Abstract
Feeding growing-finishing pigs supplemental fat is a common practice in the swine industry and can result in improved feed efficiency and reduced feed intake; however, dietary lipids also play a key role in determining pork quality. Objectives of the study were to evaluate the effects of feeding graded levels of high oleic soybean oil (HOSO) on loin and belly quality. A total of 288 pig raised in two separate blocks (144 pigs each) were assigned to one of four diets containing either 25% dried distiller’s grains with solubles (DDGS), 2% high oleic soybean oil (HOSO2), 4% high oleic soybean oil (HOSO4), or 6% high oleic soybean oil (HOSO6). Following the conclusion of the feeding trial, 144 pigs were slaughtered at the University of Illinois Meat Science Laboratory. Following fabrication, loins were collected for the evaluation of fresh quality measurements and color stability. Belly quality and fatty acid composition were evaluated using skin-on natural fall bellies. There were no differences (P ≥ 0.11) in pH, visual color, lightness (L*), drip loss, or WBSF among dietary treatments. However, visual marbling was increased (P ≤ 0.01) in loin chops from pigs fed HOSO4 and HOSO6 treatments compared with chops from pigs fed the DDGS dietary treatment. Additionally, loin chops were more red (a*) (P ≤ 0.01) from pigs fed HOSO diets when compared with pigs fed DDGS. Extractable lipid was decreased (P ≤ 0.01) in fresh loin chops from pigs fed DDGS and HOSO2 diets compared with pigs fed HOSO6. There were no differences (P ≥ 0.75) in trained sensory tenderness, juiciness, or flavor for loin chops from pigs fed different dietary treatments. Pork fatty acid composition was altered by dietary HOSO inclusion, with pigs fed DDGS having (P ≤ 0.01) the greatest concentration of C16:0 and was decreased with increasing levels of HOSO inclusion. Inversely, the percentage of C18:1n-9 was least (P ≤ 0.01) in pigs fed DDGS and increased with increasing levels of HOSO inclusion. Pigs fed DDGS produced wider (P ≤ 0.03) and thinner (P ≤ 0.04) bellies with reduced flop distance compared with pigs fed HOSO diets. Overall, HOSO diets did not negatively affect fresh loin quality or sensory traits of loin chops. Furthermore, feeding HOSO to swine resulted in bellies containing greater percentages of oleic acid and reduced percentages of palmitic and linoleic acid.
Keywords: belly quality, fatty acid, loin quality, high oleic soybean oil, pork
High oleic soybean oil can be fed as a supplemental dietary fat source to growing-finishing pigs with little impact on fresh loin quality and palatability. Feeding diets containing high oleic soybean oil resulted in carcasses with thicker bellies and increased flop distance compared with an industry-reference diet.
Introduction
Due to availability of dried distiller’s grains with solubles (DDGS) and growing consumers’ health concerns, feeding growing-finishing pigs supplemental unsaturated fats is a common practice in the swine industry (Thaler, 2002; Wood et al., 2004). However, dietary fats are also a key determinant of pork fat composition (Azain, 2001) and influence product quality. Increased levels of polyunsaturated fatty acids (PUFA) in pork fat resulted in greater incidences of oxidation-related off-flavors (Miller et al., 1990; Shackelford et al., 1990) or decreased shelf life (Wood et al., 2004), as well as belly and bacon processing challenges (Wahlstrom, Libal, and Berns, 1971; Ellis and McKeith, 1999; White et al., 2009; Xu et al., 2010). Although some have attempted to mitigate these challenges by including, in finishing diets, oil sources modified to contain increased monounsaturated fatty acids (MUFA) levels, many of these oils still contain high levels of linoleic acid, a PUFA, which may negatively affect pork fat quality and can reduce fat firmness (Ellis and Isbell, 1926; Wood and Enser, 1982).
High oleic soybean oil (HOSO) is a novel soy product with improved functional and nutritional characteristics due to its increased oleic acid (C18:1) content. Providing food manufacturers and foodservice operators an extended product shelf life and fry life due to its increased oxidative stability, HOSO also has potential applications as novel dietary fat source for swine diets. Whereas commodity soybean oil contains approximately 51% linoleic acid and 23% oleic acid (National Research Council [NRC], 2012), HOSO contains approximately 75% oleic acid and only approximately 7% linoleic acid (United Soybean Board, 2021). This increased oleic acid content sets HOSO apart from not only conventional soybean oil, but also other oil-containing ingredients such as DDGS which typically contains 4% to 10% corn oil with approximately 54% linoleic acid, 26% oleic acid, and 14% palmitic acid (Shurson, 2019). Therefore, HOSO contains a substantial increase in MUFA and reduced proportion of PUFA compared with other dietary lipids commonly used in swine diets. Given projected food service industry demand for HOSO, high oleic soybeans are expected to increase in availability, leading to new opportunities for use within the swine industry. However, in order for HOSO to be considered as an ingredient in growing-finishing diets, its effect on pork loins and bellies needs to be established.
Considering the well-documented potential for dietary fats to inhibit de novo lipogenesis and influence pork fatty acid composition (Allee et al., 1971; Azain, 2001), the primary objective of the study was to determine the effects of feeding HOSO to growing-finishing pigs on fresh belly and fat quality as well as loin quality. Furthermore, the study aimed to determine the optimal inclusion level of HOSO in growing-finishing pig diets to maximize growth potential and limit negative effects on loin and belly quality. Overall, it was hypothesized that feeding HOSO as a dietary substitute for DDGS would improve fresh belly and pork loin quality through improved fat quality.
Materials and Methods
All animal care and use procedures were approved by the Institutional Animal Care and Use Committee (Protocol # 18231) at the University of Illinois and followed standard practices described in the Guide for the Care and Use of Agricultural Animals in Research and Teaching (American Society of Animal Science [ASAS], 2020).
Dietary treatments
Four dietary treatments (Supplementary Table S1) were formulated, including an industry-typical reference diet containing 25% DDGS, plus treatments containing 2% high oleic soybean oil (HOSO2), 4% high oleic soybean oil (HOSO4), or 6% high oleic soybean oil (HOSO6). The reference diet was formulated with 25% DDGS as evidence suggests that feeding below 30% DDGS does not negatively impact growth rate or feed efficiency (Xu et al., 2010). The intent was to permit a general comparison between the HOSO2 diet and DDGS reference at similar oil inclusion, though the DDGS sourced for the study was analyzed to contain 5.6% extractable lipid (Gaffield et al., 2022). High oleic soybean oil and DDGS ingredients were analyzed by a commercial laboratory (Barrow-Agee; Memphis, TN) using the method outlined by Gaffield et al. (2022). The composition of DDGS and HOSO are available in Supplementary Table S2.
Multiple batches of feed for each of the three phases were sampled after mixing and then pooled within each feeding phase to create composite samples for analysis. All samples were stored at 0 °C prior to analyses (Supplementary Table S3). Composite samples of the reference and treatment diets for the three phases were analyzed for fatty acid profiles at the University of Illinois (Urbana, IL) using procedures outlined in Gaffield et al. (2022).
Animal housing and experimental design
A total of 288 pigs were raised in two separate blocks of equal size. For each block, pigs were housed in same-sex pens with four pigs per pen (36 pens/block). A total of nine pens per dietary treatment were represented in each block. The experimental design was a 2 × 4 factorial arrangement of sex and dietary treatments. Pigs were weighed and allocated to treatments by sex and body weight to minimize variation among pens at study initiation. The experimental design used in this study is described in detail by Gaffield et al. (2022).
The first block of pigs (PIC 1050, Line 02 × Line 03) had initial BW of 35.7 ± 4.51 kg. These pigs were housed in three separate barns containing fully slated floors. Each pen was 1.60 m × 3.96 m (1.58 m2/pig) and contained a double-door, dry-box feeder fastened to the side gate and one nipple drinker. In each barn, there were three replicates of each dietary treatment. For the second block, pigs (PIC 359 sires × PIC Camborough females) had an initial body weight of 25.1 ± 4.59 kg. These pigs were housed in a single barn containing all 36 pens with partially slatted flooring. Each pen was 1.83 m × 2.59 m (1.18 m2/pig) and contained a single-space, dry-box feeder, and a nipple drinker. For both blocks, temperature in barns was maintained using fan ventilation and controlled heaters using age-appropriate protocols.
Pigs were fed for 98 d using a three-phase feeding system. On day 98, the heaviest pig from each pen was selected resulting in 36 pigs from each block (72 total) being transported to the University of Illinois Meat Science Laboratory (Urbana, IL) for slaughter on day 99. The second heaviest pig was transported on day 100, which included 36 pigs from each block (72 total) and slaughtered on day 101 at the University of Illinois Meat Science Laboratory.
Carcass fabrication
The method outlined by Boler et al. (2011) was used to fabricate the left side of each carcass at 1 d postmortem to meet the specifications as described in the North American Meat Institute Meat Buyer’s Guide (North American Meat Institute [NAMI], 2014). The loins were separated into anterior and posterior portions between the 10th and 11th rib and were skinned and trimmed to the specifications of NAMP #410 bone-in loin. Both portions were used to determine the weight of the whole, skinless, bone-in loin. Each portion was then fabricated into a NAMP #414 Canadian back loin, NAMP #415A tenderloin, and NAMP #413D sirloin. The posterior portion of the Canadian back was used for loin quality analyses. The skin-on natural fall belly (NAMP #408) was fabricated to meet specifications and used for both fresh belly quality and fatty acid analyses.
Early postmortem chop quality evaluation
At 1 d postmortem, loins were re-faced at the longissimus thoracis (LTL) surface posterior to the 10th rib. The portion of muscle removed during re-facing was later used for the evaluation of drip loss through suspension method outlined by Boler et al. (2014). At the re-faced 10th-rib surface, consecutive 2.54-cm chops were hand cut for the evaluation of proximate composition (moisture and extractable lipid), cook loss, Warner–Bratzler shear force (WBSF), trained sensory panels, and initial and final thiobarbituric acid reactive substances (TBARS) evaluations. Quality measurements were also collected on the most anterior chop after resting at 4 °C for approximately 20 min after cutting to allow for oxygenation of myoglobin to occur. Instrumental CIE L* (lightness), a* (redness), and b* (yellowness) measurements (Commission Internationale de l’Eclairage [CIE], 1976) were collected using a Minolta CR-400 Chroma Meter (Minolta Camera Co., Ltd., Osaka, Japan) with a D65 illuminant, closed 8 mm aperture, 2° observer, and calibrated with a white tile specific to the machine. Ultimate pH was measured in geometric center of the chop using a glass electrode fitted to a MPI pH meter probe (MPI pH-Meter, Topeka, KS) in block 1 or with a Hanna Foodcare Portable pH Meter fitted with a Hanna electrode (Hanna 4198163 pH meter, −2.0 to 20.0 pH/ ± 2,000.0 mV; Hanna FC2323 meat specific electrode) in block 2. Both pH meters were calibrated using pH 4 and pH 7 buffer at 4 °C. Visual color and marbling scores (National Pork Producers Council [NPPC], 1999), and subjective firmness scores (National Pork Producers Council [NPPC], 1991) were collected by the same technician on both blocks of pigs. After chop quality evaluations were complete, chops for proximate composition were trimmed of subcutaneous fat and secondary muscles, vacuum packaged, and aged for 13 d at 4 °C. Then, chops were stored at −2 °C until determination of moisture and extractable lipid. Chops used for WBSF and trained sensory panels were vacuum packaged and aged at 4 °C to 13 d postmortem. After day 13, chops were stored at −2 °C until shearing. Chops used to evaluate lipid oxidation were vacuum packaged and aged 12 ± 1 d at 4 °C. After aging, the chop used for initial lipid oxidation determination was homogenized and stored at −80 °C until evaluation. For evaluation of final lipid oxidation, one chop from each loin was individually packaged in polystyrene trays, overwrapped with polyvinylchloride film (oxygen transmission rate = 1,627.9 cc/m2/d; moisture vapor transmission rate = 170.5 g/m2/d) and placed into simulated retail display conditions for 9 d. Packages were arranged in a single layer on wire shelves lined with butcher paper. Two 122-cm-long 32-W fluorescent bulbs (Ecolux with Starcoat, 3000 K, General Electric, Boston, MA) were suspended approximately 38 cm above the packages. Temperature of the room was maintained at 4 °C. After 9 d of display, chops were homogenized and stored at −80 °C until evaluation.
Loin proximate composition
Loin chops were thawed at 25 °C and then homogenized in a Cuisinart (East Windsor, NJ) food processor. Duplicate 10 g samples from each loin chop were placed in a drying oven set at 110 °C for at least 24 h. Moisture and extractable lipid content were determined using the chloroform-methanol solvent method described by Novakofski et al. (1989).
Cook loss and WBSF
Pork chops were removed from the freezer at a minimum of 24 h prior to analysis to thaw at approximately 1 °C. Chops were individually weighed and then cooked on a Farberware Open Hearth grill (model 455N, Walter Kidde, Bronx, NY). Internal temperature was monitored during cooking through copper-constantan thermocouples (Type T, Omega Engineering, Stamford, CT) placed in the geometric center of each chop. The thermocouples were connected to a digital data logger (Omega HH378, Stamford, CT). Chops were cooked to an internal temperature of 35 °C, flipped, and then cooked until they reached an internal temperature of 70 °C. Chops were then removed and allowed to cool to approximately 25 °C. After properly cooling, chops were weighed to determine cook loss percentage. Five 1.25-cm-diameter cores were removed parallel to the orientation of the muscle fibers and sheared using a Texture Analyzer TA.HD Plus (Texture Technologies Corp., Scarsdale, NY/Stable Mirosystems, Godalming, UK) with a blade speed of 3.33 mm/s and a load cell capacity of 100 kg. The shear force value for the five cores were averaged and the average was reported as WBSF.
Trained sensory panels
Trained sensory panels consisted of six individuals evaluating cooked pork chop tenderness, juiciness, and pork flavor. Panelists were selected from personnel trained using the Sensory Guidelines from the American Meat Science Association (2016) from the University of Illinois Meat Science Laboratory (Urbana, IL). For each panel, panelists were seated at breadbox-style stations with red light to mask color differences. Panelists were provided water and unsalted crackers to cleanse the palate between samples. Panelists evaluated tenderness, juiciness, and flavor, using a 15-cm line scale anchored at 7.5 cm where 0 = extremely tough, extremely dry, no flavor and 15 = extremely tender, extremely juicy, and very intense flavor.
Chops were assigned to sensory panel sessions using an incomplete randomized block schedule of chops for each sensory panel. Two sensory sessions occurred per day with a minimum of an hour between and each session contained four samples representing one from each dietary treatment.
Chops were thawed at approximately 1 °C for a minimum of 24 h. Chops were cooked in the same manner as WBSF to an internal temperature of 70 °C. After cooking, chops were trimmed of subcutaneous fat, squared, and cut into 1-cm cubes using an acrylic guide. Two cubes from the chop were then randomly served to each of the six panelists. Data from panelists were averaged for statistical analyses.
Lipid oxidation
Lipid oxidation was measured using the TBARS assay of Leick et al. (2010) using chops to represent initial and final oxidation during simulated retail display. Duplicate 5-g samples from each chop were combined with 1 mL of 0.2 mg/mL butylated hydroxytoluene and 45.5-mL 10% trichloroacetic acid in 0.2 M phosphoric acid. This mixture was blended for 30 s (Waring Products, Torrington, CT) and filtered through Whatman No. 1 filter paper into flasks. Two separate 5-mL samples of filtrate were inserted into 15-mL conical tubes. In one of the conical tubes, 5 mL of 0.02 M thiobarbituric acid was added, while in the other conical tube, 5 mL of deionized water was added. Additionally, two loin chop samples were randomly selected, from both 0 and 9 d, to act as spike samples. Spike samples were used to estimate percent recovery. Spike samples were prepared in the same manner, except the addition of 12 mL of 10 µM 1, 1, 3, 3-tetramethoxypropane in substitution for 10% trichloroacetic acid in 0.2 M phosphoric acid. Additionally, a standard curve was prepared. This curve represented 0, 1.25, 2.5, 5, and 7.5 mg malondialdehyde/mL using 25 µM 1, 1, 3, 3-tetramethoxypropane. A portion of the 25 µM 1,1,3,3-tetramethoxypropane was combined with 5 mL of 0.02 M thiobarbituric acid and volumized to 10 mL by 10% trichloroacetic acid in 0.2 M phosphoric acid solution. Each tube was sealed, inverted, and then incubated in the dark for 16 h at approximately 23 °C. Next, 150 µL from each tube was transferred to a 96-well round bottom plate and absorbance at 530 nm was measured with a Synergy HT Multi-Detection Microplate Reader (BioTek Instruments, Inc., Winooski, VT). TBARS were reported as mg MDA/g wet tissue.
Fresh belly characteristics
Procedures outlined by Kyle et al. (2014) were used in the determination of fresh belly characteristics. A sample for fatty acid profile analyses which contained all 3 layers of adipose tissue and was free of lean tissue was removed from the dorsal edge of the anterior end of each belly. Bellies were measured at the midpoint of the latitudinal axis for the evaluation of length and at the midpoint of the longitudinal axis for the evaluation of width. Fresh belly thickness was measured at eight individual locations along the belly. Thickness was evaluated by inserting a probe through the lean side of each belly. Measurements 1 to 4 were collected at the midpoint between the latitudinal axis and the dorsal edge at 20%, 40%, 60%, and 80% of the length of the belly, respectively, starting at the anterior end. Measurements 5 to 8 were collected at the midpoint between the longitudinal axis and the ventral edge at 20%, 40%, 60%, and 80% of the length of the belly, respectively, starting at the anterior end. Additionally, flop distance was measured by placing the bellies, skin side down, over a bar. The distance was then measured between the inside edges of the bellies.
Fatty acid profile
Adipose tissue samples, taken from the dorsal edge of the anterior end of each belly, were used to prepare fatty acid methyl esters (FAME) using the procedure outlined by Lepage and Roy (1986). Samples were analyzed in duplicate and followed standardized procedures. The long-chain fatty acids (LCFA) were analyzed using Hewlett-Packard 5890 Series II and Hewlett-Packard 6890 gas chromatography equipment. A glass column (Supelco SP-2560, 100 M × 0.25 mm × 0.2 µm film) was used in each chromatographer. The oven temperature, detector temperature, and injector temperature were 240 °C, 245 °C, and 240 °C, respectively. The concentrations of LCFA were calculated as the LCFA content of substrate-containing tubes minus the LCFA content of blank tubes divided by substrate weight expressed on a dry matter basis. Values were corrected for differences in total FA content of each sample by expressing them as g LCFA per 100 g of FAME. Iodine values were calculated using industry standard equations as outlined by the AOCS (2009) and Meadus et al. (2010).
Statistical analysis
Data were analyzed using the MIXED procedure of SAS (SAS Inst. In., Cary, NC) as a 2 × 4 factorial arrangement of treatments. Pig (N = 144) served as the experimental unit for all fixed variables. Fixed effects were diet, sex, and the interaction between diet and sex. Block, barn nested within block, and kill day served as random effects. Additionally, for trained sensory panels, session number served as a random effect. Effect of diet, sex, and the interaction between diet and sex was considered significant at P < 0.05. Least squares means were separated using the PDIFF statement in the MIXED procedure of SAS. Normality of residuals was tested using the UNIVARIATE procedure of SAS. Homogeneity of variances was tested using the Levene’s hovtest option in the GLM procedure of SAS.
Results
Fatty acid analysis
Total saturated fatty acid content was different (P ≤ 0.01) among all treatments with DDGS having the greatest concentration (33.5%) and SFA content decreasing with increasing levels of HOSO inclusion in pig diets (Table 1). Following the same pattern as the total SFA content, both C16:0 and C18:0 differed (P ≤ 0.01) between all treatments with DDGS-fed pigs having the greatest concentrations and HOSO6-fed pigs having the least. The percentage of C14:0 was decreased (P ≤ 0.01) in pigs fed HOSO6 compared with pigs fed DDGS or other HOSO diets. The percentage of C20:0 was increased (P ≤ 0.01) for pigs fed DDGS and HOSO2 diets, with pigs fed HOSO4 intermediate, and HOSO6 having the smallest concentration. Concentrations of C8:0, C10:0, C12:0, C15:0, C19:0, C21:0, C22:0, and C24:0 were each less than 0.1% of total fatty acids across all treatments and C17:0 comprised less than 1% of total fatty acids for all treatments (Supplementary Table S4).
Table 1.
Main effects of diet and sex on fatty acid profile (g/100 g FAME) of belly adipose tissue from barrows and gilts1
| Item3 | Dietary treatment2 | SEM | Sex | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DDGS | HOSO2 | HOSO4 | HOSO6 | Barrow | Gilt | Diet | Sex | Diet × Sex | |||
| Pens, n | 18 | 18 | 18 | 18 | 36 | 36 | |||||
| SFA | |||||||||||
| C14:0 | 1.21a | 1.23a | 1.18a | 1.07b | 0.129 | 1.20 | 1.14 | 0.128 | <0.001 | 0.04 | 0.66 |
| C16:0 | 21.83a | 20.94b | 19.58c | 17.66d | 1.157 | 20.45 | 19.55 | 1.149 | <0.001 | <0.001 | 0.41 |
| C18:0 | 9.44a | 8.58b | 7.69c | 6.35d | 0.264 | 8.18 | 7.85 | 0.205 | <0.001 | 0.16 | 0.64 |
| C20:0 | 0.20a | 0.20a | 0.18b | 0.17c | 0.017 | 0.19 | 0.18 | 0.017 | <0.001 | <0.001 | 0.34 |
| Total SFA4 | 33.47a | 31.70b | 29.36c | 25.98d | 1.469 | 30.78 | 29.47 | 1.443 | <0.001 | <0.01 | 0.65 |
| MUFA | |||||||||||
| C16:1 | 2.68a | 2.58ab | 2.42bc | 2.31c | 0.176 | 2.49 | 2.50 | 0.172 | <0.001 | 0.91 | 0.34 |
| C18:1n-9 (Oleic) | 41.31d | 47.60c | 50.68b | 54.40a | 0.909 | 48.93 | 48.06 | 0.817 | <0.001 | 0.13 | 0.91 |
| Total MUFA5 | 48.17d | 54.19c | 57.57b | 61.14a | 0.391 | 55.41 | 55.12 | 0.303 | <0.001 | 0.41 | 0.86 |
| PUFA | |||||||||||
| C18:2n-6 | 16.12a | 12.03b | 10.98c | 10.62c | 1.039 | 11.74 | 13.13 | 1.030 | <0.001 | <0.001 | 0.10 |
| C18:3n-6 | 0.04a | 0.03b | 0.03c | 0.03c | 0.002 | 0.03 | 0.03 | 0.002 | <0.001 | <0.001 | 0.01 |
| C18:3n-3 | 0.87d | 0.99c | 1.09b | 1.29a | 0.197 | 1.00 | 1.12 | 0.196 | <0.001 | <0.001 | <0.01 |
| C20:2n-6 | 0.76a | 0.52b | 0.45c | 0.44c | 0.033 | 0.53 | 0.56 | 0.032 | <0.001 | 0.02 | 0.27 |
| C20:3n-6 | 0.12a | 0.09b | 0.08c | 0.08c | 0.003 | 0.09 | 0.10 | 0.002 | <0.001 | <0.001 | 0.62 |
| C20:3n-3 | 0.14c | 0.15bc | 0.15b | 0.17a | 0.009 | 0.16 | 0.15 | 0.009 | <0.001 | 0.77 | 0.70 |
| C20:4n-6 | 0.24a | 0.21b | 0.19c | 0.17c | 0.008 | 0.19 | 0.22 | 0.007 | <0.001 | <0.001 | 0.05 |
| Total PUFA6 | 18.37a | 14.11b | 13.05c | 12.88c | 1.288 | 13.80 | 15.40 | 1.278 | <0.001 | <0.001 | 0.09 |
| Calculations | |||||||||||
| UFA:SFA7 | 2.01d | 2.19c | 2.43b | 2.88a | 0.181 | 2.31 | 2.45 | 0.179 | <0.001 | <0.001 | 0.64 |
| PUFA:SFA8 | 0.56a | 0.46c | 0.45c | 0.51b | 0.069 | 0.45 | 0.53 | 0.069 | <0.001 | <0.001 | 0.32 |
| MUFA:PUFA9 | 2.68d | 3.93c | 4.46b | 4.83a | 0.328 | 4.19 | 3.76 | 0.325 | <0.001 | <0.001 | 0.03 |
Different superscript letters within the same row reflect dietary treatment differences (P ≤ 0.05).
DDGS, dried distillers’ grains with solubles; FAME, fatty acid methyl esters; HOSO2, high oleic soybean oil 2%; HOSO4, high oleic soybean oil 4%; HOSO6, high oleic soybean oil 6%
MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; UFA, unsaturated fatty acids.
Total SFA = [(C8:0) + (C10:0) + (C12:0) + (C14:0) + (C15:0) + (C16:0) + (C17:0) + (C18:0) + (C19:0) + (C20:0) + (C21:0) + (C22:0) + (C24:0)]; brackets indicate concentration.
Total MUFA = [(C14:1) + (C16:1) + (C18:1trans-9) + (C18:1n-9) + (C18:1n-7) + (C19:1) + (C20:1) + (C21:1)]; brackets indicate concentration.
Total PUFA = [(C18:2n-6) + (C18:3n-6) + (C18:3n-3) + (C20:2n-6) + (C20:3n-6) + (C20:3n-3) + (C20:4n-6) + (C20:5n-3) + (C22:5n-3) + (C22:6n-3)]; brackets indicate concentration.
Unsaturated fatty acids (UFA):SFA = (total MUFA + total PUFA)/total SFA.
PUFA:SFA = total PUFA/total SFA.
MUFA:PUFA = total MUFA/total PUFA.
Total MUFA content was different (P ≤ 0.001) between all dietary treatments with pigs fed HOSO6 having the greatest MUFA concentration (61.1%) and DDGS-fed pigs (48.2%) having the least. Adipose tissue from DDGS-fed pigs had the least C18:1n-9 (P ≤ 0.01) and C18:1n-9 increased with increasing levels of HOSO inclusion (HOSO2—47.6%, HOSO4—50.7%, HOSO6—54.4%). The percentage of C16:1 in pigs fed DDGS was greater (P ≤ 0.01) than in pigs fed HOSO4 and HOSO6. Concentrations of C14:1, C18:1 trans-9, C19:1, C20:1n-9, C20:1n-7, and C21:1 were each less than 1% of total fatty acids for all treatments. For the percentage of C18:1trans-9, all dietary treatments differed (P ≤ 0.01) with pigs fed DDGS having the greatest percentage and pigs fed HOSO6 having the lowest percentage. Inversely, the percentage of C20:1n-9 was increased (P ≤ 0.01) for pigs fed HOSO6 compared with pigs fed DDGS. However, pigs fed HOSO2 and HOSO4 were intermediate (P > 0.05) in percentage of C20:1n-9 with pigs fed the DDGS and HOSO6 diets. Adipose tissue concentrations of C18:1n-7 did not differ (P = 0.92) among treatments.
The total PUFA concentration was greater (P ≤ 0.01) in fat from pigs fed the DDGS diet compared with pigs fed HOSO diets. However, between HOSO diets, fat from HOSO2-fed pigs contained a greater (P ≤ 0.01) concentration of total PUFA than pigs fed HOSO4 and HOSO6 diets. The PUFA present in the greatest concentration in pork fat followed this same pattern with adipose from pigs fed DDGS (16.1%) having greater (P ≤ 0.01) C18:2n-6 concentration compared with pigs fed HOSO diets. However, between HOSO diets, pigs fed HOSO2 (12.0%) contained a greater (P ≤ 0.01) concentration of C18:2n-6 than pigs fed HOSO4 (11.0%) and HOSO6 (10.6%) diets. The percentage of C18:3n-3 was different (P ≤ 0.01) between all dietary treatments, with fat from pigs fed DDGS having the least and C18:3n-3 concentration increasing with increased HOSO inclusion. The PUFA C20:2n-6, C20:3n-3, and C20:4n-6 followed similar patterns, with fat from pigs fed DDGS having greater (P ≤ 0.01) concentrations compared with pigs fed HOSO diets. Similarly, fat from pigs fed the smallest concentration of HOSO (2%) contained a greater (P ≤ 0.05) concentration of each PUFA compared with fat from pigs fed HOSO4 and HOSO6 diets. With the exception of C18:2n-6 and C18:3n-3, all PUFA displayed in Table 1 each comprised less than 0.1% of total fatty acid concentration for all treatments. There were significant interactions between dietary treatment and sex for γ-linolenic acid (C18:3n-6), α-linolenic acid (C18:3n-3), and arachidonic acid (C20:4n-6) concentrations; however, differences were minimal (data not shown).
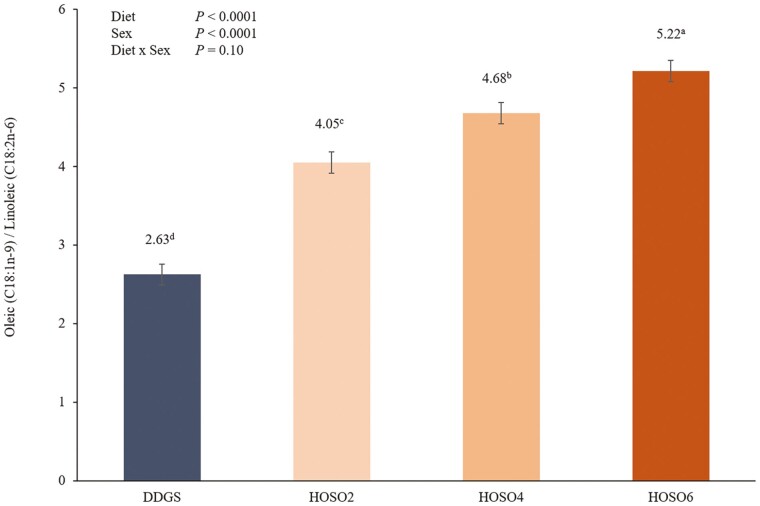
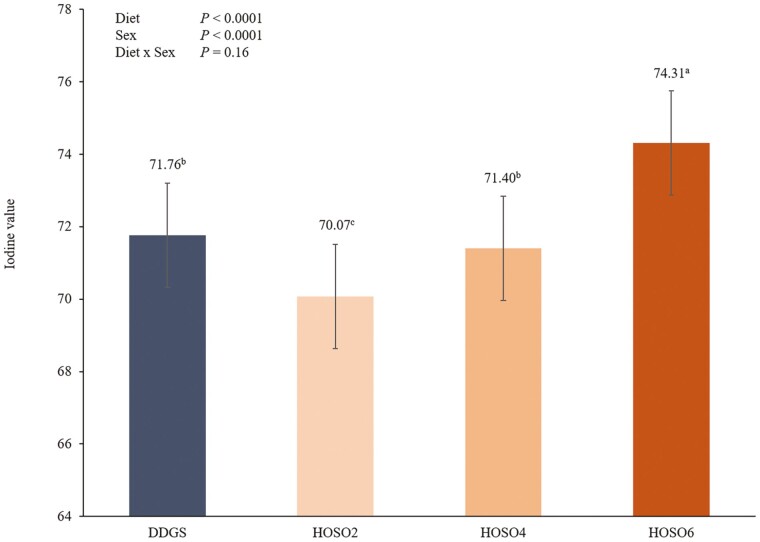
Overall, adipose tissue from pigs fed DDGS was more saturated than pigs fed the diets containing HOSO as indicated by decreased (P ≤ 0.01) UFA:SFA ratios. This change was likely the result of increased MUFA oleic acid concentration and decreased SFA palmitic and stearic acid concentrations in HOSO diets. However, fat from pigs fed the DDGS diet also had an increased (P ≤ 0.01) PUFA:SFA ratio compared with pigs fed the HOSO diets. Similarly, fat from pigs fed the DDGS diet also had a decreased (P ≤ 0.01; Figure 1) MUFA oleic acid:PUFA linoleic acid ratio compared with HOSO-fed pigs. This increased degree of unsaturation, however, was not reflected in differences in iodine value (IV; Figure 2). The IV of fat from pigs fed DDGS was similar to that of pigs fed the HOSO4 diet and reduced (P ≤ 0.01) compared with pigs fed the HOSO6 diet. Furthermore, pigs fed HOSO2 had a reduced (P ≤ 0.01) IV compared with pigs fed the other dietary treatments.
Figure 1.
Effect of dietary treatment on the ratio of oleic acid to linoleic acid in belly adipose tissue. Different superscript letters reflect dietary treatment differences (P ≤ 0.05). DDGS, dried distillers’ grains with solubles; HOSO2, high oleic soybean oil 2%; HOSO4, high oleic soybean oil 4%; HOSO6, high oleic soybean oil 6%.
Figure 2.
Effect of dietary treatment on AOCS iodine value (AOCS, 2009) of belly adipose tissue. Different superscript letters reflect dietary treatment differences (P ≤ 0.05). DDGS, dried distillers’ grains with solubles; HOSO2, high oleic soybean oil 2%; HOSO4, high oleic soybean oil 4%; HOSO6, high oleic soybean oil 6%.
Fresh belly and processed belly characteristics
Belly length did not differ (P = 0.78) among pigs fed the four different dietary treatments (Table 2). Bellies from pigs fed DDGS were wider (P ≤ 0.03) compared with pigs fed HOSO2 and HOSO4. However, belly width did not differ (P > 0.05) among pigs fed HOSO diets. Bellies from pigs fed DDGS were thinner (P ≤ 0.04) and had decreased flop distances (P ≤ 0.02) compared with pigs fed all HOSO diets, regardless of HOSO inclusion level.
Table 2.
Main effects of diet and sex on fresh belly and bacon processing characteristics1
| Item | Dietary treatment2 | Sex | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| DDGS | HOSO2 | HOSO4 | HOSO6 | Barrow | Gilt | Diet | Sex | Diet × Sex | |
| Pens, n | 18 | 18 | 18 | 18 | 36 | 36 | |||
| Length, cm | 70.81 | 70.33 | 70.80 | 70.49 | 71.75 | 69.47 | 0.78 | <0.001 | 0.94 |
| Width, cm | 27.84a | 26.20b | 26.72b | 26.97ab | 27.30 | 26.56 | 0.01 | 0.04 | 0.20 |
| Thickness,3 cm | 3.25b | 3.46a | 3.64a | 3.63a | 3.74 | 3.25 | <0.001 | <0.001 | 0.05 |
| Flop, cm | 16.14b | 22.45a | 23.01a | 20.18a | 22.91 | 17.98 | <0.001 | <0.001 | 0.10 |
| Initial weight,4 kg | 7.13b | 7.12b | 7.58a | 7.79a | 7.98 | 6.83 | <0.001 | <0.001 | 0.10 |
| Pump uptake, % | 13.23a | 12.14b | 11.56b | 12.02b | 11.67 | 12.81 | <0.01 | <0.001 | 0.19 |
| Cooked weight,4 kg | 7.27b | 7.22b | 7.72a | 7.96a | 8.12 | 6.96 | <0.001 | <0.001 | 0.04 |
Different superscript letters within the same row reflect dietary treatment differences (P ≤ 0.05).
DDGS, dried distillers’ grains with solubles; HOSO2, high oleic soybean oil 2%; HOSO4, high oleic soybean oil 4%; HOSO6, high oleic soybean oil 6%.
Thickness was an average of measurements from eight locations from the anterior to posterior, with four measurements on each of the dorsal and ventral edges, respectively.
Initial and cooked weight was taken with skin on. Skin, teatline, and bootjack were removed after processing.
Interactions between dietary treatment and sex were observed for both belly thickness (P = 0.05) and flop distance (P = 0.01). In gilts, belly thickness did not differ between dietary treatments (P ≥ 0.07). However, belly thickness was reduced in barrows fed DDGS compared with barrows fed HOSO diets (P < 0.01; data not shown). Belly flop distances were reduced in gilts fed DDGS diets compared to gilts fed HOSO4 diets (P < 0.01); however, flop distances of bellies from gilts fed HOSO2 and HOSO6 diets did not differ from either extreme (P ≥ 0.11). Differently, flop distance was reduced for barrows fed DDGS compared with barrows fed HOSO diets. As expected, barrows had longer, wider, and thicker bellies with greater flop distances (P ≤ 0.04) than gilts.
Pump uptake was increased (P ≤ 0.01) for pigs fed DDGS compared with pigs fed HOSO. Despite differences in pump uptake, cook yield did not differ (P = 0.11) among pigs fed DDGS or HOSO dietary treatments. Additionally, there were differences between sexes with barrows producing heavier (P ≤ 0.01) bellies, while greater pump uptake (P ≤ 0.04) occurred in gilt bellies. Initial belly weight (green weight) was reduced in pigs fed DDGS and HOSO2 compared with pigs fed HOSO4 and HOSO6 (P < 0.01).
Loin quality
There were no interactions (P ≥ 0.08) between dietary treatment and sex for any fresh quality measurement (Table 3). Ultimate pH did not differ (P = 0.82) between pigs fed DDGS and pigs fed HOSO diets. Visual color and firmness did not differ (P ≥ 0.11) between pigs fed any dietary treatment; however, visual marbling was increased (P ≤ 0.01) in loin chops from pigs fed HOSO4 and HOSO6 treatments compared with chops from pigs fed the DDGS dietary treatment. Additionally, extractable lipid was increased (P ≤ 0.01) in fresh loin chops from pigs fed HOSO6 compared with DDGS and HOSO2 diets. Despite differences in belly adipose tissue fatty acid composition, no differences (P ≥ 0.92) in initial or final lipid oxidation of loin chops were observed. For objective color measurements, lightness (L*) and yellowness (b*) did not differ (P ≥ 0.14) between loin chops from pigs fed DDGS compared with pigs fed HOSO. However, loin chops from pigs fed HOSO diets were more red (a*; P ≤ 0.01), regardless of inclusion level, when compared with DDGS-fed pigs. Drip loss did not differ (P = 0.50) between pigs fed different dietary treatments. In contrast, extractable moisture was increased (P ≤ 0.01) in fresh loin chops from pigs fed DDGS and HOSO2 compared with pigs fed HOSO6. Both cook loss and WBSF did not differ (P ≥ 0.32) among pigs fed the four different dietary treatments.
Table 3.
Main effects of diet and sex on loin chop quality traits1,2
| Item | Dietary treatment3 | SEM | Sex | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DDGS | HOSO2 | HOSO4 | HOSO6 | Barrow | Gilt | Diet | Sex | Diet × Sex | |||
| Pens, n | 18 | 18 | 18 | 18 | 36 | 36 | |||||
| pH | 5.61 | 5.62 | 5.63 | 5.62 | 0.06 | 5.65 | 5.59 | 0.06 | 0.83 | <0.01 | 0.52 |
| Drip loss, % | 4.04 | 3.86 | 4.16 | 3.48 | 0.33 | 3.81 | 3.95 | 0.24 | 0.50 | 0.67 | 0.40 |
| Color score4 | 3.5 | 3.5 | 3.7 | 3.6 | 0.33 | 3.6 | 3.6 | 0.33 | 0.11 | 0.67 | 0.59 |
| Marbling score4 | 1.7b | 1.9ab | 2.1a | 2.1a | 0.36 | 2.1 | 1.8 | 0.35 | 0.03 | <0.01 | 0.45 |
| Firmness score4 | 2.6 | 2.5 | 2.6 | 2.5 | 0.10 | 2.6 | 2.4 | 0.07 | 0.73 | 0.04 | 0.75 |
| Lightness5, L* | 51.13 | 51.31 | 50.05 | 51.66 | 0.77 | 51.47 | 50.61 | 0.63 | 0.30 | 0.17 | 0.23 |
| Redness5, a* | 7.41b | 8.17a | 8.68a | 8.46a | 0.72 | 8.27 | 8.08 | 0.71 | <0.001 | 0.37 | 0.91 |
| Yellowness5, b* | 4.71 | 5.10 | 4.98 | 5.51 | 0.66 | 5.23 | 4.91 | 0.63 | 0.14 | 0.19 | 0.09 |
| Extractable lipid, % | 2.40b | 2.56b | 2.76ab | 3.10a | 0.16 | 3.01 | 2.40 | 0.13 | <0.01 | <0.001 | 0.30 |
| Moisture, % | 74.16a | 73.97a | 73.84ab | 73.51b | 0.48 | 73.70 | 74.05 | 0.47 | <0.01 | <0.01 | 0.37 |
| WBSF6, kg | 3.03 | 3.02 | 3.24 | 3.05 | 0.10 | 2.95 | 3.22 | 0.07 | 0.32 | <0.01 | 0.41 |
| Cook loss, % | 23.07 | 23.31 | 22.89 | 22.18 | 0.60 | 22.50 | 23.22 | 0.45 | 0.53 | 0.21 | 0.08 |
| Initial TBARS7, mg MDA/g tissue | 0.07 | 0.04 | 0.06 | 0.06 | 0.09 | 0.03 | 0.09 | 0.09 | 0.97 | 0.07 | 0.25 |
| Final TBARS7, mg MDA/g tissue | 0.13 | 0.11 | 0.14 | 0.14 | 0.08 | 0.12 | 0.14 | 0.08 | 0.92 | 0.38 | 0.26 |
Different superscript letters within the same row reflect dietary treatment differences (P ≤ 0.05).
Early postmortem loin quality traits were evaluated at 1 d postmortem.
DDGS, dried distillers’ grains with solubles; HOSO2, high oleic soybean oil 2%; HOSO4, high oleic soybean oil 4%; HOSO6, high oleic soybean oil 6%; MDA, malondialdehyde; TBARS, thiobarbituric acid reactive substances; WBSF, Warner–Bratzler shear force.
NPPC subjective scoring system for color (NPPC, 1999), marbling (NPPC, 1999), and firmness (NPPC, 1991). Visual color was scored on a 1–6 scale in half units with 1 being the lightest. Visual marbling was scored on a 1–10 scale in half units with 1 being the least. Firmness was scored on a 1–5 scale in whole units with 1 being the least firm.
L* measures darkness to lightness (greater L* indicates a lighter color), a* measures redness (greater a* indicates a redder color), b* measures yellowness (greater b* indicates a more yellow color).
WBSF evaluated on chops cooked to 70 °C after 14 d aging.
Initial TBARS evaluated at day 1 of simulated retail display (day 12 postmortem). Final TBARS evaluated at day 9 of simulated retail display (day 21 postmortem).
Despite minimal differences between treatments, there were multiple differences between sexes for fresh chop quality. Ultimate pH was increased (P < 0.01) in barrows compared to gilts. Furthermore, barrows produced firmer, more marbled chops (P ≤ 0.04) compared with gilts. Additionally, loin chops from barrows had decreased (P ≤ 0.01) moisture but increased (P ≤ 0.01) extractable lipid compared to gilts. Finally, loin chops from gilts had increased WBSF values (P ≤ 0.01) compared to barrows.
Trained sensory panels
There were no interactions (P ≥ 0.17) between diet and sex for any of the palatability attributes measured by trained sensory panelists (Table 4). Additionally, there were no differences (P ≥ 0.75) in tenderness, juiciness, or flavor measured by trained panelist for loin chops from pigs fed the four different dietary treatments. There were also no differences (P ≥ 0.26) for tenderness, juiciness, or flavor between barrows and gilts.
Table 4.
Main effects of diet and sex on trained taste panel characteristics1
| Item | Dietary treatment2 | SEM | Sex | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DDGS | HOSO2 | HOSO4 | HOSO6 | Barrow | Gilt | Diet | Sex | Diet × Sex | |||
| Pens, n | 18 | 18 | 18 | 18 | 36 | 36 | |||||
| Tenderness3 | 8.55 | 8.42 | 8.47 | 8.43 | 0.17 | 8.54 | 8.40 | 0.14 | 0.86 | 0.26 | 0.52 |
| Juiceness3 | 8.27 | 8.32 | 8.32 | 8.24 | 0.30 | 8.24 | 8.33 | 0.28 | 0.96 | 0.50 | 0.17 |
| Flavor3 | 2.11 | 2.15 | 2.12 | 2.14 | 0.26 | 2.12 | 2.14 | 0.26 | 0.75 | 0.42 | 0.30 |
Different superscript letters within the same row reflect dietary treatment differences (P ≤ 0.05).
DDGS, dried distillers’ grains with solubles, HOSO2, high oleic soybean oil 2%, HOSO4, high oleic soybean oil 4%, HOSO6, high oleic soybean oil 6%.
Sensory scores with a greater value represent a greater degree of tenderness, juiciness, or flavor. Scores were recorded on a 15-point line scale.
Discussion
The main objective for the present study was to investigate the potential benefits of feeding HOSO to growing-finishing pigs due to the predicted increase in MUFA and decrease in PUFA content of pork fat. High oleic soybean oil was selected due to its high oleic acid content (75%) and low linoleic acid content (7%). This dietary fatty acid manipulation has the potential to reduce saturation of pork fat to meet consumer health concerns, while mitigating the negative impacts of high PUFA content on pork fat quality.
The three most prevalent fatty acids within pork fat in this study were palmitic acid (C16:0), oleic acid (C18:1n-9), and linoleic acid (C18:2n-6). Together, these fatty acids comprise between 79% and 83% of all fatty acids. Saturated fatty acids were hypothesized to decrease with the inclusion of higher HOSO levels due to the inhibition of de novo fatty acid synthesis, ultimately reducing deposition of palmitic acid in pork fat (Azain, 2001). As expected, the two SFA present in the greatest concentration in pork fat (C16:0 and C18:0) decreased as the inclusion level of HOSO increased. Reductions in both palmitic and stearic acid were also reported in past literature feeding high MUFA in pig diets (West and Myer, 1987; Rhee et al., 1988; Miller et al., 1990; Shackelford et al., 1990). Reductions in saturated fatty acids as a result of feeding increasing levels of HOSO may appeal to the health-conscious consumer segment. Considering United States, Canadian, and European national recommendations have urged citizens to decrease consumption of saturated fats (Mapiye et al., 2012), feeding HOSO to growing-finishing pigs has the potential to help consumers meet those goals. However, it is also important to recognize the potential ramifications of reduced saturated fatty acid concentrations in pork fat. Such challenges include bacon-manufacturing difficulties, oily product appearance, decreased shelf life, and greater potential for quality defects associated with lipid and protein oxidation (Xu et al., 2010).
In addition to changes in saturated fatty acids, it was hypothesized that MUFA would increase with graded HOSO inclusion as oleic acid represents over 75% of the total FA composition of HOSO. It was thought that by inhibiting de novo synthesis, the addition of HOSO should allow for greater oleic acid deposition in pork fat (Azain, 2001). As hypothesized, the percentage of oleic acid increased as higher inclusions of HOSO were fed to pigs. Therefore, feeding HOSO did influenced the fatty acid profile of the pork fat. This finding was important as historical studies have indicated manipulation of saturated fatty acids and MUFA in pork fat were less likely to occur due to pigs’ ability to synthesize both de novo (Wood and Enser, 1982; Navarro et al., 2021). However, the increase in pork fat oleic acid observed in the present study indicates successful manipulation of saturated fatty acid and MUFA composition through dietary supplementation. Furthermore, linoleic acid was expected to decrease with dietary HOSO inclusion vs feeding DDGS. This was expected due to the high proportion of linoleic acid (approximately 44%) in corn oil present in DDGS (Navarro et al., 2021). In comparison, HOSO is reported to have linoleic acid concentrations as low as 7%, therefore limiting the potential for C18:2n-6 transfer to pork fat through dietary supplementation. In line with this expectation, linoleic acid was reduced in pork fat when pigs were fed HOSO compared with DDGS. The manipulation of linoleic acid is important as it is considered a key determinant in pork fat firmness and bacon quality (Azain, 2001). It has also been largely associated with the alteration of pork flavor important when understanding bacon sensory characteristics (Navarro et al., 2021).
The Pork Composition and Quality Assessment Procedures (National Pork Producers Council (NPPC), 2000) suggested specific fatty acid concentrations for ‘high quality’ pork fat including less than 15% PUFA, more than 15% stearic acid, and less than 14% linoleic acid. However, even with conventional dietary ingredients like corn, these pork fat standards are often not met (Rentfrow et al., 2003). In the present study, fat from pigs fed the DDGS diet failed to meet these guidelines having contained, on average, 9% stearic acid, 18% PUFA and 16% linoleic acid. Thus, reduced flop distance in bellies from DDGS-fed pigs, a common measure of fat quality, was expected. However, dietary HOSO inclusion in pig diets did not result in fat quality that met this standard either. Fat from pigs fed HOSO at any inclusion level contained less than 10% stearic acid, though both PUFA and linoleic acid contents met these guidelines. This improvement in fat quality is reflected in the greater flop distances in bellies from pigs fed HOSO diets.
Iodine value, often used in industry as a primary indicator of fat and belly quality, is reported as a single measure. However, it is important to recognize IV is calculated from the percentages of various unsaturated fatty acids. In general, reduced IV is thought to result in firmer fat. In the present study, while fat from HOSO2-fed pigs had the lowest IV, interestingly fat from HOSO6-fed pigs had greater iodine values than fat from DDGS-fed pigs. These IV do not align, however, with belly flop or MUFA/PUFA concentrations. All HOSO treatments resulted in similar belly flop that was greater than that of the DDGS treatment. Further, feeding HOSO6 nearly doubled the ratio of oleic acid to linoleic acid in pork fat compared to DDGS. Therefore, although fat from DDGS-fed pigs resulted in similar IV to some HOSO treatments, belly fat of DDGS-fed pigs was softer. This discrepancy could potentially be attributed to the fact that saturated fatty acid concentrations are not included in IV calculations. Although the concentrations of unsaturated fatty acids, such as oleic acid and linoleic acid, are crucial for understanding pork fat quality, differences in the concentration of saturated fats relative to unsaturated fatty acids as well as MUFA relative to PUFA also greatly impacts fat firmness. Therefore, it is important that multiple belly and bacon quality traits are evaluated to fully characterize the impacts dietary treatments on fat quality.
While fat composition and firmness have many pork quality implications, bacon manufacturing is often cited as one important outcome influenced by fat quality (Xu et al., 2010). Feeding diets containing HOSO increased the thickness of fresh bellies, an important trait as many processors desire thicker bellies as these bellies are typically associated with increased processing yields and greater total profitability (Soladoye et al., 2015). However, improvements in thickness and flop distance of HOSO bellies did not translate into improvements in bacon processing yields. While pump uptake was improved in pigs fed the DDGS diet, cook yields were not different. This is contradictory to past literature that has reported no differences in pump uptake, yet an increasing cook yield when dietary fat was added to the diet (St. John et al., 1987; Shackelford et al., 1990).
As fresh bellies are comprised largely of adipose tissue, the potential for dietary treatment effects to influence belly and bacon processing is well recognized and extremely important. Nonetheless, fresh quality and color stability of lean cuts like the loin can also be influenced by fatty acid composition. The use of HOSO did not substantially change fresh loin quality parameters such as ultimate pH, visual color, subjective firmness, instrumental lightness, instrumental yellowness, WBSF, cook loss, or lipid oxidation. This general lack of differences is in alignment with past literature reporting few loin quality differences when feeding various dietary lipids (West and Myer, 1987; Rhee et al., 1988; Martin et al., 2008).
Despite the general lack of differences, the present study did observe a small increase in loin chop redness of pigs fed HOSO. Differences in redness have not been reported in past literature investigating feeding high levels of MUFA to growing-finishing pigs (West and Myer, 1987; Rhee et al., 1988; Martin et al., 2008). However, when put into the context of visually distinguishable changes in redness (a* differences must typically exceed 0.6 units to be visually detectable; Zhu and Brewer, 1999), differences in loin redness between diets are likely to observed by consumers. Marbling was also increased in chops from pigs fed increased levels of HOSO. This contradicts past literature when feeding unsaturated dietary fats with studies reporting either a decrease in marbling from pigs fed increasing levels of dietary fats (West and Myer, 1987; Miller et al., 1990; Myer et al., 1992) or no difference in marbling or intramuscular fat (Rhee et al., 1988; Martin et al., 2008). The increases in redness and marbling through the dietary inclusion of HOSO indicates a potential loin quality improvement. This is attributed to color typically being thought to have the largest influence over consumer purchasing decisions (Mancini and Hunt, 2005). Additionally, visual marbling has also proven to be one of the largest influencers for consumer purchasing decision for many countries (Levy and Hanna, 1994). Greater redness and marbling in loins from pigs fed HOSO may also be slightly more appealing in U.S. export markets than loins from conventionally fed pigs as Taiwanese, Japanese, and Korean consumers prefer darker, more highly marbled pork (Ngapo et al., 2007). Similar to the results for fresh quality characteristics, there were no differences between dietary treatments for tenderness, juiciness, or flavor as assessed by trained taste panelists similar to previous reports of feeding increased MUFA to growing-finishing pigs (West and Myer, 1987; Miller et al., 1990; Myer et al., 1992).
In conclusion, results from the present study demonstrate that HOSO-containing diets did not have adverse effects on loin quality including fresh quality, palatability, and discoloration and oxidation during display. Bellies from pigs fed the HOSO2 diet consistently produced thicker, firmer bellies that translated into bacon with similar processing yields when compared with pigs fed the DDGS diet. However, as HOSO inclusion levels were increased to 4% and 6%, pigs continued to produce thicker bellies with similar flop distances, despite increased IV. Overall, the study indicates positive implications for HOSO in the pork industry, specifically at a 2% inclusion level. However, more extensive research on bacon slicing yield and shelf life need to be conducted on a commercial-application scale to fully understand the impacts higher HOSO inclusion levels may have within the pork industry.
Supplementary Material
Glossary
Abbreviations
- DDGS
dried distiller’s grains with soluble
- FAME
fatty acid methyl esters
- GLM
generalized linear model
- HOSO
high oleic soybean oil
- HOSO2
2% dietary inclusion (as fed) rate of high oleic soybean oil
- HOSO4
4% dietary inclusion (as fed) rate of high oleic soybean oil
- HOSO6
6% dietary inclusion (as fed) rate of high oleic soybean oil
- IV
iodine value
- LCFA
long-chain fatty acid
- LTL
longissimus thoracis
- MUFA
monounsaturated fatty acid
- NAMP
North American Meat Processors
- PDIFF
probability of difference
- PUFA
polyunsaturated fatty acid
- TBARS
thiobarbituric acid reactive substances
- WBSF
Warner–Bratzler Shear Force
Contributor Information
Katelyn N Gaffield, Department of Animal Sciences, University of Illinois, Urbana–Champaign, IL, USA.
Dustin D Boler, Department of Animal Sciences, University of Illinois, Urbana–Champaign, IL, USA.
Ryan N Dilger, Department of Animal Sciences, University of Illinois, Urbana–Champaign, IL, USA.
Anna C Dilger, Department of Animal Sciences, University of Illinois, Urbana–Champaign, IL, USA.
Bailey N Harsh, Department of Animal Sciences, University of Illinois, Urbana–Champaign, IL, USA.
Funding
Financial support for this project was provided by the United Soybean Board (award USB 1930-362-0602-B).
Conflict of Interest Statement
The authors have no conflicts of interest.
Literature Cited
- Allee, G. L., Baker D. H., and Leveille G. A.. . 1971. Influence of level of dietary fat on adipose tissue lipogenesis and enzymatic activity in the pig. J. Anim. Sci. 33:1248–1254. doi: 10.2527/jas1971.3361248x [DOI] [PubMed] [Google Scholar]
- American Meat Science Association. 2016. Research guidelines for cookery, sensory evaluation, and instrumental tenderness measurements of meat. 4th ed. Champaign (IL): AMSA. [Google Scholar]
- American Society of Animal Science (ASAS). 2020. Guide for the care and use of agricultural animals in research and teaching. 4th ed. Champaign (IL): ASAS. [Google Scholar]
- AOCS. 2009. Official methods and recommended practices of the American oil chemist society. Champaign (IL): AOCS. [Google Scholar]
- Azain, M. 2001. Fat in swine nutrition. In: Lewis J. and Southern L., editors. Swine nutrition. Boca Raton (FL): CRC Press; p. 95–105. [Google Scholar]
- Boler, D. D., Kutzler L. W., Meeuwse D. M., King V. L., Campion D. R., McKeith F. K., and Killefer J.. . 2011. Effects of increasing lysine on carcass composition and cutting yields of immunologically castrated male pigs. J. Anim. Sci. 89:2189–2199. doi: 10.2527/jas.2010-3640 [DOI] [PubMed] [Google Scholar]
- Boler, D. D., Puls C. L., Clark D. L., Ellis M., Schroeder A. L., Matzat P. D., Killefer J., McKeith F. K., and Dilger A. C.. . 2014. Effects of immunological castration (Improvest) on changes in dressing percentage and carcass characteristics of finishing pigs. J. Anim. Sci. 92:359–368. doi: 10.2527/jas.2013-6863 [DOI] [PubMed] [Google Scholar]
- Commission Internationale de l’Eclairage (CIE). 1976. Colorimetry – Part 4: CIE 1976 L*a*b* colour space. Vienna (Austria): CIE Central Bureau. [Google Scholar]
- Ellis, N. R., and Isbell H. S.. . 1926. Soft pork studies: III. The effect of food fat upon body fat, as shown by the separation of the individual fatty acids of the body fat. J. Biol. Chem. 69:239–248. doi: 10.1016/S0021-9258(18)84609-9 [DOI] [Google Scholar]
- Ellis, M., and McKeith F.. . 1999. Nutritional influences on pork quality. In: Pork fact sheets. American Meat Science Association. p. 1–8. Champaign (IL): AMSA. [Google Scholar]
- Gaffield, K. N., Boler D. D., Dilger R. N., Dilger A. C., and Harsh B. N.. . 2022. Effects of feeding high oleic soybean oil to growing-finishing pigs on growth performance and carcass characteristics. J. Anim. Sci. 100:skac071. doi: 10.1093/jas/skac071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. John, L. C., Young C. R., Knabe D. A., Thompson L. D., Schelling G. T., Grundy S. M., and Smith S. B.. . 1987. Fatty acid profiles and sensory and carcass traits of tissues from steers and swine fed an elevated monounsaturated fat diet. J. Anim. Sci. 64:1441–1447. doi: 10.2527/jas1987.6451441x [DOI] [PubMed] [Google Scholar]
- Kyle, J. M., Bohrer B. M., Schroeder A. L., Matulis R. J., and Boler D. D.. . 2014. Effects of immunological castration (Improvest) on further processed belly characteristics and commercial bacon slicing yields of finishing pigs. J. Anim. Sci. 92:4223–4233. doi: 10.2527/jas.2014-7988 [DOI] [PubMed] [Google Scholar]
- Leick, C. M., Puls C. L., Ellis M., Killefer J., Carr T. R., Scramlin S. M., England M. B., Gaines A. M., Wolter B. F., Carr S. N., . et al. 2010. Effect of distillers dried grains with solubles and ractopamine (Paylean) on quality and shelf-life of fresh pork and bacon. J. Anim. Sci. 88:2751–2766. doi: 10.2527/jas.2009-2472 [DOI] [PubMed] [Google Scholar]
- Lepage, G., and Roy C. C.. . 1986. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 27:114–120. doi: 10.1016/S0022-2275(20)38861-1 [DOI] [PubMed] [Google Scholar]
- Levy, S., and Hanna M.. . 1994. Consumer quality audit summary. Des Moines (IA): National Pork Producers Council. [Google Scholar]
- Mancini, R. A., and Hunt M. C.. . 2005. Current research in meat color. Meat Sci. 71:100–121. doi: 10.1016/j.meatsci.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Mapiye, C., Aldai N., Turner T. D., Aalhus J. L., Rolland D. C., Kramer J. K. G., and Dugan M. E. R.. . 2012. The labile lipid fraction of meat: from perceived disease and waste to health and opportunity. Meat Sci. 92:210–220. doi: 10.1016/j.meatsci.2012.03.016 [DOI] [PubMed] [Google Scholar]
- Martin, D., Muriel E., Gonzalez E., Viguera J., and Ruiz J.. . 2008. Effect of dietary conjugated linoleic acid and monounsaturated fatty acids on productive, carcass and meat quality traits of pigs. Livest. Sci. 117:155–164. doi: 10.1016/j.livsci.2007.12.005 [DOI] [Google Scholar]
- Meadus, W. J., Duff P., Uttaro B., Aalhus J. L., Rolland D. C., Gibson L. L., and Dugan M. E. R.. . 2010. Production of docosahexaenoic acid (DHA) enriched bacon. J. Agric. Food Chem. 58:465–472. doi: 10.1021/jf9028078 [DOI] [PubMed] [Google Scholar]
- Miller, M. F., Shackelford S. D., Hayden K. D., and Reagan J. O.. . 1990. Determination of the alteration in fatty acid profiles, sensory characteristics and carcass traits of swine fed elevated levels of monounsaturated fats in the diet. J. Anim. Sci. 68:1624. doi: 10.2527/1990.6861624x [DOI] [PubMed] [Google Scholar]
- Myer, R. O., Johnson D. D., Knauft D. A., Gorbet D. W., Brendemuhl J. H., and Walker W. R.. . 1992. Effect of feeding high-oleic-acid peanuts to growing-finishing swine on resulting carcass fatty acid profile and on carcass and meat quality characteristics. J. Anim. Sci. 70:3734–3741. doi: 10.2527/1992.70123734x [DOI] [PubMed] [Google Scholar]
- National Pork Producers Council (NPPC). 1991. Procedures to evaluate market hogs. 3rd ed. Des Moines (IA): NPPC. [Google Scholar]
- National Pork Producers Council (NPPC). 1999. Official color and marbling standards. Des Moines (IA): NPPC. [Google Scholar]
- National Pork Producers Council (NPPC). 2000. Pork composition and quality assessment procedures. Des Moines (IA): NPPC. [Google Scholar]
- National Research Council (NRC). 2012. Nutrient Requirements of Swine. 11th rev. Washington (DC): National Academies Press. [Google Scholar]
- Navarro, M., Dunshea F. R., Lisle A., and Roura E.. . 2021. Feeding a high oleic acid (C18:1) diet improves pleasing flavor attributes in pork. Food Chem. 357:129770. doi: 10.1016/j.foodchem.2021.129770 [DOI] [PubMed] [Google Scholar]
- Ngapo, T. M., Martin J. F., and Dransfield E.. . 2007. International preferences for pork appearance: I. Consumer choices. Food Qual. Prefer 18:26–36. doi: 10.1016/j.foodqual.2005.07.001 [DOI] [Google Scholar]
- North American Meat Institute (NAMI). 2014. The meat buyer’s guide. 8th ed. Washington (DC): North American Meat Association. [Google Scholar]
- Novakofski, J., Park S., Bechtel P. J., and McKeith F. K.. . 1989. Composition of cooked pork chops: effect of removing subcutaneous fat before cooking. J. Food Sci. 54:15–17. doi: 10.1111/j.1365-2621.1989.tb08556.x [DOI] [Google Scholar]
- Rentfrow, G., Sauber T., Allee G., and Berg E.. . 2003. The influence of diets containing either conventional corn, conventional corn with choice white grease, high oil corn, or high oil high oleic corn on belly/bacon quality. Meat Sci. 64:459–466. doi: 10.1016/S0309-1740(02)00215-2 [DOI] [PubMed] [Google Scholar]
- Rhee, K. S., Ziprin Y. A., Ordonez G., and Bohac C. E.. . 1988. Fatty acid profiles of the total lipids and lipid oxidation in pork muscles as affected by canola oil in the animal diet and muscle location. Meat Sci. 23:201–210. doi: 10.1016/0309-1740(88)90034-4 [DOI] [PubMed] [Google Scholar]
- Shackelford, S. D., Miller M. F., Haydon K. D., Lovegren N. V., Lyon C. E., and Reagan J. O.. . 1990. Acceptability of bacon as influenced by the feeding of elevated levels of monounsaturated fats to growing-finishing swine. J. Food Sci. 55:621–624. doi: 10.1111/j.1365-2621.1990.tb05191.x [DOI] [PubMed] [Google Scholar]
- Shurson, J. 2019. Corn DDGS is a High-Value Feed Ingredient for Swine: Part 7. Minneapolis (MN): Univ. Minnesota Dep. Anim. Sci. https://blog-swine.extension.umn.edu/. Accessed May 2022. [Google Scholar]
- Soladoye, P. O., Shand P. J., Aalhus J. L., Gariépy C., and Juárez M.. . 2015. Review: pork belly quality, bacon properties and recent consumer trends. Can. J. Anim. Sci. 95:325–340. doi: 10.4141/cjas-2014-121 [DOI] [Google Scholar]
- Thaler, B. 2002. Use of distillers dried grains with solubles (DDGS) in swine diets. SDSU Ext. Extra Arch 60. Accessed May 2022. https://openprairie.sdstate.edu/extension_extra/60. [Google Scholar]
- United Soybean Board. 2021. Use Soy-Benefits. United Soybean Board. Accessed May 2022. https://soynewuses.org/benefits. [Google Scholar]
- Wahlstrom, R. C., Libal G. W., and Berns R. J.. . 1971. Effect of cooked soybeans on performance, fatty acid composition and pork carcass characteristics. J. Anim. Sci. 32:891–894. doi: 10.2527/jas1971.325891x [DOI] [PubMed] [Google Scholar]
- West, R. L., and Myer R. O.. . 1987. Carcass and meat quality characteristics and backfat fatty acid composition of swine as affected by the consumption of peanuts remaining in the field after harvest. J. Anim. Sci. 65:475–480. doi: 10.2527/jas1987.652475x [DOI] [PubMed] [Google Scholar]
- White, H. M., Richert B. T., Radcliffe J. S., Schinckel A. P., Burgess J. R., Koser S. L., Donkin S. S., and Latour M. A.. . 2009. Feeding conjugated linoleic acid partially recovers carcass quality in pigs fed dried corn distillers grains with solubles. J. Anim. Sci. 87:157–166. doi: 10.2527/jas.2007-0734 [DOI] [PubMed] [Google Scholar]
- Wood, J. D., and Enser M.. . 1982. Comparison of boars and castrates for bacon production 2. Composition of muscle and subcutaneous fat, and changes in side weight during curing. Anim. Sci. 35:65–74. doi: 10.1017/s0003356100000829 [DOI] [Google Scholar]
- Wood, J. D., Richardson R. I., Nute G. R., Fisher A. V., Campo M. M., Kasapidou E., Sheard P. R., and Enser M.. . 2004. Effects of fatty acids on meat quality: a review. Meat Sci. 66:21–32. doi: 10.1016/S0309-1740(03)00022-6 [DOI] [PubMed] [Google Scholar]
- Xu, G., Baidoo S. K., Johnston L. J., Bibus D., Cannon J. E., and Shurson G. C.. . 2010. Effects of feeding diets containing increasing content of corn distillers dried grains with solubles to grower-finisher pigs on growth performance, carcass composition, and pork fat quality. J. Anim. Sci. 88:1398–1410. doi: 10.2527/jas.2008-1404 [DOI] [PubMed] [Google Scholar]
- Zhu, L. G., and Brewer M. S.. . 1999. Relationship between instrumental and visual color in a raw, fresh beef and chicken model system. J. Musc. Foods 10:131–146. doi: 10.1111/j.1745-4573.1999.tb00391.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.