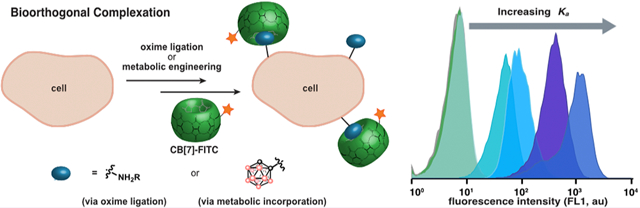
Abstract
The widespread adoption of the bioorthogonal chemical reporter strategy revolutionized chemical biology. However, its translation to living mammals has been challenging, due to the size/stability properties of the chemical reporter group and/or the reaction kinetics of the labeling step. While developing new bioorthogonal reactions has been the traditional approach to optimizing the bioorthogonal chemical reporter strategy, here we present a different avenue, leveraging intermolecular interactions, to create bioorthogonal host–guest pairs. This approach, deemed “bioorthogonal complexation, does not rely on activated functional groups or second-order rate constants. We utilize the cucurbit[7]uril (CB[7]) scaffold to showcase bioorthogonal complexation and determine that medium-affinity (Ka ≈ 108–109 M–1) guests efficiently label cell surfaces and outperform the strain-promoted azidealkyne cycloaddition. Finally, we implement bioorthogonal complexation in the chemical reporter strategy through the metabolic incorporation of ortho-carborane into cell-surface glycans and detection with a CB[7]-fluorescein conjugate.
Graphical Abstract
Advances in chemical biology have resulted in unique methods to probe and manipulate biomolecules in cells and animals.1,2 A classic chemical biology method is the bioorthogonal chemical reporter strategy (Figure 1A)—a two-step approach to biomolecule labeling involving the incorporation of an unnatural metabolite, followed by a selective covalent chemical reaction.3,4 The bioorthogonal chemical reporter strategy expanded the scope of biomolecules analyzed in their native environments by introducing unnatural functionality through metabolic incorporation.5–9 A challenge that has arisen with this method surrounds its reliance on second-order reaction kinetics that often require high concentrations of a labeling reagent (Y), primed for reactivity with the chemical reporter group (X) (see Figure 1B, as well as Figure S1 in the Supporting Information). The high concentration of activated functionality leads to high background, especially in animal models.10–12 To overcome these challenges, the field has developed new bioorthogonal chemistries focusing on increased second-order rate constants (k).13–15 Significant success resulted in the tetrazine ligation with reaction kinetics 7–10 orders of magnitude faster than early bioorthogonal chemistries.16,17 However, the size and stability17–19 of the chemical reporters/labeling reagents in the fastest bioorthogonal chemistries limit opportunities for metabolic incorporation.
Figure 1.
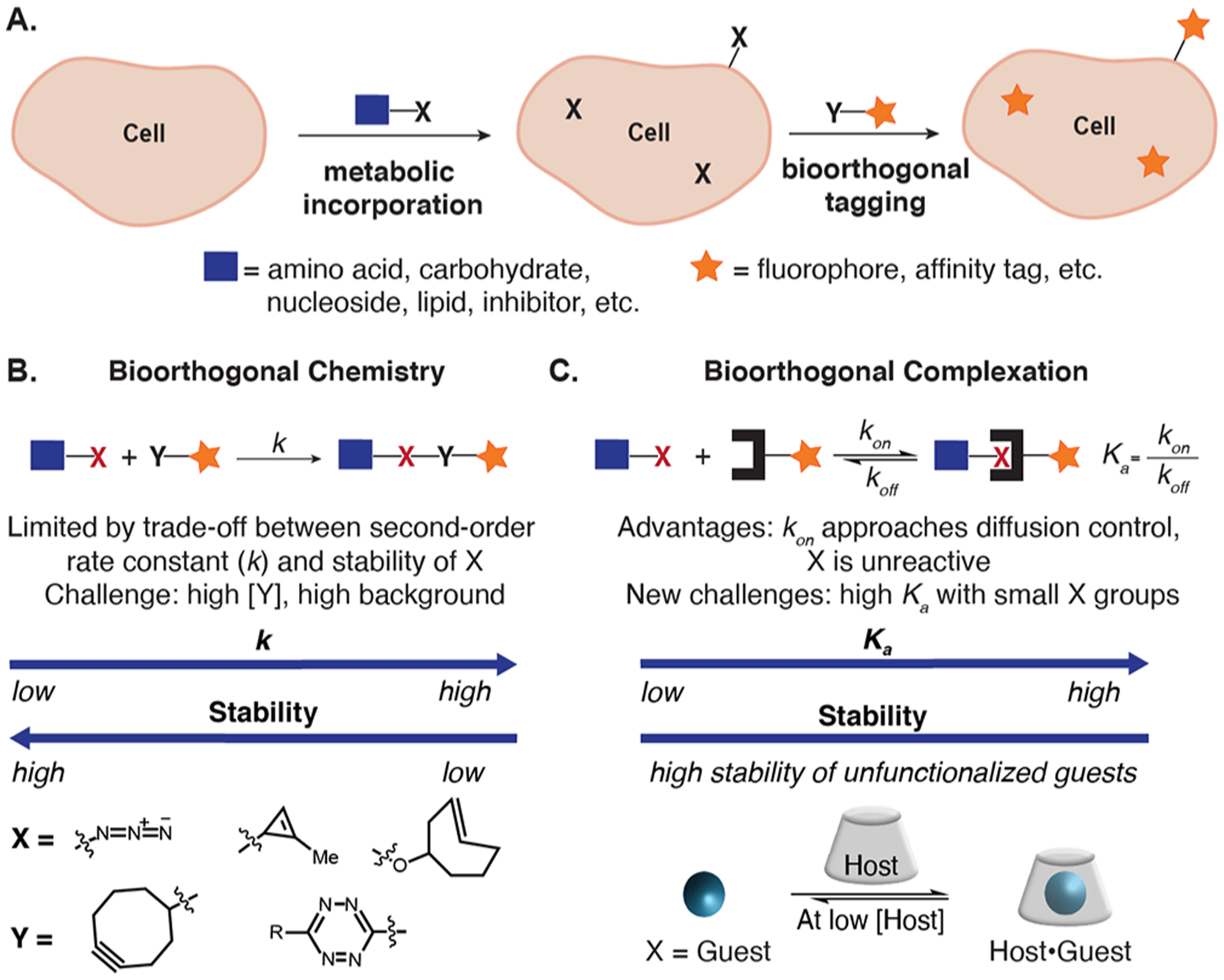
A complementary method to biomolecular tagging in living systems. (A) The bioorthogonal chemical reporter strategy. (B, C) Advantages, challenges, and examples of bioorthogonal chemistry (panel (B)) and complexation (panel (C)). See Figure S1 for further details.
A complementary approach to detect chemical reporter groups is to leverage host–guest chemistry.20–23 We give this approach using bioorthogonal noncovalent pairs the name bioorthogonal complexation (Figure 1C) to clearly differentiate it from bioorthogonal chemistry, which relies on covalent chemistry. The advantages of bioorthogonal complexation include (1) high on-rates (often approaching diffusion control in water),24 (2) absence of activated functional groups mitigating side reactivity, and (3) reversibility allowing undesired labeling to be corrected. We envision that these advantages will be particularly relevant for detection of chemical reporter groups in dilute conditions and complex environments.25,26 The widespread utility of the biotin–(strept)avidin pair showcase the power of noncovalent chemistries; however, their endogenous presence and large size is limiting.26
The challenge of bioorthogonal complexation is the development of selective host–guest pairs with small guests that can be metabolically incorporated yet have high enough Ka values for efficient labeling (Figure 1C). With the long-term goal of designing bioorthogonal host–guest pairs that fulfill these requirements, we sought to determine the minimum Ka necessary to efficiently label cell surfaces. We utilized the cucurbit[7]uril (CB[7]) scaffold, a synthetic biotin–avidin mimic, due to the impressive binding affinities that can be achieved.27–29 CB[7] and high-affinity guests adamantylamine and ferrocene amine have been used to isolate proteins30,31 and label proteins in live32,33 and fixed cells.34 While this work suggested that CB[7] would successfully detect high-affinity (Ka ≥ 1012 M–1) guests presented on the surface, we were particularly intrigued by CB[7]’s low to medium-affinity guests (Ka = 105–1010 M–1). Their smaller size offers more opportunities for metabolic incorporation, an advantage of the bioorthogonal chemical reporter strategy. Furthermore, medium-affinity complexes are more accessible, setting the stage for custom design of bioorthogonal host–guest complexes.
To explore the limits of bioorthogonal complexation, we designed a flow cytometry-based assay where guests were introduced on Jurkat cell surfaces via oxime ligation, followed by tagging with a direct CB[7]-fluorescein conjugate (CB[7]-FITC) (Figure 2A). The oxime ligation was necessary to ensure compatibility with the amine functionality present in CB[7] guests, and Jurkat cells were chosen for their high sialic acid content.35 We chose CB[7] guests spanning a Ka range of 106–1014 M–1 to determine the minimum Ka value and lowest concentration required to achieve statistically significant labeling using CB[7]-FITC (Figure 2B). CB[7]-FITC was prepared via functionalizing CB[7]-N3 with 1 through strain-promoted azide–alkyne cycloaddition (SPAAC; see Figure 2B, as well as Scheme S1 in the Supporting Information).34 The requisite amino-oxy functionalized guests 2–6 were prepared in four steps (Figure 2C, as well as Scheme S2A in the Supporting Information).36 After optimizing the cell-surface oxidation and oxime ligation conditions with an amino-oxy fluorophore (Figure S2, as well as Scheme S3 in the Supporting Information), we presented guests 2–6 on the cell surface.
Figure 2.
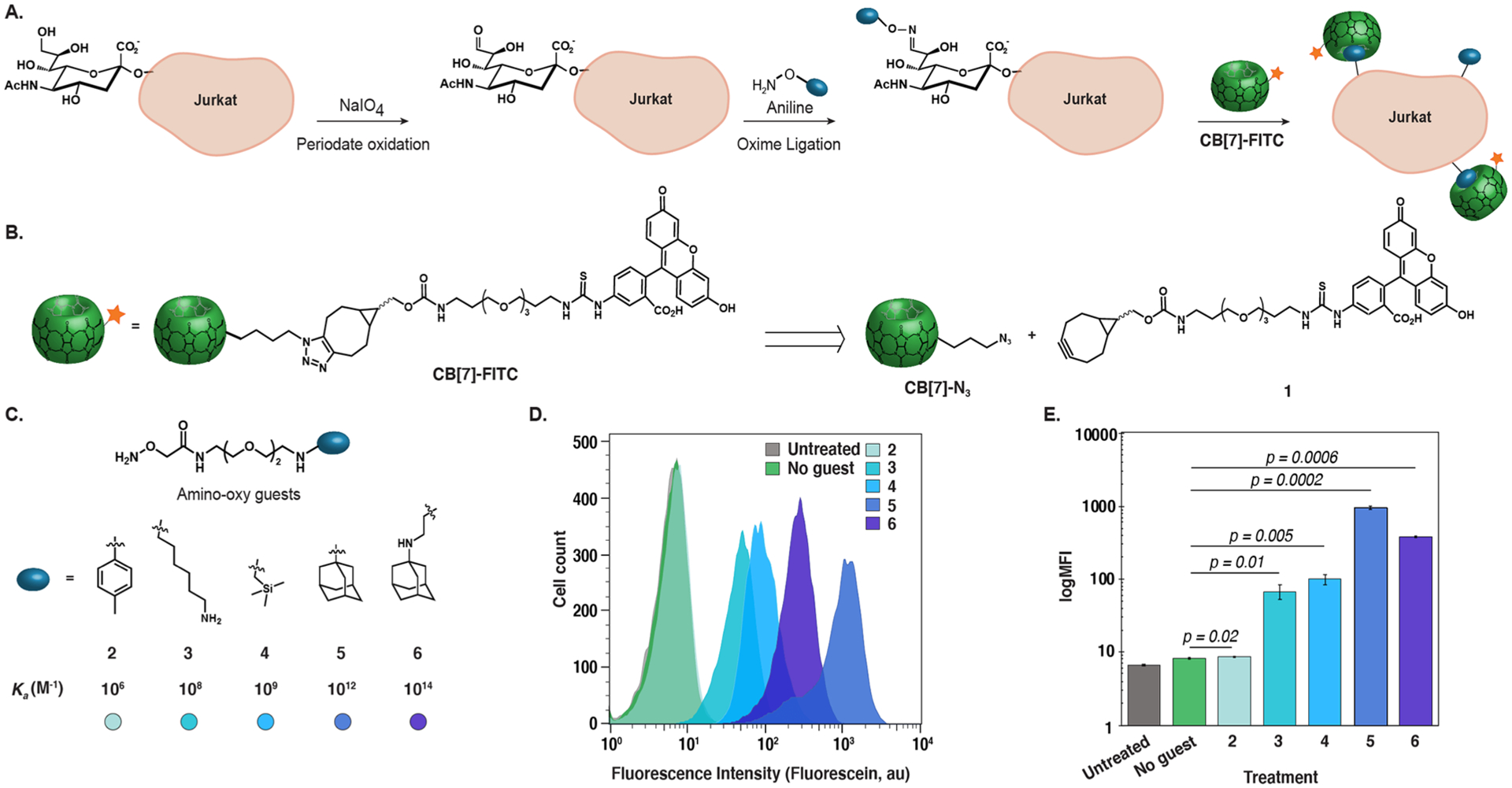
Determination of the minimum Ka required for cell-surface bioorthogonal complexation. (A) Bioorthogonal complexation assay developed to assess the minimum Ka requirement of the strategy (B) CB[7]-FITC conjugate prepared from CB[7]-N3 and 1. (C) Amino-oxy-guest compounds (2–6) with varying Ka values used in the assay. (D) Histogram and (E) bar graph of flow cytometry analysis of bioorthogonal complexation with CB[7]-FITC and different guests. Jurkat cells were oxidized using NaIO4 (1 mM) for 10 min at 0 °C, followed by incubation with 2–6 (500 μM), and aniline (10 mM) for 1 h at 0 °C. Cells were then treated with CB[7]-FITC (1 μM) for 30 min at 0 °C and analyzed by flow cytometry. See Figures S3 and S4 in the Supporting Information for replicates. Error bars represent the standard deviation of the mean of triplicates in a single experiment.
Excitingly, treatment of guest-functionalized cells with 1 μM CB[7]-FITC (Figures 2D and 2E, as well as Figures S3 and S4 in the Supporting Information) displayed excellent cell-surface labeling for four of the five guests tested with signal-to-noise (S/N) values ranging from 8.4 ± 0.2 to 122 ± 8 (3–6, Ka = 108–1014 M–1). The lowest Ka guest tested (2, Ka = 106 M–1)27 did not display statistically significant labeling at any concentration of CB[7] tested (up to 20 μM; see Figure S5 in the Supporting Information), establishing 108 M–1 as a benchmark Ka requirement for noncovalent labeling approaches that are not enhanced via avidity. It is possible that this Ka threshold could be lowered if alternative methods to increase guest concentrations on the cell surface or the use of hosts with even lower background binding were available. We did observe variability in the high-binding affinity guests (5 and 6), suggesting that Ka values of >1012 M–1 may not be necessary.
We further explored bioorthogonal complexation requirements with dose- and time-dependent experiments. Interestingly, labeling efficiency did not improve significantly at higher CB[7]-FITC concentrations (2–10 μM) (Figure S6 in the Supporting Information). Probing the lower concentration limit of guests 4–6 demonstrated that statistically significant labeling at low nanomolar concentrations could be obtained (10 nM for 4, 1 nM for 5 and 6; see Figures S6–S8 in the Supporting Information). These values are 2–3 orders of magnitude lower than concentrations used for labeling cell surface chemical reporter groups with bioorthogonal chemistry and detection by flow cytometry.13,37–40 Time-dependent experiments with guest 6 and 1 μM CB[7]-FITC showed immediate statistically significant labeling with a signal-to-noise (S/N) ratio of 9.1 ± 0.2, which increased to 22 ± 3 after 30 min. Interestingly, the flow cytometry histograms showed two clear populations (Figure S9 in the Supporting Information), suggesting that there is heterogeneity in the guest accessibility on the cell surface. Further studies are necessary to fully understand the biomodal distributions observed, which we also believe will provide insight on the differences in the high binding (Ka > 1012 M−1) guests.
The low concentration limits of bioorthogonal complexation prompted us to perform a comparative study with bioorthogonal chemistry.17 Besides higher concentrations needed for bioorthogonal chemistry with flow cytometry detection, a two-step labeling where the bioorthogonal chemistry introduces a cell-impermeable biotin that is then detected by fluorescently labeled (strept)avidin is commonly used. This secondary labeling step amplifies the signal by delivering many fluorophores per biotin. To compare bioorthogonal chemistry and complexation labeling, we chose to compare the widely used SPAAC between an azide and bicyclononyne (BCN)37 to the medium-affinity host–guest complex of (trimethylsilyl)methylamine and CB[7]-FITC.27 We introduced a consistent amount of chemical reporters onto cell surfaces using the oxime ligation with 7 (Scheme S2B in the Supporting Information) and 4, respectively. The cells were then treated with a fluorescein-BCN conjugate (1) or CB[7]-FITC at 10 μM (Figure 3A). While labeling with 4 and CB[7]-FITC resulted in a robust S/N ratio of 6 ± 2, treatment with 10 μM 1 did not produce statistically significant results (see Figures 3B and 3C, as well as Figure S10 in the Supporting Information). Applying the traditionally employed signal amplification step through treatment with an α-fluorescein IgG-CF640R conjugate, resulted in statistically significant labeling (see Figure 3D, as well as S9 in the Supporting Information); however, the S/N remained below bioorthogonal complexation (2.2 ± 0.5). Increasing the concentration of 1 (100 μM) (Figure S10 in the Supporting Information) to match previous reports37,40 improved the S/N to 3.5 ± 0.2. Note that the conditions in this comparison experiment were optimized for bioorthogonal chemistry, resulting in lower S/N ratios than previously observed with bioorthogonal complexation (Figure S6 in the Supporting Information). Similar experiments with 5 demonstrated even larger differences between bioorthogonal chemistry and complexation (see Figures S11–S13 in the Supporting Information).
Figure 3.
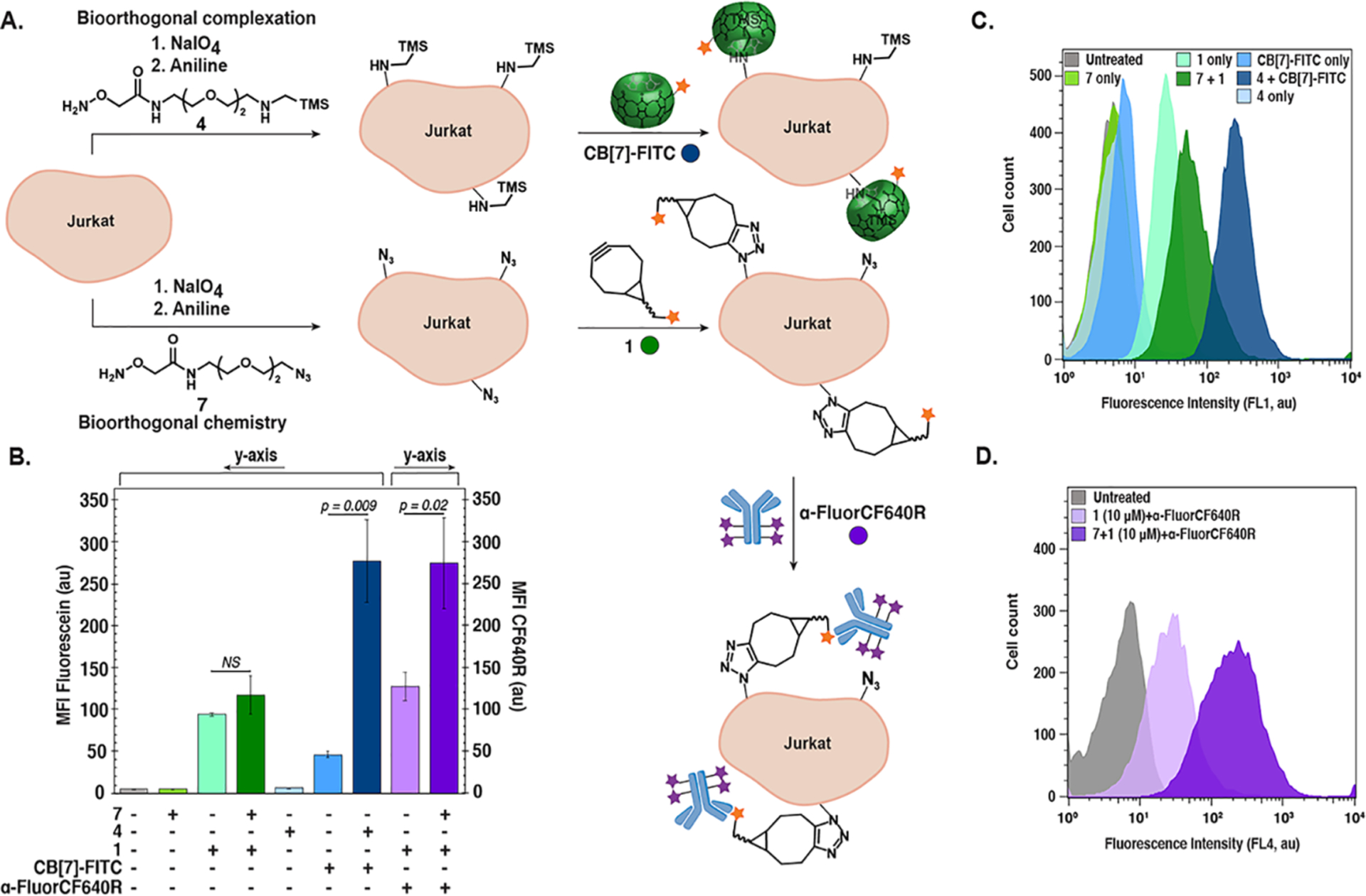
Comparative study between bioorthogonal complexation and bioorthogonal chemistry. (A) Schematic of experiment comparing bioorthogonal chemistry (SPAAC) and bioorthogonal complexations (Ka = 109 M–1). (B–D) Jurkat cells were oxidized using NaIO4 (1 mM) for 10 min at 0 °C followed by incubation with 4, 7 (500 μM), and aniline (10 mM) for 1 h at 0 °C. Fluorescence labeling occurred with 1 or CB[7]-FITC (10 μM) for 45 min at 0 °C. Cells treated with 7 and 1 were labeled with α-Fluorescein IgG-CF640R (0.002 mg mL–1) twice for 15 min at 0 °C and analyzed by flow cytometry. Data are represented as a bar graph of mean fluorescence intensities (MFI in arbitrary units, au). (B) Representative histograms for (C) fluorescein (FL1) and (D) CF640R (FL4) emission. See Figure S10 in the Supporting Information for replicate experiments.
Here, we have presented one point of comparison and we note that exchanging bioorthogonal reactions and/or fluorescent payloads14,15 could yield improved labeling. However, the demonstration of the superior labeling provided by bioorthogonal complexation at micromolar concentrations and the finding that it can be successful at nanomolar concentrations with a direct fluorophore conjugate showcase the potential for labeling chemical reporter groups with high S/N ratios in living systems. Future generations of host–guest pairs with smaller size, higher cell permeability and faster diffusion would translate bioorthogonal complexation to intracellular targets that can currently be labeled with bioorthogonal chemistries.
While the oxime ligation allowed direct comparison of bioorthogonal complexation and chemistry, ultimately for bioorthogonal complexation to be employed in the bioorthogonal chemical reporter strategy, guests must be metabolically incorporated. Metabolic oligosaccharide engineering allows guests to be appended to sialic acid residues on the cell surface when modified mannosamine or sialic acid monosaccharides are introduced. Given that a majority of known CB[7] guests are bulky molecules and cationic under physiological conditions, the incorporation of most guests in Figure 2 would be challenging.41,42 Indeed, our efforts to incorporate the smallest guest, (trimethylsilyl)methylamine, as a sialic acid derivative were unsuccessful (see Scheme S3 and Figures S14 and S15 in the Supporting Information). Considering the large chemical reporters previously incorporated in the 9-position of sialic acid,42 we attributed the lack of incorporation to the cationic nature of the (trimethylsilyl)methylamine guest.
Thus, to apply bioorthogonal complexation to the chemical reporter strategy, we turned to a recently reported medium-affinity neutral guest for CB[7]: ortho-carborane.43 Orthocarborane ethanoic acid was appended to 9-aminosialic acid (Figure 4A, as well as Scheme S4 in the Supporting Information) to produce 10 that was found to bind CB[7] with Ka = 2 × 109 M–1 in 50 mM NaOAc buffer (Figure S16 in the Supporting Information). Successful incorporation in engineered, hyposialylated BJAB K20 cells was determined using a flow cytometry-based lectin assay with 11 (the peracetylated derivative of 10) (see Figures S17 and S18 in the Supporting Information). Upon incorporation of 11, CB[7]-FITC was used for cell-surface labeling via bioorthogonal complexation (see Figures 4B–D, as well as Figures S19 and S20 in the Supporting Information). The higher CB[7]-FITC concentrations (20 μM) required were attributed to higher background due to the hyposialylated cell line, although a lower Ka value of 10 in cellulo could also be responsible. This confirms previous work on the suitability of carboranes as chemical reporters for cell-surface processes.44 We envision that the unique properties of carborane will allow for expanded applications of bioorthogonal complexation.43 Further guest optimization strives to allow the incorporation of smaller, neutral guests that would allow use of wild-type cell lines, such as Jurkat.
Figure 4.
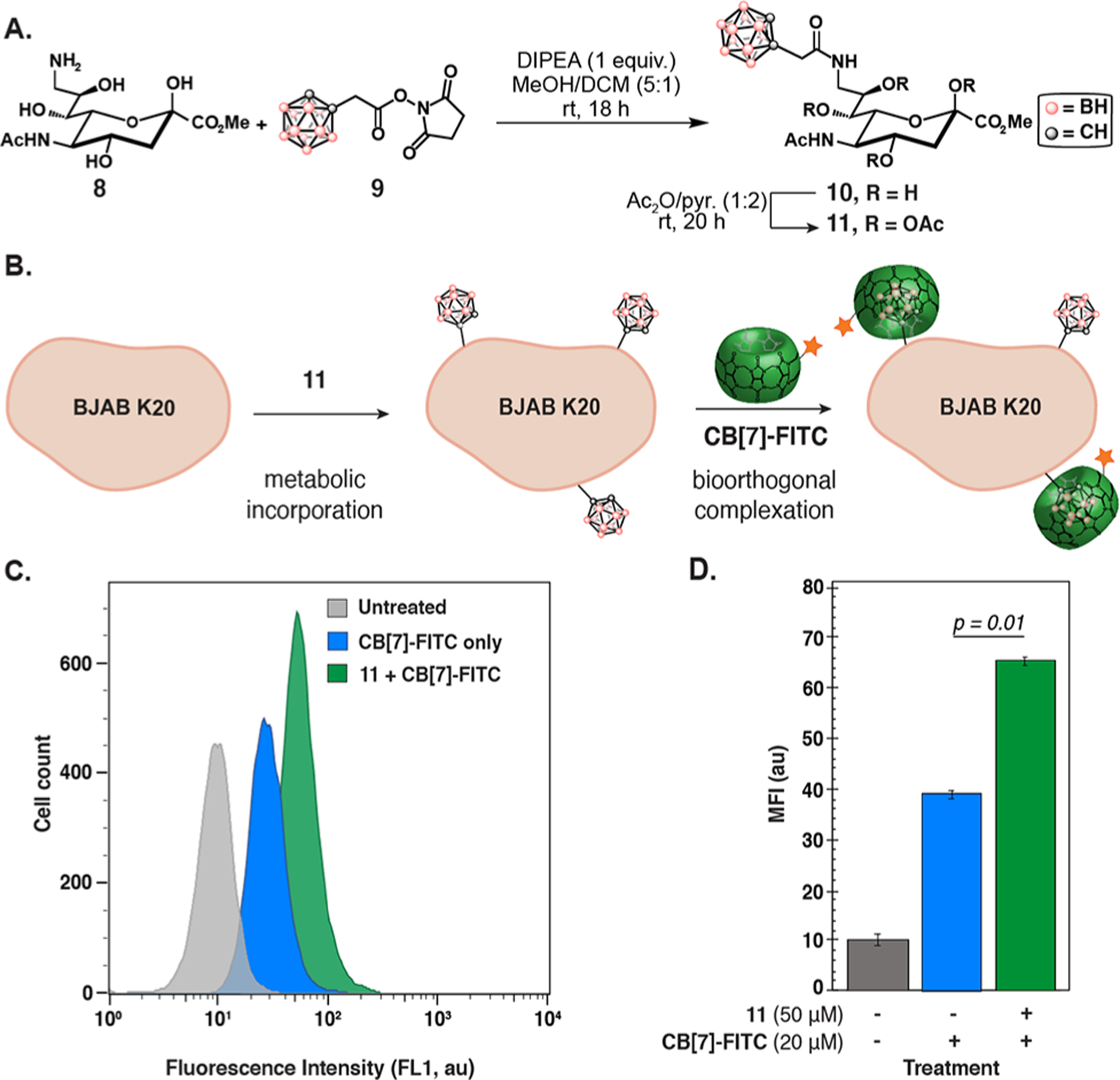
Metabolic incorporation of a CB[7] guest and labeling via bioorthogonal complexation. (A) Preparation of 11 from sialic acid precursor 8 and activated 9. (B–D) BJAB K20 cells were grown in 11 (0–50 μM) for 3 days in 90% nutridoma/FBS RPMI media. After washing, the cells were incubated with CB[7]-FITC (20 μM) for 30 min at 0 °C and analyzed by flow cytometry. A representative histogram is shown in panel (C) and an averaged MFI bar graph is shown in panel (D). Error bars represent the standard deviation of the mean of triplicates in a single experiment. [See Figures S19 and S20 for replicate experiments.]
In this report, we introduce bioorthogonal complexation as a complementary strategy to bioorthogonal chemistry for labeling chemical reporter groups. Bioorthogonal complexation efficiency relies on thermodynamics rather than kinetics, and we determined that a Ka value of ≥ 108 M–1 is necessary for robust detection of cell-surface chemical reporter groups. This is a threshold that is achievable through molecular design, providing opportunities for the development of custom bioorthogonal host–guest pairs. Concentration-dependent studies and a direct comparison to SPAAC confirmed that lower concentrations of reagent were necessary to achieve high S/N labeling with bioorthogonal complexation than bioorthogonal chemistry. The nM concentrations necessary and the decreased propensity for covalent background labeling overcome long-standing challenges for bioorthogonal chemistry.45 Finally, the metabolic incorporation of an ortho-carborane sialic acid derivative and its detection using CB[7]-FITC achieved the use of bioorthogonal complexation in the chemical reporter strategy.
These findings expand the chemical toolbox for labeling unnatural functionality in biological systems. We expect bioorthogonal complexation to be particularly relevant for direct imaging experiments, where a covalent bond is not required, such as flow cytometry and microscopy experiments. Notably, in some instances, such as activity-based protein profiling, a covalent bond is essential to the experiment and bioorthogonal chemistry will still be the preferred choice. Other applications where noncovalent chemistries are suitable, such as in pull-down experiments, may have different Ka requirements, which remains an area of exploration. Overall, we believe bioorthogonal complexation will be an advantageous approach for translating the bioorthogonal chemical reporter strategy to complex organisms.
METHODS
Details of experimental material and methods are provided in the Supporting Information.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Pawlita and T. Waterboer for their permission to use the BJAB K20 cell line and J. Kohler for providing us with the cells.
Funding
This work was supported by NIH Grant No. 1DP2GM13268 to E.M.S. NMR data were obtained on instruments funded by NSF (No. MRI CHE-1048804).
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acschembio.1c00494.
Figures S1–S20, Schemes S1–S5, and additional experimental details including synthetic and experimental procedures, NMR and mass spectra, and flow cytometry data (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acschembio.1c00494
Contributor Information
Anna Kataki-Anastasakou, Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, California 90095, United States.
Selena Hernandez, Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, California 90095, United States; Present Address: Department of Chemistry, University of Illinois Urbana–Champaign, 600 South Mathews Ave., Urbana, IL, 61801, USA.
Ellen M. Sletten, Department of Chemistry and Biochemistry, University of California Los Angeles, Los Angeles, California 90095, United States.
REFERENCES
- (1).Agatemor C; Buettner MJ; Ariss R; Muthiah K; Saeui CT; Yarema KJ Exploiting Metabolic Glycoengineering to Advance Healthcare. Nat. Rev. Chem 2019, 3, 605–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Liu CC; Jewett MC; Chin JW; Voigt CA Toward an Orthogonal Central Dogma. Nat. Chem. Biol 2018, 14, 103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Prescher JA; Bertozzi CR Chemistry in Living Systems. Nat. Chem. Biol 2005, 1, 13–21. [DOI] [PubMed] [Google Scholar]
- (4).Saxon E; Bertozzi CR Cell Surface Engineering by a Modified Staudinger Reaction. Science 2000, 287, 2007–2009. [DOI] [PubMed] [Google Scholar]
- (5).Dube DH; Bertozzi CR Glycans in Cancer and Inflammation - Potential for Therapeutics and Diagnostics. Nat. Rev. Drug Discovery 2005, 4, 477–488. [DOI] [PubMed] [Google Scholar]
- (6).Dieterich DC; Link AJ; Graumann J; Tirrell DA; Schuman EM Selective Identification of Newly Synthesized Proteins in Mammalian Cells Using Bioorthogonal Noncanonical Amino Acid Tagging (BONCAT). Proc. Natl. Acad. Sci. U. S. A 2006, 103, 9482–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Salic A; Mitchison TJ A Chemical Method for Fast and Sensitive Detection of DNA Synthesis in Vivo. Proc. Natl. Acad. Sci. U. S. A 2008, 105, 2415–2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ganz D; Harijan D; Wagenknecht H-A Labelling of DNA and RNA in the Cellular Environment by Means of Bioorthogonal Cycloaddition Chemistry. RSC Chem. Biol 2020, 1, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Flores J; White BM; Brea RJ; Baskin JM; Devaraj NK Lipids: Chemical Tools for Their Synthesis, Modification, and Analysis. Chem. Soc. Rev 2020, 49, 4602–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Rossin R; Robillard MS Pretargeted Imaging Using Bioorthogonal Chemistry in Mice. Curr. Opin. Chem. Biol 2014, 21, 161–169. [DOI] [PubMed] [Google Scholar]
- (11).Chang P; Prescher JA; Sletten EM; Baskin JM; Miller IA; Agard NJ; Lo A; Bertozzi CR Copper-Free Click Chemistry in Living Animals. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Vugts DJ; Vervoort A; Stigter-Van Walsum M; Visser GWM; Robillard MS; Versteegen RM; Vulders RCM; Herscheid JDM; van Dongen GAMS Synthesis of Phosphine and Antibody-Azide Probes for in Vivo Staudinger Ligation in a Pretargeted Imaging and Therapy Approach. Bioconjugate Chem. 2011, 22, 2072–2081. [DOI] [PubMed] [Google Scholar]
- (13).Jewett JC; Sletten EM; Bertozzi CR Rapid Cu-Free Click Chemistry with Readily Synthesized Biarylazacyclooctynones. J. Am. Chem. Soc 2010, 132, 3688–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Blackman ML; Royzen M; Fox JM Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels–Alder Reactivity. J. Am. Chem. Soc 2008, 130, 13518–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Devaraj NK; Weissleder R; Hilderbrand SA Tetrazine-Based Cycloadditions: Application to Pretargeted Live Cell Imaging. Bioconjugate Chem. 2008, 19, 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Selvaraj R; Fox JM trans-Cyclooctene—a Stable, Voracious Dienophile for Bioorthogonal Labeling. Curr. Opin. Chem. Biol 2013, 17, 753–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Patterson DM; Nazarova LA; Prescher JA Finding the Right (Bioorthogonal) Chemistry. ACS Chem. Biol 2014, 9, 592–605. [DOI] [PubMed] [Google Scholar]
- (18).Rossin R; van den Bosch SM; ten Hoeve W; Carvelli M; Versteegen RM; Lub J; Robillard MS Highly Reactive Trans-Cyclooctene Tags with Improved Stability for Diels–Alder Chemistry in Living Systems. Bioconjugate Chem. 2013, 24, 1210–1217. [DOI] [PubMed] [Google Scholar]
- (19).Rossin R; Verkerk PR; van den Bosch SM; Vulders RCM; Verel I; Lub J; Robillard MS Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew. Chem., Int. Ed 2010, 49, 3375–3378. [DOI] [PubMed] [Google Scholar]
- (20).Agasti SS; Liong M; Tassa C; Chung HJ; Shaw SY; Lee H; Weissleder R Supramolecular Host-Guest Interaction for Labeling and Detection of Cellular Biomarkers. Angew. Chem., Int. Ed 2012, 51, 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tannous BA; Grimm J; Perry KF; Chen JW; Weissleder R; Breakefield XO Metabolic Biotinylation of Cell Surface Receptors for in Vivo Imaging. Nat. Methods 2006, 3, 391–396. [DOI] [PubMed] [Google Scholar]
- (22).Houk KN; Leach AG; Kim SP; Zhang X Binding Affinities of Host–Guest, Protein–Ligand, and Protein–Transition-State Complexes. Angew. Chem., Int. Ed 2003, 42, 4872–4897. [DOI] [PubMed] [Google Scholar]
- (23).Rood MTM; Spa SJ; Welling MM; Ten Hove JB; van Willigen DM; Buckle T; Velders AH; van Leeuwen FWB Obtaining control of cell surface functionalizations via pre-targeting and supramolecular host guest interactions. Sci. Rep 2017, 7, 39908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Tang H; Fuentealba D; Ko YH; Selvapalam N; Kim K; Bohne C Guest Binding Dynamics with Cucurbit[7]Uril in the Presence of Cations. J. Am. Chem. Soc 2011, 133, 20623–20633. [DOI] [PubMed] [Google Scholar]
- (25).Schreiber CL; Smith BD Molecular Conjugation Using Non-Covalent Click Chemistry. Nat. Rev. Chem 2019, 3, 393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Liu W; Samanta SK; Smith BD; Isaacs L Synthetic Mimics of Biotin/(Strept)Avidin. Chem. Soc. Rev 2017, 46, 2391–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Liu S; Ruspic C; Mukhopadhyay P; Chakrabarti S; Zavalij PY; Isaacs L The Cucurbit[n]Uril Family: Prime Components for Self-Sorting Systems. J. Am. Chem. Soc 2005, 127, 15959–15967. [DOI] [PubMed] [Google Scholar]
- (28).Cao L;Šekutor M; Zavalij PY; Mlinarić-Majerski K; Glaser R; Isaacs L Cucurbit[7]Uril·Guest Pair with an Attomolar Dissociation Constant. Angew. Chem., Int. Ed 2014, 53, 988–993. [DOI] [PubMed] [Google Scholar]
- (29).Cao L; Isaacs L Absolute and Relative Binding Affinity of Cucurbit[7]uril towards a Series of Cationic Guests. Supramol. Chem 2014, 26, 251–258. [Google Scholar]
- (30).Lee D-W; Park KM; Banerjee M; Ha SH; Lee T; Suh K; Paul S; Jung H; Kim J; Selvapalam N; et al. Supramolecular Fishing for Plasma Membrane Proteins Using an Ultrastable Synthetic Host–Guest Binding Pair. Nat. Chem 2011, 3, 154–159. [DOI] [PubMed] [Google Scholar]
- (31).Lee SB; Kim HL; Jeong H-J; Lim ST; Sohn M-H; Kim DW Mesoporous Silica Nanoparticle Pretargeting for PET Imaging Based on a Rapid Bioorthogonal Reaction in a Living Body. Angew. Chem., Int. Ed 2013, 52, 10549–10552. [DOI] [PubMed] [Google Scholar]
- (32).Kim KL; Sung G; Sim J; Murray J; Li M; Lee A; Shrinidhi A; Park KM; Kim K Supramolecular Latching System Based on Ultrastable Synthetic Binding Pairs as Versatile Tools for Protein Imaging. Nat. Commun 2018, 9, 1712–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Sasmal R; das Saha N; Pahwa M; Rao S; Joshi D; Inamdar MS; Sheeba V; Agasti SS Synthetic Host–Guest Assembly in Cells and Tissues: Fast, Stable, and Selective Bioorthogonal Imaging via Molecular Recognition. Anal. Chem 2018, 90, 11305–11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bockus AT; Smith LC; Grice AG; Ali OA; Young CC; Mobley W; Leek A; Roberts JL; Vinciguerra B; Isaacs L; et al. Cucurbit[7]Uril–Tetramethylrhodamine Conjugate for Direct Sensing and Cellular Imaging. J. Am. Chem. Soc 2016, 138, 16549–16552. [DOI] [PubMed] [Google Scholar]
- (35).Pham ND; Fermaintt CS; Rodriguez AC; McCombs JE; Nischan N; Kohler JJ Cellular Metabolism of Unnatural Sialic Acid Precursors. Glycoconjugate J. 2015, 32, 515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Previous work did not show diminishing Ka values upon conjugation of adamantylamine to similar structures; hence, we hypothesized that 2–6 would bind CB[7] with Ka values of the same order of magnitude as the free amines.
- (37).Dommerholt J; Schmidt S; Temming R; Hendriks LJA; Rutjes FPJT; van Hest JCM; Lefeber DJ; Friedl P; van Delft FL Readily Accessible Bicyclononynes for Bioorthogonal Labeling and Three-Dimensional Imaging of Living Cells. Angew. Chem., Int. Ed 2010, 49, 9422–9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Leunissen EHP; Meuleners MHL; Verkade JMM; Dommerholt J; Hoenderop JGJ; van Delft FL Copper-Free Click Reactions with Polar Bicyclononyne Derivatives for Modulation of Cellular Imaging. ChemBioChem 2014, 15, 1446–1451. [DOI] [PubMed] [Google Scholar]
- (39).Devaraj NK; Upadhyay R; Haun JB; Hilderbrand SA; Weissleder R Fast and Sensitive Pretargeted Labeling of Cancer Cells through a Tetrazine/Trans-Cyclooctene Cycloaddition. Angew. Chem., Int. Ed 2009, 48, 7013–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Patterson DM; Nazarova LA; Xie B; Kamber DN; Prescher JA Functionalized Cyclopropenes As Bioorthogonal Chemical Reporters. J. Am. Chem. Soc 2012, 134, 18638–18643. [DOI] [PubMed] [Google Scholar]
- (41).Oetke C; Brossmer R; Mantey LR; Hinderlich S; Isecke R; Reutter W; Keppler OT; Pawlita M Versatile Biosynthetic Engineering of Sialic Acid in Living Cells Using Synthetic Sialic Acid Analogues. J. Biol. Chem 2002, 277, 6688–6695. [DOI] [PubMed] [Google Scholar]
- (42).Moons SJ; Adema GJ; Derks MT; Boltje TJ; Büll C Sialic Acid Glycoengineering Using N-Acetylmannosamine and Sialic Acid Analogs. Glycobiology 2019, 29, 433–445. [DOI] [PubMed] [Google Scholar]
- (43).Kataki-Anastasakou A; Axtell JC; Hernandez S; Dziedzic RM; Balaich GJ; Rheingold AL; Spokoyny AM; Sletten EM Carborane Guests for Cucurbit[7]Uril Facilitate Strong Binding and On-Demand Removal. J. Am. Chem. Soc 2020, 142, 20513–20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Chen X Nanotechnologies and Chemical Tools for Cell Biology. Ph.D. Thesis, University of California, Berkeley, CA, 2007. [Google Scholar]
- (45).Devaraj NK The Future of Bioorthogonal Chemistry. ACS Cent. Sci 2018, 4, 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


