Abstract
PURPOSE OF REVIEW:
This article describes the spectrum of neurologic complications associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, their underlying pathology and pathogenic mechanisms, gaps in knowledge, and current therapeutic strategies.
RECENT FINDINGS:
COVID-19 is the clinical syndrome caused by the novel coronavirus SARS-CoV-2. It can affect the entire neuraxis, and presentations in the acute phase are variable, although anosmia is a common manifestation. Encephalopathy is common in patients who are hospitalized and is often associated with multiorgan involvement. Immune-mediated encephalitis is probably underrecognized; however, viral encephalitis is rare. Other manifestations include stroke, seizures, myelitis, and peripheral neuropathies, including Guillain-Barré syndrome, which sometimes has atypical manifestations. Treatment is symptomatic, and immunotherapies have been used successfully in some patients. Long-term complications include dysautonomia, exercise intolerance, malaise, sleep disturbances, cognitive impairment, and mood disorders.
SUMMARY:
Neurologic manifestations of COVID-19 may occur in the acute setting and may be independent of respiratory manifestations. Immune-mediated syndromes and cerebrovascular complications are common. Large populations of patients are expected to have long-term neurologic complications of COVID-19, many of which may emerge only after recovery from the acute illness.
INTRODUCTION
Coronaviruses are known causes of respiratory, enteric, and systemic infections. Most human coronaviruses cause mild symptoms that resolve spontaneously. Coronaviruses are enveloped viruses with a positive-sense single-stranded RNA genome. The Latin word corona means “crown” and describes the spikelike proteins projecting from the surface of the virus. Coronaviruses are classified into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus, and Deltacoronavirus. Alphacoronavirus, Betacoronavirus, and Deltacoronavirus infect mammals. Deltacoronavirus and Gammacoronavirus infect avian species. However, the virus is able to jump between species with dire consequences, causing the emergence of Middle East respiratory syndrome (MERS) coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV)-1, and SARS-CoV-2.1 To date, seven human coronaviruses have been identified. SARS-CoV-1 and SARS-CoV-2 originated from bats. Both these viruses use spike proteins to attach to angiotensin-converting enzyme receptor type 2 (ACE2), which is highly expressed in the respiratory tract.2 Neuropilin acts as a coreceptor for the virus. The virus has four major structural proteins. Spike (S) protein is a trimeric protein and is made up of two separate polypeptides, S1 (binding domain) and S2 (stalk). The membrane protein is the most abundant structural protein in the virion. The envelope protein facilitates assembly and release of the virus. The ion channel activity in the envelope protein plays a critical role in pathogenesis. The N protein is the nucleocapsid that binds the viral RNA.
The SARS-CoV-2 virus was first discovered in December 2019 and emerged in Wuhan, China. Within a few months, it had spread to every country in the world, causing paralysis of the global economy, devastation of health care systems, and isolation of large populations. COVID-19, the clinical syndrome caused by SARS-CoV-2, is the biggest challenge that humanity has faced in over a century, affecting every aspect of society. History teaches us that many prior pandemics have each killed more people on the planet than all wars combined. For example, in the 1300s, the plague is said to have killed 25 million people in Europe. In the 1600s, smallpox killed 20 million indigenous people in North America. The influenza epidemic of 1918–1919 killed about 30 million to 50 million people. Similarly, millions of people have been killed by yellow fever, polio, measles, and acquired immunodeficiency syndrome (AIDS). Unfortunately, SARS-CoV-2 is following the same pattern of devastation, with the number of infections and deaths rising exponentially. As of January 1, 2021, the United States was seeing nearly 200,000 new infections per day and nearly 400,000 people had died, but the rate of new infections in the United States has steadily declined as vaccination rates have increased. It is estimated that more than 800,000 people have died from the infection in United States.3 Nearly 10% to 35% of survivors have persistent and disabling neurologic symptoms. With nearly 200 million people infected worldwide, the long-term socioeconomic consequences are unfathomable. Although effective vaccines have been developed and are being distributed, millions of people will have long-term complications from the infection, some of which will be neurologic. The burden of care for these patients will be the responsibility of neurologists; hence, we must be prepared to take care of them.
Symptoms of COVID-19 infection may initially resemble influenza. Fever is present in almost 90% of patients, and cough is present in approximately 70%. The median incubation period is 4 to 7 days,4 during which the patients may be infectious. Myalgia and fatigue are seen in about 50% of patients and may persist even after recovery from other symptoms. Headache occurs in 8% of patients Diarrhea occurs in less than 5% of patients and, in some patients, might be the major symptom.4 Anosmia and ageusia may be heralding manifestations.4 On CT of the chest, more than 50% of patients have a ground-glass opacity. Many patients develop superimposed bacterial pneumonia. Older patients have more severe disease. In a study from China, mechanical ventilation was required in 6% of patients.4 In patients who are hospitalized, rates of acute respiratory distress syndrome are as high as 29%. A study of 262 confirmed cases revealed that 18% had severe disease, 73% were mild, 4% were nonpneumonic, and 5% were asymptomatic5; widespread antibody testing will likely show far higher numbers of asymptomatic or mildly symptomatic people. Lymphopenia is common in patients who are critically ill.6 Mortality is higher with advanced age and underlying comorbidities, including diabetes, cardiac and respiratory disorders, and immunosuppressed states. Several acute neurologic syndromes have been associated with coronaviruses (TABLE 11-1). About 13.5% of hospitalized patients have neurologic manifestations. Of these, nearly half have metabolic abnormalities or hypoxic brain injury.7
TABLE 11-1.
Neurologic Complications of Coronavirus Infections
|
Parainfectious syndromes ◆ Anosmia and ageusia ◆ Encephalopathy (metabolic/hypoxic) ◆ Viral meningoencephalitis ◆ Central hypoventilation ◆ Stroke ◆ Acute necrotizing hemorrhagic encephalopathy ◆ Myositis Postinfectious syndromes ◆ Acute disseminated encephalomyelitis (ADEM) ◆ Brainstem encephalitis ◆ Myelitis ◇ Transverse myelitis ◇ Acute flaccid myelopathy ◇ Necrotizing myelitis ◆ Guillain-Barré syndrome ◇ Miller Fisher syndrome ◇ Cranial neuropathies ◆ Long-haul COVID ◇ Multi-systemic inflammatory syndrome |
NEUROLOGIC COMPLICATIONS OF COVID-19
The neurologic manifestations of COVID-19 can be broadly divided into two categories: those that occur during the acute phase of the infection (parainfectious complications) and the postviral manifestations that occur following the acute phase (post–acute phase complications).
Anosmia
Anosmia and ageusia are the most common early symptoms of the infection. Nearly 40% to 60% of patients develop loss of smell,8 and, upon testing, nearly 90% have an alteration of smell.9 The loss of taste is secondary to anosmia but can lead to anorexia and weight loss. Many patients recover their sense of smell; others may develop hyposmia, parosmia, or permanent anosmia. The virus is thought to invade the sustentacular (also called support) cells in the vicinity of the olfactory nerve endings in the nasal mucosa, which express the SARS-CoV-2 receptor ACE2.10 Transient obstruction of the olfactory clefts11 and olfactory bulb edema have been seen on MRI in patients with COVID-19–associated anosmia.12 To date, no direct evidence of infection of the olfactory nerve has been seen.
Encephalopathy
Encephalopathy is the most common neurologic manifestation in patients who are hospitalized with COVID-19, with nearly one-third of patients who are hospitalized developing encephalopathic symptoms ranging from alteration in consciousness to delirium and seizures. Patients with encephalopathy have prolonged hospitalization, and two-thirds are unable to manage activities of daily living at the time of discharge.13 Encephalopathy is more common in older adults. The underlying causes of encephalopathy are complex and require careful evaluation and investigation (CASE 11-1). In patients who have significant pulmonary or multiorgan involvement, hypoxic or metabolic abnormalities should be considered as major contributors to the encephalopathy. In some critically ill patients, the MRI may show diffuse, bilaterally symmetrical high-signal-intensity lesions suggestive of a delayed posthypoxic leukoencephalopathy. This may be associated with microhemorrhagic lesions in the corpus callosum and juxtacortical regions.14 Delirium in patients hospitalized with COVID-19 has been commonly described. Rarely, it is present at onset and may be associated with sepsis. In the critical care setting, the causes are multifactorial. In one series, 84% of patients with COVID-19 in the critical care unit had delirium with impairment of attention, awareness, and cognition.15 In this setting, delirium may also arise from medications such as sedative-hypnotics, anticholinergics, and corticosteroids. Prolonged mechanical ventilation and isolation from the health care team and family members may also be contributory factors.
CASE 11-1.
A 32-year-old woman developed a low-grade fever for 2 days with a stuffy nose and hyposmia. She tested positive for SARS-CoV-2 but was sent home. The next day she developed visual hallucinations and became agitated. Upon admission, her oxygen saturation was 88%. She appeared delirious but could follow commands. Only a partial neurologic assessment could be performed, but it did not show any focal deficits. The patient had to be sedated for an MRI of the brain, which was normal. EEG showed some focal slowing in the left temporal lobe. CSF was normal, including polymerase chain reaction (PCR) for SARS-CoV-2. Chest CT showed bilateral infiltrates in the lower lobes of the lungs.
COMMENT
A broad differential diagnosis should be considered in hospitalized patients with COVID-19 who are encephalopathic. Metabolic abnormalities should be corrected, and medications and drugs of abuse should be considered as potential etiologies; psychosocial aspects, including social isolation, can be contributory factors. Neurologic etiologies to consider include posterior reversible encephalopathy syndrome (PRES) or autoimmune encephalitis, as a wide variety of autoantibody syndromes are being reported in this patient population.
Viral Encephalitis
Direct viral invasion of the brain in COVID-19 is rare. A case of SARS-CoV-2 meningoencephalitis was reported in Japan; the patient presented with generalized seizures and was found to have lesions of the temporal lobe with adjoining ventriculitis as well as paranasal sinusitis. The virus was detected in the CSF but not by nasal swab. CSF showed a mild pleocytosis with 12 cells/mm3.16 Other case reports of encephalitis with confirmed SARS-CoV-2 in CSF have been rare,17,18 so it does not seem to be a common manifestation of infection. Most autopsy studies have been unable to detect the virus in the brain.19–21 Although low copy numbers of the virus have been detected in the medulla and frontal lobe of an occasional patient, they were considered a blood contaminant.20 A postmortem neuropathologic study of 43 patients found evidence of SARS-CoV-2 RNA and proteins in the brain tissue of 53% of patients, but rare infected cells with low copy numbers of the virus were detected and the presence of the virus was not associated with the severity of neuropathologic changes, such as astrogliosis, ischemic lesions, and inflammatory lesions.22
Acute Necrotizing Hemorrhagic Encephalopathy
Acute necrotizing hemorrhagic encephalopathy is a feared complication of several viruses, most notably influenza. It is thought to result from cytokine release syndrome rather than direct viral invasion of brain parenchyma,23 which is especially salient given the propensity of SARS-CoV-2 for causing similar cytokine storms in the lungs. Patients with this condition develop bilateral symmetric lesions of the thalami with hemorrhagic foci; the temporal lobes, brainstem, and other regions of the brain may also be involved. Usually, no contrast enhancement is seen. Patients present with several days of altered mental status in addition to more typical COVID-19 symptoms.24 Acute necrotizing hemorrhagic encephalopathy does not respond to corticosteroids,25,26 but response to plasma exchange and IV immunoglobulin (IVIg) has been described.27
Another cytokine release neurologic syndrome has been described with COVID-19 in which patients develop confusion, tremor, cerebellar ataxia, behavioral alterations, aphasia, pyramidal syndrome, coma, cranial nerve palsy, dysautonomia, and central hypothyroidism. This syndrome has responded to corticosteroid therapy or IVIg in some patients.28
Acute Disseminated Encephalomyelitis
Acute disseminated encephalomyelitis (ADEM) is a rare demyelinating disease; it is often postviral and is more common in children than adults. However, in patients with COVID-19, it has been mainly described in adults.29,30 Clinically, the presentation is heterogeneous, usually causing an encephalopathy and multifocal deficits. It has been described following a mild flulike illness31,32 and following severe COVID-19 requiring admission to the critical care unit.29,33,34 MRI typically demonstrates T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities in deep white matter and at the gray-white matter interface. Contrast enhancement is not always present; when seen, it may be punctate or ring-enhancing or have an open-ring pattern. Hemorrhagic changes with ADEM have been a striking observation in some patients.30 Virus has not been detected in the CSF of patients with COVID-19 who develop ADEM.30 ADEM in patients with COVID-19 generally responds to treatment with high-dose corticosteroids.35
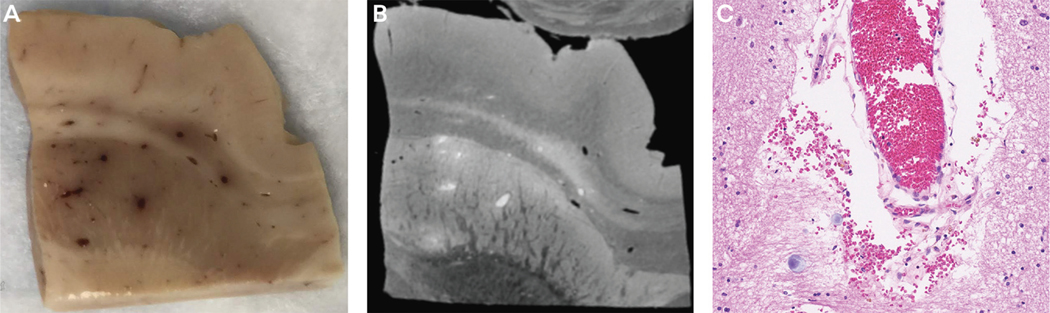
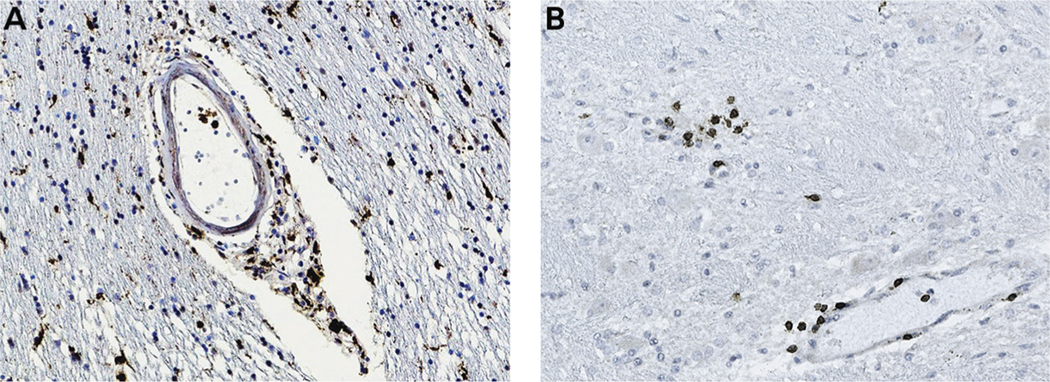
ADEM pathology is characterized by perivenular sleeves of demyelination associated with inflammatory infiltrates. Eventually, larger areas of demyelination may occur secondary to coalescence of perivenous demyelinating lesions. Postmortem neuropathologic findings in one patient with COVID-19 with ADEM revealed features suggestive of combined demyelinating and vascular mechanisms; hemorrhagic white matter lesions, aggregates of macrophages associated with axonal injury, and an ADEM-like appearance were present in the subcortical white matter.36 Congested blood vessels are a common finding in these patients, may give the appearance of punctate lesions on the MRI, and may represent perivascular leakage of red blood cells (FIGURE 11-1). Hemorrhagic transformation may occur with ADEM, which may complicate treatment with anticoagulants for coexisting hypercoagulable states. In autopsy of some patients, infiltration of macrophages and lymphocytes were also seen (FIGURE 11-2). These clinical and pathologic observations suggest that inflammatory syndromes are common in patients with COVID-19 and may respond to immunotherapies.
FIGURE 11-1. Microvascular disease in a patient with COVID-19. A, Autopsy tissue from the patient shows congested blood vessels in the cortical white matter. B, Postmortem MRI of the same tissue shows a hyperintense signal in the blood vessels. C, Microscopic examination shows blood vessels full of red blood cells in the lumen and periluminal region.
Figure courtesy of Rebecca Folkerth, MD (provided autopsy tissue), Govind Nair, PhD (performed MRI), and Myounghwa Lee, PhD (performed immunostaining).
FIGURE 11-2. Central nervous system inflammation in COVID-19. Autopsy brain tissue was stained with monoclonal antibodies to immune cellular markers. Diaminobenzidine was used as a chromogen, which gives a brown-colored precipitate. Panel A shows infiltration of macrophages staining for CD68 in the perivascular region and parenchyma, and panel B shows infiltration of CD3 T cells in foci around the blood vessels and the parenchyma.
Figure courtesy of Rebecca Folkerth, MD (provided autopsy tissue) and Myounghwa Lee, PhD (performed immunostaining).
Ondine’s Curse
Some patients report forgetting to breathe (unpublished observations), and many others have decreased oxygen saturation levels but are not breathless and have normal respiratory rates. Sudden death has also been reported with COVID-19, although this has been most often attributed to cardiac disease.37 These symptoms suggest the possibility of central hypoxia or Ondine’s curse.38 However, to date, objective evidence is lacking. Autopsy studies suggest that the pathology is particularly prominent in the olfactory system and the brainstem.22 The author’s own observations show the presence of brainstem lesions in patients with sudden death.39 Although concrete evidence for neuroinvasion by SARS-CoV-2 is lacking, it is intriguing to consider the possibility of viral brainstem invasion via transneuronal spread (as has been shown in mouse models with other coronaviruses), either via the olfactory system or the vagus nerve as it innervates the respiratory and gastrointestinal tracts.40
Stroke
Patients with COVID-19 develop a hypercoagulable syndrome causing both arterial and venous occlusions in the brain vasculature (TABLE 11-2). In a single-center case series of 219 hospitalized patients, 4.6% developed an ischemic stroke and 0.5% developed intracerebral hemorrhage.41 Ischemic stroke, hemorrhagic stroke, and cerebral venous sinus thrombosis have all been reported.42,43 Some patients may develop microhemorrhages44,45 and have other signs of microvascular injury.46 Spinal cord infarcts have also been reported.47,48
TABLE 11-2.
Strokes in Patients With COVID-19
|
Presentation ◆ Cerebral venous thrombosis ◆ Ischemic stroke with multiple arterial occlusions ◆ Microhemorrhages Pathophysiology ◆ Coagulopathy ◆ Antiphospholipid antibodies ◆ Cardiac embolism ◆ Endothelitis Risk factors ◆ Myocarditis ◆ Known vascular risk factors ◆ Acute respiratory distress syndrome ◆ Multiorgan impairment |
Cerebrovascular complications of COVID-19 are likely due to altered coagulation pathways, as demonstrated by observations of elevated D-dimer, increased prothrombin time, and activated partial thromboplastin time, and disseminated intravascular coagulation49; anticoagulants are frequently used in hospitalized patients with elevated D-dimer levels (CASE 11-2). The Virchow triad consists of endothelial cell injury, hypercoagulable state, and immobility. All three states are applicable to patients with COVID-19, putting them at risk for strokes. Older patients with preexisting cardiovascular risk factors are at risk of developing vascular occlusive syndromes, including ischemic strokes, deep vein thrombosis, and pulmonary embolism.50 Some patients may develop myocarditis, which may be an added risk factor for stroke.51,52 In severe cases that require extracorporeal membrane oxygenation (ECMO) support with continuous anticoagulation, intraparenchymal hemorrhage with a poor prognosis may occur.53
CASE 11-2.
A 60-year-old man with long-standing well-controlled hypertension presented to the emergency department with fever, loss of smell, and shortness of breath. Chest CT showed bilateral infiltrates in the lungs, and he tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) from a nasal swab. Over the next 12 hours, his respiratory status decreased further, requiring treatment with extracorporeal membrane oxygenation (ECMO). His D-dimer level was elevated to 620 ng/mL, and serum creatinine was 3.4 mg/dL. The patient was treated with heparin. However, the next day he developed Broca aphasia with right-sided hemiparesis. He underwent a thrombectomy of the left internal carotid and had good neurologic recovery.
COMMENT
Early in the pandemic, strokes were often missed in patients admitted to the critical care unit, since the focus was primarily on respiratory symptoms. As awareness of the hypercoagulable state induced by COVID-19 has increased, prophylactic anticoagulation is now provided to patients with highly elevated D-dimer levels. However, this carries the risk of hemorrhagic cerebral complications since patients with COVID-19 often have inflammation and microvascular disease in the brain with microhemorrhages. Hence, careful monitoring of these patients is necessary.
Strokes may also occur in individuals who have no risk factor other than COVID-19. Potential causes include interaction between the virus and ACE2 receptor on endothelial cells, including the cerebral vasculature and antiphospholipid antibodies.54 In patients with COVID-19 with acute stroke who are eligible for thrombolysis, some practitioners may prefer tenecteplase over alteplase given the rapidity of effect and shorter infusion time that minimizes the risk of health care workers’ exposure.
Neurodegenerative Diseases
Rare cases of acute parkinsonism and a single case of Creutzfeldt-Jakob disease have been reported in patients with COVID-19 infection.55,56 It remains unclear if this co-occurrence is coincidental or causative or whether the infection and associated inflammatory response led to precipitation or acceleration of a preexisting underlying neurodegenerative condition.
Myelitis
Several forms of myelitis have been described with COVID-19, and reports of transverse myelitis are the most common. These patients have involvement of at least three segments of the spinal cord that includes the gray and white matter, although not all patients had neuroimaging.57,58 In some patients, the clinical presentation was consistent with transverse myelitis, although the MRI was normal59; this has been reported in both adults and children.60 An isolated report described a longitudinally extensive acute necrotizing myelitis with an acute motor axonal neuropathy that responded to high-dose steroids and plasma exchange.61 A case of acute flaccid myelitis with MRI hyperintensity on T2-weighted images involving the ventral gray matter of the thoracic cord has been described, which occurred during the acute phase of the illness. This patient had a lymphocytic pleocytosis in the CSF, but the virus was not detected in the CSF by PCR.62
Guillain-Barré Syndrome
A report from Italy described five patients with Guillain-Barré syndrome (GBS) symptom onset 5 to 10 days after they first developed COVID-19 symptoms.63 The virus could not be detected in the CSF of any of the patients, and two did not have albuminocytologic dissociation in the CSF. Neurophysiologic features were consistent with the axonal variant in three of the patients and with the demyelinating variant in the other two. Two patients required mechanical ventilation, two had flaccid paraplegia, and one was able to walk independently. A subsequent review of 73 cases reported that the age range for GBS with COVID-19 was 11 to 94 years; 68.5% of cases were in male patients. Most patients had symptoms of COVID-19 with pulmonary involvement. The distribution of clinical variants resembled those of classic GBS. CSF albuminocytologic dissociation was present in 71% of cases, and CSF SARS-CoV-2 was absent by PCR in all patients tested. Seventy percent of cases had a good outcome with the use of IVIg.64 Several patients with the Miller Fisher variant of GBS with ophthalmoparesis have been described.65,66 Other patients with cranial neuropathies have also been reported.65 Such variants of GBS seem to be more common when associated with preceding COVID-19 infection.
Myositis
Myositis can occur at any time during the course of the illness and can be quite extensive, associated with myalgia and muscle weakness that can persist after recovery of other symptoms.67 It can involve the paraspinal muscles, causing patients to report back or chest pain.68 MRI may show evidence of myonecrosis, and inflammatory infiltrates have been described on histology, which responded to treatment with IV corticosteroids67; some have argued that these reports represent dermatomyositis.69 Occasionally, rhabdomyolysis may occur, increasing the risk for renal toxicity.70,71 Patients with rhabdomyolysis require careful monitoring and treatment with hydration. In severe cases of myositis, rhabdomyolysis may occur.
Long-Haul COVID or Post–acute COVID Syndrome
A distinct postviral syndrome that is independent of the severity of the acute phase of the illness is seen in some patients with COVID-19. This syndrome can emerge even in patients who have relatively mild symptoms during the acute phase. This syndrome has been termed long-haul COVID or long COVID,72 and its manifestations overlap with myalgic encephalomyelitis/chronic fatigue syndrome. Often these symptoms first manifest after the acute phase of the illness. These manifestations are 4 times more common in women and young adults. Long-haul COVID can be broadly divided into three clinical subtypes. The first subtype manifests predominantly with dysautonomia, which may include palpitations, tachycardia upon mild exercise or standing, hypotension or hypertension, gastroparesis, constipation or loose stools, and peripheral vasoconstriction (CASE 11-3). Some patients report low-grade fever, which is also thought to be due to autonomic dysfunction. Patients with the second subtype have extreme exercise intolerance, and those with the third subtype have cognitive dysfunction. In some patients, the cognitive dysfunction may be related to postural hypotension, as some have noticed that their ability to think and concentrate is better when they are lying down. Others may develop symptoms of distortion of time, short-term memory loss, and depression. Sleep disturbance is also a common symptom. Although some patients improve spontaneously over several weeks, it is anticipated that 10% to 30% of individuals may have persistent symptoms. A distinction needs to be made between patients with long-haul COVID and patients who were hospitalized, who often have a large number of lingering symptoms from respiratory disease, other organ damage, and prolonged hospitalization that may overlap with the syndrome described above.73 The full extent of this syndrome, including its prevalence and the duration of symptoms, remains unknown. More research is needed to understand the complete nature of the infection and its lasting effects. Because the majority of these patients had relatively mild symptoms during the acute phase of the illness and did not require hospitalization, it is possible that they may not have cleared the virus completely and may have a persistent or restricted viral replication that could be driving the syndrome. In support of this hypothesis, viral RNA but not viable virus has been recovered from seminal fluid, and sexual transmission of SARS-CoV-2 has not been documented.74 Similarly, viral RNA has been detected in tears, vomitus, and bile fluid, but its clinical significance has yet to be determined.75,76,77 Viral antigen has also been found in intestinal mucosa 3 months after infection.78
CASE 11-3.
A 35-year-old woman developed a sore throat, loss of smell, and pain in the supraorbital regions over a period of 2 days. This was followed by nausea, diarrhea, and a feeling of extreme fatigue. She did not develop any cough, dyspnea, or a drop in oxygen saturation levels. Polymerase chain reaction (PCR) by nasal swab was positive for SARS-CoV-2. These symptoms gradually improved over the next 5 days. However, at the same time, she developed orthostatic hypotension and became almost bedbound. She also had palpitations and decreased sweating but no new urinary symptoms. Over the next few days, she developed burning pain in the face, chest, and trunk, sparing the distal extremities.
Neurologic evaluation confirmed a drop in blood pressure with tachycardia upon standing for 2 minutes. The rest of the examination was normal. MRI brain, CSF analysis, EMG, and nerve conduction velocities were normal. Complete blood cell count and chemistry profile were normal. A course of treatment with IV immunoglobulin (IVIg) and high-dose corticosteroids with a prolonged taper showed only a mild improvement in symptoms.
COMMENT
This patient has a postural tachycardia syndrome (POTS)–like presentation following COVID-19 due to dysautonomia. The palpitations are a compensatory phenomenon caused by the drop in blood pressure from pooling of the blood in the abdomen, pelvis, and lower limbs. The use of β-adrenergic receptor blockers can make the symptoms worse. Increased fluid intake, abdominal binders, and tight stockings should be considered. Mineralocorticoids may also help. Her pain may represent a small fiber neuropathy. Many patients report a similar pain syndrome after COVID-19. It is currently unknown whether this is an immune-mediated phenomenon involving the nerves or the sensory ganglia.
Multisystem Inflammatory Syndrome in Children
It is being increasingly recognized that some children can develop systemic symptoms including neurologic manifestations about 2 to 3 weeks after recovery from the acute syndrome. These may manifest as an encephalopathy and generalized weakness and dysarthria and dysphagia. MRI may show restricted diffusion on diffusion-weighted imaging in the splenium of the corpus callosum. CSF is normal; however, blood may show signs of acute inflammation such as elevated CRP, D-dimer, and ferritin. The pathophysiology of this postviral syndrome is not entirely clear, but the patients show response to treatment with IVIg and corticosteroids.79
NEUROLOGIC COMPLICATIONS OF SARS-COV-2 VACCINES
Several different types of vaccines have been developed against the virus. Most of them are based on immunization against the spike protein of the virus delivered as DNA in a viral vector, mRNA, or protein. The first two mRNA-based vaccines to be approved have excellent safety profiles. However, several cases of Bell’s palsy and a few cases of anaphylaxislike reactions have been reported. Few cases of stroke, transverse myelitis, acute disseminated encephalomyelitis, GBS, vertigo, and unilateral facial paresthesia have also occurred.80 Currently, it is unclear whether these side effects are related to the mRNA vaccines or coincidental.
Another vaccine delivered in an adeno-associated virus vector resulted in transient myelopathic symptoms in two patients, and clinical trials were temporarily halted for this reason.81 The major difference between the mRNA and the adeno-associated virus vaccines is the sequence of the spike protein used. It remains unknown if some homology might exist between the spike protein and central nervous system antigens. If so, it would have to be a conformational-based homology since no sequence homology has been identified. Several cases of cerebral venous thrombosis and splanchnic vein thrombosis have been reported with the two vaccines that use the adeno-associated viral vector for delivery. These patients have a vaccine-induced thrombotic thrombocytopenia with antibodies to platelet factor 4. These patients should not be treated with heparin because heparin can induce such immune phenomena. IVIg and nonheparin anticoagulation should be used.
ETHICAL DILEMMA
The COVID-19 pandemic has brought to light numerous ethical issues involving neurologists and their patients. Notably, many of our patients may be unable to advocate for themselves because of neurologic disease and, as a result, could be denied rationed health care resources. For example, patients with COVID-19 and comorbid dementia could be unable to advocate for themselves and thus may be more likely to be deprived of scarce resources such as ventilator support.84 As neurologists, our role is certainly to continue to advocate for our patients, especially when they are at their most vulnerable.
CONCLUSION
Neurologic complications of SARS-CoV-2 infection can occur during the acute phase of the illness from multiorgan involvement presenting as an encephalopathy. These patients often have a prothrombotic state and can develop occlusion of multiple arteries and the venous system simultaneously. This can be further complicated with hemorrhagic lesions. Viral encephalitis is rare; however, some may develop immune-mediated syndromes such as ADEM, transverse myelitis, GBS, or myositis. Some patients are developing a constellation of chronic symptoms, termed long-haul COVID, that resembles myalgic encephalomyelitis/chronic fatigue syndrome. Even though most children develop mild symptoms from the infection, a multisystemic inflammatory syndrome that includes neurologic manifestations is being recognized. Early recognition and treatment are key to effective management of these patients.
KEY POINTS.
Coronaviruses are enveloped viruses with a positive-sense single-stranded RNA genome.
Although effective vaccines have been developed for COVID-19 and are being distributed, millions of people will have long-term complications from the infection, some of which will be neurologic.
Myalgia and fatigue are seen in about 50% of patients with COVID-19 and may persist even after recovery from the other symptoms. Headache occurs in 8% of patients.
Anosmia and ageusia may be heralding manifestations of COVID-19.
Mortality in COVID-19 is higher with advanced age and underlying comorbidities, including diabetes, cardiac and respiratory disorders, and immunosuppressed states.
The neurologic manifestations of COVID-19 can be broadly divided into two categories: those that occur during the acute phase of the infection (parainfectious complications) and the postviral manifestations that occur following the acute phase (post–acute phase complications).
Anosmia and ageusia are the most common early symptoms of COVID-19 infection. Nearly 40% to 60% of patients develop loss of smell, and, upon testing, nearly 90% have alterationof smell.
Encephalopathy is the most common neurologic manifestation in patients who are hospitalized with COVID-19, with nearly one-third of patients who are hospitalized developing encephalopathic symptoms ranging from alteration in consciousness to delirium and seizures.
Direct viral invasion of the brain in COVID-19 is rare.
Acute necrotizing hemorrhagic encephalopathy is a feared complication of several viruses, most notably influenza. It is thought to result from cytokine release syndrome rather than direct viral invasion of brain parenchyma, which is especially salient given the propensity of SARS-CoV-2 for causing similar cytokine storms in the lungs.
Acute disseminated encephalomyelitis is a rare demyelinating disease; it is often postviral and is more common in children than adults. However, in patients with COVID-19, it has been described mainly in adults.
Patients with COVID-19 develop a hypercoagulable syndrome causing both arterial and venous occlusions in the brain vasculature. Ischemic stroke, hemorrhagic stroke, and cerebral venous sinus thrombosis have all been reported.
Cerebrovascular complications of COVID-19 are likely due to altered coagulation pathways as demonstrated by observations of elevated D-dimer, increased prothrombin time and activated partial thromboplastin time, and disseminated intravascular coagulation.
Variants of Guillain-Barré syndrome seem to be more common when associated with preceding COVID-19 infection.
Myositis can occur at any time during the course of COVID-19; it can be quite extensive and associated with myalgia and muscle weakness that can persist after recovery of the other symptoms.
Long-haul COVID is a distinct postviral syndrome that is independent of the severity of the acute phase of the illness. This syndrome can emerge even in patients who have relatively mild symptoms during the acute phase.
A distinction needs to be made between patients with long-haul COVID and patients who were hospitalized, who often have a large number of lingering symptoms from respiratory disease, other organ damage, and prolonged hospitalization.
ACKNOWLEDGMENTS
Funding/Support:
This article was supported by intramural funding from the National Institute of Neurological Disorders and Stroke at the National Institutes of Health (NS03130).
Footnotes
Disclaimer: This article was written by Dr Avindra Nath in his personal capacity. The views expressed are his own and do not necessarily represent the views of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
USEFUL WEBSITES AND RESOURCES
NEUROLOGY PODCAST
This provides a discussion of the neurologic complications of COVID-19 and the various forms of COVID-19 vaccines.
http://neurology.libsyn.com/website/specialreport-looking-ahead-at-covid-in-2021-with-avinath-part-1
JOHNS HOPKINS UNIVERSITY & MEDICINE’S CORONAVIRUS RESOURCE CENTER GLOBAL MAP
This provides up-to-date information on the worldwide incidence of COVID-19. coronavirus.jhu.edu/map.html
US CENTERS FOR DISEASE CONTROL AND PREVENTION COVID-19 INFORMATION PAGE
This provides the epidemiology of COVID-19 in the United States and guidelines for prevention and treatment.
BRAIN INFECTIONS GLOBAL COVID-NEURO NETWORK
This provides a comprehensive collection of the published literature of neurologic manifestations of COVID-19 and tools for collection of data for epidemiological studies.
RELATIONSHIP DISCLOSURE: Dr Nath has served on the editorial board for Brain, as a section editor for Frontiers of Neurology, and as an associate editor for the Journal of Neurovirology and has received research grants from the National Institutes of Health (NS03130).
UNLABELED USE OF PRODUCTS/INVESTIGATIONAL USE DISCLOSURE:
Dr Nath reports no disclosure.
REFERENCES
- 1.Li Y, Li H, Fan R, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology 2016;59(3):163–169. doi: 10.1159/000453066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu Y, Gong EC, Zhang QY, et al. Expression of SARS-CoV in various types of cells in lung tissues [in Chinese]. Beijing Da Xue Xue Bao Yi Xue Ban 2005;37(5):453–457. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. COVID data tracker. Accessed April 28, 2021. covid.cdc.gov/covid-data-tracker/
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382(18):1708–1720.doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian S, Hu N, Lou J, et al. Characteristics of COVID-19 infection in Beijing. J Infect 2020;80(4): 401–406. doi: 10.1016/j.jinf.2020.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Q, Meng M, Kumar R, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis. Int J Infect Dis 2020;96:131–135. doi: 10.1016/j.ijid.2020.04.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized patients with COVID-19 in New York City. Neurology 2021;96:e575–e586. doi: 10.1212/WNL.0000000000010979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lechien JR, Chiesa-Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hornuss D, Lange B, Schröter N, et al. Anosmia in COVID-19 patients. Clin Microbiol Infect 2020; 26(10):1426–1427. doi: 10.1016/j.cmi.2020.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brann DH, Tsukahara T, Weinreb C, et al. Nonneuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv 2020;6(31). doi: 10.1126/sciadv.abc5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliezer M, Hamel AL, Houdart E, et al. Loss of smell in patients with COVID-19: MRI data reveal a transient edema of the olfactory clefts. Neurology 2020;95(23):e3145–e3152. doi: 10.1212/WNL.0000000000010806 [DOI] [PubMed] [Google Scholar]
- 12.Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulb edema during COVID-19-related anosmia. Neurology 2020; 95(5):224–225. doi: 10.1212/WNL.0000000000009850 [DOI] [PubMed] [Google Scholar]
- 13.Liotta EM, Batra A, Clark JR, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clin Transl Neurol 2020; 7(11):2221–2230. doi: 10.1002/acn3.51210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radmanesh A, Derman A, Lui YW, et al. COVID-19associated diffuse leukoencephalopathy and microhemorrhages. Radiology. 2020;297(1): E223–E227. doi: 10.1148/radiol.2020202040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Helms J, Kremer S, Merdji H, et al. Delirium and encephalopathy in severe COVID-19: a cohort analysis of ICU patients. Crit Care 2020;24(1):491. doi: 10.1186/s13054-020-03200-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARScoronavirus-2. Int J Infect Dis 2020;94:55–58. doi: 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YH, Jiang D, Huang JT. SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun 2020;87:149. doi: 10.1016/j.bbi.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Guennec L, Devianne J, JalinL, et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia 2020;61(8):e90–e94. doi: 10.1111/epi.16612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantonen J, Mahzabin S, Mäyränpää MI, et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol 2020;30(6):1012–1016. doi: 10.1111/bpa.12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid-19. N Engl J Med 2020;383(10):989–992. doi: 10.1056/NEJMc2019373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen MP, Le Quesne J, Officer-Jones L, et al. Neuropathological findings in two patients with fatal COVID-19. Neuropathol Appl Neurobiol 2020. doi: 10.1111/nan.12662 [DOI] [PubMed] [Google Scholar]
- 22.Matschke JL, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 2020;19(11):919–929. doi: 10.1016/S14744422(20)30308-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krett JD, Jewett GAE, Elton-Lacasse C, et al. Hemorrhagic encephalopathy associated with COVID-19. J Neuroimmunol 2020;346:577326. doi: 10.1016/j.jneuroim.2020.577326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poyiadji N, Shahin G, Noujaim D, et al. COVID-19associated acute hemorrhagic necrotizing encephalopathy: imaging features. Radiology 2020;296(2):E119–E120. doi: 10.1148/radiol.2020201187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixon L, Varley J, Gontsarova A, et al. COVID-19related acute necrotizing encephalopathy with brain stem involvement in a patient with aplastic anemia. Neurol Neuroimmunol Neuroinflamm 2020;7(5):e789. doi: 10.1212/NXI.0000000000000789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh R, Dubey S, Finsterer J, et al. SARS-CoV-2-associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44-year-old woman without comorbidities: a case report. Am J Case Rep 2020; 21:e925641.1-e925641.5. doi: 10.12659/AJCR.925641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virhammar J, Kumlien E, Fallmar D, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology 2020;95(10):445–449. doi: 10.1212/WNL.0000000000010250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perrin P, Collongues N, Baloglu S, et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur J Neurol 2021;28(1): 248258. doi: 10.1111/ene.14491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons T, Banks S, Bae C, et al. COVID-19associated acute disseminated encephalomyelitis (ADEM). J Neurol 2020;267(10): 2799–2802. doi: 10.1007/s00415-020-09951-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain 2020; 143(10):3104–3120. doi: 10.1093/brain/awaa240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang TR, Rodricks MB, Hirsh E. COVID-19associated acute disseminated encephalomyelitis: a case report. Med Rxiv 2020:1–7. doi: 10.1101/2020.04.16.20068148 [DOI] [Google Scholar]
- 32.Novi G, Rossi T, Pedemonte E, et al. Acute disseminated encephalomyelitis after SARSCoV-2 infection. Neurol Neuroimmunol Neuroinflamm 2020;7(5):e797. doi: 10.1212/NXI.0000000000000797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCuddy M, Kelkar P, Zhao Y, et al. Acute demyelinating encephalomyelitis (ADEM) in COVID-19 infection: a case series. Neurol India 2020;68(5):1192–1195. doi: 10.4103/0028-3886.299174 [DOI] [PubMed] [Google Scholar]
- 34.Lopes CCB, Brucki SMD, Passos Neto CEB, et al. Acute disseminated encephalomyelitis in COVID-19: presentation of two cases and review of the literature. Arq Neuropsiquiatr 2020;78(12): 805–810. doi: 10.1590/0004-282X20200186 [DOI] [PubMed] [Google Scholar]
- 35.Langley L, Zeicu C, Whitton L, Pauls M. Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. BMJ Case Rep 2020; 13(12):e239597. doi: 10.1136/bcr-2020-239597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reichard RR, Kashani KB, Boire NA, et al. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shirazi S, Mami S, Mohtadi N, et al. Sudden cardiac death in COVID-19 patients, a report of three cases. Future Cardiol 2020;17(1):113–118. doi: 10.2217/fca-2020-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orrego-Gonzalez E, Medina-Rincon GJ, Martinez-Gil S, Botero-Meneses JS. Ondine’s curse: the origin of the myth. Arq Neuropsiquiatr 2020;78(4):238–240. doi: 10.1590/0004-282X20190162 [DOI] [PubMed] [Google Scholar]
- 39.Lee M-H, Perl D, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med 2021;384(5):481–483. doi: 10.1056/NEJMc2033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dube M, Le Coupanec A, Wong AHM, et al. Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J Virol 2018;92(17):e00404-e00418. doi: 10.1128/JVI.00404-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y, Li M, Wang M, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol 2020;5(3):279–284. doi: 10.1136/svn-2020-000431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med 2020;383(25):2451–2460. doi: 10.1056/NEJMcp2009575 [DOI] [PubMed] [Google Scholar]
- 43.Dakay K, Cooper J, Bloomfield J, et al. Cerebral venous sinus thrombosis in COVID-19 infection: a case series and review of the literature. J Stroke Cerebrovasc Dis 2021;30(1):105434. doi: 10.1016/j.jstrokecerebrovasdis.2020.105434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannac O, Martinez-Almoyna L, Hraiech S. Critical illness-associated cerebral microbleeds in COVID-19 acute respiratory distress syndrome. Neurology 2020;95(11):498–499. doi: 10.1212/WNL.0000000000010537 [DOI] [PubMed] [Google Scholar]
- 45.Agarwal S, Jain R, Dogra S, et al. Cerebral microbleeds and leukoencephalopathy in critically ill patients with COVID-19. Stroke 2020; 51(9):2649–2655. doi: 10.1161/STROKEAHA.120.030940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conklin J, Frosch MP, Mukerji S, et al. Cerebral microvascular injury in severe COVID-19. medRxiv 2020. doi: 10.1101/2020.07.21.20159376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eissa M, Abdelhady M, Alqatami H, et al. Spinal cord infarction in a 41-year-old male patient with COVID-19 [published online January 22, 2021]. Neuradiol J. doi: 10.1177/1971400921988925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khedr EM, Karim AA, Soliman RK. Case report: acute spinal cord myelopathy in patients with COVID-19. Front Neurol 2020;11:610648. doi: 10.3389/fneur.2020.610648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost 2020;18(7):1559–1561. doi: 10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan 2005;14(3):113–119. [PubMed] [Google Scholar]
- 51.Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis. Herz 2020;45(3):230–232. doi: 10.1007/s00059-020-04909-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim IC, Kim JY, Kim HA, Han S. COVID-19-related myocarditisin a21-year-old female patient. Eur Heart J 2020:41(19):1859. doi: 10.1093/eurheartj/ehaa288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zahid MJ, Baig A, Galvez-Jimenez N, Martinez N. Hemorrhagic stroke in setting of severe COVID-19 infection requiring extracorporeal membrane oxygenation (ECMO). J Stroke Cerebrovasc Dis 2020;29(9):105016. doi: 10.1016/j.jstrokecerebrovasdis.2020.105016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo Y, Estes SK, Ali RA, et al. Prothrombotic autoantibodies in serum from patients hospitalized with COVID-19. Sci Transl Med 2020;12(570): eabd3876. doi: 10.1126/scitranslmed.abd3876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brundin P, Nath A, Beckham JD. Is COVID-19 a perfect storm for Parkinson’s disease? Trends Neurosci 2020;43(12):931–933. doi: 10.1016/j.tins.2020.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Young MJ, O’Hare M, Matiello M, Schmahmann JD. Creutzfeldt-Jakob disease in a man with COVID-19: SARS-CoV-2-accelerated neurodegeneration? Brain Behav Immun 2020;89:601–603. doi: 10.1016/j.bbi.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baghbanian SM, Namazi F. Post COVID-19 longitudinally extensive transverse myelitis (LETM)—a case report. Acta Neurol Belg 2020:1–2. doi: 10.1007/s13760-020-01497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munz M, Wessendorf S, Koretsis G, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol 2020;267(8):2196–2197. doi: 10.1007/s00415-020-09934-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zachariadis A, Tulbu A, Strambo D, et al. Transverse myelitis related to COVID-19 infection. J Neurol 2020;267(12):3459–3461. doi: 10.1007/s00415-020-09997-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaur H, Mason JA, Bajracharya M, et al. Transverse myelitis in a child with COVID-19. Pediatr Neurol 2020;112:5–6. doi: 10.1016/j.pediatrneurol.2020.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maideniuc C, Memon AB. Acute necrotizing myelitis and acute motor axonal neuropathy in a COVID-19 patient. J Neurol 2020:1–3. doi: 10.1007/s00415-020-10145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abdelhady M, Elsotouhy A, Vattoth S. Acute flaccid myelitis in COVID-19. BJR Case Rep 2020; 6(3):20200098. doi: 10.1259/bjrcr.20200098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med 2020;382(26): 2574–2576. doi: 10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abu-Rumeileh S, Abdelhak A, Foschi M, et al. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol 2021;268(4):1133–1170. doi: 10.1007/s00415-020-10124-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinkin M, Gao V, Kahan J, et al. COVID-19 presenting with ophthalmoparesis from cranial nerve palsy. Neurology 2020;95(5):221–223. doi: 10.1212/WNL.0000000000009700 [DOI] [PubMed] [Google Scholar]
- 66.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology 2020;95(5):e601–e605. doi: 10.1212/WNL.0000000000009619 [DOI] [PubMed] [Google Scholar]
- 67.Zhang H, Charmchi Z, Seidman RJ, et al. COVID19-associated myositis with severe proximal and bulbar weakness. Muscle Nerve 2020;62(3): E57–E60. doi: 10.1002/mus.27003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehan WA, Yoon BC, Lang M, et al. Paraspinal myositis in patients with COVID-19 infection. AJNR Am J Neuroradiol 2020;41(10):1949–1952. doi: 10.3174/ajnr.A6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tanboon J, Nishino I COVID-19-associated myositis may be dermatomyositis. Muscle Nerve 2021;63(1):E9–E10. doi: 10.1002/mus.27105 [DOI] [PubMed] [Google Scholar]
- 70.Jin M, Tong Q. Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis 2020;26(7):1618–1620. doi: 10.3201/eid2607.200445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh B, Kaur P, Mechineni A, Maroules M. Rhabdomyolysis in COVID-19: report of four cases. Cureus 2020;12(9):e10686. doi: 10.7759/cureus.10686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nath A Long-haul COVID. Neurology 2020;95(13): 559–560. doi: 10.1212/WNL.0000000000010640 [DOI] [PubMed] [Google Scholar]
- 73.Mandal S, Barnett J, Brill SE, et al. ‘Long-COVID’: a cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2020; thoraxjnl-2020–215818. doi: 10.1136/thoraxjnl-2020-215818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Massarotti C, Garolla A, Maccarini E, et al. SARS-CoV-2 in the semen: where does it come from? Andrology 2021;9:39–41. doi: 10.1111/andr.12839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Chan JF, Li KK, et al. Detection of SARS-CoV-2 in conjunctival secretions from patients without ocular symptoms. Infection 2021;49:257–265. doi: 10.1007/s15010-020-01524-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaya H, Caliskan A, Okul M, Sari T, Akbudak IH. Detection of SARS-CoV-2 in the tears and conjunctival secretions of Coronavirus disease 2019 patients. J Infect Dev Ctries 2020;14:977–981. doi: 10.3855/jidc.13224 [DOI] [PubMed] [Google Scholar]
- 77.Han D, Fang Q, Wang X. SARS-CoV-2 was found in the bile juice from a patient with severe COVID-19. J Med Virol 2021;93:102–104. doi: 10.1002/jmv.26169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gaebler C, Wang Z, Lorenzi JCC, et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021;591:639–644. doi: 10.1038/s41586-021-03207-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abdel-Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol 2020;77(11):1–6. doi: 10.1001/jamaneurol.2020.2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Ann Neurol 2021;89(5): 856–857. doi: 10.1002/ana.26065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mallapaty S, Ledford H. COVID-vaccine results are on the way - and scientists’ concerns are growing. Nature 2020;586(7827):16–17. doi: 10.1038/d41586-020-02706-6 [DOI] [PubMed] [Google Scholar]
- 82.See I, Su Jr, Lale A, et al. US case reports of cerebral venous sinus thrombosis with thrombocytopenia after Ad26.COV2.S vaccination, March 2 to April 21, 2021. JAMA 2021; 325(24):2448–2456. doi: 10.1001/jama.2021.7517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med 2021;(384): 2201–2211. doi: 10.1056/NEJMoa2105385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim SYH, Grady C. Ethics in the time of COVID: what remains the same and what is different. Neurology 2020;94(23):1007–1008. doi: 10.1212/WNL.0000000000009520 [DOI] [PMC free article] [PubMed] [Google Scholar]