Abstract
Underexpression of p53 is considered the leading cause of the decreased miR-1246 expression in B cells of systemic lupus erythematosus (SLE) patients, yet the exact mechanism of action still remains unclear. To further explore the molecular mechanism of p53 upregulating miR-1246 expression, we targeted the methylation and acetylation of histone H3 in the miR-1246 promoter region of SLE B cells. We found that increased histone H3 trimethylation at Lys27 (H3K27me3) and decreased histone H3 acetylation at Lys9 and Lys14 (H3K9/K14ac) in the miR-1246 promoter region are essential for the low expression of miR-1246 in SLE B cells. p53 can promote miR-1246 transcription by recruiting Jumonji domain–containing protein 3 (JMJD3), E1A-binding protein p300 (EP300), and CREB-binding protein (CBP) to bind to the miR-1246 promoter, downregulating H3K27me3 and upregulating H3K9/K14ac. Furthermore, early B cell factor 1 (EBF1), CD40, CD38, and X box binding protein-1 (XBP-1) expression levels in SLE B cells transfected with p53 expression plasmid were significantly decreased, whereas autoantibody IgG production in autologous CD4+ T cells cocultured with overexpressed p53 SLE B cells was reduced. Collectively, our data suggest that the reduction of p53 decreases miR-1246 expression via upregulation of H3K27me3 and downregulation of H3K9/14ac, which in turn results in SLE B cell hyperactivity.
Key Points
p53 directly upregulates miR-1246 expression in B cells.
p53 regulates histone H3K27me3 and H3K9/K14ac in the miR-1246 promoter.
p53 recruits JMJD3 and EP300/CBP to bind to the miR-1246 promoter.
Introduction
Systemic lupus erythematosus (SLE), as a complicated chronic autoimmune disease, is characterized by multiple organ or system damage caused by abnormalities in immune activity, including the innate immune response, autoreactive T cells, and, especially, abnormally active B cells (1). In the pathogenesis of SLE, the extremely active abnormal B cells are involved in almost all stages of disease development, including the release of cytokines, the presentation of Ags, and the production of autoantibodies (2–4). Therefore, exploring the molecular mechanism of B cell overactivation is essential to reveal the pathogenesis of SLE.
MicroRNAs (miRNAs), which are a class of noncoding small RNAs, can downregulate the expression of target genes at the posttranscriptional level (5). Recently, studies have reported that altered expression of miRNAs in B cells is closely related to the occurrence of SLE (6–8). In our previous study, we found that reduced expression of miR-1246 in the B cells of patients with active SLE could enhance the expression of early B cell factor 1 (EBF1), then promoting further activation of B cells (8). The deficiency of p53 is considered to be the main reason for the decreased expression of miR-1246 (8), but the molecular mechanism of p53 regulating miR-1246 expression still remains unclear.
To elucidate the molecular mechanism of p53 upregulating miR-1246 expression, we targeted the methylation and acetylation of histone H3 in the miR-1246 promoter region. Histone H3 trimethylation at Lys27 (H3K27me3), histone H3 acetylation at Lys9 (H3K9ac), and histone H3 acetylation at Lys14 (H3K14ac) were used as targets. H3K27me3 is a modification that is closely related with inactive gene promoters (9, 10). H3K9ac, as an important histone acetylation modification, is highly correlated with the active promoter state (11). H3K14ac shows a high co-occurrence with H3K9ac, which is considered as the hallmark of active gene promoters (12). Briefly, our data indicated that p53 could recruit Jumonji domain–containing protein 3 (JMJD3), E1A-binding protein p300 (EP300), and CREB-binding protein (CBP) into the miR-1246 promoter and upregulate the expression of miR-1246 by reducing the methylation of H3K27 and increasing the acetylation of H3K9/K14. Our results provide an explanation for p53 regulation of miR-1246 expression in B cells, and they further improve our understanding of the molecular mechanism of miR-1246 low expression in SLE B cells.
Materials and Methods
Subjects
Twenty female active SLE patients who fulfilled at least four of the SLE classification criteria of the American College of Rheumatology were recruited from the outpatient clinic and inpatient ward of the Department of Dermatology of The Second Xiangya Hospital (13). Table I shows the relevant clinical and laboratory information of patients. Lupus disease activity was assessed using the SLE Disease Activity Index (14). Additionally, we recruited 20 sex- and age-matched healthy controls from the medical staff at The Second Xiangya Hospital. The Human Ethics Committee of The Second Xiangya Hospital of Central South University approved this study, and written informed consent was obtained from all participants.
Table I.
Clinical and laboratory characteristics of patients with SLE in the study
| Characteristics | Characteristics of SLE (n = 20) |
|---|---|
| Sex, male/female (n) | 0/20 |
| Age (y), median (range) | 30 (15–55) |
| SLEDAI score, median (range) | 12 (8–22) |
| Anti-dsDNA (IU/ml), median (range) | 378.45 (82–801) |
| C3 (g/l), median (range) | 0.66 (0.4–0.95) |
| C4 (g/l), median (range) | 0.15 (0.06–0.29) |
| Medications (n) | |
| Prednisolone or methylprednisolone | 20 |
| Hydroxychloroquine | 16 |
| Cyclosporine-A | 4 |
| Mycophenolate mofetil | 2 |
SLEDAI, Systemic Lupus Erythematosus Disease Activity Index.
Isolation, culture, and transfection of B cells
According to our previous protocols (7), B cells were extracted from peripheral venous blood using human CD19 beads (Miltenyi Biotec, Bergisch Gladbach, Germany), and were cultured in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% FBS and 1% penicillin/streptomycin. B cells were transfected with gene expression plasmid pCMV6 or gene interference plasmid pRS using human B cell Nucleofector kits and Amaxa Nucleofector (Lonza, Basel, Switzerland). In short, B cells were harvested and resuspended in 100 μl of human B cell Nucleofector solution, and then the cell suspension was mixed with the plasmid. The mix was then electrotransfected using Nucleofector program U-015 in the Amaxa Nucleofector. The transfected cells were cultured in RPMI 1640 culture medium and harvested for 48 h. p53 expression (pCMV6-p53), negative control (pCMV6), p53 interference plasmid (pRS-p53), and scrambled sequence plasmid (pRS-scrambled sequence) were obtained from OriGene Technologies (Rockville, MD).
RNA isolation and real-time PCR
Total RNA, which was isolated from B cells using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA), was synthesized to cDNAs using a miScript II RT (reverse transcriptase) kit (Qiagen, Valencia, CA). A real-time PCR reaction using a fluorescent dye SYBR Green PCR kit (Qiagen) was performed in triplicate using ABI Prism 7500 (Thermo Fisher Scientific). The expression of target miRNA was normalized to RNU6-2. The fold change was calculated using the equation 2−ΔΔCt: ΔΔCt = (Cttarget gene − Ctinternal control)sample − (Cttarget gene − Ctinternal control)control, where Ct indicates threshold cycle. Primers for miR-1246 and RNU6-2 were obtained from Qiagen.
Western blot
B cells were lysed in protein lysis buffer containing proteinase inhibitor (Thermo Fisher Scientific). Lysates were centrifuged for 15 min at 14,000 × g at 4°C, and protein concentration was determined by a Bradford protein assay (Thermo Fisher Scientific). Proteins were separated by SDS-PAGE using 10% polyacrylamide gels and then transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA). Membranes were blocked with 5% nonfat dry milk and immunoblotted with primary Abs including anti-p53, anti-JMJD3, anti-EP300, anti-CBP, anti-EBF1, anti-CD40, anti-CD38, anti–X box binding protein-1 (XBP-1), and anti-GAPDH. All Abs were purchased from Abcam (Cambridge, U.K.). Band intensity was quantified using Quantity One software (Bio-Rad, Hercules, CA).
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) analysis was performed according to the instructions provided by a SimpleChIP Plus sonication ChIP kit (Cell Signaling Technology, Danvers, MA). First, B cells were incubated in media with 1% formaldehyde for 20 min at room temperature, and then to stop crosslinking with glycine with a final concentration of 0.125 M for 5 min. Pellet cells were collected after washing twice and suspended in cold RIPA buffer (10 mM Tris-Cl [pH 8.0], 150 mM NaCl, 0.1% SDS, 0.1% deoxycholate, 1% Triton X-100, 5 mM EDTA), and sonicated to shear the genomic DNA into 500- to 1000-bp fragments. We then added anti-p53, anti-JMJD3, anti-EP300, anti-CBP, anti-H3K27me3, anti-H3K9ac, anti-H3K14ac, or control rabbit IgG and incubated overnight. All Abs were obtained from Cell Signaling Technology. The immune complexes were precipitated with protein A–agarose beads, washed, and then eluted in 100 μl of TE (Tris-EDTA) buffer. Purified DNA was used to amplify the target fragment by PCR or real-time PCR. Primers used were as follows: forward 1 (−263 to −243), 5′-TTTATTCCTGGAGCAGATGG-3′, reverse 1 (−80 to −58), 5′-GGTTCACATTACTAAGTTCCCT-3′; forward 2 (−911 to −894), 5′-ATGGGAGCAAGAACATG-3′, reverse 2 (−664 to −646), 5′-AAGCAGCACTAGACAAGG-3′; forward 3 (−1947 to −1930), 5′-TGTTGGCAGAGGAGGTT-3′, reverse 3 (−1674 to −1654), 5′-GAGGCAATAGTTTCAGGTTC3′.
Coimmunoprecipitation
Nuclear proteins from B cells were extracted using NE-PER nuclear and cytoplasmic extraction reagents (Thermo Fisher Scientific). Nuclear extracts were incubated overnight with anti-p53 or control rabbit IgG at 4°C. Protein A–agarose beads (Cell Signaling Technology) were added to each IP reaction and incubated for 2 h at 4°C with rotation. Agarose beads were collected by centrifugation at 3000 × g for 2 min.
The protein complex was eluted with loading buffer and analyzed by Western blot. Primary Abs included anti-p53, anti-JMJD3, anti-EP300, and anti-CBP.
Plasmid construction and luciferase activity assay
DNA fragments encompassing the −263- to −58-bp, −911- to −646-bp, and −1947- to −1654-bp regions in the miR-1246 promoter were amplified by PCR from normal human B cell genomic DNA and then cloned into the pGL3-promoter vector, respectively. These constructs were then cotransfected into BJAB cells together with p53 expression (pCMV6-p53) or negative control (pCMV6) plasmid for the luciferase reporter assay. Relative luciferase activity was normalized to Renilla luciferase activity for each transfected well. Experiments were performed in triplicate in three independent trials.
T–B cell costimulation and ELISA
SLE B cells were transfected with pCMV6-p53 or pCMV6. At 24 h posttransfection, transfected B cells were cultured in six-well plates (1 × 106/ml) and stimulated with anti-IgM (2 μg/ml) (8). Autologous CD4+ T cells were isolated by positive selection using CD4 magnetic beads (Miltenyi Biotec). Purified CD4+ T cells were cultured in 24-well plates (1 × 106/ml) and stimulated with plate-bound anti-CD3 Ab (eBioscience, San Diego, CA), followed by the addition of soluble anti-CD28 Ab (eBioscience) and incubation at 37°C for 12 h. Anti-IgM–stimulated B cells were cocultured with activated autologous CD4+ T cells at a ratio of 1:4 in 96-well round-bottom plates according to the previously described procedures (15). The medium was supplemented on day 4, and the supernatants were collected on day 8 to measure the IgG concentrations. IgG concentration was measured using a human IgG ELISA kit (Abcam) according to the manufacturer’s instructions. Three replicate wells were quantified for every sample, and all experiments were performed in triplicate. OD values were read at 450 nm using an ELx800 absorbance microplate reader (BioTek, Winooski, VT).
Statistical analysis
All of the diagrams and graphs report cumulative data as the means ± SEM. Variables were compared using a Student t test (data from different transfections were compared by a paired t test, and other data were compared by a two-group t test). Correlation between two groups was analyzed using Pearson’s correlation coefficient. Significance was set as p ≤ 0.05. All statistical analyses were conducted using SPSS 22.0 software.
Results
p53 binds to the miR-1246 promoter and regulates its expression
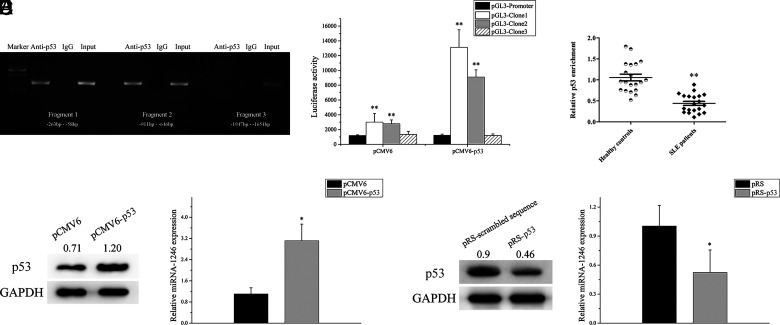
To confirm whether p53 can directly bind to the miR-1246 promoter, we performed a ChIP-PCR analysis in B cells from healthy donors using a p53 Ab. Several pairs of primers that covered the miR-1246 promoter −1947- to −58-bp region were observed. As shown in (Fig. 1A, the results indicated that p53 binds to the miR-1246 promoter −911- to −58-bp region in B cells. Consequently, DNA fragments encompassing the −263- to −58-bp, −911- to −646-bp, and −1947 to −1654-bp region in the miR-1246 promoter were cloned into pGL3-promoter vectors. These constructs were then cotransfected into BJAB cells together with pCMV6-p53 or pCMV6 for a luciferase reporter assay. The highest luciferase activities were detected in the group cotransfected with pCMV6-p53, reporting the carrier containing the −911- to −58-bp region (Fig. 1B). Additionally, we detected the binding levels of p53 in the promoter region of miR-1246 by ChIP–quantitative PCR (qPCR) in B cells from SLE patients and healthy controls. The binding level of p53 in the miR-1246 promoter in SLE B cells was clearly weakened compared with healthy controls (Fig. 1C).
FIGURE 1.
p53 binds to the miR-1246 promoter and regulates its expression. (A) ChIP-PCR showed that p53 binds in the −911- to −58-bp region of the miR-1246 promoter in B cells. Data represent the mean of three independent experiments. (B) Relative firefly luciferase activity in BJAB cells cotransfected with p53 expression plasmid (pCMV6-p53) or negative control plasmid (pCMV6), together with indicated miR-1246 promoter constructs. Clone 1, −263 to −58 bp; clone 2, −911 to −646 bp; clone 3, −1947 to −1654 bp. (C) ChIP-qPCR analysis of the enrichment of p53 in the miR-1246 promoter in B cells of SLE patients (n = 20) and healthy controls (n = 20). (D) Relative miR-1246 level in SLE B cells transfected with pCMV6 or pCMV6-p53 were assessed by real-time PCR and normalized to RNU6-2. Data represent the mean of three independent experiments. (E) Relative miR-1246 level in normal B cells transfected with p53 interference plasmid (pRS-p53) or scrambled sequence plasmid (pRS-scrambled sequence) was assessed by real-time PCR and normalized to RNU6-2. Data represent the mean of three independent experiments. *p < 0.05, **p < 0.01.
We transfected pCMV6-p53 or pCMV6 into SLE B cells. The miR-1246 expression was increased after overexpression of p53 in SLE B cells compared with the negative control group (Fig. 1D). Furthermore, we transfected the p53 interference plasmid (pRS-p53) or scrambled sequence plasmid (pRS-scrambled sequence) into normal B cells. The miR-1246 expression was decreased after p53 interference in normal B cells compared with the negative control group (Fig. 1E). These findings indicate that p53 can directly bind to the miR-1246 promoter and upregulate its transcription.
p53 regulates histone H3K27me3 and H3K9/K14ac in the miR-1246 promoter region
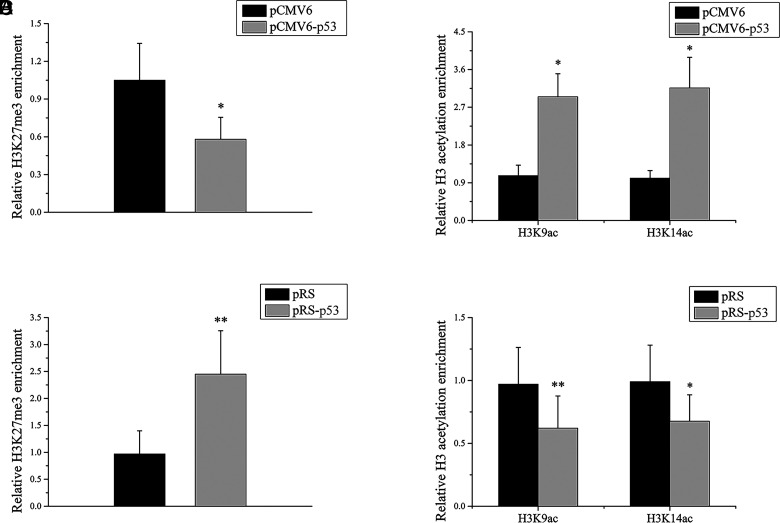
To explore the relationship between p53 and histone methylation/acetylation of the miR-1246 promoter, we transfected pCMV6-p53 or pCMV6 into SLE B cells. The results of ChIP-qPCR showed that the level of H3K27me3 was significantly lower (Fig. 2A) and the levels of H3K9/K14ac were noticeably higher in the miR-1246 promoter of SLE B cells after p53 overexpression (Fig. 2B). Additionally, the pRS-p53 or pRS-scrambled sequence was transfected into normal B cells. After p53 interference, the H3K27me3 level increased (Fig. 2C) and the H3K9/K14ac levels decreased in the promoter region (Fig. 2D). These findings indicate that p53 upregulates miR-1246 expression by decreasing the H3K27me3 level and increasing the H3K9/K14ac level in B cells.
FIGURE 2.
p53 regulates histone H3K27me3 and H3K9/K14ac in the miR-1246 promoter region. (A and B) ChIP-qPCR analysis of the enrichment of histone H3K27me3 (A) and H3K9/K14ac (B) in the miR-1246 promoter in SLE B cells transfected with pCMV6 or pCMV6-p53. Results are presented relative to those obtained with input DNA prepared from untreated chromatin. Data represent the mean of three independent experiments. (C and D) ChIP-qPCR analysis of the enrichment of histone H3K27me3 (C) and H3K9/K14ac (D) in the miR-1246 promoter in normal B cells transfected with pRS-scrambled sequence or pRS-p53. Results are presented relative to those obtained with input DNA prepared from untreated chromatin. Data represent the mean of three independent experiments. *p < 0.05, **p < 0.01.
Enrichment of histone H3K27me3 and H3K9/K14ac in the miR-1246 promoter in SLE B cells
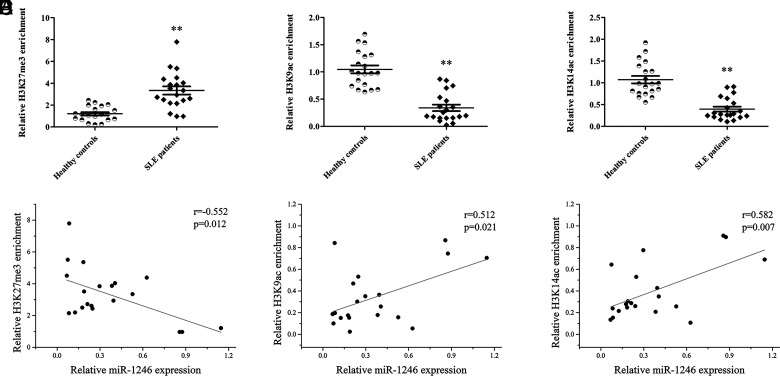
To explain the epigenetic mechanism of low expression of miR-1246 in B cells of SLE patients, we detected H3K27me3 and H3K9/14ac of the miR-1246 promoter in B cells from 20 SLE patients and 20 healthy controls. The ChIP-qPCR results showed that the level of H3K27me3 of the miR-1246 promoter was significantly higher, whereas the levels of H3K9/K14ac were significantly lower in SLE patients compared with healthy controls (Fig. 3A–C). In addition, to explore the relationship between H3K27me3/H3K9ac/H3K14ac and miR-1246 expression, we measured the expression level of miR-1246 in SLE B cells by real-time PCR and performed correlation analyses between H3K27me3/H3K9ac/H3K14ac and miR-1246 expression in SLE B cells. The results of the correlation analyses showed a significant negative correlation between the H3K27me3 and miR-1246 expression levels, as well as a significant positive correlation between the H3K9/K14ac and miR-1246 expression levels (Fig. 3D–F).
FIGURE 3.
Enrichment of histone H3K27me3 and H3K9/K14ac in the miR-1246 promoter in SLE B cells. (A–C) ChIP-qPCR analysis of the enrichment of histone H3K27me3 (A) and H3K9/K14ac (B and C) in the miR-1246 promoter of B cells isolated from SLE patients or healthy controls (n = 20 for each group). The results are presented relative to those obtained with input DNA prepared from untreated chromatin. (D) Correlation between histone H3K27me3 enrichment and miR-1246 expression level (n = 20). (E) The correlation between histone H3K9ac enrichment and miR-1246 expression level (n = 20). (F) Correlation between histone H3K14ac enrichment and miR-1246 expression level (n = 20). **p < 0.01.
p53 recruits JMJD3 and EP300/CBP to bind to the miR-1246 promoter
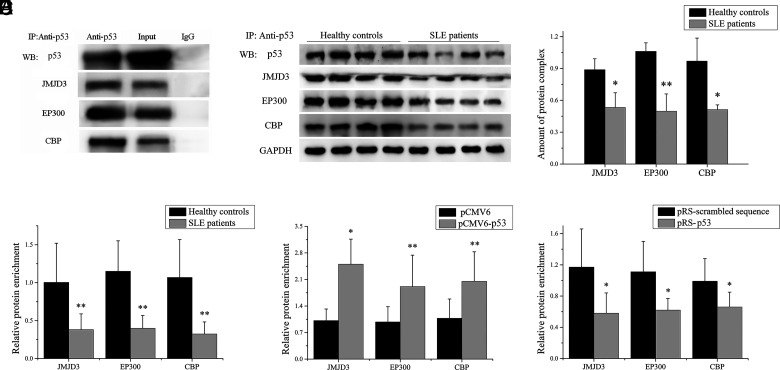
To explain p53 regulating histone methylation and acetylation in the miR-1246 promoter region, we used the STRING database (https://string-db.org/cgi/input.pl) to predict the proteins that might interact with p53 and where multiple proteins were predicted, including JMJD3 (a histone H3K27 demethylase) and EP300 and CBP (two histone acetyltransferases). We tested whether p53 could form complexes with JMJD3, EP300, and CBP. Briefly, JMJD3, EP300, and CBP expression plasmids were cotransfected into BJAB cells together with a p53 expression plasmid for coimmunoprecipitation assay. As shown in (Fig. 4A, the results of coimmunoprecipitation indicated that p53 coprecipitates with JMJD3, EP300, and CBP. Furthermore, we measured the amount of p53/JMJD3/EP300/CBP protein complex in B cells of SLE patients and healthy controls, and the results of coimmunoprecipitation showed that the amount of p53/JMJD3/EP300/CBP protein complex in B cells of SLE patients was significantly lower compared with healthy controls (Fig. 4B, 4C).
FIGURE 4.
p53 recruits JMJD3 and EP300/CBP to bind to the miR-1246 promoter. (A) A coimmunoprecipitation assay using anti-p53 in BJAB cells was cotransfected with p53, JMJD3, EP300, and CBP expression plasmids, with detection of the combination of p53 and JMJD3/EP300/CBP by Western blot. (B and C) Lysates from B cells isolated from SLE patients (n = 4) or healthy controls (n = 4) were coimmunoprecipitated using the p53 Ab, followed by Western blot with p53, JMJD3, EP300, and CBP Abs. GAPDH as input in lysates. One representative blot is shown (B). The intensity of bands was semiquantitated and normalized to GAPDH (C). (D) ChIP-qPCR analysis of the enrichment of JMJD3, EP300, and CBP in the miR-1246 promoter in B cells from SLE patients and healthy controls. Results are presented relative to those obtained with input DNA prepared from untreated chromatin (n = 10 for each group). (E) ChIP-qPCR analysis of the enrichment of JMJD3, EP300, and CBP in the miR-1246 promoter in SLE B cells transfected with pCMV6-p53 or pCMV6. Results are presented relative to those obtained with input DNA prepared from untreated chromatin. Data represent the mean of three independent experiments. (F) ChIP-qPCR analysis of the enrichment of JMJD3, EP300, and CBP in the miR-1246 promoter in normal B cells transfected with pRS-p53 or pRS-scrambled sequence. Results are presented relative to those obtained with input DNA prepared from untreated chromatin. Data represent the mean of three independent experiments. *p < 0.05, **p < 0.01.
In addition, we detected the binding levels of JMJD3, EP300, and CBP in the miR-1246 promoter region of miR-1246 by ChIP-qPCR in B cells from SLE patients and healthy controls. The results showed that the binding levels of JMJD3, EP300, and CBP were significantly decreased in the miR-1246 promoter in SLE B cells compared with healthy controls (Fig. 4D). Furthermore, pCMV6-p53 or pCMV6 was transfected into SLE B cells. JMJD3, EP300, and CBP binding levels in the promoter region of miR-1246 were increased after p53 expression (Fig. 4E). In addition, the pRS-p53 or pRS-scrambled sequence was transfected into normal B cells. JMJD3, EP300, and CBP binding levels in the promoter region of miR-1246 were decreased after p53 interference (Fig. 4F). These data strongly suggest that p53 has a crucial role in recruiting JMJD3, EP300, and CBP to the miR-1246 promoter region.
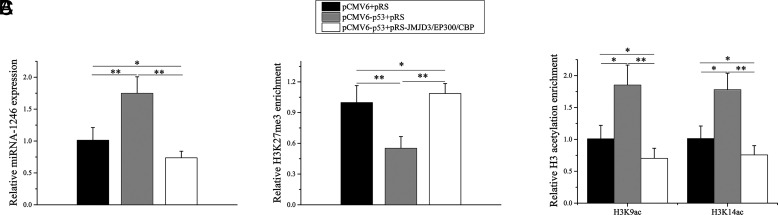
p53 regulates histone H3K27me3 and H3K9/K14ac in the miR-1246 promoter dependent on JMJD3/EP300/CBP
To confirm the importance of JMJD3/EP300/CBP in p53-mediated regulation of histone methylation and acetylation in the miR-1246 promoter region, we transfected the p53 expression plasmid and JMJD3/EP300/CBP interference plasmid into normal B cells. Compared with the negative control group, the expression level of miR-1246 was significantly increased in the p53 overexpression group (Fig. 5A). Moreover, the expression level of miR-1246 in p53 overexpression combined with the JMJD3/EP300/CBP interference group was significantly decreased compared with the p53 overexpression group and negative control group (Fig. 5A). Compared with the negative control group, the H3K27me3 level was decreased and the H3K9/K14ac levels were increased in the p53 overexpression group (Fig. 5B, 5C). The H3K27me3 level was increased and the H3K9/K14ac levels were decreased in p53 overexpression combined with the JMJD3/EP300/CBP interference group compared with p53 overexpression group and negative control group (Fig. 5B, 5C). These findings indicate that p53 regulates histone H3K27me3 and H3K9/K14ac in the miR-1246 promoter dependent on JMJD3/EP300/CBP.
FIGURE 5.
p53 regulates histone H3K27me3 and H3K9/K14ac in miR-1246 promoter dependent on JMJD3/EP300/CBP. (A) Real-time PCR analysis of the expression levels of miR-1246 in SLE B cells transfected with p53 expression plasmid and JMJD3/EP300/CBP interference plasmid. Data represent the mean of three independent experiments. (B and C) ChIP-qPCR analysis of the enrichment of H3K27me3 and the H3K9/K14ac in the miR-1246 promoter in SLE B cells transfected with p53 expression plasmid and JMJD3/EP300/CBP interference plasmid. Data represent the mean of three independent experiments. *p < 0.05, **p < 0.01.
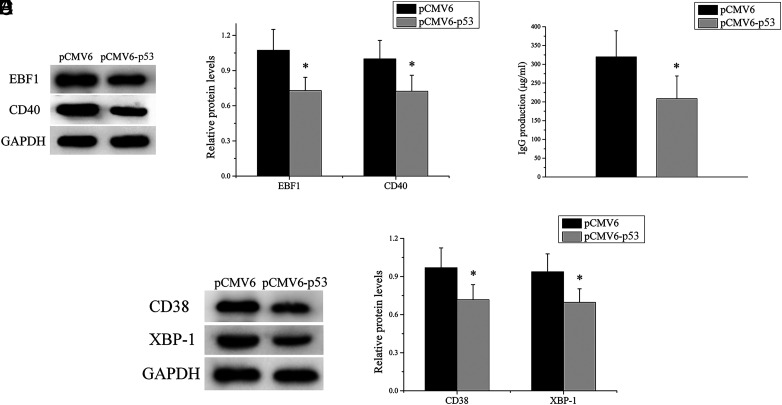
Upregulation of p53 expression alleviates the self-reactivity of SLE B cells
SLE B cells were transfected with p53 expression plasmid or negative control. The transfected B cells were stimulated with anti-IgM for 12 h and then the stimulated cells were taken for Western blot analysis to detect the expression levels of EBF1 and CD40, indicators of early activation of B cells. As expected, we observed a significant decrease of EBF1 and CD40 expression levels in SLE B cells after p53 expression (Fig. 6A, 6B). In addition, other stimulated cells were cocultured with purified autologous CD4+ T cells. ELISA was used to measure the concentration of IgG in coculture supernatants. As shown in (Fig. 6C, IgG Ab production in the p53 expression group was clearly lower than in the negative control group. Furthermore, we detected the expression levels of CD38 and XBP-1, markers of plasma cells, in cocultured cells and found that the expression levels of CD38 and XBP-1 in the p53 expression group were significantly lower than those in the negative control group (Fig. 6D, 6E). Taken together, these results suggested that the upregulation of p53 expression inhibited the self-reactivity of SLE B cells, which can reduce autoantibody production.
FIGURE 6.
The upregulation of p53 expression alleviates the self-reactivity of SLE B cells. (A and B) EBF1 and CD40 expression levels in SLE B cells transfected with p53 expression plasmid or negative control were analyzed by Western blot. One representative blot is shown (A). The intensity of the bands was semiquantitated and normalized to GAPDH (B). (C) SLE B cells transfected with p53 expression plasmid or negative control, where the transfected cells were cocultured with purified autologous CD4+ T cells, and IgG levels in the culture supernatants were measured by ELISA. (D and E) CD38 and XBP-1 expression levels in cocultured cells were analyzed by Western blot. One representative blot is shown (D). The intensity of the bands was semiquantitated and normalized to GAPDH (E). Data represent the mean of three independent experiments per group. *p < 0.05.
Discussion
p53 has a central role in tumor suppression by inducing apoptosis, cell cycle arrest, senescence, and DNA repair (16, 17). Recently, many studies have confirmed that p53 is related to the suppression of inflammatory and autoimmune diseases (18). p53 and p53-inducible molecules, such as p21 and Gadd45a, are inhibitors of T cell proliferation and activation (19–21). The decrease of Treg cells and the increase of Th17 cells are crucial in the course of autoimmune diseases (22, 23). p53 enhances the transcription of Foxp3 by binding to the promoter and conserved noncoding DNA sequence-2 of the Foxp3 gene and then promotes regulatory T cell induction (24). In contrast, p53 can suppress Th17 cell differentiation by inhibiting Stat3 phosphorylation (25). p53 gene mutations in synovial cells (26) and lower p53 expression in lymphocytes (27) have been found in patients with rheumatoid arthritis. In addition, our recent data suggested that lower expression of p53 is an important cause of the insufficient expression of miR-1246 in SLE B cells, which may lead to the overexpression of the miR-1246 target gene EBF1, and in turn overactivation of SLE B cells (8). EBF1 is required for the proliferation, survival, and signaling of pro–B cells and peripheral B cell subsets, including B1 cells and marginal zone B cells (28). Elevation of the expression level of miR-1246 in SLE B cells could decrease the expression of EBF1, causing the downregulation of CD40, CD80, and CD86, which are costimulatory molecules required for full B cell activation and IgG secretion (8). Taken together, the above data suggested that p53 suppresses autoimmune diseases through several mechanisms.
JMJD3 is a histone demethylase that explicitly removes the trimethyl group from the H3K27 lysine residue (29); H3K27me3 is a hallmark of gene silencing, which has an important role in the transcriptional repression of target genes (30). Therefore, JMJD3-mediated demethylation of H3K27me3 triggers the activation of target gene transcription. Yin et al. (31) found that the increased JMJD3 binding decreases H3K27me3 enrichment within the CD11a promoter, thus resulting in overexpression of CD11a in SLE CD4+ T cells. Moreover, Zhang et al. (32) have reported that the inhibited hematopoietic progenitor kinase 1 (HPK1, a negative regulator of T cell–mediated immune responses) expression in SLE CD4+ T cells is associated with loss of JMJD3 binding and increased H3K27me3 enrichment at the HPK1 promoter, which contributes to T cell overactivation and B cell overstimulation in SLE.
EP300 functions as histone acetyltransferase and regulates transcription via chromatin remodeling. It acetylates all four core histones in nucleosomes, thus giving an epigenetic tag for transcriptional activation. EP300 mediates cAMP gene regulation by specifically binding to phosphorylated CREB protein (33). CBP is another protein that can acetylate histones, giving a specific tag for transcriptional activation. It specifically binds to phosphorylated CREB and enhances its transcriptional activity toward cAMP-responsive genes (34). CBP and EP300 define a unique family of histone acetyltransferase that is often referred to as a single entity (CBP/EP300) due to the extensive sequence homology and functional similarity (35). H3K9ac is an important histone acetylation modification that is highly correlated with active promoters. A previous study has shown that H3K9ac has high co-occurrence with H3K14ac; both H3K9ac and H3K14ac are considered the hallmarks of active gene promoters (12). Ding et al. (15) have found that in SLE CD4+ T cells, increased BCL-6 upregulates H3K27me3 and downregulates H3K9/14ac at the miR-142 promoter. These factors induce a decline in miR-142-3p/5p expression, consequently resulting in CD4+ T cell hyperactivity. All of the above studies mainly focused on CD4+ T cells, thus failing to elucidate whether abnormal expression of related molecules in SLE B cells is related to JMJD3, CBP, and EP300.
To further reveal the specific molecular mechanism of p53 regulating miR-1246 expression, we analyzed the histone H3K27me3 and H3K9/K14ac modifications in the miR-1246 promoter region in B cells of SLE patients and healthy controls. We found that the upregulation of H3K27me3 and downregulation of H3K9/K14ac in the miR-1246 promoter region could be a relevant regulator for the expression of miR-1246 in SLE B cells. We also found that p53 promotes miR-1246 transcription by recruiting JMJD3, EP300, and CBP, thereby downregulating H3K27me3 and upregulating H3K9/K14ac. In addition, the upregulation of p53 expression in SLE B cells could increase the expression of miR-1246 and inhibit the self-reactivity of SLE B cells, thus reducing the autoantibody production.
In summary, our findings improve the molecular mechanisms of p53 regulating miR-1246 expression and provide a reasonable explanation for the insufficient expression of miR-1246 in SLE B cells. As a result, p53 may be used as a new therapeutic target for lupus.
Acknowledgments
We are grateful to all patients and healthy donors for donating their samples.
This work was supported by the National Natural Science Foundation of China Grants 81974477, 81872533, and 82073448 and by the Hunan Provincial Natural Science Foundation of China Grant 2021JJ40848.
Q.Z., Y.L., P.Z., and S.L. contributed to the design and planning of the experiments; J.L. and S.L. provided samples; Q.Z., Y.L., R.W., Y.Z., P.Z., and S.L. conducted the laboratory experimental work; and Q.Z. and S.L. contributed to the reporting of findings and writing of the manuscript. All authors critically revised the manuscript and gave final approval of the version to be submitted.
- CBP
- CREB-binding protein
- ChIP
- chromatin immunoprecipitation
- EBF1
- early B cell factor 1
- EP300
- E1A-binding protein p300
- H3K9ac
- histone H3 acetylation at Lys9
- H3K14ac
- histone H3 acetylation at Lys14
- H3K27me3
- histone H3 trimethylation at Lys27
- JMJD3
- Jumonji domain–containing protein 3
- miRNA
- microRNA
- qPCR
- quantitative PCR
- SLE
- systemic lupus erythematosus
- XBP-1
- X box binding protein-1
Disclosures
The authors have no financial conflicts of interest.
References
- 1. Frieri M. 2013. Mechanisms of disease for the clinician: systemic lupus erythematosus. Ann. Allergy Asthma Immunol. 110: 228–232. [DOI] [PubMed] [Google Scholar]
- 2. Anolik J. H. 2013. B cell biology: implications for treatment of systemic lupus erythematosus. Lupus 22: 342–349. [DOI] [PubMed] [Google Scholar]
- 3. Chu V. T., Enghard P., Schürer S., Steinhauser G., Rudolph B., Riemekasten G., Berek C.. 2009. Systemic activation of the immune system induces aberrant BAFF and APRIL expression in B cells in patients with systemic lupus erythematosus. Arthritis Rheum. 60: 2083–2093. [DOI] [PubMed] [Google Scholar]
- 4. Chan V. S., Tsang H. H., Tam R. C., Lu L., Lau C. S.. 2013. B-cell-targeted therapies in systemic lupus erythematosus. Cell. Mol. Immunol. 10: 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carthew R. W., Sontheimer E. J.. 2009. Origins and mechanisms of miRNAs and siRNAs. Cell 136: 642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ren D., Liu F., Dong G., You M., Ji J., Huang Y., Hou Y., Fan H.. 2016. Activation of TLR7 increases CCND3 expression via the downregulation of miR-15b in B cells of systemic lupus erythematosus. Cell. Mol. Immunol. 13: 764–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luo S., Ding S., Liao J., Zhang P., Liu Y., Zhao M., Lu Q.. 2019. Excessive miR-152-3p results in increased BAFF expression in SLE B-cells by inhibiting the KLF5 expression. Front. Immunol. 10: 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luo S., Liu Y., Liang G., Zhao M., Wu H., Liang Y., Qiu X., Tan Y., Dai Y., Yung S., et al. 2015. The role of microRNA-1246 in the regulation of B cell activation and the pathogenesis of systemic lupus erythematosus. Clin. Epigenetics 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cao R., Wang L., Wang H., Xia L., Erdjument-Bromage H., Tempst P., Jones R. S., Zhang Y.. 2002. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 298: 1039–1043. [DOI] [PubMed] [Google Scholar]
- 10. Pan M. R., Hsu M. C., Chen L. T., Hung W. C.. 2018. Orchestration of H3K27 methylation: mechanisms and therapeutic implication. Cell. Mol. Life Sci. 75: 209–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gates L. A., Shi J., Rohira A. D., Feng Q., Zhu B., Bedford M. T., Sagum C. A., Jung S. Y., Qin J., Tsai M. J., et al. 2017. Acetylation on histone H3 lysine 9 mediates a switch from transcription initiation to elongation. J. Biol. Chem. 292: 14456–14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Karmodiya K., Krebs A. R., Oulad-Abdelghani M., Kimura H., Tora L.. 2012. H3K9 and H3K14 acetylation co-occur at many gene regulatory elements, while H3K14ac marks a subset of inactive inducible promoters in mouse embryonic stem cells. BMC Genomics 13: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hochberg M. C. 1997. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40: 1725. [DOI] [PubMed] [Google Scholar]
- 14. Bombardier C., Gladman D. D., Urowitz M. B., Caron D., Chang C. H., The Committee on Prognosis Studies in SLE . 1992. Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum. 35: 630–640. [DOI] [PubMed] [Google Scholar]
- 15. Ding S., Zhang Q., Luo S., Gao L., Huang J., Lu J., Chen J., Zeng Q., Guo A., Zeng J., Lu Q.. 2020. BCL-6 suppresses miR-142-3p/5p expression in SLE CD4+ T cells by modulating histone methylation and acetylation of the miR-142 promoter. Cell. Mol. Immunol. 17: 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Green D. R., Kroemer G.. 2009. Cytoplasmic functions of the tumour suppressor p53. Nature 458: 1127–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vousden K. H., Prives C.. 2009. Blinded by the light: the growing complexity of p53. Cell 137: 413–431. [DOI] [PubMed] [Google Scholar]
- 18. Takatori H., Kawashima H., Suzuki K., Nakajima H.. 2014. Role of p53 in systemic autoimmune diseases. Crit. Rev. Immunol. 34: 509–516. [DOI] [PubMed] [Google Scholar]
- 19. Leech M., Xue J. R., Dacumos A., Hall P., Santos L., Yang Y., Li M., Kitching A. R., Morand E. F.. 2008. The tumour suppressor gene p53 modulates the severity of antigen-induced arthritis and the systemic immune response. Clin. Exp. Immunol. 152: 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santiago-Raber M. L., Lawson B. R., Dummer W., Barnhouse M., Koundouris S., Wilson C. B., Kono D. H., Theofilopoulos A. N.. 2001. Role of cyclin kinase inhibitor p21 in systemic autoimmunity. J. Immunol. 167: 4067–4074. [DOI] [PubMed] [Google Scholar]
- 21. Salvador J. M., Hollander M. C., Nguyen A. T., Kopp J. B., Barisoni L., Moore J. K., Ashwell J. D., Fornace A. J. Jr. 2002. Mice lacking the p53-effector gene Gadd45a develop a lupus-like syndrome. Immunity 16: 499–508. [DOI] [PubMed] [Google Scholar]
- 22. Sakaguchi S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22: 531–562. [DOI] [PubMed] [Google Scholar]
- 23. Korn T., Bettelli E., Oukka M., Kuchroo V. K.. 2009. IL-17 and Th17 cells. Annu. Rev. Immunol. 27: 485–517. [DOI] [PubMed] [Google Scholar]
- 24. Kawashima H., Takatori H., Suzuki K., Iwata A., Yokota M., Suto A., Minamino T., Hirose K., Nakajima H.. 2013. Tumor suppressor p53 inhibits systemic autoimmune diseases by inducing regulatory T cells. J. Immunol. 191: 3614–3623. [DOI] [PubMed] [Google Scholar]
- 25. Zhang S., Zheng M., Kibe R., Huang Y., Marrero L., Warren S., Zieske A. W., Iwakuma T., Kolls J. K., Cui Y.. 2011. Trp53 negatively regulates autoimmunity via the STAT3-Th17 axis. FASEB J. 25: 2387–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamanishi Y., Boyle D. L., Rosengren S., Green D. R., Zvaifler N. J., Firestein G. S.. 2002. Regional analysis of p53 mutations in rheumatoid arthritis synovium. Proc. Natl. Acad. Sci. USA 99: 10025–10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maas K., Westfall M., Pietenpol J., Olsen N. J., Aune T.. 2005. Reduced p53 in peripheral blood mononuclear cells from patients with rheumatoid arthritis is associated with loss of radiation-induced apoptosis. Arthritis Rheum. 52: 1047–1057. [DOI] [PubMed] [Google Scholar]
- 28. Györy I., Boller S., Nechanitzky R., Mandel E., Pott S., Liu E., Grosschedl R.. 2012. Transcription factor Ebf1 regulates differentiation stage-specific signaling, proliferation, and survival of B cells. Genes Dev. 26: 668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiang Y., Zhu Z., Han G., Lin H., Xu L., Chen C. D.. 2007. JMJD3 is a histone H3K27 demethylase. Cell Res. 17: 850–857. [DOI] [PubMed] [Google Scholar]
- 30. Kondo Y., Shen L., Cheng A. S., Ahmed S., Boumber Y., Charo C., Yamochi T., Urano T., Furukawa K., Kwabi-Addo B., et al. 2008. Gene silencing in cancer by histone H3 lysine 27 trimethylation independent of promoter DNA methylation. Nat. Genet. 40: 741–750. [DOI] [PubMed] [Google Scholar]
- 31. Yin H., Wu H., Zhao M., Zhang Q., Long H., Fu S., Lu Q.. 2017. Histone demethylase JMJD3 regulates CD11a expression through changes in histone H3K27 tri-methylation levels in CD4+ T cells of patients with systemic lupus erythematosus. Oncotarget 8: 48938–48947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang Q., Long H., Liao J., Zhao M., Liang G., Wu X., Zhang P., Ding S., Luo S., Lu Q.. 2011. Inhibited expression of hematopoietic progenitor kinase 1 associated with loss of jumonji domain containing 3 promoter binding contributes to autoimmunity in systemic lupus erythematosus. J. Autoimmun. 37: 180–189. [DOI] [PubMed] [Google Scholar]
- 33. Allis C. D., Berger S. L., Cote J., Dent S., Jenuwien T., Kouzarides T., Pillus L., Reinberg D., Shi Y., Shiekhattar R., et al. 2007. New nomenclature for chromatin-modifying enzymes. Cell 131: 633–636. [DOI] [PubMed] [Google Scholar]
- 34. Bedford D. C., Kasper L. H., Fukuyama T., Brindle P. K.. 2010. Target gene context influences the transcriptional requirement for the KAT3 family of CBP and p300 histone acetyltransferases. Epigenetics 5: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ogryzko V. V., Schiltz R. L., Russanova V., Howard B. H., Nakatani Y.. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87: 953–959. [DOI] [PubMed] [Google Scholar]