential for mutual correlations and confounding effects thereof, and we were not able to identify the sources of heterogeneity.
Despite high heterogeneity, we found a significantly greater increase in blood BHB and a greater decrease in blood glucose following ingestion of ketone monoesters compared with ketone salts across all comparisons. Exploratory analysis revealed peak blood BHB to occur between ∼30–60 min following ketone monoester consumption, which was mirrored by the greatest decrease in blood glucose at ∼60 min. Additionally, we found average and peak blood BHB concentrations to moderate the study-level effect of exogenous ketones on blood glucose, suggesting that studies achieving higher blood BHB lead to a greater glucose-lowering effect.
Potential mechanisms
Glucose-lowering effects of exogenous ketones are likely the result of multiple mechanisms that might (inter)act simultaneously. While we observed an overall increase in insulin concentrations following consumption of exogenous ketones across trials conducted in a fasted state, not all studies have found this effect. In the presence of sub-stimulatory glucose concentration, BHB stimulates the G-protein–coupled receptors expressed on pancreatic β-cells, which elevates intracellular cyclic adenosine monophosphate (cAMP) and calcium, leading to accelerated exocytosis of insulin (71, 72). Therefore, the glucose-lowering effect of exogenous ketones, at least in individuals with preserved β-cell function, could be related to increased insulin secretion via direct stimulatory action by BHB (27, 73). However, the effect of ketones does not appear to be fully mediated by an increase in insulin secretion, as infusion of ketones in the absence of insulin secretory function lowers blood glucose as well (74).
The glucose-lowering effects of exogenous ketone ingestion may also be mediated through reduced nonesterified fatty acid concentrations, as BHB can act through the GPR109A (G-protein-coupled receptor 109A) (or HCAR2; Hydroxycarboxylic acid receptor 2) receptor on adipocytes to inhibit lipolysis (75, 76). Decreased nonesterified fatty acid concentrations are associated with reduced gluconeogenesis and hepatic glucose output (77), metabolic mechanisms that align with the consistent glucose-lowering effect of exogenous ketones seen in the studies with various designs (i.e., ketone ingestion only, ketone ingestion combined with nutrient ingestion, and ketone ingestion in conjunction with exercise) reported in this meta-analysis. It is also possible that reducing nonesterified fatty acids could improve peripheral insulin sensitivity (78–80), which could facilitate the acute glucose-lowering effects of exogenous ketones (e.g., through increased glucose uptake by skeletal muscle).
Other potential mechanisms whereby elevated BHB could lower glucose include reducing circulating gluconeogenic precursors such as l-alanine (60), altering incretin hormones [e.g., glucose-dependent insulinotropic peptide (27)], or via sympathetic nervous system modulation (47, 81). However, it is clear that the underlying mechanisms of the observed glucose-lowering effects of exogenous ketones have yet to be fully elucidated.
We found the effects of exogenous ketones on blood BHB and glucose to be relatively consistent across both within-group (i.e., after compared with before ingestion of ketones in a fasted state) and between-condition (i.e., after ingestion of ketones compared with a comparator supplement across fasted and fed states) analyses. However, it is important to note that different (yet likely interacting) mechanisms may underlie the effect seen in different metabolic contexts—that is, the immediate glucose-lowering effect of exogenous ketones when consumed in a fasted state may potentially be orchestrated by 1 primary set of mechanisms (e.g., reduced hepatic glucose output), while the attenuation of postprandial increases in glucose seen with co-ingestion of nutrients alongside exogenous ketones or exogenous ketones consumed in a fed state could potentially be mediated by other mechanisms (e.g., increased insulin sensitivity).
Future studies are needed to investigate exactly how raising BHB decreases blood glucose across different populations. In particular, the currently available literature on longer-term supplementation with exogenous ketones is limited, as is the evidence on ketone supplementation in populations in whom lowering glucose may have clinical benefit (e.g., individuals with prediabetes or T2D). While the above-mentioned mechanisms suggest an acute glucose-lowering effect that may compound over time (and thereby lead to decreased mean daily glucose and, eventually, HbA1c), more research is needed to determine if supplementing with exogenous ketones is a viable therapeutic option for improving glycemic control.
Other limitations
The majority of evidence was obtained in acute, single-dose studies conducted in controlled laboratory-based environments, and there was limited evidence on prolonged supplementation with exogenous ketones, limiting our ability to draw conclusions about longer-term or clinically relevant effects. Of note, our study included only 2 types of ketone supplements—namely, ketone salts and the (R)-3-hydroxybutyl (R)-3-hydroxybutyrate ketone monoester. In the future, other exogenous ketone supplements that are currently under investigation in clinical studies (e.g., using different precursor molecules) may add to the evidence base on how exogenous ketones affect blood glucose (82).
Furthermore, many of the included studies may have had potential for a conflict of interest (e.g., due to affiliation of authors to the ketone industry or provision of ketone supplement free of charge to study authors). While we have assessed the potential for risk of bias to the best of our abilities, we cannot exclude the possibility that results may have been affected by this in some way.
Across analyses, we observed a large degree of heterogeneity alongside wide prediction intervals, except for the between-condition analysis on the effects of exogenous ketones on decreases in blood glucose. This suggests uncertainty around the effect estimate, which could indicate that certain populations may not experience the observed (BHB-raising and glucose-lowering) effect.
Finally, while we aimed to provide a comprehensive overview of the available literature and synthesize the available evidence in a (clinically and statistically) meaningful way, our approach relies on a number of assumptions (as outlined in the Methods section) that may influence the results of our statistical analyses. The conducted sensitivity analyses support the validity of our approach, but despite our best efforts to minimize biases and transparently document our statistical approach with its associated limitations, the robustness against any underlying assumptions remains unknown.
Conclusions
In conclusion, our findings show that exogenous ketone supplements, in particular ketone monoesters, are a viable method to acutely decrease blood glucose across a variety of study settings. In light of the growing burden of T2D and insulin resistance, these results provide promising evidence on the potential therapeutic use of exogenous ketones.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the study authors that, upon request, shared their raw data with the researchers. The authors’ responsibilities were as follows—KF, AD, SCF, and JPL: designed the research; KF and AD: conducted the research; KF: analyzed data with oversight from an external statistician; KF and JPL: wrote the manuscript; AD and SCF: critically revised the manuscript; JPL: had primary responsibility for the final content; and all authors: read and approved the final manuscript.
Notes
JPL is supported by a Michael Smith Foundation for Health Research (MSFHR) Scholar Award (no. 16890).
Author disclosures: JPL is volunteer Chief Scientific Officer for the not-for-profit Institute for Personalized Therapeutic Nutrition. JPL holds founder shares in Metabolic Insights, Inc., a for-profit company that developed noninvasive metabolic monitoring devices. The other authors report no conflicts of interest.
Supplemental Figures 1–10 and Supplemental Tables 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: BHB, β-hydroxybutyrate; HbA1c, glycated hemoglobin; MD, mean difference; SMD, standardized mean difference; T2D, type 2 diabetes.
Contributor Information
Kaja Falkenhain, School of Health and Exercise Sciences, University of British Columbia Okanagan, Kelowna, British Columbia, Canada.
Ali Daraei, School of Health and Exercise Sciences, University of British Columbia Okanagan, Kelowna, British Columbia, Canada.
Scott C Forbes, Department of Physical Education Studies, Faculty of Education, Brandon University, Brandon, Manitoba, Canada.
Jonathan P Little, School of Health and Exercise Sciences, University of British Columbia Okanagan, Kelowna, British Columbia, Canada.
Data Availability
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at: https://osf.io/tv2mg/?view_only=cafa270bc93d4ee4b7e1af74df41216f.
References
- 1. Paoli A, Rubini A, Volek J, Grimaldi K. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67(8):789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huntriss R, Campbell M, Bedwell C. The interpretation and effect of a low-carbohydrate diet in the management of type 2 diabetes: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2018;72(3):311–25. [DOI] [PubMed] [Google Scholar]
- 3. Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25(1):42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JL, Faull OKet al. . On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walsh JJ, Myette-Côté É, Neudorf H, Little JP. Potential therapeutic effects of exogenous ketone supplementation for type 2 diabetes: a review. Curr Pharm Des. 2020;26(9):958–69. [DOI] [PubMed] [Google Scholar]
- 6. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, et al. . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Falkenhain K, Daraei A, Forbes CS, Little JP. Effect of exogenous ketone supplementation on blood glucose: a systematic review and meta-analysis. PROSPERO 2021 CRD42021260201 [Internet]. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021260201 (accessed 4 November 2021). [DOI] [PMC free article] [PubMed]
- 8. Covidence systematic review software [Internet]. Veritas Health Innovation, Melbourne, Australia. Available from: https://www.covidence.org (accessed 4 November 2021). [Google Scholar]
- 9. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron Iet al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;28:l4898. [DOI] [PubMed] [Google Scholar]
- 10. Sterne JAC, Hernán MA, Reeves BC, Savovic J, Berkman ND, Viswanathan Met al. . ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Canales RA, Wilson AM, Pearce-Walker JI, Verhougstraete MP, Reynolds KA. Methods for handling left-censored data in quantitative microbial risk assessment. Appl Environ Microbiol. 2018;84(20)e01203–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rohatgi A. WebPlotDigitizer [Internet]. Available from: https://automeris.io/WebPlotDigitizer (accessed 4 November 2021).
- 13. Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis in R: a hands-on guide. [Internet]. 2019. Available from: https://zenodo.org/badge/latestdoi/152492192 (accessed 4 November 2021).
- 14.R Core Team. A language and environment for statistical computing [Internet]. R Foundation for Statistical Computing;. 2020. Available from: https://www.r-project.org/ (accessed 4 November 2021).
- 15. Balduzzi S, Ruecker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. EBMH. 2019,22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 17. Harrer M, Cuijpers P, Furukawa T, Ebert DD. Dmetar: companion R package for the guide: ‘Doing meta-analysis in R’. R package version 0.0.9000. [Internet]. Available from: http://dmetar.protectlab.org/ (accessed 4 November 2021). [Google Scholar]
- 18. Luedecke D. esc: effect size computation for meta analysis (version 0.5.1). [Internet]. Available from: https://CRAN.R-project.org/package=esc (accessed 4 November 2021).
- 19. Ruecker G, Krahn U, Koenig J, Efthimiou O, Schwarzer G. netmeta: network meta-analysis using frequentist methods. R package version 1.2–1. [Internet]. Available from: https://CRAN.R-project.org/package=netmeta (accessed 4 November 2021). [Google Scholar]
- 20. IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random-effects meta-analysis is straightforward and considerably outperforms the standard Dersimonian-Laird method. BMC Med Res Method. 2014;14(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101. [Google Scholar]
- 23. Olkin I, Dahabreh IJ, Trikalinos TA. GOSH—a graphical display of study heterogeneity. Res Synth Methods. 2012;3(3):214–23. [DOI] [PubMed] [Google Scholar]
- 24. Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. [DOI] [PubMed] [Google Scholar]
- 25. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, al. et, editors. Cochrane handbook of systematic reviews of interventions. Version 6.2. [Internet](updated February 2021). Cochrane Collaboration;. 2021. Available from www.training.cochrane.org/handbook (accessed 4 November 2021). [Google Scholar]
- 27. Bharmal SH, Cho J, Ramos GCA, Ko J, Cameron-Smith D, Petrov MS. Acute nutritional ketosis and its implications for plasma glucose and glucoregulatory peptides in adults with prediabetes: a crossover placebo-controlled randomized trial. J Nutr. 2021;151(4):921–9. [DOI] [PubMed] [Google Scholar]
- 28. Clark D., Munten S, Herzig KH, Gagnon DD. Exogenous ketone salt supplementation and whole-body cooling do not improve short-term physical performance. Front Nutr. 2021;8:663206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith Aet al. . Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24(2):256–68. [DOI] [PubMed] [Google Scholar]
- 30. Crabtree CD, Kackley ML, Buga A, Fell B, LaFountain RA, Hyde PNet al. . Comparison of ketogenic diets with and without ketone salts versus a low-fat diet: liver fat responses in overweight adults. Nutrients. 2021;13(3):966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dearlove DJ, Faull OK, Rolls E, Clarke K, Cox PJ. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front Physiol. 2019;10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dearlove DJ, Harrison OK, Hodson L, Jefferson A, Clarke K, Cox PJ. The effect of blood ketone concentration and exercise intensity on exogenous ketone oxidation rates in athletes. Med Sci Sports Exercise. 2021;53(3):505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dearlove D, Holdsworth D, Kirk T, Hodson L, Charidemou E, Kvalheim Eet al. . β-Hydroxybutyrate in exercise is impaired by low-carbohydrate and high-fat availability. Front Med. 2021;8:721673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Evans M, Patchett E, Nally R, Kearns R, Larney M, Egan B. Effect of acute ingestion of β-hydroxybutyrate salts on the response to graded exercise in trained cyclists. Eur J Sport Sci. 2018;18(3):376–86. [DOI] [PubMed] [Google Scholar]
- 35. Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exercise. 2018;50(11):2330–38. [DOI] [PubMed] [Google Scholar]
- 36. Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sports Exercise. 2019;51(12):2506–15. [DOI] [PubMed] [Google Scholar]
- 37. Fischer T, Och U, Klawon I, Och T, Grueneberg M, Fobker Met al. . Effect of a sodium and calcium DL-β-hydroxybutyrate salt in healthy adults. J Nutr Metabol. 2018;2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greaves G, Xiang R, Rafiei H, Malas A, Little JP. Prior ingestion of a ketone monoester supplement reduces postprandial glycemic responses in young healthy-weight individuals. Appl Physiol Nutr Metab. 2021;46(4):309–17. [DOI] [PubMed] [Google Scholar]
- 39. Holdsworth DA, Cox PJ, Kirk T, Stradling H, Impey SG, Clarke K. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sports Exercise. 2017;49(9):1789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. James S, Kjerulf Greer B. Influence of exogenous β-hydroxybutyrate on walking economy and rating of perceived exertion. J Diet Suppl. 2019;16(4):463–9. [DOI] [PubMed] [Google Scholar]
- 41. McCarthy DG, Bostad W, Powley FJ, Little JP, Richards DL, Gibala MJ. Increased cardiorespiratory stress during submaximal cycling after ketone monoester ingestion in endurance-trained adults. Appl Physiol Nutr Metab. 2021;46(8):986–93. [DOI] [PubMed] [Google Scholar]
- 42. Monzo L, Sedlacek K, Hromanikova K, Tomanova L, Borlaug BA, Jabor Aet al. . Myocardial ketone body utilization in patients with heart failure: the impact of oral ketone ester. Metabolism. 2021;115:154452. [DOI] [PubMed] [Google Scholar]
- 43. Moore AR, Holland-Winkler AM, Ansley JK, Boone EDH, Schulte MKO. Reliability and diagnostic performance of a new blood ketone and glucose meter in humans. J Int Soc Sports Nutr. 2021;18(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mose M, Brodersen K, Rittig N, Schmidt J, Jessen N, Mikkelsen URet al. . Anabolic effects of oral leucine-rich protein with and without β-hydroxybutyrate on muscle protein metabolism in a novel clinical model of systemic inflammation—a randomized crossover trial. Am J Clin Nutr. 2021;114(3):1159–72. [DOI] [PubMed] [Google Scholar]
- 45. Mujica-Parodi LR, Amgalan A, Sultan SF, Clarke K. Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc Natl Acad Sci. 2020;117(11):6170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Myette-Côté É, Neudorf H, Rafiei H, Clarke K, Little JP. Prior ingestion of exogenous ketone monoester attenuates the glycaemic response to an oral glucose tolerance test in healthy young individuals. J Physiol. 2018;596(8):1385–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Myette-Côté É, Caldwell HG, Ainslie PN, Clarke K, Little JP. A ketone monoester drink reduces the glycemic response to an oral glucose challenge in individuals with obesity: a randomized trial. Am J Clin Nutr. 2019;110(6):1491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakagata T, Tamura Y, Kaga H, Sato M, Yamasaki N, Someya Yet al. . Ingestion of an exogenous ketone monoester improves the glycemic response during oral glucose tolerance test in individuals with impaired glucose tolerance: a cross-over randomized trial. J Diabetes Investig. 2021;12(5):756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Connor A, Chang JL, Brownlow M, Contractor N. Acute oral intake of β-hydroxybutyrate in a pilot study transiently increased its capillary levels in healthy volunteers. J Nutr Health Food Eng. 2018;8(4):324–8. [Google Scholar]
- 50. O'Malley T, Myette-Cote E, Durrer C, Little JP. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab. 2017;42(10):1031–5. [DOI] [PubMed] [Google Scholar]
- 51. Poffé C, Ramaekers M, Van Thienen R, Hespel P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol. 2019;597(12):3009–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Poffé C, Ramaekers M, Bogaerts S, Hespel P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J Appl Physiol. 2020;128(6):1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Poffé C, Ramaekers M, Bogaerts S, Hespel P. Bicarbonate unlocks the ergogenic action of ketone monoester intake in endurance exercise. Med Sci Sports Exercise. 2021;53(2):431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Poffé C, Wyns F, Ramaekers M, Hespel P. Exogenous ketosis impairs 30-min time-trial performance independent of bicarbonate supplementation. Med Sci Sports Exercise. 2021;53(5):1068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Poffe C, Robberechts R, Podlogar T, Kusters M, Debevec T, Hespel P. Exogenous ketosis increases blood and muscle oxygenation but not performance during exercise in hypoxia. Am J Physiol Regul Integr Comp Physiol. 2021;321(6):R844–57. [DOI] [PubMed] [Google Scholar]
- 56. Rittig N, Svart M, Thomsen HH, Vestergaard ET, Rehfeld JF, Hartmann Bet al. . Oral D/L-3-hydroxybutyrate stimulates cholecystokinin and insulin secretion and slows gastric emptying in healthy males. J Clin Endocrinol Metab. 2020;105(10):e3597. [DOI] [PubMed] [Google Scholar]
- 57. Rodger S, Plews D, Laursen P, Driller M. Oral β-hydroxybutyrate salt fails to improve 4-minute cycling performance following submaximal exercise. J Sci Cycling. 2017;6(1):26–31. [Google Scholar]
- 58. Soto-Mota A, Vansant H, Evans RD, Clarke K. Safety and tolerability of sustained exogenous ketosis using ketone monoester drinks for 28 days in healthy adults. Regul Toxicol Pharm. 2019;109:104506. [DOI] [PubMed] [Google Scholar]
- 59. Soto-Mota A, Norwitz NG, Evans R, Clarke K, Barber TM. Exogenous ketosis in patients with type 2 diabetes: safety, tolerability and effect on glycaemic control. Endocrinol Diabetes Metab. 2021;4(3):e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Soto-Mota A, Norwitz NG, Evans RD, Clarke K. Exogenous D-β-hydroxybutyrate lowers blood glucose in part by decreasing the availability of L-alanine for gluconeogenesis. Endocrinol Diabetes Metab. 2022:5(1):e00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stubbs BJ, Cox PJ, Evans RD, Cyranka M, Clarke K, de Wet H. A ketone ester drink lowers human ghrelin and appetite. Obesity (Silver Spring). 2018;26(2):269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stubbs BJ. Cox PJ, Evans RD. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Svart M, Rittig N, Pedersen SB, Jessen N, Møller N. Oral 3-hydroxybutyrate ingestion decreases endogenous glucose production, lipolysis, and hormone-sensitive lipase phosphorylation in adipose tissue in men: a human randomized, controlled, crossover trial. Diabet Med. 2021;38(2):e14385. [DOI] [PubMed] [Google Scholar]
- 64. Vandoorne T, De Smet S, Ramaekers M, Van Thienen R, De Bock K, Clarke Ket al. . Intake of a ketone ester drink during recovery from exercise promotes mTORC1 signaling but not glycogen resynthesis in human muscle. Front Physiol. 2017;8:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vestergaard ET, Zubanovic NB, Rittig N, Moller N, Kuhre RE, Holst JJet al. . Acute ketosis inhibits appetite and decreases plasma concentrations of acyl ghrelin in healthy young men. Diabetes Obesity Metab. 2021;23(8):1834–42. [DOI] [PubMed] [Google Scholar]
- 66. Waldman HS, Basham SA, Price FG, Smith JEW, Chander H, Knight ACet al. . Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl Physiol Nutr Metab. 2018;43(7):711–7. [DOI] [PubMed] [Google Scholar]
- 67. Waldman HS, Shepherd BD, Egan B, McAllister MJ. Exogenous ketone salts do not improve cognitive performance during a dual-stress challenge. Int J Sport Nutr Exerc Metab. 2020:30(2):1–8. [DOI] [PubMed] [Google Scholar]
- 68. Walsh JJ, Neudorf H, Little JP. 14-Day ketone supplementation lowers glucose and improves vascular function in obesity: a randomized crossover trial. J Clin Endocrinol Metab. 2021;106(4):e1738–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Whitfield J, Burke LM, McKay AKA, Heikura IA, Hall R, Fensham Net al. . Acute ketogenic diet and ketone ester supplementation impairs race walk performance. Med Sci Sports Exercise. 2021;53(4):776–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Neudorf H, Durrer C, Myette-Côté É, Makins C, O'Malley T, Little JP. Oral ketone supplementation acutely increases markers of NLRP3 inflammasome activation in human monocytes. Mol Nutr Food Res. 2019;63(11):1801171. [DOI] [PubMed] [Google Scholar]
- 71. Besse-Patin A, Montastier E, Vinel C, Castan-Laurell I, Louche K, Dray Cet al. . Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. Int J Obes. 2014;38(5):707–13. [DOI] [PubMed] [Google Scholar]
- 72. Fridlyand LE, Philipson LH. Pancreatic β cell G-protein coupled receptors and second messenger interactions: a systems biology computational analysis. PLoS One. 2016;11(5):e0152869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Madison LL, Mebane D, Unger RH, Lochner A. The hypoglycemic action of ketones. II. Evidence for a stimulatory feedback of ketones on the pancreatic β cells. J Clin Invest. 1964;43(3):408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sherwin RS, Hendler RG, Felig P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes. 1976;25(9):776–84. [DOI] [PubMed] [Google Scholar]
- 75. Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito Met al. . (D)-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280(29):26649–52. [DOI] [PubMed] [Google Scholar]
- 76. Senior B, Loridan L. Direct regulatory effect of ketones on lipolysis and on glucose concentrations in man. Nature. 1968;219(5149):83–4. [DOI] [PubMed] [Google Scholar]
- 77. Reaven G. All obese individuals are not created equal: insulin resistance is the major determinant of cardiovascular disease in overweight/obese individuals. Diabetes Vasc Dis Res. 2005;2(3):105–12. [DOI] [PubMed] [Google Scholar]
- 78. Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJet al. . Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes. 1999;48(9):1836–41. [DOI] [PubMed] [Google Scholar]
- 79. Saloranta C, Groop L, Ekstrand A, Franssila-Kallunki A, Eriksson J, Taskinen MR. Different acute and chronic effects of Acipimox treatment on glucose and lipid metabolism in patients with type 2 diabetes. Diabet Med. 1993;10(10):950–7. [DOI] [PubMed] [Google Scholar]
- 80. Worm D, Henriksen JE, Vaag A, Thye-Ronn P, Melander A, Beck-Nielsen H. Pronounces blood glucose-lowering effect of the antilipolytic drug Acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab. 1994;78(3):717–21. [DOI] [PubMed] [Google Scholar]
- 81. Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi Set al. . Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci. 2011;108(19):8030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made publicly and freely available without restriction at: https://osf.io/tv2mg/?view_only=cafa270bc93d4ee4b7e1af74df41216f.