Abstract
Objectives
To study the role of combined CTPA and indirect CT venogram to diagnose venous thromboembolism (VTE) in patients with COVID-19 pneumonia and to compare the clinical characteristics, laboratory parameters, CT findings and clinical outcomes between the VTE positive and negative groups.
Methods
In this retrospective study, 131 patients with COVID-19 pneumonia who underwent CTPA and venogram between August 2020 and January 2021 were included. Relevant demographical, clinical and laboratory data and CT images were collected. Two thoracic radiologists independently reviewed the CTPA and venogram images.
Results
VTE was identified in 29 patients (22% of the study population). CT venogram identified DVT in 9 patients. No statistical difference was observed between the two groups with respect to age, gender, BMI and presence of comorbidities. There was a significant difference in the hospital stay duration, which is increased in the VTE positive group. The number of patients who were dependent on oxygen and mortality were also high in the positive group. There was statistically significant difference in the mean D-dimer value and the mean Neutrophil/lymphocyte ratio, which were higher in the VTE positive group.
Conclusion
Combined CTPA and venogram can be used as a one-stop investigation for diagnosing PE and DVT of lower limbs in patients with COVID-19 pneumonia. CTPA with venogram should be performed in patients with D-dimer value in the range of 1000 – 1200 μg/L and above to rule out VTE as the hospital stay duration and final outcomes vary between the positive and negative groups.
Keywords: venous thromboembolism, venogram, pulmonary angiogram
Introduction
Coronavirus disease 2019 (COVID-19), caused by the novel coronavirus 2019 (nCoV), is one of the widespread and worst pandemics that the human race has witnessed. COVID-19 started as a cluster of cases with severe acute respiratory tract infection (SARI) and has revealed a multitude of clinical manifestations since then. Reverse-transcriptase polymerase chain reaction (RT-PCR) remains the gold standard for the diagnosis of COVID-19 infection. Multimodality imaging including radiography and CT play a crucial role in the rapid identification and early diagnosis of patients presenting with respiratory symptoms.
Many studies have shown that venous thromboembolism (VTE) is a significant cause of mortality in these patients. VTE encompasses deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE). Kollias et al,1 in their meta-analysis found the overall prevalence of VTE in hospitalized patients to be around 30%. The pathogenesis of thrombosis in vivo is explained by Virchow’s triad of hypercoagulability, endothelial injury and stasis. Prothrombotic predisposition and DVT/PTE in COVID-19 has been well documented and is believed to be due to a combination of the inflammatory response induced endothelial injury, hypoxia and immobilization.
Clinical tools like pulmonary embolism rule-out criteria (PERC) and Wells criteria are used to assess a patient’s risk for developing VTE. However, COVID-19 patients even without VTE can present with tachycardia, chest pain and hypoxia, rendering the clinical criteria unreliable. D-dimer elevation is being traditionally used to diagnose VTE; however, being an acute phase reactant, it is non-specific and also elevated in severe pneumonia making it a less reliable marker in COVID-19.2
Computed tomography pulmonary angiography (CTPA) has been a reliable modality to diagnose PTE. Studies from various countries have shown PTE incidence to be in the range of 15% to 44% of COVID-19 patients who underwent CTPA.3,4
The appropriateness of the lower extremity compression ultrasound (USG) to exclude DVT in COVID patients is debatable, due to the undue exposure of healthcare workers to the virus and its overall specificity in picking clinically relevant DVTs. Staff exposure, increased turnaround time, strenuous protocols, machine quality and availability pose hurdle in the performance of USG studies.5 CT venogram offers the advantage of diagnosing DVT while avoiding undue exposure and additional cost of PPE.
Therefore, we planned to analyze the clinical characteristics, laboratory parameters, parenchymal severity and outcomes between the VTE positive and negative groups, in those who underwent combined CTPA and venogram.
Materials and Methods
Patient Selection
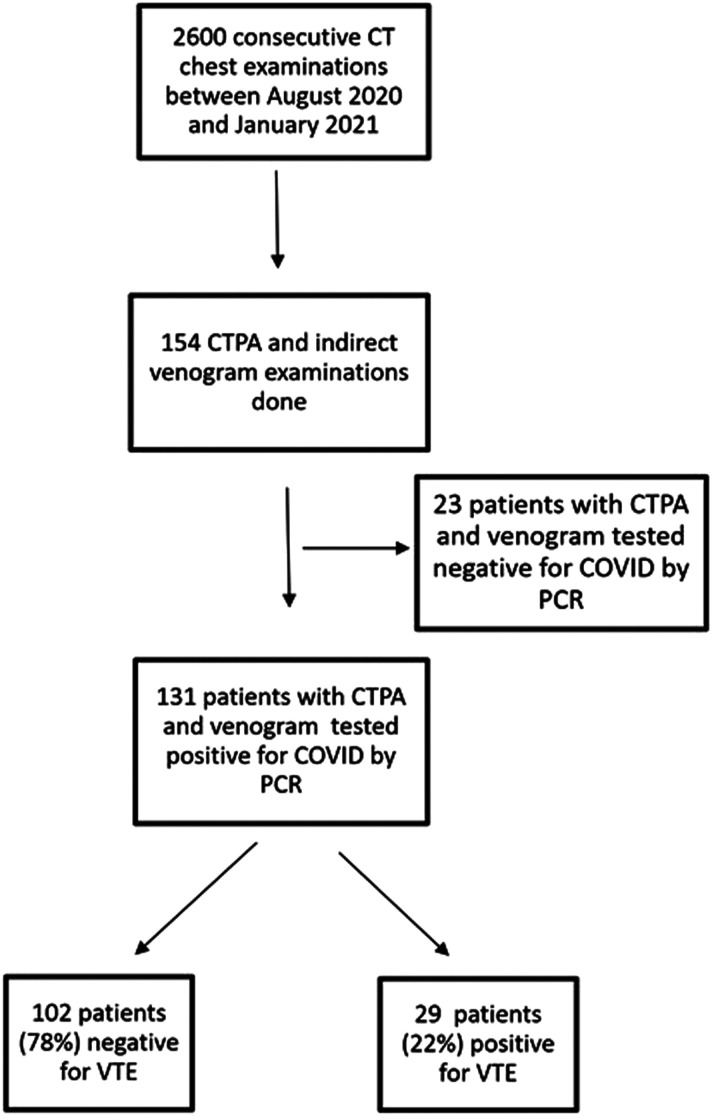
This study was approved by the local institutional ethics committee. The study was conducted as a single centre retrospective study. We included 131 consecutive patients who underwent CTPA and indirect CT venogram between August 2020 and January 2021 (Figure 1). All the patients were diagnosed to be positive for COVID-19 infection by reverse transcriptase polymerase chain reaction (RT-PCR) from the nasopharyngeal and throat swabs. Following initial triage at admission, patients in the mild category were administered a prophylactic dose of enoxaparin 1 mg/kg once daily. The moderate to severe category were given a therapeutic dose of enoxaparin (1 mg/kg twice daily) or equivalent dose of any other heparin analogue as clinically indicated.
Figure 1.
Consort diagram depicting the patient enrolment for the study.
CT Image Acquisition and Interpretation
CT examinations were performed with patients in the supine position on a 128 slice multidetector CT scanner (Siemens Perspective, China). CT pulmonary angiogram phase was acquired after injecting 80-100 mL (based on the bodyweight) of non-ionic iodinated contrast medium (Iopramide, Ultravist 370 mg) at a flow rate of 4-5 mL/second followed by a 50-mL saline flush using a pressure injector. The acquisition timing was optimized using the bolus-tracking technique with the region of interest placed at the superior venacava with a trigger threshold of 100 Hounsfield Units (HU). The entire chest was scanned in the caudocranial direction in a single breath-hold. The scan parameters were tube voltage of 80 kv, tube current of 100-120 mAs, pitch of 1.5 and gantry rotation time of .6 s. Images were reconstructed with a thickness of 1 mm, and coronal and sagittal reformats were obtained. Indirect CT venogram was acquired from the level of diaphragm to the proximal legs at a time delay of 3 minutes post contrast injection. 1 mm thin axial sections were acquired and multiplanar reformats were obtained.
Two thoracic radiologists of 10 and 7 years’ experience respectively reviewed all CTPA and venogram images. Plain chest images were analysed to evaluate the disease extent of COVID pneumonia. The amount of lung involvement by ground-glass opacities and consolidation is quantitatively measured by the CT severity score, where both the lungs are categorized into 20 segments; a score of 0 is given for each segment if there is no parenchymal involvement, a score of 1 is given if there is <50% involvement and a score of 2 is given if there is >50% involvement. The highest possible score for each patient is 40. The CTPA images were assessed for the presence or absence of PTE and if thrombus was detected, the location was categorized into main, lobar, segmental and subsegmental pulmonary arterial branches. CT venogram was assessed for the presence of DVT and the location of the thrombus. DVT was defined as a hypodense partial or complete filling defect seen on at least two consecutive axial images. Chronic DVT, seen as eccentric wall thickening was excluded from the study.
Clinical and Laboratory Data
Patient’s demographical, clinical and laboratory data were collected retrospectively from the medical records. Demographical and clinical data included age, gender, body mass index (BMI), history of active malignancy, trauma or surgery in the last four weeks and previous VTE, comorbidities (diabetes mellitus, hypertension, coronary/ischemic heart disease and chronic obstructive pulmonary disease), indications for CTPA, duration of hospital stay and the final outcome. The patient’s outcome was classified as a) improved with oxygen support and discharged; b) clinically worsened/required mechanical ventilation and subsequently discharged; c) expired. The relevant laboratory values such as D-dimer, platelets, C-reactive protein (CRP), ferritin and neutrophil-lymphocyte ratio (NLR) were collected. The time interval between the selected laboratory data and the CTPA examination was kept to less than 24 hours.
Statistical Analysis
The collected data were analysed with SPSS version 23.0 (IBM SPSS, Chicago, IL, USA). Categorical variables were expressed as the frequencies and proportions (%) and continuous variables were presented as the mean (standard deviation). To find the significant difference between the bivariate samples in independent groups, the unpaired sample t-test was used, and for the multivariate analysis, the one-way ANOVA with Turkey’s Post-Hoc test was used. To assess the relationship between the variables, Pearson’s correlation was used. To find the significance in categorical data, the Chi-Square test was used. To find the D-dimer’s efficacy, the Receiver Operating Curve [ROC] was used. In all the above statistical tools, the probability value .05 is considered as a significant level.
Results
From August 2020 to January 2021, 2600 consecutive CT chest were performed in our centre for those with RT-PCR positive COVID-19 and for those with clinical suspicion of COVID-19, but RT-PCR negative. Of these CT examinations, 154 (6%) patients had both CTPA and indirect CT venogram, of which 131 (85%) were COVID-19 RT-PCR positive and were included for the final analysis. Out of the 131, 29 patients (22%) were diagnosed radiologically to have VTE, while 102 patients (78%) were negative for VTE. Among the 29 patients who were VTE positive, 20 patients (69% of positive patients) showed radiological findings suggestive of isolated PTE, 5 patients (17% of positive patients) showed findings of combined PTE and lower limb DVT, 4 patients (14% of positive patients) with isolated DVT.
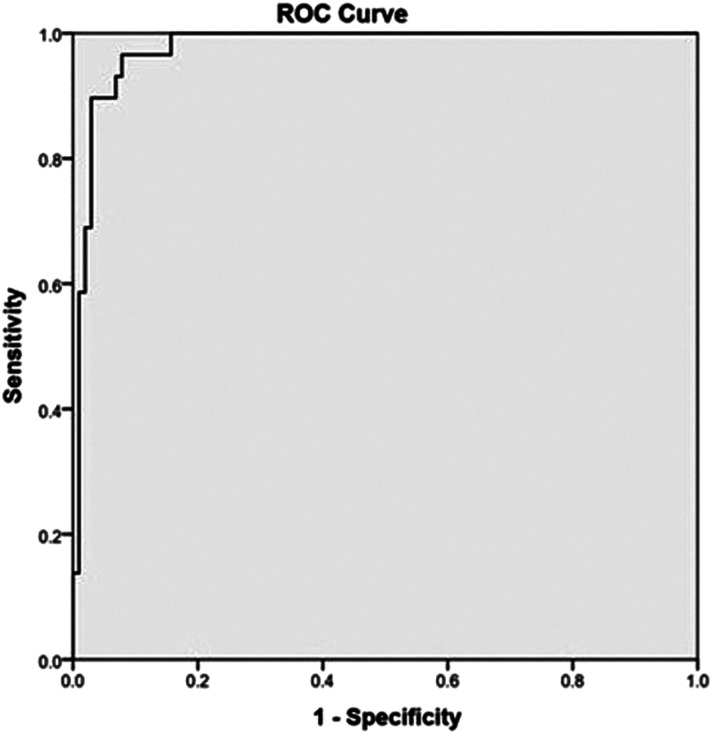
There was no statistical difference between the two groups in terms of mean age, gender, BMI at admission, frequency of patients with risk factors of VTE. (Table 1). The indications for CTPA and venogram study included hypoxia (<94%), tachycardia (HR > 120/min), respiratory distress, clinical signs of DVT and elevated D-dimer values. Of these, the frequency of positive clinical signs of DVT and D-dimer values are statistically higher in the VTE positive group. The mean D-dimer value and the mean NLR were significantly higher in the VTE positive group, when compared to VTE negative group and is statistically significant. No significant difference was noted in the CRP and Ferritin values. On ROC analysis, the D-Dimer cut off obtained in our study group for diagnosing VTE in COVID-19 was 1170.50 μg/L with high sensitivity (96.6%) and specificity (92.2%) (Figure 2).
Table 1.
Demographic and Clinical Characteristics of the COVID-19 Patients Included in the Study.
| Total | VTE Positive | VTE Negative | P-value | |
|---|---|---|---|---|
| Number of patients | 131 | 29 (22%) | 102 (78%) | |
| Age (years) | 62 ± 15 | 57 ± 13 | .124# | |
| Gender | .464# | |||
| Males | 97 (74%) | 23 (79%) | 74 (73%) | |
| Females | 34 (26%) | 6 (21%) | 28 (27%) | |
| Co-morbidities and risk factors for VTE | ||||
| Body mass index | 26.5 ± 3.6 | 26.7 ± 4.5 | .822# | |
| Obesity (BMI ≥30) | 29 (22%) | 8 (28%) | 21 (21%) | .423# |
| Diabetes mellitus | 56 (43.1%) | 15 (51.7%) | 41 (40.6%) | .286# |
| Hypertension | 47 (35.9%) | 12 (41.4%) | 35 (34.3%) | .484# |
| COPD/Asthma | 4 (3.1%) | 1 (3.4%) | 3 (2.9%) | 1.000# |
| CAD/IHD | 11 (8.4%) | 2 (6.9%) | 9 (8.8%) | 1.000# |
| Hypothyroidism | 10 (7.6%) | 1 (3.4%) | 9 (8.8%) | .457# |
| History of previous VTE | 3 (2.3%) | 1 (3.4%) | 2 (2.0%) | .531# |
| History of active malignancy | 2 (1.5%) | 1 (3.4%) | 1 (1.0%) | .395# |
| Trauma/surgery during the past 4 weeks | 4 (3.1%) | 1 (3.4%) | 3 (2.9%) | 1.000# |
| Indications for CTPA | ||||
| Hypoxia | 76 (58%) | 21 (72.4%) | 55 (53.9%) | .090# |
| Respiratory distress | 102 (77.9%) | 23 (79.3%) | 79 (77.5%) | 1.000# |
| Chest pain | 3 (2.3%) | 0 | 3 (2.9%) | 1.000# |
| Tachycardia | 10 (7.6%) | 2 (6.9%) | 8 (7.8%) | 1.000# |
| Elevated d-dimer | 29 (22.1%) | 12 (41.4%) | 17 (16.7%) | .010* |
| Clinical signs of DVT | 4 (3.1%) | 3 (10.3%) | 1 (1.0%) | .034* |
| Laboratory parameters | ||||
| D-dimer | 10 864 ± 11 580 | 378 ± 491 | .0005** | |
| CRP | 69.9 ± 80.1 | 59 ± 54 | .396# | |
| Ferritin | 784 ± 1073 | 532 ± 645 | .117# | |
| Neutrophil/lymphocytes ratio | 16.2 ± 14.9 | 6.3 ± 5.4 | .001** | |
| Clinical course | ||||
| No of hospital stay days | 10.8 ± 4.6 | 8.3 ± 3.9 | .004** | |
#No Significance at P > .05 and *Significance at P < .05 and **Highly Significance at P < .01.
Figure 2.
Receiver Operating Characteristics curve for D-dimer values to derive a cut-off value to differentiate the positive and negative groups.
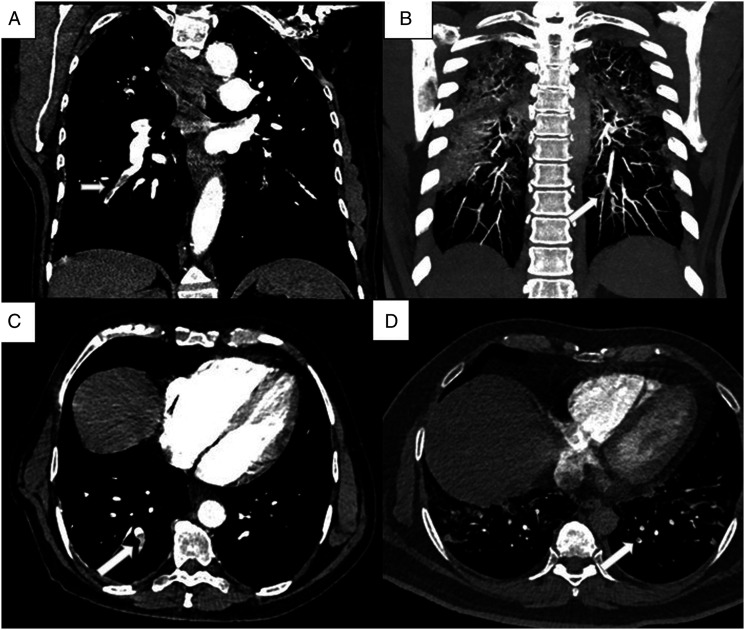
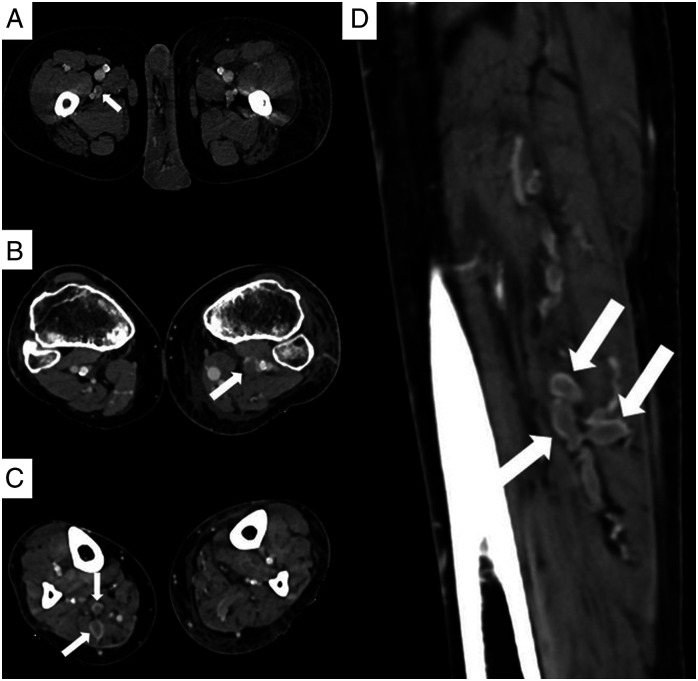
The average CT severity score among the 29 VTE positive patients in our study was 26.52, and among the 102 negative patients was 23.2, which was not significant statistically. Among the 25 patients with PTE, 16 patients (64%) showed isolated subsegmental thrombi, 5 patients (20%) showed segmental thrombi extending into subsegmental branches, 2 patients (8%) had thrombi in the lobar artery extending into segmental and subsegmental branches (Figure 3 A- D). Only one patient (4%) had a central thrombus, seen in main pulmonary artery, extending to the lobar and segmental branches. Of the 9 patients with DVT, 4 patients (44.4%) had thrombi in both proximal and distal veins of the lower extremity, 3 patients (33.3%) had isolated thrombi in distal veins, and 2 (22.2%) patients had proximal venous thrombus (Figure 4 A- D).
Figure 3.
(A - D): CTPA images of a 54-year-old male with respiratory distress. Coronal (A) and axial (C) CT images show hypodense filling defect consistent with thrombus (arrow) in the segmental and subsegmental branches of right lower lobe. CTPA images of a 38-year-old male with elevated d-dimer value. Coronal maximum intensity projection (B) and axial (D) images show thrombi in the subsegmental branches (arrow).
Figure 4.
(A - D): CT venogram images of three different patients. Axial (A) CT image of a 54-year-old male shows a hypodense partial thrombus in the right profunda femoris vein (arrow). Axial (D) CT image of a 61-year-old female shows hypodense filling defect in the left popliteal vein (arrow). Axial (C) and sagittal (D) images of the leg acquired in the venogram images of a 49-year-old male show expanded lumen with filling defects consistent with DVT in the muscular veins of the posterior compartment of the right leg (arrows).
The patients with VTE had a longer stay in the hospital with a mean duration of 10.8 days, compared to 8.3 days in the negative group (P < .01). About 41% of the patients in positive VTE group had a fatal outcome compared to 6% in the negative group, which is statistically highly significant (P < .001) (Table 2). There were also more patients dependent on oxygen in the VTE positive group who recovered subsequently.
Table 2.
Statistical analysis of the outcome of the patients in the positive and negative groups.
| Outcome | Total | Positive | Negative | P-value |
|---|---|---|---|---|
| Expired | 18(13.7%) | 12(41.4%) | 6(5.9%) | .0005** |
| Improved | 97(74.0%) | 11(37.9%) | 86(84.3%) | |
| Needed oxygen | 16(12.2%) | 6(20.7%) | 10(9.8%) |
**Highly Significant at P < .01.
Discussion
In our study, VTE incidence including PTE and DVT, among the COVID-19 patients in the Indian cohort who underwent CTPA and venogram was 22%. Different studies and research letters from various Chinese, European and US cohorts have reported PTE incidence between 15% and 44%.3,4 This is the first study reporting the incidence of COVID associated VTE in the Indian population to the best of our knowledge.
Many research papers have shown that demographic factors such as older age and male gender are at a higher risk for poor outcomes and mortality among COVID-19 patients.6-11 In our cohort, there was no significant difference between the VTE positive and negative groups with respect to age and gender distribution, which are concordant with most published studies. Our study showed that the extent of parenchymal involvement on plain CT, assessed by the CT severity score showed no statistically significant difference between the positive and negative groups. The results are concordant, with most published studies sparing Ooi et al4,9,12-15 In patients with PE, akin to the previous studies, our study also showed distribution of thrombi predominantly in the subsegmental and segmental branches, supporting the hypothesis of in situ microvascular thrombosis as the likely etiology of PE.16
In the meta-analysis by Young Joo et al, the pooled incidence of lower extremity DVT in COVID patients from various studies was found to be 14.8%.17 Bin et al reported a very high incidence of DVT, about 85% in patients with severe COVID.18 The presence of DVT is a poor prognostic indicator and such patients were shown to have adverse outcomes, including more ICU admissions and deaths.19 Guidelines for therapeutic decision making in these patients with DVT recommend a minimum duration of anticoagulation in positive cases for up to 90 days.20 In our study, DVT was detected in 6.8% of the total sample size and in 31% of the positive group. Isolated DVT was observed in 4 patients (14% of the positive group). CT venogram plays a vital role in identifying this subset of patients, where performing CTPA alone will yield false-negative results for VTE. These radiological findings were crucial in deciding the further course of management in these patients regarding the anticoagulation duration.
Many previous studies have validated the use of CT venogram in diagnosing DVT and combining it with CTPA in non-COVID settings.21,22 The PIOPED II study performed with a sample size of 711 patients concluded that the compression ultrasound and CT venography are diagnostically equivalent in excluding DVT with about 96% agreement between these modalities.23 The role of CT venogram in COVID-19 setting is an unexplored territory. The addition of venographic phase allowed evaluation of abdominal, pelvic, femoropopliteal and distal leg veins with only a minimal increase in the scan duration. This combined technique can exclude DVT with a total duration of less than 15 minutes without the requirement of any additional resources or cost. Besides, the radiologist’s personnel exposure was significantly minimized with CT venogram, compared to performing a separate lower limb Doppler for these COVID patients to rule out DVT. However, there is an additional radiation dose to the pelvis and lower extremities.
In our study, patients with VTE had a longer hospital stay and had worse outcomes than the patients without VTE. CTPA and venogram potentially identifies the patients with VTE, thereby influencing the decision to start therapeutic anticoagulation on them.
D-dimer is one of several breakdown products formed during fibrinolysis, and identifying D-dimer confirms the ongoing coagulation cascade with thrombin in action.24 Due to its non-specific nature, elevated D-dimer levels in a patient with suspected VTE necessitates imaging confirmation of VTE and further management accordingly.
The D-Dimer threshold is a number sought after by many researchers to optimize the diagnosis of true positive VTE patients. Optimal D-Dimer cut-off defined by few authors included 2903 μg/L by Ventura Diaz et al, 2660 μg/L by Leonard-Lorant et al, 2500 μg/L by Alonso-Fernández et al and 2969 μg/L by Hoffer J et al25-28 However, the D-Dimer cut-off obtained in our group was 1170.50 μg/L, which had very high sensitivity (96.6%) and specificity (92.2%). This cut-off value is concordant to the observations in the meta-analysis by Young Joo et al who suggested the conventional cut-off value 1000 μg/L could be used for PTE screening that serves as an indication for CTPA.17 Contrary to the other previous studies, the specificity of d-dimer was very high in our study, which could be multifactorial. The addition of venographic phase in our study diagnosed more patients with VTE, who would have been labeled negative if CTPA alone was performed. Most published studies correlated the d-dimer values with CTPA study and did not perform venous Doppler or CT venogram to exclude out DVT. So, patients with asymptomatic DVT in these studies would have been missed with CTPA and were included in the negative group. Selection bias could have also contributed to the high specificity since many patients with severe disease and high D-dimer values were not subjected for CT examination as they were already ventilated or hemodynamically unstable. Since D-dimer is a screening tool for VTE, keeping a low cut-off value is advantageous, as these patients could be subjected to CTPA and venogram for further evaluation to exclude VTE.
Of the other laboratory markers, NLR was statistically different between the two groups in our cohort. Many studies have shown that a higher NLR at hospital admission was associated with a more severe outcome; in particular, a NLR of >4 was a predictor of admission to the ICU.29 Recent study by Petito et al30 showed that Neutrophil activation is associated with thrombotic complications more than the platelet activation.
Our study has a few limitations. The data were collected retrospectively which could have resulted in selection bias. DVT was assessed only with indirect CT venogram and lower limb venous Doppler was not performed to confirm the findings. The estimation of additional radiation dose to the patient due to CT venogram was beyond the scope of the study design. However, there is need for more prospective studies to validate the role of CT venogram in this COVID-19 setting.
Conclusion
During this COVID-19 pandemic, as imaging and management protocols are continually evolving along with our understanding of the pathogenesis and complications of this disease. We have put forth our experience in combining indirect CT venography with pulmonary angiography in COVID patients suspected of VTE. Using this approach, diagnosis of any combination of DVT and PE is possible, along forming a one-stop shot investigation for VTE that can help decide further management. Keeping a low D-dimer threshold value for VTE screening by CTPA and venogram is vital as early identification of the VTE positive patients will help to modify the duration and the dosage of anticoagulation.
Acknowledgments
The authors thank Mr. Naveen Kumar for his contribution in reformatting and processing the CT images and authors also thank Mr. Venkatesan for his statistical work.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Committee: We have got institutional ethical committee approval from Dr.Rela Institute Ethics Committee -Ref. No. ECR/1276/Inst/TN/2019/052.
ORCID iD
Dillibabu Ethiraj https://orcid.org/0000-0002-4892-1939
References
- 1.Kollias A, Kyriakoulis KG, Lagou S, Kontopantelis E, Stergiou GS, Syrigos K. Venous thromboembolism in COVID-19: A systematic review and meta-analysis. Vasc Med. 2021;26(4):415-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramadan L, Koziatek CA, Caldwell JR, et al. Pulmonary thromboembolism in COVID-19: Evaluating the role of D-dimer and computed tomography pulmonary angiography results. Am J Emerg Med. 2020:S0735-6757(20)30790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freund Y, Drogrey M, Miró Ò, et al. Association between pulmonary embolism and COVID-19 in emergency department patients undergoing computed tomography pulmonary angiogram: The PEPCOV international retrospective study. Acad Emerg Med. 2020;27(9):811-820. [DOI] [PubMed] [Google Scholar]
- 4.Fang C, Garzillo G, Batohi B, et al. Extent of pulmonary thromboembolic disease in patients with COVID-19 on CT: Relationship with pulmonary parenchymal disease. Clin Radiol. 2020;75(10):780-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gogna A, Yogendra P, Lee SHE, et al. Diagnostic ultrasound services during the coronavirus disease (COVID-19) Pandemic. AJR Am J Roentgenol. 2020;215(5):1130-1135. [DOI] [PubMed] [Google Scholar]
- 6.Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: Focus on severity and mortality. Front Public Health. 2020;8:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gervaise A, Bouzad C, Peroux E, Helissey C. Acute pulmonary embolism in non-hospitalized COVID-19 patients referred to CTPA by emergency department. Eur Radiol. 2020;30(11):6170-6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grillet F, Behr J, Calame P, Aubry S, Delabrousse E. Acute pulmonary embolism associated with COVID-19 pneumonia detected with pulmonary CT angiography. Radiology. 2020;296(3):E186-E188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauvel C, Weizman O, Trimaille A, et al. Critical Covid-19 france investigators. Pulmonary embolism in COVID-19 patients: A French multicentre cohort study. Eur Heart J. 2020;41(32):3058-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaminetzky M, Moore W, Fansiwala K, et al. Pulmonary embolism on CTPA in COVID-19 patients. RadiolCardiothorac Imaging. 2020;2(4):e200308. Published 2020 Jul 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jalaber C, Revel MP, Chassagnon G, et al. Role of upfront CT pulmonary angiography at admission in COVID-19 patients. Thromb Res. 2020;196:138-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espallargas I, Rodríguez Sevilla JJ, Rodríguez Chiaradía DA, et al. CT imaging of pulmonary embolism in patients with COVID-19 pneumonia: A retrospective analysis. Eur Radiol. 2020:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ooi MWX, Rajai A, Patel R, Gerova N, Godhamgaonkar V, Liong SY. Pulmonary thromboembolic disease in COVID-19 patients on CT pulmonary angiography - Prevalence, pattern of disease and relationship to D-dimer. Eur J Radiol. 2020;132:109336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desborough MJR, Doyle AJ, Griffiths A, Retter A, Breen KA, Hunt BJ. Image-proven thromboembolism in patients with severe COVID-19 in a tertiary critical care unit in the United Kingdom. Thromb Res. 2020;193:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh YJ, Hong H, Ohana M, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: A systematic review and meta-analysis. Radiology. 2021;298(2):E70-E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ren B, Yan F, Deng Zet al. Extremely high incidence of lower extremity deep venous thrombosis in 48 patients with severe COVID-19 in Wuhan. Circulation. 2020;142(2):181-183. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: Prevalence, risk factors, and outcome. Circulation. 2020;142(2):114-128. [DOI] [PubMed] [Google Scholar]
- 20.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST guideline and expert panel report. Chest. 2020;158(3):1143-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garg K, Kemp JL, Wojcik Det al. Thromboembolic disease: Comparison of combined CT pulmonary angiography and venography with bilateral leg sonography in 70 patients. AJR Am J Roentgenol. 2000;175(4):997-1001. [DOI] [PubMed] [Google Scholar]
- 22.Taffoni MJ, Ravenel JG, Ackerman SJ. Prospective comparison of indirect CT venography versus venous sonography in ICU patients. AJR Am J Roentgenol. 2005;185(2):457-462. [DOI] [PubMed] [Google Scholar]
- 23.Goodman LR, Stein PD, Matta Fet al. CT venography and compression sonography are diagnostically equivalent: Data from PIOPED II. Am J Roentgenol. 2007;189(5):1071-1076. [DOI] [PubMed] [Google Scholar]
- 24.Yao Y, Cao J, Wang Qet al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J Intensive Care. 2020;8:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ventura-Díaz S, Quintana-Pérez JV, Gil-Boronat A, et al. A higher D-dimer threshold for predicting pulmonary embolism in patients with COVID-19: A retrospective study. Emerg Radiol. 2020;27(6):679-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Léonard-Lorant I, Delabranche X, Séverac F, et al. Acute pulmonary embolism in patients with COVID-19 at CT angiography and relationship to d-dimer levels. Radiology. 2020;296(3):E189-E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso-Fernández A, Toledo-Pons N, Cosío BG, et al. Prevalence of pulmonary embolism in patients with COVID-19 pneumonia and high D-dimer values: A prospective study. PLoS One. 2020;15(8):e0238216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffer J, Stewart L, Pettit K, Kline J. Venous Thromboembolism and D-dimer in patients with COVID-19. Proceedings of IMPRS. 2020;3(1). [Google Scholar]
- 29.Imran MM, Ahmad U, Usman U, Ali M, Shaukat A, Gul N. Neutrophil/lymphocyte ratio-A marker of COVID-19 pneumonia severity. Int J Clin Pract. 2021;75(4):e13698. [DOI] [PubMed] [Google Scholar]
- 30.Petito E, Falcinelli E, Paliani U, et al. Association of Neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J Infect Dis. 2021;223(6):933-944. [DOI] [PMC free article] [PubMed] [Google Scholar]