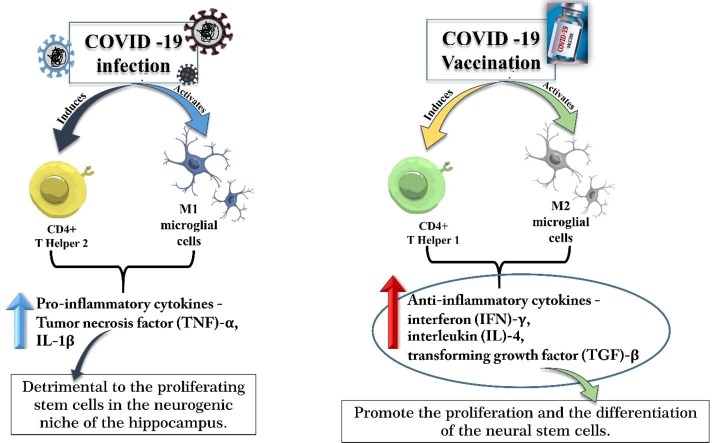
Graphical abstract
Keywords: COVID-19 vaccination, Acquired immunity, Hippocampus, Neurogenesis, Adult
Abstract
Emerging evidence suggests a detrimental impact of COVID-19 illness on the continued hippocampal neurogenesis in adults. In contrast, the existing literature supports an enhancing effect of COVID-19 vaccination on adult hippocampal neurogenesis. Vaccines against respiratory infections, including influenza, have been shown to enhance hippocampal neurogenesis in adult-age animals. We propose that a similar benefit may happen in COVID-19 vaccinated adults. The vaccine-induced enhancement of the hippocampal neurogenesis in adults thus may protect against age-related cognitive decline and mental disorders. It also hints at an added mental health benefit of the COVID-19 vaccination programs in adults.
1. Introduction
The continued hippocampal neurogenesis in adults is crucial for adapting to the new life circumstances and protecting against age-related cognitive decline (Kumar et al., 2019). Emerging evidence suggests a detrimental impact of COVID-19 illness on adult hippocampal neurogenesis (AHN) not only during the acute illness phase (Fernández-Castañeda et al., 2022, Klein et al., 2021) but also as a long-term impact (Guo et al., 2022). On the contrary, the available literature on respiratory system diseases, such as influenza and tuberculosis, suggests that vaccine-induced adaptive immunity creates a neurotrophic milieu that can not only block the detrimental influence of acute infection on the AHN but also may enhance it (Qi, 2015).
The subgranular zone of the dentate gyrus part of the hippocampus is one of the sites where neurogenesis occurs in the adult brain (Kumar et al., 2019). The newly born neurons in the adult hippocampus integrate into the local neuronal circuitry (Kumar et al., 2019). They set a lower threshold for the long-term potentiation of the memory-related pathways in the hippocampus. Moreover, they are more excitable than the older neurons. Therefore, resulting in a more coordinated hippocampal network function (Snyder and Cameron, 2012). The AHN contributes to cerebral plasticity—a key determinant of cognitive adaptability in the adult brain (Kumar et al., 2019). Newly born neurons potentially enhance overall hippocampal functions, including learning, memory, and spatiomotor performances. Moreover, they provide a neural substrate to accommodate new experiences, resilience to stress and anxiety and protect from attrition and neurodegeneration (Kumar et al., 2019).
The AHN occurs in a highly permissive milieu and depends significantly on neuro-immune interactions (Qi, 2015). A systemic infection like that in COVID-19 and the vaccination will likely impact the ongoing AHN (Qi, 2015). The available literature suggests that the adaptive immune response against acute infections and that developed from the vaccines are characteristically different, with a distinct impact on hippocampal neurogenesis. The infections induce a (CD4+) T helper-2 (Th2) cell-mediated adaptive immune response added with the M1 microglial activation inducing elevated levels of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and IL-1β, which are known to be detrimental to the proliferating NSCs in the neurogenic niche of the hippocampus (Fig. 1 a) (Qi, 2015). In contrast, the vaccination induces a (CD4+) T helper-1 (Th1) cell-mediated adaptive immune response, added with the M2 microglial activation, thus causing elevated levels of the anti-inflammatory cytokines, such as interferon (IFN)-γ, interleukin (IL)-4, transforming growth factor (TGF)-β, which promote proliferation and differentiation of the NSCs (Fig. 1b) (Qi et al., 2017, Qi et al., 2016, Song et al., 2020, Xia et al., 2014, Yang et al., 2016). The vaccination also induces elevated levels of neurotrophic molecules, such as BDNF and insulin-like growth factor (IGF)-1, which can further promote AHN (Qi, 2015).
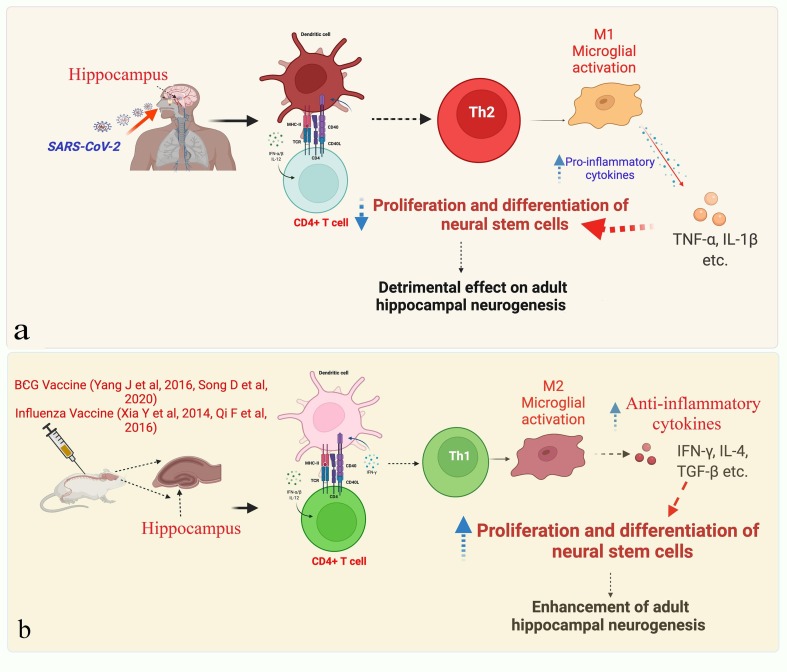
Fig. 1.
Plausible effects of SARS-CoV-2 infection and/or vaccination on adult hippocampal neurogenesis, a. Infection induces a (CD4+) T helper-2 (Th2) cell-mediated adaptive immune response added with the M1 microglial activation inducing elevated levels of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α and IL-1β, which are known to reduce proliferation and differentiation of the neural stem cells, thus prove detrimental to the adult hippocampal neurogenesis, b. The vaccine induces a (CD4+) T helper-1 (Th1) cell-mediated adaptive immune response, added with the M2 microglial activation, thus inducing elevated levels of the anti-inflammatory cytokines, such as interferon (IFN)-γ, interleukin (IL)-4, transforming growth factor (TGF)-β, which promote proliferation and differentiation of the neural stem cells, thus leads to enhancement of adult hippocampal neurogenesis.
There is substantial empirical evidence supporting the potential neurotrophic influence of the COVID-19 vaccination. Noteworthy to mention is the animal model studies showing the effects of vaccinations against respiratory tract infections such as influenza and tuberculosis (Qi et al., 2017, Qi et al., 2016, Song et al., 2020, Xia et al., 2014, Yang et al., 2016).
In a study in pregnant mice, Xia et al. showed that vaccination against influenza type A (H1N1) increased spatial working memory performance compared to the controls. A transient increase in both cell proliferation and neuronal differentiation in the neurogenic niche of the hippocampus was observed in the vaccinated mice. IL-4 and IFN-γ levels also increased in the serum and hippocampus of the vaccinated group, whereas the expression of IL-6 and TNF-α, the proinflammatory factors, were significantly reduced. Thus, the test groups showed a T helper-1 (Th1) like response compared to the controls, which showed a T helper-2 (Th2) like response (Xia et al., 2014). In another study on the influenza vaccine, Qi et al. found that T lymphocytes were recruited from the periphery to the choroid plexus (CP) of the lateral and third (3rd) ventricles in pregnant mice, given with influenza type A (H1N1) vaccination. The microglia found skewed towards an M2-like phenotype (increased Arginase-1 and Ym1 mRNA levels) and elevated BDNF and insulin-like growth factor-1 (IGF-1) levels in the hippocampus in the vaccinated mice. In contrast, these effects were obliterated by anti-TCR antibody treatment. Further, intra-cerebroventricular delivery of anti-TCR and intravenous delivery of anti-CD4 antibodies to the periphery markedly down-regulated the AHN (Qi et al., 2016).
In a further study, Song et al. wanted to see the effects of BCG vaccination via the adoptive transfer of T lymphocytes from wild-type mice into naive mice. The authors observed that naive mice with adoptive BCG-induced lymphocytes showed anxiolytic and antidepressant-like performance on given tasks. Increased cell proliferation and newborn neurons than the controls were observed in the hippocampus. IFN-γ and IL-4 levels in the serum of BCG vaccinated naive mice also increased; in contrast, TNF-α and IL-1β levels reduced. The alterations in ratios of splenic CD4+ and CD8+ memory T cells most likely affected the expression of correlative cytokines in the serum, accounting for the shift in behavioral results (Song et al., 2020).
In another BCG vaccine study, Yang et al. investigated the effect of neonatal vaccination on brain development during early postnatal life. Newborn mice were injected subcutaneously with BCG or phosphate-buffered saline (PBS), and their mood status, spatial cognition, and hippocampal neurogenesis were observed at specified intervals. The authors noted that the BCG-vaccinated mice showed better behavioral performances and showed elevated neurogenesis, M2 microglial activation, and a neurotrophic profile of neuroimmune molecules [increased interferon (IFN)-γ, interleukin (IL)-4, transforming growth factor (TGF)-β, brain-derived neurotrophic factor (BDNF) and insulin-like growth factor (IGF)-1 and decreased tumor necrosis factor (TNF)-α and IL-1β] in the hippocampus (Yang et al., 2016). In a further study conducted in mice, Qi et al. reported that combined neonatal BCG vaccination and exposure to an enriched environment-induced anti-inflammatory meningeal macrophage polarization and increased expression of the neurotrophic factors BDNF/IGF-1 and the M2 microglial phenotype in the hippocampus leading to enhanced neurogenesis (Qi et al., 2017).
Notably, these studies showed considerable enhancement of hippocampus-dependent cognitive functions, such as working memory and spatial learning abilities, in the vaccinated animals (Qi et al., 2017, Qi et al., 2016, Song et al., 2020, Xia et al., 2014, Yang et al., 2016).
The review of indirect evidence from the animal studies conducted for influenza and BCG vaccines hints that COVID-19 vaccination may protect against acute infection-induced deterioration and enhance ongoing hippocampal neurogenesis in adults. The animal model studies using the immunohistochemical and transcriptomic approaches may optimally validate this hypothesis. The behavioral tests can be applied to assess the impact of vaccine-induced neurogenesis on hippocampus-dependent cognitive functions (Sierra et al., 2011). Although there are severe constraints to evaluating AHN in living humans, behavioral tests can reasonably differentiate between vaccinated and non-vaccinated cohorts. In addition, MRI-based cerebral blood volume (CBV) measurement can efficiently see a correlated increase in hippocampal angiogenesis in the case of enhanced neurogenesis in vaccinated individuals (Sierra et al., 2011). The ongoing pandemic of COVID-19 is likely to increase the global burden of mental illness as its long-term impact. As a hope, millions have received single or repeat doses of a COVID-19 vaccine. Whether these vaccine doses may have a neurogenic benefit hence improving the mental health of the recipients, will be an exciting phenomenon to unfold.
Funding
There was no dedicated funding for this project.
Author (s) contributions
AK conceived the idea, performed a literature review, and wrote the first draft. RKN and PP prepared figures/graphs. CK, SK, RKN, PP, RKJ, and MAF reviewed and edited the manuscript. All authors consented to the submission of the final draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- Fernández-Castañeda A., Lu P., Geraghty A.C., Song E., Lee M.-H., Wood J., Yalçın B., Taylor K.R., Dutton S., Acosta-Alvarez L., Ni L., Contreras-Esquivel D., Gehlhausen J.R., Klein J., Lucas C., Mao T., Silva J., Peña-Hernández M.A., Tabachnikova A., Takahashi T., Tabacof L., Tosto-Mancuso J., Breyman E., Kontorovich A., McCarthy D., Quezado M., Hefti M., Perl D., Folkerth R., Putrino D., Nath A., Iwasaki A., Monje M. Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. bioRxiv Prepr. Serv. Biol. 2022 doi: 10.1101/2022.01.07.475453. [DOI] [Google Scholar]
- Guo P., Benito Ballesteros A., Yeung S.P., Liu R., Saha A., Curtis L., Kaser M., Haggard M.P., Cheke L.G. COVCOG 2: cognitive and memory deficits in long COVID: A second publication from the COVID and cognition study. Front. Aging Neurosci. 2022;14:204. doi: 10.3389/FNAGI.2022.804937/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Soung A., Sissoko C., Nordvig A., Canoll P., Mariani M., Jiang X., Bricker T., Goldman J., Rosoklija G., Arango V., Underwood M., Mann J.J., Boon A., Dowrk A., Boldrini M. COVID-19 induces neuroinflammation and loss of hippocampal neurogenesis. Res. Sq. 2021 doi: 10.21203/RS.3.RS-1031824/V1. [DOI] [Google Scholar]
- Kumar A., Pareek V., Faiq M.A., Ghosh S.K., Kumari C. Adult neurogenesis in humans: A review of basic concepts, history, current research, and clinical implications. Innov. Clin. Neurosci. 2019;16:30. [PMC free article] [PubMed] [Google Scholar]
- Qi F. Immune-based modulation of adult hippocampal neurogenesis, link to systemic Th1/Th2 balance. J. Vaccines Vaccin. 2015;06 doi: 10.4172/2157-7560.1000274. [DOI] [Google Scholar]
- Qi F., Yang J., Xia Y., Yuan Q., Guo K., Zou J., Yao Z. A(H1N1) vaccination recruits T lymphocytes to the choroid plexus for the promotion of hippocampal neurogenesis and working memory in pregnant mice. Brain Behav. Immun. 2016;53:72–83. doi: 10.1016/J.BBI.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Qi F., Zuo Z., Yang J., Hu S., Yang Y., Yuan Q., Zou J., Guo K., Yao Z. Combined effect of BCG vaccination and enriched environment promote neurogenesis and spatial cognition via a shift in meningeal macrophage M2 polarization. J. Neuroinflammation. 2017;14 doi: 10.1186/S12974-017-0808-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Encinas J.M., Maletic-Savatic M. Adult human neurogenesis: from microscopy to magnetic resonance imaging. Front. Neurosci. 2011;5 doi: 10.3389/FNINS.2011.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J.S., Cameron H.A. Could adult hippocampal neurogenesis be relevant for human behavior? Behav. Brain Res. 2012;227(2):384–390. doi: 10.1016/j.bbr.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D., Qi F., Liu S.S., Tang Z., Duan J., Yao Z., Li X.-J. The adoptive transfer of BCG-induced T lymphocytes contributes to hippocampal cell proliferation and tempers anxiety-like behavior in immune deficient mice. PLoS One. 2020;15(4):e0225874. doi: 10.1371/JOURNAL.PONE.0225874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y., Qi F., Zou J., Yao Z. Influenza A(H1N1) vaccination during early pregnancy transiently promotes hippocampal neurogenesis and working memory. Involvement of Th1/Th2 balance. Brain Res. 2014;1592:34–43. doi: 10.1016/J.BRAINRES.2014.09.076. [DOI] [PubMed] [Google Scholar]
- Yang J., Qi F., Gu H., Zou J., Yang Y., Yuan Q., Yao Z. Neonatal BCG vaccination of mice improves neurogenesis and behavior in early life. Brain Res. Bull. 2016;120:25–33. doi: 10.1016/J.BRAINRESBULL.2015.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.