Abstract
A general mechanism in bacteria to rescue stalled ribosomes involves a stable RNA encoded by the ssrA gene. This RNA, termed tmRNA, encodes a proteolytic peptide tag which is cotranslationally added to truncated polypeptides, thereby targeting them for rapid proteolysis. To study this ssrA-mediated mechanism in Bacillus subtilis, a bipartite detection system was constructed that was composed of the HrcA transcriptional repressor and the bgaB reporter gene coding for a heat-stable β-galactosidase fused to an HrcA-controlled promoter. After the predicted proteolysis tag was fused to HrcA, the reporter β-galactosidase was expressed constitutively at a high level due to the instability of the tagged HrcA. Replacement of the two C-terminal alanine residues of the tag by aspartate rendered the repressor stable. Replacement of the hrcA stop codon by a transcriptional terminator sequence rendered the protein unstable; this was caused by trans translational addition of the proteolytic tag. Inactivating the B. subtilis ssrA or smpB (yvaI) gene prevented the trans translational tagging reaction. Various protease-deficient strains of B. subtilis were tested for proteolysis of tagged HrcA. HrcA remained stable only in clpX or clpP knockouts, which suggests that this ATP-dependent protease is primarily responsible for the degradation of SsrA-tagged proteins in B. subtilis.
What happens to translating ribosomes arriving at the 3′ end of an mRNA molecule lacking a stop codon? Will they fall off (dissociate) or remain stalled? Work carried out over the last 5 years with Escherichia coli has shown that the ribosomes first are stalled and then are released by a novel mechanism designated trans translation (for a recent review, see reference 13). This mechanism requires a small stable RNA (SsrA RNA), also termed 10Sa RNA or tmRNA, which serves both as a tRNA and as an mRNA. When translating ribosomes arrive at the 3′ end of a truncated mRNA without encountering a stop codon, the process of trans translation is initiated. A tmRNA charged with alanine at its 3′ end (17, 28) enters the acceptor position of the ribosome and this is followed by transfer of the alanine residue to the nascent polypeptide chain. Next, the ribosome switches to the tmRNA to continue translation.
The possibility that tmRNA also functions as mRNA was first suggested by Tu and coworkers (27), who showed that a fraction of mouse interleukin 6 expressed in E. coli carried an additional 11-amino-acid tag sequence. This tag sequence was not encoded by the interleukin 6 mRNA, but the last 10 of the 11 amino acids were coded for by the E. coli tmRNA gene. Later, this tag sequence was also found attached to other polypeptides when they were translated from mRNAs lacking a termination codon (16), when poly(U) mimicking a truncated mRNA was translated in vitro in the presence of tmRNA (9), and when clusters of rare codons were present in the mRNA (24). In E. coli, at least four proteases, Tsp, FtsH, ClpXP, and ClpAP, recognize the C-terminal tag and subsequently degrade polypeptide chains with the proteolytic tag (7, 8, 16).
The tmRNA is encoded by the ssrA gene (23), and it has been shown that a protein encoded by the smpB gene is also essential for the trans translation process (14). While the exact role of this protein remains elusive, it has been suggested that the SmpB protein directly or indirectly facilitates stable association of tmRNA with 70S ribosomes. As of November 2000, 103 complete tmRNA sequences were known from species of Eubacteria and certain plastids (31). In the case of Caulobacter crescentus, the tmRNA is synthesized as a precursor which is processed by removal of an internal fragment, and the two flanking RNA fragments form a two-piece tmRNA (15).
Bacillus subtilis serves as a model organism for the gram-positive bacteria, and the tmRNA of B. subtilis was isolated and sequenced several years ago (28). Sequencing of the complete chromosome revealed the presence of both the ssrA gene and a gene with homology to smpB (yvaI) (18). Recently, it was shown that growth of ssrA knockout strains of B. subtilis is impaired at elevated temperatures. This defect is caused by blocking of ribosomes and not by accumulation of truncated polypeptides. Furthermore, it has been shown that the ssrA gene is heat inducible (22).
Although the sequence of the proteolytic tag encoded by the B. subtilis ssrA gene has been predicted, the ability of this tag to label polypeptides for proteolysis has not been tested yet. In addition, it is not known which of the various cytoplasmic proteases of B. subtilis is involved in degradation of ssrA-tagged proteins. We are interested in the biological significance of the ssrA-mediated tagging mechanism in B. subtilis, and as a first step in an analysis of the role of this mechanism in protein quality control, we devised an experimental system to demonstrate its activity. Using this system, we identified the protease responsible for degradation of cytoplasmic proteins with the proteolytic tag.
MATERIALS AND METHODS
B. subtilis strains.
Strains clpP::spec and clpE::spec (2), strain clpC::spec (20), and strain MP01 (ftsH::cat) (3) are all derivatives of strain 1012 (leuA8 metB5 trpC2 hsrM1) (25).
Construction of the HrcA-BgaB reporter system.
The hrcA gene encoding the repressor of class I heat shock genes (26) was generated by PCR by using chromosomal DNA of B. subtilis SW01 (with the CIRCE element in front of the dnaK operon deleted [12]) as the template. The resulting amplicon flanked by SacI and BamHI-SphI-SacII sites was digested with SacI and SacII and ligated into integration vector pAX01 (which allows integration at the lacA locus [unpublished data]) cut with the same enzymes; this resulted in pA-hrcA. Next, hrcA was fused to three different sequences, the predicted ssrA tagging sequence (which codes for the amino acid sequence [A]GKTNSFNQNVALAA), mutated tagging sequence (A)GKTNSFNQNVALDD, and the E. coli trpA transcriptional terminator (which adds amino acid residues AARLMSG) (16) without a stop codon, by using appropriate complementary oligonucleotides flanked by BamHI and SphI restriction sites. The sequences of the three resulting hybrid hrcA genes (hrcA-AA, hrcA-DD, and hrcA-ter) were verified by DNA sequencing. Then, B. subtilis AM20 with a chromosomal deletion of part of the hrcA gene and a transcriptional fusion of the dnaK promoter that included the region from the CIRCE operator sequence to the heat-stable β-galactosidase gene (bgaB) in the amyE locus was transformed with chromosomal DNA of strain IHA01 (lacA::spec). Transformants were selected for spectinomycin and neomycin resistance and were screened for the absence of HrcA by Western blotting. The resulting strain, TW11, was used as a recipient for the plasmids carrying the three hybrid hrcA genes, and transformants were selected on plates containing neomycin and erythromycin and screened for spectinomyin sensitivity. The four new strains were designated TW12 (wild-type hrcA), TW13 (hrcA-AA), TW14 (hrcA-DD), and TW15 (hrcA-ter).
Construction of B. subtilis knockout strains.
Different B. subtilis knockout strains were constructed by PCR amplification of the appropriate genes with flanking regions using chromosomal DNA of B. subtilis 1012 as a template, cloning into the pUC19 plasmid, deletion of internal parts of the genes, and insertion of a cat gene. These constructs were PCR amplified with the pUC/M13 universal and reverse sequencing primers, and the PCR products were directly transformed into B. subtilis. Chloramphenicol-resistant clones were checked by PCR and, in case of ssrA::cat and smpB::cat by Southern blotting. The clpX gene with flanking regions was PCR amplified with primers clpX5′ and clpX3′ (the sequences of all oligonucleotides are available upon request). The 2.4-kb PCR product was cloned via BamHI-PstI restriction sites into pUC19. The resulting pUC-clpX plasmid was cut with Eco47III and NruI to remove a substantial part of the clpX coding region, and the cat cassette of pUC18Cm (30) was inserted as an EcoRV fragment. The ssrA chromosomal region was PCR amplified with primers ssrA5′ and ssrA3′, which yielded a 1.3-kb product that was cloned via EcoRI and HindIII into pUC19. pUC19ssrA was digested with SacI and ClaI to remove a substantial part of ssrA. The cat cassette of pUC18Cm was isolated as a SacI-ClaI fragment and ligated to pUC-ssrA SacI/ClaI. The smpB (yvaI) gene of B. subtilis was PCR amplified with primers yvaI5′ and ssrA3′ and cloned via EcoRI and HindIII into pUC19. An internal portion of smpB was removed by deleting a NaeI-StuI fragment and inserting the cat cassette restricted with EcoRV.
Media.
The media used were Luria broth and S7 minimal medium (29) with the appropriate antibiotics for growing plasmid-bearing cells.
Immunoblot analysis and pulse-chase experiments.
Samples were prepared for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis as described previously (10); 5 μg of total cellular protein was applied per lane. Polyclonal serum against HrcA, DnaK, or HtpG, a donkey anti-rabbit immunoglobulin G-horseradish peroxidase conjugate (Amersham), and a chemiluminescence reaction (ECL system; Amersham) were used for detection. The stability of the HrcA repressor protein was determined by pulse-chase experiments essentially as described previously (29), using polyclonal serum against HrcA for immunoprecipitation.
β-Galactosidase assay.
β-Galactosidase activities were assayed at 55°C as described previously (19) by using o-nitrophenyl-β-d-galactopyranoside as the substrate. All assays were repeated at least three times, and the replicates yielded comparable results. Mean values are given below together with the standard deviations.
RESULTS AND DISCUSSION
Predicted proteolysis tag encoded by the ssrA gene mediates rapid degradation of HrcA protein.
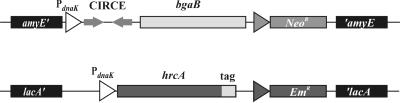
To analyze the ssrA-mediated degradation mechanism, we constructed a reporter system that allowed fast and easy detection of ssrA-mediated activity. This bipartite system consisted of the hrcA repressor gene, to which the predicted proteolysis tag and a variant assumed not to be recognized by the protease(s) (16) was added, and a promoter-operator system fused to the bgaB reporter gene coding for a thermostable β-galactosidase. Both parts of the detection system were integrated into the B. subtilis chromosome; the hrcA gene was expressed from a constitutive promoter at the lacA locus, and the transcriptional fusion was expressed from a promoter at the amyE locus (Fig. 1). The HrcA repressor negatively controls class I heat shock genes of B. subtilis, including the heptacistronic dnaK operon and the bicistronic groE operon, by binding to the operator sequence, designated CIRCE. Upon heat shock, the repressor becomes transiently inactive, which results in increased transcription of the groE and dnaK operons (26).
FIG. 1.
Experimental system used to verify SsrA-mediated tagging. The promoter-operator region of the dnaK operon was fused to the bgaB reporter gene coding for a heat-stable β-galactosidase and integrated at the amyE locus. The hrcA repressor gene and its three variants were fused to the promoter of the dnaK gene lacking its operator, termed CIRCE, and integrated at the lacA locus.
To test the validity of the system, different strains carrying the transcriptional fusion and either no hrcA gene, the wild-type allele, or the tagged variants were grown in Luria broth medium to the mid-exponential phase, and samples were taken in order to measure β-galactosidase activity. The enzymatic activity measured in the absence of HrcA was defined as 100 relative units. In parallel, cultures were heat shocked from 37° to 48°C for 30 min to verify HrcA functionality. For unknown reasons, even in the absence of the HrcA repressor, there was an approximately twofold increase in specific β-galactosidase activity after the heat shock from 37° to 48°C (Table 1), which has been described previously (11). In the presence of the hrcA gene, the enzymatic activity decreased by a factor of about 20 due to the repressor protein interacting with its operator (Fig. 1 and Table 1). Upon heat shock, the repressor became inactivated, which resulted in a dramatic increase in β-galactosidase activity. These data clearly show that the reporter system works properly.
TABLE 1.
Relative β-galactosidase activities
| Gene | Heat shock | β-Galactosidase activity (relative units) ofa:
|
|||||
|---|---|---|---|---|---|---|---|
| Wild-type strain | Strain clpC::spec | Strain clpE::spec | Strain clpP::spec | Strain clpX::cat | Strain ftsH::cat | ||
| hrcA | − | 100 | 100 | 100 | 100 | 100 | 100 |
| + | 234 ± 2 | 195 ± 31 | 150 ± 20 | 167 ± 10 | 165 ± 25 | 155 ± 2 | |
| hrcA+ | − | 5.3 ± 0.3 | 5.8 ± 0.2 | 4.6 ± 0.2 | 6.0 ± 0.8 | 3.9 ± 1.5 | 3.5 ± 0.3 |
| + | 97 ± 30 | 79 ± 24 | 75 ± 6 | 63 ± 15 | 65 ± 15 | 46 ± 6 | |
| hrcA-AA | − | 89 ± 3 | 95 ± 5 | 71 ± 10 | 7.1 ± 0.8 | 3.6 ± 1.2 | 74 ± 14 |
| + | 211 ± 8 | 172 ± 34 | 141 ± 22 | 49 ± 13 | 66 ± 5 | 146 ± 11 | |
| hrcA-DD | − | 5.6 ± 0.4 | 6.2 ± 1.5 | 4.4 ± 0.5 | 5.7 ± 1.6 | 4.0 ± 1.4 | 4.0 ± 0.2 |
| + | 81 ± 22 | 72 ± 16 | 82 ± 7 | 63 ± 5 | 67 ± 4 | 54 ± 3 | |
| hrcA-ter | − | 99 ± 2 | 96 ± 2 | 64 ± 3 | 10.3 ± 0.4 | 5.3 ± 0.9 | 78 ± 22 |
| + | 219 ± 16 | 171 ± 26 | 144 ± 20 | 51 ± 11 | 75 ± 19 | 145 ± 12 | |
Relative β-galactosidase activities of B. subtilis strains with a transcriptional fusion of PdnaK-bgaB expressing no hrcA (TW11), wild-type hrcA (TW12), hrcA-AA (TW13), hrcA-DD (TW14), or hrcA-ter (TW15) in wild-type and knockout backgrounds for clpC, clpE, clpP, clpX, and ftsH. To verify HrcA functionality, samples were taken from cultures grown at 37°C and from cultures that were heat shocked from 37 to 48°C for 30 min. The β-galactosidase activities of hrcA strains that were not heat shocked were defined as 100 relative units.
In the second step, strains synthesizing the two tagged versions of HrcA were analyzed. When hrcA-AA was expressed, the β-galactosidase activity increased 16-fold in the absence of heat stress compared to wild-type hrcA activity (Table 1), indicating that the predicted proteolysis tag rendered the repressor protein either inactive or unstable (see below). When the two C-terminal alanine residues were replaced by aspartate residues (HrcA-DD), the repressor remained active and was able to repress the operon fusion to the same extent as the wild-type protein. The HrcA-DD repressor in cells that were heat shocked lost its activity, which resulted in an increase in β-galactosidase activity comparable to that measured with the wild-type repressor (Table 1). These results further demonstrate that the proteolysis tag can be poisoned by replacement of the two terminal alanine residues by aspartate residues to the same extent that has been reported for the E. coli tag (16).
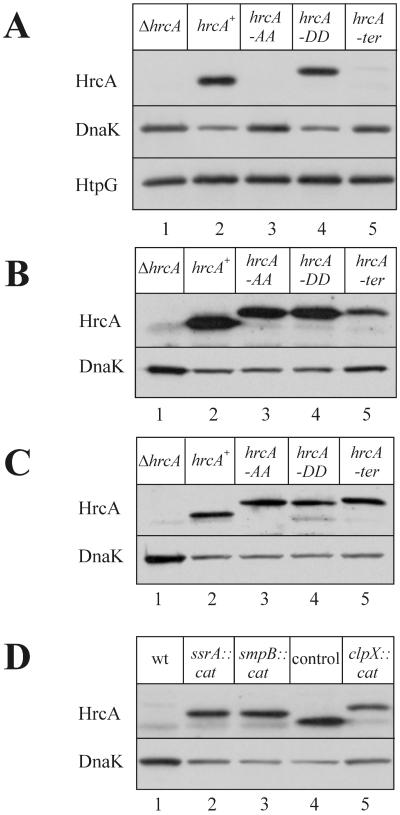
It is tempting to assume that the inactivity of HrcA-AA is caused by degradation rather than by inappropriate folding due to the foreign amino acid residues added to its C terminus. To test this assumption, the presence of HrcA protein in whole-cell extracts was directly analyzed by immunoblotting. While HrcA-AA was clearly absent from crude cell extracts, HrcA-DD was present and moved slower than the wild-type protein due to the additional amino acid residues at its C terminus (Fig. 2A). The activity of HrcA was also reflected by its effect on expression of the dnaK gene. In the presence of wild-type HrcA or HrcA-DD, the amount of DnaK was reduced compared to the amount in cells lacking HrcA or HrcA-AA (Fig. 2A). The level of the heat shock protein HtpG served as another internal control. The htpG gene is not regulated by HrcA (26), and therefore, the amount of HtpG remained constant.
FIG. 2.
Western blots of cell extracts of different B. subtilis strains grown at 37°C and developed with polyclonal antibodies against HrcA, DnaK, or HtpG. Five micrograms of total protein was applied to each lane. (A) B. subtilis TW11 (hrcA) (lane 1), TW12 (hrcA+) (lane 2), TW13 (hrcA-AA) (lane 3), TW14 (hrcA-DD) (lane 4), and TW15 (hrcA-ter) (lane 5). (B) Same strains as in panel A with the clpP::spec background. (C) Same strains as in panel A with the clpX::cat background. (D) Strain TW15 (hrcA-ter) with the wild-type background (lane 1), ssrA::cat (lane 2), and smpB::cat (lane 3). Samples of strains TW12 (hrcA+) (lane 4) and TW15 (hrcA-ter clpX::cat) served as controls.
To corroborate the hypothesis that the failure of HrcA-AA to carry out repression at its operator is based on instability of the protein, a pulse-chase experiment was carried out. Proteins were labelled for 1 min, and samples were taken at chase times of 15 s and 5, 20, and 60 min. While wild-type HrcA remained stable for at least 60 min (Fig. 3A, lanes 1 to 4), HrcA-AA had largely disappeared 15 s after the chase (lane 6). In contrast, HrcA-DD turned out to be as stable as the wild-type repressor (lanes 9 to 12).
FIG. 3.
Stability of HrcA and two of its variants as determined by pulse-chase experiments. Strains were grown in S7 medium to the early logarithmic phase and were labelled for 1 min with [35S]methionine, after which a 1,000-fold excess of nonradioactive methionine was added. Samples were withdrawn at chase times of 15 s and 5, 20, and 60 min and subjected to immunoprecipitation with antiserum against HrcA, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fluorography. (A) Strains TW12 (hrcA+) (lanes 1 to 4), TW13 (hrcA-AA) (lanes 5 to 8), and TW14 (hrcA-DD) (lanes 9 to 12). (B) Strain TW13 (hrcA-AA) (lanes 1 to 4) and its congenic ftsH::cat (lanes 5 to 8) and clpP::spec (lanes 9 to 12) derivatives.
In summary, cotranslational addition of the AGKTNSFNQNVALAA residues to the HrcA repressor protein almost completely destroyed its activity due to extreme instability, while replacement of the two C-terminal AA residues by DD residues made the protein as stable as the wild-type form. These experiments also demonstrated that addition of 15 foreign amino acid residues to HrcA does not impair either its activity as a repressor or its response to heat stress.
Replacement of the stop codon of hrcA by a terminator structure results in in vivo tagging of the repressor protein.
It has been reported that replacement of the stop codon of the λ repressor gene by the trpA transcriptional terminator leads to rapid degradation of the repressor protein due to addition of the tmRNA-encoded proteolytic tag (16). To investigate whether this also occurs in B. subtilis, the stop codon at the end of the coding region of the hrcA gene was replaced by the trpA transcriptional terminator (hrcA-ter). Next, the β-galactosidase activity was measured and was found to be comparable to the chromosome-borne hrcA-AA activity (Table 1). Direct Western blotting analysis of samples of the strain expressing hrcA-ter revealed a faint band that moved slower than the HrcA-DD band (Fig. 2A, compare lanes 4 and 5). This indicates that hrcA-ter is translated to HrcA extended by seven additional amino acids encoded by the terminator sequence and then ssrA tagged by 15 additional amino acids in vivo, which makes the protein unstable. Thus, in accordance with the data of Keiler et al. (16), proteins translated from the mRNAs that lacked a termination codon appeared to be modified by the ssrA-dependent peptide tag.
ClpXP is the major protease involved in degradation of HrcA protein with the proteolytic tag.
Since it was shown that the stability of the HrcA-AA repressor is greatly impaired, we asked which protease(s) is responsible for degradation of this repressor. In E. coli, three ATP-dependent proteases have been identified which recognize cytoplasmic proteins with the proteolytic tag; these proteases are FtsH (8), ClpXP, and ClpAP (7). The Clp proteases consist of two multimeric components, an ATPase and a proteolytic component, both of which are required for proteolysis (6). In B. subtilis, three Clp ATPases (ClpC, ClpE, and ClpX) and one proteolytic component (ClpP) have been described (2, 4, 5, 21). Therefore, knockouts of all four clp genes and the ftsH gene were tested to determine whether they conferred stability to the tagged HrcA variants. ClpC, ClpE, and FtsH null mutants were indistinguishable from the isogenic wild-type strain and did not influence the activity of HrcA-AA and HrcA-ter (Table 1). In clpP and clpX knockouts, HrcA-AA- and HrcA-ter-repressing activities were significantly enhanced, as revealed by clearly reduced β-galactosidase activities in non-heat-shocked samples (Table 1). These results suggest that the ClpXP protease is responsible for degradation of HrcA-AA.
To confirm this finding, the presence of the different HrcA variants was checked by immunoblotting. It turned out that both HrcA-AA and HrcA-ter were detectable in both clpP and clpX knockouts (Fig. 2B and C). In addition, the stability of HrcA-AA was monitored in a pulse-chase experiment. While the HrcA-AA protein disappeared rapidly in the wild type and the ftsH knockout, it was stable in the clpP null mutant (Fig. 3B). We concluded from all these results that ClpXP is the major (if not the only) ATP-dependent protease responsible for recognition and degradation of the HrcA repressor protein with the proteolytic tag. However, we cannot rule out the possibility that another protease(s) is responsible for degradation of additional substrates.
ssrA and smpB are both needed for addition of the proteolytic tag to HrcA.
Besides the tmRNA encoded by the ssrA gene, a second gene, smpB, coding for an 18-kDa protein, has been reported to be essential for adding the proteolytic tag; in E. coli, the two genes form a bicistronic operon (14). In B. subtilis, the two genes are also adjacent but most probably form two transcriptional operons (18). To find out whether these two genes are responsible for the tagging reaction in B. subtilis, they were independently inactivated and transferred into the strain carrying the hrcA gene with the stop codon replaced by the transcriptional terminator sequence (HrcA-ter).
First, we monitored the activity of the repressor by measuring the β-galactosidase activity. It turned out that HrcA-ter was active in both null mutants (Table 2). Immunoblot experiments detected stable HrcA repressor in both the ssrA::cat and smpB::cat strains (Fig. 2D, lanes 2 and 3). The repressor variants moved between wild-type HrcA (Fig. 2D, lane 4) and stable HrcA-ter expressed in clpX::cat (Fig. 2D, lane 5) due to seven additional amino acid residues encoded by the trpA terminator and the absence of in vivo ssrA tagging in the ssrA::cat and smpB::cat background. Again, as mentioned above, the presence of HrcA, whether extended at its C terminus by the foreign amino acid residues encoded by the transcriptional terminator or extended by the SsrA tag, remained active since it was able to repress expression of the dnaK gene (Fig. 2D). Most interestingly, these results indicate that stalled ribosomes are able to dissociate from an mRNA molecule even in the absence of a stop codon and in the absence of a functional SsrA-tagging mechanism. This probably occurs by a competing slower mechanism involving either passive dissociation or some unknown factor(s).
TABLE 2.
Relative β-galactosidase activities with the hrcA-ter gene
| Heat shock | β-Galactosidase activity (relative units) ofa:
|
||
|---|---|---|---|
| Wild-type | Strain ssrA::cat | Strain smpB::cat | |
| − | 100 | 6.5 ± 0.4 | 6.3 ± 0.5 |
| + | 229 ± 5 | 140 ± 5 | 130 ± 15 |
Relative β-galactosidase activities of B. subtilis strains with a transcriptional fusion of Pdnak-bgaB expressing hrcA with the trpA terminator and without a stop codon (hrcA-ter; TW15) in wild-type and knockout backgrounds for ssrA or smpB (yvaI). Strains were subjected to a heat shock, and β-galactosidase activities were expressed as described in Table 1, footnote a.
B. subtilis is the second species in which the SsrA-mediated tagging mechanism has been studied in detail and the proteolytic activity responsible for degradation of a protein with the proteolytic tag added either cotranslationally or trans translationally has been identified. Experiments are in progress to find additional conditions that lead to tagging of proteins. We are also investigating whether the SsrA-mediated tagging mechanism can be used to eliminate essential proteins in a short time to create a new form of depletion assay. Last but not least, we are interested in finding out whether this tagging mechanism is used as a regulatory mechanism by B. subtilis to limit the amounts of certain proteins. The possibility that such a mechanism is indeed being exploited by bacteria has recently been discussed by Abo and coworkers (1). These workers showed that the LacI repressor is a natural target for SsrA-mediated tagging, which thus fine tunes the amount of available repressor protein.
ACKNOWLEDGMENTS
Financial support was provided by EU project QLRT-1999-00413 and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Abo T, Inada T, Ogawa A, Aiba H. SsrA-mediated tagging and proteolysis of LacI and its role in the regulation of lac operon. EMBO J. 2000;19:3762–3769. doi: 10.1093/emboj/19.14.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derré I, Rapoport G, Devine K, Rose M, Msadek T. ClpE, a novel type of HSP100 ATPase, is part of the CtsR heat shock regulon of Bacillus subtilis. Mol Microbiol. 1999;32:581–593. doi: 10.1046/j.1365-2958.1999.01374.x. [DOI] [PubMed] [Google Scholar]
- 3.Deuerling E, Mogk A, Richter C, Purucker M, Schumann W. The ftsH gene of Bacillus subtilis is involved in major cellular processes such as sporulation, stress adaptation and secretion. Mol Microbiol. 1997;23:921–933. doi: 10.1046/j.1365-2958.1997.2721636.x. [DOI] [PubMed] [Google Scholar]
- 4.Gerth U, Krüger E, Derré I, Msadek T, Hecker M. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol Microbiol. 1998;28:787–802. doi: 10.1046/j.1365-2958.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 5.Gerth U, Wipat A, Harwood C R, Carter N, Emmerson P T, Hecker M. Sequence and transcriptional analysis of clpX, a class-III heat-shock gene of Bacillus subtilis. Gene. 1996;181:77–83. doi: 10.1016/s0378-1119(96)00467-2. [DOI] [PubMed] [Google Scholar]
- 6.Gottesman S. Proteases and their targets in Escherichia coli. Annu Rev Genet. 1996;30:465–506. doi: 10.1146/annurev.genet.30.1.465. [DOI] [PubMed] [Google Scholar]
- 7.Gottesman S, Roche E, Zhou Y N, Sauer R T. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman C, Thévenet D, Bouloc P, Walker G C, D'Ari R. Degradation of carboxy-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB (FtsH) Genes Dev. 1998;12:1348–1355. doi: 10.1101/gad.12.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Himeno H, Sato M, Tadaki T, Fukushima M, Ushida C, Muto A. In vitro trans translation mediated by alanine-charged 10Sa RNA. J Mol Biol. 1997;268:803–808. doi: 10.1006/jmbi.1997.1011. [DOI] [PubMed] [Google Scholar]
- 10.Homuth G, Heinemann M, Zuber U, Schumann W. The genes lepA and hemN form a bicistronic operon in Bacillus subtilis. Microbiology. 1996;142:1641–1649. doi: 10.1099/13500872-142-7-1641. [DOI] [PubMed] [Google Scholar]
- 11.Homuth G, Masuda S, Mogk A, Kobayashi Y, Schumann W. The dnaK operon of Bacillus subtilis is heptacistronic. J Bacteriol. 1997;179:1153–1164. doi: 10.1128/jb.179.4.1153-1164.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Homuth G, Mogk A, Schumann W. Post-transcriptional regulation of the Bacillus subtilis dnaK operon. Mol Microbiol. 1999;32:1183–1197. doi: 10.1046/j.1365-2958.1999.01428.x. [DOI] [PubMed] [Google Scholar]
- 13.Karzai A W, Roche E D, Sauer R T. The SsrA-SmpB system for protein tagging, directed degradation and ribosome rescue. Nat Struct Biol. 2000;7:449–455. doi: 10.1038/75843. [DOI] [PubMed] [Google Scholar]
- 14.Karzai A W, Susskind M M, Sauer R T. SmpB, a unique RNA-binding protein essential for the peptide-tagging activity of SsrA (tmRNA) EMBO J. 1999;18:3793–3799. doi: 10.1093/emboj/18.13.3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keiler K C, Shapiro L, Williams K P. tmRNAs that encode proteolysis-inducing tags are found in all known bacterial genomes: a two-piece tmRNA functions in Caulobacter. Proc Natl Acad Sci USA. 2000;97:7778–7783. doi: 10.1073/pnas.97.14.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keiler K C, Waller P R H, Sauer R T. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- 17.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H. A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci USA. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunst F, Ogasawara N, Moszer I, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 19.Mogk A, Hayward R, Schumann W. Integrative vectors for constructing single-copy transcriptional fusions between Bacillus subtilis promoters and various reporter genes encoding heat-stable enzymes. Gene. 1996;182:33–36. doi: 10.1016/s0378-1119(96)00447-7. [DOI] [PubMed] [Google Scholar]
- 20.Msadek T, Dartois V, Kunst F, Herbaud M-L, Denizot F, Rapoport G. ClpP of Bacillus subtilis is required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol Microbiol. 1998;27:899–914. doi: 10.1046/j.1365-2958.1998.00735.x. [DOI] [PubMed] [Google Scholar]
- 21.Msadek T, Kunst F, Rapoport G. MecB of Bacillus subtilis, a member of the ClpC ATPase family, is a pleiotropic regulator controlling competence gene expression and growth at high temperature. Proc Natl Acad Sci USA. 1994;91:5788–5792. doi: 10.1073/pnas.91.13.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muto A, Fujihara A, Ito K, Matsuno J, Ushida C, Himeno H. Requirement of transfer-messenger RNA for the growth of Bacillus subtilis under stresses. Genes Cells. 2000;5:627–635. doi: 10.1046/j.1365-2443.2000.00356.x. [DOI] [PubMed] [Google Scholar]
- 23.Oh B-K, Chauhan A K, Isono K, Apirion D. Location of a gene (ssrA) for small, stable RNA (10Sa RNA) in the Escherichia coli chromosome. J Bacteriol. 1990;172:4708–4709. doi: 10.1128/jb.172.8.4708-4709.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roche E D, Sauer R T. SsrA-mediated peptide tagging caused by rare codons and tRNA scarcity. EMBO J. 1999;18:4579–4589. doi: 10.1093/emboj/18.16.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito H, Shibata T, Ando T. Mapping of genes determining nonpermissiveness and host-specific restriction to bacteriophages in Bacillus subtilis Marburg. Mol Gen Genet. 1979;170:117–122. doi: 10.1007/BF00337785. [DOI] [PubMed] [Google Scholar]
- 26.Schumann W. Function and regulation of temperature-inducible bacterial proteins on the cellular metabolism. In: Scheper T, editor. Advances in biochemical engineering biotechnology. Berlin, Germany: Springer-Verlag; 2000. pp. 1–33. [DOI] [PubMed] [Google Scholar]
- 27.Tu G-F, Reid G E, Zhang J-G, Moritz R L, Simpson R J. C-terminal extension of truncated recombinant proteins in Escherichia coli with a 10Sa RNA decapeptide. J Biol Chem. 1995;270:9322–9326. doi: 10.1074/jbc.270.16.9322. [DOI] [PubMed] [Google Scholar]
- 28.Ushida C, Himeno H, Watanabe T, Muto A. tRNA-like structures in 10Sa RNAs of Mycoplasma capricolum and Bacillus subtilis. Nucleic Acids Res. 1994;22:3392–3396. doi: 10.1093/nar/22.16.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Dijl J M, De Jong A, Smith H, Bron S, Venema G. Non-functional expression of Escherichia coli signal peptidase I in Bacillus subtilis. J Gen Microbiol. 1991;137:2073–2083. doi: 10.1099/00221287-137-9-2073. [DOI] [PubMed] [Google Scholar]
- 30.Versteeg S, Mogk A, Schumann W. The Bacillus subtilis htpG gene is not involved in thermal stress management. Mol Gen Genet. 1999;261:582–588. doi: 10.1007/s004380051004. [DOI] [PubMed] [Google Scholar]
- 31.Williams K P. The tmRNA website. Nucleic Acids Res. 2000;28:168. doi: 10.1093/nar/28.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]