Abstract
Immunotherapy has dramatically revolutionized the therapeutic landscape for patients with cancer. Although immune checkpoint inhibitors are now accepted as effective anticancer therapies, they introduce a novel class of toxicity, termed immune‐related adverse events, which can lead to the temporary or permanent discontinuation of immunotherapy and life‐threatening tumor progression. Therefore, the effective prevention and treatment of immune‐related adverse events is a clinical imperative to maximize the utility of immunotherapies. Immune‐related adverse events are related to the intestinal microbiota, baseline gut microbiota composition is an important determinant of immune checkpoint inhibitor‐related colitis, and antibiotics exacerbate these undesirable side‐effects. Supplementation with specific probiotics reduces immune checkpoint inhibitor‐related colitis in mice, and fecal microbiota transplantation has now been shown to effectively treat refractory immune checkpoint inhibitor‐related colitis in the clinic. Hence, modifying the microbiota holds great promise for preventing and treating immune‐related adverse events. Microbiomes and their metabolites play important roles in the potential underlying mechanisms through interactions with both innate and adaptive immune cells. Here we review the gut microbiota and immune regulation; the changes occurring in the microbiota during immune checkpoint inhibitor therapy; the relationship between the microbiota and immune‐related adverse events, antibiotics, probiotics/prebiotics, and fecal microbiota transplantation in immune checkpoint inhibitor‐related colitis; and the protective mechanisms mediated by the microbiome and metabolites in immune‐related adverse events.
Keywords: fecal microbiota transplantation, immune checkpoint inhibitors, immune‐related adverse events, intestinal microbiota, probiotics
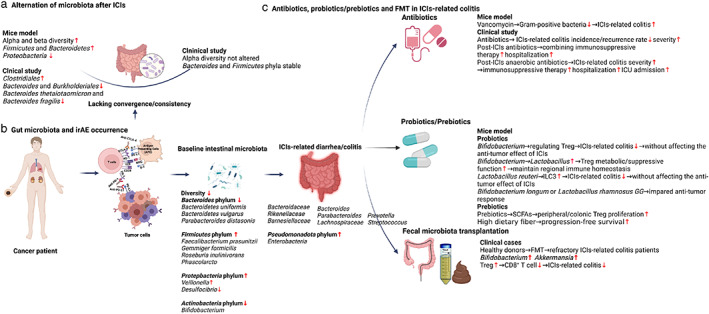
(A) Whether the intestinal microbiota changes after ICI therapy is still uncertain. (B) The baseline gut microbiota composition is an important determinant of ICIs‐related colitis. (C) After the onset of ICIs‐related colitis, the antibiotic application is a harmful factor, particularly those with anaerobic activity. The supplementation of specific probiotics e.g., Bifidobacterium and Lactobacillus could reduce ICIs‐related colitis in mice. FMT could effectively treat refractory ICIs‐related colitis in clinical cases.

INTRODUCTION
Immunotherapy, especially when combined with other therapies, continues to revolutionize anticancer therapy and improve outcomes for cancer patients. Immune checkpoint inhibitors (ICIs) are now widely used in cancer management and include antiprogrammed cell death protein‐1 (PD‐1) or antiprogrammed cell death ligand 1 (PD‐L1) inhibitors and cytotoxic T lymphocyte‐associated antigen‐4 (CTLA‐4) inhibitors, mainly as monoclonal antibody therapies. ICIs are now being used to treat many different types of cancer, especially melanoma and non‐small cell lung cancer (NSCLC). 1
Although efficacious, ICIs also introduce a novel class of toxicity termed immune‐related adverse events (irAEs). IrAEs occur due to overactivation of the immune system against both tumor and self antigens, resulting in autoimmune phenomena and consequent damage to normal tissues or organs. 2 IrAEs range from self‐limiting (e.g., rash) to life‐threatening (e.g., myocarditis, pneumonitis) and frequently preclude the continuation of ICIs in patients who are otherwise experiencing clinical responses. Furthermore, patients experiencing irAEs in fact demonstrate better progression‐free survival (PFS), overall survival (OS), and overall response rates (ORR) compared with those not experiencing toxicity. 3 Specifically, any grade (severity) of gastrointestinal irAE is associated with an improved OS and PFS. 4 However, severe irAEs lead to the temporary or permanent discontinuation of immunotherapy, resulting in life‐threatening tumor progression. Therefore, the effective prevention and treatment of irAEs are essential to ensure the continuing success of immunotherapy.
The efficacy of immunotherapy is mediated by ICI‐elicited immune responses. In 2018, three ground‐breaking studies indicated that ICI efficacy is related to the intestinal microbiota. These studies showed that, at baseline, responders have higher baseline α‐diversity (species richness in the gut microbiome within a single sample) and relative abundance of Ruminococcaceae family members than non‐responders. ICI treatment has been shown to be more effective in germ‐free mice treated by fecal microbiota transplantation (FMT) from ICI responders than those treated with FMT from ICI nonresponders. 5 , 6 Furthermore, microbiota‐based interventions (e.g., supplementation with Akkermansia muciniphila) reversed ICI resistance or restored ICI efficacy in mice initially failing to respond to FMT. 7 Recently, two clinical FMT studies demonstrated that allogeneic FMT from responders reversed primary anti‐PD‐1 resistance in 6/15 and 3/10 melanoma patients. 8 , 9 These results are promising, and modifications to the diverse baseline gut microbiota is likely to impact ICI efficacy through complex interactions between host immunity, diet, probiotics, prebiotics, postbiotics, and/or FMT. 10
However, IrAEs are the “dark” side of the immune response stimulated by ICIs. As with ICI responses, there is mounting evidence that irAEs are similarly associated with the intestinal microbiota. Therefore, microbiota‐based interventions may be promising not only to improve ICI efficacy but also for the prevention and treatment of irAEs, thereby ensuring the optimal and maximal use of immunotherapy and significantly improving outcomes for cancer patients. Here,we review the role of the gut microbiota in immune regulation, alterations occurring in the microbiota during ICI therapy, the relationship between the microbiota and irAEs, antibiotics, probiotics/prebiotics, and FMT in ICI‐related colitis, and the putative protective mechanisms exerted by the microbiome and metabolites in irAEs.
THE GUT MICROBIOTA AND IMMUNE REGULATION
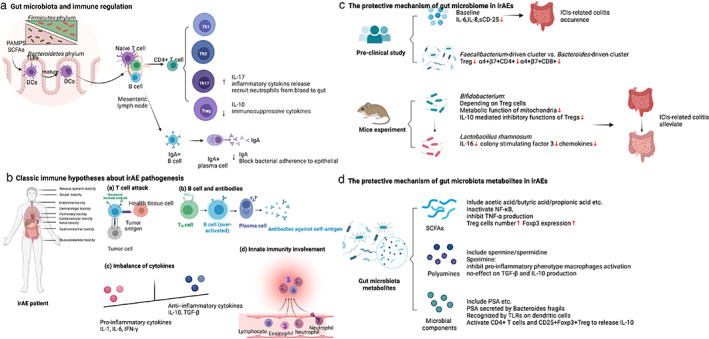
Thousands of microbial species live in the human gastrointestinal tract, mainly from the Bacteroidetes and Firmicutes phyla, which form a gut microbiota that plays crucial roles in numerous host functions. 11 The interactions between the gut microbiota and host cells not only allow for nutrient metabolism and antigen tolerance but also regulate innate and adaptive immunity both locally and systemically, contributing to immune homeostasis and thus a healthy host. 12 , 13 , 14 Briefly, pathogen‐associated molecular patterns (PAMPs) on/in bacteria or bacterial metabolites (e.g., short‐chain fatty acids; SCFAs) are transported by M cells for subsequent recognition by toll‐like receptors (TLRs) on dendritic cells in the lamina propria of the gut mucosa, facilitating the dendritic cell maturation. Mature dendritic cells migrate to mesenteric lymph nodes, where they initiate adaptive immune responses by stimulating naïve T cell differentiation into CD4+ T cells, specifically CD4+ T regulatory cells (Tregs) and T helper 17 (Th17) cells, and B cell maturation into plasma cells. 15 The Treg/Th17 axis balances host immunity: Th17 cells produce interleukin (IL)‐17, which stimulates the release of other inflammatory cytokines and recruits neutrophils from the bloodstream into the gut, while Treg cells produce IL‐10, which functions as an immunosuppressive cytokine. Moreover, plasma cells produce IgA to block bacterial adherence to intestinal epithelial cells. The crosstalk between diverse gut microbiomes and host immune systems prevents bacterial invasion and infection and modulates tumor growth (Figure 1a).
FIGURE 1.
Mechanisms of gut microbiota and immune‐related adverse events. (a) Gut microbiota and immune regulation. (b) Classic immune hypotheses about irAE pathogenesis. (c) The protective mechanism of the gut microbiome in irAEs. (d) The protective mechanisms of gut microbiota metabolites in irAEs. DCs, dendritic cells; Foxp3, forkhead box P3; ICIs, immune checkpoint inhibitors; IFN‐γ, interferon‐γ; IgA, immunoglobulin A;IL, interleukin; irAE, immune‐related adverse events; NF‐κB, nuclear factor‐κB; PAMPs, pathogen‐associated molecular patterns; PSA, polysaccharide A; sCD, soluble CD; SCFAs, short‐chain fatty acids; TGF‐β, transforming growth factor‐β; Th, T helper; TLRs, toll‐like receptors; TNF‐α, tumor necrosis factor‐α; Treg, T regulatory cell.
CHANGES IN THE GUT MICROBIOTA DURING ICI THERAPY
ICIs lack efficacy without an intact intestinal microbiota, at least experimentally. Antibiotic‐treated or germ‐free tumor‐bearing mice do not respond to anti‐CTLA‐4 antibody therapy. Furthermore, ICI treatment may alter the intestinal microbiota. In 2,4,6‐trinitrobenzene sulfonic acid (TNBS)‐treated mice, a classic inflammatory bowel disease (IBD) model, anti‐PD‐1 therapy improved the α‐diversity and restored the β‐diversity of the intestinal microbiota. It also significantly enriched for SCFA‐producing Firmicutes and Bacteroidetes bacteria but depopulated Proteobacteria. 16 In clinical studies, there is still uncertainty about if and how the intestinal microbiota changes after ICI therapy. Vetizou et al. found that only a single injection of anti‐CTLA‐4 induced rapid underrepresentation of both Bacteroides and Burkholderiales and a relative increase in Clostridiales in fecal samples. Additionally, the relative abundance of Bacteroides thetaiotaomicron and Bacteroides fragilis increased in the small intestine mucosa, but there was no increase in Bacteroides fragilis in the colonic mucosa. 17 However, Chaput et al. demonstrated relatively stable microbiota composition after anti‐CTLA‐4 treatment, except in the case of ICI‐related colitis. The α‐diversity was not altered, and the main Firmicutes and Bacteroidetes phyla remained stable. While the genera proportions differed at different time points, these changes were not significantly different. 18 However, the sample size was very small and further, large scale studies that account for confounders are now required for validation (Figure 2a).
FIGURE 2.
An overview of the gut microbiota and immune‐related adverse events. (a) Changes in the microbiota after ICIs. (b) Gut microbiota and irAE occurrence. (c) Antibiotics, probiotics/prebiotics, and FMT in ICI‐related colitis. CTLA‐4, cytotoxic T lymphocyte‐associated antigen; FMT, fecal microbiota transplantation; ICIs, immune checkpoint inhibitors; ICU, intensive care unit; ILC3, group 3 innate lymphoid cell; irAE, immune‐related adverse event; PD‐1, programmed cell death‐1; SCFAs, short‐chain fatty acids; Treg, T regulatory cell
THE GUT MICROBIOTA AND IRAE OCCURRENCE
IrAEs can affect different organs, and in general Firmicutes are associated with a higher incidence of irAEs and Bacteroidetes, Bifidobacterium of the Actinobacteria phylum, and Desulfovibrio of the Proteobacteria phylum are associated with a lower incidence. 19 , 20 Intuitively, gastrointestinal irAEs might be expected to be related to the intestinal microbiota. Indeed, ICI‐related colitis does not occur in germ‐free mice because its onset relies on the gut microbiota. 17 In humans, the baseline gut microbiota composition is an important determinant of whether ICI‐related adverse events occur. Patients who develop ICI‐related colitis have associated decreased diversity and a shift in gut microbiota composition. More specifically, they have a relative high abundance of Firmicutes (e.g., Faecalibacterium prausnitzii, Gemmiger formicilis, Roseburia inulinivorans, butyrate‐producing bacteria), while those without colitis have a high abundance of Bacteroidetes (e.g., Bacteroidetes uniformis, Bacteroidetes vulgatus, and Parabacteroides distasonis). 18 , 21 Dubin et al. analyzed baseline microbiota from 34 metastatic melanoma patients prior to ipilimumab treatment, and conclusively established that patients who did not develop ICI‐related colitis also had a higher abundance of Bacteroidetes, including Bacteroidaceae, Rikenellaceae, and Barnesiellaceae. Andrews et al. found that a more varied peripheral T cell repertoire was associated with a higher risk of developing irAEs. 22 Bacteroides intestinalis were significantly enriched in baseline gut microbiomes and the peripheral T cell repertoire was more diverse in melanoma patients who developed grade 3–5 irAEs, which was shown to be related to the upregulation of mucosal IL‐1β in murine models. 22 Furthermore, deficiencies in polyamine transport and B vitamin biosynthesis pathways were associated with a risk of colitis 21 , 23 (Table 1).
TABLE 1.
Current data on microbiota and irAEs
| Reference | Species | Disease | Intervention | irAE organ affected | Microbiota analysis method | Significant outcome | Potential mechanism |
|---|---|---|---|---|---|---|---|
|
Chaput et al. 18 |
26 patients | Metastatic melanoma | Anti‐CTLA‐4 | Colitis | 16 s rRNA |
Baseline phyla level: enriched Firmicutes in patients with colitis, high proportions of Bacteroidetes in patients without colitis 15 bacterial OTUs were detected as potential biomarkers of colitis onset. 5/6 OTUs (e.g., B. uniformis, B. vulgatus, Parabacteroides distasonis, Prevotella) from the Bacteroidetes phylum were associated with an absence of colitis. 8/9 OTUs (e.g., Faecalibacterium prausnitzii, Gemmiger formicilis, Roseburia inulinivorans, Fusicatenibacter saccharivorans, Blautia obeum, Clostridiales bacterium, butyrate‐producing bacteria from the Firmicutes phylum) were associated with colitis. |
Low proportion of peripheral blood regulatory T cells (Tregs), α4+β7+ CD4+ T cells, and α4+β7+ CD8+ T cells in patients enriched with Faecalibacterium and other Firmicutes. Patients with irAE colitis tended to have significantly higher CD4+ T cells, IL‐6, IL‐8 and sCD25. Higher inducible T cell costimulator induction on CD4+ T cells and serum CD25 in patients who enriched with Faecalibacterium. |
| Dubin et al. 23 | 34 patients | Metastatic melanoma | Anti‐CTLA‐4 | Colitis | 16 s rRNA; shotgun sequencing |
Patients with or without colitis shared many bacterial taxa belonging to the Firmicutes phylum. Patients without colitis harbored a greater proportion of the Bacteroidaceae family. Patients without colitis had a higher abundance of Bacteroidaceae, Rikenellaceae, and Barnesiellaceae from the Bacteroidetes phylum. |
Genetic pathways involved in polyamine transport and B vitamin biosynthesis were associated with an increased risk of colitis. |
| Liu et al. 24 | 26 patients | Advanced lung cancer | Anti‐PD‐1 | Colitis | 16 s rRNA |
Species abundance and diversity tended to be lower in patients with diarrhea/colitis, but without a statistical difference. Firmicutes and Bacteroidetes phylum were the most abundant in patients with or without diarrhea/colitis, respectively. Probable risk factors for diarrhea/colitis: Bacteroides and Parabacteroides from the Bacteroidetes phylum, Phascolarcto bacterium from the Firmicutes phylum, and Veillonella from the Proteobacteria phylum. |
NA |
| Chau et al. 20 | 13 patients | Lung cancer | Anti‐PD‐1/PD‐L1 | Rash/colitis/myositis/pneumonitis/thrombocytopenia | 16 s rRNA | Enrichment of Bifidobacterium of Actinobacteria phylum and Desulfovibrio of Proteobacteria phylum were significantly associated with lower incidence and grade of irAEs. | NA |
| SaKai et al. 25 | 18 patients | Lung/stomach/kidney/ovary cancer | ICIs | Colitis | 16 s rRNA | Decreased abundance of Bacteroides species and enriched Enterobacteria in inflamed regions of irAE colitis. | Pathways associated with molecular transport systems, including fatty acids, were enriched in irAE colitis. |
| McCulloch et al. 26 | 57 patients | Melanoma | Anti‐PD‐1 | Pneumonitis/colitis/hepatitis/nephritis/arthritis/throid/adrenal/dermatologic/neurologic | 16 s rRNA | Enriched for Lachnospiraceae spp. and Streptococcus spp. | NA |
Abbreviations: CTLA‐4, cytotoxic T lymphocyte antigen‐4; ICIs, immune checkpoint inhibitors; irAEs, immune‐related adverse events; NA, not applicable; OTUs, operational taxonomic units; PD‐1, programmed cell death‐1; qPCR, quantitative polymerase chain reaction; TNBS, 2,4,6‐trinitrobenzene sulfonic acid.
It is particularly worth mentioning a Chinese clinical study that explored the baseline microbiota of 26 advanced NSCLC patients receiving anti‐PD‐1 treatment. Eight of these patients experiencing diarrhea had low microbiota diversity and species abundance compared with the nondiarrhea patients. It was a pity that the difference did not achieve statistical significance, probably due to the limited number of patients. However, there was a significant difference in microbiota composition, with a higher abundance of Bacteroidetes and lower abundance of Firmicutes in non‐diarrhea patients. Bacteroides (Parabacteroides belonging to the Bacteroidetes phylum) and Phascolarctobacterium of Firmicutes were more abundant, whereas Veillonella of the Proteobacteria phylum was sparse. 24 Moreover, the decreased abundance of Bacteroides species and fatty acid pathways were observed not only in inflamed regions but also the non‐inflamed regions of irAE colitis, where immune cells are reconstituted. 25 The latest meta‐analysis showed two taxonomic groups with opposing effects on anti‐PD‐1 response, and enrichment with either Lachnospiraceae spp. or Streptococcus spp. was associated with irAEs 26 (Table 1, Figure 2b).
Thus, it may be possible to predict the risk of irAEs based on the baseline intestinal microbiota composition. Furthermore, supplementation with probiotics and FMT appear to alleviate or reduce the risk of irAEs without affecting current therapeutic efficacy.
ANTIBIOTICS, PROBIOTICS/PREBIOTICS, AND FMT IN ICI‐RELATED COLITIS
Antibiotics
In real world clinical practice, up to 68.9% patients with cancer, including melanoma, solid tumors, and hematological tumors, receive antibiotic treatment before or after ICI therapy. Antibiotics targeting anaerobic bacteria were prescribed in 51% of cases. 27 Antibiotics are likely to change the intestinal microbiota composition, so how they affect ICI‐related colitis is a further interesting question. In mouse experiments, oral administration of vancomycin, a broad‐spectrum antibiotic with activity against Gram‐positive bacteria, significantly aggravated ICI‐related colitis. 28 , 29 In clinical studies, antibiotics given before ICIs do not seem to influence the baseline dominant microbiota, potentially predictive operational taxonomic units (OTUs), or bacterial taxons, although prescription of antibiotics may alter microbiota composition. 18 Prescribing antibiotics after ICIs was associated with a higher rate of ICI‐related diarrhea/colitis accompanied by an increased risk of requiring immunosuppressive therapy and hospitalization compared with patients given antibiotics either before ICIs or both before and after ICIs. In particular, patients given antibiotics with activity against anaerobes after anti‐PD‐1 or anti‐CTLA‐4 treatment may experience severe ICI‐related diarrhea/colitis with a requirement for immunosuppressive therapy, hospitalization, and intensive care unit admission. After the onset of ICI‐related diarrhea/colitis, patients who received prophylactic antibiotics also had severe diarrhea/colitis requiring immunosuppressive therapy, intravenous glucocorticoids, and infliximab/vedolizumab and a high risk of hospitalization, longer hospital duration, and higher rate of recurrence. Thus, after the onset of ICI‐related diarrhea/colitis, antibiotics (especially those targeting anaerobic microbiomes) appear to be an adverse risk factor for irAEs and, overall, prescribing antibiotics (especially those targeting anaerobic microbiomes) risks ICI‐related diarrhea/colitis. 27 Certain anaerobes (e.g., Akkermansia muciniphila) may attenuate colitis. 7 Therefore, the unfavorable gut microbiota changes driven by the application of antibiotics targeting anaerobes may significantly affect the host immune system, facilitating immune dysregulation and ICI‐related diarrhea/colitis. 4 , 27 Adding further complexity, a recent study showed that baseline coadministration of antibiotics, glucocorticoids, proton pump inhibitors, and nonsteroidal anti‐inflammatory drugs (NSAIDs) was associated with a decreased occurrence of irAEs 30 (Table 2, Figure 2c).
TABLE 2.
Current findings on interventions targeting the microbiota and irAEs
| Section | Reference | Species | Disease/intervention | Microbiota analysis method | Significant outcome |
|---|---|---|---|---|---|
| Antibiotics in ICIs‐related colitis | Wang et al. 28 | Mouse | DSS + anti‐CTLA‐4 | 16 s rRNA |
|
| Wang et al. 29 | Mouse | DSS + anti‐PD‐1 + anti‐CTLA‐4 | 16 s rRNA |
|
|
| Abu‐Sbeih H et al. 27 | 826 patients | Anti‐PD‐1, anti‐CTLA‐4, anti‐PD‐1 + anti‐CTLA‐4 | NA |
|
|
| Kostine et al. 30 | 276 patients | ICIs | NA |
|
|
| Probiotics/prebiotics in ICIs‐related colitis | Wang F et al. 28 | Mouse | DSS + anti‐CTLA‐4 | 16 s rRNA |
|
| Sun et al. 31 | Mouse | DSS + anti‐CTLA‐4 | NA |
|
|
| Wang et al. 29 | Mouse | DSS + anti‐PD‐1 + anti‐CTLA‐4 | 16 s rRNA |
|
|
| FMT in refractory ICI‐related colitis | Wang et al. 37 | Two patients with refractory ICI‐related colitis | First patient: anti‐PD‐1 + anti‐CTLA‐4; second patient: anti‐CTLA‐4 | 16 s rRNA |
|
| Fasanello et al. 38 | One patient with refractory ICI‐related colitis | Anti‐PD‐1 | NA |
|
Abbreviations: CSF3, colony stimulating factor 3; CTLA‐4, cytotoxic T lymphocyte antigen‐4; DSS, dextran sulfate sodium; FMT, fecal microbiota transplantation; ICIs, immune checkpoint inhibitors; IFN‐γ, interferon‐γ; IL‐6, interleukin‐6; irAEs, immune‐related adverse events; KC, chemokines; OTUs, operational taxonomic units; PD‐1, programmed cell death‐1; TNF‐α, tumor necrosis factor‐α.
Probiotics/prebiotics
Probiotics are living microorganisms that provide health benefits when consumed. Probiotics may contain a variety of bacteria, such as well‐known Lactobacillus and Bifidobacterium. With respect to irAEs, supplementing diets with specific probiotics has been shown to effectively reduce the risk of ICI‐related colitis in mice. Wang et al. first established the anti‐CTLA‐4 colitis mouse model, and, in this model, the colitis is aggravated after oral administration of vancomycin and the Gram‐positive component of the microbiota appears to have a mitigating effect. Indeed, supplementation with the widely used Gram‐positive probiotic Bifidobacterium significantly reduced colitis in this model without affecting the antitumor effect of ICIs. The protective effect of Bifidobacterium was abrogated in Treg‐knockout mice, suggesting that the protective role of Bifidobacteria on ICI‐related colitis depended on Tregs. Thus, modulating the abundance of specific gut microbiomes and the presence of Tregs may maximize the efficacy of immunotherapy and minimize the risk of irAEs. If applied to humans, this strategy to regulate the intestinal microbiota could be used to prevent and treat irAEs without diminishing the anticancer responses of ICIs. 28 Interestingly, a follow‐up study demonstrated that Bifidobacterium altered the composition of the gut microbiota by increasing the abundance of probiotic Lactobacillus and Treg cells. Both the metabolic and immune‐inhibitory functions of Tregs in the gut were intensified by probiotics and the adjusted commensal microbiota, thereby maintaining regional immune homeostasis after the application of CTLA‐4 inhibitor. 31 The result was also confirmed in a subsequent mouse experiment. Wang et al. found that the microbiota was altered after the establishment of ICI‐related colitis in mice, and principal component analysis showed an obvious reduction in the abundance of Lactobacillus. The ICI‐related colitis was augmented by oral administration of vancomycin and was associated with complete depletion of Lactobacillus. Finally, supplementation with the oral, widely available probiotic Lactobacillus reuteri eliminated the ICI‐related colitis. Mechanistically, the protective effect of Lactobacillus reuteri was associated with a decrease in the population of group 3 innate lymphocytes induced by ICI‐related colitis. 29 Conversely, Spencer et al. found that probiotic supplementation (Bifidobacterium longum or Lactobacillus rhamnosus GG) significantly decreased the frequency of interferon‐γ‐positive CD8+ T cells in mice, suggesting an impaired antitumor response. 32 Hence, the multifaceted roles of probiotics in immunotherapy efficacy and irAE still require further investigation (Table 2, Figure 2c).
Prebiotics are various fibers that can be digested by gut commensals, and can be obtained from high‐fiber sources such as fruits, vegetables, nuts, and certain grains. Prebiotics promote proliferation of commensal bacteria and production of their metabolites such as SCFAs including propionate, acetate, butyrate, and succinate. 33 Prebiotics are generally accepted as a safe, noninvasive supplement to modulate the microbiota. 34 High intake of dietary fiber has been shown to be a beneficial supplement in patients taking ICIs, prolonging PFS in melanoma patients. 32 There is no direct research on the protective effect of prebiotics on irAEs. However, two studies have shown that SCFAs, as metabolites of gut bacteria, could promote the proliferation of colonic Tregs to exert immunomodulatory effects. Smith et al. demonstrated that the ability of SCFAs to induce colonic Treg proliferation in germ‐free mice depended on free fatty acid receptor 2. 35 Another study by Arpaia et al. showed that butyrate induced peripheral Treg proliferation. 36 These findings suggest that prebiotics might play a protective role in ICI‐related colitis by regulating Treg cells via SCFA production (Table 2, Figure 2c).
Fecal microbiota transplantation (FMT)
Therefore, the occurrence of ICI‐related colitis is related to the composition of the intestinal microbiota, and antibiotics or supplementation with specific probiotics can augment or reduce ICI‐related colitis. These data promoted the hypothesis that altering the intestinal microbiota by fecal transplantation could be a promising method to effectively treat irAEs, especially refractory irAEs resistant to glucocorticoids and biologics. FMT is a process in which stools from healthy donors (allo‐FMT) or previous stools from the same individual (auto‐FMT) are transplanted into the gastrointestinal tract of recipients to balance or restore gut microbial composition. FMT is already used to treat refractory Clostridium difficile colitis and IBD, which have similar manifestations and immune dysregulation to ICI‐related colitis.
In the first application of FMT in the ICI setting, Wang et al. reported two cases of refractory ICI‐related colitis which were successfully treated by FMT from healthy donors. The intestinal microbiota of the recipient was dynamically monitored, and it became most similar to the donor after the first FMT, after which it gradually returned to baseline. This result indicated that multiple FMTs might be needed to guarantee microbiota stability after FMT. Moreover, dynamic changes in T cell immunity were also monitored. FMT treatment significantly reduced the density of CD8+ T cells by increasing Treg density in the colonic mucosa. In addition, beneficial Bifidobacterium and Akkermansia were also significantly enriched after FMT, perhaps playing a protective role in preventing colitis. 7 , 37 Fasanello et al. also reported that patients with refractory ICI‐related colitis resistant to corticosteroids and biologic therapy could achieve clinical and colonoscopic remission after FMT 38 (Table 2, Figure 2c).
As a result, the 2020 National Comprehensive Cancer Network (NCCN) guidelines included FMT as an optional treatment for immunosuppressant‐refractory colitis based on institutional availability and expertise. 39
THE PROTECTIVE MECHANISM OF THE GUT MICROBIOTA IN IRAES
Classic immune hypotheses about irAE pathogenesis
Given the role of gut microbiota in mediating host immune responses, either microbiomes themselves or their metabolites may significantly mediate not only response but also toxicity to immunotherapy. To our knowledge, studies about the potential mechanisms underlying the relationship between the intestinal microbiota and irAEs are very limited. There are several classical hypotheses about irAE pathogenesis: (1) T cell attack: T cells stimulated by ICIs attack both tumor cells and healthy tissues due to similar antigenic epitopes 40 ; (2) B cell and autoantibody involvement: T cell expansion induced by ICIs may over‐activate B cells to produce antibodies against self‐antigens, leading to tissue or organ damage; (3) cytokine release: the imbalance between proinflammatory cytokines (e.g., IL‐1, IL‐6, interferon‐γ [IFN‐γ]) and anti‐inflammatory cytokines (e.g., IL‐10 and transforming growth factor‐β [TGF‐β]) may lead to irAEs, where Th17 activation and IL‐17 secretion favor the Treg/Th17 axis; and (4) innate immunity involvement: neutrophil‐mediated inflammatory responses, a high level of circulating eosinophils, and an increased neutrophil/lymphocyte ratio are associated with irAE development 41 (Figure 1b).
Gut microbiomes
In their preclinical study, Chaput et al. analyzed the correlation between baseline immune populations and microbiota composition. They demonstrated that patients who ultimately developed colitis had decreased baseline serum IL‐6, IL‐8, and soluble CD (sCD)‐25. 17 IL‐6 and IL‐8 are proinflammatory cytokines, and sCD‐25 plays an essential role in IL‐2 production and thereby the development of Tregs. 42 Patients belonging to the Faecalibacterium‐driven cluster had a low proportion of baseline Tregs (α4+β7+CD4+, α4+β7+CD8+) compared with the Bacteroides‐driven cluster. 18 In mouse experiments, Bifidobacterium altered the composition of the gut microbiota depending on the existence of Tregs. Moreover, this adjusted commensal microbiota improved both the metabolic function of mitochondria and the IL‐10–mediated inhibitory functions of intestinal Tregs, facilitating the amelioration of ICI‐related colitis. Interestingly, Bifidobacterium breve might be the key functional strain responsible for alleviating colitis, since there was a correlation between the abundance of Bifidobacterium and Lactobacillus at the genus level, and these two bacteria lead to decreased serum inflammatory cytokines IL‐6, colony stimulating factor 3, and chemokines. Thus, Bifidobacterium breve and Lactobacillus rhamnosum are potential key influencing bacteria to improve gastrointestinal adverse effects during ICI treatment. 31 Akkermansia muciniphila is currently the leading bacteria related to ICI efficacy, and it is also significantly reduced in IBD patients and mouse colitis models. Supplementation with Akkermansia muciniphila could improve colitis and reduce infiltrating macrophages and CD8+ T cells in the colon 43 (Figure 1c).
Gut microbiota metabolites
In addition to gut microbiomes, metabolites (e.g., SCFAs, polyamines, and microbial fragments such as polysaccharide A) can potentially induce or relieve irAEs. SCFAs, including acetic acid, butyric acid, and propionic acid, are primary metabolic end‐products of gut microbiota; the SCFA concentration in the gut depends on the host diet, especially fiber content. SCFAs not only acts as an energy source for the gut microbiota and intestinal epithelial cells but also affect host immunity and thereby irAEs after ICI therapy in cancer patients. In the blood, the interaction between peripheral blood mononuclear cells and SCFAs inactivates nuclear factor‐κB (NF‐κB) and inhibits the production of TNF‐α, which is a pro‐inflammatory cytokine. 44 , 45 The inhibition of histone deacetylases by SCFAs increases the expression of forkhead box P3 (Foxp3) and Treg numbers. In mice, Tao et al. found that enhanced Treg function mitigates colitis in mice. 46 Accordingly, microbe‐related SCFAs function as a suppressive factor against inflammation and therefore a potential mediator of irAEs. In addition, bacteria produce polyamines essential for host cell function and immunity, including spermine and spermidine. Zhang et al. found that spermine inhibits the proinflammatory macrophage phenotype without affecting the production of anti‐inflammatory TGF‐β and IL‐10. Consequently, a lack of polyamine production driven by dysbiosis may lead to over‐production of inflammatory cytokines and thereby the development of irAEs.
Finally, microbial components such as polysaccharide A also play an important role in host immunity and the development of irAEs. Polysaccharide A is secreted by Bacteroides fragilis in the colon and is recognized by TLR2 on dendritic cells, which activate CD4+ T cells and CD25+Foxp3+ Tregs to release IL‐10, which suppresses inflammation. 47 , 48 Collectively, microbial components and metabolites, including SCFAs, polysaccharide A, and polyamines, are anti‐inflammatory mediators that may suppress the development of irAEs by interacting with both innate and adaptive immune cells (Figure 1d).
CONCLUSIONS
The occurrence of IrAEs relies on the presence of the gut microbiota. Baseline gut microbiota composition is an important determinant of ICI‐related colitis, especially the proportion of Firmicutes and Bacteroidetes. After the onset of ICI‐related colitis, administering antibiotics is harmful, particularly those targeting anaerobic organisms. Supplementation with specific probiotics, e.g., Bifidobacterium and Lactobacillus, seems to reduce the severity of ICI‐related colitis in mice. FMT is emerging as an effective treatment for refractory ICI‐related colitis in the clinic. Our knowledge about the potential underlying mechanisms is still limited, but the microbiomes and their metabolites appear to play important roles by interacting with both innate and adaptive immune cells.
CONFLICT OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the Youth Program of National Natural Science Foundation of China (82000526, 81702292); and the General Program of Natural Science Foundation of Beijing Municipality (7192172). The authors are grateful to the BioRender software for aiding the creation of figures.
Tan B, Liu Y, Tang H, Chen D, Xu Y, Chen M, et al. Gut microbiota shed new light on the management of immune‐related adverse events. Thorac Cancer. 2022;13(19):2681–2691. 10.1111/1759-7714.14626
Funding information National Natural Science Foundation of China, Grant/Award Number: 82000526; Natural Science Foundation of Beijing Municipality, Grant/Award Number: 7192172
Contributor Information
Min‐jiang Chen, Email: minjiangchen@163.com.
Yue Li, Email: yuelee76@gmail.com.
REFERENCES
- 1. Vaddepally RK, Kharel P, Pandey R, Garje R, Chandra AB. Review of indications of FDA‐approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence. Cancers. 2020;12:738. 10.3390/cancers12030738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:1–11. 10.1038/212276-018-0191-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Das S, Johnson DB. Immune‐related irAE events and anti‐tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. 10.1186/240425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abu‐Sbeih H, Ali FS, Qiao W, Lu Y, Patal S, Diab A, et al. Immune checkpoint inhibitor‐induced colitis as a predictor of survival in metastatic melanoma. Cancer Immunol Immunother. 2019;68:553–61. 10.1007/s00262-019-02303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Matson V, Fessier J, Bao R, Chongsuwat T, Zha Y, Alegre ML, et al. The commensal microbiome is associated with anti‐PD‐1 efficacy in metastatic melanoma patients. Science. 2018;359:104–8. 10.1126/science.aao3290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, et al. Gut microbiome modulates response to anti‐PD‐1 immunotherapy in melanoma patients. Science. 2018;359:97–103. 10.1126/science.aan4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou M, Daillere R, et al. Gut microbiome influences efficacy of PD‐1‐based immunotherapy against epithelial tumors. Science. 2018;359:91–7. 10.1126/science.aan3706 [DOI] [PubMed] [Google Scholar]
- 8. Davar D, Dzutsev AK, MuCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti‐PD‐1 therapy in melanoma patients. Science. 2021;371:595–602. 10.1126/science.abf3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baruch EN, Youngster I, Ben‐Betzalel G, Ortenberg R, Lahat A, Katz L, et al. Fecal microbiota transplant promotes response in immunotherapy‐refractory melanoma patients. Science. 2021;371:602–9. 10.1126/science.abb5920 [DOI] [PubMed] [Google Scholar]
- 10. Zhou Y, Liu Z, Chen T. Gut microbiota: a promising milestone in enhancing the efficacy of PD1/PD‐L1 blockade therapy. Front Oncol. 2022;12:847350. 10.3389/fonc.2022.847350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Human Microbiome Project Consortium . Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. 10.1038/nature11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lozupone CA, Stombaugh J, Gordon J, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–30. 10.1038/nature11550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lloyd‐Price J, Mahurkar A, Rahnavard G, Crabtree J, Orvis J, Hall AB, et al. Strains, functions and dynamics in the expanded human microbiome project. Nature. 2017;550:61–6. 10.1038/nature23889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. 10.1038/nature18848 [DOI] [PubMed] [Google Scholar]
- 15. Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4. 10.1038/nature10434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu HM, Zhou YL, Xu J, Li YF, Zhao C, Huang HL, et al. Inhibition of PD‐1 protects against TNBS‐induced colitis via alteration of enteric microbiota. Biomed Res Int. 2021;2021:4192451. 10.1155/2021/4182451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vetizou M, Pitt JM, Daillere R, Lepage P, Waldschmitt N, Flament C, et al. Anticancer immunotherapy by CTLA‐4 blockade relies on the gut microbiota. Science. 2015;350:1079–84. 10.1126/science.aad1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chaput N, Lepage P, Coutzac C, Soularue E, Roux KL, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368–79. 10.1093/annonc/mdx108 [DOI] [PubMed] [Google Scholar]
- 19. Huang C, Li M, Liu B, Zhu H, Dai Q, Fan X, et al. Relating gut microbiome and its modulating factors to immunotherapy in solid tumors: a systematic review. Front Oncol. 2021;11:642110. 10.3389/fonc.2021.642110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chau J, Yadav M, Liu B, Furqan M, Dai Q, Shahi S, et al. Prospective correlation between the patient microbiome with response to and development of immune‐mediated adverse effects to immunotherapy in lung cancer. BMC Cancer. 2021;21:808. 10.1186/s12885-021-08530-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pierrard J, Seront E. Impact of the gut microbiome on immune checkpoint inhibitor efficacy‐a systematic review. Curr Oncol. 2019;26:395–403. 10.3747/co.26.5177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andrews MC, Duong CPM, Gopalakrishnan V, Iebba V, Chen WS, Derosa L, et al. Gut microbiota signatures are associated with toxicity to combined CTLA‐4 and PD‐1 blockade. Nat Med. 2021;27:1432–41. 10.1038/s41591-021-01406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dubin K, Callahan MK, Ren B, Khanin R, Viale A, Ling L, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint‐blockade‐induced colitis. Nat Commun. 2016;7:10391. 10.1038/ncomms10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu T, Xiong Q, Li L, Hu Y. Intestinal microbiota predicts lung cancer patients at risk of immune‐related diarrhea. Immunotherapy. 2019;11:385–96. 10.2217/imt-2018-0144 [DOI] [PubMed] [Google Scholar]
- 25. Sakai K, Sakurai T, De Velasco MA, Nagai T, Chikugo T, Ueshima K, et al. Intestinal microbiota and gene expression reveal similarity and dissimilarity between immune‐mediated colitis and ulcerative colitis. Front Oncol. 2021;11:763468. 10.3389/fonc.2021.763468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCulloch JA, Davar D, Rodrigues RR, Badger JH, Fang JR, Cole AM, et al. Intestinal microbiota signatures of clinical response and immune‐related adverse events in melanoma patients treated with anti‐PD‐1. Nat Med. 2022;28:545–56. 10.1038/s41591-022-01698-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abu‐Sbeih H, Herrera LN, Tang T, Altan M, Chaftari AMP, Okhuysen PC, et al. Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor‐mediated diarrhea and colitis. J Immunother Cancer. 2019;7:242. 10.1186/s40425-019-0714-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang F, Yin Q, Chen L, Davis MM, et al. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA‐4 blockade. Proc Natl Acad Sci U S A. 2018;115:157–61. 10.1073/pnas.1712901115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang T, Zheng N, Luo Q, Jiang L, He B, Yuan X, et al. Probiotics Lactobacillus reuteri abrogates immune checkpoint blockade‐associated colitis by inhibiting group 3 innate lymphoid cells. Front Immunol. 2019;10:1235. 10.3389/fimmu.2019.01235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kostine M, Mauric E, Tison A, Barnetche T, Barre A, Nikolski M, et al. Baseline co‐medications may alter the anti‐tumoural effect of checkpoint inhibitors as well as the risk of immune‐related adverse events. Eur J Cancer. 2021;157:474–84. 10.1016/j.ejca.2021.08.036 [DOI] [PubMed] [Google Scholar]
- 31. Sun S, Luo L, Liang W, Yin Q, Cuo J, Rush AM, et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc Natl Acad Sci U S A. 2020;117:27509–15. 10.1073/pnas.1921223117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spencer CN, McQuade JL, Gopalakrishnan V, McCulloch JA, Vetizou M, et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science. 2021;374:1632–40. 10.1126/science.aaz7015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham T, et al. A gut commensal‐produced metabolite mediates colonization resistance to salmonella infection. Cell Host Microbe. 2018;24:296–307.e7. 10.1016/j.chom.2018.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schwartz DJ, Rebeck ON, Dantas G. Complex interactions between the microbiome and cancer immune therapy. Crit Rev Clin Lab Sci. 2019;56:567–85. 10.1080/10408363.2019.1660303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly‐Y M, et al. The microbial metabolites, short‐chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341(6145):569–73. 10.1126/science.1241165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arpaia N, Campbell C, Fan X, Dikiy S, Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T‐cell generation. Nature. 2013;504:451–5. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Wiesnoski DH, Helmink BA, Gopalakrishnan V, Choi K, Dupont HL, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor‐associated colitis. Nat Med. 2018;24:1804–8. 10.1038/s41591-018-0238-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fasanello MK, Robillard KT, Boland PM, Bain AJ, Kanehira K. Use of fecal microbial transplantation for immune checkpoint inhibitor colitis. ACG Case Rep J. 2020;7:e00360. 10.14309/crj.0000000000000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Thompson JA, Schneider BJ, Brahmer J, Andrews S, Armand P, Bhatia S, et al. NCCN guidelines insights: management of immunotherapy‐related toxicities, version 1.2020. J Natl Compr Cancer Network. 2020;18:230–41. [DOI] [PubMed] [Google Scholar]
- 40. Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, et al. Genetic basis for clinical response to CTLA‐4 blockade in melanoma. N Engl J Med. 2014;371:2189–99. 10.1056/NEJMoa1406498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drobni ZD, Zafar A, Zubiri L, Zlotoff DA, Alvi MR, Lee C, et al. Decreased absolute lymphocyte count and increased neutrophil/lymphocyte ratio with immune checkpoint inhibitor‐associated myocarditis. J Am Heart Assoc. 2020;9:e018306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bell CJ, Sun Y, Nowak UM, Clark J, Howlett S, Pekalski ML, et al. Sustained in vivo signaling by long‐lived IL‐2 induces prolonged increases of regulatory T cells. J Autoimmun. 2015;56:66–80. 10.1016/j.haut.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang L, Tang L, Feng Y, Zhao SY, Han M, Zhang C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourgenesis by modulation of CD8+ T cells in mice. Gut. 2020;69:1988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Usami M, Kishimoto K, Ohata A, Miyoshi M, Aoyama M, Fueda Y, et al. Butyrate and trichostatin a attenuate nuclear factorκB activation and tumor necrosis factor α secretion and increase prostaglandin E2 secretion in human peripheral blood mononuclear cells. Nutr Res. 2008;28:321–8. 10.1016/j.nutres.2008.02.012 [DOI] [PubMed] [Google Scholar]
- 45. Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short‐chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22:849–55. 10.1016/j.jnutbio.2010.07.009 [DOI] [PubMed] [Google Scholar]
- 46. Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–307. 10.1038/nm1652 [DOI] [PubMed] [Google Scholar]
- 47. Wang Q, McLoughlin RM, Cobb BA, Charrel‐Dennis M, Zaleski KJ, Golenbock D, et al. A bacterial carbohydrate links innate and adaptive responses through Toll‐like receptor 2. J Exp Med. 2006;203:2853–63. 10.1084/jem.20062008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, et al. The Toll‐like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–7. 10.1126/science.1206095 [DOI] [PMC free article] [PubMed] [Google Scholar]


